Abstract
Interleukin (IL)-12 plays a critical role in modulating the activities of natural killer (NK) cells and T lymphocytes. In animal models, IL-12 has potent antitumor effects that are likely mediated by its ability to enhance the cytotoxic activity of NK cells and cytotoxic T lymphocytes, and to induce the production of interferon (IFN)-γ by NK and T cells. In addition to IL-12, NK cells are responsive to IL-2, and may mediate some of the antitumor effects of IL-2. In this study, we examine the interaction between IL-2 and the signaling events induced by IL-12 in NK cells. We find that IL-2 not only up-regulates the expression of IL-12Rβ1 and IL-12Rβ2, it also plays an important role in up-regulating and maintaining the expression of STAT4, a critical STAT protein involved in IL-12 signaling in NK cells. In contrast to the effects of IL-2 alone, expression of IL-12 receptors and STAT4 are unaffected or decreased by IL-12 or the combination of IL-2 and IL-12. Through expression of high levels of IL-12 receptors and STAT4, IL-2–primed NK cells show enhanced functional responses to IL-12 as measured by IFN-γ production and the killing of target cells. NK cells from cancer patients who received low-dose IL-2 treatment also exhibited increased expression of IL-12 receptor chains, suggesting that IL-2 may enhance the response to IL-12 in vivo. These findings provide a molecular framework to understand the interaction between IL-2 and IL-12 in NK cells, and suggest strategies for improving the effectiveness of these cytokines in the immunotherapy of cancer.
Interleukin (IL)-12 is a heterodimeric cytokine consisting of p35 and p40 subunits. It was originally identified as natural killer (NK) cell stimulatory factor (NKSF),1 and is produced mainly by antigen-presenting cells. IL-12 plays an important role in moduating both innate and adaptive immune responses. It induces the production of interferon (IFN)-γ from human NK cells and T cells, and also plays a key role in promoting the development of the Th1-type immune response.2 In addition, IL-12 enhances the cytotoxicity of NK cells and cytotoxic T lymphocytes (CTL). NK cells activated by IL-12 exhibit enhanced cytotoxic activity against NK cell sensitive and resistant target cells, both in vitro and in vivo.3-6
IL-12 exerts its functions by binding to specific cell surface receptors and signaling through the Jak-STAT pathway. Two IL-12 receptor subunits, IL-12Rβ1 and IL-12Rβ2, have been identified. Each receptor subunit has a low IL-12 binding affinity, and neither is capable of transmitting an IL-12 signal on its own.7,8 The functional IL-12 receptor, which binds to IL-12 with high affinity, is the heterodimeric receptor formed by IL-12Rβ1 and IL-12Rβ2.8 IL-12Rβ1 is constitutively expressed on a large portion of resting T cells and is relatively unaffected by cytokines that induce Th1 or Th2 differentiation. On the other hand, the expression of IL-12Rβ2 is only detectable on activated T cells and is enhanced by cytokines that promote Th1 development and inhibited by cytokines that promote Th2 development.8-12 Therefore, regulating the expression of IL-12Rβ2 may be a key step in modulating IL-12 function. In addition to forming the high-affinity receptor with IL-12Rβ1, IL-12Rβ2 also plays a unique role in IL-12 signaling. Unlike IL-12Rβ1, the cytoplasmic domain of IL-12Rβ2 becomes tyrosine phosphorylated on IL-12 binding. In addition, IL-12Rβ2 provides the binding sites for Tyk2 and Jak2, the kinases responsible for activating STAT proteins.13 Several STAT proteins, including STAT1, STAT3, STAT4, and STAT5, have been implicated in IL-12 signaling in T cells.8,14 Among these STATs, STAT4 is essential in mediating IL-12 function in T and NK cells.13,15-17 All major functions of IL-12 are abrogated in STAT4-deficient mice, including production of IFN-γ and NK cell cytotoxic activity.18 19 This suggests that regulating the expression of STAT4 may also be an important control point in modulating IL-12 function in NK and T cells.
The ability of IL-12 to augment the cytotoxicity of NK cells and CTL suggested a therapeutic role for this cytokine in cancer treatment. In preclinical studies using several murine tumor models, IL-12 demonstrated potent antitumor activity. Administration of IL-12 to tumor-bearing mice resulted in the regression of established tumors, systemic antitumor activity, and long-term survival.20-22IL-12–induced augmentation of cytolytic activity and proliferation of lymphocytes from cancer patients has also been demonstrated both in vitro and in vivo.23 NK cells have been implicated as playing a major role in the antitumor effect of IL-12. Depletion of NK cells abolishes IL-12–induced cytotoxicity and IFN-γ production in mouse models, and abrogates the antimetastatic effect of IL-12 in these mice.24
Although the antitumor effect of IL-12 in murine models is promising, the use of IL-12 as a therapeutic anticancer agent in humans has been disappointing because of its toxicity and decreased responsiveness over time.25 Several approaches have been considered to improve the effectiveness of IL-12 in cancer therapy, including the combination of IL-12 with other immunomodulatory cytokines such as IL-2. IL-2 has been shown to enhance the proliferation and cytotoxicity of NK cells in vitro and in vivo.26-28 In vitro studies demonstrate that the combination of IL-2 and IL-12 has a synergistic effect in promoting IFN-γ production by T and NK cells, and the cytotoxic activity of NK cells.29-31 Although the combination of IL-12 and IL-2 has been associated with severe toxicity in mouse models,32,33the treatment of mice bearing metastatic Renca tumors with IL-12 and pulse IL-2 was shown to be less toxic and more effective than IL-12 or IL-2 alone in inducing tumor regression.32 This suggests that the anticancer activity of the combination of IL-12 and IL-2 may rely on the strategic design of a regimen, which ultimately should be based on an understanding of the mechanisms by which IL-2 affects IL-12 function.
In light of the important role that NK cells play in IL-12–induced antineoplastic effects and the potential importance of combination therapy with IL-12 and IL-2, we investigated the effect of IL-2 on IL-12 activated signaling pathways and functions in NK cells. Our data show that pretreatment with IL-2, but not IL-12 or the combination of IL-12 and IL-2, significantly enhances IL-12 signaling in NK cells. Priming NK cells with IL-2 before IL-12 treatment leads to elevated expression of both the IL-12 receptor chains and STAT4, and enhances the response of NK cells to IL-12. These findings provide evidence that IL-2, when used alternately with IL-12 in immunotherapy, may significantly enhance the antitumor activity of IL-12.
Materials and methods
Cytokines, antibodies, and reagents
Recombinant human IL-2 (specific activity 6 × 106 U/mL) was generously provided by Amgen (Thousand Oaks, CA). Human recombinant IL-12 (specific activity 1.7 × 107 U/mg) was kindly provided by Genetics Institute (Cambridge, MA). Purified unconjugated, phycoerythrin (PE) or PC5-conjugated murine monoclonal antibodies (mAbs), including B1 (CD20, IgG2a), My4 (CD14, IgG2b), NKH1 (CD56, IgG1), N901 (CD56, IgG1), T1 (CD5, IgG2a), T3 (CD3, IgG1), and isotype control MsIgG1, were obtained from Coulter Immunology (Hialeah, FL). PE-conjugated goat antimouse IgG1 was purchased from Southern Biotechnology Associates Inc (Birmingham, AL). The generation of anti–IL-12 receptor β1 subunit, β44 was described previously.11 STAT1, STAT5, and STAT4 antibodies were purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA). Expression vector pRC42, which contains an EF-1α promoter and hygromycin resistant marker, was kindly provided by Dr Gordon Freeman (Dana-Farber Cancer Institute).
Generation of antihuman IL-12 receptor β2 chain antibodies
Human IL-12 receptor β2 subunit (IL-12Rβ2) cDNA was generated by reverse transcriptase–polymerase chain reaction (RT-PCR) with IL-12Rβ2 specific primers based on the sequence information in GenBank (Accession U64198). The cDNA was then cloned into expression vector pRC42. The newly constructed IL-12Rβ2–expressing plasmid RC-Rb2 was transfected into the murine cell line 300-19 and CHO cell line. Transfected cells were selected with hygromycin B. Single clones from hygromycin B–resistant cells were then screened for their IL-12 binding activity by labeling the cells with IL-12, mouse anti–IL-12 antibody (IgG1, Genetics Institute), and PE-conjugated goat antimouse IgG1 sequentially. The fluorescence of each clone was analyzed on a Coulter EPICS XL cytometer (Coulter, Miami, FL). IL-12Rβ2–expressing 300-19 clones were used to immunize BALB/c mice to generate anti–IL-12Rβ2 antibodies. Two monoclonal antibodies against IL-12Rβ2, 5A7 (IgG1) and 7A8 (IgG1), were generated and bind to the RC-Rb2 transfected CHO cells but not to CHO cells transfected with the vector alone. Both antibodies also bind to PHA-activated T cells but not resting T cells. The 5A7 but not 7A8 blocked IL-12–induced STAT4 activation in activated T cells, and IL-12 induced proliferation in activated T cells (data not shown).
Immunofluorescence analysis of the binding specificity of anti–IL-12 receptor antibodies to IL-12 receptor chains
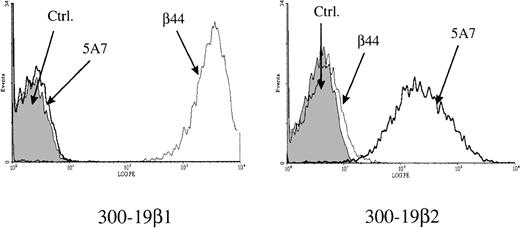
Murine cell line 300-19 transfected with IL-12Rβ1 (300-19β1) or IL-12Rβ2 (300-19β2) were labeled with isotype control mouse IgG1, anti–IL-12Rβ1 antibody β44, or anti–IL-12Rβ2 antibody 5A7. All samples were then labeled with secondary antibody PE-conjugated goat antimouse IgG1. The binding of the antireceptor antibodies to cell surface receptors was analyzed by flow cytometry. As shown in Figure1, 5A7 interacted only with 300-19β2 cells and β44 interacted only with 300-19β1 cells. This indicates that the 5A7 monoclonal antibody we developed for these experiments specifically interacts with IL-12Rβ2 and does not cross-react with IL-12Rβ1.
Binding of anti–IL-12 receptor antibodies to IL-12 receptors.
The 300-19 cell lines transfected with IL-12Rβ1 (300-19β1) or IL-12Rβ2 (300-19β2) were labeled with mouse IgG1, anti–L-12Rβ1 antibody, β44 or anti-IL-12Rβ2 antibody, 5A7 and analyzed by flow cytometry.
Binding of anti–IL-12 receptor antibodies to IL-12 receptors.
The 300-19 cell lines transfected with IL-12Rβ1 (300-19β1) or IL-12Rβ2 (300-19β2) were labeled with mouse IgG1, anti–L-12Rβ1 antibody, β44 or anti-IL-12Rβ2 antibody, 5A7 and analyzed by flow cytometry.
Purification and culture of natural killer cells
Blood samples enriched with white blood cells were obtained from healthy donors undergoing platelet pheresis in the Dana-Farber Cancer Institute Blood Donor Center. Peripheral blood mononuclear cells (PBMCs) were isolated from blood samples by Ficoll-Hypaque (Pharmacia LKB) gradient centrifugation. Primary NK cells (more than 85% purity) were obtained by depleting monocytes, B cells, and T cells from PBMCs with B1 (CD20, IgG2a), My4 (CD14, IgG2b), T1 (CD5, IgG2a), and T3 (CD3, IgG1) antibodies and immunomagnetic anti-IgG beads (PerSeptive Biosystems, Framingham, MA). Purified NK cells were cultured at 2 × 106 cells per millilter in RPMI 1640 medium containing 15% fetal calf serum, with or without cytokines as indicated. All cytokines were used at 100 U/mL, if not specified.
Immunofluorescence analysis of IL-12 receptor expression
Primary or cultured T cells and NK cells were incubated with 20% goat serum in RPMI to reduce the background binding by the secondary antibody, goat antimouse IgG1-PE. Cells were then labeled with IgG1 isotype control, anti–IL-12Rβ1 antibody β44, or anti–IL-12Rβ2 antibody 5A7 and secondary antibody PE-conjugated goat antimouse IgG1. Samples were incubated with PC5-conjugated CD56 for testing on NK cells or PC5-conjugated CD3 for T cells. The expression of IL-12 receptors on NK cells was analyzed by flow cytometry by gating on the CD56+ population, and the expression on T cells was analyzed by gating on the CD3+ population. In the IL-12 competition assay, NK cells were first incubated with IL-12 (100 U/mL) for 15 minutes, washed, and then labeled with antibodies as describe above.
Whole-cell extracts and Western blotting
Whole-cell extracts were prepared as described previously.17 Protein was separated on a 9% gel by SDS-PAGE, then electrotransferred to nitrocellulose. The membrane was blocked with 5% dry milk in TBST (100 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, and 0.05% Tween-20) and probed with primary antibody diluted 1:10 000 in 5% bovine serum albumin (BSA). After incubation with horseradish peroxidase–conjugated secondary antibody, specific protein was detected by chemiluminescence (ECL, NEN Life Science Products).
Nuclear extracts and electrophoretic mobility shift assay
The STAT binding oligonucleotide used, 5′-GAGCCTGATTTCCCCGAAATGATGAGC-3′ and its complement, is derived from the IFN-γ responsive factor 1 gene promoter.34 NK cells were primed as indicated for 3 days. Cells were then washed and treated with IL-12 for 30 minutes. Nuclear extracts were prepared and 5 μg of nuclear extract was used in the electrophoretic mobility shift assay (EMSA) as described previously.17
Interferon-γ enzyme-linked immunosorbent assay
The NK cells in equal numbers were primed for 3 days with or without cytokines as indicated, and supernatants were collected on the third day. Cells were then washed and plated at 30 000 cells per well in duplicate into a 96-well U-bottom plate and cultured with or without IL-12 for 3 days, after which the supernatants were collected. The concentration of IFN-γ in all supernatants was determined by IFN-γ enzyme-linked immunosorbent assay (ELISA) (Endogen, Boston, MA).
Cytotoxicity assay
The NK cells were primed for 3 days with or without cytokines as indicated. Cells were then washed and cultured with or without IL-12 overnight. 51Cr-labeled Colo target cells were plated into a 96-well plate with overnight cultured NK cells at a 5:1 effector-to-target ratio and incubated for 4 hours at 37°C. Supernatants were then harvested and the release of 51Cr was measured with a gamma counter. The percentage of specific cytotoxicity was calculated as previously described.3
Analysis of IL-12 receptor expression on natural killer cells from patients receiving IL-2 treatment
Samples of PBMCs were cryopreserved from patients with metastatic cancer enrolled in a clinical trial to examine the toxicity and efficacy of continuous infusion IL-2, followed by bolus infusion of IL-2.35 This clinical trial was approved by the Human Subjects Protection Committee of the Dana-Farber Cancer Institute and informed consent was obtained from each patient. Samples obtained after 4 weeks of continuous infusion IL-2 contained increased numbers of CD56+ NK cells and were used in the current experiments to examine the expression of IL-12 receptors. All samples were labeled with anti–IL-12Rβ1 or anti–IL-12Rβ2 antibodies as described above and with PC5-conjugated CD56. The expression of IL-12Rβ1 and IL-12Rβ2 on NK cells from these samples was determined with flow cytometry by gating on the CD56+ population.
Results
Natural killer cells differ from T cells in IL-12 receptor chain expression.
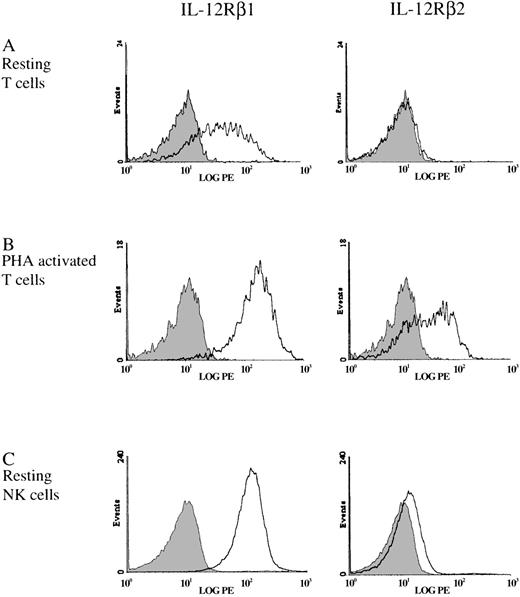
With the use of the monoclonal antibodies to IL-12Rβ1 and IL-12β2 that we developed, the expression of these receptor chains on human NK and T cells was examined. Resting T cells showed significant expression of IL-12Rβ1 as detected by the binding of anti–IL-12Rβ1 antibody, β44, whereas the expression of IL-12Rβ2 was not detectable (Figure2A). This result is in agreement with previous reports, which showed that, although a large portion of resting PBMCs express IL-12Rβ1, they do not bind IL-12, and the mRNA for the IL-12Rβ2 is not detectable in this population.8,9The expression of IL-12Rβ2 on T cells was markedly enhanced after activation with the mitogen phytohemagglutinin (PHA) (Figure 2B), consistent with the known increase in mRNA for this subunit.8 The expression of IL-12Rβ1 was also elevated by PHA activation. In comparison with resting T cells, the expression of IL-12Rβ1 and IL-12Rβ2 on resting NK cells showed notable differences. The level of IL-12Rβ1 expression on resting NK cells was significantly higher than that on resting T cells, and was comparable to the expression on PHA-activated T cells (Figure 2B and C). Unlike resting T cells, resting NK cells express IL-12Rβ2 (Figure 2C), which correlates with our previous observations that IL-12 induces STAT4 activation, specific cytotoxic killing, and proliferation in resting NK cells.3 17 This suggests that the expression of IL-12Rβ2 on resting NK cells, although at low level, is sufficient for resting NK cells to respond to IL-12. In both resting T cells and resting NK cells, which have a high expression of IL-12Rβ1, IL-12Rβ2 appeared to be the limiting component of the functional IL-12 receptor.
Expression of IL-12Rβ1 and IL-12Rβ2 on T cells and NK cells.
Cell surface IL-12Rβ1 and IL-12Rβ2 was measured by flow cytometry on purified resting T cells (A), PHA-activated T cells (B), or purified resting NK cells (C). The shaded area represents the isotype negative control.
Expression of IL-12Rβ1 and IL-12Rβ2 on T cells and NK cells.
Cell surface IL-12Rβ1 and IL-12Rβ2 was measured by flow cytometry on purified resting T cells (A), PHA-activated T cells (B), or purified resting NK cells (C). The shaded area represents the isotype negative control.
IL-2 and IL-12 modulate the expression of IL-12 receptor chains on NK cells.
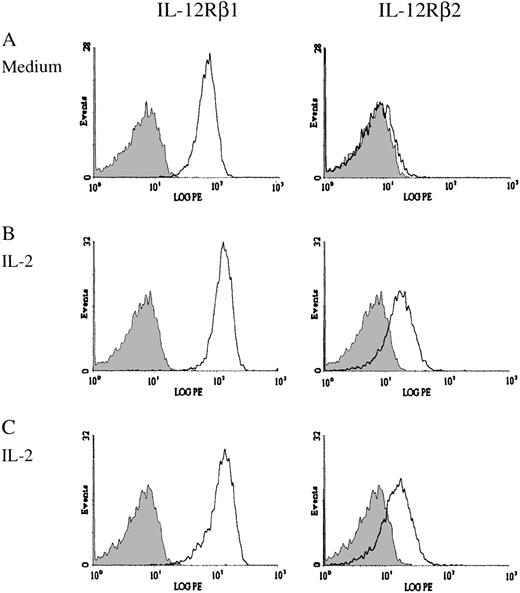
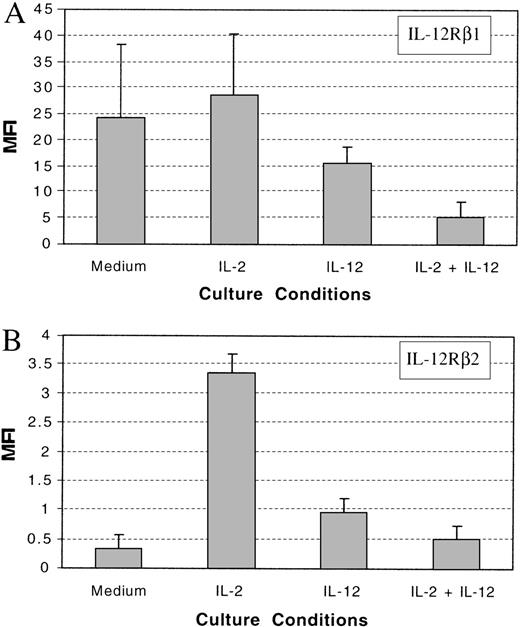
Resting NK cells express both IL-12Rβ1 and IL-12Rβ2 and are responsive to IL-12. Resting NK cells also express intermediate-affinity IL-2 receptors and respond directly to IL-2 without prior activation.36 37 To investigate the effect of IL-2 and IL-12 on the expression of IL-12Rβ1 and IL-12Rβ2 on NK cells, we purified NK cells from the PBMCs of healthy donors and cultured them in vitro with medium alone or supplemented with IL-2, IL-12, or IL-2 plus IL-12. After 3 days of incubation, the expression of IL-12Rβ1 and IL-12Rβ2 was tested by immunofluorescence analysis. The expression of IL-12Rβ1 was slightly increased in NK cells treated with IL-2 in comparison with medium alone (Figure3A and B and Figure4A). By contrast, IL-12 or the combination of IL-2 and IL-12 reduced the expression of IL-12Rβ1 (Figure 4A). To test whether IL-12, which was present in these culture conditions, would compete with the anti–IL-12R antibodies in binding to the IL-12 receptor, we incubated IL-2–cultured NK cells with IL-12 before antibody labeling. The addition of IL-12 did not significantly affect the binding of either anti–IL-12Rβ1 antibody or anti–IL-12Rβ2 antibody to the cell surface receptor (Figure 3B and C). This indicated that the reduced expression of IL-12Rβ1 in IL-12– or IL-2 plus IL-12–cultured NK cells was not due to competition between IL-12 in the culture medium and the anti–IL-12Rβ1 antibody. Although the expression of IL-12Rβ1 was reduced by IL-12 or the combination of IL-2 and IL-12, there was still significant expression of IL-12Rβ1 on NK cells (Figure 4A). It is not clear whether the reduction in the expression of IL-12Rβ1 would significantly impact IL-12 signaling in NK cells. The expression of IL-12Rβ2 on NK cells was slightly increased by priming with IL-12 or the combination of IL-2 and IL-12, but it was significantly enhanced when NK cells were primed with IL-2 (Figure 4B). The expression of IL-12Rβ2 on NK cells cultured with IL-2 increased 10-fold in comparison with that on NK cells cultured without cytokines, and 3-fold over NK cells primed with IL-12 (Figure 4B).
Expression of IL-12Rβ1 and IL-12Rβ2 on cytokine-primed NK cells.
Purified NK cells were incubated for 3 days in the absence or presence of IL-2. Expression of IL-12 receptor chains was then determined by flow cytometry. Representative histograms demonstrate the expression of IL-12Rβ1 and IL-12Rβ2 on NK cells cultured without IL-2 (A) or with IL-2 (B). In (C), NK cells cultured with IL-2 were incubated with IL-12 before antibody labeling. The shaded area represents the isotype negative control.
Expression of IL-12Rβ1 and IL-12Rβ2 on cytokine-primed NK cells.
Purified NK cells were incubated for 3 days in the absence or presence of IL-2. Expression of IL-12 receptor chains was then determined by flow cytometry. Representative histograms demonstrate the expression of IL-12Rβ1 and IL-12Rβ2 on NK cells cultured without IL-2 (A) or with IL-2 (B). In (C), NK cells cultured with IL-2 were incubated with IL-12 before antibody labeling. The shaded area represents the isotype negative control.
Summary of the expression of IL-12Rβ1 and IL-12Rβ2.
A summary of the expression of IL-12Rβ1 (A) and IL-12Rβ2 (B) on cultured NK cells with or without cytokines from 3 healthy donors. MFI: mean fluorescent intensity.
Summary of the expression of IL-12Rβ1 and IL-12Rβ2.
A summary of the expression of IL-12Rβ1 (A) and IL-12Rβ2 (B) on cultured NK cells with or without cytokines from 3 healthy donors. MFI: mean fluorescent intensity.
IL-2 and IL-12 modulate the expression of STAT4 in natural killer cells.
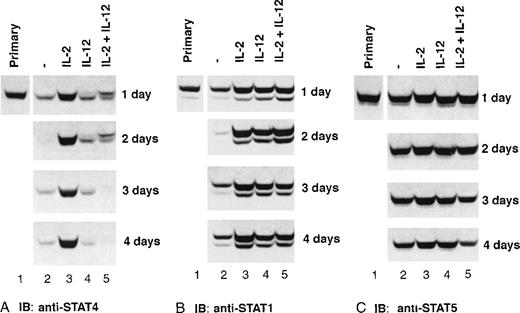
Because STAT4 is the only known STAT protein that mediates IL-12 signaling in NK cells, we investigated whether IL-2 treatment would alter the expression of STAT4. After culture with or without cytokines, cell lysates were prepared from highly purified NK cells, and the expression of STAT4 in the cells was monitored by Western blot. The expression of STAT4 was enhanced by IL-2, compared with freshly isolated NK cells, and was maintained at a similar level over 4 days of culture (Figure 5A). In NK cells cultured with medium alone, IL-12, or IL-2 plus IL-12, the level of STAT4 was dramatically reduced in 1 day and further decreased over the time, indicating that the expression of STAT4 is dependent on IL-2 but not IL-12. The slower migrating band that appears in IL-12– or IL-2 plus IL-12–treated NK cells is the phosphorylated STAT4 induced by IL-2 or IL-12 in NK cells.17 In contrast to the dependency of STAT4 on IL-2, the level of STAT5 is stable in NK cells in all culture conditions examined over the 4 days (Figure 5C). The expression of STAT1 appeared to be dependent on either IL-2 or IL-12, and STAT1 decreased in cells cultured without either cytokine (Figure 5B). These data suggest that there are different mechanisms regulating the expression of these STAT proteins in NK cells.
Expression of STAT proteins in cytokine- primed NK cells.
Purified NK cells were primed with cytokines as indicated. Cells were collected at the indicated times, whole-cell extracts were prepared, and Western blots were performed with anti-STAT4 (A), and reprobed with anti-STAT1 (B) and anti-STAT5 (C). Representative data from 1 of 3 experiments is shown.
Expression of STAT proteins in cytokine- primed NK cells.
Purified NK cells were primed with cytokines as indicated. Cells were collected at the indicated times, whole-cell extracts were prepared, and Western blots were performed with anti-STAT4 (A), and reprobed with anti-STAT1 (B) and anti-STAT5 (C). Representative data from 1 of 3 experiments is shown.
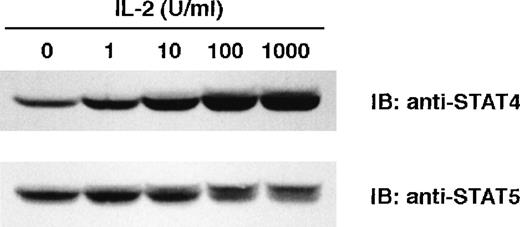
When used at a concentration of 100 U/mL, IL-2 consistently promotes the expression of STAT4 in our culture systems. To determine whether lower concentrations of IL-2 also promote the expression of STAT4, we incubated NK cells with different concentrations of IL-2 for 2 days, and examined the expression of STAT4 by Western blot. As shown in Figure 6, enhanced expression of STAT4 was noted at an IL-2 concentration of 1 U/mL and reached a maximum at 100 U/mL (Figure 6 upper panel). IL-2 induced the phosphorylation of STAT5, resulting in the appearance of a slower migrating band. The level of phospho-STAT5 increased with higher concentrations of IL-2 but the overall expression of STAT5 showed no increase with higher concentrations of IL-2 (Figure 6 lower panel). These results indicate that very low levels of IL-2 can promote the expression of STAT4, and this promotion is dose-dependent.
Dose response of IL-2–induced STAT4 expression.
NK cells were cultured with IL-2 at the indicated concentration for 2 days. Whole-cell extracts were prepared, and Western blots were probed with anti-STAT4 (A) and reprobed with anti-STAT5 (B).
Dose response of IL-2–induced STAT4 expression.
NK cells were cultured with IL-2 at the indicated concentration for 2 days. Whole-cell extracts were prepared, and Western blots were probed with anti-STAT4 (A) and reprobed with anti-STAT5 (B).
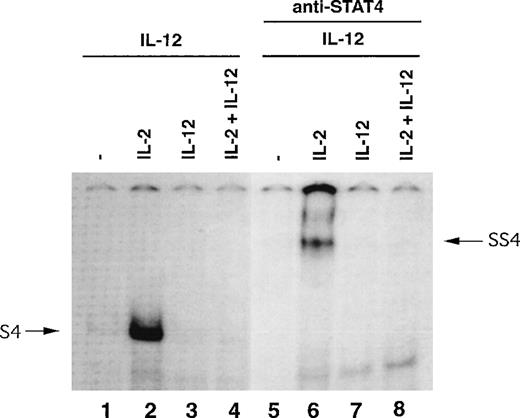
IL-12–induced STAT4-DNA–binding activity is enhanced in natural killer cells primed with IL-2.
IL-12 exerts its functional effects by inducing tyrosine phosphorylation of STAT4, which leads to its translocation to the nucleus, binding specific promoter regions, and activation of its target genes. Because IL-2–primed NK cells express high levels of IL-12 receptors and STAT4, we investigated whether this would affect the IL-12–induced STAT4-DNA–binding activity in NK cells. Purified NK cells were primed for 3 days with or without cytokines, and IL-12–induced DNA-binding activity was examined by EMSA with an oligo-ucleotide containing a STAT4 binding site.17IL-12 induced a prominent STAT4-DNA complex only in IL-2–primed NK cells (Figure 7, lane 2, S4). The involvement of STAT4 in this complex was confirmed by the disappearance of complex S4 and the appearance of a slower migrating complex, SS4, after the anti-STAT4 antibody was introduced into the binding reaction (Figure 7, lane 6). The IL-12–induced STAT4-DNA complex S4 was nearly undetectable in NK cells primed under all other conditions. The presence of a prominent IL-12–induced STAT4-DNA complex in IL-2–primed NK cells suggests that the priming of NK cells with IL-2 may have a prominent impact on the functional response of these cells to IL-12.
IL-12–induced STAT4-DNA–binding activity in NK cells primed with cytokines.
NK cells were primed with or without cytokines as indicated for 3 days and then all cells were treated with IL-12 for 30 minutes. Nuclear extracts were prepared and EMSA was performed in the absence or presence of antibody to STAT4, as indicated.
IL-12–induced STAT4-DNA–binding activity in NK cells primed with cytokines.
NK cells were primed with or without cytokines as indicated for 3 days and then all cells were treated with IL-12 for 30 minutes. Nuclear extracts were prepared and EMSA was performed in the absence or presence of antibody to STAT4, as indicated.
Enhanced IL-12–induced natural killer cell function after priming with IL-2.
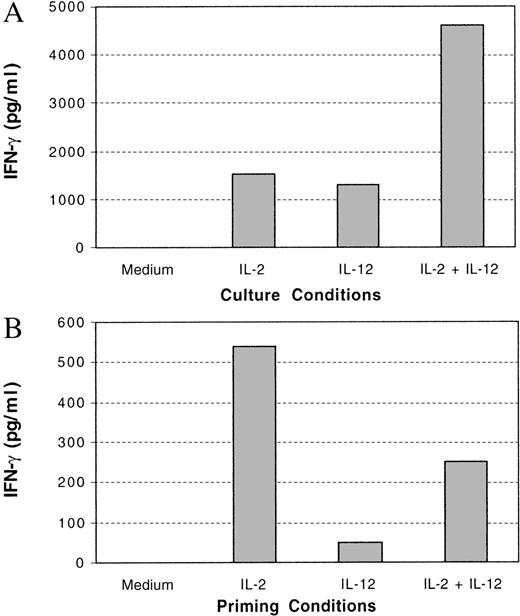
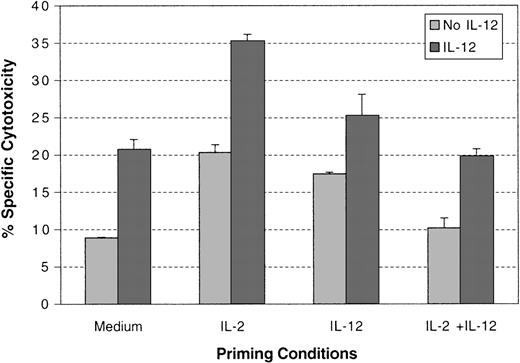
Previous evidence suggests that the antitumor effect of IL-12 is mediated by the ability of IL-12 to induce IFN-γ production in NK cells and to enhance the cytotoxicity of NK cells toward target cells. To investigate the effect of IL-2 priming on the functions of IL-12–treated NK cells, purified NK cells were primed with or without cytokines, and IL-12–induced IFN-γ production and cytotoxicity were tested. Both IL-2 and IL-12 induced IFN-γ production in NK cells during the priming period and, as previously reported, the combination of IL-2 and IL-12 induced synergistic IFN-γ production in NK cells (Figure 8A).29 30 Although a significant amount of IFN-γ was produced in all primed cultures with cytokines, the majority of the IFN-γ induced by these cytokines was produced within the first 24 hours (data not shown). When these primed NK cells were then treated with IL-12, maximal production of IFN-γ was detected only in the NK cells primed with IL-2 (Figure 8B). The NK cells primed with IL-12 showed a significant reduction in IFN-γ production in response to additional IL-12 (Figure 8B). This indicates that continued IL-12 treatment of NK cells does not lead to continued production of IFN-γ. The specific cytotoxicity of the primed NK cells in response to IL-12 was also tested with Colo target cells. IL-2–primed NK cells showed stronger cytotoxic killing against these target cells, compared with NK cells primed under other conditions, particularly in the presence of IL-12 (Figure9). IL-12 priming of NK cells led only to a weak enhancement of cytotoxic killing in response to IL-12. IL-2– and IL-12–primed NK cells did not exhibit any enhancement of specific cytotoxic killing in response to IL-12 compared with cells primed with medium alone. Similar results were obtained at varying effector-to-target ratios (data not shown). Thus, in addition to potentiating IL-12–mediated signaling events, IL-2 priming enhances the functional stimulation of NK cells by IL-12.
IFN-γ production in cytokine-primed NK cells.
NK cells were primed with or without cytokines as indicated for 3 days. The INF-γ produced in the medium at the end of 3 days was quantified by ELISA (A). These primed cells were then washed and treated with or without IL-12 for 3 days. The IFN-γ produced from the cells was quantified by ELISA (B). Net IFN-γ production, representing the difference between that produced in the presence and absence of IL-12, is shown.
IFN-γ production in cytokine-primed NK cells.
NK cells were primed with or without cytokines as indicated for 3 days. The INF-γ produced in the medium at the end of 3 days was quantified by ELISA (A). These primed cells were then washed and treated with or without IL-12 for 3 days. The IFN-γ produced from the cells was quantified by ELISA (B). Net IFN-γ production, representing the difference between that produced in the presence and absence of IL-12, is shown.
IL-12 induced cytotoxicity in cytokine-primed NK cells.
NK cells were primed with or without cytokines as indicated for 3 days. Cells were then washed and incubated with or without IL-12 for 18 hours. Specific cytotoxicity of the NK cells against Colo cells at a 5:1 ratio was tested. A summary of experiments with NK cells from 3 different donors is presented.
IL-12 induced cytotoxicity in cytokine-primed NK cells.
NK cells were primed with or without cytokines as indicated for 3 days. Cells were then washed and incubated with or without IL-12 for 18 hours. Specific cytotoxicity of the NK cells against Colo cells at a 5:1 ratio was tested. A summary of experiments with NK cells from 3 different donors is presented.
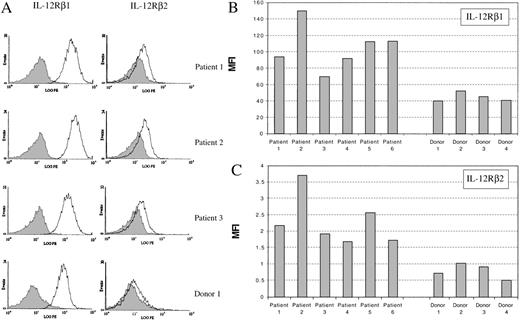
Patients treated with low-dose IL-2 show increased expression of IL-12 receptor chains on natural killer cells.
Our data have shown that IL-2 can enhance the expression of IL-12Rβ1, IL-12Rβ2, and STAT4 in NK cells in vitro, and lead to enhanced IL-12–induced function of NK cells. This suggests that the immune response of NK cells to IL-12 could be modulated by exposure to IL-2. To explore whether IL-2 has a similar effect on NK cells in vivo, we examined the expression of IL-12 receptors on NK cells from patients treated with IL-2. These patients had metastatic cancer and received low-dose continuous infusion of IL-2 for at least 4 weeks.35 The NK cell population in these patients was expanded during the treatment and constitutes about 45% to 85% of the PBMCs (data not shown; Soiffer et al35). The expression of IL-12Rβ1 and IL-12Rβ2 was assessed on NK cells obtained from 6 patients after continuous IL-2 treatment and compared with enriched NK cells from 4 healthy donors. NK cells from these 6 patients exhibited elevated expression of both IL-12Rβ1 and IL-12Rβ2, compared with the NK cells from the healthy donors (Figure10A, B, and C). The expression of IL-12Rβ1 in the 6 patients treated with IL-2 was twice that in the healthy donors. The expression of IL-12Rβ2 on the NK cells from the patients treated with IL-2 was comparable to that on IL-2–primed NK cells in vitro, and was 3-fold higher than the expression level on NK cells from healthy donors (Figures 4B and 10C).
Expression of IL-12Rβ1 and IL-12Rβ2 on NK cells from healthy donors and patients treated with IL-2.
(A) Flow cytometric histograms of the expression of IL-12 receptor chains on NK cells from 3 patients and 1 healthy control. (B) and (C) Summary of the expression of IL-12Rβ1 and IL-12Rβ2 on NK cells from 6 patients and 4 healthy donors. MFI: mean fluorescent intensity.
Expression of IL-12Rβ1 and IL-12Rβ2 on NK cells from healthy donors and patients treated with IL-2.
(A) Flow cytometric histograms of the expression of IL-12 receptor chains on NK cells from 3 patients and 1 healthy control. (B) and (C) Summary of the expression of IL-12Rβ1 and IL-12Rβ2 on NK cells from 6 patients and 4 healthy donors. MFI: mean fluorescent intensity.
Discussion
Previous studies have demonstrated that the response of T cells to IL-12 can be modulated by several other cytokines, such as IL-4, IL-18, IFN-α, and IFN-γ. These cytokines modulate IL-12 signaling primarily by altering the expression of IL-12Rβ2.10,11,38 39 In this report, we investigated the regulation of IL-12 signaling in human NK cells and demonstrated that IL-2, a cytokine that directly modulates NK cell function both in vitro and in vivo, has a critical effect on the ability of these cells to respond to IL-12. IL-2 not only enhances the expression of IL-12 receptor β1 and β2, but also enhances the expression of STAT4, a critical STAT protein that is known to be associated with IL-12 signaling in NK cells. The up-regulation of both IL-12 receptors and STAT4 by IL-2 leads to an augmented response of NK cells to IL-12 in both cell-mediated cytotoxicity and IFN-γ production.
Our results show that the expression of STAT4 in NK cells is regulated independently from that of STAT1 and STAT5, particularly with respect to dependency on exogenous cytokines. The expression of STAT5 in NK cells appears to be independent of cytokines. By contrast, the expression of STAT1 is dependent on either IL-2 or IL-12, because only NK cells cultured without IL-2 or IL-12 express low levels of STAT1. The expression of STAT4 is unique in that it is dependent only on IL-2, and the addition of IL-12 resulted in decreased expression of STAT4. These 3 STATs participate in many cytokine signaling pathways. STAT4 is involved in the signaling of IL-12, IFN-α, and IL-2 in NK cells. STAT1 is associated with IL-2, IFN-α, IFN-γ, and IL-15 signaling, and STAT5 can be activated by IL-2, IL-3, IL-5, and granulocyte-macrophage colony-stimulating factor.15,17,40-44 Although all 3 STATs are involved in multiple cytokine pathways in NK cells, the unique IL-2–dependent expression of STAT4 may allow a more complex mechanism of regulation. For example, the signals mediated by STAT4 in NK cells may be more dependent on the microenvironment in the immune system and could be more precisely controlled by other factors, compared with the signals relayed by other STATs. In the current study, we demonstrate that IL-2 can promote the expression of STAT4. Other cytokines such as IL-15 may have a similar effect. IL-15 utilizes the IL-2 β and γ receptor chains and has many modulating effects on NK cells that are similar to those of IL-2, such as inducing NK cell proliferation, enhancing target specific cytotoxicity, and synergizing with IL-12 in inducing IFN-γ production.45-47 Because IL-15 is present in human bone marrow where NK cells are derived,48 IL-15 may play a role in promoting the expression of STAT4 and in priming NK cells to respond to IL-12 in vivo.
It has previously been shown that IL-12 can synergize with IL-2 in inducing IFN-γ and TNF-α production from NK cells and up-regulating the expression of several cell surface molecules on NK cells,29,33 but IL-12 has also been shown to inhibit the proliferative effect of IL-2 on NK cells.3,49 Our results indicate that the expression of IL-12Rβ1 and STAT4 is significantly reduced in NK cells treated with IL-2 plus IL-12, compared with cells treated with IL-2 alone. This suggests that the addition of IL-12 should have an inhibitory effect on IL-2–induced functions rather than a synergistic effect. This apparent contradiction was further examined in our functional experiments in which we also demonstrated enhanced production of IFN-γ from NK cells stimulated by both IL-2 and IL-12. However, the synergy between IL-12 and IL-2 in inducing IFN-γ production from NK cells largely occurred in the first 24 hours of treatment, and diminished thereafter (data not shown). We have previously demonstrated that, in addition to STAT1 and STAT5, STAT4 is also activated by IL-2 in NK cells.17 The synergy between IL-2 and IL-12 in inducing IFN-γ production in NK cells is likely due to the initial enhanced activation of STAT4 by both cytokines. Although the expression of STAT1 and STAT5 remains the same, the expression of STAT4 in NK cells treated with both IL-2 and IL-12 is significantly decreased after 24 hours, compared with that in NK cells treated with IL-2 alone. The correlation between the reduced expression of STAT4 and the inhibitory effects of IL-12– on IL-2–induced functions in NK cells suggests that STAT4 may play an important role in IL-2–induced functions such as proliferation and IFN-γ production in this unique population. NK cells are known to play a critical role in the antineoplastic effects of IL-12. In some animal models, the major factors that contribute to the antitumor effect of IL-12 are NK cell cytotoxicity and IFN-γ production.20,24 As a result of the observation that IL-2 and IL-12 are synergistic in inducing IFN-γ production and cytotoxic activity of NK cells in vitro, the combination of IL-2 and IL-12 has become a more attractive alternative to the use of IL-12 alone in models of cancer immunotherapy.29,31,50,51 However, our data suggest that the simultaneous use of IL-2 and IL-12 is unlikely to be effective in cancer therapy because of the loss of STAT4 expression in NK cells treated by both IL-12 and IL-2 and the subsequent loss of the responsiveness to these cytokines. Therefore, improving the antitumor effect of IL-12 in combination with IL-2 will likely require strategies that would maintain the responsiveness of NK cells to IL-12. IL-2 has been shown to enhance the proliferation of NK cells both in vitro and in vivo, and patients who receive low-dose IL-2 have a greatly expanded NK population in their peripheral blood.28 35 In this study, we have demonstrated that in vitro priming of NK cells with IL-2 up-regulates the expression of IL-12 receptors and STAT4 and enhances the response of NK cells to IL-12 in both cell-mediated cytotoxicity and IFN-γ production. More importantly, NK cells from patients who receive low-dose IL-2 treatment also exhibited enhanced expression of IL-12 receptors, indicating the potential of enhanced response to IL-12 in vivo. The results of clinical trials using IL-2 or IL-12 alone have generally been disappointing, with few sustained responses in patients with metastatic cancer. These results provide further insights into the functional interactions between IL-2 and IL-12 that can be further evaluated in animal models and used in the design of future clinical immunotherapy trials. For example, continuous low-dose IL-2 treatment can expand the NK cell population and also enhance the NK cell response to IL-12. A treatment schedule using continuous low-dose IL-2, followed by an intermittent dose of IL-12, may therefore be a more effective strategy for enhancing the antitumor activity of these cytokines, and this can be tested in future clinical trials.
Acknowledgments
We thank Dr Gordon Freeman for providing materials and advice in generating receptor expressing cell lines, and Dr Edward Greenfield and Khuong Nguyen for their assistance in generating monoclonal antibodies.
Supported by NIH grant CA41619.
Reprints:Jerome Ritz, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; email: jerome_ritz@dfci.harvard.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal