Abstract
Fas/Apo-1 (CD95) triggers programmed cell death (PCD) and is involved in immune response control and cell-mediated cytotoxicity. In the autoimmune/lymphoproliferative syndrome (ALPS), inherited loss-of-function mutations of the Fas gene cause nonmalignant lymphoproliferation and autoimmunity. We have recently identified an ALPS-like clinical pattern (named autoimmune lymphoproliferative disease [ALD]) in patients with decreased Fas function, but noFas gene mutation. They also displayed decreased PCD response to ceramide, triggering a death pathway partially overlapping that used by Fas, which suggests that ALD is caused by downstream alterations of the Fas signaling pathway. Decreased Fas function is also involved in tumor development, because somatic mutations hitting the Fas system may protect neoplastic cells from immune surveillance. This work assessed the inherited component of the ALD defect by evaluating Fas- and ceramide-induced T-cell death in both parents and 4 close relatives of 10 unrelated patients with ALD. Most of them (22 of 24) displayed defective Fas- or ceramide-induced (or both) cell death. Moreover, analysis of the family histories showed that frequencies of autoimmunity and cancer were significantly increased in the paternal and maternal line, respectively. Defective Fas- or ceramide-induced T-cell death was also detected in 9 of 17 autoimmune patients from 7 families displaying more than a single case of autoimmunity within first- or second-degree relatives (multiple autoimmune syndrome [MAS] patients). Autoimmune diseases displayed by ALD and MAS families included several organ-specific and systemic forms. These data suggest that ALD is due to accumulation of several defects in the same subject and that these defects predispose to development of cancer or autoimmune diseases other than ALPS/ALD.
Fas/Apo-1 (CD95), a transmembrane molecule belonging to the tumor necrosis factor receptor (TNFR) superfamily, binds FasL (CD95L) belonging to the tumor necrosis factor (TNF) superfamily.1 2 Fas/FasL interaction triggers programmed cell death (PCD) in several cell types and may play a role in immune response control, lymphocyte life span regulation, and peripheral tolerance induction. Moreover, Fas is involved in cytotoxic T lymphocyte (CTL) and TH1 cell cytotoxicity, which is partially due to interaction of FasL expressed by activated cytotoxic cells with Fas expressed by target cells. In lymphocytes, Fas triggering does not induce PCD in resting and recently activated T cells, but the PCD-inducing pathway is connected to Fas several days after cell activation.
In lpr/lpr mice and in patients with the autoimmune lymphoproliferative syndrome (ALPS), inherited loss-of-function mutations of the Fas gene have been associated with a clinical picture characterized by nonmalignant lymphoproliferation with lymphadenopathy or splenomegaly and peripheral expansion of T-cell receptor (TCRαβ+) T cells that are double negative for CD4 and CD8 (DN T cells), and autoimmune phenomena.1 3-9Lpr/lpr mice develop hypergammaglobulinemia, autoantibody production, glomerulonephritis, arthritis, and vasculitis, whereas patients with ALPS display hemolytic anemia, thrombocytopenia, neutropenia, or their combinations, recurrent urticaria consistent with immune vasculitis, and glomerulonephritis. Despite their shared clinical and molecular pattern, the human and mouse diseases display different genetic inheritance. In the mouse, expression of the disease requires homozygous mutations of the Fas gene, whereas most ALPS patients are heterozygous for Fas mutations. Intriguingly, their parents who are heterozygous for the Fas mutation are generally healthy, which suggests that either patients carry mutations in other complementary genes or specific environmental factors are required to trigger the disease.
In a previous work, we identified 6 unrelated patients with an ALPS-like clinical pattern, though with no DN cell expansion.10 T cells displayed reduced Fas capacity to induce PCD, but no Fas gene mutation, together with decreased PCD response to ceramide, which triggers a death pathway partially overlapping that used by Fas. These data showed that clinical pictures with lymphoproliferation and autoimmunity may involve not only Fas itself, as in patients with ALPS, but also its signaling pathway, and suggest that Fas gene mutations and DN cell expansion are not the only signs of a defective Fas system. Because the disease displayed by our patients did not fit the crucial parameters required to diagnose ALPS, we operatively named it autoimmune lymphoproliferative disease (ALD).
Decreased function of Fas has also been suggested to play a role in tumor development.11-16 Fresh tumor cells and tumor cell lines, in fact, are often resistant to Fas-induced cell death, because they down-regulate Fas expression, secrete soluble forms of Fas, or express a Fas molecule with decreased signaling activity. These alterations may be ascribed to somatic mutations hitting the Fas system in the multistep cancerogenesis process and may protect neoplastic cells from immune surveillance. Moreover in a recent work, somatic mutations of the Fas gene have been associated with development of a clinical picture with lymphoma and autoimmunities.17Development of lymphomas has also been anecdotally reported in some families of ALPS patients.1 3-5
The aim of this work was to test the hypothesis that ALD is a genetic disease and to evaluate whether this genetic pattern predisposes to development of neoplasia and autoimmune diseases that are different from typical ALPS/ALD. We found that T cells from the parents of all the ALD patients displayed defective Fas- or ceramide-induced death (or both). Frequencies of autoimmune diseases and death from cancer were increased in these families in the paternal and maternal line, respectively. Moreover, defective Fas- or ceramide-induced T-cell death was detected in autoimmune patients from families displaying a high frequency of autoimmunity.
Materials and methods
Patients
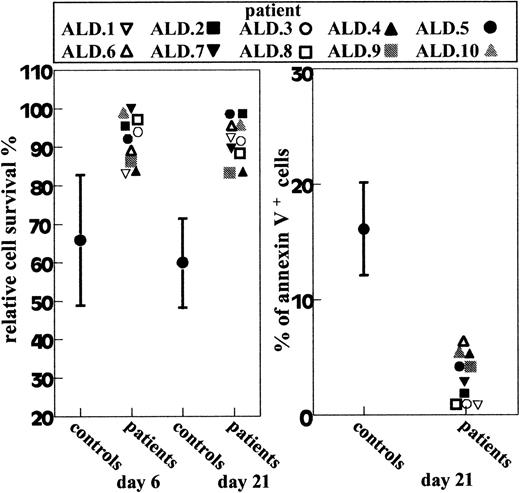
The ALD patients were those with chronic hematologic autoimmunities (anemia, thrombocytopenia, or neutropenia), lymphadenopathy or splenomegaly, and T-cell resistance to cell death induced in vitro by anti-Fas monoclonal antibodies (mAb) (Figure1) in the absence of mutations of theFas gene. ALD patients 1, 2, 3, 5, 6, and 7 were previously described.10 Control families were those of 21 consecutive children observed in the general practitioner outpatient clinic. The main clinical data for each patient are listed below.
Fas-induced T-cell death in ALD patients and 15 normal donors.
Left panel shows T cells cultured for 6 or 21 days with IL-2 and treated with anti-Fas mAb; relative cell survival was assessed after 18 hours. Right panel shows day-21 T-cells that were treated with anti-Fas mAb. Cell apoptosis was assessed after 6 hours by annexin V staining. Results are expressed as percentage of annexin V+ cells in the Fas-treated well corrected for the percentage of annexin V+ cells in the Fas-untreated well. The percentage of annexin V+ cells in the Fas-untreated well was always less than 10%. Results from normal donors (controls) are shown as mean ± SD.
Fas-induced T-cell death in ALD patients and 15 normal donors.
Left panel shows T cells cultured for 6 or 21 days with IL-2 and treated with anti-Fas mAb; relative cell survival was assessed after 18 hours. Right panel shows day-21 T-cells that were treated with anti-Fas mAb. Cell apoptosis was assessed after 6 hours by annexin V staining. Results are expressed as percentage of annexin V+ cells in the Fas-treated well corrected for the percentage of annexin V+ cells in the Fas-untreated well. The percentage of annexin V+ cells in the Fas-untreated well was always less than 10%. Results from normal donors (controls) are shown as mean ± SD.
ALD.1 family.
Man, aged 24 years, was observed at the age of 13 for thrombocytopenia, splenomegaly, and lymphadenopathy. Subsequently he also developed neutropenia. Mild thrombocytopenia and neutropenia persisted after splenectomy.
ALD.2 family.
Boy, aged 5 years, was observed at 4 months of age for anemia, thrombocytopenia, and splenomegaly. He is in remission after intravenous immunoglobulin and steroids; splenomegaly persists.
ALD.3 family.
Boy, aged 10 years, presented at age 3 years with thrombocytopenia and neutropenia with generalized lymph node enlargement. At present, he is in hematologic remission, but shows mild splenomegaly and adenomegaly.
ALD.4 family:
Boy, aged 12 years, was treated at the age of 7 years for subdiaphragmatic stage IIb Hodgkin disease. Since the age of 9 he has been followed for chronic thrombocytopenia responsive to high steroid doses.
ALD.5 family.
Girl, aged 13 years, was hospitalized at the age of 8 months for persistent fever and substantial splenomegaly. She has always presented anemia and high levels of IgG, which persist after splenectomy performed at the age of 12.
ALD.6 family.
Man, aged 18 years, has thrombocytopenia and large axillary and inguinal lymph nodes. Adenopathy and thrombocytopenia persist after splenectomy.
ALD.7 family.
Boy, aged 6 years, has very large spleen and thrombocytopenia.
ALD.8 family.
Girl, aged 13 years, was admitted to our department at the age of 7 for severe hemolytic anemia and splenomegaly. She presents recurrent episodes of thrombocytopenia or hemolytic anemia, both responsive to a high dose of steroids.
ALD.9 family.
Girl, aged 12 years, was observed at the age of 10 for severe thrombocytopenia and splenomegaly. Thrombocytopenia persists after splenectomy.
ALD.10 family.
Boy, aged 6 years, was hospitalized for cervical lymph node enlargement; he also displayed polyarthritis involving small joints of the hand and thrombocytopenia, both responsive to steroids.
All the patients displayed anticardiolipin and antinuclear antibodies (ANA), whereas none of them displayed anti-DNA antibodies. The family histories for autoimmunity and cancer were collected by interviewing both parents.
Multiple autoimmune syndrome (MAS) families are those with more than 1 case of organ-specific or systemic autoimmune disorder within the first- or second-degree relatives.18 All patients attending the outpatient clinic of the Division of Clinical Immunology and suffering from connective tissue diseases (CTD) were routinely questioned about family members; a group of consecutive multicase families (2 or more CTD) has been selected for the study. Seventeen subjects with autoimmune manifestations from 7 MAS families were studied. Some members of these families were not included because they were not available.
MAS.1 family.
Patient MAS.1a is a 55-year-old woman with mixed CTD; MAS1b is a 24-year-old woman with undifferentiated connective tissue disease (UCTD), niece of MAS1a; MAS.1c is a 45-year-old woman with UCTD, niece of MAS.1a.
MAS.2 family.
MAS.2a is a 70-year-old woman with Sjögren syndrome (SS); MAS.2b is a 37-year-old woman with SS, daughter of MAS.2a.
MAS.3 family.
MAS.3a is a 43-year-old woman with UCTD; MAS.3b is a 12-year-old girl with insulin-dependent diabetes mellitus (IDDM), daughter of MAS.3a; MAS.3c is a 21-year-old woman with systemic lupus erythematosus (SLE), sister of MAS.3a (not available).
MAS.4 family.
MAS.4a is a 60-year-old man with SLE; MAS.4b is a 31-year-old man, healthy but ANA positive, son of MAS.4a; MAS.4c is a 30-year-old woman with UCTD, daughter of MAS.4a.
MAS.5 family.
MAS.5a is a 34-year-old woman with SS; MAS.5b is a 34-year-old woman with SS, homozygous twin of MAS.5a (not available); MAS.5c is an 8-year-old boy with autoimmune thrombocytopenia, son of MAS.5b.
MAS.6 family.
MAS.6a is an 11-year-old girl with SLE; MAS.6b, MAS.6c, and MAS.6d were the mother, the father, and the 14-year-old sister of MAS.6a, and displayed serum ANA without clinical manifestations. MAS.6d also displayed high titer of anti-dsDNA and anticardiolipin antibodies.
MAS.7 family.
MAS.7a is a 70-year-old woman with rheumatoid arthritis (RA); MAS.7b is a 30-year-old man with SLE, son of MAS.7a (not available).
Serologic positivities were reported in the family history and subsequently confirmed by our clinical pathology laboratory.
Informed consent was obtained from all subjects analyzed or from their parents.
Immunophenotype analysis
Expression of surface molecules was evaluated by direct immunofluorescence and cytofluorimetric analysis (FACScan. Becton Dickinson, San Jose, CA). The following mAb were used: anti-CD3 (Leu-4), -CD4 (Leu-3a), -CD8 (Leu-2a), -TCRαβ (Becton Dickinson), and -Fas (Immunotech, Marseilles, France). CD4 and CD8 DN TCRαβ-positive cells were detected by 2-color immunofluorescence, using fluorescein isothiocyanate (FITC)-conjugated anti-TCRαβ mAb and phycoerythrin (PE)-conjugated anti-CD4 and anti-CD8 mAbs. Fas was detected by 2-color immunofluorescence on resting or activated T cells, using PE-conjugated anti-CD3 mAb and FITC-conjugated anti-Fas mAb (Chemicon, Temecula, CA). Nonspecific background fluorescence was established with the appropriate isotype-matched control mAb (Becton Dickinson). Antigenic density was expressed as median fluorescence intensity ratio (MFI-R) of total lymphocytes according to the following formula: MFI-R = MFI of sample histogram (arbitrary units)/MFI of control histogram (arbitrary units).
Cell death assay
Cell death induced by Fas or C2-ceramide was evaluated as previously reported10 on T-cell lines obtained by activating peripheral blood mononuclear cells (PBMC) with phytohemagglutinin (PHA) at days 0 (1 μg/mL) and 15 (0.2 μg/mL) and cultured in RPMI 1640 + 10% FCS + recombinant IL-2 (5 U/mL) (Biogen, Geneva, Switzerland). Fas function was assessed 6 days after the second stimulation (day-21 T cells). In some experiments Fas function was also evaluated 6 days after the first stimulation (day-6 T cells). Cells were incubated with control medium or anti-Fas mAb (CH11, IgM isotype) (1 μg/mL) (UBI, Lake Placid, NY) in the presence of recombinant interleukin 2 (rIL-2) (5 U/mL) to minimize spontaneous cell death. Cell survival was evaluated after 18 hours by counting live cells in each well by the trypan blue exclusion test. The same conditions were used to measure cell death induced by methyl-prednisolone (100 μmol/L) (PDN) (Upjohn, Puurs, Belgium) or C2-ceramide (50 μmol/L) (N-acetyl-d-sphingosine) (Sigma, St Louis, MO). Assays were performed in triplicate and analyzed by a blind observer. Cells from 2 normal donors were included in each experiment as a positive control. Results were expressed as relative cell survival percentage, calculated as follows: (total live cell count in the assay well/total live cell count in the control well) × 100. Spontaneous cell loss in the control well was always less than 10% of the seeded cells and similar in cultures from the patients and normal donors. This protocol was chosen in preliminary experiments, when several anti-Fas mAb concentrations (10, 1, 0.1 μg/mL) and incubation times (1, 4, 8, 18, 48, 72 hours) were used to induce cell death in T-cell lines cultured for 3, 6, 9, 15, 18, 21, and 24 days with PHA+IL-2. Cell death was evaluated both indirectly, by counting total surviving cells by the trypan blue exclusion test, or directly, by FACS determination of the proportion displaying shrunken/hypergranular morphology or those displaying DNA fragmentation after staining with propidium iodide or those stained by annexin V. The protocol chosen was found to give the most reproducible results. It evaluates the overall cell survival at each time point and was more sensitive than the other techniques detecting the instantaneous proportion of dying cells at each time. Fas-induced cell death was always less striking in these polyclonal T-cell lines than in stabilized tumor cell lines, because it was slower and more asynchronous.10
The normal range of responses of day-21 T cells to Fas-, ceramide- and PDN-induced T-cell survival, defined as the mean ± 2 SD of data obtained from 75 normal donors, were 60 ± 22 (Fas), 58 ± 32 (ceramide), and 49 ± 26 (PDN), respectively (relative cell survival percentage).
Evaluation of cell staining with annexin V was performed using the Annexin-V-Fluos kit (Boehringer Mannhein, Gmbh, Germany). Briefly, 106 cells were stained with annexin-V 1:50 and propidium iodide (1 μg/mL) in 10 mmol/L Hepes/NaOH pH7.4, 140 mmol/L NaCl, 5 mmol/L CaCl2 and analyzed by flow cytometry gating on propidium iodide-negative cells.
Analysis of the Fas gene
Mutation analysis of the Fas gene was performed by single-strand conformation polymorphism (SSCP) and cDNA sequencing, as previously reported.10 Total RNA was extracted from fresh PBMC with the Ultraspec kit (Biotex, Houston, TX). Total RNA (2 μg) was used as a template for cDNA synthesis with the Promega cDNA synthesis kit. The entire coding sequence of the Fas gene was amplified in a single 1114 bp segment and in overlapping 203-302 bp segments. Polymerase chain reaction (PCR) was performed with 25 pmol of each primer and one fourth of the cDNA synthesis reaction (35 cycles per fragment) and using the primers previously reported.10SSCP analysis was performed for each PCR product using 32P labeling and a protocol previously reported.10 Sequencing of cDNA was performed with a dye terminator DNA sequencing kit (Applied Biosystems, Perkin-Elmer, Foster City, CA) on an ABI Model 373A automated DNA sequencer (Applied Biosystems, Perkin-Elmer). Each DNA fragment was sequenced twice using primers from both ends and PCR products from 2 independent PCR reactions. Only patients with ALD were analyzed for mutations.
Statistical analysis
Cancer frequency was analyzed by comparing the observed cases of cancer in patients' families with expected cases based on site, age and calendar period, regional-specific incidence, and mortality rates multiplied by observed number of person-time at risk. Person-time of grandparents cumulated only starting from the year of birth of father (or mother) who generated the sick child; person-time of father (or mother) cumulated only from the year of birth of the sick child. Observed and expected cases were compared by means of standardized mortality (SMR) and standardized incidence (SIR) ratios and their 95% confidence intervals (CI).19 To compare frequencies of autoimmune subjects the chi-square with Yates correction or Fisher exact tests were used. Control families were obtained from 21 consecutive children observed in the general practitioner outpatient clinic. They were interviewed using the same criteria and by the same person who interviewed the families of the patients with ALD.
Results
Deficiency of the Fas cell death pathway in families of ALD patients
Figure 1 shows the death responses to anti-Fas mAb of T-cell lines from 10 ALD patients. All patients were resistant to Fas-induced cell death. The defect was displayed by both day-6 and day-21 cultures. Moreover, it was detected by evaluation of both cell survival by the trypan blue exclusion test and cell apoptosis by annexin V staining. However, cell survival on day-21 T cells detected the maximal difference between patients and normal donors. Fas function was not abolished because cell death increased when incubation was prolonged to 48 hours or Fas triggering was potentiated by the anti-IgM serum (reference 10 and data not shown). Search for DN T-cell expansion in fresh PBMC detected expansion only in patient 9 (21% DN T cells), whereas DN cells were less than 1% in the other patients. In line with our previous findings, lymphocyte subset distribution and proliferative response to mitogens were normal. Expression of CD38, HLA-DR, and Fas was slightly increased, which indicated presence of activated cells and was in line with what is observed in other autoimune diseases (reference 10 and data not shown). Search for mutations of the Fas gene by SSCP analysis and sequencing of the entire coding region did not detect any causal mutation (data not shown).
To assess the inherited component of the Fas apoptosis pathway deficiency, we evaluated cell death induction in T-cell lines derived from both parents. Moreover, we analyzed 4 close relatives (3 siblings and 1 aunt) who were available. All these subjects were healthy except the aunt of patient 5, who displayed chronic splenomegaly, hypergammaglobulinemia, and autoimmune thrombocytopenia. We also evaluated the response to ceramide, whose pathway partially overlaps that of Fas,20 21 and to PDN, which does not involve the Fas system. As shown in Figure 2, 9 of 10 mothers and 8 of 10 fathers were resistant to Fas-induced cell death. Moreover, the 3 parents with normal response (ie, fathers 1 and 3, and mother 10) were near the upper limit of the normal range. Seven patients and 8 parents (4 of 10 mothers and 4 of 10 fathers) were also resistant to ceramide. Father 3 and mother 10 also displayed a normal response to ceramide, whereas father 1 was resistant to ceramide. All 4 relatives were resistant to either Fas- or ceramide-induced cell death; 2 were resistant to both Fas and ceramide, 1 to Fas only, and 1 to ceramide only. By contrast, all subjects displayed a normal cell death response to PDN. Similar data were obtained by annexin V staining (data not shown).
Fas-, ceramide-, and PDN-induced T-cell death in 10 ALD families.
Members of each family are marked with the indicated symbols and were evaluated in the same experiment (different from that shown in Figure1). Two or 3 normal controls were included in each experiment and are shown in lane C. Day-21 T cells were treated with the indicated reagent and survival was assessed after 18 hours. Results are expressed as specific cell survival percentage. The horizontal lines indicate the upper limit of the normal range calculated as the mean + 2 SD from data obtained from 75 normal donors. Among the close relatives, the aunt was selected because she displayed an ALPS-like clinical pattern, whereas the 3 healthy siblings were casually selected. All relatives analyzed are included in the text. C indicates normal controls; F, fathers; M, mothers; Pt, ALD patients; R, close relatives.
Fas-, ceramide-, and PDN-induced T-cell death in 10 ALD families.
Members of each family are marked with the indicated symbols and were evaluated in the same experiment (different from that shown in Figure1). Two or 3 normal controls were included in each experiment and are shown in lane C. Day-21 T cells were treated with the indicated reagent and survival was assessed after 18 hours. Results are expressed as specific cell survival percentage. The horizontal lines indicate the upper limit of the normal range calculated as the mean + 2 SD from data obtained from 75 normal donors. Among the close relatives, the aunt was selected because she displayed an ALPS-like clinical pattern, whereas the 3 healthy siblings were casually selected. All relatives analyzed are included in the text. C indicates normal controls; F, fathers; M, mothers; Pt, ALD patients; R, close relatives.
Statistical analysis showed that frequencies of resistance to Fas and ceramide were significantly higher in families of ALD patients than in normal controls. By contrast, frequency of resistance to PDN was not significantly increased (Table 1). Search for DN T-cell expansion in fresh PBMC did not detect expansion in any parent or close relative. Moreover, lymphocyte subset distribution and expression of CD38, HLA-DR, and Fas were always normal.
Frequency of Fas-, ceramide-, and PDN-resistant subjects in normal controls and different patient groups
| Subjects . | Total n . | Fas-Resistant* . | Ceramide-Resistant* . | PDN-Resistant* . | |||
|---|---|---|---|---|---|---|---|
| n . | (%) . | n . | (%) . | n . | (%) . | ||
| Total controls† | 75 | 2 | (3) | 2 | (3) | 3 | (4) |
| ALD controls‡ | 20 | 1 | (5) | 0 | (0) | 0 | (0) |
| ALD relatives | 24 | 20 | (83)1-153,1-155 | 11 | (46)1-153,1-155 | 0 | (0) |
| MAS controls1-154 | 15 | 0 | (0) | 1 | (7) | 1 | (7) |
| MAS families | 17 | 8 | (47)1-153# | 2 | (14) | 2 | (14) |
| Subjects . | Total n . | Fas-Resistant* . | Ceramide-Resistant* . | PDN-Resistant* . | |||
|---|---|---|---|---|---|---|---|
| n . | (%) . | n . | (%) . | n . | (%) . | ||
| Total controls† | 75 | 2 | (3) | 2 | (3) | 3 | (4) |
| ALD controls‡ | 20 | 1 | (5) | 0 | (0) | 0 | (0) |
| ALD relatives | 24 | 20 | (83)1-153,1-155 | 11 | (46)1-153,1-155 | 0 | (0) |
| MAS controls1-154 | 15 | 0 | (0) | 1 | (7) | 1 | (7) |
| MAS families | 17 | 8 | (47)1-153# | 2 | (14) | 2 | (14) |
Number (n) and percentage (%) of subjects displaying a specific cell survival higher than the mean + 2 SD displayed by total controls.
Total controls run in this study.
Controls run in the experiments testing ALD relatives.
Significantly different from total controls (P < .0001, Fisher exact test).
Significantly different from ALD controls (P < .0001 for Fas and P = .0007 for ceramide, Fisher exact test).
Controls run in the experiments testing MAS families.
#Significantly different from MAS controls (P = .0042, Fisher exact test).
Fas expression was evaluated in each T-cell line by direct immunofluorescence on the same day in which the cell death assay was performed and was always in the normal range; moreover, search for DN T cells in fresh PBMC by 2-color immunofluorescence did not reveal expansion of these cells in any of these subjects (data not shown).
Increased frequency of autoimmune diseases and cancer in the families of ALD patients
The decreased Fas- or ceramide-induced cell death displayed by most members of the patients' families supports the possibility that ALD has a genetic component. Because decreased Fas function may favor development of autoimmunity and cancer, we determined whether frequency of these diseases was increased in these families. Family histories were collected up to patients' grandparents and pedigrees are shown in Figure 3.
Pedigrees of the ALD families.
Black symbols mark subjects with cancer. Arabic numbers indicate the type of cancer: 1, gastric carcinoma; 2, lymphoma; 2B, Hodgkin lymphoma; 3, small cell lung cancer; 4, polycythemia vera; 5, colon carcinoma; 6, breast carcinoma; 7, lung carcinoma; 8, kidney adenocarcinoma; 9, squamous head carcinoma. Gray symbols mark subjects with autoimmune disease. In each family the ALD patient is the single affected individual in the third generation. Lower-case letters indicate the type of autoimmune disease: a, IDDM; b, chronic hemolytic anemia, c, autoimmune thrombocytopenia; d, neutropenia; e, SLE; f, multiple sclerosis; g, RA; h, autoimmune hepatitis; i, ankylosing spondylitis; j, polyarthritis.
Pedigrees of the ALD families.
Black symbols mark subjects with cancer. Arabic numbers indicate the type of cancer: 1, gastric carcinoma; 2, lymphoma; 2B, Hodgkin lymphoma; 3, small cell lung cancer; 4, polycythemia vera; 5, colon carcinoma; 6, breast carcinoma; 7, lung carcinoma; 8, kidney adenocarcinoma; 9, squamous head carcinoma. Gray symbols mark subjects with autoimmune disease. In each family the ALD patient is the single affected individual in the third generation. Lower-case letters indicate the type of autoimmune disease: a, IDDM; b, chronic hemolytic anemia, c, autoimmune thrombocytopenia; d, neutropenia; e, SLE; f, multiple sclerosis; g, RA; h, autoimmune hepatitis; i, ankylosing spondylitis; j, polyarthritis.
Analysis of autoimmune disease was performed by comparing frequencies of autoimmune diseases in the patients' families and in those of 21 consecutive children observed in the general practitioner outpatient clinic (Table 2) (control families). We found that families of ALD patients displayed a significantly higher frequency of autoimmune diseases than control families (9.3% versus 2.6%, P = .014). The difference was mostly due to males (11.1% versus 0.8%, P = .0035), because frequency of autoimmune diseases in females was similar in the 2 groups (7.4% versus 4.5%). To investigate the inheritance pattern, we evaluated frequency of autoimmunity in paternal and maternal lineages. In the maternal lineage, no difference was found between ALD patients and controls (1.9% versus 3.9%), whereas in the paternal lineage, frequency of autoimmune diseases was significantly higher in the families of ALD patients (19.6% versus 1.7%, P = .0002). The significantly increased frequency was displayed by both males (18.8% versus 1.4%, P = .0036) and females (21.4% versus 2.1%, P = .035).
Frequency of autoimmune diseases in families of ALD patients
| . | ALD Families . | Control Families . | P Value . | ||
|---|---|---|---|---|---|
| Cases/Total* . | %† (CI)‡ . | Cases/Total* . | %† . | ||
| Total | 10/108 | 9.3 (4.5-16.4) | 6/235 | 2.6 (0.9-5.5) | .0142-153 |
| Males | 6/54 | 11.1 (4.2-22.6) | 1/123 | 0.8 (0.02-4.4) | .00352-153 |
| Females | 4/54 | 7.4 (2.1-17.9) | 5/112 | 4.5 (1.5-10.1) | |
| Paternal lineage | 9/46 | 19.6 (9.4-33.9) | 2/117 | 1.7 (0.2-6.1) | .00022-155 |
| Males | 6/32 | 18.8 (7.2-36.4) | 1/70 | 1.4 (0.03-7.7) | .00362-155 |
| Females | 3/14 | 21.4 (4.7-50.8) | 1/47 | 2.1 (0.05-11.3) | .0352-155 |
| Maternal lineage | 1/52 | 1.9 (0.04-10.3) | 4/102 | 3.9 (1.1-9.7) | |
| Males | 0/17 | 0 (0-19.5) | 0/44 | 0 (0-8.1) | |
| Females | 1/35 | 2.8 (0.1-14.9) | 4/58 | 6.7 (1.9-16.7) | |
| . | ALD Families . | Control Families . | P Value . | ||
|---|---|---|---|---|---|
| Cases/Total* . | %† (CI)‡ . | Cases/Total* . | %† . | ||
| Total | 10/108 | 9.3 (4.5-16.4) | 6/235 | 2.6 (0.9-5.5) | .0142-153 |
| Males | 6/54 | 11.1 (4.2-22.6) | 1/123 | 0.8 (0.02-4.4) | .00352-153 |
| Females | 4/54 | 7.4 (2.1-17.9) | 5/112 | 4.5 (1.5-10.1) | |
| Paternal lineage | 9/46 | 19.6 (9.4-33.9) | 2/117 | 1.7 (0.2-6.1) | .00022-155 |
| Males | 6/32 | 18.8 (7.2-36.4) | 1/70 | 1.4 (0.03-7.7) | .00362-155 |
| Females | 3/14 | 21.4 (4.7-50.8) | 1/47 | 2.1 (0.05-11.3) | .0352-155 |
| Maternal lineage | 1/52 | 1.9 (0.04-10.3) | 4/102 | 3.9 (1.1-9.7) | |
| Males | 0/17 | 0 (0-19.5) | 0/44 | 0 (0-8.1) | |
| Females | 1/35 | 2.8 (0.1-14.9) | 4/58 | 6.7 (1.9-16.7) | |
Subjects with autoimmune disease/total subjects observed. These counts do not include the patients.
Percentage of subjects with autoimmune disease.
95% confidence interval.
Chi-square test.
Fisher exact test.
Cancer frequency was analyzed by comparing the observed cases of cancer in these families with the frequency expected from the mortality rates for our region (ie, Piedmont, Italy) because official registers were available (Table 3). In the maternal family line, ALD patients displayed significantly higher incidence (SIR = 1.87; CI, 0.99-3.2) and mortality (SMR = 2.75; CI, 1.26-5.23) for cancer than expected. The significantly increased frequency was displayed by females (SIR = 2.68; CI, 1.15-5.27; SMR = 4.63; CI, 1.49-10.83) and not by males. No increased frequency was found in the paternal family line. These data support the possibility that genetic alterations of the Fas signaling pathway may be a novel genetic factor predisposing to cancer development and suggest that the trait is controlled by the maternal line.
Incidence and mortality for cancer in ALD patients' families
| Incidence . | Observed/Expected . | Standardized Incidence Ratio (95% confidence interval) . | ||||
|---|---|---|---|---|---|---|
| Total . | Males . | Females . | Total . | Males . | Females . | |
| Both parents | 18/14.8 | 10/9 | 8/5.8 | 1.22 (0.72-1.92) | 1.11 (0.53-2.04) | 1.38 (0.59-2.72) |
| Paternal lineage | 4/7.8 | 4/5 | 0/2.8 | 0.51 (0.14-1.31) | 0.80 (0.22-2.05) | 0 (0-1.31) |
| Maternal lineage | 13/6.93-150 | 5/3.9 | 8/3.03-150 | 1.88 (1-3.22) | 1.28 (0.41-2.99) | 2.67 (1.15-5.25) |
| Mortality | Observed/Expected | Standardized Mortality Ratio (95% confidence interval) | ||||
| Total | Males | Females | Total | Males | Females | |
| Both parents | 11/7.4 | 6/5 | 5/2.3 | 1.49 (0.74-2.66) | 1.20 (0.44-2.61) | 2.17 (0.7-5.07) |
| Paternal lineage | 2/4.1 | 2/2.8 | 0/1.3 | 0.49 (0.05-1.76) | 0.71 (0.08-2.58) | 0 (0-2.83) |
| Maternal lineage | 9/3.33-150 | 4/2.2 | 5/1.13-150 | 2.73 (1.24-5.18) | 1.82 (0.49-4.65) | 4.55 (1.46-10.61) |
| Incidence . | Observed/Expected . | Standardized Incidence Ratio (95% confidence interval) . | ||||
|---|---|---|---|---|---|---|
| Total . | Males . | Females . | Total . | Males . | Females . | |
| Both parents | 18/14.8 | 10/9 | 8/5.8 | 1.22 (0.72-1.92) | 1.11 (0.53-2.04) | 1.38 (0.59-2.72) |
| Paternal lineage | 4/7.8 | 4/5 | 0/2.8 | 0.51 (0.14-1.31) | 0.80 (0.22-2.05) | 0 (0-1.31) |
| Maternal lineage | 13/6.93-150 | 5/3.9 | 8/3.03-150 | 1.88 (1-3.22) | 1.28 (0.41-2.99) | 2.67 (1.15-5.25) |
| Mortality | Observed/Expected | Standardized Mortality Ratio (95% confidence interval) | ||||
| Total | Males | Females | Total | Males | Females | |
| Both parents | 11/7.4 | 6/5 | 5/2.3 | 1.49 (0.74-2.66) | 1.20 (0.44-2.61) | 2.17 (0.7-5.07) |
| Paternal lineage | 2/4.1 | 2/2.8 | 0/1.3 | 0.49 (0.05-1.76) | 0.71 (0.08-2.58) | 0 (0-2.83) |
| Maternal lineage | 9/3.33-150 | 4/2.2 | 5/1.13-150 | 2.73 (1.24-5.18) | 1.82 (0.49-4.65) | 4.55 (1.46-10.61) |
Statistically significant.
To confirm these data, we compared SIR and SMR for cancer in families of ALD patients and in the control families and calculated the corresponding ratios. Moreover, we applied the Kaplan-Meier method with the log-rank test. Both analyses gave results consistent with those shown in Table 3 (data not shown).
Deficiency of Fas-induced cell death in families with high frequency of autoimmunity
To further assess the possibility that deficiency of Fas-induced cell death may predispose to development of autoimmune diseases different from typical ALD, we evaluated Fas- and ceramide-induced cell death in T cells from 17 patients displaying autoimmune diseases from 7 families with a high frequency of autoimmunity. In each family, several cases of different autoimmune diseases were diagnosed, with the exception of the MAS.6 family, which displayed 1 subject with frank autoimmunity and 3 first-degree relatives with high amounts of serum autoantibodies in the absence of overt autoimmune manifestations. We found that 8 of 17 subjects were resistant to Fas-induced cell death and 2 of 17 were resistant to ceramide; 7 were resistant to Fas only, 1 was resistant to ceramide only, and 1 was resistant to both Fas and ceramide; 2 patients were also resistant to PDN (Figure4). Similar data were obtained by evaluating cell death by annexin V staining (data not shown). Statistical analysis showed that frequency of resistance to Fas was significantly higher in families of ALD patients than in normal controls. By contrast, frequencies of resistance to ceramide and PDN were not significantly increased (Table 1). Fas expression in all T-cell lines was always in the normal range, and no expansion of DN T cells was detected in PBMC (data not shown).
Fas-, ceramide-, and PDN-induced T-cell death in autoimmune subjects from 7 MAS families.
Black symbols mark subjects with autoimmune disease and gray symbols subjects with serologic autoimmunity without overt disease. Results are expressed as in Figure 1. Two or 3 normal controls were included in each experiment and are shown in Table 1.
Fas-, ceramide-, and PDN-induced T-cell death in autoimmune subjects from 7 MAS families.
Black symbols mark subjects with autoimmune disease and gray symbols subjects with serologic autoimmunity without overt disease. Results are expressed as in Figure 1. Two or 3 normal controls were included in each experiment and are shown in Table 1.
Discussion
Fas function defects due to inherited loss-of-function mutations of the Fas gene are responsible for the pattern of autoimmunity/lymphoproliferation displayed by patients with ALPS andlpr mice.1-11 Both the human and mouse immunopathologic patterns are characterized by peripheral expansion of DN T cells. Interestingly, most ALPS patients are heterozygous for Fas mutations, but their parents who are heterozygous for the mutation are generally healthy. Therefore, it has been suggested that heterozygous symptomatic ALPS patients inherit a single mutation in the Fasgene from 1 parent and a second abnormality, not directly involving theFas gene, from the other. We have recently reported altered Fas-induced cell death in patients displaying an ALPS-like clinical pattern in the absence of Fas mutation and peripheral expansion of DN T cells. The ALD abnormality seems to involve the Fas signaling pathway downstream from Fas. This possibility has been recently confirmed in 2 patients with an ALPS-like clinical pattern who displayed mutations of caspase-10 in the absence of mutations of Fas and FasL.22 A recent classification uses the terms ALPS type Ia and Ib for the disease with mutations of Fas and FasL, respectively, and ALPS type II for the disease without such mutations.23 Our patients differ from the ALPS type II patients reported by Wang et al22 because only one of them displayed DN cell expansion. Our data did not rule out the possibility that the Fas defect was the outcome of a lymphocyte functional state induced by unknown environmental factors. However, the pediatric onset, the similarities with ALPS, the consanguineous parents of 1 patient, and the family history with autoimmunity of some patients favored a genetic component. Our present finding that 17 of 20 parents of the ALD patients display defective Fas-induced cell death strongly supports this model. The fact that most were healthy suggests that ALD patients inherited 2 mutations of the Fas signaling pathway and that presence of both mutations is required for ALD expression. Interestingly, we detected 3 patterns of cell death deficiency. Most subjects displayed dual resistance to Fas- and ceramide-induced cell death, but some were resistant to Fas and not to ceramide, or vice versa. These data suggest that different subjects carry different genetic alterations hitting different levels of the Fas signaling pathway and that ALD expression requires accumulation of several hits in the same subject. Moreover, these data suggest that the death pathways triggered by Fas and ceramide are in part distinct. The current opinion is that Fas triggering activates a caspase cascade. Hydrolysis of the phospholipid sphingomyelin is an alternative pathway that seems to boost the caspase pathway, because it is triggered by caspases and potentiates caspase activation.20,21 However, the role played by ceramide production in Fas signaling is debated.24 25 Therefore, it is noteworthy that some patients were resistant to ceramide, but responded normally to Fas triggering, because this shows that Fas can also function normally even when the ceramide pathway is nonfunctional.
Autoimmune diseases displayed by patients with ALPS and ALD are generally hematologic autoimmunities, that is, autoimmune anemia, thrombocytopenia, or neutropenia. Patients with ALPS, but not ALD, may also display vasculitis. Moreover, 1 ALPS patient reported by Pensati et al displayed autoimmune hepatitis.7 We now report 2 lines of evidence showing that decreased function of Fas may also predispose to development of autoimmune patterns different from the rare ALPS/ALD pattern. The former line of evidence was suggested by the higher frequency of autoimmune diseases in families of ALD patients than in control families. Interestingly, this increased frequency was selectively displayed by the paternal lineage. The latter line of evidence was obtained by showing that resistance to Fas-induced cell death was detectable in a substantial proportion of autoimmune patients belonging to families with a high frequency of autoimmune diseases.
Interestingly, the pattern of autoimmune diseases displayed by these patients and by families of ALD patients was heterogeneous, because it comprised multiple sclerosis, IDDM, SS, RA, SLE, autoimmune hepatitis, and ankylosing spondylitis.
The possibility that genetically based deficiencies of Fas may predispose to development of organ-specific cell-mediated autoimmune diseases, such as IDDM and multiple sclerosis, apparently contradicts the model suggested by several authors that development of these diseases involves cytotoxic mechanisms mediated by the Fas/FasL system. This model was suggested by the observation that high levels of Fas and FasL are expressed in pancreatic islet, thyroid, and central nervous tissue in IDDM, thyroiditis, and multiple sclerosis, respectively.26 Moreover, the lpr character seems to protect animals from development of autoimmunity in experimental models of IDDM and multiple sclerosis.27-29 However, it must be emphasized that we detected decreased Fas function in selected patients, which does not imply that decreased Fas function is a common cause of autoimmunity. In line with this possibility, Fas function has been reported to be increased in patients with SLE,30,31and we did not detect defective Fas function in random patients with autoimmune thyroiditis (n = 19) or IDDM (n = 13) (unpublished data). Moreover, predisposition to autoimmune diseases is multifactorial and may involve factors controlling autoantigen expression, lymphocyte responsiveness, and immune effector functions. Therefore, decreased function of the Fas system may protect from development of autoimmunity in certain genetic contexts and models of autoimmunity, but may be detrimental in others. Moreover, the expression pattern of the disease may be influenced by residual Fas function and function of other systems involved in apoptosis induction, such as the TNF and TRAIL systems, which use signaling pathways partially overlapping that of Fas.1,2,32 33
Our data also show that the ALD genetic trait also predisposes to development of cancer. The ALD families displayed several types of cancers without apparent predominance of any particular type. They did not display predominance of lymphoid neoplasia, as might have been expected from anecdotal report of lymphoma development in families of ALPS patients. However, immune alterations are partly different in ALD and ALPS patients, as shown by the expansion of DN cells in ALPS only, and these differences might influence development of lymphoid neoplasia. Interestingly, increased predisposition to development of cancer was inherited only via the maternal lineage and was significant in females and not in males. Moreover, analysis of each pedigree suggests that ALD families are heterogeneous with respect to predisposition to cancer and autoimmunity, because only families 5 and 7 displayed several cases of both autoimmune diseases and cancers, whereas families 1, 8, 9, and 10 displayed several cases of autoimmune disease only, and families 3, 4, and 6 several cases of cancers only. Interestingly, pedigrees of families 1, 3, 4, 5, 6, and 7 showed an inheritance pattern compatible with X-linked inheritance, assuming that ALD and tumors are manifestations of the same disease. These data support the possibility that genetic alterations of the Fas signaling pathway may be a novel genetic trait predisposing to cancer development and suggest that it is controlled by a gene on the X chromosome.
In conclusion, our data suggest that ALD development requires accumulation of several genetic defects hitting cell apoptosis in the same subjects. Some of these defects are also associated with increased development of common autoimmune diseases, others to increased development of cancer. These increased predispositions seem to be inherited independently because they are differently displayed by ALD families and inherited through different family lines.
Supported by Telethon grant No E566 (Rome), Associazione Italiana Ricerca sul Cancro (A.I.R.C., Milan), MURST ex-40% (Rome), AIDS Project (Istituto Superiore di Sanità, Rome). S.B. was supported by Lega Italiana Lotta contro i Tumori (Novara).
U.R. and S.B. contributed equally to this work.
Reprints:Umberto Dianzani, Dipartimento di Scienze Mediche, Via Solaroli, 17, I-28100 Novara, Italy; e-mail:dianzani@med.no.unipmn.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal