Abstract
Rituximab, a chimeric antibody that targets CD20+ B cells, produces a 48% response rate in patients with refractory low-grade non-Hodgkin lymphoma. In this phase II trial, patients with low-grade non-Hodgkin lymphoma who had previously received no systemic therapy were treated with rituximab, 375 mg/m2, administered by IV infusion for 4 consecutive weeks. Patients with objective response or stable disease received repeat 4-week courses of rituximab at 6-month intervals. At the time of initial reevaluation at 6 weeks, 21 of 39 patients (54%) had objective response to treatment, and an additional 14 patients (36%) had stable disease or minor response. Response rates were similar in patients with follicular and small lymphocytic (CLL-type) lymphoma (52% versus 57%, respectively). At present, follow-up is short and only 13 patients have undergone a second course of rituximab treatment. However, 4 additional responses were documented either prior to the second course of rituximab (2 patients) or following the second course (2 patients) and 4 patients improved from partial to complete response. The current response rate is 64%, with 6 complete responses (15%). Treatment with rituximab was well tolerated, with only 1 patient experiencing grade 3/4 infusion-related toxicity. Rituximab is well tolerated and highly active in patients with low-grade non-Hodgkin lymphoma previously untreated with systemic therapy. Although further follow-up is required, the demonstration of minimal toxicity and considerable activity of this new biologic agent represents an important beginning of more specific, less toxic treatment for this important group of cancer patients.
The treatment of low-grade non-Hodgkin lymphoma has changed very little during the last 20 years. Although these tumors are responsive to a number of standard chemotherapeutic agents, the large majority of patients remain incurable. Patients with low-grade non-Hodgkin lymphoma usually receive a series of treatments, beginning with low-dose, relatively well-tolerated oral agents. However, remissions become shorter with each subsequent treatment, and treatments often become increasingly toxic. Treatment with intensive, combination chemotherapy results in a higher complete response rate; however, any impact of this intensive approach on long-term survival has been difficult to demonstrate.1 2
In 1982, Miller et al reported the first successful treatment of a nodular B-cell lymphoma with a monoclonal antibody raised against the unique surface immunoglobulin produced by this clonal B-cell proliferation.3 This “anti-idiotype” antibody produced a long-term remission in a patient refractory to chemotherapy and demonstrated the promise of tumor-specific therapy. Lymphomas have provided the largest number of potential targets for monoclonal antibody therapy; in addition to the unique idiotype of each B-cell lymphoma clone, a number of surface protein-specific various lymphocyte subpopulations have been recognized. However, in the 17 years since the first demonstration of activity, a variety of problems have prevented targeted therapy from becoming a reality. These have included difficulties in identifying a target antigen with ubiquitous expression on tumor cells, problems with delivery of the monoclonal antibody to the target and subsequent tumor cell destruction, and toxicity from the monoclonal antibody itself.
Rituximab is a chimeric monoclonal antibody directed against the CD20 antigen, expressed on the surface of most B-cell lymphomas. Although this antigen is also expressed by normal B cells, it is not found on other normal cell types; therefore, the rituximab monoclonal antibody provides targeted treatment but is not entirely tumor specific. After attaching to the cell surface, the antibody activates the cell-mediated and complement-mediated cytotoxicity mechanisms, resulting in cell death.
Because the CD20 antigen is expressed by a variety of B-cell non-Hodgkin lymphomas, rituximab has a number of potential therapeutic applications. Initial trials focused on previously treated patients with low-grade non-Hodgkin lymphoma. In a group of patients who had previously received one or more chemotherapy regimens, treatment with 4 doses of this antibody, administered weekly, produced a response rate of 45% to 50% and a median response duration of 12 months.4,5 Treatment-related toxicity was mild and limited primarily to infusion-related events. Although data are limited, responding patients can be retreated with rituximab at relapse and have a second response rate of 40% without increased toxicity.6
The favorable toxicity profile, short treatment duration, and high response rate in refractory patients suggest that first-line treatment with rituximab may provide an excellent treatment option in patients with low-grade non-Hodgkin lymphoma. Periodic retreatment, or “maintenance” therapy at scheduled intervals may be effective in prolonging remissions and avoiding the necessity for treatment with cytotoxic agents. In this phase II trial, we report the preliminary results of treatment with rituximab in a group of patients with low-grade non-Hodgkin lymphoma previously untreated with chemotherapy. As part of this ongoing trial, we are also evaluating the duration of remission with maintenance courses of rituximab administered every 6 months for a total of 2 years. In this preliminary report, we detail the initial response rate and toxicity of rituximab treatment in previously untreated patients with low-grade non-Hodgkin lymphoma. Because follow-up and maintenance therapy are continuing in most patients, the final assessment of the efficacy of this treatment approach awaits further follow-up.
Patients and methods
Accrual to this phase II trial was initiated in March 1998 by participating sites in the Minnie Pearl Cancer Research Network (see Acknowledgments). All patients were required to have biopsy-proven low-grade B-cell non-Hodgkin lymphoma. The following histologic subtypes, as defined by the Revised European-American Lymphoma classification, were eligible: follicular small-cleaved cell; follicular mixed small-cleaved and large cell; plasmacytoid; and small lymphocytic (chronic lymphocytic leukemia [CLL] type). Patients with stages II, III, or IV disease at diagnosis were eligible, as were patients with early stage disease (stage I, II) who had relapsed after previous radiation therapy. No previous chemotherapy or monoclonal antibody therapy was permitted. Additional eligibility criteria included measurable or evaluable disease; ECOG performance status 0, 1, or 2; age over 18 years; white blood cell (WBC) count 3000/μL or higher and platelets 100 000/μL or more; and adequate liver and kidney function. Patients who had severe lymphoma-related symptoms requiring a rapid response to therapy (eg, bowel obstruction, chylous ascites, respiratory compromise due to large effusions or airway obstruction) were not eligible; standard chemotherapeutic approaches were recommended for this patient subset. Patients with central nervous system involvement (brain or meningeal) were ineligible. All patients were required to provide written informed consent before entering the study. This study was approved by the Institutional Review Board at Centennial Medical Center and by the review boards at participating sites.
Before beginning therapy, all patients underwent staging procedures including history, physical examination, complete blood counts, chemistry profile, computerized tomography of the chest and abdomen, and bone marrow aspiration/biopsy. All patients were treated with weekly doses of rituximab, 375 mg/m2, administered IV for 4 consecutive weeks. Rituximab was mixed with normal saline at a final concentration of no more than 1 to 4 mg/mL. The first dose of rituximab was infused at an initial rate of 50 mg/h. If no hypersensitivity or infusion-related events occurred, the infusion rate was increased by 50-mg/h increments every 30 minutes, to a maximum of 400 mg/h. Subsequent rituximab infusions were administered at an initial rate of 100 mg/h and escalated by 100-mg/h increments at 30-minute intervals to a maximum of 400 mg/h. Thirty minutes before each dose of rituximab, patients received premedication with oral acetaminophen 650 mg and diphenhydramine 50 mg. Patients who experienced any treatment-related nausea or vomiting with the first treatment received subsequent premedication with a serotonin receptor antagonist. Administration of dexamethasone was avoided. Serum levels of rituximab were not measured as part of this study.
Although myelosuppression was not an expected treatment-related toxicity, parameters for dose modification were established as follows: WBC 2000/μL or more and platelets 75 000/μL or higher, full dose administered; WBC less than 2000/μL or platelets 75 000/μL, dose withheld, blood counts repeated after 1 week, and full dose resumed if WBC was 2000/μL or higher and platelets 75 000/μL or higher.
Infusion-related events were expected to be the most common toxicity in this trial. If serious events occurred (ie, hypotension, angioedema, bronchospasm), infusion of rituximab was stopped, and then restarted at very low rates. The rate of infusion was subsequently escalated as previously described, until a maximum tolerated infusion rate was reached. Treatment of infusion-related symptoms was at the discretion of the treating physician, but could include additional diphenhydramine, 25 to 50 mg IV, bronchodilators, or IV normal saline. Meperidine, 25 to 50 mg IV, was given for moderate to severe chills or rigors. If severe hypersensitivity occurred even after maximum premedication and slowing of the infusion rate, rituximab infusion was terminated and the patient was removed from study.
If any other grade 3 or 4 nonhematologic toxicity occurred, rituximab was held for 1 week or until the toxicity had decreased to grade 2 or less. Rituximab was then reinstituted at full dose. If nonhematologic toxicity had not resolved after 2 weeks, therapy was discontinued and the patient removed from study. All courses of rituximab consisted of 4 doses, whether or not any treatment delays were necessary.
Patients were evaluated for response to treatment 2 weeks after completing the 4-week course of rituximab. Restaging included a repeat of all previously abnormal staging tests. Patients with progressive disease were removed from the study and considered treatment failures. Patients with objective response or stable disease remained in the study. Courses of rituximab were repeated in these patients at 6-month intervals for a maximum of 4 courses (2 years of therapy), as long as disease progression did not occur. Prior to each maintenance course of rituximab, restaging was performed to document that remission was continuing. The dose and schedule of rituximab used in the maintenance courses were identical to the initial course.
Patients who had an initial objective response to rituximab but showed evidence of progressive disease prior to the 6-month interval were eligible to receive the next course of rituximab prior to the scheduled 6-month interval. This decision was made by the treating physician, based on the patient's initial response and symptomatic benefit from treatment. The intervals between courses of rituximab could never be less than 4 months; all patients progressing earlier than 4 months were removed from trial.
Following completion of the initial course of rituximab, all patients were assigned a response category using standard definitions of response. Complete response required the total disappearance of clinically and radiologically detectable disease for at least 4 weeks. Partial response required at least 50% reduction of all measurable lesions, as measured by the product of the perpendicular diameters of the greatest dimensions of tumor size, with no new lesions appearing. For patients with evaluable disease, partial response required objective improvement in evaluable lesions, with accompanying symptomatic improvement. Patients had stable disease if tumor size reduced by less than 50% or increased by less than 25%, with no new lesions appearing. For patients with evaluable disease, stable disease required no change in the evaluable lesions, with no new lesions appearing, and no worsening of objective symptoms. Progressive disease was defined as an increase of more than 25% in any measurable or evaluable lesion or the appearance of any new lesion.
All patients who completed 4 weeks of therapy were considered evaluable for response. Patients who received at least 1 dose of rituximab were included in the analysis of toxicity. Time to progression was calculated from the first day of treatment until the time progressive disease was documented.
Between March 1998 and February 1999, 41 patients entered this trial and are evaluable for response to initial treatment. Patient characteristics are summarized in Table 1. The median age of the patients was 65 years, typical of the population of patients with low-grade non-Hodgkin lymphoma. Most patients (71%) had stage III or IV disease, and 11 patients (27%) had systemic symptoms. Distribution of the various low-grade histologic subtypes was also typical of the population of low-grade lymphoma patients; 26 patients (64%) had follicular lymphoma. Four patients (2 with stage I and 2 with stage II lymphoma at diagnosis) had relapsed after previous radiation therapy. Thirty-seven patients (90%) had received no previous treatment. Eighteen patients (44%) received treatment at the Sarah Cannon Cancer Center, and the remaining 56% were treated at other sites in the Minnie Pearl Cancer Research Network.
Patient characteristics (n = 41)
| Characteristic . | Number of Patients (%) . |
|---|---|
| Median, age, y (range) | 65 (43-89) |
| Gender | |
| Male | 19 (46%) |
| Female | 22 (54%) |
| ECOG Performance Status | |
| 0 | 26 (63%) |
| 1 | 13 (32%) |
| 2 | 2 (5%) |
| Histology | |
| Follicular, small cleaved | 13 (32%) |
| Follicular, mixed small/large cleaved | 13 (32%) |
| Small lymphoid (CLL type) | 15 (36%) |
| Stage at diagnosis | |
| I | 2 (5%) |
| II | 10 (24%) |
| III | 11 (27%) |
| IV | 18 (44%) |
| B symptoms | 11 (27%) |
| Previous radiation therapy | 4 (10%) |
| Characteristic . | Number of Patients (%) . |
|---|---|
| Median, age, y (range) | 65 (43-89) |
| Gender | |
| Male | 19 (46%) |
| Female | 22 (54%) |
| ECOG Performance Status | |
| 0 | 26 (63%) |
| 1 | 13 (32%) |
| 2 | 2 (5%) |
| Histology | |
| Follicular, small cleaved | 13 (32%) |
| Follicular, mixed small/large cleaved | 13 (32%) |
| Small lymphoid (CLL type) | 15 (36%) |
| Stage at diagnosis | |
| I | 2 (5%) |
| II | 10 (24%) |
| III | 11 (27%) |
| IV | 18 (44%) |
| B symptoms | 11 (27%) |
| Previous radiation therapy | 4 (10%) |
Results
Thirty-nine of 41 patients (95%) completed the first 4-week course of rituximab and were evaluable for response. One patient developed flushing, dyspnea, and chest pain radiating to the jaw shortly after the first dose of rituximab was initiated. An electrocardiogram (ECG) revealed ischemic changes; the patient was admitted to the hospital for observation, and all symptoms resolved completely. This patient was removed from study and not rechallenged with rituximab. A second patient, an 89-year-old man, was put in a nursing home by his family after completing the first course (4 weeks) of rituximab and could not continue follow-up.
Thirty-nine patients were evaluated for response 2 weeks after completing the first course of rituximab. Twenty-one of 39 patients (54%) had objective responses with 2 complete responses (5%) and 19 partial responses (49%). Fourteen additional patients (36%) had minor response or stable disease and continued treatment with rituximab per protocol. Only 4 patients (10%) had progression during the first course of treatment; these patients were removed from study and received treatment with standard chemotherapy. Initial response rates were similar in the various histologic subtypes; 13 of 25 patients (52%) with follicular lymphoma responded, and 8 of 14 patients (57%) with a small lymphocytic (CLL type) lymphoma responded.
Because some delayed responses were expected to occur after the first reevaluation at 6 weeks, all patients with initial objective response or stable disease were reevaluated at 6 months, prior to the second scheduled course of rituximab. Of the 35 patients who remained in the study after the first reevaluation, 2 patients (1 stable, 1 partial responder) progressed during the first 6 months and were removed from study. An additional patient, who had stable disease as best response, progressed after the second course of rituximab. Restaging at 6 months (prior to the second scheduled course of rituximab) resulted in reclassification of response category in 5 patients; 2 additional patients achieved partial remission, and 3 patients converted from partial to complete response. Three patients had further decrease in tumor size during this interval, but remained in partial response.
Thirteen patients have completed a second 4-week course of rituximab. Following the second rituximab course, 3 patients had improvement in their response category (stable to partial response, 2 patients; partial to complete response, 1 patient).
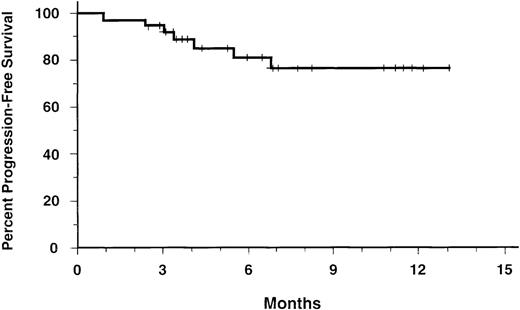
When the additional responses after initial (6-week) reevaluation are included, 25 of 39 patients (64%) achieved a response, with 6 complete responses (15%). After a median follow-up of 8 months, 32 of 39 patients are free of disease progression; actuarial 1-year progression-free survival is 77% (Figure1).
Progression-free survival for 39 evaluable patients.
The actuarial 12-month progression-free survival is 77%.
Progression-free survival for 39 evaluable patients.
The actuarial 12-month progression-free survival is 77%.
Toxicity encountered during the first course of rituximab therapy is summarized in Table 2. Most of the toxicity related to rituximab was infusion-related and of brief duration. One 67-year-old woman with follicullar small cleaved-cell lymphoma developed severe flushing, dyspnea, chest pain, and ischemic ECG changes during the first infusion, and was removed from study. This patient had stage IV lymphoma with bone marrow involvement, but had a normal leukocyte count and no bulky lymph nodes. No other grade 3 or 4 toxicity was observed, and all other patients completed the first treatment course without dose delays or reductions. Grade 1 and 2 infusion-related toxicities were the only common side effects: fever (22%), chills/rigors (29%), flushing (10%), and nausea/vomiting (12%). The incidence of grade 1 and 2 infusion-related toxicities was highest with the first infusion of rituximab: week 1, 37%; week 2, 2%; week 3, 0%; week 4, 0%. Three of 12 patients over 70 years of age (25%) had grade 1/2 infusion-related toxicity, versus 14 of 29 patients (48%) who were less than 70 years old. Mild fatigue was reported by 11 patients (27%). There have been no hospitalizations for treatment of infections, no recognized opportunistic infections, and no fevers of undetermined etiology. Four patients had circulating malignant lymphocyte counts of more than 50 000/μL, and 4 patients had counts of 10 000 to 50 000/μL when they began treatment. None of these patients had grade 3 infusion-related toxicity, and 4 of 8 (50%) had grade 1/2 toxicity.
Treatment-related toxicity during the initial course of therapy (41 patients, 161 rituximab doses)
| Toxicity . | Number of Patients (%) . | |
|---|---|---|
| Grade 1/2 . | 3/4 . | |
| Infusion-related | ||
| Fever | 9 (22%) | 0 |
| Chills/rigors | 12 (29%) | 0 |
| Flushing | 4 (10%) | 1 (2%) |
| Nausea/vomiting | 5 (12%) | 0 |
| Hypotension | 1 (2%) | 0 |
| Angioedema | 1 (2%) | 0 |
| Bronchospasm | 1 (2%) | 0 |
| Chest pain/cardiac ischemia | 0 | 1 (2%) |
| Other toxicity | ||
| Fatigue | 11 (27%) | 0 |
| Leukopenia | 2 (5%) | 0 |
| Anemia | 3 (7%) | 0 |
| Toxicity . | Number of Patients (%) . | |
|---|---|---|
| Grade 1/2 . | 3/4 . | |
| Infusion-related | ||
| Fever | 9 (22%) | 0 |
| Chills/rigors | 12 (29%) | 0 |
| Flushing | 4 (10%) | 1 (2%) |
| Nausea/vomiting | 5 (12%) | 0 |
| Hypotension | 1 (2%) | 0 |
| Angioedema | 1 (2%) | 0 |
| Bronchospasm | 1 (2%) | 0 |
| Chest pain/cardiac ischemia | 0 | 1 (2%) |
| Other toxicity | ||
| Fatigue | 11 (27%) | 0 |
| Leukopenia | 2 (5%) | 0 |
| Anemia | 3 (7%) | 0 |
The second course of rituximab therapy was tolerated well in all 13 patients, with no new or unusual toxicities recognized. Grade 1 and 2 infusion-related events during the first rituximab dose of the second course occurred in 3 of 13 patients (23%). At the time the second rituximab course was initiated, only 1 of 13 patients had an elevated circulating lymphocyte count (13 500/μL). There were no grade 3/4 toxicities during the second treatment course.
Discussion
The recent introduction of rituximab into clinical practice was based on its demonstrated efficacy in the treatment of refractory low-grade non-Hodgkin lymphoma. In this role, rituximab has provided an additional treatment option, producing a response rate of 48% in patients previously treated with standard chemotherapy.5However, the optimal role of rituximab in the treatment of non-Hodgkin lymphoma remains undefined. In the initial trials, the dose and schedule of rituximab administration were chosen empirically, and very little information with alternate doses or different schedules of administration exists. The efficacy of rituximab in the initial treatment of low-grade non-Hodgkin lymphoma has not been defined. Additionally, the relative activity in various low-grade lymphoma subtypes was not well defined, although, the small lymphocytic (CLL) subtype responded less well in the refractory disease trials.5 The use of rituximab in other types of CD20-expressing lymphomas (eg, large-cell lymphoma) is also incompletely defined, although responses have been observed in refractory patients.7
The trial reported here was designed to address several of the unresolved issues regarding the use of rituximab in low-grade non-Hodgkin lymphoma. Initial treatment with rituximab, rather than chemotherapy, has not been previously investigated. However, the use of a highly effective, short duration treatment with low toxicity would certainly be attractive to patients, when compared to a 4- to 6-month course of induction chemotherapy. The possibility of a higher response rate in previously untreated patients is suggested by subset analysis of previous trials with rituximab in previously treated patients. Patients who had received only 1 previous chemotherapy regimen had a response rate of 57%, versus a response rate of 38% in patients who had received 3 previous treatment regimens.5 In addition to assessing response rates in previously untreated patients, this trial evaluates the feasibility of administering periodic maintenance courses of rituximab to prolong remission duration. Maintenance therapy has not been previously investigated, although retreatment at the time of progression in refractory patients previously responding to rituximab produced a second remission rate of 40%.6 We selected a 6-month interval for the time between maintenance courses of rituximab, based on the continued detection of antibody for up to 6 months following a 4-week initial treatment, as well as the median response duration of 12 months observed in previously treated patients.
The preliminary results of this trial document a high response rate to a 4-week course of rituximab in patients previously untreated for low-grade non-Hodgkin lymphoma. When assessed at 6 weeks, the overall objective response rate was 54%, with an additional 36% of patients having stable disease or minor response, and only 10% experiencing disease progression. Continued shrinkage of measurable lymphoma occurred in several patients between the first reevaluation at 6 weeks and the reevaluation at 6 months, and improvement in response also occurred following the second course of rituximab. When the additional responses are included, overall response rate has already improved from 54% to 64%, and complete response rate has risen from 5% to 15%. As additional patients are reevaluated, and receive maintenance courses of rituximab, it seems likely that additional responses will be documented.
Preliminary results of a similar trial have recently been reported by Solal-Celigny et al.8 Fifty patients with previously untreated low-grade follicular non-Hodgkin lymphoma received a standard 4-week course of rituximab. Patients were required to have low tumor burden (no tumor mass > 7 cm, no B symptoms, no organ compression or effusion, normal lactic dehydrogenase), and 92% had World Health Organization performance status 0. Results of treatment were similar to our results. Initial response rate (assessed at day 50) was 65%; with additional follow-up, 3 patients with stable disease improved to partial response, for a final response rate of 71%. Treatment was well tolerated, with no grade 3 or 4 toxicity. Response duration is not yet available.
As anticipated, patients with objective response to the initial course of rituximab rarely experienced disease progression prior to the scheduled 6-month retreatment. Early progression occurred in only 1 partial responder, and in 2 of 14 patients with stable disease at first reevaluation. The initial course of treatment was well tolerated; 40 of 41 patients received the entire scheduled treatment, with only 1 patient being withdrawn from treatment due to severe hypersensitivity symptoms.
In this trial, the initial response rates were similar in all subtypes of low-grade non-Hodgkin lymphoma. The achievement of major responses in 8 of 14 evaluable patients (57%) with small lymphocytic (CLL type) lymphoma is of particular interest. In previously treated patients with this histologic subtype, rituximab produced a response rate of only 13%.5 Low-density expression of surface CD20 antigen has been postulated as the cause of lower response rates in these patients. Further evaluation of rituximab in previously untreated patients with small lymphocytic lymphoma, and with classic CLL, is indicated to confirm these preliminary observations.
Although the high initial response rate in previously untreated patients is noteworthy, several important questions regarding this treatment approach remain unanswered by these preliminary data. Due to the short follow-up, the current report allows only a preliminary assessment of the efficacy of maintenance therapy. Already, the improved response to therapy in some patients has been noted, but the frequency of such additional response remains undetermined. Most importantly, the median duration of response remains unknown, and in large part will determine the desirability of this new treatment approach. The efficacy of subsequent chemotherapy in patients progressing after initial rituximab is also unknown. Although there is no strong rationale to suspect decreased efficacy, further experience is necessary to definitively address this question.
The high initial response rate to rituximab suggests a number of new possibilities for the treatment of low-grade non-Hodgkin lymphoma. In addition to providing an excellent treatment option for patients who may have difficulty tolerating chemotherapy, the high single-agent response rate suggests that addition of rituximab to standard chemotherapy may be effective in prolonging remission duration or survival in these patients. Although limited data exist, the concurrent administration of CHOP chemotherapy and rituximab has been proved feasible and highly active in previously untreated patients with low-grade non-Hodgkin lymphoma. In a phase II study recently reported by Czuczman et al, 38 of 40 patients (95%) obtained objective response after combination therapy, and 55% of patients had complete response.9 In this trial, cytogenetics were assessed in a small cohort of patients. Seven patients with clinical complete response also had clearance of bc1-2 translocations detected by polymerase chain reaction examination of the bone marrow. Although no definitive conclusions can be made from these small numbers of patients, further evaluation of concurrent chemotherapy and rituximab, with particular attention to cytogenetics, is of great importance.
The optimal role of rituximab in the treatment of low-grade non-Hodgkin lymphoma continues to be defined. In addition to treatment of refractory disease, rituximab produces a high response rate with minimal toxicity in patients previously untreated with systemic therapy. In some patients who are elderly or who have poor performance status, initial treatment with rituximab may provide an attractive treatment option. The efficacy of initial treatment with rituximab as compared to standard chemotherapy, as well as the value of maintenance courses of rituximab, await clarification by further clinical trials, as well as additional follow-up of patients in this trial. However, additional indications for the use of rituximab are likely to be defined in the near future.
The use of a relatively specific target-directed biologic therapy represents an important beginning for mechanistic-based therapy for patients with neoplasms. The demonstration of substantial antitumor effects with minimal toxicity in previously untreated patients represents an exciting clinical success derived from a rational target-based approach. These preliminary results provide encouragement for further development of targeted biologic agents for patients with various advanced cancers.
Acknowledgments
Minnie Pearl Cancer Research Network Participating Sites include: Tennessee Oncology, PLLC, Nashville, TN; Consultants in Blood Disorders and Cancer, Louisville, KY; Graves-Gilbert Clinic, Bowling Green, KY; Florida Oncology Associates, Orange Park, FL; Louisiana Oncology Associates, Lafayette, LA; The Medical Oncology Group, Gulfport, MS; Upstate Carolina CCOP, Spartanburg, SC; King's Daughters' Medical Center, Ashland, KY; Greenview Hospital, Bowling Green, KY; McLeod Cancer and Blood Center, Johnson City, TN; University Oncology Associates, Chattanooga, TN; The Hematology and Oncology Clinic, Hattiesburg, MS; Northeast Alabama Regional Medical Center, Anniston, AL; Atlanta Cancer Care, Atlanta, GA; Oncology and Hematology of Southwest Virginia, Roanoke, VA; Oncology Hematology of South Florida, Miami, FL; Winter Park Memorial Hospital, Winter Park, FL; Hematology Oncology Services, New Orleans, LA; and Medical Oncology, LLC Baton Rouge, LA.
Supported in part by grants from Genentech Inc and the Minnie Pearl Foundation.
Reprints:John D. Hainsworth, Sarah Cannon Cancer Center, 250 25th Avenue, North, Suite 412, Nashville, TN 37203.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal