Abstract
Stimulation of the T-cell receptor (TCR) alters a number of intracellular signaling pathways including one that involves protein tyrosine kinases, phospholipase C-γ1 (PLC-γ1), diacylglycerol (DAG), and calcium messengers. By a divergent pathway, TCR-stimulated protein tyrosine kinase activity is thought to result independently in recruitment of the Ras activator Sos to the plasma membrane, leading to Ras activation. Here we show that RasGRP, a Ras activator that contains calcium-binding EF hands and a DAG-binding domain, is expressed in T cells. A PLC-γ1 inhibitor diminished activation of Ras following TCR stimulation. Membranes from TCR-stimulated Jurkat T cells exhibited increased RasGRP and increased Ras-guanyl nucleotide association activity that was inhibited by antibodies directed against RasGRP. Overexpression of RasGRP in T cells enhanced TCR-Ras-Erk signaling and augmented interleukin-2 secretion in response to calcium ionophore plus DAG analogues phorbol ester myristate or bryostatin-1. Thus, RasGRP links TCR and PLC-γ1 to Ras-Erk signaling, a pathway amenable to pharmacologic manipulation.
A key step in T-cell activation is the physical interaction between the T-cell receptor (TCR) on the surface of the T cells and peptide antigen presented in the context of a major histocompatibility molecule on an antigen-presenting cell. This interaction results in the rapid modulation of several biochemical processes within the T cells that ultimately lead to T-cell proliferation.1-3 TCR-proximal events include the activation of cytoplasmic protein tyrosine kinases such as the CD4-associated protein tyrosine kinase lck. Tyrosine phosphorylation of the intracellular domains of the small TCR subunits by lck leads to the recruitment to the TCR of a second protein tyrosine kinase, ZAP-70.4-6 TCR-associated ZAP-70 phosphorylates adaptor proteins such as LAT and SLP-76, which may be complexed at the plasma membrane.7-10
Within minutes of TCR stimulation, several divergent signaling systems are thought to come into play. Phospholipase C-γ1 (PLC-γ1) is activated by a combination of tyrosine phosphorylation and recruitment to the tyrosine phosphorylated adaptor proteins.11 Cleavage of membrane phosphoinositides by PLC-γ1 generates membrane diacylglycerol (DAG) messenger, which activates protein kinase C (PKC). PLC-γ1 also generates inositol triphosphate, which facilitates a rise in free cytoplasmic calcium, stimulation of the calcium-activated phosphatase calcineurin, and engagement of the nuclear factor of activated T cells (NF-AT) transcription system.12
Independently, early protein tyrosine kinase activity is thought to activate the small GTPase Ras by promoting the formation of Ras bound to GTP. Ras-GTP activates the Raf-Mek-Erk protein kinase cascade and this pathway controls the level of the Jun/Fos dimeric transcription factor known as AP1.13 The coordinated activation of AP1 transcription factors by the Ras-Erk signaling system and the NF-AT transcription factor results in efficient transcription of the interleukin-2 (IL-2) gene, the protein product of which drives T-cell proliferation.12 Additional signaling systems contribute to the synthesis of the IL-2 receptor, changes in cell shape, and other aspects of T-cell activation.14-16
Despite considerable effort, the mechanism whereby Ras is activated after TCR stimulation has not been fully elucidated. In principle, the equilibrium between the GDP-bound “off” and GTP-bound “on” states of Ras could be regulated by controlling the rates of GTP hydrolysis and guanyl nucleotide exchange. The hydrolysis reaction is controlled by GTPase activating proteins (GAPs). The exchange rate is limited by the activity of guanyl nucleotide exchange factors (GEFs). Early work with T cells indicated that Ras activation following TCR stimulation involved a PKC-dependent inhibition of Ras GAPs.17-19 The ability of the DAG analogue phorbol ester myristate (PMA) to activate Ras in T cells has also been attributed to PKC-dependent inhibition of Ras GAPs. However, in most cell types, Ras is positively regulated by the recruitment of Ras GEFs such as Sos to the plasma membrane through tyrosine phosphorylated adaptor proteins.20,21 The results from a number of studies suggest that Sos may activate Ras in T cells by such a mechanism.22-24
We recently described a novel Ras GEF called RasGRP (Ras guanyl nucleotide releasing protein25). In addition to the catalytic domain responsible for catalyzing nucleotide release, RasGRP contains a pair of calcium-binding EF hands and a DAG-binding domain. Treatment of engineered fibroblasts with PMA resulted in increased association of RasGRP with membranes and Ras activation.25Although normal expression was initially detected only in brain, subsequent studies revealed that RasGRP RNA was expressed in a variety of blood cells including T cells.26 27 These observations led to the hypothesis that TCR stimulation, PLC-γ1 activity, and DAG and possibly calcium messengers are linked by RasGRP to Ras-Erk signaling.
Materials and methods
Antibodies and plasmids
H176 is derived from rabbits immunized with the amino-terminal rat RasGRP peptide (residues 1-15). J32 was raised in rabbits by injecting the catalytic domain of rat RasGRP (residues 49-473); these antibodies react with both rat and human RasGRP in an immunoblot protocol. Antibodies were purified by affinity selection using immobilized recombinant rat RasGRP. Full-length human RasGRP complementary DNA (cDNA) was cloned into the SalI site of pBMGNeo.28 The plasmid was introduced into Jurkat T cells by electroporation and Neo+ cells were selected in 1 mg/mL G418.
Detection of RasGRP by immunoblotting
Protein lysates were prepared from various cell lines and from thymocytes freshly isolated from the thymus of a 6-week-old female Balb/c mouse. H176 was used in an immunoblot protocol with goat antirabbit IgG conjugated to horseradish peroxidase and enhanced chemiluminescence detection (ECL kit, Pierce, Rockford, IL) to detect p90 RasGRP (100 μg/mL total cellular protein per sample).
Ras activation assay
Ras activation was assayed by comparing the amount of Ras-GTP that could be precipitated using GST-Raf fusion protein and the amount of total Ras in cell lysates as described,29 with minor modification. Jurkat were grown to 1 × 106/mL in RPMI containing 10% heat-inactivated fetal bovine serum plus penicillin and streptomycin. Cells were incubated in herbimycin A (1.0 μg/mL) for 18 hours. Alternatively, cells were treated with U73343 orU73122 (1.0 μmol/L) for the last 15 minutes of incubation. Cells were then concentrated by centrifugation, suspended in 1.0 mL of the original medium in a plastic centrifuge tube (5 × 106 cells/assay), and incubated at 37°C for 5 minutes. After addition of OKT3 at 10 μg/mL or 0.156 μg/mL for a further 5 minutes, the cells were immediately centrifuged at 1400g for 2 minutes at 4°C. (Note that soluble OKT3 was used throughout these experiments.) Cell pellets were then lysed in 25 mmol/L HEPES pH 7.5, 150 mmol/L NaCl, 1.0% NP40, 10% glycerol, 25 mmol/L NaF, 10 mmol/L MgCl2, 1.0 mmol/L EDTA, and 1.0 mmol/L sodium ortho-vanadate plus protease inhibitors. After centrifugation to remove nuclei, 90% of each supernatant was incubated with GST-RAF (Ras-binding domain) fusion protein bound to glutathione beads to collect GTP-bound Ras. Bead-associated Ras was detected using an anti–K-ras antibody (Santa Cruz no. F234, Santa Cruz, CA) in an immunoblotting protocol. The remaining 10% of each lysate was probed with the anti-Ras antibody to verify that similar amounts of total Ras were present in each lysate and with phospho-Erk antibody (New England Biolabs no. 9101, Beverly, MA) to assess the level of Erk activation. Inhibitors were purchased from Calbiochem (San Diego, CA).
Subcellular fractionation
Jurkat T cells were suspended in hypotonic buffer and disrupted in a glass homogenizer.25 After removing nuclei and unbroken cells by centrifugation at 2000g, cell homogenates were separated into P100 and S100 by ultracentrifugation at 100 000g. For detection of RasGRP, samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with H176 IgG.
Membrane Ras-guanyl nucleotide association assay
To assay guanyl nucleotide association with membrane-bound Ras, membrane fractions were suspended in 20 mmol/L Tris pH 7.5, 100 mmol/L NaCl, 1 mmol/L MgCl2 (2.5 × 105 cell equivalents/μL). Membranes (25 μL) were then incubated with 1 μL [α32P]GTP (3000 Ci/mmol final concentration 66 nmol/L) in a final volume of 50 μL. After incubation at 30°C for 1 minute to allow exchange of resident guanyl nucleotide with the radiolabeled GTP, the reaction was diluted in ice-cold buffer (50 mmol/L Tris pH 7.5, 150 mmol/L NaCl, 20 mmol/L MgCl2, 0.5% v/v NP40) containing 1 μg anti-Ras antibody Y13-259. After incubation at 0°C for 1 hour, Ras-guanyl nucleotide complexes were recovered using protein A-Sepharose beads coated with rabbit antirat IgG. Guanyl nucleotide was released by heating at 80°C in 1.0 mol/L potassium phosphate (pH 3.4). Following chromatography of polyethylenimine plates, total guanyl nucleotide (GTP plus GDP) was quantified by phosphor-imager analysis. Background values obtained when no Y13-259 antibody was present were used to correct the experimental values. To determine the maximal degree of Ras activation in these preparations, membranes were exposed to 5.0 mmol/L EDTA for 5 minutes at 30°C followed by addition of excess MgCl2, before lysis and precipitation. To demonstrate the specificity of the above assay, membrane preparations were preincubated with antibodies (J32) raised against the catalytic domain of RasGRP (residues 49-473) or with preimmune antibodies from the same rabbit. To demonstrate that J32 antibodies specifically inhibited RasGRP, exchange assays were performed with purified proteins in buffer containing 20 mmol/L Tris pH 7.5, 100 mmol/L NaCl, 1.0 mmol/L DTT, 1.0 mmol/L MgCl2, 10% v/v glycerol, and either 1.6 pmol recombinant full-length Sos or 1.96 pmol RasGRP catalytic domain. These low amounts of Ras GEF were used to increase the likelihood that the antibody was in molar excess. Total IgG was 62 pmol/reaction but the amount of neutralizing antibody is an unknown fraction of the total.
IL-2 assays
Where indicated, cells were incubated with 5.0 μmol/L CdCl2 for 24 hours to induce RasGRP expression from the metallothionein promoter. Cells (5 × 105) of each type were then exposed in 1.0 mL fresh medium to calcium ionophore and DAG agonist for a further 48 hours. IL-2 in supernatants was measured using a colorimetric cell survival bioassay using the IL-2–dependent HT2 mouse T-cell line and units were defined as described.30 31
Results
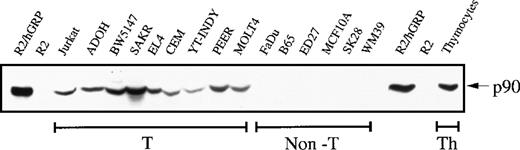
Using an antibody (H176) directed against the amino-terminal peptide sequence of RasGRP, we documented expression of this Ras activator in a variety of human and mouse T-cell lines, but not in other cell types (Figure 1). RasGRP was also detected in primary mouse thymocytes. As controls, we showed that p90 RasGRP was present in rat2 cells engineered to express human RasGRP cDNA, but not in parental rat2 cells.
RasGRP expressed in T cells.
Protein lysates were prepared from T-cell lines (T; Jurkat, human acute T-cell leukemia; ADOH, mouse T-helper cell hybridoma; BW5147, mouse T-cell lymphoma; SAKR, mouse T-cell lymphoma; EL4, mouse T-cell lymphoma; CEM, human acute T-cell lymphoblastic leukemia; YT-INDY, human NK-like T-cell leukemia; PEER, human T-cell lymphoblastic leukemia; MOLT4, human acute lymphoblastic T-cell leukemia), and from non–T-cell lines (non-T: FaDu, human pharynx squamous cell carcinoma; B65, mouse neuroblastoma; ED27, human chroriocarcinoma; MCF10A, human mammary epithelium; SK28 and WM39, human melanoma). Freshly isolated mouse thymocyctes (Th) were also examined. As controls, we studied rat2 cells and rat2 cells engineered to express RasGRP. Protein extracts were resolved by SDS/PAGE followed by immunoblotting with an antibody (H176) directed against the amino-terminus of RasGRP.
RasGRP expressed in T cells.
Protein lysates were prepared from T-cell lines (T; Jurkat, human acute T-cell leukemia; ADOH, mouse T-helper cell hybridoma; BW5147, mouse T-cell lymphoma; SAKR, mouse T-cell lymphoma; EL4, mouse T-cell lymphoma; CEM, human acute T-cell lymphoblastic leukemia; YT-INDY, human NK-like T-cell leukemia; PEER, human T-cell lymphoblastic leukemia; MOLT4, human acute lymphoblastic T-cell leukemia), and from non–T-cell lines (non-T: FaDu, human pharynx squamous cell carcinoma; B65, mouse neuroblastoma; ED27, human chroriocarcinoma; MCF10A, human mammary epithelium; SK28 and WM39, human melanoma). Freshly isolated mouse thymocyctes (Th) were also examined. As controls, we studied rat2 cells and rat2 cells engineered to express RasGRP. Protein extracts were resolved by SDS/PAGE followed by immunoblotting with an antibody (H176) directed against the amino-terminus of RasGRP.
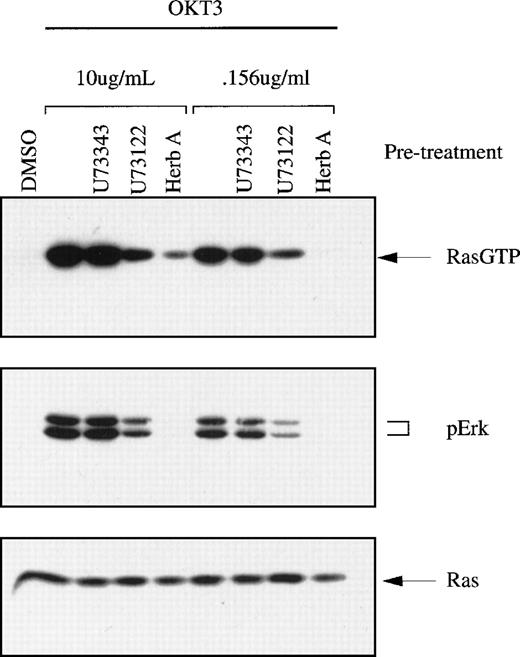
Jurkat T cells can be stimulated with antibodies such as OKT3, which recognizes the extracellular region of the ε chain of CD3, a component of TCR. Treatment with soluble OKT3 activates Ras within 5 minutes in Jurkat T cells. The tyrosine kinase inhibitor herbimycin A interferes with early T-cell signaling events, including Ras activation, probably by inhibiting lck and ZAP-70.32 We confirmed that herbimycin A strongly diminished Ras activation (Figure2). The compound U73122 has been shown to inhibit PLC-γ1 and to block the proliferative response in stimulated T cells.33 We found that U73122 diminished OKT3-stimulated Ras activation in Jurkat T cells. Inhibition was 2.8-fold when a saturating concentration of OKT3 was used and 5.3-fold when a suboptimal concentration of OKT3 was used. The specificity of this inhibition was demonstrated by showing that the inert analogue U73343had no effect.
TCR-stimulated Ras activation in Jurkat T cells and the effects of pharmacologic inhibitors.
Jurkat T cells were preincubated with herbimycin A (protein tyrosine kinase inhibitor), U73343 (inert analogue) or U73122 (PLC-γ1 inhibitor), and then stimulated with OKT3 at either a saturating concentration (10 μg/mL) or at a suboptimal concentration (0.156 μg/mL). Cell lysates were assayed for Ras-GTP using GST-Raf affinity selection followed by immunoblotting to detect precipitated Ras. Phospho-Erk and total Ras in lysates were also detected using immunoblot methods.
TCR-stimulated Ras activation in Jurkat T cells and the effects of pharmacologic inhibitors.
Jurkat T cells were preincubated with herbimycin A (protein tyrosine kinase inhibitor), U73343 (inert analogue) or U73122 (PLC-γ1 inhibitor), and then stimulated with OKT3 at either a saturating concentration (10 μg/mL) or at a suboptimal concentration (0.156 μg/mL). Cell lysates were assayed for Ras-GTP using GST-Raf affinity selection followed by immunoblotting to detect precipitated Ras. Phospho-Erk and total Ras in lysates were also detected using immunoblot methods.
Using subcellular fractionation techniques, RasGRP from untreated cells was found in both the soluble and particulate fractions. After TCR stimulation, a reproducible (n = 7) decrease in the relative amount of soluble RasGRP was detected. In a time-course experiment, this change in RasGRP fractionation behavior paralleled the activation of Ras (Figure 3A and 3B).
RasGRP in membranes of activated T cells.
(A) In a time-course experiment, Jurkat T cells were stimulated with OKT3 for various times and aliquots were assayed for Ras-GTP and total Ras using immunoblotting methods. (B) Homogenates of Jurkat T cells from the various time points were separated into particulate (P) and soluble (S) fractions and RasGRP was immunoblotted with the H176 anti-RasGRP peptide antibody. (C) Membranes from untreated cells, or from cells treated for 10 minutes with OKT3, were assayed for their ability to promote transfer of exogenous guanyl nucleotide to membrane-bound Ras and the sensitivity of this reaction to antibodies raised against the catalytic domain of RasGRP was determined. Membranes were preincubated with IgG prepared from immune (I) or preimmune (pre-I) serum for 12 minutes at 30°C, then [α32P]GTP was added for 1 minute. Ras was then extracted with detergent and precipitated with an anti-Ras monoclonal antibody. Coprecipitated, Ras-associated guanyl nucleotides were resolved by chromatography and quantified. Representative chromatographic data are shown. The graphed values are the average of triplicate assays. Values were normalized to the value obtained when full association was achieved by incubation with EDTA, followed by addition of excess MgCl2 to stabilize the Ras-guanyl nucleotide complex before immune-precipitation. (D) In vitro Ras guanyl nucleotide exchange reactions were performed with recombinant Sos and RasGRP in the presence of IgG to demonstrate the specificity of the J32 antibodies.
RasGRP in membranes of activated T cells.
(A) In a time-course experiment, Jurkat T cells were stimulated with OKT3 for various times and aliquots were assayed for Ras-GTP and total Ras using immunoblotting methods. (B) Homogenates of Jurkat T cells from the various time points were separated into particulate (P) and soluble (S) fractions and RasGRP was immunoblotted with the H176 anti-RasGRP peptide antibody. (C) Membranes from untreated cells, or from cells treated for 10 minutes with OKT3, were assayed for their ability to promote transfer of exogenous guanyl nucleotide to membrane-bound Ras and the sensitivity of this reaction to antibodies raised against the catalytic domain of RasGRP was determined. Membranes were preincubated with IgG prepared from immune (I) or preimmune (pre-I) serum for 12 minutes at 30°C, then [α32P]GTP was added for 1 minute. Ras was then extracted with detergent and precipitated with an anti-Ras monoclonal antibody. Coprecipitated, Ras-associated guanyl nucleotides were resolved by chromatography and quantified. Representative chromatographic data are shown. The graphed values are the average of triplicate assays. Values were normalized to the value obtained when full association was achieved by incubation with EDTA, followed by addition of excess MgCl2 to stabilize the Ras-guanyl nucleotide complex before immune-precipitation. (D) In vitro Ras guanyl nucleotide exchange reactions were performed with recombinant Sos and RasGRP in the presence of IgG to demonstrate the specificity of the J32 antibodies.
Stimulation of Jurkat T cells with OKT3 also resulted in a prompt 5.9-fold increase in the ability of subsequently isolated membranes to support the transfer of [α32P]GTP to endogenous, membrane-bound Ras (Figure 3C). In both the stimulated and untreated membranes, much of this newly associated guanyl nucleotide was converted to GDP during the course of the reaction, a phenomenon that we attribute to the presence of Ras GAPs in these membrane preparations. Ras is constitutively associated with the plasma membrane and we found TCR stimulation does not affect the amount of Ras in T-cell membrane preparations (data not shown). Significantly, we found that stimulated labeling of Ras could be blocked by adding purified antibodies (J32) directed against RasGRP catalytic domain (Figure 3C). In control experiments with purified proteins in vitro, we demonstrated that J32 antibodies specifically inhibit the Ras GEF activity of recombinant RasGRP but not that of Sos (Figure 3D).
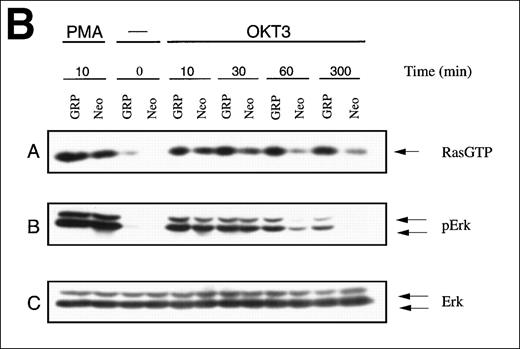
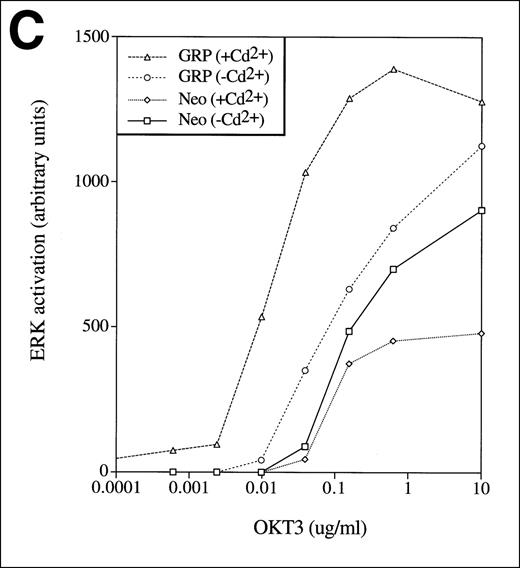
To assess the effects of excess RasGRP on TCR signaling, Jurkat T cells were engineered to overexpress the human RasGRP cDNA using a bovine papilloma-based vector with an inducible promoter (Figure4A). Overexpression of RasGRP resulted in higher OKT3-induced levels of Ras-GTP and Ras activation was relatively sustained, compared to the case with normal RasGRP levels (Figure 4B). Overexpression also modestly enhanced acute activation of Ras by PMA. The level of phospho-Erk closely paralleled the level of Ras-GTP in this experiment. In a separate experiment, we examined the effects of RasGRP overexpression on the level of Erk phosphorylation achieved at various concentrations of OKT3. RasGRP overexpression increased the absolute amount of phosphorylated Erk observed at a saturating concentration of stimulating antibody and it decreased the concentration of OKT3 required to elicit a given level of Erk phosphorylation (Figure 4C).
TCR-Ras-Erk signaling and agonist-induced IL-2 secretion in Jurkat T cells overexpressing RasGRP.
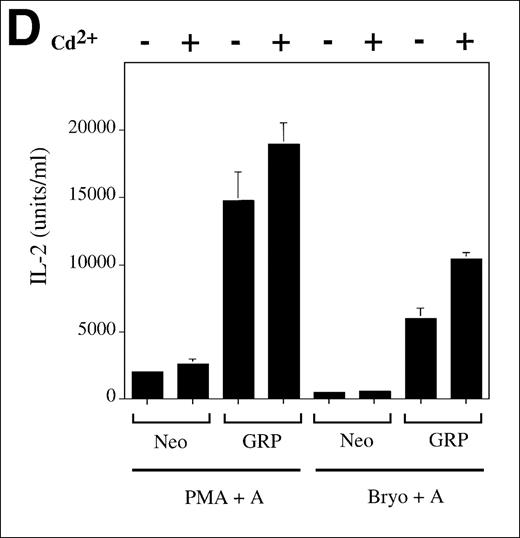
(A) Jurkat cells transformed with either the empty vector pBMGNeo (Neo) or the same vector containing full-length human RasGRP (GRP) were induced with 5.0 μmol/L CdCl2 for 24 hours, as indicated, then examined for RasGRP expression by immunoblotting using the H176 antibody. (B) Cells expressing BMGNeo or RasGRP were induced with CdCl2 for 24 hours then stimulated with either 50 ng/mL PMA for 10 minutes or 0.156 μg/mL OKT3 for various periods. Cells were then harvested, lysed, and assayed for Ras-GTP, phospho-Erk and total Erk. (C) Cells from the same cultures as in (A) were stimulated with various concentrations of OKT3 for 15 minutes at 37°C and then directly lysed in SDS sample buffer. The lysates were then probed for phospho-Erk. The bands representing phospho-Erk were quantified by densitometry and plotted versus the concentration of OKT3. Samples were also probed with an anti-Erk antibody to verify that similar amounts of total Erk protein were present (data not shown). (D) Cells were induced with CdCl2 as indicated for 24 hours then stimulated for 48 hours with the calcium ionophore A23187 (A, 0.5 μmol/L) and either PMA (24 nmol/L) or bryostatin-1 (Bryo, 10 nmol/L). IL-2 in the medium was then measured. Values are the average of quadruplicate samples, with the standard error of the mean indicated.
TCR-Ras-Erk signaling and agonist-induced IL-2 secretion in Jurkat T cells overexpressing RasGRP.
(A) Jurkat cells transformed with either the empty vector pBMGNeo (Neo) or the same vector containing full-length human RasGRP (GRP) were induced with 5.0 μmol/L CdCl2 for 24 hours, as indicated, then examined for RasGRP expression by immunoblotting using the H176 antibody. (B) Cells expressing BMGNeo or RasGRP were induced with CdCl2 for 24 hours then stimulated with either 50 ng/mL PMA for 10 minutes or 0.156 μg/mL OKT3 for various periods. Cells were then harvested, lysed, and assayed for Ras-GTP, phospho-Erk and total Erk. (C) Cells from the same cultures as in (A) were stimulated with various concentrations of OKT3 for 15 minutes at 37°C and then directly lysed in SDS sample buffer. The lysates were then probed for phospho-Erk. The bands representing phospho-Erk were quantified by densitometry and plotted versus the concentration of OKT3. Samples were also probed with an anti-Erk antibody to verify that similar amounts of total Erk protein were present (data not shown). (D) Cells were induced with CdCl2 as indicated for 24 hours then stimulated for 48 hours with the calcium ionophore A23187 (A, 0.5 μmol/L) and either PMA (24 nmol/L) or bryostatin-1 (Bryo, 10 nmol/L). IL-2 in the medium was then measured. Values are the average of quadruplicate samples, with the standard error of the mean indicated.
Although TCR signaling can lead to IL-2 synthesis and secretion in a normal T-cell response, Jurkat T cells do not synthesize IL-2 after treatment with soluble OKT3. Conventionally, a calcium ionophore and a DAG analogue such as PMA, which are thought to activate calcineurin and PKC, respectively, are used to stimulate IL-2 in Jurkat T cells. We found that RasGRP overexpressing Jurkat T cells produced substantially more IL-2 than the control cells on dual stimulation (Figure 4D). This effect was also seen with the combination of calcium ionophore and bryostatin-1, a heterocyclic lactone that also acts as a DAG analogue.34-37 Note that for each DAG analogue, the levels of IL-2 measured roughly correlate with the levels of RasGRP expressed in these cells (Figure 4A). Negligible IL-2 was detected in the medium when no agonist or only 1 agonist was used (data not shown).
Discussion
The presence of RasGRP in T cells leads to the hypothesis that it positively regulates Ras during TCR signaling. This hypothesis is supported by the following observations: (1) TCR-induced Ras activation is diminished by a PLC-γ1 inhibitor, (2) RasGRP is differentially associated with membranes after TCR stimulation, (3) the increased Ras guanyl nucleotide association activity in membranes from TCR-stimulated cells is inhibited by anti-RasGRP antibodies, and (4) Jurkat T cells that overexpress RasGRP are hypersensitive to TCR-Ras-Erk signaling and hypersensitive to agonist-induced IL-2 secretion. These studies support a model of T-cell signaling whereby receptor stimulation leads to activation of PLC-γ1 followed by the generation of membrane DAG, RasGRP membrane recruitment, and Ras activation. Our model may help explain several puzzling facts about TCR signaling including the observation that stimulation of PLC-γ1 by ectopically expressed G-protein–coupled receptors leads to T-cell activation.38One could propose that PLC-γ1–generated free cytoplasmic calcium regulates RasGRP through its EF hands. However, we observed that RasGRP can activate Ras in vivo even in the presence of EGTA plus BAPTA/AM, compounds that sequester extracellular and intracellular calcium, respectively (data not shown).
Diacylglycerol is known to activate PKC and independent evidence indicates that PKC function is required for TCR signaling.39 Indeed, PMA-stimulated PKC probably activates phospholipase D, which can indirectly generate DAG at later stages of T cell activation.40 Regardless of this complexity, the demonstration here that DAG analogues can also directly activate RasGRP and thereby promote Ras signaling in T cells is exciting. Bryostatins activate PKC, have potent growth inhibitory effects on certain cell types, and are currently being tested in clinical trials as antineoplastic agents.41 Like PMA, however, bryostatin-1 also activates Ras in Jurkat T cells and in rat2 cells that ectopically express RasGRP (Stang and Stone, unpublished data). Furthermore, in this latter situation, bryostatin-1 activates Ras-Erk by a mechanism that depends on the DAG-binding domain of RasGRP. Taken together, our results suggest that DAG analogues could be exploited to regulate RasGRP and thereby modulate the immune response.
It should be noted that Ras-Erk signaling is thought to play important roles at a number of stages in the life of a T cell. Early growth and maturation in the thymus are dependent on a pre-TCR complex42 and on Ras-Erk signaling.43,44 After T-cell maturation, autocrine and paracrine action of IL-2 is thought to contribute to Ras activation.45 46 RasGRP could facilitate Ras activation during these stages, as well.
Our findings do not address the role of Sos in TCR signaling, nor do they preclude a role for PKC down-regulation of Ras GAPs. Multiple forms of Ras regulation might allow a common Ras switch to differentially respond to diverse external stimuli and developmental cues, and thereby orchestrate distinct biochemical changes and biologic responses. For example, the amplitude or duration of Ras signaling might be varied according to the combination of regulatory mechanisms deployed.
Acknowledgments
We thank Gideon Bollag for recombinant Sos, Ed Chan for useful suggestions, and Nancy Dower for careful reading of the manuscript.
Supported by grants to J.C.S., H.L.O., and R.C.B. from the Medical Research Council and the National Cancer Institute of Canada.
Reprints:James C. Stone, Department of Biochemistry, University of Alberta, Edmonton, Alberta, Canada T6G 2H7; e-mailjim.stone@ualberta.ca.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 3. RasGRP in membranes of activated T cells. / (A) In a time-course experiment, Jurkat T cells were stimulated with OKT3 for various times and aliquots were assayed for Ras-GTP and total Ras using immunoblotting methods. (B) Homogenates of Jurkat T cells from the various time points were separated into particulate (P) and soluble (S) fractions and RasGRP was immunoblotted with the H176 anti-RasGRP peptide antibody. (C) Membranes from untreated cells, or from cells treated for 10 minutes with OKT3, were assayed for their ability to promote transfer of exogenous guanyl nucleotide to membrane-bound Ras and the sensitivity of this reaction to antibodies raised against the catalytic domain of RasGRP was determined. Membranes were preincubated with IgG prepared from immune (I) or preimmune (pre-I) serum for 12 minutes at 30°C, then [α32P]GTP was added for 1 minute. Ras was then extracted with detergent and precipitated with an anti-Ras monoclonal antibody. Coprecipitated, Ras-associated guanyl nucleotides were resolved by chromatography and quantified. Representative chromatographic data are shown. The graphed values are the average of triplicate assays. Values were normalized to the value obtained when full association was achieved by incubation with EDTA, followed by addition of excess MgCl2 to stabilize the Ras-guanyl nucleotide complex before immune-precipitation. (D) In vitro Ras guanyl nucleotide exchange reactions were performed with recombinant Sos and RasGRP in the presence of IgG to demonstrate the specificity of the J32 antibodies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/10/10.1182_blood.v95.10.3199/5/m_bloo01037003aw.jpeg?Expires=1769113227&Signature=Bo9eQzykqPXrivcbFSLI7c3C6jOhb8U0c8CQ1uopA-NSwLjCphsxmyF4~YqPgmifDvJ5v91SZzWhMetMMi~0MHA~GF2H5OjXEoVegkm8vogkHCUoxWnCPm8ezUtn~fduyTIZWP6uHHLJB2jzJebocZ4w7VMihIH1MqAukdEvSPE-clJFu6z8P3thzQKAv2h2H9NqCQRGpH5SHJqGKOXHqTkfWftGixC-mIteke3okCXUTSLP2BUqB3Dijlw6HLn~v94jL-Cr6~V-wCVmw4bv6NeBN4AvfU1C~5tP-hBwWAkDMND5ez4nDI8jmaQsIAvAka4U3yhMz-exiQhxQdB70Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. RasGRP in membranes of activated T cells. / (A) In a time-course experiment, Jurkat T cells were stimulated with OKT3 for various times and aliquots were assayed for Ras-GTP and total Ras using immunoblotting methods. (B) Homogenates of Jurkat T cells from the various time points were separated into particulate (P) and soluble (S) fractions and RasGRP was immunoblotted with the H176 anti-RasGRP peptide antibody. (C) Membranes from untreated cells, or from cells treated for 10 minutes with OKT3, were assayed for their ability to promote transfer of exogenous guanyl nucleotide to membrane-bound Ras and the sensitivity of this reaction to antibodies raised against the catalytic domain of RasGRP was determined. Membranes were preincubated with IgG prepared from immune (I) or preimmune (pre-I) serum for 12 minutes at 30°C, then [α32P]GTP was added for 1 minute. Ras was then extracted with detergent and precipitated with an anti-Ras monoclonal antibody. Coprecipitated, Ras-associated guanyl nucleotides were resolved by chromatography and quantified. Representative chromatographic data are shown. The graphed values are the average of triplicate assays. Values were normalized to the value obtained when full association was achieved by incubation with EDTA, followed by addition of excess MgCl2 to stabilize the Ras-guanyl nucleotide complex before immune-precipitation. (D) In vitro Ras guanyl nucleotide exchange reactions were performed with recombinant Sos and RasGRP in the presence of IgG to demonstrate the specificity of the J32 antibodies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/10/10.1182_blood.v95.10.3199/5/m_bloo01037003bw.jpeg?Expires=1769113227&Signature=CfuGvdX4vUw7RcYoHvKGkZW1naWmttv7Vgcn0wf9mFuYsWvtbIIVRD-i8yyn~V40GEHiymR7f3Ky3Erv5tenlZ89w0DGsdAqNpz45zWzEDb5EGKfhufIjluDv2UVgZ13Y~zQdhtBgeVPHC0rwls5Mm5ZPoU~n34mazfMy3m8Dkq91q7SqrwyRnaxCnRdBzQGZyxDPmPN2MdS-Zr1ptrvC~wFstJBQIe8GwaKMHPAw8~bnIO~S~fDRp9UlSAnmpB4QbtgJJl7R7RJI5wT6G26gKCxF3m7WRu1K81uOpA-5u9jY3LweBhYJyUu1zdSpz5tEP6A4AIStGfInRi6PvBpng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. RasGRP in membranes of activated T cells. / (A) In a time-course experiment, Jurkat T cells were stimulated with OKT3 for various times and aliquots were assayed for Ras-GTP and total Ras using immunoblotting methods. (B) Homogenates of Jurkat T cells from the various time points were separated into particulate (P) and soluble (S) fractions and RasGRP was immunoblotted with the H176 anti-RasGRP peptide antibody. (C) Membranes from untreated cells, or from cells treated for 10 minutes with OKT3, were assayed for their ability to promote transfer of exogenous guanyl nucleotide to membrane-bound Ras and the sensitivity of this reaction to antibodies raised against the catalytic domain of RasGRP was determined. Membranes were preincubated with IgG prepared from immune (I) or preimmune (pre-I) serum for 12 minutes at 30°C, then [α32P]GTP was added for 1 minute. Ras was then extracted with detergent and precipitated with an anti-Ras monoclonal antibody. Coprecipitated, Ras-associated guanyl nucleotides were resolved by chromatography and quantified. Representative chromatographic data are shown. The graphed values are the average of triplicate assays. Values were normalized to the value obtained when full association was achieved by incubation with EDTA, followed by addition of excess MgCl2 to stabilize the Ras-guanyl nucleotide complex before immune-precipitation. (D) In vitro Ras guanyl nucleotide exchange reactions were performed with recombinant Sos and RasGRP in the presence of IgG to demonstrate the specificity of the J32 antibodies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/10/10.1182_blood.v95.10.3199/5/m_bloo01037003cw.jpeg?Expires=1769113227&Signature=eyFSsywJlNYeWfY1TLk7oGhBK1k91EtiWNeUN6EWMK~a303dQe~BT837anBz8NByv2D0QIFNiJqragJdy0RcOmkowjqiVUZPgY5s3sUUZhCKPV60k9hc4ZUDBXTIe4l3n3F02tUS82aMvoaIVtxEbtVFEvFc2E~ltYeAaOLH3Wp6cy06OoHCq2yo9Nv-qp-KOA5ub9Z7OII11ieLsiaYHpZ-3ledH6wJdmOhjcyDXAz3efuOsYhMPDYpwnNHbizTZOM1mDFYsM~RzoW5sTV0tIykO~LczEjcOT2kvheIEPRfOhZNayJqX4PlKgm~1ZABj~L-WLONHfa6t8CfWgc7ww__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. RasGRP in membranes of activated T cells. / (A) In a time-course experiment, Jurkat T cells were stimulated with OKT3 for various times and aliquots were assayed for Ras-GTP and total Ras using immunoblotting methods. (B) Homogenates of Jurkat T cells from the various time points were separated into particulate (P) and soluble (S) fractions and RasGRP was immunoblotted with the H176 anti-RasGRP peptide antibody. (C) Membranes from untreated cells, or from cells treated for 10 minutes with OKT3, were assayed for their ability to promote transfer of exogenous guanyl nucleotide to membrane-bound Ras and the sensitivity of this reaction to antibodies raised against the catalytic domain of RasGRP was determined. Membranes were preincubated with IgG prepared from immune (I) or preimmune (pre-I) serum for 12 minutes at 30°C, then [α32P]GTP was added for 1 minute. Ras was then extracted with detergent and precipitated with an anti-Ras monoclonal antibody. Coprecipitated, Ras-associated guanyl nucleotides were resolved by chromatography and quantified. Representative chromatographic data are shown. The graphed values are the average of triplicate assays. Values were normalized to the value obtained when full association was achieved by incubation with EDTA, followed by addition of excess MgCl2 to stabilize the Ras-guanyl nucleotide complex before immune-precipitation. (D) In vitro Ras guanyl nucleotide exchange reactions were performed with recombinant Sos and RasGRP in the presence of IgG to demonstrate the specificity of the J32 antibodies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/10/10.1182_blood.v95.10.3199/5/m_bloo01037003dx.jpeg?Expires=1769113227&Signature=kJYJNurg-CWkqCBOv599xYwuYg~vhSvQjrxtDi7sDKHMOTrbd-ZuF1IkYyw5bqDXQNGvIdIrh9peA~WLmpLfHLDfN8C1VU0R9fizLQ2juPABVW8W9xq~u3QiyRMFfVseq4-ylE6wqlZPWnKcfnLiFOS8qc88tQDCRKhwkwXHssN~YJGmtw0oQgT22GD2c1RS07WyEmwMS2p3HnKZktp9DmAdoL3md1yXvyGEKolKWK5ih8aKVm3m-EI9qg~w4L5rZhQ~04o0Pcn3LsuoxtAPhez7e9hkvRxE9nlHwfhKdyNYPxtfU6lmzObulQwj1tOZf058ikEN83Ch~h7L~47xgQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal