Abstract
Bacterial endotoxin (lipopolysaccharide, or LPS) has potent proinflammatory properties by acting on many cell types, including endothelial cells. Secretion of the CXC-chemokine interleukin-8 (IL-8) by LPS-activated endothelial cells contributes substantially to the inflammatory response. Using human umbilical vein endothelial cells (HUVECs), we analyzed the role of small GTP-binding Rho proteins and p38 mitogen-activated protein kinase (MAPK) for LPS-dependent IL-8 expression in endothelial cells. Specific inactivation of RhoA/Cdc42/Rac1 by Clostridium difficile toxin B-10463 (TcdB-10463) reduced LPS-induced tyrosine phosphorylation, nuclear factor (NF)-κB–dependent gene expression, IL-8 messenger RNA, and IL-8 protein accumulation but showed no effect on LPS-dependent p38 MAPK activation. Inhibition of p38 MAPK by SB 202190 also blocked LPS-induced NF-κB activation and IL-8 synthesis. Furthermore, selective activation of the p38 MAPK pathway by transient expression of a constitutively active form of MAPK kinase (MKK)6, the upstream activator of p38, was as effective as LPS with respect to IL-8 expression in HUVECs. In summary, our data suggest that LPS-induced NF-κB activation and IL-8 synthesis in HUVECs are regulated by both a Rho-dependent signaling pathway and the MKK6/p38 kinase cascade.
A common and serious consequence of gram-negative infection is the development of septic shock and associated organ failure, which accounts for 50 000 to 100 000 deaths annually in the United States.1
The endothelium lines the inner surface of blood vessels and functions as an interactive barrier between blood and tissue. Exposed to the blood flow, it is a primary target for inflammatory agents during local or systemic inflammation. Endotoxin, or lipopolysaccharide (LPS), is a glycolipid that constitutes the major portion of the outermost membrane of gram-negative bacteria.2 Exposure of endothelial cells to LPS results in a complex activation of endothelial cells in vivo and in vitro: LPS-induced up-regulation of adhesion-molecules on the surface of endothelial cells mediates rolling, adhesion, and transmigration of white blood cells into surrounding tissue.3 LPS-related procoagulant activity,4enhanced endothelial permeability,5 and secretion of proinflammatory mediators4 contribute to the inflammatory response.
An important endothelial cell–derived cytokine represents the CXC-chemokine interleukin (IL)-8,6 a potent neutrophil chemotactic factor.7,8 In endothelial cells, IL-8 expression is tightly controlled by the transcription factors nuclear factor (NF)-κB and activator protein (AP)-1.9,10 Besides the transcriptional control, IL-8 apparently is stored in Weibel-Palade bodies of human microvascular and umbilical vein endothelial cells.11 12
The precise mechanisms of LPS-induced signaling in endothelial cells are not yet fully understood. Endothelial cells do not express CD14 but respond very well to LPS in the presence of soluble CD14 and LPS-binding protein.13,14 Increasing evidence suggests that LPS stimulation of toll-like receptors (TLR), especially TLR2, leads to activation of the NF-κB signaling cascade.15-17
Exposure of endothelial cells to LPS increased protein tyrosine phosphorylation and resulted in activation of several mitogen-activated protein kinases (MAPK).13,14 Stimulation of p38 MAPK14,18 appears to be of specific importance. At least 3 members of the MAPK kinase (MKK) superfamily (MKK3, MKK4, and MKK6) are capable of activating p38 MAPK when overexpressed in cell lines.19,20 Activated p38 MAPK in turn phosphorylates downstream kinases such as MAPK-associated kinases 2/320(MAPKAP-kinases 2/3) or 3pK-kinase.21 Moreover, direct phosphorylation of transcription factors such as activated transcription factor-2 (ATF2)22 or myocyte enhancer factor-2 (MEF2)23 may also contribute to p38 MAPK-dependent signaling.
Subsequent activation of NF-κB and AP-1 is considered to be an essential prerequisite for altered gene expression in LPS-stimulated endothelial cells.4,9,10 The link between kinase activation and transcription factor translocation/activation is controversial and depends on the cell types investigated and stimuli used.24-29
An interaction between NF-κB and the Rho family of small GTPases was suggested recently by the demonstration that Rho, Cdc42, and Rac proteins promote NF-κB–activation.30,31 Rho proteins are members of the Ras superfamily of small GTP-binding proteins and function as key regulators of distinct important signal transduction pathways as well as of microfilament organization.32 33
Recently, we demonstrated the requirement of functionally intact Rho proteins for translocation and activation of protein kinase C (PKC) in human endothelial and epithelial cells.34 Rho proteins may also participate in the regulation of p44 MAPK35 and p42 MAPK,35 c-Jun N-terminal kinase/stress-activated protein kinase,36 phosphatidylinositol 3-kinase,37phosphatidylinositol 4-phosphate 5-kinase,38,39 Rho kinase,40,41 or phosphatases.41
Large clostridial toxins turned out to be effective tools for studying the role of small GTP-binding proteins in cellular processes.Clostridium difficile toxin B-10463 (TcdB-10463), a single-chained 270-kd molecule that easily enters cells by receptor-mediated endocytosis, glucosylates Rho proteins at threonine 37 (RhoA) or threonine 35 (Rac/Cdc42), thereby specifically inactivating them.42,43 In a previous study using this toxin, we demonstrated glucosylation of Rho proteins in endothelial cells, an effect that was accompanied by loss of endothelial barrier function44 and impaired PKC translocation and activation.34C. difficile toxin B-1470 (TcdB-1470) primarily targets Rac.13 Proteins of the Ras GTPases subfamily were inactivated by C. sordellii lethal toxin (TcsL)-induced UDP-glucosylation.45 Using these toxins, we analyzed the role of small GTPases in LPS-induced signaling in endothelial cells.
Here we show that inhibition of Rho proteins blocked LPS-induced protein tyrosine phosphorylation, NF-κB activation, and IL-8 expression in human endothelial cells, while p38 kinase activation was unrelated to Rho proteins. Inhibition of p38 kinase also reduced LPS-dependent NF-κB activation and IL-8 expression. Furthermore, selective activation of p38 kinase by overexpression of a constitutively active mutant of its upstream kinase MKK6 was sufficient to induce endothelial IL-8 production. Overall, the data suggest that 2 LPS-activated signaling pathways independently mediate IL-8 expression in human umbilical vein endothelial cells (HUVECs): a Rho-dependent signaling pathway and the MKK6/p38 MAPK cascade.
Material and methods
Materials
MCDB 131, fetal calf serum (FCS), Hank's balanced salt solution, phosphate-buffered saline (PBS), trypsin-EDTA-solution, HEPES, Igepal CA-650, and antibiotics were obtained from Life Technologies (Karlsruhe, Germany). Excell 400 medium was from Biochrom (München, Germany). Collagenase (CLS type II) was purchased from Worthington Biochemical Corp (Freehold, NJ). Gelatin from porcine skin type I, leupeptin, pepstatin A, antipain, DTT, Triton X-100, PMSF, 4-dichloroisocumarin, and Tween-20 were purchased from Sigma Chemical Co (Munich, Germany). 32P-γ-ATP was purchased from Amersham (Braunschweig, Germany). LPS fromSalmonella abortus equii was a gift of Prof Dr C. Galanos (Max Planck Institute for Immunbiology, Freiburg, Germany). All other chemicals used were of analytical grade and obtained from commercial sources.
Preparation of bacterial toxins
Preparation of human umbilical cord vein endothelial cells
Cells were isolated from umbilical cord veins and identified as described previously.34,44,46 47 Briefly, cells obtained from collagenase digestions were washed, resuspended in MCDB 131 supplemented with 5% FCS, and seeded into 6- or 96-well plates. Confluent monolayers of primary cultures, only, were used.
Stimulation of endothelial cells
In all experiments presented, cells were stimulated with LPS in the presence of 2% FCS. FCS was heated for 45 minutes at 65°C to inactivate complement factors.
Interleukin-8 ELISA
HUVEC monolayers were stimulated for 12 hours, as indicated, in a humidified atmosphere. After incubation, supernatants were collected, centrifuged, and processed for IL-8 quantification by enzyme-linked immunosorbent assay (ELISA). Briefly, microtiterplates were coated with anti–IL-8 monoclonal antibody (mAb) 208 (R & D Systems, Wiesbaden, Germany), washed, and blocked with 0.2% (v/v) casein buffer; 100 μL of supernatant was then added. After incubation with a second biotin-labeled anti–IL-8 mAb (R & D Systems), detection was performed using a streptavidin peroxidase-conjugated antibody complex (DAKO, Glostrup, Denmark) at 450 nm.
Immunecomplex kinase assays
HUVEC monolayers were exposed to TcdB-10463 for 1 hour in a humidified atmosphere, stimulated as indicated, and lysed in TLB buffer21 containing 20mM Tris, pH 7.4; 50mM sodium β-glycerophosphate; 20mM sodium pyrophosphate; 137mM NaCl; 10% (v/v) glycerol; 1% (v/v) Triton X-100; 2mM EDTA; 1mM Pefabloc; 1mM sodium orthovanadate; 5mM benzamidine; 5 μg/mL aprotinin; and 5 μg/mL leupeptin on ice for 30 minutes. Cell debris was removed by centrifugation at 10 000 g rpm for 20 minutes. After protein quantification (Bio-Rad Protein Assay, Bio-Rad, Munich, Germany), equal amounts were incubated with 25 μL of protein A agarose (Boehringer Mannheim, Mannheim, Germany) and 1 μL/mL rabbit antisera against p38 (kindly provided by Dr J. Han, La Jolla, CA) for 2 hours at 4°C. After washing in TLB buffer supplemented with 500mM NaCl and kinase buffer (10mM MgCl2; 25mM β-glycerophosphate; 25mM HEPES, pH 7.5; 5mM benzamindine; 0.5mM DDT; and 1mM sodium orthovanadate), each sample was incubated with 3pK/MAPKAP-K3 as substrate for p38 MAPK in the presence of 100μM unlabeled ATP, 18.5 · 107 Bq32P-γ-ATP, and kinase buffer in a volume of 20 μL for 15 minutes at 30°C. Thereafter, reactions were terminated by boiling in 5 × Laemmli sodium dodecyl sulfate (SDS) sample buffer for 4 minutes. Samples were subsequently subjected to SDS-polyacrylamide gel electrophoresis (PAGE), blotted onto nitrocellulose, and visualized by autoradiography. Western blot analysis was performed to confirm equal loading of p38 proteins as described.21
Plasmids and transient transfection procedures
NF-κB reporter gene assay was performed as described previously.46,47 Briefly, 6 NF-κB DNA binding sites (5′-GGG GAC TTT CCC T-3′) were inserted into SmaI site in a pGL3 basic vector (Promega, Mannheim, Germany). Downstream of this 6 NF-κB binding region, a minimal β-globin promoter (containing a TATA box) was inserted into theXhoI/HindIII sites followed by the luciferase gene (pGL3.BG.6kB). HUVECs were transiently transfected with 2 μg of NF-κB plasmid. Transfected HUVECs were stimulated, harvested in reporter lysis buffer (Promega), and total-protein quantified. Luciferase assays were performed using a commercial kit (Promega) in a Lumat LB 9501 luminometer (Berthold, Wildbad, Germany). Relative luminescence activities were normalized to total protein and expressed as fold activation relative to control ± SEM. A control plasmid was created by inserting 6 mutated NF-κB sites (5′-GGC CAC TTT CCC T-3′) into the same vector (pGL3.BG.6kB.mut) as described.46 47
MKK6 and green fluorescence protein expression
Plasmids employed included the KRSPA eukaryotic expression vector, pGreen Lantern for expression of green fluorescent protein (GFPS65T; Life Technologies, Eggenstein, Germany), and KRSPA expression plasmids for constitutively active MKK6(Glu) (kindly provided by Dr R. J. Davies, Worchester, MA). Briefly, subconfluent HUVEC cultures grown in 10-cm plates were washed twice with 1mM HEPES/PBS and incubated with 20 μg of DNA and 250 μg/mL DEAE-dextran (Pharmacia-Amersham, Freiburg, Germany) in 4 mL of 1mM HEPES/PBS for 30 minutes at 37°C. Thereafter, EGM medium containing 0.15mM chloroquine was added to each plate, and cells were incubated for another 2.5 hours. Medium was then removed, and cells were treated with 10% (v/v) DMSO in EGM medium for 2.5 minutes. HUVECs were subsequently cultured for 36 hours in EGM medium and finally stimulated as indicated. Expression and function of transfected kinase mutants were confirmed by immunecomplex kinase assay and Western blots.24
Flow cytometry
To determine the expression of IL-8 by flow cytometry analysis, an intracellular staining procedure was applied.24 Confluent HUVEC monolayers were treated with LPS, toxins, or both, as indicated, in the presence of 2μM monesin. Cells were washed, fixed with 4% (w/v) paraformaldehyde in PBS at 4°C for 20 minutes, incubated with mouse mAb against human IL-8 (mouse immunoglobulin G2; clone G265-8) or corresponding isotype control mAb (Pharmingen, Hamburg, Germany) in permeabilization buffer containing 1% FCS and 0.1% (w/v) saponin. Cells were successively stained with biotin-SP–conjugated goat antimouse immunoglobulin G (F(ab)2 and streptavidin-Cy-Chrome (Pharmingen). Fluorescence was measured with a FACScan (Becton Dickinson, Heidelberg, Germany). In HUVEC cotransfected with pGreen Lantern and either empty expression vector or vector expressing constitutively active MKK6, only cells that expressed GFPS65T (as detected in the FL-1 channel) were considered for the detection of IL-8 (as measured in the FL-3 channel). Nonviable cells were excluded employing forward scatter and side scatter parameters.
Northern blot
RNA was extracted and processed as described previously.46,47 Briefly, complementary DNA (cDNA) probes were labeled with 32P-γ-dCTP by random priming (Rediprime DNA labeling system, Pharmacia-Amersham), added to the prehybridization chambers, and incubated for 12 to 16 hours at 42°C. A human IL-8–cDNA probe base pair (bp) 19–bp 338 (320-bp probe) of the IL-8 sequence as deposited in the GenBank (accession number: M26383) was used for detection of IL-8 messenger RNA (mRNA). The 598-bp cDNA fragment of glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) was obtained as previously described.46 47
Western blot
For determination of protein tyrosine phosphorylation, HUVECs were starved for 6 hours in serum-free medium, stimulated as indicated, and washed twice in HEPES buffer, pH 7.4, containing 100mM sodium fluoride, 2mM sodium vanadate, and 15mM sodium pyrophosphate.34,47Cells were then harvested by scraping them on ice in ice-cold wash-buffer supplemented with 1mM PMSF, leupeptin, pepstatin and antipain, 2 μg/mL each, and afterward lysed in buffer supplemented with 1% (v/v) Triton X-100. After removal of cell debris by centrifugation, cell lysates were subjected to SDS-PAGE on a 7.5% gel (40 μg of protein per lane) and then blotted on Hybond-ECL membrane (Pharmacia-Amersham). Immunodetection of tyrosine phosphorylated proteins was carried out by incubating membranes with RC-20 mAb (Transduction Laboratories, Lexington, KY). Proteins were visualized by ECL (Pharmacia-Amersham).34 47
Release of lactate dehydrogenase
Endothelial cell monolayers were exposed to stimuli for 24 hours. Lactate dehydrogenase (LDH) activity in the supernatants was determined by the colorimetric measurement of the reduction of sodium pyruvate in the presence of NADH as described.34,44,46 47 Enzyme release was expressed as the percentage of total enzyme activity liberated from endothelial cells in the presence of 100 μg/mL mellitin.
Statistical methods
A one-way analysis of variance (ANOVA) was used for data of Figures1, 2, 3A, 3B, 4, 5B, 6C, and 7. Main effects were then compared by an F probability test.48P < .05 was considered to be significant.
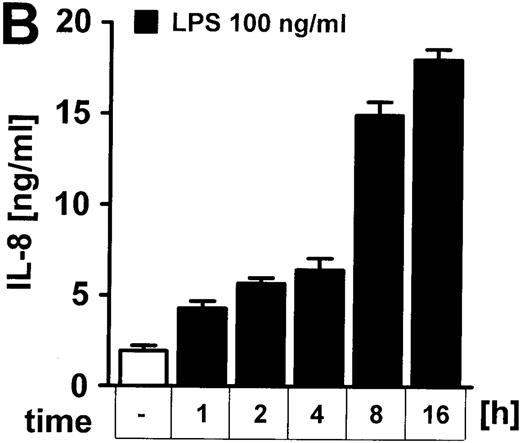
LPS induces IL-8 secretion of human endothelial cells in a dose- and time-dependent manner.
(A) Cells were incubated with 1 to 100 ng/mL LPS for 8 hours or (B) exposed to 100 ng/mL LPS for 1 to 16 hours. IL-8 in the supernatant was quantified by ELISA technique. Data presented are mean ± SEM of 5 separate experiments.
LPS induces IL-8 secretion of human endothelial cells in a dose- and time-dependent manner.
(A) Cells were incubated with 1 to 100 ng/mL LPS for 8 hours or (B) exposed to 100 ng/mL LPS for 1 to 16 hours. IL-8 in the supernatant was quantified by ELISA technique. Data presented are mean ± SEM of 5 separate experiments.
Results
Inhibition of small GTP-binding Rho proteins blocked LPS-dependent IL-8 expression in human endothelial cells
Exposure of cultured human endothelial cell monolayers to LPS resulted in a dose (0-100 ng/mL)– and time (0-16 hours)–dependent increase of IL-8 secretion by endothelial cells (Figure 1A and 1B). Within the dose and time frame studied, no significant LDH release was noted, ie, there was no evidence of overt cell damage (data not shown).
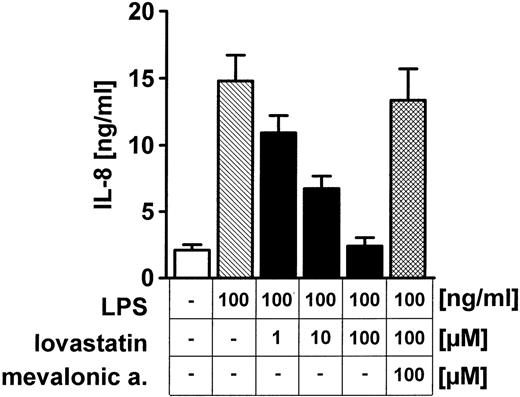
Prenylation of Rho proteins is required for membrane binding and Rho-dependent signaling. We used 0μM to 100μM lovastatin to inhibit HMG–coenzyme A reductase to block protein prenylation. This resulted in decreased LPS-dependent IL-8 production by HUVECs (Figure2). Addition of the HMG–coenzyme A reductase product mevalonic acid (100 μM) restored the ability of HUVECs to produce IL-8 upon exposure to LPS (Figure 2).
Inhibition of protein prenylation blocks LPS-induced IL-8 production by HUVECs.
Exposure of endothelial monolayers to 1μM to 100μM HMG–coenzyme A reductase inhibitor lovastatin 2 hours before and during stimulation with 100 ng/mL LPS inhibited IL-8 synthesis in a dose-dependent manner; 100μM mevalonic acid restored the ability of lovastatin-exposed HUVECs to secrete IL-8 upon LPS stimulation. Data presented are mean ± SEM of 4 separate experiments.
Inhibition of protein prenylation blocks LPS-induced IL-8 production by HUVECs.
Exposure of endothelial monolayers to 1μM to 100μM HMG–coenzyme A reductase inhibitor lovastatin 2 hours before and during stimulation with 100 ng/mL LPS inhibited IL-8 synthesis in a dose-dependent manner; 100μM mevalonic acid restored the ability of lovastatin-exposed HUVECs to secrete IL-8 upon LPS stimulation. Data presented are mean ± SEM of 4 separate experiments.
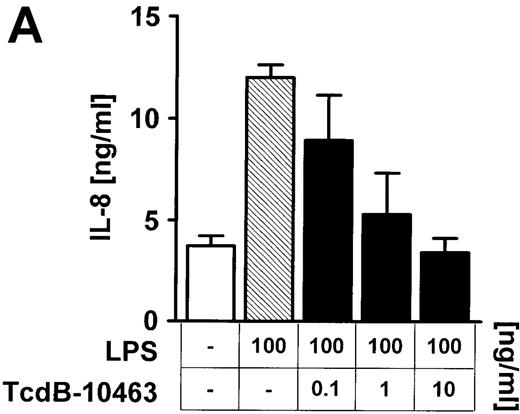
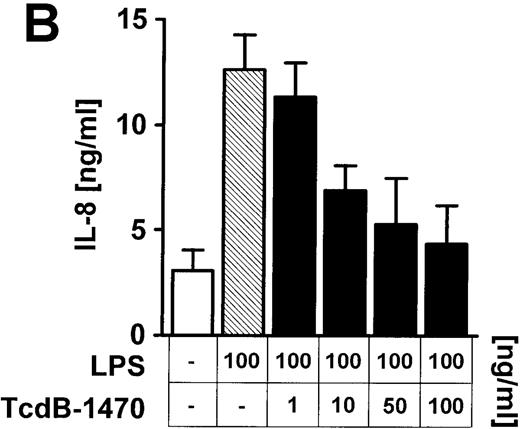
To address more directly the role of Rho proteins for LPS-mediated IL-8 expression in endothelial cells, Rho GTPases were selectively inhibited by large clostridial cytotoxins. Preincubation (60 minutes) of endothelial cells with 10 ng/mL TcdB-10463, which blocks RhoA, Cdc42, and Rac but not other members of the Ras superfamily of small GTP-binding proteins, dose-dependently (0.1-10 ng/mL) reduced LPS-related IL-8 expression with respect to IL-8 protein and mRNA (Figure 3A-C). Inhibition of Cdc42 and Rac, but not RhoA, by 60 minutes of preincubation of cells with 1 to 100 ng/mL TcdB-1470 also reduced IL-8 production in LPS-treated endothelial cells, indicating that Cdc42 and Rac are essential elements of LPS-activated signaling cascades (Figure 3B).
Inhibition of Rho proteins blocks LPS-induced IL-8 expression by endothelial cells.
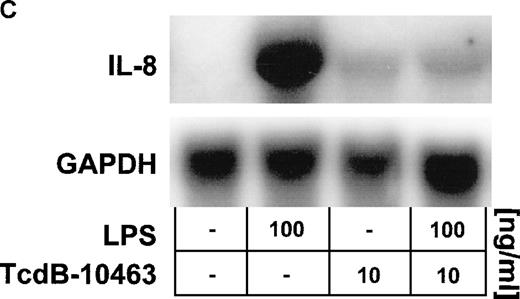
Cells were preincubated with (A) 0.1 to 10 ng/mL TcdB-10463 (inhibitor of RhoA, Cdc42, and Rac) or (B) 1 to 100 ng/mL TcdB-1470 (inhibitor of Cdc42 and Rac) for 60 minutes prior to stimulation with 100 ng/ml LPS for 8 hours. Both toxins inhibited LPS-related IL-8 production in HUVEC cultures in a dose-dependent manner. (C) Inhibition of Rho proteins by 10 ng/mL TcdB-10463 prior to stimulation of cells with 100 ng/mL LPS for 4 hours reduced LPS-dependent IL-8 mRNA accumulation, as evidenced by Northern blot. Constitutively expressed message of GAPDH was shown to confirm equal RNA loading. Data presented are mean ± SEM of 4 separate experiments. A representative autoradiograph out of 3 independent experiments (C) is shown.
Inhibition of Rho proteins blocks LPS-induced IL-8 expression by endothelial cells.
Cells were preincubated with (A) 0.1 to 10 ng/mL TcdB-10463 (inhibitor of RhoA, Cdc42, and Rac) or (B) 1 to 100 ng/mL TcdB-1470 (inhibitor of Cdc42 and Rac) for 60 minutes prior to stimulation with 100 ng/ml LPS for 8 hours. Both toxins inhibited LPS-related IL-8 production in HUVEC cultures in a dose-dependent manner. (C) Inhibition of Rho proteins by 10 ng/mL TcdB-10463 prior to stimulation of cells with 100 ng/mL LPS for 4 hours reduced LPS-dependent IL-8 mRNA accumulation, as evidenced by Northern blot. Constitutively expressed message of GAPDH was shown to confirm equal RNA loading. Data presented are mean ± SEM of 4 separate experiments. A representative autoradiograph out of 3 independent experiments (C) is shown.
In contrast, inactivation of Ras by exposure of cells to 0 to 200 ng/mL TcsL 2 hours prior to LPS stimulation had no effect on LPS-induced IL-8 synthesis (data not shown). Activity of toxins was always controlled by the observation of typical morphologic alterations in endothelial cells (data not shown). Exposure of cells to clostridial toxins did not result in enhanced LDH release (data not shown).
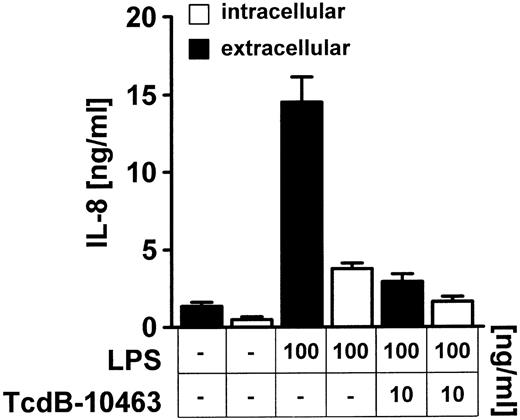
Because Rho protein inhibition is accompanied by alterations of the endothelial cytoskeleton, the possibility of an intracellular accumulation of preformed IL-8 was explored (Figure4). Analysis of intracellular IL-8 in permeabilized TcdB-10463–treated endothelial cells stimulated with 100 ng/mL LPS showed no significant increase in intracellular IL-8 levels (Figure 4).
TcdB-10463–mediated inhibition of LPS-induced IL-8 secretion is not due to intracellular accumulation of IL-8.
Cells were incubated with 10 ng/ml TcdB-10463 for 1 hour prior to LPS stimulation; 100 ng/mL LPS was added for 8 hours as indicated. Supernatant was collected, and cells were washed three times, permeabilized with 100 ng/mL saponin, and resuspended. Supernatants and cell extracts representing the intracellular fraction were subjected to ELISA analysis. Data presented are mean ± SEM of 3 separate experiments.
TcdB-10463–mediated inhibition of LPS-induced IL-8 secretion is not due to intracellular accumulation of IL-8.
Cells were incubated with 10 ng/ml TcdB-10463 for 1 hour prior to LPS stimulation; 100 ng/mL LPS was added for 8 hours as indicated. Supernatant was collected, and cells were washed three times, permeabilized with 100 ng/mL saponin, and resuspended. Supernatants and cell extracts representing the intracellular fraction were subjected to ELISA analysis. Data presented are mean ± SEM of 3 separate experiments.
Rho protein– and p38 MAPK–related signaling in LPS-stimulated endothelial cells
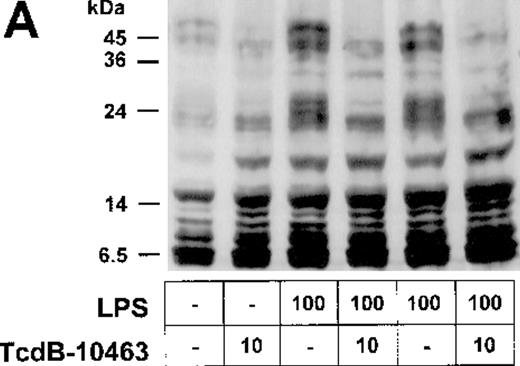
Stimulation of endothelial cells with 100 ng/mL LPS for 15 minutes was accompanied by increased tyrosine phosphorylation of different proteins (Figure 5A), an effect that was blocked by inhibition of RhoA, Cdc42, and Rac via TcdB-10463 (Figure5A). Moreover, the tyrosine kinase inhibitor geldanamycin dose-dependently reduced LPS-related IL-8 expression in HUVECs (Figure5B), suggesting that Rho protein–dependent activation of tyrosine kinases is part of the LPS–IL-8 signaling pathway.
Inhibition of Rho proteins blocks protein tyrosine phosphorylation in LPS-stimulated endothelial cells.
(A) Cells were preexposed to 10 ng/mL TcdB-10463 for 60 minutes as indicated and stimulated with 100 ng/mL LPS for 15 minutes. Cell lysates were obtained as described in “Materials and methods” and were subjected to Western blot analysis (7.5% SDS-PAGE). Membranes were labeled with antibodies against tyrosine-phosphorylated proteins. Note reduction of protein tyrosine phosphorylation in LPS-treated cells after inhibition of Rho proteins with TcdB-10463. (B) Endothelial cells were incubated with 25nM to 500nM geldanamycin 30 minutes prior to stimulation with 100 ng/mL LPS for 8 hours. Supernatants were subsequently analyzed by IL-8 ELISA. A representative gel (1 of 3 separate experiments) is shown in (A). Data presented in (B) are mean ± SEM of 3 separate experiments.
Inhibition of Rho proteins blocks protein tyrosine phosphorylation in LPS-stimulated endothelial cells.
(A) Cells were preexposed to 10 ng/mL TcdB-10463 for 60 minutes as indicated and stimulated with 100 ng/mL LPS for 15 minutes. Cell lysates were obtained as described in “Materials and methods” and were subjected to Western blot analysis (7.5% SDS-PAGE). Membranes were labeled with antibodies against tyrosine-phosphorylated proteins. Note reduction of protein tyrosine phosphorylation in LPS-treated cells after inhibition of Rho proteins with TcdB-10463. (B) Endothelial cells were incubated with 25nM to 500nM geldanamycin 30 minutes prior to stimulation with 100 ng/mL LPS for 8 hours. Supernatants were subsequently analyzed by IL-8 ELISA. A representative gel (1 of 3 separate experiments) is shown in (A). Data presented in (B) are mean ± SEM of 3 separate experiments.
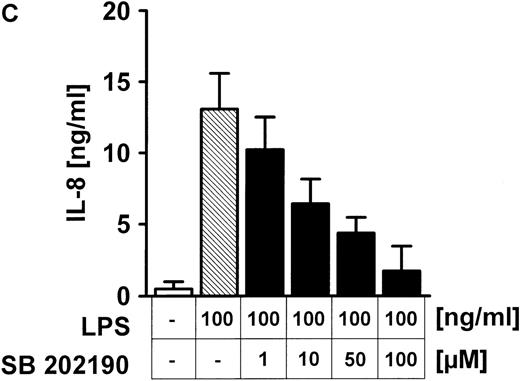
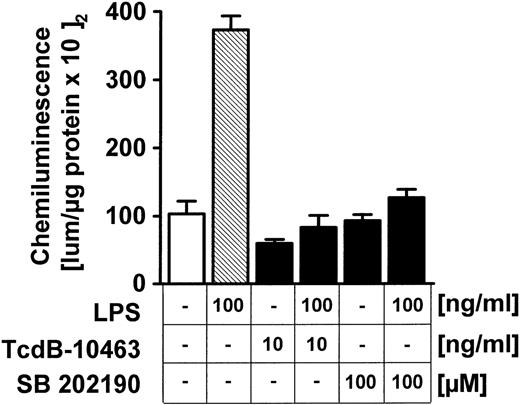
Besides tyrosine kinases, serine/threonine p38 kinase activation upon LPS exposure was also studied; 100 ng/mL LPS induced a time-dependent increase of p38 MAPK activity within 60 minutes (Figure6A) in HUVECs, as shown by phosphorylation of the target 3pK/MAPKAP-K3. In the next step, the role of Rho proteins for LPS-related p38 activation was tested (Figure 6B). Pretreatment of endothelial cells for 60 minutes with 100 ng/mL TcdB-10463, 100 ng/mL TcdB-1470, or 200 ng/mL TcsL did not affect LPS-related p38 kinase activity (Figure 6B). The toxins' effectiveness was confirmed by induction of typical morphologic alterations (data not shown). On the other hand, the specific p38 kinase inhibitor SB 202190 dose-dependently (0-100 μM) reduced LPS-induced IL-8 synthesis in HUVEC cultures (Figure 6C). Because Rho protein inhibition did not modify LPS-induced p38 kinase activity (Figure 6B), effects were tested at the NF-κB level. Inhibition of p38 kinase by SB 202190 (Figure7) or blocking of Rho proteins (Figure 7) by TcdB-10463 was very effective in reducing LPS-dependent expression of an NF-κB–dependent reporter gene.
LPS-induced activation of p38 MAPK does not depend on Rho proteins, but p38 MAPK inhibition blocks IL-8 expression in endothelial cells.
(A) HUVECs were exposed to 100 ng/mL LPS for the times indicated; p38 activity was assessed by immunecomplex kinase assay, as described in “Materials and methods,” employing 3pK/MAPKAP-K3 as substrate. Equal gel loading was confirmed by p38 immunoblot. (B) Endothelial cells were pretreated with 10 ng/mL TcdB-10463, 100 ng/mL TcdB-1470, or 200 ng/mL TcsL, each for 1 hour prior to addition of 100 ng/mL LPS for another hour. Inhibition of Rho or Ras proteins had no effect on LPS-induced p38 kinase activity. (C) HUVECs were pretreated with the p38 kinase inhibitor SB 202190 for 30 minutes before stimulation with 100 ng/mL LPS. SB 202190 inhibited IL-8 secretion in a dose-dependent manner. Representative gels (1 of 3 separate experiments) are shown in (A) and (B). Data presented in (C) are mean ± SEM of 4 separate experiments.
LPS-induced activation of p38 MAPK does not depend on Rho proteins, but p38 MAPK inhibition blocks IL-8 expression in endothelial cells.
(A) HUVECs were exposed to 100 ng/mL LPS for the times indicated; p38 activity was assessed by immunecomplex kinase assay, as described in “Materials and methods,” employing 3pK/MAPKAP-K3 as substrate. Equal gel loading was confirmed by p38 immunoblot. (B) Endothelial cells were pretreated with 10 ng/mL TcdB-10463, 100 ng/mL TcdB-1470, or 200 ng/mL TcsL, each for 1 hour prior to addition of 100 ng/mL LPS for another hour. Inhibition of Rho or Ras proteins had no effect on LPS-induced p38 kinase activity. (C) HUVECs were pretreated with the p38 kinase inhibitor SB 202190 for 30 minutes before stimulation with 100 ng/mL LPS. SB 202190 inhibited IL-8 secretion in a dose-dependent manner. Representative gels (1 of 3 separate experiments) are shown in (A) and (B). Data presented in (C) are mean ± SEM of 4 separate experiments.
Inhibition of Rho proteins or p38 MAPK activity blocks LPS-dependent activation of an NF-κB–dependent reporter gene.
HUVECs transiently transfected with an NF-κB–luciferase construct were pretreated with 10 ng/mL TcdB-10463 for 1 hour or 100μM SB 202190 for 30 minutes as indicated. Expression of reporter gene was measured by chemiluminescence as described in “Materials and methods.” Data presented are mean ± SEM of 3 separate experiments.
Inhibition of Rho proteins or p38 MAPK activity blocks LPS-dependent activation of an NF-κB–dependent reporter gene.
HUVECs transiently transfected with an NF-κB–luciferase construct were pretreated with 10 ng/mL TcdB-10463 for 1 hour or 100μM SB 202190 for 30 minutes as indicated. Expression of reporter gene was measured by chemiluminescence as described in “Materials and methods.” Data presented are mean ± SEM of 3 separate experiments.
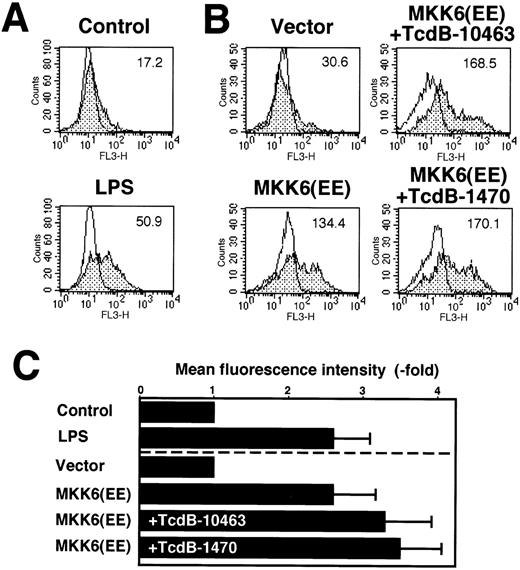
To assess whether activation of p38 kinase alone is sufficient to induce IL-8 expression in endothelial cells, we transiently transfected HUVECs with a constitutively active MKK6 kinase that activates p38 kinase (Figure 8B and 8C). With respect to IL-8 synthesis, kinase overexpression was as effective as stimulation of nontransfected cells with 100-ng/mL LPS (Figure 8A, 8C). In MKK6-transfected endothelial cells, inhibition of RhoA, Cdc42, and Rac by 10 ng/mL TcdB-10463 or Cdc42 and Rac by 100-ng/mL TcdB-1470 did not inhibit IL-8 production, suggesting that p38 MAPK–mediated expression of the cytokine is independent of Rho function (Figure 8A, 8B).
IL-8 synthesis in HUVECs expressing constitutively active MKK6(Glu).
(A) HUVECs were either left untreated or incubated with 100 ng/mL LPS for 8 hours. IL-8 synthesis was measured by flow cytometry as described in “Materials and methods.” Flow cytometry profiles of 1 representative experiment are shown. Open profiles represent isotype controls; shaded profiles, cells labeled for IL-8 expression. Bold letters indicate mean fluorescence intensities. A total of 10 000 cells of each sample were analyzed. (B) HUVECs were transfected in a 1:3 ratio with pGreenLantern expressing GFPS65T and plasmids expressing either empty expression vector KRSPA or KRSPA MKK6(Glu). Cells were left untreated or treated 36 hours after transfection with 10 ng/mL TcdB-10463 or 100 ng/mL TcdB-1470 for 8 hours. Successfully transfected cells were identified by expression of GFPS65T and analyzed for IL-8 synthesis by flow cytometry as described. Flow cytometry profiles of 1 representative experiment are shown. A total of 5000 transfected cells were assessed of each sample. (C) Mean fluorescence intensities ± SD in fold of unstimulated or vector-transfected control cells of at least 2 experiments as described in (A) and (B) are shown.
IL-8 synthesis in HUVECs expressing constitutively active MKK6(Glu).
(A) HUVECs were either left untreated or incubated with 100 ng/mL LPS for 8 hours. IL-8 synthesis was measured by flow cytometry as described in “Materials and methods.” Flow cytometry profiles of 1 representative experiment are shown. Open profiles represent isotype controls; shaded profiles, cells labeled for IL-8 expression. Bold letters indicate mean fluorescence intensities. A total of 10 000 cells of each sample were analyzed. (B) HUVECs were transfected in a 1:3 ratio with pGreenLantern expressing GFPS65T and plasmids expressing either empty expression vector KRSPA or KRSPA MKK6(Glu). Cells were left untreated or treated 36 hours after transfection with 10 ng/mL TcdB-10463 or 100 ng/mL TcdB-1470 for 8 hours. Successfully transfected cells were identified by expression of GFPS65T and analyzed for IL-8 synthesis by flow cytometry as described. Flow cytometry profiles of 1 representative experiment are shown. A total of 5000 transfected cells were assessed of each sample. (C) Mean fluorescence intensities ± SD in fold of unstimulated or vector-transfected control cells of at least 2 experiments as described in (A) and (B) are shown.
Discussion
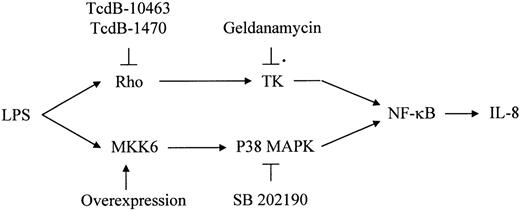
Here we present data indicating that LPS-induced IL-8 synthesis in human endothelial cells depends on 2 parallel signaling pathways converging at the level of NF-κB activation (Figure9): one via p38 MAPK and one via Rho proteins and tyrosine kinases. These conclusions are based on the following observations: Inhibition of Rho proteins by clostridial toxins blocked LPS-related protein tyrosine phosphorylation, NF-κB activation, and IL-8 synthesis but displayed no effect on LPS-induced p38 activity. Overexpression of the upstream kinase MKK6 alone was sufficient to induce endothelial IL-8 production and was not inhibited by blocking of Rho protein activity.
Proposed scheme of 2 parallel LPS-stimulated signaling pathways leading to IL-8 expression in HUVECs upon LPS stimulation: Both pathways are required for sufficient IL-8 expression.
Please see “Discussion” for details. TK indicates tyrosine kinase.
Proposed scheme of 2 parallel LPS-stimulated signaling pathways leading to IL-8 expression in HUVECs upon LPS stimulation: Both pathways are required for sufficient IL-8 expression.
Please see “Discussion” for details. TK indicates tyrosine kinase.
Role of Rho proteins
GTP-binding Rho proteins are considered to play an essential role in different important cellular systems, ranging from regulation of the microfilament network to kinase activation.30-41,49 The study of Rho protein function remains difficult. Available tools such as C. botulinum C3 toxin (which ADP-ribosylates Rho proteins at Asn-41) poorly enter mammalian cells,50 while constitutively activated p21Rho must be microinjected into target cells.33 Overexpression of GTPases has been useful to study Rho protein function, although this method suffers limitations.30,31 Alternatively, C. difficiletoxins such as TcdB-10463, which renders Rho proteins inactive by glucosylation (RhoA at threonine 37; Cdc42/RAC at threonine 35), can be used. These clostridial toxins are highly selective and easily enter mammalian cells. They were used to demonstrate the requirement of Rho proteins for maintenance of endothelial barrier function,44PKC activation and translocation,34 myosin light chain phosphorylation,51 phospholipase D activation,39,49 as well as for evaluation of lysophosphatidic acid–mediated signal transduction pathways.52 Inhibition of Rho proteins by TcdB-10463 dose-dependently blocked LPS-related IL-8 secretion in HUVECs, but inhibition of H-Ras by TcsL did not.
Rho proteins are implicated in the regulation of the microfilament system,32,33 and inhibition of these proteins markedly altered endothelial cytoskeleton.44 Therefore, an intracellular accumulation of IL-8 protein was possible but ruled out.
Rho protein inhibition resulted in a reduced expression of IL-8 mRNA in LPS-treated endothelial cells. Because the transcription factor NF-κB is considered to be essential for IL-8-expression,9,10 we tested and verified by reporter gene assay that Rho inhibition blocked LPS-induced activation of NF-κB–dependent genes. A link between NF-κB and the Rho family of small GTPases had already been suggested by the demonstration that expression of constitutively active Rho, Cdc42, and Rac proteins in NIH-3T3 cells or COS-7 cells promotes NF-κB activation.30 31 Taken together, it seems reasonable to suppose that Rho proteins are essential for LPS-induced NF-κB activation in endothelial cells.
Increased protein tyrosine phosphorylation is implicated in LPS-dependent endothelial cell activation.13 14 We made use of TcdB-14630–pretreated cells to study the role of Rho proteins in LPS-related tyrosine phosphorylation and noted that LPS-induced protein tyrosine phosphorylation of 40 to 37 kd and 26 to 21 kd was markedly reduced in cells without Rho function. In addition, inhibition of tyrosine kinases blocked LPS-related IL-8 synthesis, suggesting that Rho-dependent tyrosine phosphorylation plays an important role in the LPS-signaling pathway.
Role of p38 MAPK
Increased p38 MAPK activity appears to be instrumental in LPS-induced endothelial activation. For this reason, we verified p38 activation by LPS in our cells. Moreover, LPS-induced p38 activation was assessed in endothelial cells without Rho function. However, Rho or Ras protein inhibition did not alter p38 kinase activity in LPS-treated HUVECs. The role of Rho proteins for p38 MAPK regulation remains controversial and appears to depend on cell type, stimulus, and assay procedures.
In one study using Rat-1 cells, inhibition of RhoA, RhoB, and RhoC by botulinum C3 toxin activated p38 signaling pathway.53 In other studies using transfection procedures, a Cdc42-Pak–dependent activation of p38 kinase was demonstrated.54-56 Zhang et al also described a relationship between the Rac/Cdc42/Pak1 pathway and IL-1β–induced p38 kinase activation.56
In the study presented, inactivation of GTPases did not impair LPS-induced p38 activation, while inhibition of p38 kinase blocked LPS-related IL-8 production in HUVECs. Moreover, LPS-dependent expression of an NF-κB–dependent reporter gene was suppressed by p38 inhibition. In line with these observations, a p38 MAPK–dependent NF-κB transactivation was reported in HeLa cells, embryonic kidney cells, or mouse fibrosarcoma cell line L929.27 29
The central role of p38 for endothelial IL-8 expression was further demonstrated by using cells transiently transfected with constitutively active MKK6. Cells overexpressing MKK6(Glu),21 which in turn activates p38, showed IL-8 synthesis comparable to that found in LPS-stimulated endothelial cells. Inhibition of Rho proteins in MKK6(Glu)-transfected cells by clostridial toxins did not alter kinase-dependent IL-8 synthesis.
Moreover, the central role of p38-dependent signaling for LPS-induced cell activation was highlighted by the recent study of Kotlyarov et al,57 which demonstrated a central role of p38-dependent MAPKAP kinase 2 for LPS-induced tumor necrosis factor-α biosynthesis.
Hitherto, there is limited knowledge about the structures involved in LPS-induced signal transduction in endothelial cells. These cells do not express CD14 receptors but respond to LPS in the presence soluble CD14 and LPS-binding protein.13,14 Recent evidence suggests that members of the TLR family transmit LPS signals by using an analogous molecular framework for signaling as IL-1 containing MyD88, IRAK, IRAK2, and TRAF6 proteins.15-17 From this, it seems reasonable to suggest that Rho proteins and p38 MAPK are part of the LPS-TLR signaling pathway acting in parallel to transmit LPS-dependent signals in endothelial cells.
In summary, the data presented illustrate the complex LPS-dependent signaling resulting in IL-8 secretion in human endothelial cells (Figure 9). On one hand, LPS increases protein tyrosine phosphorylation, NF-κB activity, and IL-8 expression, steps that require functionally active Rho proteins while, on the other hand, LPS-related activation of p38 MAPK was not dependent on Rho GTPases. Besides Rho inactivation, inhibition of p38 MAPK signaling also reduced LPS-dependent NF-κB activation as well as IL-8 expression. Selective activation of p38 kinase signaling pathway was sufficient and as effective as LPS stimulation to induce IL-8 production in endothelial cells. Taken together, the data presented suggest the existence of at least 2 parallel LPS-induced pathways in endothelial cells that lead to NF-κB–dependent IL-8 expression: one via p38 kinase and one via Rho proteins and tyrosine kinases.
Supported by the Deutsche Forschungsgemeinschaft (SFB 547/B2 to N.S., Ei 206/8-2 to C.v.E.-S., and Go 811/1-1 to M.G. and S.L.).
Reprints:Norbert Suttorp, Charité, Department of Internal Medicine/Infectious Diseases, Humboldt-University, Augustenburgerplatz 1, 13353 Berlin, Germany; e-mail:norbert.suttorp@charite.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal