Abstract
Highly-active antiretroviral therapy (HAART) has lead to a dramatic decrease in the morbidity of patients infected with the human immunodeficiency virus (HIV). However, metabolic side effects, including lipodystrophy-associated (LD-associated) dyslipidemia, have been reported in patients treated with antiretroviral therapy. This study was designed to determine whether successful HAART was responsible for a dysregulation in the homeostasis of tumor necrosis factor- (TNF-), a cytokine involved in lipid metabolism. Cytokine production was assessed at the single cell level by flow cytometry after a short-term stimulation of peripheral blood T cells from HIV-infected (HIV+) patients who were followed during 18 months of HAART. A dramatic polarization to TNF- synthesis of both CD4 and CD8 T cells was observed in all patients. Because it was previously shown that TNF- synthesis by T cells was highly controlled by apoptosis, concomitant synthesis of TNF- and priming for apoptosis were also analyzed. The accumulation of T cells primed for TNF- synthesis is related to their escape from activation-induced apoptosis, partly due to the cosynthesis of interleukin-2 (IL-2) and TNF-. Interestingly, we observed that LD is associated with a more dramatic TNF- dysregulation, and positive correlations were found between the absolute number of TNF- CD8 T-cell precursors and lipid parameters usually altered in LD including cholesterol, triglycerides, and the atherogenic ratio apolipoprotein B (apoB)/apoA1. Observations from the study indicate that HAART dysregulates homeostasis of TNF- synthesis and suggest that this proinflammatory response induced by efficient antiretroviral therapy is a risk factor of LD development in HIV+ patients.
Highly-active antiretroviral therapy (HAART) combines 1 protease inhibitor (PI) and 2 nucleoside analogue reverse transcriptase inhibitors (bi-RTI). It is highly efficient at reducing plasma human immunodeficiency virus–1 (HIV-1) RNA to undetectable concentrations and increasing CD4 T-cell numbers. Recent studies of the immune system reconstitution during antiretroviral therapy have pointed out the complexity of the mechanisms involved. The increase in CD4 T cells after HAART could result from a combination of several mechanisms including the release of sequestered cells from lymphoid compartments to peripheral blood,1 increased peripheral cell survival,2-4 increased peripheral proliferation,5 and central renewal of lymphocytes.6,7 Apart from these changes in CD4 T-cell numbers, in vitro improvement in the proliferative response of T cells to recall antigens,8 partial restoration of the frequency of cytomegalovirus-specific CD4 memory T lymphocytes,9,10and evolution of the T-cell receptor repertoire11,12 have also been demonstrated, all of which suggest at least a partial restoration of the immune system in response to HAART. However, standard HAART regimens are not able to eradicate HIV-1 infection,13 and specific immunity to HIV-1 antigens is not preserved by antiretroviral therapy.10 As a result, prolonged intensive therapeutic regimens might be required to maintain suppression of viral replication.
Long-term HAART has been associated with a unique and unexpected syndrome consisting of metabolic abnormalities and body fat redistribution, called lipodystrophy (LD), which associates various levels of peripheral fat wasting in face and limbs; hyperlipidemia; insulin resistance; and central adiposity (accumulation of visceral fat, breast hypertrophy, and cervical fat-pads).14-16Development of this syndrome is linked to new potent antiretroviral regimens, and although it was first recognized in patients submitted to PI therapy,15-17 it was recently reported in patients receiving only RTIs.18-20 The pathogenesis of this syndrome, which may be linked to premature coronary artery disease recently reported in HAART-treated HIV-infected (HIV+) patients,21,22 remains unknown, although direct effects of antiretroviral drugs, such as PIs and RTIs, on lipid metabolism have been hypothesized.23,24 Proinflammatory cytokines, such as tumor necrosis factor–alpha (TNF–α), induce a number of metabolic alterations including hyperlipidemia and insulin resistance.25 26 At the single cell level we analyzed the influence of HAART on TNF-α synthesis in an 18-month longitudinal study and asked whether modifications in the frequency of TNF-α producers may contribute to alterations in lipid metabolism observed in patients with LD.
Patients, materials, and methods
Patients studied
We conducted a longitudinal 18-month follow-up study of 15 HIV+ patients naı̈ve of PI therapy. Peripheral blood samples were obtained the day before initiation of PI, at month 0 (M0), and then at regular intervals (M1, M2, M6, M9, M12, M15, and M18). Clinical and biological characteristics of these patients are given in Table 1. In parallel, a cross-sectional study was performed on HIV+ patients treated for at least 9 months with PI (n = 41). Of these patients, 17 presented with LD+, assessed according to a standardized clinical score (Table 2). Points were assigned to the following symptoms: peripheral facial loss (Bichat fat-pad lipoatrophy) (2 points), peripheral fat loss with skin-fold thickness less than 6 mm on arms or legs (1 point), prominent superficial veins on arms or legs (1 point), abdominal obesity with waist:hip ratio greater than 0.9 (2 points), cervical fat-pad enlargement (“buffalo hump”), or adipomasty (2 points).20 We also included 32 HIV-1+ bi-RTI persons receiving 2 RTIs but no PI therapy and 19 HIV− healthy blood donors (Centre National de Transfusion Sanguine, Paris, France). Informed consent was obtained from all the patients.
Baseline characteristics and evolution of PI− patients receiving HAART
| Patient . | Baseline values . | Antiretroviral regimen . | Follow-up, months . | VL after PI therapy, log10 RNA copies/mm3† . | CD4 change . | CD8 change . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD4, cells/mm3 . | CD8, cells/mm3 . | VL, log10 RNA copies/mm3* . | M6, cells/mm3 . | M15, cells/mm3 . | M6, cells/mm3 . | M15, cells/mm3 . | ||||
| 1 | 53 | 747 | 4.73 | ZDV, ddC, IDV | 18 | <2.30 | +251 | +275 | +1782 | +929 |
| 2 | 99 | 412 | 2.63 | D4T, ddI, IDV | 9 | 3.14 | +204 | ND | +439 | ND |
| 3 | 319 | 778 | 5.44 | ZDV, 3tC, IDV | 15 | <2.3 | +152 | +461 | −311 | +89 |
| 4 | 493 | 493 | 5.51 | ZDV, 3tC, IDV | 18 | <2.30 | +362 | +252 | −14 | −79 |
| 5 | 622 | 876 | 4.48 | ZDV, 3tC, IDV‡ | 18 | 2.98 | +99 | +240 | −358 | −410 |
| 6 | 159 | 935 | 5.06 | ZDV, 3tC, IDV | 9 | <2.30 | +180 | ND | −50 | ND |
| 7 | 210 | 955 | 4.99 | D4T, ddI, IDV | 15 | 3.78 | +83 | +140 | +120 | +567 |
| 8 | 210 | 724 | 3.94 | ZDV, 3tC, IDV | 18 | <2.30 | +74 | +100 | +50 | +215 |
| 9 | 230 | 2556 | 5.15 | ZDV, 3tC, IDV | 18 | <2.30 | +20 | +170 | −646 | −976 |
| 10 | 247 | 433 | 4.44 | D4T, 3tC, IDV | 15 | 3.40 | +95 | +108 | +102 | +201 |
| 11 | 267 | 954 | <2.30 | d4T, ddI, IDV‡ | 9 | <2.30 | +39 | ND | −450 | ND |
| 12 | 312 | 800 | 3.56 | D4T, 3tC, IDV | 18 | <2.30 | −32 | +102 | −412 | −481 |
| 13 | 380 | 1520 | 2.69 | ZDV, 3tC, IDV | 6 | <2.30 | +127 | ND | −675 | ND |
| 14 | 383 | 709 | <2.30 | ZDV, 3tC, IDV | 18 | <2.30 | +361 | +522 | −4 | −278 |
| 15 | 600 | 1622 | 5.24 | ZDV, ddC, IDV | 18 | <2.30 | +120 | +140 | −530 | −770 |
| Patient . | Baseline values . | Antiretroviral regimen . | Follow-up, months . | VL after PI therapy, log10 RNA copies/mm3† . | CD4 change . | CD8 change . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD4, cells/mm3 . | CD8, cells/mm3 . | VL, log10 RNA copies/mm3* . | M6, cells/mm3 . | M15, cells/mm3 . | M6, cells/mm3 . | M15, cells/mm3 . | ||||
| 1 | 53 | 747 | 4.73 | ZDV, ddC, IDV | 18 | <2.30 | +251 | +275 | +1782 | +929 |
| 2 | 99 | 412 | 2.63 | D4T, ddI, IDV | 9 | 3.14 | +204 | ND | +439 | ND |
| 3 | 319 | 778 | 5.44 | ZDV, 3tC, IDV | 15 | <2.3 | +152 | +461 | −311 | +89 |
| 4 | 493 | 493 | 5.51 | ZDV, 3tC, IDV | 18 | <2.30 | +362 | +252 | −14 | −79 |
| 5 | 622 | 876 | 4.48 | ZDV, 3tC, IDV‡ | 18 | 2.98 | +99 | +240 | −358 | −410 |
| 6 | 159 | 935 | 5.06 | ZDV, 3tC, IDV | 9 | <2.30 | +180 | ND | −50 | ND |
| 7 | 210 | 955 | 4.99 | D4T, ddI, IDV | 15 | 3.78 | +83 | +140 | +120 | +567 |
| 8 | 210 | 724 | 3.94 | ZDV, 3tC, IDV | 18 | <2.30 | +74 | +100 | +50 | +215 |
| 9 | 230 | 2556 | 5.15 | ZDV, 3tC, IDV | 18 | <2.30 | +20 | +170 | −646 | −976 |
| 10 | 247 | 433 | 4.44 | D4T, 3tC, IDV | 15 | 3.40 | +95 | +108 | +102 | +201 |
| 11 | 267 | 954 | <2.30 | d4T, ddI, IDV‡ | 9 | <2.30 | +39 | ND | −450 | ND |
| 12 | 312 | 800 | 3.56 | D4T, 3tC, IDV | 18 | <2.30 | −32 | +102 | −412 | −481 |
| 13 | 380 | 1520 | 2.69 | ZDV, 3tC, IDV | 6 | <2.30 | +127 | ND | −675 | ND |
| 14 | 383 | 709 | <2.30 | ZDV, 3tC, IDV | 18 | <2.30 | +361 | +522 | −4 | −278 |
| 15 | 600 | 1622 | 5.24 | ZDV, ddC, IDV | 18 | <2.30 | +120 | +140 | −530 | −770 |
ND indicates not determined; ZDV, zidovudine; ddC, zalcitabine; IDV, indinavir; d4T, stavudine; ddI, didanosine; and 3TC, lamivudine. Patients 1-5 were naı̈ve of previous antiretroviral therapy, and patients 6-15 had previously been treated by RTIs.
Amplicor HIV-1 Monitor test (Roche, Branchburg, NJ) was used for a threshold of detection of 200 copies/mm3.
Values are given for the last point of follow-up.
Therapy was changed from IDV to saquinavir (SQV) at M2.
Clinical parameters of bi-RTI, PI+LD−, and PI+LD+ patients
| Parameters . | Bi-RTI, n = 10 . | Pl+LD−, n = 24 . | PI+LD+, n = 17 . |
|---|---|---|---|
| Age | 35.5 ± 10.4 | 37.5 ± 5.7 | 44.2 ± 9.9* |
| Sex, M/F (ratio) | 10/0 | 20/4 | 14/3 |
| CD4 | |||
| N/mm3 | 467 ± 161 | 517 ± 234 | 471 ± 225 |
| Range | 248-774 | 290-990 | 284-925 |
| CD8 | |||
| n/mm3 | 955 ± 347 | 911 ± 498 | 1235 ± 501*,† |
| Range | 538-1572 | 341-2040 | 616-2620 |
| Viral load, log10 DNA copies/mm3‡ | 3.45 ± 0.80 | 2.67 ± 0.55† | 2.49 ± 0.38† |
| Length of PI therapy, months | 0.0 ± 0.0 | 13.5 ± 3.6 | 20.7 ± 8.3* |
| CD4 increase using PI, n/mm3 | — | 229 ± 175 | 386 ± 243 |
| CD8 increase using PI, n/mm3 | — | 12 ± 484 | 508 ± 623* |
| Parameters . | Bi-RTI, n = 10 . | Pl+LD−, n = 24 . | PI+LD+, n = 17 . |
|---|---|---|---|
| Age | 35.5 ± 10.4 | 37.5 ± 5.7 | 44.2 ± 9.9* |
| Sex, M/F (ratio) | 10/0 | 20/4 | 14/3 |
| CD4 | |||
| N/mm3 | 467 ± 161 | 517 ± 234 | 471 ± 225 |
| Range | 248-774 | 290-990 | 284-925 |
| CD8 | |||
| n/mm3 | 955 ± 347 | 911 ± 498 | 1235 ± 501*,† |
| Range | 538-1572 | 341-2040 | 616-2620 |
| Viral load, log10 DNA copies/mm3‡ | 3.45 ± 0.80 | 2.67 ± 0.55† | 2.49 ± 0.38† |
| Length of PI therapy, months | 0.0 ± 0.0 | 13.5 ± 3.6 | 20.7 ± 8.3* |
| CD4 increase using PI, n/mm3 | — | 229 ± 175 | 386 ± 243 |
| CD8 increase using PI, n/mm3 | — | 12 ± 484 | 508 ± 623* |
Bi-RTI patients received ZDV plus ddI or ZDV plus ddC for 3-6 months. PI+ patients received a combination of bi-RTI and 1 PI including ZDV, ddI, d4T, 3tC, IDV, RTV, or SQV. The only difference between LD+ vs LD− regimens concerned the treatment of LD+ patients, who received RTV more frequently and ZDV less frequently (P < .02 andP < .003, respectively, for the X2test). Values are given as the mean plus or minus SD.
P < .05 vs PI+LD− group.
P < .05 vs bi-RTI group.
Amplicor HIV-1 Monitor test (Roche) was used.
Monoclonal antibodies
The following monoclonal antibodies (mAbs) specific for human surface antigens were directly coupled either to fluorescein isothiocyanate (FITC) or peridinin chlorophyll protein (PerCP): anti-CD3 immunoglobulin G1κ (IgG1κ, clone SK7); anti-CD4 (IgG1κ, clone SK3); and anti-CD8 (IgG1κ, clone SK1) (Becton Dickinson, Pont de Claix, France). Control antibodies were IgG1κ or IgG2aκ FITC- or PerCP-conjugated (Becton Dickinson). For intracellular detection of cytokines, we used phycoerythrin-conjugated (PE-conjugated) or FITC-conjugated anti–TNF-α (clone Mab11) and PE-conjugated anti–IL-2 mAb (clone MQ1-17H12) (PharMingen, La Jolla, CA).
Cytokine production by flow cytometry
Peripheral blood mononuclear cells (PBMCs) from healthy donors or HIV+ patients were isolated from heparinized blood by centrifugation on a Ficoll-Hypaque density gradient (Pharmacia Biotech, Uppsala, Sweden). The cells were then resuspended in Roswell Park Memorial Institute–1640 medium (RPMI-1640) (Bio-Whittaker, Verviers, Belgium) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Institut Jacques Boy, Reims, France), 10 IU/mL penicillin, 10 mg/mL streptomycin, 20 mmol/L 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and 2 mmol/L L-glutamine. Next the cells were stimulated for 16 hours with 50 ng/mL PMA (Sigma Aldrich, France), 100 ng/mL PHA (Murex Diagnostic, Paris, France), and 300 ng/mL ionomycin (Sigma Aldrich). Brefeldin A (Sigma Aldrich) was added at the concentration of 10 μg/mL during the last 12 hours to inhibit cytokine release. Enumeration at the single cell level of cytokine-producing peripheral T cells was performed as previously described.27 Briefly, cells were stained externally with FITC-conjugated anti-CD3 or anti-CD8 mAbs, washed in phosphate-buffered serum–bovine serum albumin–sodium nitrogen (PBS-BSA-NaN3), and fixed in PBS-BSA-NaN3 containing 1% paraformaldehyde (PFA) for 15 minutes at 4°C. Intracellular cytokine staining was then performed with PE-conjugated anticytokine mAbs in saponin buffer (Sigma Aldrich) for 30 minutes at 4°C. Double-stained cells were washed in PBS-BSA-NaN3 and fixed with 1% PFA in PBS-BSA-NaN3. Immediate acquisition of 20 000 cells on a fluorescence-activated cell sorter (FACS) flow cytometer (FACScan, Becton Dickinson, San Jose, CA) was followed by analysis with CellQuest software (Becton Dickinson). Due to the down-regulation of the CD4 molecule following PMA stimulation, CD4+ T cells were determined as CD3+ CD8− cells.
Cytokine production by enzyme-linked immunosorbent assays
TNF-α and TNF-RII concentrations were determined by enzyme-linked immunosorbent assays (QUANTIKINE ELISA; R&D Systems, Minneapolis, MN) on plasma previously stored at −80°C. Thresholds of detection were 4 pg/mL for TNF-α and 1 pg/mL for TNF-RII.
Susceptibility to apoptosis of cytokine-producing T cells
The relationship between the propensity to undergo apoptosis, the synthesis of a given cytokine, and the membrane phenotyping was performed as previously described.27 28 Briefly, [PMA + PHA + ionomycin]-stimulated PBMCs were dual-stained with FITC-conjugated anti-CD3 or anti-CD8 mAbs and with 20 μg/mL nuclear dye 7-amino-actinomycin D (7-AAD) (Sigma-Aldrich). Cytokine staining was then performed as described above, and 20 000 events were immediately analyzed on a FACScan flow cytometer. Apoptotic cells were quantified among cytokine-producing cells according to their 7-AAD staining.
Lipid measurements
Total cholesterol and triglyceride plasmatic concentrations were determined in the fasted state by the usual enzymatic method (Kone Optima; Kone Instruments, Espoo, Finland). After precipitation of other lipid components with dextran sulfate–magnesium, high-density lipoprotein (HDL) cholesterol was measured enzymatically. Apolipoprotein A1 (apoA1) and apoB concentrations were measured by enzyme-linked immunonephelometric assay (BNA; Behring, Marburg, Germany). Calibration standards from the National Federation of French Clinical Biochemistry were used.
Statistical analyses
The impact of PI therapy on immunological parameters was evaluated with intention to treat. Nonparametric measures of associations were used including the Mann-Whitney U test, Wilcoxon signed rank test, and Spearman rank correlation. P < .05 was considered significant.
Results
Accumulation of TNF-–producing T cells during HAART
The HIV+ patients who participated in this study had CD4 T-lymphocyte counts of at least 50 cells per μL (mean CD4 T cells 306 ± 167 cells per μL) and plasmatic HIV RNA levels ranging from 2.3-5.5 log10 copies per mL (mean viral load 4.2 ± 1.2 log10 copies per mL). Upon entering the study, all patients were naive of PIs, although two-thirds of these patients had previously received a bi-RTI combination (Table 1). All patients in the study received triple combination therapy with bi-RTI and the PI indinavir (IDV). Using this combined therapy, plasma HIV RNA was decreased to levels below the limit of detection in a large majority of patients (73% and 87% of patients at M3 and M18, respectively), the mean reduction being −1.8 log10copies per mL. A concomitant increase in the CD4 T-cell count was detected in the blood of all patients, and the mean increase was 206 ± 142 CD4 T cells per μL at M12 (P < .02 vs baseline) (Figure1). The mean absolute number of CD8 T cells progressively decreased by a mean of 448 ± 231 CD8 T cells per μL at M12 (P < .05 vs baseline) (Table 1). The increase in CD4 counts was correlated to the baseline viral load (r = 0.65, P < .02) and to the decrease in viral load (r = −0.59, P < .04). None of these patients developed LD. The body mass index (BMI) of patients was unchanged during the follow-up (M0, 22.5 ± 2.4 kg/m2, and M18, 22.8 ± 1.9 kg/m2).
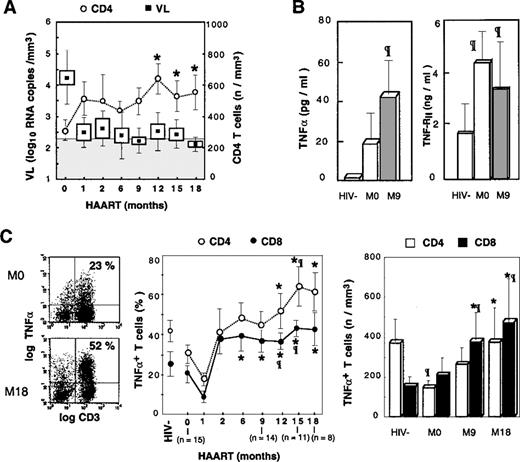
Accumulation of TNF-–producing T cells following HAART.
(A) Evolution of plasmatic viral load and ex vivo CD4 T-cell counts is depicted in treated patients. Boxes and Whisker plots indicate mean values, SEM and SD, respectively; vertical bars, SEM; and shaded rectangle, threshold of viral load detection. (B) TNF-α and TNF-RII plasmatic concentrations are given, with open box indicating HIV− controls; the shaded box, HIV+PI− patients; and the solid box, HIV+PI+ patients after 9 months of treatment including PIs. (C) PBMCs were stimulated 16 hours with PMA, PHA, and ionomycin. Dot-plots from a representative patient are given before and after 18 months of treatment (left panel); percentages and absolute counts (middle and right panel, respectively) of TNF-α–producing T cells following HAART. Asterisk indicates P < .05 vs baseline level (Wilcoxon signed rank test); paragraph symbol, P < .05 vs HIV− controls (Mann-Whitney U test).
Accumulation of TNF-–producing T cells following HAART.
(A) Evolution of plasmatic viral load and ex vivo CD4 T-cell counts is depicted in treated patients. Boxes and Whisker plots indicate mean values, SEM and SD, respectively; vertical bars, SEM; and shaded rectangle, threshold of viral load detection. (B) TNF-α and TNF-RII plasmatic concentrations are given, with open box indicating HIV− controls; the shaded box, HIV+PI− patients; and the solid box, HIV+PI+ patients after 9 months of treatment including PIs. (C) PBMCs were stimulated 16 hours with PMA, PHA, and ionomycin. Dot-plots from a representative patient are given before and after 18 months of treatment (left panel); percentages and absolute counts (middle and right panel, respectively) of TNF-α–producing T cells following HAART. Asterisk indicates P < .05 vs baseline level (Wilcoxon signed rank test); paragraph symbol, P < .05 vs HIV− controls (Mann-Whitney U test).
TNF-α is a potent inducer of HIV replication and thus may be involved in controlling the pool of latently infected cells.29 As a result, it was important to determine the evolution of TNF-α production during HAART. We previously reported that the chronic phase of HIV infection is associated with a moderate decrease in the proportion of T cells producing TNF-α, determined by single cell analysis of intracellular cytokine expression following PMA + PHA + ionomycin activation, and correlated with progression of HIV disease.27 We confirmed that baseline proportions of CD3+TNF-α+ T cells in HIV+patients were lower than the baseline proportions of control donors (23.2% ± 16.4% vs 37.4% ± 23.4%, respectively). This same decrease in the proportion of TNF-α+ T cells was observed in both CD4 and CD8 subsets. A reduction in the absolute number of CD4 T cells producing TNF-α was detected in untreated patients compared with healthy donors (121 ± 98 cells per μL vs 374 ± 201 cells per μL [P < .01]), whereas the absolute number of CD8+TNF-α+ T cells was not altered (Figure 1C). In parallel, baseline plasma levels of TNF-α and TNF-RII were increased in patients compared with controls (Figure 1B), and single cell analysis of TNF-α production by monocytes suggested that in patients not receiving PIs, plasma TNF-α originated from monocytes (M. Bocchino et al, unpublished data, May 1999).
Following PI administration, a dramatic increase in the percentage and absolute number of T cells synthesizing TNF-α was observed in all the patients. Proportions of CD3+TNF-α+ T cells increased from 23.2% ± 16.4% to 46.1% ± 17.9% at M12 (P < .01 vs control donors). Analysis of CD4 and CD8 T cells revealed that both subsets were highly polarized to TNF-α synthesis. Indeed, the proportions of CD4 T cells producing TNF-α increased progressively and became statistically significant compared with baseline values following M12, and the absolute number of T cells increased from 121 ± 98 cells per μL at M12 to 376 ± 206 cells per μL at M18, thereby reaching the range of normal values (Figure 1C). A similar increase was observed for TNF-α–synthesizing CD8 T cells, which became over-represented because their absolute number increased more than 3-fold compared with control donors and increased 2-fold compared with baseline values: M18, 431 ± 460 cells per μL vs 147 ± 79 cells per μL for controls (P < .01) and 206 ± 239 cells per μLat M0 (P < .03) (Figure 1C). This polarization of T cells to TNF-α synthesis was associated with increased concentrations of TNF-α and TNF-RII in the sera of PI-treated patients (Figure 1B).
The contribution of monocytes to the rise in plasma TNF-α was excluded following single cell analysis because the proportion of monocytes producing TNF-α was not increased as the result of HAART (M. Bocchino et al, unpublished data, May 1999). It is noteworthy that this accumulation of TNF-α T-cell producers occurred in the context of efficient suppression of HIV RNA in plasma (Figure 1A), and no correlation was found between the viral load (VL) at baseline or during the follow-up and the percentage or the absolute number of CD4 or CD8 TNF-α producers. In contrast, the change in the number of CD8+ TNF-α+ T cells correlated to the change in VL (r = −0.56, P < .05). Plasmatic TNF-RII concentrations and viral load were correlated at baseline (r = 0.81, P < .02), but there were no further correlations following PI administration. Altogether these data suggest that the regulation of the TNF-α system is altered following successful antiretroviral therapy, which leads to the dramatic accumulation of T cells polarized for the synthesis of this proinflammatory cytokine.
Defective control by apoptosis on TNF-+ T cells following HAART
We previously reported that the decreased proportion of TNF-α–producing T cells in untreated or RTI-treated patients was correlated with an increased rate of apoptosis in this subset.27 Because apoptosis, physiological anti-inflammatory process,30 is dramatically reduced following HAART,2-4 we asked whether the accumulation of TNF-α producers in patients receiving HAART was related to their increased survival. We first confirmed that HAART is associated with a significant decrease in activation-induced apoptosis in CD3+ T cells and noted 18.6% ± 11.6% at M12 vs 39.0% ± 17.9% at M0 (P < .01), leading to values close to those of control donors (21.8% ± 13.5%). This drop in apoptosis concerned all T-cell subsets including TNF-α–synthesizing CD3 T cells. Indeed, analysis of apoptosis at the single cell level within TNF-α CD3 T-cell producers, as shown in Figure2A, indicates a remarkable increased survival of this subset following HAART. In this representative patient, the proportion of apoptotic cells within CD3+TNF-α+ T cells decreased from 58% at M0 to 16% at M12, whereas the percentage of TNF-α+ cells within CD3+ T cells increased from 26% to 49% (Figure2A).
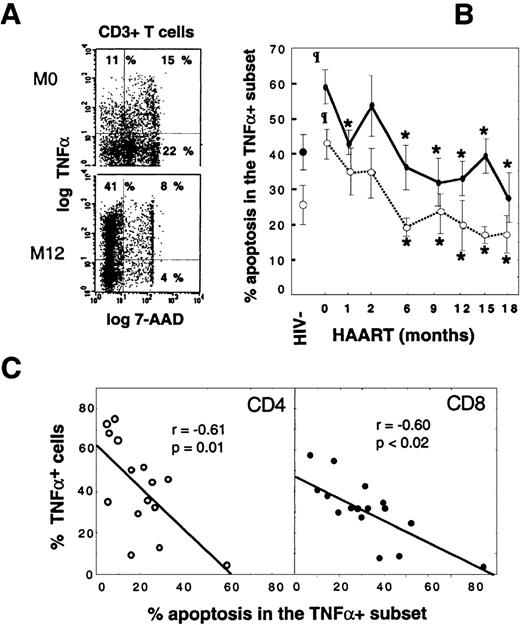
The peripheral accumulation of TNF-–producing T cells is linked to their escape from apoptosis.
Apoptosis susceptibility of TNF-α+ T cells was determined by concomitant intracellular TNF-α+and 7-AAD stainings. (A) Dot-plots of gated CD3+ T cells from a representative HIV+ patient before HAART and at M12. (B) Follow-up of susceptibility to activation-induced apoptosis of TNF-α+T cells during HAART. Vertical bars indicate SEM. (C) Scatter-plots of apoptosis rates in TNF-α+ T cells and percentages of TNF-α+ peripheral cells in 16 patients who received at least 9 months of HAART (Spearman rank correlations). Values are given as the mean plus or minus SD. Shaded box indicates HIV+PI− patients; open circle, CD4 T cells; and closed circle, CD8 T cells. Asterisk indicates P < .05 vs baseline values (Wilcoxon signed rank test); paragraph symbol,P < .05 vs HIV− controls (Mann-Whitney U test).
The peripheral accumulation of TNF-–producing T cells is linked to their escape from apoptosis.
Apoptosis susceptibility of TNF-α+ T cells was determined by concomitant intracellular TNF-α+and 7-AAD stainings. (A) Dot-plots of gated CD3+ T cells from a representative HIV+ patient before HAART and at M12. (B) Follow-up of susceptibility to activation-induced apoptosis of TNF-α+T cells during HAART. Vertical bars indicate SEM. (C) Scatter-plots of apoptosis rates in TNF-α+ T cells and percentages of TNF-α+ peripheral cells in 16 patients who received at least 9 months of HAART (Spearman rank correlations). Values are given as the mean plus or minus SD. Shaded box indicates HIV+PI− patients; open circle, CD4 T cells; and closed circle, CD8 T cells. Asterisk indicates P < .05 vs baseline values (Wilcoxon signed rank test); paragraph symbol,P < .05 vs HIV− controls (Mann-Whitney U test).
The 18-month follow-up of susceptibility to apoptosis of CD4 and CD8 TNF-α–producing T cells revealed that the initially high level of apoptosis in both subsets declined progressively during HAART: 19.3% ± 11.6% in CD4+TNF-α+ T cells at M18 vs 41.4% ± 16.7% at baseline (P < .003) and 58.4% ± 18.7% in CD8+TNF-α+ T cells at M18 vs 30.5% ± 10.0% at baseline (P < .001). At M18, values were below those of controls (Figure 2B). Furthermore, the accumulation of TNF-α–producing T cells within both CD4 and CD8 subsets was inversely correlated with the level of activation-induced apoptosis in these cells (Figure 2C), which supports an important role of apoptosis in the homeostasis of TNF-α+ T cells. It is noteworthy that only patient No. 10, who showed a persistent high rate of T-cell apoptosis in TNF-α–producers (60% and more than 80% in CD4 and CD8 T cells, respectively), had a concomitant very low proportion (less than 10%) of TNF-α T cells and was not efficiently controlled at the viral level (Figure 2C and Table 1). From these data, we conclude that peripheral accumulation of TNF-α–synthesizing T cells in patients receiving HAART is the direct consequence of a defective physiological control by apoptosis.
Escape from activation-induced apoptosis of TNF-–producing cells partly related to cosynthesis of IL-2
Regulation by endogenous cytokines of lymphocyte survival can be studied by flow cytometry, which allows the simultaneous analysis at the single cell level of the coproduction of several cytokines and the susceptibility to activation-induced apoptosis. Because IL-2 is able to prevent ex vivo apoptosis of T cells from HIV+patients,31 we tested whether increased survival of TNF-α producers in HAART-treated patients was related to the cosynthesis of IL-2 by these cells. We show that a significant fraction of TNF-α+ cells coproduce IL-2 in both control donors and HIV+ patients (Figure 3A). Indeed, the proportion of double-positive CD3 T cells in these representative donors was 55% of the total T cells in the control donor, and the proportion was reduced to 15% in the PI− patient and restored to 48% in the PI+ patient. The decrease in the proportion of IL-2+TNF-α+ cells in the PI− patient was mainly due to an alteration within the CD8 subset (21.5% ± 11.1% CD8+TNF-α+ T cells in PI−patients vs 48.8% ± 16.9% cells in controls (P < .001), and it was restored during PI therapy (38.5% ± 15.2% cells, nonsignificant vs controls).
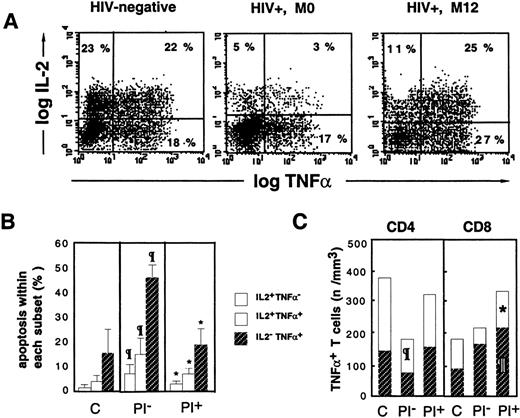
Escape from apoptosis of TNF-+ T cells is associated with cosynthesis of IL-2.
Following 16 hours of PMA+PHA+ionomycin stimulation, dual intracellular staining of PBMCs was performed with anti–IL-2-PE and anti–TNF-α-FITC mAbs. (A) Dot-plots, gated on CD3+ T cells, from a representative HIV− control, an HIV+ PI− patient (M0), and an HIV+ PI+ patient treated for 12 months (M12). (B) Differential susceptibility to activation-induced apoptosis of IL-2+TNF-α−, IL-2+TNF-α+, and IL-2−TNF-α+ lymphocytes. (C) Relative contribution of IL-2+TNF-α+ and IL-2−TNF-α+ cells to the expansion of total TNF-α+ T cells following HAART. The letter C indicates healthy subjects (n = 7); PI−, HIV+ PI− patients naı̈ve of PI therapy (n = 12); and PI+, HIV+ PI+patients with at least 9 months of PI therapy (n = 13). Paragraph symbol indicates P < .05 vs HIV− controls; asterisk, P < .05 vs HIV+ PI− patients (Mann-Whitney U test).
Escape from apoptosis of TNF-+ T cells is associated with cosynthesis of IL-2.
Following 16 hours of PMA+PHA+ionomycin stimulation, dual intracellular staining of PBMCs was performed with anti–IL-2-PE and anti–TNF-α-FITC mAbs. (A) Dot-plots, gated on CD3+ T cells, from a representative HIV− control, an HIV+ PI− patient (M0), and an HIV+ PI+ patient treated for 12 months (M12). (B) Differential susceptibility to activation-induced apoptosis of IL-2+TNF-α−, IL-2+TNF-α+, and IL-2−TNF-α+ lymphocytes. (C) Relative contribution of IL-2+TNF-α+ and IL-2−TNF-α+ cells to the expansion of total TNF-α+ T cells following HAART. The letter C indicates healthy subjects (n = 7); PI−, HIV+ PI− patients naı̈ve of PI therapy (n = 12); and PI+, HIV+ PI+patients with at least 9 months of PI therapy (n = 13). Paragraph symbol indicates P < .05 vs HIV− controls; asterisk, P < .05 vs HIV+ PI− patients (Mann-Whitney U test).
Concomitant analysis of apoptosis within cytokine producers indicated the existence of a gradient of susceptibility related to the cytokine synthesized: IL-2− TNF-α+ cells were more susceptible to apoptosis than IL-2+TNF-α+ cells, which were more susceptible than IL-2+TNF-α− cells (Figure 3B). This gradient of apoptosis was observed in lymphocytes from control donors and in HIV+ PI−patients, although the rate of apoptosis was higher in all 3 subsets (Figure 3B). A decreased susceptibility to activation-induced apoptosis was observed in the 3 subsets in PI+ patients, and in spite of this, the IL-2+TNF-α+ subset was still less susceptible to apoptosis than the single TNF-α+subset (Figure 3B). A relationship between susceptibility to apoptosis of TNF-α producers and their absolute number in the blood was observed. Indeed, in PI− patients, a dramatic reduction in the number of double-positive cells within both the CD4 subset (110 ± 37 cells per μL in PI− patients vs 244 ± 131 cells per μL in controls [P < .03]) and the CD8 subset (44 ± 12 cells per μL in PI− patients vs 73 ± 30 cells per μL in controls, NS) was observed (Figure 3B). With PI therapy, the enrichment in TNF-α T-cell producers was associated with an increase in the number of both CD4 and CD8 double-positivecells (171 ± 82 cells per μL IL-2+TNF-α+ CD4 T cells (NS vs M0) and 105 ± 55 cells per μL IL-2+TNF-α+ CD8 T cells (P < .02 vs M0) and also an increase in the number of single positive cells in both subsets (P < .01 vs controls for the CD8 subset) (Figure 3B).We conclude from these data that accumulation of CD4 and CD8 T cells producing TNF-α during HAART results from the escape from physiological apoptosis of TNF-α T-cell producers, partly due to an increased proportion of apoptosis-resistant IL-2+TNF-α+ T cells.
Lipid alterations in PI− and PI+HIV+ patients
LD, recently reported in HIV-treated patients, is characterized by abnormal fat redistribution (peripheral loss of fatty tissue and central fat accumulation) together with insulin resistance and dyslipidemia.15,16 In a cross-sectional study, we analyzed the lipid parameters in 20 healthy HIV-negative (HIV−) controls, 10 HIV+ patients receiving bi-RTI (zidovudine [ZDV] and didanosine [ddI] or ZDV and zalcitabine [ddC]) for 3-6 months, and 30 HIV+ men receiving HAART including PIs for at least 9 months. LD was assessed by a clinical score of facial and limb fat wasting and central fat deposition as previously reported.20 Clinical characteristics of patients are given in Table 2. In patients receiving bi-RTI, a decrease in the concentration of HDL cholesterol and an increase in the concentration of triglycerides, as compared with HIV− healthy controls, was observed. Nevertheless, in these patients the apolipoprotein fractions were not significantly altered (Table 3). In PI+LD− patients, no alterations were observed in total cholesterol, HDL cholesterol, and triglycerides, but a trend toward an increase in the atherogenic ratio apoB/apoA1 was observed. In PI+LD+ patients, dyslipidemia consists of dramatically increased concentrations of cholesterol and triglycerides together with a strong increase in the atherogenic fraction apoB and in the apoB/apoA1 ratio,20 which is significantly different compared with other groups of donors (Table 3).
Lipid parameters of controls, bi-RTI, PI+LD−, and PI+LD+ patients
| Parameters . | HIV− Controls, n = 12 . | Bi-RTI, n = 10 . | PI+LD−, n = 13 . | PI+LD+, n = 17 . |
|---|---|---|---|---|
| Cholesterol, mmol/L (g/L) | 4.8 ± 0.9 | 4.4 ± 1.2 | 5.0 ± 1.3 | 6.9 ± 0.173-152 |
| (1.85 ± 0.34) | (1.70 ± 0.46) | (1.94 ± 0.51) | (2.68 ± 0.653-152) | |
| HDL cholesterol, mmol/L (g/L) | 1.3 ± 0.18 | 0.54 ± 0.133-150 | 1.6 ± 0.853-151 | 1.2 ± 0.443-151 |
| (0.52 ± 0.07) | (0.21 ± 0.053-150) | (0.62 ± 0.333-151) | (0.46 ± 0.173-151) | |
| Triglycerides, mmol/L (g/L) | 1.06 ± 0.42 | 2.2 ± 2.03-150 | 2.2 ± 0.5 | 4.6 ± 4.43-152 |
| (0.94 ± 0.37) | (1.99 ± 1.753-150) | (1.09 ± 0.44) | (4.13 ± 3.923-152) | |
| ApoB, g/L | 0.85 ± 0.20 | 1.08 ± 0.17 | 0.75 ± 0.23 | 1.57 ± 0.363-152 |
| ApoB/apoA1 ratio | 0.54 ± 0.12 | 0.54 ± 0.11 | 0.72 ± 0.24 | 1.15 ± 0.343-152 |
| Parameters . | HIV− Controls, n = 12 . | Bi-RTI, n = 10 . | PI+LD−, n = 13 . | PI+LD+, n = 17 . |
|---|---|---|---|---|
| Cholesterol, mmol/L (g/L) | 4.8 ± 0.9 | 4.4 ± 1.2 | 5.0 ± 1.3 | 6.9 ± 0.173-152 |
| (1.85 ± 0.34) | (1.70 ± 0.46) | (1.94 ± 0.51) | (2.68 ± 0.653-152) | |
| HDL cholesterol, mmol/L (g/L) | 1.3 ± 0.18 | 0.54 ± 0.133-150 | 1.6 ± 0.853-151 | 1.2 ± 0.443-151 |
| (0.52 ± 0.07) | (0.21 ± 0.053-150) | (0.62 ± 0.333-151) | (0.46 ± 0.173-151) | |
| Triglycerides, mmol/L (g/L) | 1.06 ± 0.42 | 2.2 ± 2.03-150 | 2.2 ± 0.5 | 4.6 ± 4.43-152 |
| (0.94 ± 0.37) | (1.99 ± 1.753-150) | (1.09 ± 0.44) | (4.13 ± 3.923-152) | |
| ApoB, g/L | 0.85 ± 0.20 | 1.08 ± 0.17 | 0.75 ± 0.23 | 1.57 ± 0.363-152 |
| ApoB/apoA1 ratio | 0.54 ± 0.12 | 0.54 ± 0.11 | 0.72 ± 0.24 | 1.15 ± 0.343-152 |
Drug regimens are detailed in the notes of Table 2. Values are given as the mean plus or minus SD.
P < .05 vs HIV− control group.
P < .05 vs bi-RTI group.
P < .05 vs HIV− control, bi-RTI, and PI+LD− groups.
Relation between cytokine alterations and HAART-associated LD
The precise mechanisms involved in the LD syndrome still remain unclear. Because lipid metabolism is under the control of some cytokines, including TNF-α,25 we asked whether dysregulation in TNF-α synthesis during HAART was related to this syndrome. In a cross-sectional study, we analyzed 41 HIV+men who were taking HAART including PIs for at least 9 months. The LD score20 of the 17 LD+ patients was between 4 and 10. The 24 LD− patients did not present any of the scoring symptoms. We also included 19 HIV−controls in the study. Clinical and laboratory characteristics of LD+ and LD− groups are given in Table 2. BMI did not significantly differ between the male patients from the 2 groups (22.2 ± 2.4 kg/m2 and 23.8 ± 3.5 kg/m2 in LD+ and LD−patients, respectively). LD+ and LD−patients had similar CD4 T-cell numbers and a similar CD4 increase under HAART at the time of the study. It is noteworthy that the number of CD8 lymphocytes and the increase in CD8 T cells were significantly higher in LD+ patients, and the mean duration of PI therapy was longer in LD+ patients compared with LD− patients. The viral load was efficiently controlled in both groups (Table 2).
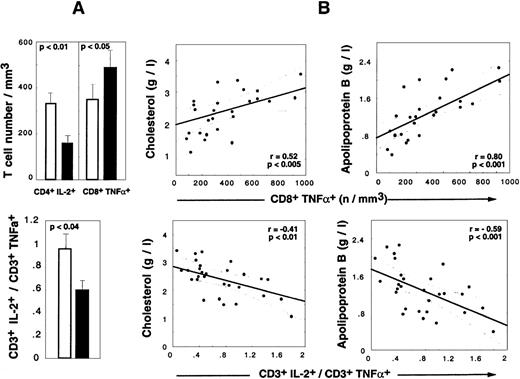
Determination of the frequency of TNF-α producers revealed that the accumulation of CD8+TNF-α+ Τ cells detected in HAART-treated LD− patients also occurred in LD+ patients but to levels higher than seen in LD− patients (mean percentage, 42.2% ± 11.1% in LD+ vs 36.7% ± 15.5% in LD−, NS; mean absolute count, 516 ± 265 in LD+ vs 350 ± 353 in LD− [P < .003]) (Figure 4A). The mean percentage of apoptotic cells in CD8+TNF-α+ Τ cells was decreased in both LD− patients (29.4% ± 15.0%) and LD+ patients (42.2% ± 22.1%) vs PI− patients (58.7% ± 15.0%) (P < .01 vs LD−, and P < .05 vs LD+). The absolute numbers of CD4+TNF-α+Τ cells were similar in LD+ vs LD− patients (data not shown), but a spectacular reduction in the mean number of IL-2–producing CD4T cells was observed (151 ± 138 cells per μL in LD+ patients vs 328 ± 265 cells per μL in LD− patients [P < .02]). The decreased capacity of IL-2 production was also observed at the CD8 T-cell level (data not shown), but the absolute number was not altered because of a compensation due to the CD8 lymphocytosis observed in LD+patients. The concomitant alteration in IL-2 production by CD4 T cells and the TNF-α polarization of CD8 T cells in LD+ patients resulted in a significant decrease in the IL-2+/TNF-α+ CD3 ratio detected in LD+ vs LD− patients. Strikingly, lipid alterations in HAART-treated patients correlated with cytokine perturbations. Indeed, the absolute numbers of CD8+TNF-α+ T cells were positively correlated with total cholesterol (P < .005) (Figure 4), total triglyceride (P < .002), and apoB concentrations (P < .001) (Figure 4) and the apoB/apoA1 ratio (P < .0001) and inversely correlated with the HDL concentration (P < .04). The absolute number of CD4+IL-2+ T cells was negatively correlated with the total triglyceride level (P < .02) and apoB concentration (P < .03) and positively correlated with HDL concentration (P < .05). Consequently, the CD3+IL-2+/CD3+TNF-α+ratio was found to be a good marker of dyslipidemia and atherogenic risks because it was negatively correlated with total cholesterol (P < .01) (Figure 4), total triglyceride (P < .009), and apoB (P < .001) concentrations (Figure 4) and the apoB/apoA1 ratio (P < .04).
Cytokine alterations during HAART are correlated to LD-associated dyslipidemia.
(A) Absolute numbers of CD4+IL-2+ T cells, CD8+TNF-α+ T cells (upper panel), and CD3+IL-2+/CD3+TNF-α+ratio (lower panel) in HIV+ PI+ patients treated for at least 9 months. Shaded box indicates LD− patients (n = 24), and darkened box, LD+ patients (n = 17). Histograms show mean values and SEM. (B) Correlations between cytokine and lipid alterations are given for 30 HIV+ PI+ patients with at least 9 months of PI therapy. The patient population includes 13 LD− patients, 17 LD+ 13 LD−, and 17 LD+ (Spearman rank correlation).
Cytokine alterations during HAART are correlated to LD-associated dyslipidemia.
(A) Absolute numbers of CD4+IL-2+ T cells, CD8+TNF-α+ T cells (upper panel), and CD3+IL-2+/CD3+TNF-α+ratio (lower panel) in HIV+ PI+ patients treated for at least 9 months. Shaded box indicates LD− patients (n = 24), and darkened box, LD+ patients (n = 17). Histograms show mean values and SEM. (B) Correlations between cytokine and lipid alterations are given for 30 HIV+ PI+ patients with at least 9 months of PI therapy. The patient population includes 13 LD− patients, 17 LD+ 13 LD−, and 17 LD+ (Spearman rank correlation).
Discussion
Recent studies have reported unusual clinical inflammatory syndromes, such as immune recovery vitritis (IRV) in CMV retinitis patients32 or focal mycobacterial lymphadenitis,33 after initiation of HAART. It was suggested that these syndromes represent immunopathological responses which occur as a result of the recovery of specific immune reactivity against microbial pathogens that are subclinically present at the time HAART is initiated.34 Our observations demonstrate that HAART is associated with the progressive accumulation of T cells producing TNF-α+ thereby creating a proinflammatory environment that might contribute to the development of these immune restoration inflammatory diseases. In addition our data suggest that this T-cell polarization to TNF-α synthesis is favoring the development of the LD syndrome by contributing to lipid metabolism alteration.
A wide number of TNF-α inducers, including HIV proteins,35 have been described. Although HIV viral load is highly suppressed by HAART, viral reservoirs still persist,29 and the release of early viral proteins, such as Tat, together with increased concentrations of soluble TNF-RII, could lead to NF-κB activation36 and subsequent induction of TNF-α production.35 Stringent control mechanisms are therefore necessary to prevent chronic production of TNF-α and subsequent adverse consequences. At the macrophage level, this control is provided by anti-inflammatory cytokines such as interleukin-4 (IL-4), IL-10, and transforming growth factor–β (TGF-β).37 We did not observe an increase in Th2 cytokine production in HIV+ patients,27 even in those patients receiving HAART. Our data argue for a role of apoptosis in the physiological regulation of TNF-α. The anti-inflammatory function of apoptosis has been reported at the macrophage level,30 and it is exploited by bacterial pathogens to suppress TNF-α production.38 Apoptosis was also shown to contribute to the resolution of inflammatory processes involved in the delayed-type hypersensitivity response.39 This study and our previous study using combined detection of intracellular cytokine synthesis and apoptosis on lymphocytes from untreated patients27 suggest that apoptosis plays an essential role in the negative regulation of TNF-α synthesis by T cells. Indeed, even in healthy donors, TNF-α producers are highly sensitive to apoptosis, and this is partly related to the decreased expression of the Bcl-2 molecule.27 During the natural progression of HIV infection, susceptibility of TNF-α producers to apoptosis progressively increases, and this is correlated to their decreased representation in patient blood samples.27 The present study shows that during combined therapy, including indinavir, apoptosis in TNF-α T-cell producers is highly suppressed and leads to their progressive accumulation. Suppression of activation-induced apoptosis with HAART 2-4is likely to be a multifactorial process. Indeed, the mechanisms involved in apoptosis susceptibility of patients' lymphocytes during the chronic phase of HIV infection, ie, the in vivo immune activation state of these lymphocytes,40 is down-regulated during HAART,8 and the production of HIV proteins with proapoptotic effects, such as gp120,41,42 is decreased, thereby restoring the global survival of patients' lymphocytes. In addition, our data show for the first time that regulation of TNF-α synthesis can occur at the single cell level through the coproduction of a survival factor. This regulation occurs because increased survival of TNF-α T-cell producers during HAART is associated with the cosynthesis of IL-2. Finally, we cannot exclude a direct immunomodulatory effect of PIs on T cells. PIs suppress physiological apoptosis by inhibition of cellular proteases4 and exert a direct impact on cytokine production.43 However, these in vitro effects of PIs were mainly observed in patients taking ritonavir.
Recently, a series of reports have associated HAART with a syndrome that includes peripheral fat wasting, dyslipidemia, insulin resistance, and central fat accumulation.15,16 The pathophysiology of LD remains unknown. The amino-acid sequence homologies between HIV protease and the low density lipid receptor–related protein or cytoplasmic retinoic-acid binding protein type 1 resulted in the suggestion that LD results from a possible interaction between PIs and these molecules.23 However, this hypothesis cannot account for the occurrence of LD in patients who never received PIs, recently reported in several studies.18-20 Our data show that hyperlipidemia and atherogenic alterations observed in LD+ patients are related to dysregulation in cytokine synthesis. Indeed, increased concentrations in circulating lipids, including cholesterol, triglycerides, and apoB, were positively correlated with the absolute number of TNF-α–producing CD8 T cells in LD+ patients. Although these associations do not prove causality, our study suggests that the progressive accumulation of TNF-α producers during HAART is a factor involved in dyslipidemia.
TNF-α inhibits the intake of free fatty acids (FFA) by adipocytes via the inhibition of lipoprotein lipase, which leads to fat wasting, and increases lipogenesis via the stimulation of the hepatic triglyceride synthetase, which leads to hyperlipidemia.25 Increased TNF-α production may also indirectly contribute to fat wasting by triggering the production of leptin, a hormone that depletes adipocyte fat content via down-regulation of the lipogenic enzymes.44 Accumulation of TNF-α could also account for insulin resistance, both indirectly via FFA increase and directly via the inhibition of signal transduction through the insulin receptor.26 Whereas the number of T-cell–producing TNF-α was strongly correlated with lipid parameters in PI-treated patients, no direct association could be observed between these lipid parameters and plasmatic TNF indices (Christeff et al, unpublished data, October 1999). These data suggest that lipid alterations may involve local cleavage of cell-borne TNF in target tissues rather than systemic diffusion of the cytokine.45-47 According to that hypothesis, Domingo et al48 recently reported that subcutaneous fat atrophy in LD+ patients is associated with focal lipogranuloma formation and adipocyte apoptosis, which is compatible with an excessive local production of TNF-α.49 It remains to be determined whether subsets other than lymphocytes, including adipocytes,50 are involved in the increased potentiality of TNF-α production following HAART. In addition, persistent high plasmatic levels of TNF-RII following HAART may also be involved in insulin resistance and dyslipidemia, as was recently proposed for another metabolic disease.51
Although prospective studies are needed, we propose that the development of the LD syndrome is a sequential and multifactorial process for the following reasons: First, as previously reported by Zangerle et al52 and reported in Table 3, lipid alterations, such as decreased HDL cholesterol and increased triglycerides, occur in HIV+ patients who received bi-RTI, while the apoB/apoA1 ratio remains normal. This later parameter is progressively altered after PI administration, in the context of increased TNF-α production by T cells, while the production of this cytokine is low in patients receiving bi-RTI.27 In addition, increased cortisol secretion and an imbalance in the cortisol/DHEA (dehydroepiandrosterone) ratio may contribute to the altered equilibrium between lipolysis and lipogenesis in LD+ patients.20,53 Interestingly, decreased serum DHEA concentration detected in LD+ patients compared with LD− patients20 may be related to the alteration in IL-2 production54 that we observed in LD+ patients. Additional mechanisms involved in central fat deposition remain to be elucidated. Although we did not observe modifications in BMI during PI therapy, including in LD+patients,20 it is possible that fat redistribution in LD+ patients masks a residual muscle wasting,55which is compatible with an excess in both TNF-α production and the catabolic/anabolic cortisol/DHEA ratio.56
Alteration in TNF-α–producing T-cell homeostasis may have other consequences, particularly at the viral level. It could favor the HIV rebound rapidly observed after interruption of HAART.29 On the other hand, it may contribute to eradication of a long decay half-life HIV reservoir from resting CD4 lymphocytes.13 29It remains to be determined whether this proinflammatory response is triggered by specific antiretroviral therapy and/or is a consequence of the sudden suppression of HIV production, which would perturb a preexisting equilibrium between the immune system and the virus. Altogether our data indicate that HAART is associated with the progressive accumulation of proinflammatory T cells, which are committed to TNF-α synthesis, due to a dramatic down-regulation of physiological apoptosis. These findings give new insights into strategies to prevent the development of LD-associated dyslipidemia in treated HIV+ patients.
Acknowledgments
The authors wish to acknowledge the nurses of Centre Hospitalier Intercommunal, Villeneuve-St-Georges, France, and Hôpital Raymond Poincaré, Garches, France, for patient care and for collecting the samples. We are indebted to Dr Ahmed Chater, Centre Hospitalier, Villeneuve-St Georges, and to Mrs Françoise Rives, Pasteur Institute, Paris, France, for their efficient help in the capture of clinical data. We particularly acknowledge Dr Luc Montagnier for his interest in this study.
Supported by grants from the Agence Nationale de Recherche sur le SIDA; the Fondation pour la Recherche Médicale (FRM); the CNRS; the Institut Pasteur, Paris, France; and by contracts BMH4-CT 97-2055 and ERB-IC15-CT97-0901 from the Union Européenne. E. L. was supported by the FRM.
Submitted October 21, 1999; accepted January 19, 2000.
Reprints:Marie-Lise Gougeon, Unité d'Oncologie Virale, Département SIDA et Rétrovirus, Institut Pasteur, 28 rue du Dr Roux, 75724 Paris, France; e-mail: mlgougeo@pasteur.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal