Abstract
Chemokines are small peptides that are potent activators and chemoattractants for leukocyte subpopulations and some nonhemopoietic cells. Their actions are mediated by a family of 7-transmembrane G-protein–coupled receptors, the size of which has grown considerably in recent years and now includes 18 members. Chemokine receptor expression on different cell types and their binding and response to specific chemokines are highly variable. Significant advances have been made in understanding the regulation of chemokine receptor expression and the intracellular signaling mechanisms used in bringing about cell activation. Chemokine receptors have also recently been implicated in several disease states including allergy, psoriasis, atherosclerosis, and malaria. However, most fascinating has been the observation that some of these receptors are used by human immunodeficiency virus type 1 in gaining entry into permissive cells. This review will discuss structural and functional aspects of chemokine receptor biology and will consider the roles these receptors play in inflammation and in infectious diseases.
Phagocytic leukocytes of the immune system undergo rapid and directed movements in chemoattractant gradients, a property that enables them to serve as the first line of cell-mediated host defense against infection. The interaction of chemoattractants with leukocytes initiates a series of coordinated biochemical and cellular events that includes alterations in ion fluxes, integrin avidity and transmembrane potential, changes in cell shape, secretion of lysosomal enzymes, production of superoxide anions, and enhanced locomotion.
Two groups of chemoattractants have been identified and extensively studied. The “classical” chemoattractants, such as bacterial-derived N-formyl peptides, complement fragment peptides C5a and C3a, and lipid molecules such as leukotriene B4 and platelet-activating factor are all chemoattractants and activators of leukocytes.1-4 Recently, a number of chemotactic cytokines in the 8- to 17-kd molecular mass range have been shown to be selective chemoattractants for leukocyte subpopulations in vitro and to elicit the accumulation of inflammatory cells in vivo.5 6 These chemotactic cytokines belong to the chemokine superfamily, which can be divided into 4 groups (CXC, CX3C, CC, and C) according to the positioning of the first 2 closely paired and highly conserved cysteines of the amino acid sequence.
The specific effects of chemokines on their target cells are mediated by members of a family of 7-transmembrane–spanning, G-protein–coupled receptors.7 These chemokine receptors are part of a much bigger superfamily of G-protein–coupled receptors that include receptors for hormones, neurotransmitters, paracrine substances, inflammatory mediators, certain proteinases, taste and odorant molecules, and even photons and calcium ions.8
To date 18 human chemokine receptors have been identified (Table1). Among the 5 receptors that selectively bind certain CXC chemokines are chemokine receptors CXCR1 to CXCR5, whereas the CC receptor family consists of 9 receptors, CCR1 to CCR9. A further receptor, designated D6, has been termed CCR10 by 1 research group, but this has yet to be officially adopted. Recently, receptors for fractalkine (CX3CR1) and lymphotactin (XCR1) have been identified. A further chemokine receptor, known as the Duffy antigen receptor for chemokines (DARC) has been shown to bind promiscuously to both CXC and CC chemokines. This review describes the characteristics of chemokine receptor gene and protein structure and includes a synopsis of each chemokine receptor identified to date. The roles that chemokine receptors play in inflammation and human disease states are then discussed.
Details of human chemokine receptors described until June 1999
| Receptor . | Amino acids . | Base pairs . | Ligand (high affinity) . | Cellular distribution . |
|---|---|---|---|---|
| XCR1 (GPR5) | 333 | 999 | Lymphotactin | T, B, NK |
| CXCR1 (IL8RA) | 350 | 1050 | IL8, GCP-2 | N, M, T, NK, Bs, Ms, En |
| CXCR2 (IL8RB) | 355 | 1065 | IL8, GRO-α, GRO-β, GRO-γ, NAP-2, ENA-78, GCP-2 | N, M, T, NK, MS, As, Nn, Ms, En |
| CXCR3 | 368 | 1104 | IP-10, Mig, I-TAC | Activated T |
| CXCR4* (LESTR, FUSIN) | 352 | 1056 | SDF-1α, SDF-1β | Myeloid, T, B, Ep, En, DC |
| CXCR5 (BLR1) | 372 | 1116 | BCA-1 | B |
| CX3CR1 (V28) | 355 | 1065 | Fractalkine | NK, M, T |
| CCR1 | 355 | 1065 | RANTES, MIP-1α, HCC-1, MCP-2, MCP-3, MIP-5, Ckβ8, | N, M, T, NK, B, Ms, As, Nn |
| CCR2A | 374 | 1122 | MCP-1, MCP-3, MCP-4 | M |
| CCR2B† | 360 | 1080 | MCP-1, MCP-2, MCP-3, MCP-4 | M, T, B, Bs |
| CCR3† | 355 | 1065 | Eotaxin, Eotaxin-2, Eotaxin-3, RANTES, MCP-2,3,4, MIP-5, | Eo, Bs, T |
| CCR4 | 360 | 1080 | TARC, MDC | T, P |
| CCR5† (ChemR13) | 352 | 1056 | RANTES, MIP-1β, MIP-1α, MCP-2 | T, M, M∅︀, DC |
| CCR6 (STRL 22, DRY-6, GPR-CY4, CKR-L3) | 374 | 1122 | MIP-3α | T, B, DC |
| CCR7 (BRL2, EBI1) | 378 | 1134 | MIP-3β, 6-C-kine, | T, B, DC |
| CCR8*,† (TER1, CKR-L1, ChemR1) | 355 | 1065 | I-309 | M, Thymus |
| CCR9 (GPR-9-6) | 369 | 1107 | TECK | T, Thymus |
| D6 (CCR10?) | 384 | 1152 | MCP-1, MCP-3 | placenta, liver |
| DARC (Duffy antigen) | 338 | 1014 | IL-8, GRO-α, RANTES, MCP-1, MCP-3, MCP-4, Eotaxin | En, RBC, T |
| Receptor . | Amino acids . | Base pairs . | Ligand (high affinity) . | Cellular distribution . |
|---|---|---|---|---|
| XCR1 (GPR5) | 333 | 999 | Lymphotactin | T, B, NK |
| CXCR1 (IL8RA) | 350 | 1050 | IL8, GCP-2 | N, M, T, NK, Bs, Ms, En |
| CXCR2 (IL8RB) | 355 | 1065 | IL8, GRO-α, GRO-β, GRO-γ, NAP-2, ENA-78, GCP-2 | N, M, T, NK, MS, As, Nn, Ms, En |
| CXCR3 | 368 | 1104 | IP-10, Mig, I-TAC | Activated T |
| CXCR4* (LESTR, FUSIN) | 352 | 1056 | SDF-1α, SDF-1β | Myeloid, T, B, Ep, En, DC |
| CXCR5 (BLR1) | 372 | 1116 | BCA-1 | B |
| CX3CR1 (V28) | 355 | 1065 | Fractalkine | NK, M, T |
| CCR1 | 355 | 1065 | RANTES, MIP-1α, HCC-1, MCP-2, MCP-3, MIP-5, Ckβ8, | N, M, T, NK, B, Ms, As, Nn |
| CCR2A | 374 | 1122 | MCP-1, MCP-3, MCP-4 | M |
| CCR2B† | 360 | 1080 | MCP-1, MCP-2, MCP-3, MCP-4 | M, T, B, Bs |
| CCR3† | 355 | 1065 | Eotaxin, Eotaxin-2, Eotaxin-3, RANTES, MCP-2,3,4, MIP-5, | Eo, Bs, T |
| CCR4 | 360 | 1080 | TARC, MDC | T, P |
| CCR5† (ChemR13) | 352 | 1056 | RANTES, MIP-1β, MIP-1α, MCP-2 | T, M, M∅︀, DC |
| CCR6 (STRL 22, DRY-6, GPR-CY4, CKR-L3) | 374 | 1122 | MIP-3α | T, B, DC |
| CCR7 (BRL2, EBI1) | 378 | 1134 | MIP-3β, 6-C-kine, | T, B, DC |
| CCR8*,† (TER1, CKR-L1, ChemR1) | 355 | 1065 | I-309 | M, Thymus |
| CCR9 (GPR-9-6) | 369 | 1107 | TECK | T, Thymus |
| D6 (CCR10?) | 384 | 1152 | MCP-1, MCP-3 | placenta, liver |
| DARC (Duffy antigen) | 338 | 1014 | IL-8, GRO-α, RANTES, MCP-1, MCP-3, MCP-4, Eotaxin | En, RBC, T |
T-lymphocyte[en]tropic HIV-1 coreceptor.
Macrophage-tropic HIV-1 coreceptor.
Previous chemokine receptor names are given in brackets.
N, neutrophil; M, monocyte/macrophage; T, T-lymphocyte; B, B-lymphocyte; NK, natural killer cell; Eo, eosinophil; Bs, basophil; Ms, mast cell; As, astrocyte; Nn, neurone; P, platelet; En, endothelial cell; Ep, epithelial cell; Hp, hepatocyte; DC, dendritic cell; M∅︀, macrophage; RBC, erythrocyte.
Structural features of chemokine receptors
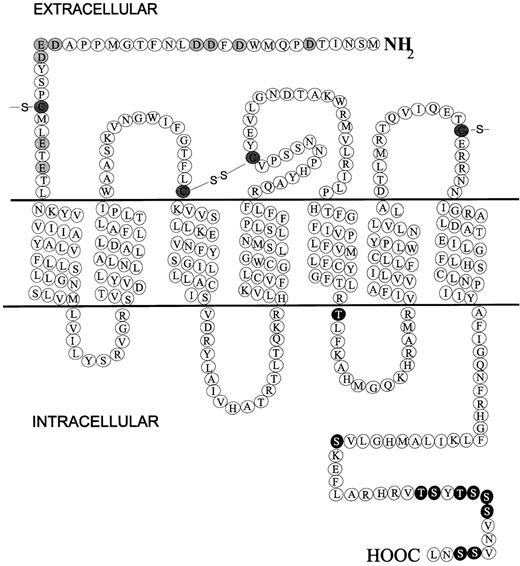
All chemokine receptors identified thus far are membrane-bound molecules composed of 7-transmembrane domains and coupled to G-proteins. Figure 1 shows a diagrammatic representation of CXCR1 that provides a good example for discussing the general chemokine receptor structure. Major hallmarks of chemokine receptors are as follows. They measure approximately 350 amino acids in length and require the introduction of few gaps in the primary sequence to be aligned to other chemokine receptors; a short extracellular N-terminus is acidic overall and may be sulfated on tyrosine residues and contain N-linked glycosylation sites; an intracellular C-terminus contains serine and threonine residues that act as phosphorylation sites for receptor regulation; 7 α-helical transmembrane domains—with 3 intracellular and 3 extracellular connecting loops composed of hydrophilic amino acids—are oriented perpendicularly to the plasma membrane; a disulfide bond links highly conserved cysteines in extracellular loops 1 and 2; G-proteins are coupled through the C-terminus segment and possibly through the third intracellular loop.
Diagrammatic representation of the chemokine receptor CXCR1.
Extracellular N-terminal acidic residues are shaded, C-terminal potential phosphorylation residues (serine and threonine) are black, and conserved cysteines are hatched.
Diagrammatic representation of the chemokine receptor CXCR1.
Extracellular N-terminal acidic residues are shaded, C-terminal potential phosphorylation residues (serine and threonine) are black, and conserved cysteines are hatched.
Human chemokine receptors
CXCR1 and CXCR2
The presence of IL-8 receptors on the surface of granulocytes was first shown by Peveri et al,9 who demonstrated that neutrophils stimulated with either fMLP, C5a, platelet-activating factor, or leukotriene B4 remain responsive to IL-8. It was further reported that sodium iodide I 125–IL-8 specifically bound human neutrophils with 2 affinities; consequently, the presence of 2 IL-8 receptors was postulated, 1 with a high affinity and 1 with a low affinity.10 Conflicting evidence was presented by Samanta et al11 and Grob et al,12who separately described single high-affinity binding sites on human neutrophils. Using chemokine competition experiments, 2 classes of IL-8 binding sites were detected, 1 of which bound NAP-2 and GRO with high affinity and 1 of which bound these chemokines with low affinity. Both classes of receptor, however, had high affinity for IL-8.13 14 The debate concerning the IL-8 binding sites was finally resolved by the cloning of 2 receptors for IL-8, CXCR1, and CXCR2.
CXCR1 (Figure 1) was first cloned by Holmes and colleagues.15 The CXCR1 cDNA contains an open reading frame encoding a protein of 350 amino acids, an N-linked glycosylation site at the N-terminus, and all the other hallmarks of a 7-transmembrane G-protein–coupled receptor. CXCR2 was simultaneously cloned by Murphy and Tiffany16 and was found to be 77% homologous to CXCR1. The CXC chemokines NAP-2, ENA-78, and GRO all bind CXCR2 with high affinity, whereas CXCR1 is selective for IL-8 only.17Recently, CXCR1 and CXCR2 were shown to bind GCP-2.18 Thus, together CXCR1 and CXCR2 bind all known N-terminal Glu-Leu-Arg (ELR)–containing CXC chemokines.
The expression of CXCR1 and CXCR2 on leukocytes has been analyzed using receptor-specific monoclonal antibodies. CXCR1 and CXCR2 are expressed on all granulocytes, monocytes, and mast cells and on some CD8+ T-cells and CD56+ natural killer (NK) cells.19 Equal amounts of CXCR1 and CXCR2 are present on neutrophils, but it appears that monocytes and positive lymphocytes express more CXCR2 than CXCR1.19 Binding of IL-8 to neutrophils rapidly down-regulates CXCR1 and CXCR2 expression by internalization, which is followed by proteolytic degradation of IL-8 by lysosomal enzymes. The receptors are then recycled back to the surface of the neutrophil cell membrane.20 The processes of receptor internalization and recycling are thought to operate for all chemokine receptors identified so far. Tumor necrosis factor and lipopolysaccharide have been shown to down-regulate the expression of both IL-8 receptors on neutrophils by activating a tyrosine kinase-dependent signaling pathway that leads to receptor proteolytic cleavage by metalloproteinases.21Granulocyte–colony-stimulating factor and the bacterial-derived molecule fMLP appear to increase the cell-surface expression of CXCR1 and CXCR2 on neutrophils.22 23
CXCR3
A human chemokine receptor that is selective for the non-ELR-containing CXC chemokines, IP-10, and Mig has been cloned, and, in accordance with other chemokine receptors, has been named CXCR3.24 The receptor shares 41% identical amino acids with CXCR1 and CXCR2 and is highly expressed on IL-2–activated T lymphocytes but not in resting T cells, B cells, monocytes, or granulocytes.24 Thus, CXCR3 seems to be involved in the selective recruitment of T cells. These data are in accordance with previous results showing that IP-10 and Mig are chemoattractants for T lymphocytes, not granulocytes.25,26 Recently, activated T cells have been shown to bind specifically and to respond to the newly described non-ELR CXC chemokine, I-TAC (interferon-inducible T-cell (chemoattractant) by CXCR3.27
CXCR4
CXCR4 was originally cloned by Loetscher et al28 as an orphan chemokine receptor (that is, a receptor whose ligand has not yet been discovered) and was given the acronym LESTR. The orphan receptor was found to be expressed on neutrophils, myeloid cells, and, in particular, T lymphocytes.28 LESTR was later identified as an essential cofactor for T-tropic human immunodeficiency virus type 1 (HIV-1) and type 2 (HIV-2) env-mediated fusion and entry into CD4+-expressing cells.29 However, it was not until the CXC chemokine SDF-1 was recognized as the biologic ligand for LESTR that the receptor was reclassified as CXCR4.30,31SDF-1α is a highly efficacious lymphocyte chemoattractant, and it inhibits HIV-1 infection of permissive CD4+ in accordance with CXCR4 expression patterns.30,31 Recently, IL-4 was found to enhance the cell-surface expression of CXCR4 on resting T-cells, whereas the receptor is down-regulated after T-cell stimulation by CD28 or CD3 and CD2.32 Mice lacking the CXCR4 gene exhibit impaired B lymphopoiesis, myelopoiesis, hematopoiesis, derailed cerebellar neurone migration, and defective formation of large vessels supplying the gastrointestinal tract.33-35 These findings suggest that SDF-1 and CXCR4 have biologic functions significantly different from those of other chemokines and chemokine receptors.
CXCR5
The orphan receptor BLR1, which is known to be highly expressed in Burkitt's lymphoma cells and B lymphocytes, was found to have significant homology with other CXC chemokine receptors.36 However, at the time no known CXC chemokines that stimulated B cells had been identified. Legler et al37recently identified and cloned a novel CXC chemokine with potent B-cell–activating capabilities that has subsequently been termed BCA-1 (B-cell–activating chemokine). Furthermore, on screening BCA-1 against a panel of putative chemokine receptors, the chemokine was found to be highly specific for BLR1.37 Consequently, because BLR1 is the fifth CXC chemokine receptor to be identified, it has been renamed CXCR5.
CX3CR1
Fractalkine, a novel class of chemokine with a unique CX3C motif, has been identified and characterized.38 Unlike other chemokines, the molecule exists as a membrane-bound glycoprotein with the chemokine atop an extended mucin-like stalk. Imai et al39 observed that fractalkine bound with high affinity to the orphan chemokine receptor V28 and subsequently renamed the V28 receptor CX3CR1.39 Fractalkine, either attached or detached from its stalk, binds to CX3CR1 and promotes adhesion of monocytes, NK cells, and T lymphocytes to endothelial, epithelial, and dendritic cells.38,40 41
CCR1
The high-affinity RANTES/MIP-1α receptor (now termed CCR1), which was first cloned by Neote et al42 has 33% similarity to CXCR2 and 31% to CXCR1. It is also 33% homologous to the 7-transmembrane cytomegalovirus protein, US28.43 Indeed, US28 can bind both RANTES and MIP-1α with high affinity, suggesting that US28 may be a CCR1 homologue acquired by viral hijack.44Xenopus oocytes injected with CCR1 cRNA acquired responsiveness to MIP-1α and RANTES but not to MIP-1β or any other CC chemokine tested.42 Cells transfected with CCR1 responded to MIP-1α, RANTES, MCP-2, and MCP-3; hence, CCR1 also binds MCP-2 and MCP-3.45-47 Recently, the novel CC chemokines MIP-5, HCC-1 (hemofiltrate CC chemokine), and CKβ8 have also been shown to bind specifically to CCR1.48 49
Lipopolysaccharide caused a rapid reduction of CCR1 mRNA levels in monocytes, leading to a reduced cellular response to ligand-specific chemokines,50 whereas monocytoid U937 cells and neutrophils stimulated with interferon exhibited increased mRNA and cell-surface expression of CCR1.51,52 IL-2 and IL-15 induced the expression of CCR1 on activated T cells,53,54 whereas IL-10 selectively up-regulated the expression of CCR1 in human monocytes by prolonging mRNA half-life.55
CCR2A and CCR2B
The monocyte chemotactic proteins function as potent activators and chemoattractants for monocytes, basophils, eosinophils, and T-lymphocyte subsets, but not for neutrophils.5 Direct binding studies with 125I–MCP-1 identified high-affinity binding sites on human monocytes.56 Monocyte, basophil, and eosinophil activation by CC chemokines, including MCP-1, are prevented by pretreatment with Bordetella pertussis toxin, suggesting that the action of MCP-1 is mediated by a G-protein–coupled receptor.57
Two independent groups simultaneously cloned CCR2. Yamagami et al58 identified CCR2B, which encodes a protein of 360 amino acids and has 56% similarity to CCR1. Cloning by Charo et al59 produced 2 positive clones corresponding to 2 forms of CCR2—CCR2A and CCR2B. Both CCR2A and CCR2B have identical 5′ untranslated and transmembrane regions, but they differ in an alternatively spliced carboxyl terminus. Consequently, the carboxy tail of CCR2B is 36% homologous to the corresponding region in CCR1, whereas the carboxy tail of CCR2A bears no similarities to any other known chemokine receptor.59 Recent studies record that MCP-2, MCP-3, and MCP-4 can also bind to CCR2B.46,47 The expression of CCR2 mRNA in monocytes has been found to be decreased by both IFN-γ60 and lipopolysaccharide,50whereas IL-2 induced the expression of CCR2 in T lymphocytes, which correlated with the response of these cells to MCP-1 in chemotaxis assays.53 Furthermore, IL-10 selectively up-regulated the expression of CCR2 in monocytes by prolonging the mRNA half-life.55
CCR3
Cross-desensitization experiments using chemokine-induced intracellular calcium mobilization have indicated that eosinophils may have specific receptors for RANTES, MCP-3, and eotaxin and a shared receptor for MIP-1α, RANTES, and MCP-3.61,62 MIP-1α, RANTES, and MCP-3 can bind to CCR1, and MCP-3 can also bind to CCR2; however, CCR2 is not expressed by eosinophils. Using this information, Daugherty et al63 and Kitaura et al64independently identified and characterized the eosinophil-selective chemokine receptor CCR3. CCR3 cDNA encodes for a protein that is expressed predominantly on eosinophils but can also be detected on basophils and T cells.63,65,66 The receptor has 63% similarity to CCR1 and 51% to CCR2B and binds several CC chemokines specifically, including eotaxin, eotaxin-2, RANTES, MCP-3, MCP-4, and MIP-5, all of which have been implicated in eosinophil recruitment and activation.63,65,67 Eotaxin and eotaxin-2 have the greatest affinity for CCR3 and, accordingly, are the most potent chemokine activators of eosinophils.68 Subsequently, CCR3 has been implicated in the progression of allergic reactions (discussed below). In association with CD4, CCR3 has also been implicated in permitting macrophage-tropic HIV-1 infection of permissive cells.69
CCR4
Power et al70 have identified a novel CC chemokine receptor called CCR4 that shares 49% identity with CCR1 and 47% with CCR2B.70 Initially, it was thought that CCR4 bound RANTES and MIP-1α. However, recent studies show that CCR4 specifically binds the CC chemokines TARC (thymus and activation-regulated chemokine) and MDC (macrophage-derived chemokine).71,72 TARC and MDC have both been shown to be selective activators of T lymphocytes, particularly CD4+ Th2 cells. These data are in agreement with the expression of CCR4, which appears to be highly expressed in T lymphocytes and platelets and weakly expressed in other peripheral mononuclear cells.70,71 Furthermore, the expression of CCR4 on Th2 cells was transiently increased after T-cell receptor and CD28 engagement. Consequently, the activity of Th2 cells to TARC was enhanced on receptor activation.73
CCR5
Originally cloned by Samson et al,74 CCR5 was found to bind the CC chemokines MIP-1β, RANTES, MIP-1, and MCP-2 specifically using transfected and peripheral blood mononuclear cells.75These studies were quickly corroborated by Raport et al,76who also demonstrated CCR5 mRNA expression in lymphoid organs such as the thymus and spleen and in peripheral T lymphocytes and macrophages.76 CCR5 is most closely related to CCR2B, with 71% identical amino acid residues. Furthermore, the gene encoding CCR5 is a localized only 18-kb pair downstream of the gene for CCR2 on chromosome 3p21,74,76 which suggest that these 2 receptors share an ancestral gene. CCR5 is also homologous to other CC receptors and shares 55%, 49%, and 48% identity with CCR1, CCR3, and CCR4, respectively.76 Recently, CCR5 has been shown to be the major coreceptor in association with CD4 for macrophage-tropic HIV-1 entry into permissive cells.69 The immunosuppressive and anti-inflammatory cytokine IL-10 selectively up-regulated the expression of CCR5 in human monocytes by prolonging the mRNA half-life.55 It appears that this increase in CCR5 expression is regulated by activation of MAP and STAT kinases.77 IL-15–stimulated T cells also increased their expression of CCR5,54 indicating that interleukins can act as modulators of chemokine receptor expression.
CCR6
CCR6 was originally cloned by several groups as an orphan chemokine receptor and was therefore provided with several different acronyms (STRL 22, DRY-6, GPR-CY4, CKR-L3). It was not until Baba et al78 discovered that the CC chemokine MIP-3α/LARC (liver and activation-regulated chemokine) specifically bound to the orphan receptor GPR-CY4 that these orphan receptors were all redesignated as CCR6. CCR6 has been detected on memory T cells, B lymphocytes, and dendritic cells but not on any other peripheral blood leukocyte.79 CCR6 mRNA has been shown to be up-regulated by treatment with IL-2.78,80 However, recent data contradict this finding79 and, as a consequence, the effect of IL-2 on CCR6 expression remains uncertain.
CCR7
By searching the expressed sequence tag (EST) database, Yoshida et al81 identified a cDNA sequence that codes for a novel CC chemokine, was termed ELC (EBI1-ligand chemokine) or MIP-3β. ELC was then screened against a panel of cells transfected with known and orphan chemokine receptors to search for its corresponding high-affinity receptor. It was found that ELC bound specifically to the orphan receptor EBI1,82 which has subsequently been renamed CCR7.81 Recently, the novel CC chemokine 6-C-kine, also known as SLC (secondary lymphoid-tissue chemokine), has been shown to be a specific agonist for CCR7.83 CCR7 is known to be expressed on activated T and B lymphocytes and dendritic cells and is strongly up-regulated in B cells infected with Epstein–Barr virus and in T cells infected with herpesvirus 6 or 7.81 84
CCR8
The human CC chemokine I-309 is a potent monocyte chemoattractant and inhibits apoptosis in thymic cell lines.85 To identify its cognate chemokine receptor, Roos et al86 used an intracellular calcium mobilization assay with I-309 to test for receptor function in cells transfected with several known orphan receptors. I-309 was found to bind specifically to and to activate cells transfected with the orphan receptor known as either TER1,87 ChemR1,88 or CKR-L1.89These findings were quickly corroborated by Tiffany et al.90 Consequently, these orphan receptors were collectively renamed CCR8, in accordance with the nomenclature system for chemokine receptors. Strong CCR8 mRNA expression was detected in the thymus and monocytes but not in other peripheral blood leukocytes.90 These data appear to be in agreement with the role of I-309 in monocyte activation and thymic cell survival. Indeed, CCR8 is preferentially expressed on Th2-polarized cells91and is transiently increased after T-cell receptor and CD28 engagement,73 suggesting that CCR8 plays a role in the control of Th2 responses and that up-regulation of CCR8 after antigen encounter may contribute to the proper positioning of activated T cells within sites of antigenic challenge or specialized areas of lymphoid tissue.73 Recently, CCR8 has been shown to serve as a cofactor, in association with CD4, to permit the infection of permissive cells with T-cell tropic and macrophage-tropic HIV-1 strains.92
CCR9
CCR9 is the most recent chemokine receptor to be identified. Zaballos et al93 found that thymus-expressed chemokine (TECK) is a specific agonist for the human orphan receptor GPR-9-6 (EMBL accession number U45 982), which has been renamed CCR9 according to the established nomenclature. CCR9 expression is high in the thymus but low in lymph nodes and spleen, and it appears to be expressed on both immature and mature T cells.93 These data are in agreement with previous results showing that TECK is an activator of dendritic cells and thymocytes, which implicates this CC chemokine in T-cell development.94
D6 (CCR10?)
D6 was simultaneously cloned by 2 independent groups and displays approximately 30% homology with other CC chemokine receptors at the amino acid level.95,96 It was originally described by Bonini et al95 as CCR10. However, because D6 does not generate an intracellular signal on ligand binding and, therefore, appears not to be functional,96 the receptor has yet to be granted a CCR number. The ligand specificity of D6 is also contentious. Bonini et al95 reported D6 to bind MCP-1 and MCP-3 with high affinity, whereas Nibbs et al96 suggest that D6 is more promiscuous because it is able to bind a number of CC chemokines with similar affinity. Northern blot analysis of several human tissues reveals that D6 is almost exclusively expressed in placenta with weak expression in the liver, lung, and thyroid.95 96 These data suggest that D6 may have a role in placental immunity or hematopoiesis.
XCR1
Yoshida et al97 have recently reported the finding that the single C motif chemokine lymphotactin binds specifically to the orphan chemokine receptor previously termed GPR5.98 In keeping with the chemokine receptor nomenclature, this receptor has now been designated XCR1. The lymphotactin receptor is strongly expressed in placenta and weakly expressed in spleen and thymus.97Because lymphotactin activity is most pronounced against lymphocytes and NK cells,99 100 it is expected that these leukocyte subpopulations will also express XCR1.
Duffy antigen receptor for chemokines
The transmembrane glycoprotein gpD had long been known to be part of the multimeric protein complex that makes up the antigen for the Duffy blood group system.101,102 However, it was not until Chaudhuri et al103 cloned the cDNA for gpD that it was found to be a 7-transmembrane receptor with significant homology to previously cloned chemokine receptors. This discovery also partly explained the phenomenon that erythrocytes could bind both the CXC chemokine IL-8 and the CC chemokine MCP-1.104,105 DARC (Duffy antigen receptor for chemokines) has also been shown to bind numerous CXC and CC chemokines specifically, including RANTES, I-309, GRO-α, MCP-3, MCP-4, and eotaxin,106 and it has been implicated in the pathogenesis of malaria102 (discussed below).
In addition to erythrocytes, DARC expression has been detected on renal and spleen capillary endothelium,107 endothelial cells of venules,108 and Purkinje cells in the brain.109It is thought that erythrocyte, endothelial, and Purkinje cell binding of CXC and CC chemokines by DARC may limit the stimulation of leukocytes by chemokines released into the bloodstream and that it may also modulate neuronal activity.104,109 DARC is expressed on endothelial cells at anatomic sites of leukocyte trafficking and diapedesis, a process highly regulated by the actions of chemokines that bind to DARC. Whether DARC actually plays a role in leukocyte trafficking remains to be determined. It is of note that DARC-dependent signal transduction has not yet been demonstrated and that DARC does not appear to be coupled to a G-protein.110 Therefore, the precise physiological role of DARC has yet to be elucidated.
Chemokine receptor signal transduction mechanisms
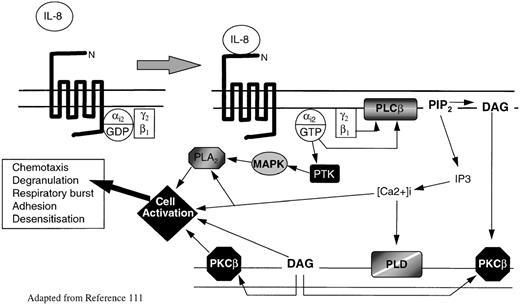
Intracellular signaling by chemokine receptors depends on coupling to Bordetella pertussis toxin-sensitive heterotrimeric G-proteins, usually of the Gi-type (for a more detailed discussion, see Murphy111 and Bokoch112). G-proteins are inactive when GDP is bound to the G-protein subunit, but they become active when GDP is exchanged for GTP. During ligand binding, chemokine receptors associate with G-proteins, facilitating the exchange of guanosine diphosphate (GDP) for guanosine triphosphate (GTP). In the active state, G-proteins dissociate into Gα and Gβ subunits; the latter are able to activate the membrane-associated enzyme phospholipase Cβ2 (PLC2), which in turn cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) to form the intracellular second messages phosphatidylinositol 1,4,5-triphosphate (IP3) and diacyl-glycerol (DAG) (Figure 2). IP3 mobilizes calcium from intracellular stores, whereas DAG acts in conjunction with calcium to activate various isoforms of protein kinase C (PKC). The activation of PKC and of various calcium-sensitive protein kinases catalyses protein phosphorylation, which activates a series of coordinated signaling events that eventually lead to cellular responses.111-114 There is increasing evidence that chemokine receptors can also activate several different intracellular effectors downstream of Gicoupling, including the low-molecular-weight proteins Ras and Rho,112,115 phospholipase A2, phosphatidylinositol-3-kinase,116 tyrosine kinases,117-119 and the MAP kinase pathway120(Figure 2). No doubt the precise signaling mechanisms by which all chemokine receptors regulate cellular function will become evident in time.
Model of chemokine receptor activation and signal transduction for IL-8 and neutrophils.
IL-8 binding to CXCR1 or CXCR2 causes guanosine triphosphate displacement of guanosine diphosphate in the Gαi2subunit, which allows dissociation of Gαi2 from Gβγ. Gβ activates phospholipase C (PLCβ), which cleaves PIP2 into the second messengers DAG and IP3. DAG activates PKCβ, whereas IP3 causes the release of calcium from intracellular stores. The rapid rise in intracellular calcium activates PLD. Meanwhile Gαi2directly activates PTK. These activate MAP kinases and phosphorylate serine and threonine residues on the C-termini of CXCR1 and CXCR2, leading to receptor inactivation. MAP kinases activate phospholipase A2. DAG, intracellular calcium, PKC, and phospholipase A2 (PLA2) all interact with specific cell activation mechanisms, leading to cell motility, degranulation, release of superoxide anions, and modification of integrin avidity.
Model of chemokine receptor activation and signal transduction for IL-8 and neutrophils.
IL-8 binding to CXCR1 or CXCR2 causes guanosine triphosphate displacement of guanosine diphosphate in the Gαi2subunit, which allows dissociation of Gαi2 from Gβγ. Gβ activates phospholipase C (PLCβ), which cleaves PIP2 into the second messengers DAG and IP3. DAG activates PKCβ, whereas IP3 causes the release of calcium from intracellular stores. The rapid rise in intracellular calcium activates PLD. Meanwhile Gαi2directly activates PTK. These activate MAP kinases and phosphorylate serine and threonine residues on the C-termini of CXCR1 and CXCR2, leading to receptor inactivation. MAP kinases activate phospholipase A2. DAG, intracellular calcium, PKC, and phospholipase A2 (PLA2) all interact with specific cell activation mechanisms, leading to cell motility, degranulation, release of superoxide anions, and modification of integrin avidity.
After activation, chemokine receptors become either partially or totally desensitized to repeated stimulation with the same or other agonists. This process is thought to involve phosphorylation of serine and threonine residues in the C-tail of the receptor by G-protein–coupled receptor kinases and receptor sequestration by internalization. Desensitization may be critical for maintaining the capacity of the cell to sense a chemoattractant gradient.111
Chemokine receptors in inflammation
Leukocyte activation in acute inflammation
To reach sites of inflammation or injury, circulating leukocytes must exit the bloodstream by traversing the endothelium. Leukocytes usually attach to the apical surface of the endothelium of postcapillary venules, where the shear stress is lowest. The first step in the process of leukocyte recruitment at sites of inflammation is the generation of transient selectin-mediated interactions that cause tethering and rolling of flowing leukocytes on the endothelial cell surface.121 The slow velocity of rolling leukocytes on selectins favors encounters with chemokines that are presented on the apical surface of the endothelium by glycosaminoglycans.122Chemokines bind to their respective chemokine receptors expressed on the leukocyte cell surface, leading to the alteration of β2 integrin avidity, especially CD11b/CD18, on the leukocyte cell surface.123 Then β2 integrins bind to their Ig counterligands, such as ICAM-1, ICAM-2, and ICAM-3, which have been up-regulated on the endothelial cell surface by proinflammatory cytokines. These interactions provide firm attachment of leukocytes to the endothelium and facilitate leukocyte haptotactic transendothelial migration.123 124 The binding of chemokines to their respective leukocyte receptors also initiates a series of cellular events, all of which aim to eradicate the infiltrating inflammatory agents. These events include changes in cell shape leading to enhanced locomotion, secretion of lysosomal enzymes, and production of superoxide anions. Once leukocytes reach the source of inflammation, a cytokine-rich milieu is generated that is sustained until the invading antigen is eliminated. In general, immune responses do not produce endothelial injury; however, on occasion acute or chronic inflammation may occur in which the endothelium and surrounding tissues become damaged (for example, by neutrophil-generated products).
Inflammation resolution and inflammatory disorders
After acute infection or injury, blood vessels may be damaged. Part of the mechanism of wound healing, the formation of new blood vessels, known as angiogenesis, is a process tightly regulated by numerous biologic mediators, among them chemokines.125 CXC chemokines, such as IL-8, GRO-α, GRO-β, PF-4, IP-10, and Mig, have been implicated in the regulation of keratinocyte and endothelial cell function, including the stimulation and inhibition of proliferation, angiogenesis, angiostasis, and cell migration.125-131However, evidence concerning the expression of chemokine receptors by endothelial cells has been conflicting.132,133 Recent data now show that endothelial and epithelial cells express several functional chemokine receptors, in particular CXCR4.134-137It has been proposed that endothelial proteoglycans can present chemokines to leukocyte and to endothelial-expressed chemokine receptors.134,136 This model is analogous to the way in which basic fibroblast growth factor is thought to bind to endothelial proteoglycans, facilitating its interaction with high-affinity fibroblast growth factor receptors on the endothelial cell surface.138 A low level of expression and responsiveness of chemokine receptors on endothelial cells may be sufficient to permit cell activation in the presence of high levels of proteoglycan-bound chemokine on the adjacent endothelial cell surface. These findings suggest that chemokines and their receptors may play an important role in the vascular remodeling and maintenance associated with inflammatory resolution and, as a consequence, may be implicated in the development of inflammatory disorders, as discussed below.
Psoriasis
The overproduction of CXC and CC chemokines has been associated with many disease states, including arthritis, multiple sclerosis, and lung disorders such as adult respiratory distress syndrome, idiopathic pulmonary fibrosis, and pneumonia.139-142 It was initially noted that large quantities of IL-8, activated neutrophils, and T lymphocytes were present in the epidermis of patients with psoriasis vulgaris.139 Consequently, IL-8 was shown to induce the expression of HLA-DR and to be chemotactic and mitogenic for keratinocytes.129,132 Furthermore, Schulz et al143 demonstrated that CXCR1 and CXCR2 mRNA levels were 10 times more abundant in lesional psoriatic epidermis than in normal epidermis.143 This has led to the suggestion that the overexpression of IL-8 receptors is responsible for the epidermal hyperplasia, leukocyte infiltration, and increased keratinocyte HLA-DR expression seen in psoriasis. Indeed, antipsoriatic drugs such as calcitriol, dithranol, cyclosporin, and FK 506 have been shown to be potent down-regulators of IL-8 receptors on keratinocytes.143 144
Atherosclerosis
The recruitment of monocytes and the migration, growth, and activation of lipid-laden macrophages, T lymphocytes, and smooth muscle cells during the development of atherosclerotic plaques are critical features of the chronic inflammatory response that typifies atherosclerosis.145 Early studies by Nelken et al146 showed that the CC chemokine MCP-1 is abundantly expressed in macrophage-rich areas of atherosclerotic plaques, suggesting that MCP-1 may be important for monocyte extravasation and for the formation of atherosclerotic lesions.
Further evidence implicating MCP-1 in the pathogenesis of atherosclerosis has come from transgenic mouse models. Apolipoprotein B/MCP-1–deficient mice that overproduce apolipoprotein B-containing lipoproteins and are therefore susceptible to atherosclerosis are protected from atherosclerotic lesion development even when fed a high-fat diet.147 Furthermore, low-density lipoprotein (LDL) receptor/MCP-1–defective mice on a high-cholesterol diet had 83% less lipid deposition throughout their arteries than controls.148 These genetic data strongly suggest that MCP-1 expression is crucial for the development of atherosclerotic plaques. Similar results have been produced using CCR2 knockout mice also deficient in the apolipoprotein E gene and therefore predisposed to severe atherosclerosis. These double-knockout mice had 50% fewer lesions, indicating that CCR2, which is the major MCP-1 receptor, is also associated with the development of early atherosclerosis.149 Interestingly, monocyte CCR2 expression was dramatically increased in hypercholesteremic patients, and native, but not oxidized, LDL significantly increases monocyte CCR2 expression and chemotaxis activity toward MCP-1.150These data imply that elevated plasma LDL levels enhance monocyte CCR2 expression and activity, contributing to increased monocyte recruitment in atherosclerotic plaques.151 Recent evidence has shown that tissue factor, a protein also found in the atherosclerotic plaques, is expressed by SMC when stimulated with MCP-1.152This can be inhibited by pertussis toxin, but the investigators failed to show CCR2 expression on SMC. However, Hayes et al153have detected the expression of CCR2 and CCR1, but not CCR3-5, by reverse transcription–polymerase chain reaction in primary cultures of SMC. Such observations have been corroborated in our laboratory. We found mRNA expression of CXCR1, CXCR2, CCR1, and CCR2B, but not CCR3-5, in vascular SMC (unpublished data). These data suggest that CCR2 expression on SMC and on monocytes is important for atherosclerosis development.
It is not only MCP-1 and CCR2 that have been implicated in atherosclerosis. Histologic studies show that activated T cells are present throughout all stages of atherosclerotic lesion development.145 Within atherosclerotic plaques, macrophages and intimal SMC express the potent chemotactic T-cell chemokines PARC, ELC, and IP-10.154,155 The latter chemokine, along with IL-8, has also been shown to induce a dose-dependent stimulation of DNA synthesis, cell proliferation, and cell migration of human SMC.155,156 Furthermore, mice lacking human homologue receptors for IL-8 are less susceptible to atherosclerosis and have fewer vascular lesions than mice expressing these receptors.157 Taken together, these data suggest that certain chemokines and their receptors play an important role in modulating the functions of leukocytes and SMC involved in the development and progression of atherosclerosis.
Allergy
Recently, chemokines have been implicated in contributing to allergic disorders, in particular to allergic airway inflammation such as allergic rhinitis and asthma.158-160 The long-term effects of these diseases have been attributed, in part, to the infiltrating leukocytes, in particular eosinophils, that surround the bronchus and infiltrate the airway.161
The CC chemokines eotaxin, eotaxin-2, and MCP-4—which are released by airway epithelial cells—preferentially attract and activate eosinophils by acting specifically through CCR3.62,68 It has been shown that levels of eotaxin and MCP-4 are substantially elevated in the lungs of persons with asthma and are correlated with the numbers of infiltrating eosinophils, implicating these chemokines in asthmatic lung inflammation.162Recently, Ying et al163 reported that the expression of CCR3 is also elevated significantly in persons with atopic asthma. There is also an increased bone marrow pool of CCR3(+) mature and immature eosinophils available for rapid mobilization to subjects with asthma.164 Thus it appears that CCR3 is substantially involved in the recruitment of inflammatory cells in allergic responses. CCR3 contains 4 nucleotide polymorphisms, 2 of which encode for an amino acid change.165 Selected polymorphisms have been shown to have dramatic effects on the manifestation of or the susceptibility to a variety of diseases. These results may have implications for those with allergic responses such as asthma, which involves CCR3. As a consequence CCR3 may be a prime therapeutic target in the spectrum of allergic diseases involving eosinophil-mediated tissue damage.
T cells with a Th2 cytokine phenotype are also prominent in the pathogenesis of allergic diseases.166 Th2 cells preferentially express CCR4 and CCR8.91 Furthermore, the ligands for these chemokine receptors (TARC, MDC, and I-309) are potent chemoattractants for Th2 cells. These observations suggest that both CCR4 and CCR8 play a role in the control of Th2 responses and may represent potential targets for the treatment of allergic diseases. The induction of experimental allergic encephalomyelitis in the rat was accompanied by increased levels of CCR2, CCR5, CXCR4 and CX3CR1 mRNA in the lumbar spinal cords of animals displaying clinical signs of the disease.167 These findings are strong evidence implicating certain chemokine receptors as mediators of allergic diseases.
Chemokine receptors in infectious diseases
Until recently little attention has been paid to the role of chemokine receptors in infectious diseases. However, it is now known that chemokine receptors participate in several disease states, either by overexpressing receptors or by facilitating viral entry into permissive cells.
Malaria
Malaria is transmitted by mosquitoes infected with 1 of 4 pathogenic parasites. These parasites bind to and invade erythrocytes eventually causing them to undergo cell lysis. It is this stage of the parasitic life cycle that is associated with clinical illness. Malaria is endemic in most tropical and subtropical areas of the world; however, it is much less prevalent in West Africa. In this geographical area, 95% of the population is resistant to the malaria parasites Plasmodium vivax and Plasmodium knowlesi.110 Miller et al168 note that erythrocytes from humans resistant toP. knowlesi infection do not express the Duffy antigen receptor for chemokines (DARC) on their red blood cells. It was subsequently shown that P. vivax could not invade erythrocytes of DARC-negative patients and that an anti-DARC antibody could block parasite invasion into DARC-positive red blood cells.169,170 A number of studies have since revealed that both P. vivax and P. knowlesi gain entry to erythrocytes by binding specifically to DARC.102,110,171The genetic difference between persons who are susceptible to P. vivax and P. knowlesi invasion and those who are not corresponds to a single G-to-A nucleotide substitution, producing a Gly44Asp substitution in the polypeptide chain that prevents parasite invasion into erythrocytes.172,173 Interestingly, in some patients DARC is not expressed on red blood cells at all but is expressed on other cell types, including the endothelium.108 It appears that this phenomenon results from a mutation in the DARC promoter region in the erythrocytes of these patients.174 Whether P. vivax or P. knowlesi can gain entry to other cell types that express DARC has yet to be determined. The physiologic roles of DARC remain only partially elucidated (see section on DARC above).
Human immunodeficiency virus
Perhaps the most exciting development in chemokine receptor-associated pathogenesis comes from the discovery that some chemokines function as HIV-1–suppressive factors.175 Feng et al29 showed that T-cell–tropic HIV-1 isolates used both CD4 and CXCR4 to support Env-mediated cell fusion and HIV-1 infection of permissive cells.29 Further evidence showed that SDF-1 could block HIV-1 entry by binding to CXCR4 expressed by lymphocytes.30,31 These discoveries promoted frantic research in the area of HIV infection. Consequently, it was soon ascertained that CC chemokine receptors CCR2, CCR3, and CCR5 could also serve as cofactors, along with CD4, to permit HIV-1 entry, this time preferentially by macrophage-tropic and dual-tropic strains of HIV-1.69,176-178 Recently, CCR8 has been identified as a cofactor, in association with CD4, to permit the infection of permissive cells either by T-cell tropic or by macrophage-tropic HIV-1 strains.92
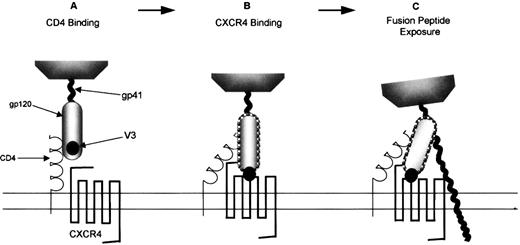
The role chemokine receptors play in permitting HIV-1 viral entry into permissive cells has been extensively reviewed.179-181Entry of HIV-1 into cells is a multistep process mediated by the viral Env protein. Env initially binds the virus particle to CD4, which induces conformational changes in the HIV gp120 subunit, exposing the third variable (V3) loop (Figure 3A). However, these conformational changes cannot generate an Env structure that is fusogenic. The fusion-inducing conformational changes are driven by certain chemokine receptors (Figure 3B, Table 1) that bind to the exposed regions of the V3-loop of gp120.182 Recent evidence suggests that after translation modification, sulfated tyrosines and acidic amino acid residues in the N-terminus of CCR5 and CXCR4 are necessary for binding to gp120 and, thus, for the formation of the HIV-1 gp120/CD4 complex.183-185 The fusogenic complex that results from this chemokine receptor/gp120 interaction comprises a coiled–coil structure in the gp41 ectodomain that displaces the hydrophobic N-terminal domain of the gp41 subunit toward the plasma membrane of the target cell.186 The fusion peptide is likely to insert into the cell membrane, making Env an integral component of the 2 membranes which eventually leads to the insertion of viral DNA into the target cell (Figure 3C). Interestingly, CXCR4 can recognize the T-tropic virus-binding site in the absence of CD4.187 The function of CD4 for these viruses may be merely to concentrate the virus and present it to the chemokine receptor. This may explain why CXCR4 is capable of permitting HIV entry into cells that do not express CD4.187
T-lymphocyte–tropic HIV-1 infection of CXCR4-expressing cells.
(A) The gp120 subunit of an env oligomer binds to CD4, inducing conformational changes including increased exposure of the V3 loop. This enables env to interact with the appropriate coreceptor, in this case CXCR4 (B). This interaction is then proposed to trigger the final conformational changes in env, including the formation of a coiled–coil structure in gp41 that ensures that the fusion peptide inserts into the cell membrane (C).
T-lymphocyte–tropic HIV-1 infection of CXCR4-expressing cells.
(A) The gp120 subunit of an env oligomer binds to CD4, inducing conformational changes including increased exposure of the V3 loop. This enables env to interact with the appropriate coreceptor, in this case CXCR4 (B). This interaction is then proposed to trigger the final conformational changes in env, including the formation of a coiled–coil structure in gp41 that ensures that the fusion peptide inserts into the cell membrane (C).
In humans it appears that during the asymptomatic stages of infection, HIV-1 binding to CCR5 predominates.188 However, as infection proceeds, HIV-1 uses other chemokine receptors, particularly CXCR4.29 An important finding for HIV biology was reported in 1996 when Liu et al189 and Samson et al190simultaneously identified a 32-bp deletion of the CCR5 gene that is present at a high frequency in white populations but is absent in African and Japanese populations.189,190 The deletion generates a rapidly degraded, nonfunctional receptor that does not support membrane fusion or infection by macrophage- or dual-tropic HIV-1 strains.189 The mutation can be heterozygous or homozygous; the latter largely protects against the acquisition of CCR5-mediated HIV-1 infection, though homozygosity does not confer absolute protection.191 Polymorphisms other than the 32-bp deletion in chemokine receptors CCR2, CCR3, and CCR5 have also been identified.165,192 193 These genomic defects generate single amino acid substitutions within the chemokine receptor. The consequences of these polymorphisms on HIV infection and pathogenesis have yet to be determined.
Recent evidence suggests that the human T-lymphotropic virus type 1 (HTLV-1) Tax protein up-regulates the cell-surface expression of CXCR4 and CCR5 by interacting with transcription factors causing transactivation of the chemokine receptor gene promoters.194 This finding suggests that HTLV-1 may contribute to the acceleration of HIV disease observed in some coinfected persons.
Summary
There has been rapid recent progress in the understanding of the biology of chemokines and their receptors. This progress has seen an increase in the association between chemokine receptors and certain human disease states. The discovery that chemokine receptors are expressed on nonhemopoietic cell types, such as endothelial and epithelial cells, will almost certainly lead to the receptors being implicated in other biologic and disease processes, such as angiogenesis, organ development, metastasis, and tumorigenesis. Future advances in chemokine receptor biology are certain to follow.
Supported by the Children's Appeal, Sheffield Children's Hospital, Sheffield, United Kingdom.
Reprints:Craig Murdoch, Division of Child Health, University of Sheffield, Sheffield Children's Hospital, Sheffield, S10 2TH, United Kingdom; e-mail: c.murdoch@sheffield.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal