Abstract
The origin and the function of HLA class I molecules present on the surface of human platelets are still unclear. In particular, it is controversial which fraction of these class I molecules represents integral membrane components derived from the megakaryocyte-platelet lineage versus soluble plasma HLA molecules acquired by adsorption. Results of the present study show that HLA-A2 ligands isolated from platelets possess the same peptide motif as described for HLA-A2-associated peptides obtained from nucleated cells. Sequencing of these platelet-derived peptides reveals that they originate mainly from ubiquitously expressed proteins also present in the megakaryocyte-platelet lineage. Moreover, one of these peptides derives from the GPIX protein, which is specifically expressed by platelets and their precursors. Platelet HLA molecules are unstable in vitro at 37°C, but can be partially stabilized by addition of exogenous β2-microglobulin and HLA class I binding peptide, suggesting that platelets cannot load HLA molecules with endogenous peptides. In in vitro experiments platelets were used to stimulate peripheral blood mononuclear cells. No allospecific cytotoxicity was observed after primary stimulation, or secondary restimulation, with allogenic resting or activated platelets, even in the presence of additional third-party helper activity. These data indicate that HLA class I molecules from platelets cannot directly induce allogenic CD8+ cytotoxic T-cell response in vitro.
Class I molecules of the major histocompatibility complex (MHC) are heterodimeric glycoproteins expressed at the cytoplasmic membrane of almost all cells. They consist of a polymorphic heavy chain of 45 kd noncovalently linked to β2-microglobulin, and contain short peptides, usually 9 amino acids in length. The HLA system is highly polymorphic, and different HLA allelic products are able to present different sets of peptides. The binding of these peptides to class I molecules depends on specific anchoring motifs that have been defined for many HLA-A, -B, and -C alleles (reviewed in reference 1). The peptides presented generally originate from endogenous proteins, but they can also derive from intracellular pathogen products when cells are infected or from aberrantly expressed proteins in tumor cells. One of the functions of class I molecules is to present these peptides to CD8+cytotoxic T lymphocytes.
Platelets express HLA molecules to a lesser extent than peripheral mononuclear cells (PBMCs); the number of HLA molecules at their surface has been reported to vary between 50,000 and 100,000.2Taking into account the number of platelets, it can be estimated that approximately two thirds of the HLA molecules from blood are carried by thrombocytes. The origin of platelet-surface class I molecules has been discussed intensively. Different groups have shown that HLA-A and -B antigens of platelets are integral membrane constituents with similar biochemical properties to HLA molecules obtained from nucleated cells.3,4 Other experiments, based on chloroquine treatment or coincubation of platelets with allogenic plasma, suggest that most HLA molecules do not have an endogenous source but are adsorbed from the plasma.5,6 Taken together, these data argue for coexpression of integral membrane and adsorbed HLA molecules on platelets, although the contribution of these 2 types is controversial.7 8
The nature of the peptides that are possibly bound to platelet HLA molecules has not been investigated, and the exact function of thrombocyte class I molecules is still unknown. However, it has been proposed that they are implicated in alloimmunization following multiple platelet transfusions. Production of anti-HLA class I alloantibodies has been reported in about half the patients receiving nonleukocyte-depleted thrombocyte concentrates, and refractoriness to platelet transfusion occurs secondarily.9-13 Numerous studies have shown that depletion of contaminant leukocytes from platelet products efficiently decreases the risk of alloimmunization (< 20%) and refractoriness (< 3%).10,14,15 However, previous pregnancies increase the risk of later immunization, even if leukocyte-depleted platelet concentrates are used.16Successful treatment of platelet refractoriness has recently been obtained by transfusing platelets matched for HLA or by using acid-stripped platelets.11,17 All these in vivo data suggest that HLA molecules present on contaminating leukocytes rather than on platelets induce primary anti-HLA alloimmunization after transfusion, although platelets may be responsible for a reactivation of secondary response in preimmunized patients. It was then hypothesized that contaminating HLA class II antigen-presenting cells (APC) present in the thrombocyte concentrates are responsible for initiating T-cell immunity and further production of alloantibodies. In contrast to these studies, several reports in mouse models showed that repeated leukocyte-depleted platelet transfusions induced CD4+ and CD8+ T-lymphocyte activation and alloantibody formation, presumably by a cross-presentation system involving responder APC.18,19 It was also reported recently that extreme leukocyte reduction can re-enhance in vivo allogenic platelet immunity in mice.20 Thus, it remains controversial whether class I molecules from platelets are efficient in inducing an in vivo immune response.
In reexamining this issue, we have studied the major functional features of thrombocyte HLA class I molecules. We could show that peptides extracted from platelet HLA-A2 molecules follow the rules of the peptide motif described for this allele. We also observed that HLA molecules are unstable when thrombocytes are incubated at 37°C, but can be partially stabilized when exogenous β2-microglobulin and HLA-binding peptide are added. Moreover, under conditions leading to in vitro allocytotoxic T lymphocyte (CTL) induction using allogenic PBMCs as a source of antigen, we observed that allogenic resting or activated platelets failed to induce and sustain CTL activity. The same results were obtained when nonspecific help was provided by adding third-party allogenic PBMCs.
Materials and methods
Platelet preparations
Platelet concentrates from healthy donors were obtained after differential centrifugation of blood containing citrate-phosphate-dextrose (CPD) as anticoagulant, and were kindly provided by the Blood Bank of Tübingen and the Blood Center of Stuttgart (leukocyte contamination < 0.2 × 106/mL). For peptide extraction experiments, platelets were centrifuged 30 minutes at 900g and 4°C, washed, and separated from plasma and as much as possible from residual leukocytes over density gradient using Nycoprep 1.063 (Nycomed Pharma AS, Oslo, Norway). Cell pellets were stored at −20°C. For peptide stabilization and allostimulation experiments, fresh thrombocyte concentrates from HLA-A2-typed donors or platelets obtained from HLA-A2+ buffy coats after Ficoll-Hypaque density gradient were used. Platelets were centrifuged at 800g for 10 minutes, washed twice, resuspended in CPD citrate buffer, pH 6.2, consisting of 0.327 g sodium citrate H2O, 2.63 g natrium citrate, 0.251 g natrium dihydrogenophosphate 2H2O, 2.55 g dextrose H2O in 100 mL water for injection (Baxter Healthcare, Maurepas, France), and counted. Leukocyte contamination was registered by microscope and was always less than 0.2 × 106/mL. When needed, platelets were stored at 4°C.
Extraction of HLA-A2 molecules from platelets and analysis of peptidic ligands
For HLA-A2 ligand extraction, 3 independent pools of outdated concentrates, and 1 pool of fresh concentrates containing between 1.3 × 1012 and 31 × 1012platelets were used. In 2 cases, peptide extraction was performed using platelet concentrate pools obtained from HLA-A2-typed donors, and in 2 cases donors were not HLA-typed (HLA-A2 is the most frequent HLA allele in the Caucasian population [P = 0.29]; thus about half of the platelets in these pools should express HLA-A2). Peptide isolation and analysis were carried out as previously described,21 22 with minor modifications. Briefly, platelets were lysed with phosphate-buffered saline (PBS) 2% NP40 for 45 minutes, and class I peptide complexes were isolated using solid-phase bound anti-HLA-A2 BB7.2 monoclonal antibody (MoAb). Peptides were eluted from purified HLA-A2 using 0.1% trifluoroacetic acid (TFA), ultrafiltrated, and separated by reversed-phase high-performance liquid chromatography HPLC (RP-HPLC). Fractions containing the peptide peaks (generally eluting between 20 and 50 minutes) were pooled and sequenced by Edman degradation using a protein sequencer 476A (Applied Biosystems, Weiterstadt, Germany). For identification of individual ligands, dominant peaks were sequenced as described above.
Analysis of platelet HLA surface expression and peptide stabilization assays
HLA-A2+ platelets (100 × 106) were incubated at 37°C in citrate buffer, pH 6.2, for different periods of time. For stripping of HLA surface molecules, a citric acid solution (pH 3.0) was prepared by mixing equal volumes of 0.263 mol/L citric acid and 0.123 mol/L Na2HPO4, as already described.23 Platelets were pelleted, gently resuspended in 500 μL of this solution, incubated at room temperature for 30 seconds under agitation, and washed twice in PBS. Peptide stabilization assays were performed as previously described,24 in citrate buffer, pH 6.2, at 37°C and 5% CO2 in the presence of human β2-microglobulin at 3 μg/mL (Biotrend, Cologne, Germany) and peptides at 100 μg/mL. Aliquots of platelets were collected at different time points and stained with MoAbs.
Synthetic peptides
Peptides were synthesized in an automated peptide synthesizer 432A (Applied Biosystems). Purity was always more than 80%. Mat 58-66 (GILGFVFTL, Influenza A matrix protein) and gp190 367-375 (SLFTDPLEL, gp190 from Plasmodium falciparum) are strong HLA-A*0201 binders.1 Peptides CH10 68-77 (VGDIILMDKY, CH10 protein from Chlamydia pneumoniae), TYBO 11-19 (ASFDKAKLK, Thymosine β10, natural ligand of HLA-A11), and Ras 8-16 (VVGAGGVGK, K-Ras, HLA-A11 binder) were used as negative controls.1
Antibodies and staining for fluorescence-activated cell sorter (FACS) analysis
W6/32 (anti-HLA class I monomorphic, IgG2a), BB7.2 (anti-HLA-A2, IgG2b), and B1.23.2 (anti-HLA-B and -C alleles, IgG2b) were used as purified MoAbs or as hybridoma culture supernatants. Optimal concentrations for staining had been previously determined. Background control stainings were evaluated with a mouse IgG2b isotype control (Coulter Immunotech, Hamburg, Germany) or OKT8 MoAb (anti-CD8, IgG2a); alternatively, fluorescein isothiocyanate (FITC)-coupled goat-antimouse IgG (GAM-FITC, Dianova, Hamburg, Germany) alone was used. There was no significant difference in the background fluorescence using these 3 methods. CD41-FITC MoAb, specific for the GpIIb protein expressed exclusively by megakaryocytes and platelets, was purchased from Coulter Immunotech.
Aliquots of 20 × 106 platelets were washed twice in PBS 0.1% sodium azide and stained with the different MoAbs for 30 minutes at 4°C. For indirect staining, GAM-FITC was added after 1 wash for another 30 minutes. Cells were washed twice and fixed in PBS 0.1% sodium azide 1% formaldehyde, or analyzed immediately without fixation. Fluorescence analysis was performed on a FACScalibur cytometer (Becton Dickinson, Heidelberg, Germany).
In vitro allostimulation of PBMCs and cytotoxic assays
The fresh PBMCs were obtained by Ficoll-Hypaque gradient centrifugation (Ficoll-Paque, Pharmacia, Oslo, Norway) of buffy coats from HLA-A2− and HLA-A2+ typed healthy donors (Blood Bank, Tübingen, Germany).
For allostimulation, PBMCs of an HLA-A2− donor were incubated with irradiated allogenic PBMCs (30 Gy) of an HLA-A2+donor at a ratio of 1:1. Alternatively, irradiated (30 Gy) HLA-A2+ resting or activated platelets autologous to the stimulator PBMCs were used at a ratio of 1 responding PBMC for 30 platelets. For activation, platelets were incubated with 0.2 U/mL of human thrombin (Roche Diagnostics, Mannheim, Germany) for 30 minutes at 37°C in α-minimum essential medium (MEM; Sigma, Munich, Germany) and washed twice before use. For the nonspecific help experiments, PBMCs from an HLA-A2−donor were mixed with PBMCs as control (ratio of 4:1), or platelets (ratios of 1:7.5 and 1:30) from a second HLA-A2+ donor in the presence or absence of PBMCs from a third HLA-A2−donor as a source of helper activity (ratio of 4:1). All allostimulations were performed in α-MEM medium, supplemented with 2 mmol/L l-glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin (Biowhittaker, Verviers, Belgium), 10% heat-inactivated human serum (Diagast, Jülich, Germany), and recombinant interleukin-2 (r-IL2) at 20 U/mL (Chiron, Marburg, Germany), at 37°C and 5% CO2 for 7 days. Allorestimulations were done weekly, with frozen PBMCs or platelets stored at 4°C, with the same ratio of responder cells to allo-PBMCs and responder cells to alloplatelets as in the first stimulation. Phytohemaggluinin (PHA) blasts as targets for the cytotoxicity assays were generated from PBMCs using phytohemagglutinin A at 0.5 μg/mL (PHA, Murex Diagnostics Ltd, Dartford, GB) and r-IL2 at 20 U/mL.
Specific cytotoxic activity of allostimulated PBMCs was measured in a standard 51Cr-release assay. PHA-induced T blasts were labeled for 90 minutes with 100 μCi Na251CrO4, washed 3 times, and plated in RPMI 1640 (Sigma) supplemented with l-glutamine, antibiotics, and 10% fetal calf serum (FCS; Sigma) at 3000 cells/well. Effector cells were added at different effector-to-target (E:T) ratios. After 5 hours of incubation at 37°C and 5% CO2, supernatant was harvested, and radioactivity was measured in a microplate format counter (1450 Microbeta Plus, Wallac, Turku, Finland) using solid-phase scintillation (Lumaplate 96, Packard, Dreieich, Germany). Spontaneous release (cells in medium alone) and maximum release (cells incubated with 1% Triton × 100) were determined for each target. Percent of specific lysis represents the mean of duplicate experiments and was calculated as (cpm experimental well − cpm spontaneous release) / (cpm maximum release − cpm spontaneous release). Spontaneous release never exceeded 20%.
Results
HLA-A2 peptide motif and identification of natural HLA class I ligands from platelets
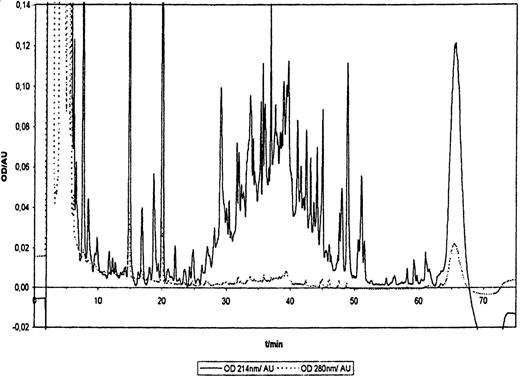
To determine whether HLA class I molecules associated to platelets contain peptides and to analyze these peptides, we performed HLA-A2 ligand extractions using platelet pool concentrates. An RP-HPLC profile obtained from one of these peptide extractions is presented in Figure1. This profile is very similar to those usually obtained from different cell types. In particular, peptides eluted between 26 minutes and 52 minutes, showing similar hydrophobicity properties to HLA-A2 peptide ligands from other cells.1,21,25 Peptide fractions were pooled and sequenced, and the results are given in Table 1. Position 2 showed a dominant preference for Leu residue, Met also being strongly represented. At position 9, Leu and Val were found almost exclusively. Thus, amino acids in positions 2 and 9 appear to be anchor residues. Moreover, the dominant residue in position 6 was Ile. Although some differences were observed for auxiliary anchor (position 6) or preferred residues, the motif found here is very similar to the classical HLA-A*0201 motif.21 Fifteen individual peptides could be identified, 12 nonamers, 1 octamer and 1 decamer, and 1 peptide longer than 11 amino acids (this peptide could not be entirely sequenced). Such length variability has been well documented for most of the natural HLA ligands.1 Among the 15 peptides, 12 were classified as HLA-A2 natural ligands; 9 of them contain 2 anchor residues at positions 2 and 9-10, and for 3 of them, 1 of the 2 anchors was present. Ile at position 6 was found in 4 peptides. Peptides YLLPAIVHI, derived from the p68 RNA helicase, and ILMEHIHKL, derived from the 60S ribosomal protein L19 were previously isolated from HLA-A*0201 and HLA-A*0214 peptide extracts, respectively.25 26 Most of the HLA-A2 ligands identified here originate from ubiquitously expressed cytosolic or nuclear proteins, and thus are not specific for the megakaryocyte-platelet lineage. To control that extracted peptides derived from platelets and not from contaminating leukocytes, several peptide extractions were also performed using the leukocyte-rich fraction obtained after density gradient separation of platelet concentrates; for the experiment with the highest amount of platelets, the amount of HLA-A2-associated peptides found in the leukocyte-rich fraction was only 5% of that obtained with the separated platelet fraction, as detected by Edman degradation (data not shown). Thus, even if the gradient-separated platelets still contain some leukocytes, these should account for less than 5% of the total peptides detected. Moreover, peptide FLLWATAEA derives from the signal sequence of the GPIX protein, which has been shown to be exclusively expressed by mature megakaryocytes undergoing fragmentation and by platelets. This peptide could be sequenced from 3 of 4 extraction experiments, and each time appears as the dominant peptide, still more abundant than the main HLA-A*0201 ligand YLLPAIVHI. We concluded that it is most likely prominent among platelet HLA-A2 ligands. We also confirmed that synthetic FLLWATAEA peptide binds to HLA-A*0201 molecules using an in vitro stabilization assay with TAP-deficient T2 cells (data not shown). Three other peptides derived from the fibrinogen α and β chains were identified. As seen in Table 1, their amino acid sequence did not fit the HLA-A*0201 motif or, indeed, any other HLA-A2 motif known. These peptides may derive from contaminating fibrinogen present during the HLA extraction procedure and may not be relevant for HLA binding. However, because nonmotif peptides have been occasionally described as MHC ligands, we do not want to exclude this possibility. Peptides 3 to 6 amino acids in length were also found occasionally, but because such short peptides did not bind to HLA-A*0201 in vitro (data not shown), they might constitute short fragments generated from longer peptides during peptide preparation. Taken together, our results show that platelet HLA-A2 molecules bind peptides derived from intracellular proteins. The peptide motif and properties of these peptides are very similar to those described for HLA-A*0201 ligands (HLA-A*0201 accounts for about 96% of all HLA-A2 alleles present in the Caucasian population). Moreover, one of the peptides identified derives from the GPIX protein, which is specifically expressed in the megakaryocyte-platelet lineage.
RP-HPLC separation profile of HLA-A2 ligands from platelets.
HLA-A2 molecules were precipitated with BB7.2 specific MoAb, and ligands extracted as described in Materials and methods. Optical densities (OD) at 214 nm (peptide bonds) and at 280 nm (aromatic side chains) are shown for 1 representative experiment of 4.
RP-HPLC separation profile of HLA-A2 ligands from platelets.
HLA-A2 molecules were precipitated with BB7.2 specific MoAb, and ligands extracted as described in Materials and methods. Optical densities (OD) at 214 nm (peptide bonds) and at 280 nm (aromatic side chains) are shown for 1 representative experiment of 4.
Motif and individual ligands of HLA-A2 molecules isolated from platelets
| . | Position . | Protein Source . | Accession N° EMBL Database . | Number of Times Found . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | . | ||||
| Anchors or auxiliary anchors | L | L | |||||||||||
| V | |||||||||||||
| Preferred residues | I | M | I | E | F | ||||||||
| P | D | ||||||||||||
| P | |||||||||||||
| Ligands | I | L | M | E | H | H | K | L | 60S Ribosomal protein L19 (137-145) | X63527 | 1 | ||
| L | L | I | E | N | V | A | S | L | Gluthatione peroxidase (38-46) | Y00483 | 1 | ||
| L | I | E | N | V | A | S | L | Gluthatione Peroxidase (39-46) | Y00483 | 1 | |||
| X | L | I | D | F | A | L | Y | L | unknown | 1 | |||
| A | L | S | N | L | E | V | K | L | unknown | 1 | |||
| I | L | F | G | H | E | N | R | V | β5-Guanine nucleotide binding (320-329) | AF017656 | 2 | ||
| I | L | D | Q | K | N | E | V | Ornithine decarboxylase (23-31) | M31061 | 1 | |||
| S | L | A | G | G | I | G | V | Ribonucleoprotein K (154-162) | S74678 | 1 | |||
| Y | L | L | P | A | V | H | I | p68 RNA helicase (148-156) | X52104 | 2 | |||
| X | L | P | D | F | G | I | S | Y | V | unknown | 2 | ||
| A | L | N | E | L | L | Q | H | V | mouse Talin (777-785) | X56123 | 1 | ||
| F | L | L | W | A | T | A | E | A | GPIX glycoprotein (8-16) | X52997 | 3 | ||
| Other peptides found | K K R E E A P S L | Fibrinogen β-chain (51-59) | J00129 | 1 | |||||||||
| L E R P G G N E I | Fibrinogen α-chain (261-269) | M64982 | 1 | ||||||||||
| S G S F R P D S P G S … | Fibrinogen α-chain (390-…) | M64982 | 1 | ||||||||||
| . | Position . | Protein Source . | Accession N° EMBL Database . | Number of Times Found . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | . | ||||
| Anchors or auxiliary anchors | L | L | |||||||||||
| V | |||||||||||||
| Preferred residues | I | M | I | E | F | ||||||||
| P | D | ||||||||||||
| P | |||||||||||||
| Ligands | I | L | M | E | H | H | K | L | 60S Ribosomal protein L19 (137-145) | X63527 | 1 | ||
| L | L | I | E | N | V | A | S | L | Gluthatione peroxidase (38-46) | Y00483 | 1 | ||
| L | I | E | N | V | A | S | L | Gluthatione Peroxidase (39-46) | Y00483 | 1 | |||
| X | L | I | D | F | A | L | Y | L | unknown | 1 | |||
| A | L | S | N | L | E | V | K | L | unknown | 1 | |||
| I | L | F | G | H | E | N | R | V | β5-Guanine nucleotide binding (320-329) | AF017656 | 2 | ||
| I | L | D | Q | K | N | E | V | Ornithine decarboxylase (23-31) | M31061 | 1 | |||
| S | L | A | G | G | I | G | V | Ribonucleoprotein K (154-162) | S74678 | 1 | |||
| Y | L | L | P | A | V | H | I | p68 RNA helicase (148-156) | X52104 | 2 | |||
| X | L | P | D | F | G | I | S | Y | V | unknown | 2 | ||
| A | L | N | E | L | L | Q | H | V | mouse Talin (777-785) | X56123 | 1 | ||
| F | L | L | W | A | T | A | E | A | GPIX glycoprotein (8-16) | X52997 | 3 | ||
| Other peptides found | K K R E E A P S L | Fibrinogen β-chain (51-59) | J00129 | 1 | |||||||||
| L E R P G G N E I | Fibrinogen α-chain (261-269) | M64982 | 1 | ||||||||||
| S G S F R P D S P G S … | Fibrinogen α-chain (390-…) | M64982 | 1 | ||||||||||
Classification of anchor residues (bold symbols), auxiliary anchor residues (underlined symbols), preferred residues, and identification of individual ligands were carried out as described in Materials and methods. Results are combined from 4 independent experiments. Amino acids are coded by their one-letter symbols. X indicates unidentified.
Platelets lose HLA surface expression after incubation at 37°C
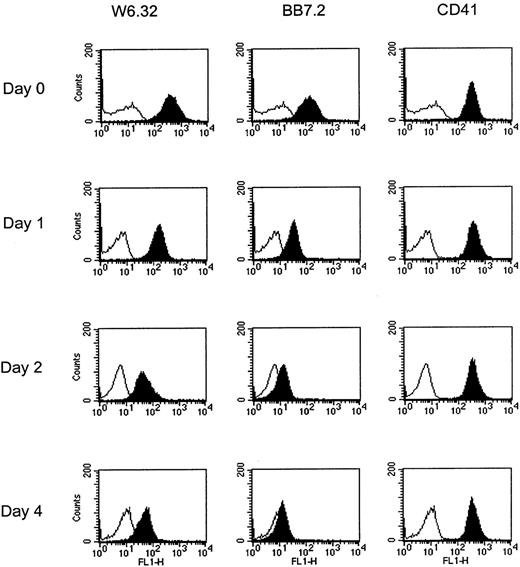
To examine the stability of thrombocyte surface HLA molecules, HLA-A2+ platelets were incubated at 37°C in citrate buffer pH 6.2, and HLA expression measured at different time points. Figure 2 shows a representative result from 5 experiments. HLA-A2+ freshly isolated platelets were stained with the conformation-dependent W6/32 (pan-anti-HLA class I molecules) and BB7.2 (HLA-A2 specific) MoAbs. This staining is weaker than that observed with PBMCs, which is in agreement with a previous report.2 After 24 hours of incubation (day 1), HLA surface expression was strongly reduced, with 34% and 20% of initial fluorescence remaining after staining with W6/32 and BB7.2, respectively. Further decrease of fluorescence was observed at day 2. Finally, after 4 days of incubation, the majority of integral HLA molecules had disappeared from the platelet surface, because less than 15% of initial surface expression was still visible. Interestingly, addition of 10% FCS to the medium could partially retain the staining with MoAbs, probably by stabilization of HLA complexes by exogenous bovine β2-microglobulin (data not shown). Finally, expression of GpIIb, as assessed by CD41 MoAb, remains stable even after 4 days at 37°C, suggesting that platelet membrane integrity was not affected by the assay conditions. These results demonstrate that class I β2-microglobulin-peptide complexes are unstable at the platelet surface and are rapidly dissociated after in vitro incubation at 37°C.
Platelet HLA surface expression decreases with time after incubation at 37°C.
Platelets were incubated at 37°C in citrate buffer pH 6.2, for the indicated times, stained for expression of HLA (W6/32 and BB7.2 MoAbs) or CD41, and analyzed by cytometry. Background fluorescence is shown in white, staining with MoAbs in black.
Platelet HLA surface expression decreases with time after incubation at 37°C.
Platelets were incubated at 37°C in citrate buffer pH 6.2, for the indicated times, stained for expression of HLA (W6/32 and BB7.2 MoAbs) or CD41, and analyzed by cytometry. Background fluorescence is shown in white, staining with MoAbs in black.
Stabilization of platelet HLA-A2 molecules by exogenous HLA-A2 binding peptides
Because we observed that properly folded HLA molecules disappear rapidly from the platelet surface after incubation at 37°C, we tested whether this dissociation could be compensated by addition of exogenous β2-microglobulin and peptide to the medium. HLA-A2+ platelets were incubated at 37°C for 24 hours, in medium alone, or in the presence of β2-microglobulin and different HLA class I binding peptides. HLA expression was then analyzed. Staining of fresh platelets before incubation at 37°C was used as control. Results of a representative experiment from 3 are shown in Table 2. After 24 hours of incubation at 37°C, HLA surface expression was strongly reduced, as already observed in Figure 2. This was detected after staining with W6/32 and BB7.2 MoAbs, as well as with B1.23.2 MoAb (anti-HLA-B and -C alleles). Addition of Mat 58-66 (HLA-A*0201 epitope) and gp190 367-375 (strong HLA-A2 binder) peptides was able to significantly retain staining of HLA-A2 molecules at the platelet surface. The effect observed with the gp190 peptide was stronger than with the Mat peptide, and about 70% of initial surface expression remained after 24 hours, as compared to 50% without peptide. This could be observed using W6/32 and BB7.2 MoAbs, but not B1.23.2 MoAb, showing that stabilization of surface HLA molecules occurred on specific binding of Mat and gp190 peptides to HLA-A2. Two other peptides, CH10 68-77 and TYBO 11-19, which do not bind to HLA-A2, could not induce any HLA stabilization, as assessed with the different MoAbs tested. Finally, as already seen in Figure 2, CD41 expression was almost stable after 24 hours and remained unchanged after incubation with the different peptides.
Stabilization of platelet HLA-A2 molecules at 37°C after addition of HLA-A2 binding exogenous peptide and β2-microglobulin
| . | Neg. Control . | W6/32 . | BB7.2 . | B1.23.2 . | CD41 . |
|---|---|---|---|---|---|
| Time 0 | 17* | 450 | 164 | 155 | 1330 |
| Incubation 24 h at 37°C† | |||||
| Medium | 15 | 215 | 86 | 54 | 930 |
| β2m + CH10 68-77‡ | 18 | 181 | 96 | 80 | 887 |
| β2m + TYBO 11-19 | 12 | 193 | 95 | 75 | 905 |
| β2m + Mat 58-66 | 12 | 271 | 110 | 49 | 913 |
| β2m + gp190 367-375 | 12 | 311 | 120 | 46 | 940 |
| . | Neg. Control . | W6/32 . | BB7.2 . | B1.23.2 . | CD41 . |
|---|---|---|---|---|---|
| Time 0 | 17* | 450 | 164 | 155 | 1330 |
| Incubation 24 h at 37°C† | |||||
| Medium | 15 | 215 | 86 | 54 | 930 |
| β2m + CH10 68-77‡ | 18 | 181 | 96 | 80 | 887 |
| β2m + TYBO 11-19 | 12 | 193 | 95 | 75 | 905 |
| β2m + Mat 58-66 | 12 | 271 | 110 | 49 | 913 |
| β2m + gp190 367-375 | 12 | 311 | 120 | 46 | 940 |
Staining with MoAbs is expressed as fluorescence mean. For W6.32 and BB7.2 stainings, fluorescence mean increases >20% are indicated in bold.
Platelets were incubated for 24 hours at 37°C in medium alone or in the presence of 100 μg/mL of exogenous peptide and 3 μg/mL of β2-microglobulin (β2m), then stained with different MoAbs as described in Materials and methods.
Mat and gp190 peptides are known HLA-A*0201 binders. CH10 and TYBO peptides, which do not contain any specific HLA-A2 motif were used as negative controls.
To confirm these results, further experiments were carried out using acid-stripped platelets. Acid treatment at pH 3 has been described to dissociate HLA-β2- microglobulin-peptide complexes present at the cell membrane. Platelets were treated for 30 seconds with stripping buffer, washed, and incubated with or without peptide for only 2 hours at 37°C. Table 3summarizes the results of a representative experiment. As expected, stripping buffer treatment followed by 2 hours of incubation at 37°C in medium alone strongly reduced HLA surface expression (41% and 28% of initial surface expression detected with W6/32 and BB7.2 MoAbs, respectively). Incubation of acid-stripped platelets for 2 hours at 4°C gave similar results (data not shown). However, addition of Mat 58-66 enhanced HLA detection considerably, almost reaching the initial level (88% with W6/32 and 73% with BB7.2 of the initial staining). The same effect was seen with the gp190 367-375 peptide, although to a lesser extent. Again, stabilization was not observed when a non-HLA-A2 binding peptide, Ras 8-16, was added. β2-Microglobulin alone slightly increased W6/32 staining, but this effect was not seen with BB7.2. Similar results were obtained in 2 additional experiments. Taken together, these data show that platelet surface HLA loss at 37°C can be partially compensated by stabilization of HLA-β2-microglobulin-peptide complexes with exogenous β2-microglobulin and HLA-binding peptide. The same effect is also obtained after acid-stripping treatment. This strongly suggests that the HLA dissociation from platelet membrane we observed occurs in a “classical” way, where HLA heavy chain is maintained anchored at the cell membrane, whereas peptides and β2-microglobulin are released into the medium.
Stabilization of HLA-A2 molecules from acid-stripped platelets after addition of exogenous HLA-A2 binding peptide and β2-microglobulin
| . | Neg. Control . | W6/32 . | BB7.2 . |
|---|---|---|---|
| Time 0 | 43-150 | 212 | 109 |
| Stripping buffer treatment3-151 | |||
| Medium | 10 | 87 | 31 |
| β2m | 9 | 116 | 28 |
| β2m + Ras 8-163-152 | 9 | 56 | 22 |
| β2m + Mat 58-66 | 20 | 186 | 80 |
| β2m + gp190 367-375 | 9 | 179 | 45 |
| . | Neg. Control . | W6/32 . | BB7.2 . |
|---|---|---|---|
| Time 0 | 43-150 | 212 | 109 |
| Stripping buffer treatment3-151 | |||
| Medium | 10 | 87 | 31 |
| β2m | 9 | 116 | 28 |
| β2m + Ras 8-163-152 | 9 | 56 | 22 |
| β2m + Mat 58-66 | 20 | 186 | 80 |
| β2m + gp190 367-375 | 9 | 179 | 45 |
Staining with MoAbs is expressed as fluorescence mean. Stabilizations induced by HLA-A2-binding peptides are indicated in bold.
Platelets were treated for 30 seconds with stripping buffer. After 2 washes, peptides (100 μg/mL) and β2-microglobulin (β2m) (3 μg/mL) were added and platelets were further incubated for 2 hours at 37°C before staining. Detailed protocol is described in Materials and methods.
Ras peptide, an HLA-A11 binder, was used as negative control.
In vitro allostimulation experiments
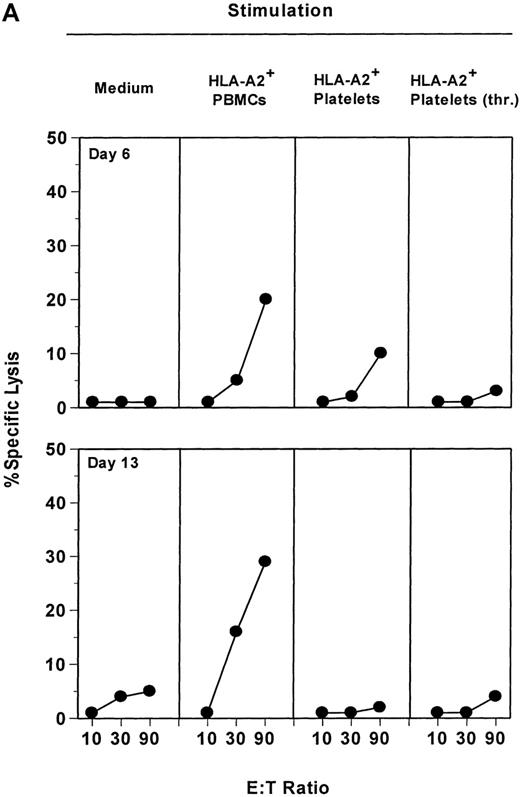
Finally, we attempted to determine whether platelet surface HLA class I molecules were able to induce T-lymphocyte alloreaction. PBMCs from an HLA-A2−donor were stimulated in vitro with PBMCs or platelets obtained from an HLA-A2+ donor. Because it has been shown that platelets express about 10 times fewer HLA molecules than lymphocytes,2 and that these surface HLA molecules, according to our results, are unstable at 37°C (see above), 30 times more platelets than PBMCs were used. Results obtained are presented in Figure 3. Primary stimulation with allogenic PBMCs induced a consistent allocytotoxicity as tested against allogenic HLA-A2+ PHA-blast cells (Figure3A). As expected, killing activity was detected as early as 6 days after stimulation (20% specific lysis at E:T ratio of 90) and increased after a single in vitro restimulation (day 13, lysis 29%). This cytotoxic activity was directed specifically against the cells used for the stimulation, because HLA-A2− PHA-blasts autologous to the responder cells were not killed under the same conditions (data not shown). In contrast, resting or thrombin-activated platelets were not able to induce significant allocytotoxicity, even after 2 rounds of in vitro stimulation. This was not due to a nonspecific inhibitory effect of citrate buffer used to store platelet because (1) platelets isolated directly from buffy coats did not stimulate alloreaction, (2) effector PBMCs resuspended in thrombocyte concentrate supernatant then washed twice responded to the same level to allostimulation as nontreated PBMCs, and (3) addition of thrombocyte concentrate supernatant up to 1:500 in the culture medium did not affect control stimulations by allogenic PBMCs. Moreover, different ratios of effector PBMCs to platelets were also tested, from 1:1 to 1:300, but none of them could induce allospecific CTL activity (data not shown).
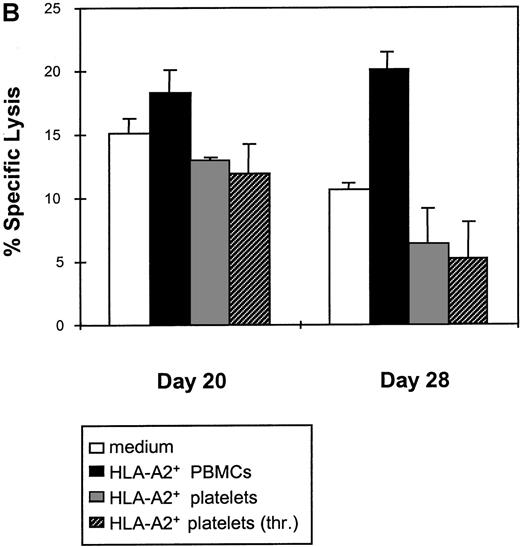
Resting or activated platelets are not able to stimulate allospecific cytotoxicity in vitro.
(A) HLA-A2− PBMCs were stimulated weekly with irradiated PBMCs, platelets, or thrombin-activated platelets from an HLA-A2+ donor, as described in Materials and methods. Allospecific cytotoxic activity was tested at day 6 (1 in vitro stimulation) and day 13 (2 in vitro stimulations) against allogenic HLA-A2+ PHA-blasts, at the indicated E:T ratios. (B) Allospecific HLA-A2− cytotoxic cell line obtained after 2 stimulations with HLA-A2+ PBMCs was split and restimulated weekly either with PBMCs or with resting or activated platelets from the same allogenic donor. As control, medium alone was added without any stimulating cells. Cytotoxicity was tested after 1 restimulation (day 20) or 2 restimulations (day 28), against allogenic PHA-blasts. Data are presented for an E:T ratio of 20.
Resting or activated platelets are not able to stimulate allospecific cytotoxicity in vitro.
(A) HLA-A2− PBMCs were stimulated weekly with irradiated PBMCs, platelets, or thrombin-activated platelets from an HLA-A2+ donor, as described in Materials and methods. Allospecific cytotoxic activity was tested at day 6 (1 in vitro stimulation) and day 13 (2 in vitro stimulations) against allogenic HLA-A2+ PHA-blasts, at the indicated E:T ratios. (B) Allospecific HLA-A2− cytotoxic cell line obtained after 2 stimulations with HLA-A2+ PBMCs was split and restimulated weekly either with PBMCs or with resting or activated platelets from the same allogenic donor. As control, medium alone was added without any stimulating cells. Cytotoxicity was tested after 1 restimulation (day 20) or 2 restimulations (day 28), against allogenic PHA-blasts. Data are presented for an E:T ratio of 20.
We then asked whether platelets could maintain alloreactivity induced by 2 allogenic PBMCs stimulations. The CTL line generated in Figure 3A was split and restimulated with allogenic PBMCs or platelets, or cultivated further with medium alone. Cytotoxic activity was tested after 1 (day 20) or 2 (day 28) rounds of restimulation. As shown in Figure 3B, restimulation with PBMCs could maintain the specific killing of the CTL line (18% lysis at day 20, 20% at day 28 for E:T ratio of 20). In contrast, after restimulation with resting platelets, allocytotoxicity dropped progressively to 13% lysis at day 20 and finally 6% at day 28. Activated platelets were also inefficient. Remaining allocytotoxicity at day 28 was not significantly different when control effector CTLs were cultivated in medium alone (10% lysis with medium, 6% with resting platelets, and 5% with activated platelets).
Finally, to determine if allocytotoxicity against platelets could be induced in the presence of helper activity, we performed experiments using a 3-party allostimulation system: PBMCs of an HLA-A2−(donor n°1) were stimulated with platelets from an HLA-A2+ donor (donor n°2) in the presence or absence of PBMCs from another HLA-A2− donor (donor n°3). As control, allostimulations against PBMCs n°2 or n°3 alone were used. Cell cultures were stimulated twice and cytotoxic activities against PHA blasts from donor n°1 (autologous control), donor n°2, and donor n°3 were tested at day 14. Results of a representative experiment are shown in Figure4. As already observed in Figure 3, allogenic PBMCs n°2 efficiently stimulated PBMCs n°1 (32% of allocytotoxicity at E:T ratio of 60), but platelets alone were unable to induce any specific killing activity at the 2 different ratios used (effector to platelets, 1:7.5 and 1:30). Addition of PBMCs n°3 as a possible source of help did not activate any significant allostimulation by platelets n°2, although allocytotoxicity against PBMCs n°3 was induced in the same conditions. As a control, no cytotoxic activity was observed against autologous target cells (PBMC-PHA n°1). To conclude, we could show that, unlike PBMCs, resting or activated platelets are generally unable to generate and maintain specific allocytotoxic activity in vitro, even in the presence of additional nonspecific helper activity.
Platelets do not stimulate allospecific cytotoxicity even in the presence of nonspecific helper activity.
Responder PBMCs n°1 were stimulated twice with PBMCs n°2 or platelets n°2 in the presence or absence of PBMCs n°3 (see Materials and methods). Allostimulation against PBMCs n°3 alone was also performed as control. Resulting cytotoxic activity was tested at day 14 against autologous PBMC-PHA n°1 (open circles), allogenic PBMC-PHA n°2 (closed circles), or n°3 (closed triangles), at the indicated E:T ratios. Stimulating conditions are indicated at the top of each graph.
Platelets do not stimulate allospecific cytotoxicity even in the presence of nonspecific helper activity.
Responder PBMCs n°1 were stimulated twice with PBMCs n°2 or platelets n°2 in the presence or absence of PBMCs n°3 (see Materials and methods). Allostimulation against PBMCs n°3 alone was also performed as control. Resulting cytotoxic activity was tested at day 14 against autologous PBMC-PHA n°1 (open circles), allogenic PBMC-PHA n°2 (closed circles), or n°3 (closed triangles), at the indicated E:T ratios. Stimulating conditions are indicated at the top of each graph.
Discussion
To investigate the origin and function of platelet MHC class I molecules, we studied platelet-derived peptides presented by the common HLA-A*0201 allelic product. We found that HLA-A2 molecules isolated from platelets are able to bind peptides of 8 to 10 amino acids, and that the binding motif of these peptides is very similar to the HLA-A*0201 motif described (HLA-A*0201 counts for 96% of HLA-A2 alleles of the Caucasian population).1,21 The classical Leu at position 2 and Leu or Val at position 8 to 10 as anchoring residues were present in 11 of 12 and 10 of 12 identified ligands, respectively (Table 1). Ile as auxiliary anchor residue at position 6, and other preferred residues identified in our study, have also been described for HLA-A*0201 ligands or T cell epitopes.1 Of 12 individual peptides that could be sequenced, 2 have already been found in other cell extracts,25,26 confirming that platelet HLA-A2 molecules bind the same kind of peptides as those from other cells. When the protein source of the peptides could be established, it was evident that most of these proteins are ubiquitously expressed (Table 1). One peptide was derived from the human actin cytoskeleton-associated protein talin, which has been shown to be highly expressed in thrombocytes, accounting for 3% to 8% of whole platelet proteins. Another abundant peptide was derived from the signal sequence of the GPIX glycoprotein, which is specifically expressed in the megakaryocyte-platelet lineage and has not been found in any other cell. These results demonstrate that HLA-A2-bound peptides from platelets are derived from endogenously processed proteins that are expressed at the megakaryocyte stage. Although we cannot exclude that a number of HLA molecules present at the platelet surface are adsorbed from the serum, our data indicate that the majority of these HLA molecules are of endogenous origin, as previously suggested.27 The stage of platelet development at which peptides to be presented by HLA molecules are generated is not known. Because platelets are anucleated cytoplasmic fragments of megakaryocytes with a short life span, it is generally thought that synthesis of proteins occurs at the megakaryocyte stage. However, endoplasmic reticulum and ribosomes have been identified in platelets.28 Protein biosynthesis, including HLA class I molecules, and presence of a functional proteasomal complex have also been reported.28-30 Thus, 2 scenarios are possible: (1) HLA molecules are synthesized and loaded with peptides at the nucleated megakaryocyte stage, or (2) remaining de novo synthesis of HLA molecules from platelet messenger RNA (mRNA) and components of the processing machinery allow the entire assembly of peptide-loaded HLA molecules at the thrombocyte stage.
To address this question, we investigated the stability of HLA molecules at the platelet surface. It has been shown that platelet HLA surface molecules are stable at 22°C.31 In contrast, we found that integral HLA surface expression is progressively lost when platelets are incubated in vitro at 37°C in citrate buffer (Figure2). This is not due to membrane alteration or platelet death, because expression of another membrane glycoprotein, CD41, was not affected, and because we did not observe any significant size modification as measured by forward scatter and side scatter cytometric analysis. This loss was irreversible, even at later time points (2 and 4 days); it seems, therefore, that platelets, even if they can synthesize new class I heavy chains as discussed above, are unable to reconstitute a detectable HLA class I membrane pool. Similar results were obtained after acid-stripping at pH 3 (Table 3), which is known to induce the release of β2-microglobulin and peptides into the medium, whereas class I heavy chains are left anchored to the cell membrane, followed by degradation. Further addition of exogenous β2-microglobulin and HLA-binding peptide allows the reconstitution and stabilization of properly folded HLA-β2-microglobulin–peptide complexes and recognition by conformational dependent MoAbs. This technique has already been used to study the binding of exogenous peptides to different HLA alleles. Acid elution has also been shown to eliminate HLA class I-β2-microglobulin–peptide complexes from the platelet surface without affecting cell integrity and viability.27,32 As expected, loss of surface HLA after platelet acid stripping could be partially compensated by addition of exogenous β2-microglobulin and peptide (Table 3). A similar effect was observed when nonstripped platelets were incubated for longer periods at 37°C in the presence of exogenous HLA-binding peptide (Table 2). This strongly suggests that the loss of detectable HLA surface molecules at 37°C is also triggered by the dissociation of peptides. Thus, we conclude that, at least in the conditions tested, platelets are unable to load HLA molecules with peptides efficiently, strongly suggesting that most platelet HLA molecules have been synthesized and loaded at the megakaryocyte stage. Moreover, we show here that platelets, which are easy to obtain, could be used as cell source for in vitro peptide binding assays. In vivo, surface HLA loss by platelets may not be detectable because of continuous renewal of the platelet pool, and due to the presence of free β2-microglobulin in the serum. The physiologic life span of a platelet has been estimated at 9 to 12 days, and it has been hypothesized that low- and high-density platelets, which express different amounts of surface HLA molecules, correspond to different age stages.33 Our observation of HLA loss by platelets would predict that aged platelets express fewer HLA molecules. Finally, the stability of platelet HLA molecules was not investigated in vivo after transfusion, but interestingly, successful transfusion of an alloimmunized refractory patient was obtained after acid treatment of platelets.17
Production of anti-HLA antibodies and refractoriness to repeated platelet transfusions is a well-known phenomenon that interferes with the therapy of patients with acute leukemia or bone marrow transplant. The use of leukocyte-depleted products decreases alloantibody production, although preimmunized patients may remain refractory.10,16 This led to the hypothesis that contaminating leukocytes present in thrombocyte concentrates are responsible for immunization, via direct or indirect (cross-priming) recipient CD4+ T-helper activation and subsequent antibody production. Induction of CD4+ and CD8+responses by an indirect presentation mechanism has also been described in a mouse model following repeated platelet transfusions.18 However, little is known about cytotoxic CD8+ T-cell induction after allogenic platelet transfusion in patients. Our in vitro experimental model allowed us to test the direct rather than indirect (ie, cross-priming) allocytotoxic stimulation. In these conditions, we did not observe any induction of allospecific killing when HLA-A2+ platelets were used in conjunction with r-IL2 to stimulate HLA-A2− PBMCs. It has also been described that human allogenic platelets do not induce any T-cell proliferation in vitro.34 One explanation could be a lack of costimulatory capacity. However, we show that thrombin-activated platelets, which are known to express increased levels of adhesion molecules and CD40L,35 also failed to stimulate direct T-cell cytotoxicity. Moreover, resting and activated platelets did not sustain allocytotoxic response efficiently, although costimulatory signals are less critical during the secondary stimulating phase. These results are consistent with the rapid loss of surface HLA class I seen after platelet incubation at 37°C. However, it should be mentioned that in 2 of 14 independent experiments, we observed an induction of specific allocytotoxic activity after stimulation with allogenic platelets. This may be explained by the presence of contaminating leukocytes, although our platelet preparations contain less than 0.1% leukocytes, or unrecognized preimmunization of the responder PBMCs. Indeed, in a humanized SCID mouse model, a weak in vitro secondary T-cell proliferation was observed when PBMCs from a preimmunized donor were stimulated with allogenic platelets; this T-cell stimulation was inhibited if platelets were pretreated with polyspecific anti-HLA serum.36
Numerous interactions of platelets with other cells have been demonstrated. It is now clear that, on activation, thrombocytes can induce endothelial cells to produce inflammatory cytokines such as IL-8 or monocyte chemotactic protein-1 (MCP-1), and to interact with monocytes.35,37 Direct interactions of platelets with neutrophils or monocytes also occur.38-41 Moreover, activated platelets, which express high levels of P-selectin, play an active role in lymphocyte delivery to lymph nodes.42Further functions of platelets in the immune response have also been described. In a rat model, it was shown that they are able, via an IgE-dependent mechanism, to display efficient antiparasite cytotoxicity against Schistosoma mansoni, and to induce protection against a subsequent challenge with the parasite.43 This platelet-mediated cytotoxicity is tightly regulated by T lymphocyte-derived cytokines, such as tumor necrosis factor-α and interferon-γ.44 45 Considered together, these data have established that platelets can play an active role in the immune response. However, it is not known if they can, in some circumstances, present antigenic peptides bound to their HLA class I surface molecules to T lymphocytes. We clearly found that platelet HLA molecules bind endogenously derived peptides. Moreover, our results show that platelets are generally unable to generate and sustain any direct alloreactivity in vitro, even if they may do so by cross-priming in vivo. This suggests that HLA molecules on platelets have no peptide presentation function, but are more a vestige of the megakaryocyte precursor stage.
Acknowledgments
We wish to thank D. Wernet, M. Schnaidt, and S. Pascolo for critically reading the manuscript; G. Mayer and H. Northoff from the Tübingen Blood Bank and A. König from the Stuttgart Blood Center for providing the platelet concentrates and buffy coats; L. Yakes for editorial help; and P. Hrstić for technical assistance.
Supported by a Marie Curie Fellowship awarded to C.G. (CT-96-1298) by the Deutsche Forschungsgemeinschaft (Leibniz Programm Ra 369/4-1), and by the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie.
Reprints:Cécile Gouttefangeas, Department of Immunology, Institute for Cell Biology, Auf der Morgenstelle 15, 72076 Tübingen, Germany.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal