Abstract
Dendritic cell (DC) activation through CD40-CD40 ligand interactions is a key regulatory step for the development of protective T-cell immunity and also plays an important role in the initiation of T-cell responses involved in autoimmune diseases and allograft rejection. In contrast to previous reports, we show that the immunosuppressive drug dexamethasone (DEX) redirects rather than simply blocks this DC activation process. We found that DCs triggered through CD40 in the presence of DEX were unable to acquire high levels of costimulatory, adhesion, and major histocompatibility complex class I and II molecules and failed to express the maturation marker CD83, whereas antigen uptake was not affected. Moreover, DEX strikingly modified the CD40-activated DC cytokine secretion profile by suppressing the production of the proinflammatory cytokine interleukin (IL)-12 and potentiating the secretion of the anti-inflammatory cytokine IL-10. Accordingly, DEX-exposed CD40-triggered DCs displayed a decreased T-cell allostimulatory potential and a dramatically impaired ability to activate cloned CD4+ T helper 1 (Th1) cells. Moreover, interaction between Th1 cells and these DCs rendered the T cells hyporesponsive to further antigen-specific restimulation. Collectively, our results demonstrate that DEX profoundly modulates CD40-dependent DC activation and suggest that the resulting alternatively activated DCs can be exploited for suppression of unwanted T-cell responses in vivo.
The remarkable immunostimulatory properties of dendritic cells (DCs) reside in their ability to transport antigens from peripheral tissues to lymphoid organs where they present these antigens to T cells in an optimal costimulatory context.1To achieve this complex sequence of events, DCs exist in different functional stages. Immature DCs behave as sentinels in peripheral tissues where they efficiently capture antigens. On pathogen invasion, induction of protective T-cell responses requires the activation of immature DCs into mature immunostimulatory cells. DC activation is triggered in inflamed tissues by cytokines, such as interleukin (IL)-1 and tumor necrosing factor α (TNF-α), and by bacterial components, such as lipopolysaccharide (LPS).2,3 Activated DCs migrate to T-cell areas in the lymph nodes while up-regulating their costimulatory capacities and optimizing their antigen-presenting functions. On interaction with antigen-specific T cells, DC activation is further completed through engagement of the receptor-ligand (L) pair CD40-CD40L, leading to the production of IL-12,4,5,6 a key cytokine for T helper type 1 (Th1) and cytotoxic T-lymphocyte priming.7
Antigen-presenting cell (APC) activation through CD40-CD40L interactions represents a crucial immunoregulatory step for the establishment of protective T-cell immunity against pathogens and tumors.8,9,10 This process also plays a key role in the onset of destructive T cell-mediated disorders, such as autoimmune diseases, allograft rejection, and graft versus host disease.11,12,13 The current treatment of these disorders largely relies on the administration of glucocorticoids (GCs), which exert potent anti-inflammatory and immunosuppressive effects. Because GCs negatively interfere with many aspects of T-cell activation, such as IL-2-driven proliferation and inflammatory cytokine production (reviewed in 14), activated T cells have long been considered as the main targets for GC action. Several lines of evidence now indicate a role for DCs in GC-induced immune suppression. Moser et al15 found that GCs prevented the spontaneous activation of murine DCs, thereby decreasing their T-cell stimulatory potential. Kitajima et al16 showed that GCs could hamper the T cell-mediated activation of a murine DC line. Viera et al17 reported that human DCs exposed to GCs were poor producers of IL-12 on LPS stimulation. These findings only concern loss of typical DC features and, therefore, favor a simple inhibitory role of GCs on DC activation. A more complex immunoregulatory action on the DC system has not been considered.
In this study, we performed detailed analysis of the impact of GCs on the CD40-mediated activation of monocyte-derived DCs. These DCs develop after culture with granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-42,18 or after transmigration through endothelial cells19 and are known to mature into the most potent human Th1-type-inducing APC on CD40 ligation.5,20Moreover, these APCs can easily be generated in large numbers and are thereby the cells of choice for DC-based modulation of T-cell immunity.21 22 In contrast to previous studies, our data show that GCs do not merely prohibit DC activation. In the presence of the GC hormone dexamethasone (DEX), CD40 ligation on human monocyte-derived DCs is transformed into an alternative activation pathway, equipping these cells with unique features that enable them to downmodulate Th1-type responses in vitro.
Materials and methods
Generation of DCs
Immature DCs were generated from peripheral blood monocyte precursors. Human peripheral blood mononuclear cells from healthy donors, isolated through Ficoll-Hypaque density centrifugation were plated at 1.5 × 107 per well in 6-well plates (Costar Corp, Cambridge, MA) in RPMI 1640 (Life Technologies, Paisley, Scotland) supplemented with 2 mmol/L glutamine, 100 UI/mL penicillin, and 10% fetal calf serum. After 2 hours at 37°C, the nonadherent cells were removed and the adherent cells were cultured in medium containing 500 U/mL IL-4 (Pepro Tech Inc, Rocky Hill, NJ) and 800 U/mL GM-CSF (kindly provided by Dr S Osanto, LUMC, Leiden, The Netherlands) for a total of 7 days.
Activation of immature DCs with a CD8-CD40L fusion protein
Activation of DCs though CD40 was performed with a fusion protein made of the extracellular domain of human CD40L and of the murine CD8α chain (CD8-CD40L). The CD8-CD40L complementary DNA described by Garrone et al23 was transferred into an eukaryotic expression vector containing the hygromycin resistance gene and used for the generation of stably transfected Chinese hamster ovary cells. Culture supernatants containing the CD8-CD40L fusion protein were concentrated with a pressurized stirred cell system (Amicon, Inc, Beverly, MA), checked for binding to CD40, and tested for optimal DC-activation conditions (not shown). DCs were incubated at 5 × 105/mL/well in a 24-well plate (Costar Corp, Cambridge, MA) and activated in the presence of 1/10 CD8-CD40L supernatant. Cells and supernatants were analyzed after 48 hours. Importantly, the specificity of CD8-CD40L supernatants was checked by comparison with control supernatants obtained from untransfected Chinese hamster ovary cells. Unlike DCs activated with CD8-CD40L supernatants, immature DCs cultured with control supernatants or with medium alone failed to up-regulate the maturation marker CD83 (respective mean fluorescence intensities: 20, 6, and 5) and to secrete IL-12 (respective IL-12 production per 2.5 × 105DC: 30 510 pg/mL, 600 pg/mL, and 250 pg/mL). The action of CD8-CD40L supernatants is, therefore, specific for the CD8-CD40L fusion protein and is not due to other factors present in the concentrated conditioned medium.
Dexamethasone and RU486 treatment of DCs
Seven-day immature DCs were treated with 10−6mol/L DEX (Sigma, St Louis, MO) in the presence of GM-CSF and IL-4 or GM-CSF alone. After 24 hours, DCs were analyzed or were further stimulated via CD40 by adding the CD8-CD40L fusion protein to the cultures as described above. In some experiments, the GC receptor antagonist RU485 (Roussel-UCLAF, Romainville, France) was used at 10 μmol/L final concentration, alone or in combination with DEX.
Analysis of DC surface phenotype by flow cytometry
Cells were stained on ice with fluorescein isothiocyanate (FITC) or phosphatidylethanolamine (PE)-conjugated mouse monoclonal antibodies for 30 minutes in phosphate-buffered saline 1% fetal calf serum and were analyzed on a FACScan® (Becton Dickinson, San Jose, CA). The following monoclonal antibodies were used: FITC-anti-CD80 (BB1), PE-anti-CD86 (FUN-1), FITC-anti-CD40 (5C3), PE-anti-CD54 (HA 58), and PE- anti-CD58 (1C3) (Pharmingen, San Diego, CA); PE-anti-CD14 (L243) and PE-anti-HLA-DR (Mφ-P9) (Becton Dickinson); PE-anti-CD83 (HB15A) (Immunotech, Marseille, France); and PE-anti-HLA class I (Tu 149) (Caltag Laboratories, Burlingame, CA).
Antigen uptake experiments
DCs were resuspended in medium buffered with 25mmol/L Hepes. FITC-beef serum albumin (BSA) and FITC-mannosylated BSA (both from Sigma) were added at 1 mg/mL final concentration, and the cells were incubated at 37°C or at 0°C to determine background uptake. After 1 hour, DCs were washed extensively with ice-cold phosphate-buffered saline and analyzed by FACS® with the use of propidium iodide to eliminate dead cells.
Cytokine detection by enzyme-linked immunosorbent assay
Culture supernatants were analyzed in serial twofold dilutions in duplicate. IL-12p70 was detected with the use of a solid phase sandwich enzyme-linked immunosorbent assay (ELISA) kit (Diaclone Research, Besancon, France) (sensitivity 3 pg/mL). For IL-12p40 and interferon (IFN)-γ detection, capture monoclonal antibodies and polyclonal biotinylated detection antibodies were obtained from Peter van de Meijde (BPRC, Rijswijk, The Netherlands) (sensitivity 10 pg/mL). IL-10 was detected with the use of the Pelikine compact human IL-10 ELISA kit (CLB, Amsterdam, The Netherlands) (sensitivity 3 pg/mL).
Allogeneic mixed lymphocyte reaction
Nonadherent allogeneic adult peripheral blood mononuclear cells from an unrelated individual were cultured in 96-well flat-bottom plates (Costar Corp, Cambridge, MA) at a density of 1.5 × 105/well with various numbers of γ-irradiated (3000 rads) DCs, in triplicates. Proliferation was assessed on day 5 by [3H]thymidine uptake (0.5 μCi/well, specific activity 5 Ci/mmol, Amersham Life Science, Buckinghamshire, UK) during a 16-hour pulse.
Th1 stimulation assays
The Mycobacterium tuberculosis and M lepraehsp65-specific, HLA-DR3-restricted CD4+ Th1 clone Rp15 1-1 used in this study recognizes an hsp65 determinant corresponding to peptide residues 3 to 13 (p3-13).24 HLA-DR-matched DEX-treated immature DCs and their DEX-untreated counterparts were pulsed with 10 μg/mL of p3-13 or with 10 μg/mL of hsp65 for 2 hours, washed extensively, and stimulated through CD40 as described above. For Ag-pulsed DEX-treated immature DCs, CD40 triggering was performed in the presence of DEX. Hsp65-specific T cells (104) were cultured with different numbers of γ-irradiated (3000 rads) DCs in 96-well flat-bottom plates (Costar Corp) in triplicates for 3 days. [3H]thymidine incorporation was measured on day 3 after a 16-hour pulse. Before the addition of [3H]thymidine, 50 μL of supernatants was collected from each well, and supernatants from triplicate wells were pooled to measure IFN-γ production. To test hsp65-specific T-cell responsiveness to a second antigenic challenge, 104 T cells were first cultured for 5 days with 104 peptide-pulsed DCs prepared as above and allowed to rest for 4 additional days in medium containing low doses of IL-2 (5 U/mL). Subsequently, 104viable T cells were restimulated with 104 peptide-pulsed DCs generated from the same donor as used for the first culture and tested for their ability to proliferate and to produce IFN-γ as previously described.
Statistical analysis
Covariance analysis was used to compare T-cell proliferation and IFN-γ production as a function of DC number, between DEX-treated CD40-triggered DCs, immature DCs, and CD40-triggered DCs (Figure 5).
Results
Impairment of CD40-CD40L-mediated phenotypic changes by DEX
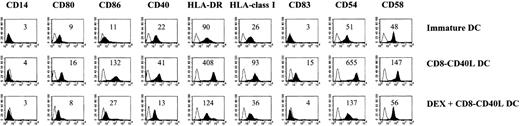
We explored the impact of DEX on the phenotypic changes induced by CD40 ligation on immature monocyte-derived DCs. In the absence of DEX, the fusion protein CD8-CD40L, which triggers DCs specifically through their CD40 receptor, induced a strong up-regulation of the costimulatory molecules CD80, CD86, and CD40; of the major histocompatibility complex (MHC) class I and II molecules; of the adhesion markers CD54 and CD58; and of the DC maturation marker CD83 (Figure 1). In the presence of DEX, these CD8-CD40L-induced phenotypic changes were dramatically impaired: the up-regulation of CD80, CD86, CD40, CD54, CD58, and of the MHC class I and II molecules was largely inhibited and CD83 was not expressed (Figure 1). Importantly, DEX-treated DCs did not revert to a monocyte/macrophage stage as shown by the lack of expression of CD14 (Figure 1). Titration of DEX showed a complete inhibition of CD40-mediated phenotypic changes at 10−6 mol/L and 10−7 mol/L, a partial blockade at 10−8 mol/L, and no effect at 10−9mol/L and 10−10 mol/L (data not shown). In addition, DEX action depended on binding to the GC receptor, since it was abolished by simultaneous addition of the GC-receptor antagonist RU486 (data not shown). In experiments performed with LPS or TNF-α as activation agents, similar results were obtained. However, the combination of DEX and TNF-α induced a massive cell death (viable cell recovery 5% to 10% of control cultures), a phenomenon that was not observed when DEX-treated DCs were stimulated with LPS or through CD40 (viable cell recovery 60%-100% of control cultures) (not shown).
Pretreatment with DEX inhibits the phenotypic changes induced by CD40 ligation.
Seven-day immature DCs were cultured for 24 hours in the absence or the presence of 10−6 mol/L DEX and activated via CD40 with the CD8-CD40L fusion protein for 48 hours. The comparison with immature DCs maintained in medium alone is shown. Empty histograms show the background staining with isotype controls monoclonal antibody, and solid histograms represent specific staining of the indicated cell-surface markers. Specific mean fluorescence intensities are indicated. Mean fluorescence intensities of isotype controls were between 3 and 4. Data are representative of 4 independent experiments.
Pretreatment with DEX inhibits the phenotypic changes induced by CD40 ligation.
Seven-day immature DCs were cultured for 24 hours in the absence or the presence of 10−6 mol/L DEX and activated via CD40 with the CD8-CD40L fusion protein for 48 hours. The comparison with immature DCs maintained in medium alone is shown. Empty histograms show the background staining with isotype controls monoclonal antibody, and solid histograms represent specific staining of the indicated cell-surface markers. Specific mean fluorescence intensities are indicated. Mean fluorescence intensities of isotype controls were between 3 and 4. Data are representative of 4 independent experiments.
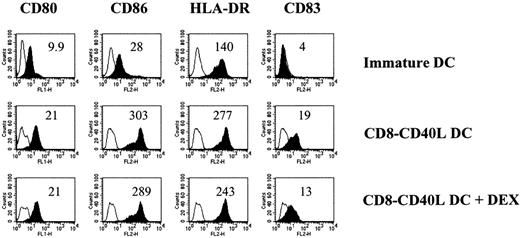
We next analyzed whether activated DCs could still be affected by DEX. DCs incubated with CD8-CD40L for 48 hours and further exposed to DEX maintained a stable activated phenotype (Figure2).
DCs triggered through CD40 maintain an activated phenotype on a subsequent DEX exposure.
Immature DCs were activated with the CD8-CD40L fusion protein. DEX (10−6 mol/L) or medium control was added 48 hours later, and cells were analyzed after 2 additional days of culture. The comparison with immature DCs maintained in medium alone is shown. Empty histograms show the background staining with isotype controls monoclonal antibody, and solid histograms represent specific staining of the indicated cell-surface markers. Specific mean fluorescence intensities are indicated. Mean fluorescence intensities of isotype controls were between 3 and 5. Data are representative of 2 independent experiments.
DCs triggered through CD40 maintain an activated phenotype on a subsequent DEX exposure.
Immature DCs were activated with the CD8-CD40L fusion protein. DEX (10−6 mol/L) or medium control was added 48 hours later, and cells were analyzed after 2 additional days of culture. The comparison with immature DCs maintained in medium alone is shown. Empty histograms show the background staining with isotype controls monoclonal antibody, and solid histograms represent specific staining of the indicated cell-surface markers. Specific mean fluorescence intensities are indicated. Mean fluorescence intensities of isotype controls were between 3 and 5. Data are representative of 2 independent experiments.
We conclude that DEX prevents the phenotypic changes induced by CD40 signals on immature DCs and that already activated DCs are resistant to DEX action.
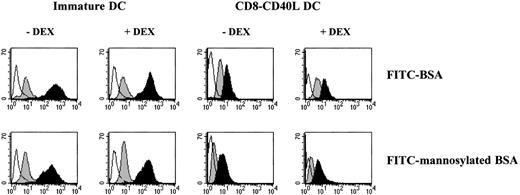
DEX does not interfere with the regulation of DC-antigen uptake machinery
Unlike activated DCs, immature DCs efficiently internalize antigens through macropinocytosis and mannose receptor-mediated endocytosis.2,3,25 26 We analyzed whether DEX could affect the DC-antigen capture machinery and its down-regulation following CD40 cross-linking. As shown in Figure 3, incorporation of FITC-BSA and FITC-mannosylated BSA by immature DCs and by DEX-treated immature DCs was comparable. On CD40 triggering, a similar decrease of FITC-BSA and FITC-mannosylated BSA uptake by both DEX-treated and untreated DCs was observed (Figure 3). These results were the first to indicate to us that DEX does not block all aspects of DC activation, since it does not interfere with the down-regulation of the DC-antigen capture machinery.
Pretreatment with DEX does not affect the regulation of DC-antigen uptake machinery.
Immature DCs were incubated in the absence or the presence of 10−6 mol/L DEX for 24 hours and further activated or not via CD40 with the CD8-CD40L fusion protein for 48 hours. Cells were pulsed for 1 hour with medium containing either 1 mg/mL FITC-BSA or 1 mg/mL FITC-mannosylated BSA. Empty histograms show the background autofluorescence, gray-filled histograms show the background uptake at 0°C, and black-filled histograms show the specific uptake at 37°C. Data are representative of 3 independent experiments.
Pretreatment with DEX does not affect the regulation of DC-antigen uptake machinery.
Immature DCs were incubated in the absence or the presence of 10−6 mol/L DEX for 24 hours and further activated or not via CD40 with the CD8-CD40L fusion protein for 48 hours. Cells were pulsed for 1 hour with medium containing either 1 mg/mL FITC-BSA or 1 mg/mL FITC-mannosylated BSA. Empty histograms show the background autofluorescence, gray-filled histograms show the background uptake at 0°C, and black-filled histograms show the specific uptake at 37°C. Data are representative of 3 independent experiments.
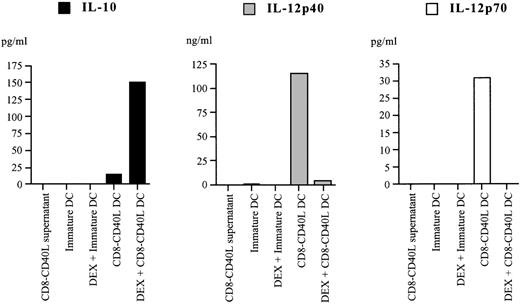
Altered cytokine secretion profile of DEX-treated CD40-triggered DCs
A key feature of CD40-triggered DCs for initiating T-cell immunity resides in their ability to produce the pro-inflammatory cytokine IL-12.5,6 27 We investigated whether DEX affected IL-12 production by DCs stimulated through CD40, and we explored the possibility that DEX could promote the secretion of the anti-inflammatory cytokine IL-10. As shown in Figure4, CD40 triggering of DCs strongly induced IL-12p40 and IL-12p70 secretion (up to 120 ng/mL and 170 pg/mL, respectively) but only poorly stimulated the production of IL-10 (up to 68 pg/mL). In contrast, CD40 triggering of DEX-treated DCs resulted in a dramatically reduced IL-12p40 production (up to 100-fold) and in the complete suppression of IL-12p70 secretion, whereas IL-10 production was strongly enhanced (up to 50-fold) (Figure 4). Immature DCs and their DEX-treated counterparts failed to secrete detectable amounts of IL-12 and IL-10 (Figure 4). Therefore, CD40 ligation of DCs in the presence of DEX triggers the secretion of high levels of the anti-inflammatory cytokine IL-10 instead of IL-12.
Pretreatment with DEX alters the cytokine secretion profile of CD40-triggered DCs.
DEX-exposed or control immature DCs were left in culture without further treatment or stimulated with the CD8-CD40L fusion protein. Culture supernatants were harvested 48 hours later, and IL-10, IL-12p40, and IL-12p70 secretion were analyzed by specific enzyme-linked immunosorbent assay. Data are representative from 6 independent experiments.
Pretreatment with DEX alters the cytokine secretion profile of CD40-triggered DCs.
DEX-exposed or control immature DCs were left in culture without further treatment or stimulated with the CD8-CD40L fusion protein. Culture supernatants were harvested 48 hours later, and IL-10, IL-12p40, and IL-12p70 secretion were analyzed by specific enzyme-linked immunosorbent assay. Data are representative from 6 independent experiments.
Modulation of DC T-cell stimulatory capacities by DEX
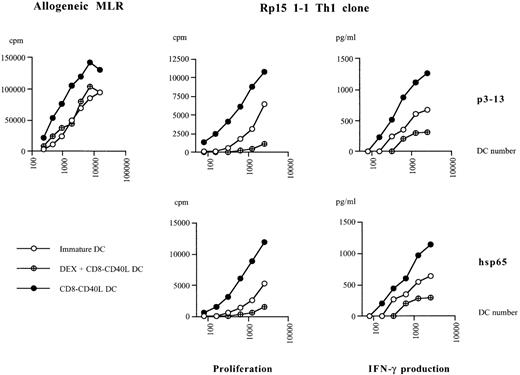
The strikingly modified response of DCs to CD40 ligation in the presence of DEX prompted us to compare the T-cell stimulatory potential of these cells with that of their DEX-untreated counterparts. In an allogeneic mixed lymphocyte reaction, CD40-triggered DCs induced a strong proliferative T-cell response, whereas the addition of DEX prior to CD40 triggering reduced their T-cell stimulatory capacity to that of immature DCs (Figure 5). When tested for their ability to stimulate an hsp65-specific CD4 ± Th1 clone, CD40-triggered DCs pulsed with the hsp65 protein or with the specific peptide epitope p3-13 were found to be highly potent inducers of both T-cell proliferation and T-cell dependent IFN-γ production (Figure5). In the presence of Ag-pulsed DEX-treated CD40-triggered DCs, T-cell proliferation and IFN-γ production were significantly lower (P ≤ .001), and the T-cell stimulatory capacity of these DCs was even lower than that of immature DCs (P ≤ .01). We next investigated whether DEX-treated CD40-triggered DCs were simply poor stimulators of Th1 cells or whether they could exert suppressive effects on these T cells. We, therefore, tested hsp65-specific T cells stimulated with p3-13-pulsed DEX-treated CD40-triggered DCs for their capacity to respond to a second potent antigenic challenge. Figure6 shows that preculturing T cells with CD40-triggered DCs or with immature DCs led to a strong T-cell proliferation and IFN-γ production on second antigen-specific restimulation. In contrast, preculture with DEX-treated CD40-triggered DCs resulted in a dramatically reduced proliferative and IFN-γ production capacity of Th1 cells. Thus, CD40 triggering of DCs in the presence of DEX results in APC that are not merely poor inducers of T-cell responses but that also induce a state of hyporesponsiveness in Th1 cells.
Pretreatment with DEX impairs the T-cell stimulatory capacities of DCs activated via CD40.
Allogeneic mixed lymphocyte reaction: nonadherent allogeneic peripheral blood mononuclear cells were cultured with different numbers of CD40-triggered DCs, DEX-treated CD40-triggered DCs, or immature DCs. The proliferative response was measured on day 5. Th1 stimulation assays: Hsp65-specific T cells were cultured with different numbers of HLA-DR matched CD40-triggered DCs or with DEX-treated CD40-triggered DCs, or with immature DCs, pulsed with the hsp65 protein or with the specific p3-13 peptide epitope. The proliferative response and the T-cell dependent IFN-γ production were analyzed on day 3. Data are representative of 3 independent experiments.
Pretreatment with DEX impairs the T-cell stimulatory capacities of DCs activated via CD40.
Allogeneic mixed lymphocyte reaction: nonadherent allogeneic peripheral blood mononuclear cells were cultured with different numbers of CD40-triggered DCs, DEX-treated CD40-triggered DCs, or immature DCs. The proliferative response was measured on day 5. Th1 stimulation assays: Hsp65-specific T cells were cultured with different numbers of HLA-DR matched CD40-triggered DCs or with DEX-treated CD40-triggered DCs, or with immature DCs, pulsed with the hsp65 protein or with the specific p3-13 peptide epitope. The proliferative response and the T-cell dependent IFN-γ production were analyzed on day 3. Data are representative of 3 independent experiments.
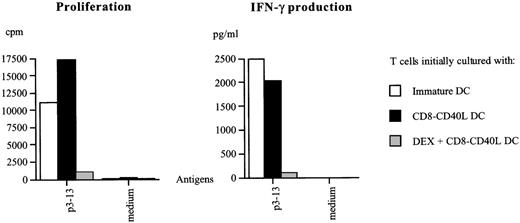
DEX-treated DCs triggered through CD40 induce a state of hyporesponsiveness in Th1 cells.
Hsp65-specific T cells precultured with CD40-triggered DCs or with immature DCs or with DEX-treated CD40-triggered DCs pulsed with the p3-13 peptide epitope were harvested after 5 days, allowed to rest in the presence of 5 U/mL IL2 for 4 days, and restimulated with p3-13-pulsed DCs or with unpulsed DCs (medium control). The proliferative response and IFN-γ production were measured on day 3. Similar results were obtained in 2 independent experiments.
DEX-treated DCs triggered through CD40 induce a state of hyporesponsiveness in Th1 cells.
Hsp65-specific T cells precultured with CD40-triggered DCs or with immature DCs or with DEX-treated CD40-triggered DCs pulsed with the p3-13 peptide epitope were harvested after 5 days, allowed to rest in the presence of 5 U/mL IL2 for 4 days, and restimulated with p3-13-pulsed DCs or with unpulsed DCs (medium control). The proliferative response and IFN-γ production were measured on day 3. Similar results were obtained in 2 independent experiments.
Discussion
In this study, we demonstrate that DEX profoundly affects the CD40-dependent maturation of human monocyte-derived DCs, not only by preventing the up-regulation of costimulatory, adhesion, and MHC surface molecules but also by causing these cells to secrete the anti-inflammatory mediator IL-10 instead of the Th1 stimulatory cytokine IL-12. In agreement with these phenotypic and functional changes, DCs triggered through CD40 in the presence of DEX are poor stimulators of Th1-type responses and are able to induce a state of hyporesponsiveness in Th1 cells, suggesting that such DCs might contribute to active suppression of Th1-type immunity.
The impact of GCs on DCs has been the subject of several previous studies by others. However, in contrast with our data, these studies only highlighted inhibitory effects of GCs on the DC system. DEX was found to block the up-regulation of CD80, CD86, and MHC class II molecules upon activation of murine spleen DCs,15,16whereas very recently DEX was demonstrated to also prevent the differentiation of DCs from monocyte precursors.28 In these studies, the inability of DCs to acquire high expression of costimulatory and MHC molecules was accompanied with a decrease in their T-cell stimulatory potential, but the effect of GCs on IL-12 production was not investigated. Conversely, Viera et al17 found that the effect of GCs on LPS-induced DC activation consisted in a 4-fold reduction of IL-12p70 synthesis. This partial effect on IL-12 secretion contrasts with the complete suppression of IL-12p70 production described in our work and can be explained by the fact that their GC-treated immature DCs were extensively washed prior to LPS stimulation. We indeed found that on removal of GCs, the effects of these drugs on immature DCs were rapidly reversible. The continuous presence of GCs during CD40 triggering of DCs was absolutely required to stably and completely modulate DC activation (data not shown). Taken together, previous findings indicated that the impact of GCs on the DC system should be merely interpreted as an inhibitory event.
Importantly, our results clearly demonstrate that GCs do not simply suppress DC activation but rather redirect this process toward a distinct functional program. Even though DCs triggered through CD40 in the presence of DEX maintain an immature-like surface phenotype, an important aspect of CD40-mediated DC activation, the shut-down of DC-antigen uptake machinery, still takes place. Furthermore, DEX does not only abrogate the production of bioactive IL-12 induced by CD40 cross-linking, but it also exerts a strong synergistic effect with CD40 signals on IL-10 secretion. A change in the IL-12/IL-10 balance in favor of IL-10 induced by GCs, although less striking than in our study, has also been observed in human peripheral blood mononuclear cells and purified monocytes upon LPS stimulation.29 30
The varying effects of DEX on the multiple aspects of DC activation suggest that DEX differentially affects the intracellular signals that are elicited when DCs are triggered through their CD40 receptor. The analysis of CD40 signaling events, although so far restricted to B cells, has shown that binding of CD40L to CD40 triggers multiple signaling pathways leading to the activation of transcription factors, such as NF-κB, AP-1, and NF-AT (reviewed in 31). These transcription factors control the expression of many genes involved in immune responses and are also major targets for GC-induced transcriptional repression. For instance, GCs inhibit NF-κB activation through the induction of IκB synthesis.32,33 The NF-κB binding site present in the IL-12p40 gene promoter region plays an important role for CD40-dependent induction of IL-12.34 35 It is, therefore, conceivable that DEX blocks IL-12 secretion by preventing NF-κB activation induced by CD40 ligation. Importantly, our data clearly show that DEX does not block all CD40-dependent aspects of DC activation since the CD40-mediated down-regulation of DC-antigen capture machinery is unaffected. In addition, GCs may even enhance some CD40 signals, as shown by the synergistic effect of DEX on CD40-mediated IL-10 secretion.
DC activation through engagement of CD40-CD40L is a key stimulatory event for the generation of effective Th1 and CD4-dependent cytotoxic T-lymphocyte responses in vivo.10,36-38 This pathway, however, is also involved in the development of unwanted T-cell responses leading to autoimmune disease or organ-transplant rejection.11-13 Until now, treatment of patients with such disorders largely relies on the systemic administration of GC hormones. This treatment does not only suppress pathogenic T-cell responses but also induces a general state of immunosuppression and metabolic and endocrine side effects. Activation of human monocyte-derived DCs through CD40 in the presence of DEX results in the generation of IL-10-producing APCs with low costimulatory capacities. Unlike immature DCs, which mature upon interaction with Th1 cells,5 and CD40-triggered DCs, these DCs are poor stimulators for Th1-type responses and even confer hyporesponsiveness to Th1 cells. Although experiments addressing the mechanisms underlying the unique phenotypic and functional features of such DCs are under way in our laboratory, it can already be envisioned that administration of such DCs loaded with appropriate antigens may be exploited as a novel approach for specifically down-regulating unwanted T-cell responses in vivo. The feasibility of this approach is currently being tested in murine autoimmune and transplantation models.
Acknowledgments
We thank Francine Briere and Pierre Garrone (Schering Plough, Dardilly, France) for kindly giving us the opportunity to use the CD8-CD40L fusion protein; Andrea Woltman (Department of Nephrology, LUMC) for expert help with the CD8-CD40L protein production; and A.H. Zwindermann for statistical analysis.
Supported by grants from the European Union, TMR contract FMRX-CT96-0053, and from the Netherlands Leprosy Foundation (NSL).
Reprints:Delphine Rea, Department of Immunohematology and Blood Bank, Leiden University Medical Center, Albinusdreef 2, Postbus 9600, 2300 RC Leiden, The Netherlands; e-mail:d.g.rea@immunohematology.medfac.leidenuniv.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal