We have used real-time video microscopy to study the mechanisms of platelet adhesion to type I collagen fibrils of distinct structure exposed to flowing blood. Electron microscopy analysis by surface replication demonstrated morphological differences between acid-insoluble fibrils, displaying a regularly repeating striated pattern (banded collagen), and acid-soluble fibrils generated by pepsin treatment of insoluble collagen, smaller in size with a helical configuration (nonbanded collagen). These structural differences proved to be related to the role of platelet integrin 2β1 in stabilizing adhesion to collagen under a variety of flow conditions. Blocking 2β1 function with a monoclonal antibody had no effect on platelet adhesion to insoluble type I collagen coated at high density on a glass surface, whereas there was an absolute dependence of 2β1 function for the initial permanent arrest of platelets and subsequent thrombus formation on pepsin-solubilized type I collagen under the same conditions. In contrast, reconstituted, banded fibrils prepared from pepsin-solubilized type I collagen supported platelet adhesion and thrombus development even when platelet 2β1 function was blocked, a process that was greatly accelerated by pre-exposure of this substrate to autologous plasma under flow. These results implicate a collagen receptor(s) on platelets other than 2β1 that can selectively engage domains in banded, but not nonbanded type I collagen when 2β1 function is blocked. In addition, collagen structure may regulate the extent and affinity of the binding under flow of plasma components such as von Willebrand factor and/or other IIbβ3 ligands.
PLATELET ADHESION TO exposed components of the subendothelium at sites of vascular injury is an essential step in hemostatic and thrombotic processes. Collagen has been recognized as a key thrombogenic component of the vessel wall, in particular types I, III, and VI.1-3 Several collagen-binding proteins are expressed on the platelet surface that may mediate collagen-induced platelet activation and/or platelet adhesion under flow, including the integrin α2β1,4-6 glycoprotein (GP) VI,7,8 GP IV (GP IIIa, CD-36),9-11 and a nonintegrin 65-kD protein specific for type I collagen.12Although multiple putative collagen receptors exist on platelets, the relative contribution of each in collagen-induced platelet activation and adhesion under flow is ill-defined. One reason for this is that the effects of distinct collagen fibril morphologies on platelet reactivity are poorly understood. For example, numerous studies of platelet adhesion under both stationary and flow conditions have used type I collagen solubilized by pepsin, which cleaves collagen in the nontriple helical regions where the covalent cross-links responsible for the typical banded structure and collagen insolubility are found. The triple helix itself is resistant to most proteases except collagenases. Because collagen monomers obtained from pepsin-digested collagen have altered nonhelical extremities,13 they may polymerize to form fibrils with helical configurations distinct from that found in native collagen fibrils.14 Furthermore, the morphology of collagen fibrils in vivo may be heterogeneous. Vascular collagen with a spiraled appearance (composed of an assembly of fibrils in a helical configuration) has been demonstrated by electron microscopic and immunohistochemical studies in both normal and pathological conditions.15 In these studies, spiraled collagen was more abundant in veins as compared with arteries and was particularly noted in the left anterior descending coronary artery beneath a myocardial bridge that had been free from atherosclerosis. Such collagens were also more evident in normal saphenous veins as compared with corresponding phlebosclerotic vessels in diseased patients. Immunohistochemical examination of matrix metalloproteinases (MMP-1, -2, and -3) showed significant expression of MMP-1 in smooth muscle cells of the vascular wall in which spiral collagens were abundant.15 Thus, spiral collagen may be formed preferentially in normal blood vessels through a physiologic degradation of normal collagen fibrils. How such structural modifications impact on the mechanisms of platelet adhesion under flow has not been previously documented. The purpose of this study was to examine the molecular mechanisms of collagen-induced platelet adhesion and thrombus formation in flowing whole blood by measuring in real-time the dynamics of platelet interaction with type I collagen fibrils having distinct morphologies.
MATERIALS AND METHODS
Blood donors.
Blood was collected through a 19-gauge needle from the antecubital vein of healthy adult donors with normal cell count profiles. The syringes contained the anticoagulant D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone dihydrochloride (PPACK; 40 μmol/L final concentration) that inhibited thrombin activation. Blood was supplemented with additional PPACK, where necessary, at approximately 2-hour intervals to ensure thrombin inactivation. All donors claimed to have abstained from taking aspirin, or other drugs known to affect platelet function, in the preceding 10 days.
Preparation of insoluble fibrillar collagen-coated coverslips.
Acid-insoluble fibrillar type I collagen from bovine achilles tendon (Sigma, St Louis, MO) in 0.5 mol/L acetic acid, pH 2.8, at a concentration of 2.5 mg/mL, was prepared as previously described16 and 200 μL was applied evenly over a horizontal glass coverslip (Corning, Inc, Corning, NY; 24 × 50 mm) covering all but the first 10 mm that remained uncoated to facilitate handling. Coated coverslips were then placed in a humid environment at room temperature (22°C to 25°C) for 60 minutes. Excess, unbound collagen was removed by 4 sequential rinses with phosphate-buffered saline (PBS), pH 7.4, and the coverslip was assembled in the flow chamber described below. Uncoated coverslips did not support platelet adhesion under the flow conditions used in these studies, and saturating the surface with bovine serum albumin (coating at 0.1 mg/mL) did not affect initial platelet adhesion or subsequent thrombus formation (unpublished observations).
Preparation of pepsin-solubilized collagen-coated coverslips.
Acid-soluble type I collagen from human placenta (Sigma) was dissolved in 0.1 mol/L acetic acid to give a stock solution at a final concentration of 2.5 mg/mL. The stock solution was diluted with PBS, pH 7.4, to a final concentration of 200 μg/mL for coating on glass coverslips as described above. Under these conditions, the surface was fully saturated with collagen; increasing the coating concentration up to 2.5 mg/mL had no effect on the extent of platelet adhesion or thrombus formation under all flow conditions examined (unpublished observations). These preparations were used in all flow studies except those experiments using a substrate derived from pepsin treatment of achilles tendon-insoluble collagen. In these studies, a suspension of acid-insoluble type I collagen from bovine achilles tendon (Sigma) at a concentration of 2.5 mg/mL was incubated with pepsin (Sigma) at an enzyme to substrate ratio of 1:1,000 (wt/wt), at 4°C for 18 hours. All insoluble material was sedimented by centrifugation at a relative centrifugal force of 100,000g for 3 hours, and the supernatant was removed. Untreated collagen suspension were also sedimented under the same conditions, and the supernatant was removed for control studies.
Preparation of reconstituted collagen fibrils from pepsin-solubilized collagen.
Fibrils of human type I collagen were formed by dialyzing acid-soluble type I collagen (from human placenta; Sigma) dissolved in 0.1 mol/L acetic acid at a concentration of 2.5 mg/mL against 4 changes of 4 L of PBS, pH 7.4, for 96 hours at 4°C. The concentration of reconstituted collagen was essentially unchanged from that of the starting material. This procedure has been used to prepare banded collagen fibers from pepsin-solubilized collagen and is based on the observations that purified solutions of collagen under physiological conditions in vitro assemble spontaneously into typical cross-striated fibrils.17 18 The resulting preparation was applied over a glass coverslip and kept in a humid environment for 60 minutes at 22°C to 24°C. Excess, unbound collagen was removed by rinsing the coverslip with PBS, pH 7.4, which was then assembled in the flow chamber.
Inhibition of platelet receptor function and binding of von Willebrand factor (vWF) to collagen.
The monoclonal antibodies used to inhibit the function of platelet receptors αIIbβ3, α2β1, and GP Ibα were purified from murine ascites using protein A (Sigma) chromatography.19These antibodies have been extensively characterized and their ability to inhibit receptor function has been well-documented. LJ-Ib1 (IgG1) reacts with the amino-terminal 45-kD domain of GP Ibα containing the vWF binding site20-22 and inhibits fully the ligation of platelet GP Ib with immobilized vWF under a variety of flow conditions.23 LJ-CP8 (IgG1) is specific for the integrin αIIbβ3 (GP IIb-IIIa complex) and blocks the activation-dependent binding of soluble ligands to this receptor24,25 as well as platelet aggregation and thrombus formation.25 MR-5 (IgG1) binds to the A3 domain of vWF and prevents the binding of vWF to collagen under a variety of experimental conditions.24,25 Antibody R2-7E4 (IgG1) was obtained from the same fusion as the previously reported antibody R2-8C826 that is specific for the α2 integrin subunit and prevents the adhesion of Chinese hamster ovary cells transfected with α2β1 to type I collagen under stationary conditions. Antibody 12F1 (IgG2a) binds to platelet α2β1, but does not inhibit receptor function.27 All antibodies were used at a final concentration of 100 μg/mL in whole blood. This concentration was shown to produce a maximal specific effect.
Real-time epifluorescence videomicroscopy and flow chamber.
A modification of the Hele-Shaw perfusion chamber, described in detail elsewhere,28,29 was used to study the interaction of platelets in flowing blood with immobilized collagen under a variety of flow conditions. A flow path height of 254 μm was determined by a silicone rubber gasket placed on a collagen-coated coverslip that formed the lower surface of the chamber. The flow chamber was assembled and filled with PBS, pH 7.4. A syringe pump (Harvard Apparatus Inc, Holliston, MA) was used to draw blood through the flow chamber. Flow rates of 1.94, 0.65, and 0.13 mL/min produced initial wall shear rates of 1,500 s−1, 500 s−1, and 100 s−1, respectively, at the inlet of the flow chamber. Measurements of platelet adhesion and thrombus formation were made at a position proximal to the inlet of the chamber, thereby avoiding pre-exposure of flowing platelets to either the substrate or to preformed platelet thrombi. The fluorescent dye mepacrine (quinacrine dihydrochloride; final concentration, 10 μmol/L) was used to label platelets in whole blood. Fluorescently labeled leukocytes were distinguished from platelets by their relatively large size, nuclear morphology, and sparsity; moreover, permanent leukocyte attachment to collagen was negligible at wall shear rates greater than 500 s−1. At a wall shear rate of 100 s−1, the contribution of adherent leukocytes to the total thrombus volume was typically less than 10% (unpublished observations). Erythrocytes were completely opaque to fluorescence detection and visualization due to fluorescence quenching by hemoglobin. Mepacrine concentrates in the dense granules of platelets and has been shown to have no effect on normal platelet function at the concentration used in these studies.30Platelet secretion after adhesion does not prevent their visualization, and mepacrine does not interfere with platelet adhesion.29The perfusion chamber was positioned on the motorized stage of an Axiovert 135M/LSM 410 inverted epifluorescence/laser scan confocal microscope (Carl Zeiss Inc, Oberkochen, Germany). The platelet adhesion process was visualized in real time and recorded on tape with a videocassette recorder (Magnavox; Philips, Eindhoven, The Netherlands).
Measurement of thrombus volumes.
The cumulative volume occupied by thrombi in an area of 102,236 μm2 was calculated from confocal sections, obtained at 1.0-μm intervals in the z axis while blood was flowing using an excitation laser wavelength of 488 nm and a scanning time of 2 seconds per section. The microscope settings, including contrast, brightness, magnification, and pinhole aperture, were maintained at constant values to facilitate comparisons between different experiments. Confocal sections were analyzed using the Metamorph software package (Universal Imaging Corp, West Chester, PA). A threshold was applied to the image stack to distinguish thrombus sections from the background; this value was then used in all subsequent analyses of confocal sections for a given experiment. The area occupied by all thrombi in a given cross-section was calculated and the volume of a 1.0-μm–thick section was estimated by multiplying the area by the height of the section (1.0 μm). The cumulative volume occupied by all thrombi was then estimated as the sum total of the sectional volumes.
Measurement of platelet motion.
Platelets were defined as moving on the surface when they were displaced by a distance exceeding their diameter.29 A series of images from the recorded experiment was digitized at a sampling rate of 6 frames per second using a computer controlled VCR (Sony 9500; Sony Corp, New York, NY) and a frame grabber (Matrox Image LC; Matrox Electronic Systems Ltd, Dorval, Quebec, Canada). A threshold was applied to the images to distinguish platelets from the background, and the images were then binarized. Time was calculated by referring to the frame number. Image analysis was performed using the Metamorph software package (version 2.76; Universal Imaging Corp). The first 2 consecutive frames in the series were then superimposed (by using the logical AND function) so that the resultant image represented only the overlapping areas of the platelet at 2 different times. The new image was then superimposed to the next frame and the process was continued until the overlapping area was equal to 0. When this occurred, a platelet had moved by a distance greater than its diameter; if this did not occur, a platelet was considered firmly attached during the period of observation. The time for individual platelet motion was computed and the process was repeated until all the platelets in the microscopic field of study moved or for a preselected time interval, whichever occurred first.
Measurement of platelet surface coverage.
Single frame images were captured from videotapes at various times after the onset of blood flow and a threshold was applied to distinguish both single platelets and platelet thrombi from the background; this value was then used in all subsequent image analyses of surface area coverage. The area occupied by all platelets and thrombi in an image was measured using the Metamorph software package (version 2.76; Universal Imaging Corp).
Platelet aggregation assay.
Aggregation induced by fibrillar type I collagen was studied in a dual channel aggregometer (Chrono-Log Corp, Havertown, PA) using 3 × 108 platelets per milliliter of autologous plasma containing citrate as anticoagulant. Turbidity changes were recorded after the addition of agonist. The pH of the collagen suspension was adjusted to 7.4 before it was added to the platelet suspension.
Gel electrophoresis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed using standard procedures.31
Electron microscopy analysis by surface replication.
Frozen samples of immobilized collagen were placed in a Balzers freeze fracture machine (BAF-400T; Balzers AG, Liechtenstein) and were shadowed at an angle of 45° with platinum. The resulting replica was supported with a backing of carbon. The replica was subsequently cleaned with bleach, picked up on carbon-coated mesh grids, and examined on a Hitachi HU600 electron microscope (Hitachi Instruments Inc, San Jose, CA). Representative areas of specific interest were documented photographically.
RESULTS
Structural features of native insoluble and pepsin-solubilized type I collagen fibrils.
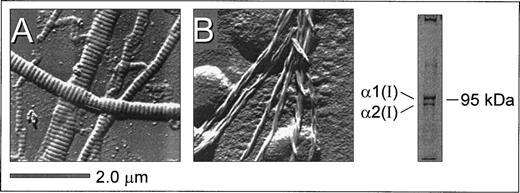
Surface replication images of surface-bound insoluble fibrillar type I collagen (from bovine achilles tendon) and microfibrils derived from pepsin-solubilized type I collagen (from human placenta) showed distinct structural features (Fig 1). A characteristic striated pattern was seen with insoluble collagen due to the regular quarter-staggering of collagen monomers with a 67 nm periodicity,32 yielding regions of alternate maximal electron density regardless of the thickness of the fiber (Fig 1A). In contrast, this banded pattern was noticeably absent from the microfibrils derived from pepsin-solubilized collagen (Fig 1B). Here, collagen monomers polymerize to form thinner microfibrils with a clearly discernible spiraled structure comprising 2 to 3 fibers in a twisted configuration. Gel electrophoresis of pepsin-solubilized type I collagen under denaturing and nonreducing conditions showed the characteristic α1(I) and α2(I) bands (in the predicted ratio of 2:1) as well as high molecular weight multimers that did not enter the gel (Fig 1). The substrates shown in Fig 1 were prepared in an identical manner to those used for evaluating platelet adhesion and thrombus formation under flow described below and are therefore representative of the surfaces presented to flowing blood in these studies. Based on these structural features, the immobilized substrates prepared from insoluble and soluble type I collagen are referred to as banded and nonbanded collagens, respectively, throughout the text. Note also that preparations of nonbanded collagen may contain monomeric collagen in addition to polymerized helical fibrils.
Structural features of native insoluble and plasmin-solubilized type I collagen as a representative example. Native, insoluble fibrillar type I collagen (from bovine achilles tendon) and pepsin-solubilized type I collagen (from human placenta) were coated on glass coverslips as described in Materials and Methods. Surface replication analysis of insoluble type I collagen (A) showed a characteristic banded pattern that was noticeably absent from the smaller fibrils derived from pepsin-solubilized type I collagen (B), where microfibril assemblies displayed a nonbanded spiraled configuration. Polyacrylamide gel electrophoresis under denaturing and nonreducing conditions showed the presence of the 1(I) and 2(I) type I collagen subunits as well as high molecular weight multimers (right).
Structural features of native insoluble and plasmin-solubilized type I collagen as a representative example. Native, insoluble fibrillar type I collagen (from bovine achilles tendon) and pepsin-solubilized type I collagen (from human placenta) were coated on glass coverslips as described in Materials and Methods. Surface replication analysis of insoluble type I collagen (A) showed a characteristic banded pattern that was noticeably absent from the smaller fibrils derived from pepsin-solubilized type I collagen (B), where microfibril assemblies displayed a nonbanded spiraled configuration. Polyacrylamide gel electrophoresis under denaturing and nonreducing conditions showed the presence of the 1(I) and 2(I) type I collagen subunits as well as high molecular weight multimers (right).
Role of platelet integrin α2β1 in platelet adhesion and thrombus formation on type I collagen under flow.
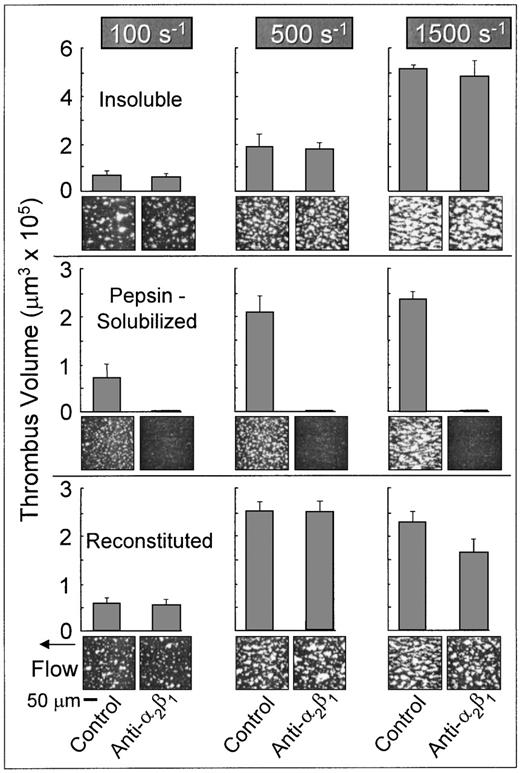
Platelets in flowing whole blood adhered to both banded and nonbanded type I collagens at wall shear rates ranging from 100 s−1 to 1,500 s−1(Fig 2). Banded collagen presented a more thrombogenic surface than nonbanded collagen to flowing control blood at 1,500 s−1, as reflected in the higher total thrombus volumes measured after 3 minutes perfusion, whereas the cumulative thrombus volumes measured with control blood at shear rates of 500 s−1 and 100 s−1 were essentially equivalent for both banded and nonbanded collagens (Fig 2). The concentrations of collagen used to coat the coverslips in these experiments produced maximal platelet adhesion: increasing the coating concentration of nonbanded collagen (up to 2.5 mg/mL) had no significant effect on the extent of platelet adhesion and thrombus development (data not shown). Furthermore, decreasing the coating concentration of banded collagen by up to 10-fold had no measurable effect on thrombus formation at the shear rates indicated.33 Therefore, differences in thrombogenicity seen at 1,500 s−1 with these substrates were not due to differences in the extent of surface saturation with collagen.
Role of platelet integrin 2β1 in platelet adhesion and thrombus formation under flow on type I collagens with distinct structural specificities. Blood containing PPACK as anticoagulant and treated with the fluorescent dye mepacrine for platelet visualization was perfused at 37°C over distinct type I collagen preparations, in a parallel plate flow chamber. The flow rate was set to produce a wall shear rate of 1,500 s−1, 500 s−1, or 100 s−1 at the inlet of the flow chamber and each experiment was recorded on tape using a videomicroscopy system. The figure shows single frame images of the surface, each corresponding to an area of 65,536 μm2, obtained after 3 minutes of perfusion. Blood was either untreated (control) or treated with a monoclonal antibody selectively inhibiting the platelet integrin 2β1 function. Note that functional inhibition of platelet 2β1 completely abolished stable platelet attachment and thrombus formation on pepsin-solubilized (nonbanded), but not on native insoluble collagen (banded) or collagen reconstituted from the same pepsin-solubilized collagen (from human placenta) used in these studies. These images are representative of the results obtained in at least 6 separate experiments with blood from different donors. After 3 minutes of perfusion, the total volume of platelet thrombi present in an area of 102,236 μm2 was measured by confocal sectioning at 1.0-μm intervals, as described in Materials and Methods. Volume measurements represent the mean ± standard error of the mean of 4 separate experiments with different blood donors. The size of single platelets can be appreciated in the panel showing the results obtained in the presence of the anti-2β1 antibody with pepsin-solubilized collagen or in Figs 3 and 7, in which platelet to platelet interactions have been prevented by blocking IIbβ3 function.
Role of platelet integrin 2β1 in platelet adhesion and thrombus formation under flow on type I collagens with distinct structural specificities. Blood containing PPACK as anticoagulant and treated with the fluorescent dye mepacrine for platelet visualization was perfused at 37°C over distinct type I collagen preparations, in a parallel plate flow chamber. The flow rate was set to produce a wall shear rate of 1,500 s−1, 500 s−1, or 100 s−1 at the inlet of the flow chamber and each experiment was recorded on tape using a videomicroscopy system. The figure shows single frame images of the surface, each corresponding to an area of 65,536 μm2, obtained after 3 minutes of perfusion. Blood was either untreated (control) or treated with a monoclonal antibody selectively inhibiting the platelet integrin 2β1 function. Note that functional inhibition of platelet 2β1 completely abolished stable platelet attachment and thrombus formation on pepsin-solubilized (nonbanded), but not on native insoluble collagen (banded) or collagen reconstituted from the same pepsin-solubilized collagen (from human placenta) used in these studies. These images are representative of the results obtained in at least 6 separate experiments with blood from different donors. After 3 minutes of perfusion, the total volume of platelet thrombi present in an area of 102,236 μm2 was measured by confocal sectioning at 1.0-μm intervals, as described in Materials and Methods. Volume measurements represent the mean ± standard error of the mean of 4 separate experiments with different blood donors. The size of single platelets can be appreciated in the panel showing the results obtained in the presence of the anti-2β1 antibody with pepsin-solubilized collagen or in Figs 3 and 7, in which platelet to platelet interactions have been prevented by blocking IIbβ3 function.
In marked contrast to banded collagen, platelet adhesion and thrombus development on nonbanded type I collagen required competent α2β1 function, because functional inhibition of this integrin completely abolished stable platelet attachment at all shear rates tested (Fig 2), resulting in a transient surface translocation of platelets mediated by platelet GP Ibα and the A1 domain of collagen-bound vWF absorbed from plasma. A nonfunction blocking anti-α2β1 monoclonal antibody had no effect on the extent of platelet adhesion and thrombus formation with either banded or nonbanded collagen (data not shown). Blocking platelet α2β1 function had no significant effect on the extent of thrombus formation on banded collagen at any shear rate tested (P > .1 for all shear rates; Fig 2). Inhibition of plasma vWF A3 domain binding to collagen, or functional blockade of platelet GP Ibα, completely abolished platelet interaction with both banded and nonbanded collagens at 1,500 s−1, but not at 500 s−1 or 100 s−1 (data not shown), in a manner similar to that previously described.33
Properties of reconstituted type I collagen derived from pepsin-solubilized collagen.
The differential role of platelet α2β1 in platelet adhesion to type I collagen fibrils having distinct structural features was confirmed with reconstituted type I collagen, produced from pepsin-solubilized type I collagen (from human placenta) by extensive dialysis of the latter against phosphate buffer, pH 7.4, at 4°C. These conditions have been shown to produce banded collagen fibers from pepsin-solubilized collagen and are based on the observation that purified solutions of collagen under physiological conditions in vitro assemble spontaneously into typical cross-striated fibrils.17 18 Reconstituted type I collagen prepared in this way supported platelet adhesion and thrombus formation in a manner that did not require competent α2β1function; blocking platelet α2β1 function with a monoclonal antibody resulted in only a slight, albeit significant (P < .05) decrease in the total thrombus volume after 3 minutes of perfusion at 1,500 s−1 compared with controls (Fig 2). Furthermore, at wall shear rates of 100 s−1 and 500 s−1, inhibition of α2β1 function had no significant effect on the extent of thrombus formation compared with control blood (P> .1 at both shear rates; Fig 2). These results contrast sharply those seen with nonbanded type I collagen, in which inhibition of α2β1 function completely abolished stable platelet attachment and thrombus formation at all shear rates. Thus, reconstituted type I collagen showed distinct functional properties compared with the nonbanded type I collagen from which it was derived, demonstrating that structural characteristics of fibril assembly have a profound effect on the mechanisms of platelet interaction with this substrate under flow.
Further confirmation of the differential role of platelet α2β1 in platelet adhesion to different type I collagen preparations was obtained by controlled pepsin-digestion of native, banded type I collagen obtained from achilles tendon. The supernatant obtained after sedimenting pepsin-treated banded type I collagen, when immobilized on glass, supported stable platelet attachment and thrombus formation at shear rates ranging from 100 s−1 to 1,500 s−1 in a manner that required intact α2β1 function; blocking α2β1 function resulted in a continuous turnover of transiently attached platelets with no further accrual of surface associated platelets, even after prolonged perfusion of blood (data not shown). Thus, pepsin-solubilized type I collagen prepared from either achilles tendon or placenta required competent α2β1 function for irreversible platelet attachment and thrombus formation.
Time course of platelet adhesion and thrombus development.
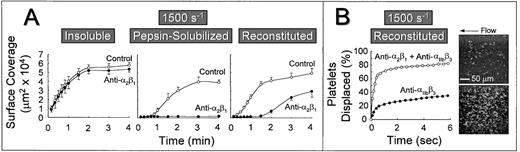
The role of platelet α2β1 was further studied by measuring the extent of platelet surface coverage as a function of time after the onset of blood flow. Platelets adhered most rapidly to banded collagen in a manner that was essentially independent of α2β1 function (Fig 3A). Under these conditions, half maximal surface coverage was achieved after approximately 30 seconds. In contrast, the rate at which the surface became covered with platelets was lower on nonbanded collagen exposed to the same control blood at this shear rate (half maximal surface coverage was achieved after ∼1.5 minutes), and blocking α2β1function resulted in the complete inhibition of stable platelet attachment and subsequent lack of surface accumulation of platelets at any time after the onset of blood flow (Fig 3A). Furthermore, reconstituted collagen presented a surface that differed substantially from both the nonbanded collagen from which it was derived and from native, banded collagen. Blocking platelet α2β1 function with this substrate resulted in a prolonged lag phase before the accrual of firmly attached platelets (Fig 3A), although after 3 minutes of perfusion, there was significant platelet adhesion and thrombus formation, as shown previously (Fig 2). Although the anti-α2β1antibody was clearly effective in blocking α2β1 function, as evidenced by its inhibitory effect at early time points after the onset of blood flow over this substrate, prolonged perfusion of blood resulted in platelet adhesion and thrombus formation in a manner that was independent of α2β1 function (Fig 3A).
Time course of platelet adhesion on type I collagens with distinct structural specificities at a wall shear rate of 1,500 s−1. (A) Control blood (containing no antibody) or blood treated with a function blocking anti-2β1monoclonal antibody was perfused through a parallel plate chamber as described in the legend to Fig 2, and the flow rate was adjusted to produce a wall shear rate of 1,500 s−1 at the inlet of the chamber. Single frame images corresponding to an area of 65,536 μm2 were captured from videotapes at various times after the onset of blood flow and analyzed for the area occupied by surface covered platelets (indicated as surface coverage). Note the differential effects of inhibiting platelet 2β1 function with type I collagens having distinct structural features, particularly the differences seen between reconstituted collagen and pepsin-solubilized collagen (nonbanded) from which it was derived. Surface coverage measurements represent the mean ± standard error of the mean of 4 separate experiments with different blood donors. (B) Blood containing an anti-2β1 monoclonal antibody, either alone or concurrently with an anti-IIbβ3monoclonal antibody, was perfused over reconstituted type I collagen at a wall shear rate of 1,500 s−1. After 3 minutes of perfusion (corresponding to time 0), consecutive images were captured from videotapes and analyzed for platelet movement (for definition, see Materials and Methods). The percentage of platelets displaced from their initial position was calculated as a function of time relative to the total number of platelets attached to the surface in the first image analyzed. Note that approximately 80% of all platelets (indicated as platelets displaced) moved on the surface within 3 seconds of observation when 2β1 and IIbβ3 were inhibited concurrently, compared with greater than 60% of the platelets remaining stationary after selective inhibition of IIbβ3. The 2 single frame images on the right, each representing an area of 65,536 μm2, depict surface coverage after 3 minutes of perfusion with selective IIbβ3 inhibition (lower) or combined IIbβ3 and 2β1 inhibition (upper). Note the differences in surface coverage by platelets, reflecting differences in the stability of platelet attachment.
Time course of platelet adhesion on type I collagens with distinct structural specificities at a wall shear rate of 1,500 s−1. (A) Control blood (containing no antibody) or blood treated with a function blocking anti-2β1monoclonal antibody was perfused through a parallel plate chamber as described in the legend to Fig 2, and the flow rate was adjusted to produce a wall shear rate of 1,500 s−1 at the inlet of the chamber. Single frame images corresponding to an area of 65,536 μm2 were captured from videotapes at various times after the onset of blood flow and analyzed for the area occupied by surface covered platelets (indicated as surface coverage). Note the differential effects of inhibiting platelet 2β1 function with type I collagens having distinct structural features, particularly the differences seen between reconstituted collagen and pepsin-solubilized collagen (nonbanded) from which it was derived. Surface coverage measurements represent the mean ± standard error of the mean of 4 separate experiments with different blood donors. (B) Blood containing an anti-2β1 monoclonal antibody, either alone or concurrently with an anti-IIbβ3monoclonal antibody, was perfused over reconstituted type I collagen at a wall shear rate of 1,500 s−1. After 3 minutes of perfusion (corresponding to time 0), consecutive images were captured from videotapes and analyzed for platelet movement (for definition, see Materials and Methods). The percentage of platelets displaced from their initial position was calculated as a function of time relative to the total number of platelets attached to the surface in the first image analyzed. Note that approximately 80% of all platelets (indicated as platelets displaced) moved on the surface within 3 seconds of observation when 2β1 and IIbβ3 were inhibited concurrently, compared with greater than 60% of the platelets remaining stationary after selective inhibition of IIbβ3. The 2 single frame images on the right, each representing an area of 65,536 μm2, depict surface coverage after 3 minutes of perfusion with selective IIbβ3 inhibition (lower) or combined IIbβ3 and 2β1 inhibition (upper). Note the differences in surface coverage by platelets, reflecting differences in the stability of platelet attachment.
In a previous study using native banded type I collagen,33the efficacy of the anti-α2β1 monoclonal antibody was confirmed by concurrently blocking platelet αIIbβ3 function with a well-characterized anti-αIIbβ3 monoclonal antibody.24 25 Because αIIbβ3function is required for platelet aggregate and thrombus formation, its selective inhibition allowed direct evaluation of individual platelet surface interactions. In the present study, we have used a similar strategy to demonstrate the inhibitory efficacy of the anti-α2β1 antibody using reconstituted type I collagen. Platelets adhered to a surface coated with this substrate in a predominantly stable manner after 3 minutes at a wall shear rate of 1,500 s−1, when αIIbβ3function alone was inhibited (Fig 3B); only 30% to 35% of attached platelets were displaced on the surface by a distance greater than their respective diameters over a period of 6 seconds. In contrast, when platelet α2β1 and αIIbβ3 function were blocked concurrently, a predominantly transient platelet attachment was seen, with approximately 85% of platelets displaced over the same time period (Fig 3B). A control, nonfunction blocking anti-α2β1 monoclonal antibody had no effect on the stability of platelet attachment when αIIbβ3 function was blocked (data not shown). These differences in the stability of platelet attachment were reflected in the number of surface-attached platelets at 3 minutes after the onset of blood flow, as shown visually in Fig 3B. Thus, when only αIIbβ3 function was blocked, the majority of platelets were irreversibly adherent through bonds involving the ligation of α2β1 with domains in collagen (after the initial tethering of platelets, mediated by platelet GP Ibα engagement of collagen-bound vWF, derived from plasma, as described below). These results confirm that the anti-α2β1 monoclonal antibody used in these studies was of sufficient inhibitory efficacy to prevent ligation of α2β1 with domains in reconstituted collagen, despite its inability to prevent stable platelet attachment and thrombus development on this substrate when αIIbβ3 function was not concurrently blocked, as shown in Figs 2, 3, and 4.
Influence of collagen-bound plasma components on the rate of platelet accrual under flow.
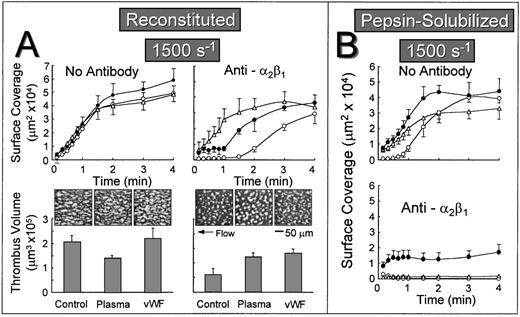
The lag phase before the onset of stable platelet attachment to reconstituted collagen when α2β1 function was inhibited may reflect the time-dependent binding of plasma components to collagen, including vWF or other αIIbβ3 ligands such as fibronectin, which can then mediate stable platelet attachment and thrombus development in a manner that does not require the participation of α2β1. To test this hypothesis, autologous plasma was perfused over reconstituted collagen at a wall shear rate of 1,500 s−1 for 5 minutes before perfusing with either control whole blood or blood containing the anti-α2β1 antibody. When α2β1 function was not blocked, the rate of surface coverage was similar, regardless of whether the substrate was previously exposed to plasma under flow or to purified vWF under stationary conditions (Fig 4A). In marked contrast, when α2β1 function was inhibited, the initial lag phase was substantially reduced when the substrate was pre-exposed to flowing plasma or precoated with purified vWF under stationary conditions (Fig 4A). In this regard, reconstituted type I collagen differed substantially from the nonbanded collagen from which it was derived, because pre-exposing nonbanded collagen to plasma under flow or to purified vWF did not ameliorate the inhibitory effect of blocking α2β1 (Fig 4B). In fact, the extent of surface coverage when nonbanded collagen was precoated with vWF, and α2β1 function was blocked, was similar to that seen with vWF coated directly onto a glass surface without collagen (data not shown). When α2β1 function was blocked, cumulative thrombus volumes measured after 2.5 minutes of perfusion were significantly higher when reconstituted collagen was pre-exposed to plasma under flow or to purified vWF under stationary conditions (Fig 4A).
Effect of exposing collagen to plasma under flow or vWF before perfusion with whole blood at a wall shear rate of 1,500 s−1. Blood containing no antibody or blood containing an anti-2β1 monoclonal antibody was perfused over reconstituted type I collagen (A) or over pepsin-solubilized (nonbanded) collagen from which it was derived (B) in a parallel plate flow chamber as described in the legend to Fig 2, and the flow rate was adjusted to produce a wall shear rate of 1,500 s−1 at the inlet of the chamber. (A) Upper figures: The time course of platelet adhesion on reconstituted collagen was measured as described in the legend to Fig 3. The collagen substrate was pre-exposed to autologous plasma at a wall shear rate of 1,500 s−1 for 5 minutes (▵) or to purified vWF under stationary conditions for 30 minutes (•) before perfusing with blood. Control blood (○) was perfused directly over reconstituted collagen without prior exposure to plasma or vWF. (A) Lower figures: After 2.5 minutes of perfusion, the total volume of platelet thrombi present in an area of 102,236 μm2 was measured by confocal sectioning at 1.0-μm intervals, as described in Materials and Methods. Measurements represent the mean ± standard error of the mean of 4 separate experiments with different blood donors. The single frame images, each representing an area of 65,536 μm2, depict surface coverage of platelet thrombi after 3 minutes of perfusion under the conditions indicated. (B) The time course of platelet adhesion on pepsin-solubilized (nonbanded) collagen. The collagen substrate was pre-exposed to autologous plasma at a wall shear rate of 1,500 s−1 for 5 minutes (▵) or to purified vWF under stationary conditions for 30 minutes (•) before perfusing with blood. Control blood (○) was perfused directly over pepsin-solubilized (nonbanded) collagen without prior exposure to plasma or vWF. Measurements represent the mean ± standard error of the mean of 4 separate experiments with different blood donors.
Effect of exposing collagen to plasma under flow or vWF before perfusion with whole blood at a wall shear rate of 1,500 s−1. Blood containing no antibody or blood containing an anti-2β1 monoclonal antibody was perfused over reconstituted type I collagen (A) or over pepsin-solubilized (nonbanded) collagen from which it was derived (B) in a parallel plate flow chamber as described in the legend to Fig 2, and the flow rate was adjusted to produce a wall shear rate of 1,500 s−1 at the inlet of the chamber. (A) Upper figures: The time course of platelet adhesion on reconstituted collagen was measured as described in the legend to Fig 3. The collagen substrate was pre-exposed to autologous plasma at a wall shear rate of 1,500 s−1 for 5 minutes (▵) or to purified vWF under stationary conditions for 30 minutes (•) before perfusing with blood. Control blood (○) was perfused directly over reconstituted collagen without prior exposure to plasma or vWF. (A) Lower figures: After 2.5 minutes of perfusion, the total volume of platelet thrombi present in an area of 102,236 μm2 was measured by confocal sectioning at 1.0-μm intervals, as described in Materials and Methods. Measurements represent the mean ± standard error of the mean of 4 separate experiments with different blood donors. The single frame images, each representing an area of 65,536 μm2, depict surface coverage of platelet thrombi after 3 minutes of perfusion under the conditions indicated. (B) The time course of platelet adhesion on pepsin-solubilized (nonbanded) collagen. The collagen substrate was pre-exposed to autologous plasma at a wall shear rate of 1,500 s−1 for 5 minutes (▵) or to purified vWF under stationary conditions for 30 minutes (•) before perfusing with blood. Control blood (○) was perfused directly over pepsin-solubilized (nonbanded) collagen without prior exposure to plasma or vWF. Measurements represent the mean ± standard error of the mean of 4 separate experiments with different blood donors.
Collagen-induced platelet activation and aggregation.
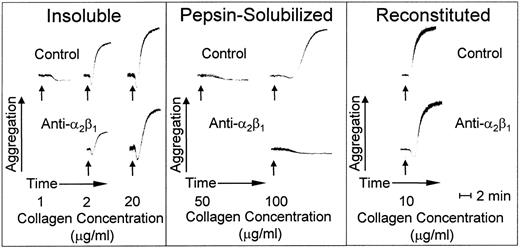
Insoluble banded collagen, when added to platelets in plasma with citrate as anticoagulant, caused irreversible platelet aggregation in a dose-dependent manner; maximal platelet aggregation response was seen at an agonist concentration of 20 μg/mL (Fig 5). In contrast, nonbanded collagen was less efficient at inducing platelet aggregation; a 50-fold increase in concentration compared with banded collagen was required for a full platelet response, and even then, there was a prolonged lag phase before the onset of full aggregation (Fig 5). Blocking platelet α2β1 function had no effect on the lag phase or extent of platelet aggregation with banded collagen at a concentration of 20 μg/mL, whereas, at suboptimal concentrations of this agonist (2.0 μg/mL), there was a partial inhibition of platelet aggregation; increasing the antibody concentration did not produce further inhibition under these conditions (data not shown), indicating the presence of an activating collagen receptor on platelets other than α2β1. In fact, simple collagen-like peptides have been synthesized that are potent platelet agonists whose activity is totally α2β1-independent.34 In contrast, blocking platelet α2β1 function completely inhibited platelet aggregation induced by nonbanded collagen (Fig 5), indicating that this integrin is essential to mediate platelet activation induced by this agonist. However, reconstituted collagen prepared from nonbanded collagen was a potent platelet agonist, promoting platelet activation and aggregation in a manner that was independent of α2β1 function when used at a concentration of 10.0 μg/mL (Fig 5).
Platelet aggregation induced by type I collagen fibrils with distinct morphologies. Aggregation at 37°C is shown as an increase in light transmittance. The arrows indicate addition of agonist at the final concentrations indicated. The pH of all collagen preparations was adjusted to 7.4 immediately before adding to platelet-rich plasma. The anti-2β1monoclonal antibody was added as indicated at a final concentration of 100 μg/mL, which was shown to produce a maximum specific effect.
Platelet aggregation induced by type I collagen fibrils with distinct morphologies. Aggregation at 37°C is shown as an increase in light transmittance. The arrows indicate addition of agonist at the final concentrations indicated. The pH of all collagen preparations was adjusted to 7.4 immediately before adding to platelet-rich plasma. The anti-2β1monoclonal antibody was added as indicated at a final concentration of 100 μg/mL, which was shown to produce a maximum specific effect.
Distinct mechanisms of platelet attachment to banded and nonbanded collagen.
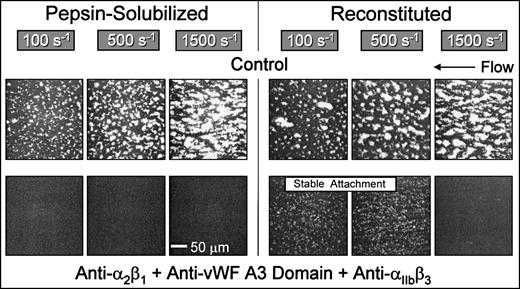
Blocking the function of platelet integrins αIIbβ3 and α2β1concurrently with inhibition of the binding of plasma vWF completely abolished platelet interaction, including initial tethering, with nonbanded collagen at wall shear rates ranging from 100 s−1 to 1,500 s−1(Fig 6). Thus, with this substrate, platelet adhesion and thrombus formation under flow require the functional integration of 3 platelet receptors at a wall shear rate of 1,500 s−1, namely GP Ibα, α2β1, and αIIbβ3. In contrast, α2β1 is not required with native banded collagen when coated at high density under the conditions used in the present study (Figs 2 and 3), although blockade of α2β1 caused significant inhibition of thrombus formation at low surface densities of this substrate.33 In contrast with the results obtained with nonbanded collagen, reconstituted collagen supported stable platelet attachment at 100 s−1 and 500 s−1when vWF binding to collagen was blocked concurrently with functional inhibition of both α2β1 and αIIbβ3 (Fig 6), implicating the participation of another collagen receptor(s) other than α2β1, possibly GP VI,7,8 GP IV,9-11 or the recently identified 65-kD collagen-binding protein.12 Results similar to those seen for reconstituted collagen were obtained with native, banded collagen (data not shown). Stable platelet attachment under these conditions was completely inhibited when divalent cations were chelated with EDTA (data not shown). Therefore, platelet adhesion to banded (native or reconstituted) collagen type I at shear rates less than 500 s−1, although cation-dependent, does not require vWF or the platelet integrins α2β1 or αIIbβ3, whereas requisite α2β1-collagen pairing leading to stable platelet attachment to nonbanded collagen was always observed at shear rates ranging from 100 s−1 to 1,500 s−1 (Fig 2). Consistent with the present findings are recent binding studies showing that platelets interact with soluble and insoluble collagens through different mechanisms, implying that α2β1 and another collagen receptor mediate collagen-platelet interactions with different specificities.35
Role of distinct collagen receptors in mediating platelet attachment under flow to reconstituted (banded) and pepsin-solubilized (nonbanded) type I collagen. Blood was perfused through the parallel plate chamber as described in the legend to Fig 2. Control blood or blood containing monoclonal antibodies to block specifically the function of platelet 2β1 and IIbβ3 concurrently with inhibition of plasma vWF binding to collagen was perfused over pepsin-solubilized (nonbanded) type I collagen or reconstituted collagen derived from the former, as indicated. Note the complete inhibition of platelet interaction with pepsin-solubilized (nonbanded) collagen when platelet 2β1 and IIbβ3function were blocked concurrently with inhibition of plasma vWF binding to collagen, compared with the stable attachment of single platelets at 100 s−1 and 500 s−1 under the same conditions with reconstituted type I collagen. Representative images of 6 separate experiments with blood from different donors are shown.
Role of distinct collagen receptors in mediating platelet attachment under flow to reconstituted (banded) and pepsin-solubilized (nonbanded) type I collagen. Blood was perfused through the parallel plate chamber as described in the legend to Fig 2. Control blood or blood containing monoclonal antibodies to block specifically the function of platelet 2β1 and IIbβ3 concurrently with inhibition of plasma vWF binding to collagen was perfused over pepsin-solubilized (nonbanded) type I collagen or reconstituted collagen derived from the former, as indicated. Note the complete inhibition of platelet interaction with pepsin-solubilized (nonbanded) collagen when platelet 2β1 and IIbβ3function were blocked concurrently with inhibition of plasma vWF binding to collagen, compared with the stable attachment of single platelets at 100 s−1 and 500 s−1 under the same conditions with reconstituted type I collagen. Representative images of 6 separate experiments with blood from different donors are shown.
Shear rate dependence of α2β1-mediated platelet attachment to nonbanded collagen.
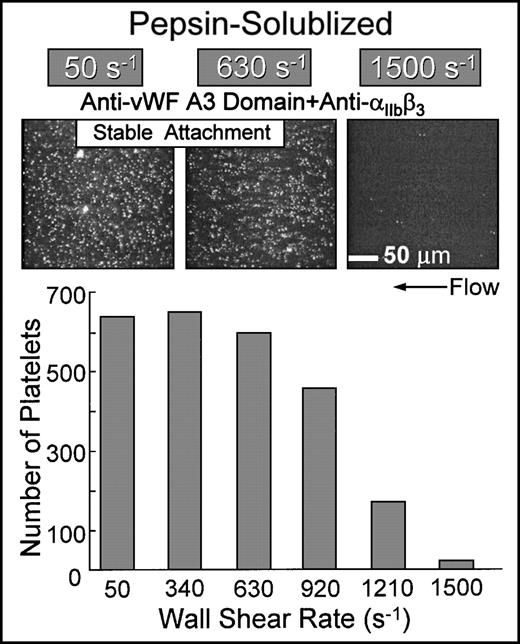
Simultaneous inhibition of platelet αIIbβ3function and the binding of plasma vWF to collagen resulted in the stable attachment of single platelets at shear rates up to approximately 1,000 s−1 when blood was perfused over nonbanded collagen (Fig 7). The extent of surface coverage was inversely related to the wall shear rate, with virtually no platelet-surface interactions at 1,500 s−1. Under these conditions, platelet attachment was contingent upon ligation of collagen domains with α2β1 on platelets; blocking α2β1 function under these conditions completely prevented platelet interaction with this substrate (Fig 6). The biomechanical properties of the α2β1-collagen bond appear to be similar to those observed for αIIbβ3-fibrinogen interactions, in which αIIbβ3-mediated platelet adhesion to surface-bound fibrinogen cannot occur above a limit wall shear rate.29 Functional inhibition of α2β1 by chelation of divalent cations with EDTA completely abolished platelet interaction under these conditions (data not shown).
Flow-dependent characteristics of 2β1-mediated platelet attachment to pepsin-solubilized (nonbanded) type I collagen. Blood containing function blocking anti-IIbβ3 and anti-vWF A3 domain monoclonal antibodies (the latter to inhibit plasma vWF binding to collagen) was perfused over immobilized pepsin-solubilized (nonbanded) type I collagen in the parallel plate flow chamber as described in the legend to Fig 2. Note that, for stable platelet attachment under these conditions, there was an absolute requirement for 2β1-collagen interactions, because additional functional inhibition of 2β1completely inhibited platelet interaction with this substrate (as shown in Fig 6). The number of single platelets attached to the surface after 3 minutes of perfusion was measured as a function of the wall shear rate.
Flow-dependent characteristics of 2β1-mediated platelet attachment to pepsin-solubilized (nonbanded) type I collagen. Blood containing function blocking anti-IIbβ3 and anti-vWF A3 domain monoclonal antibodies (the latter to inhibit plasma vWF binding to collagen) was perfused over immobilized pepsin-solubilized (nonbanded) type I collagen in the parallel plate flow chamber as described in the legend to Fig 2. Note that, for stable platelet attachment under these conditions, there was an absolute requirement for 2β1-collagen interactions, because additional functional inhibition of 2β1completely inhibited platelet interaction with this substrate (as shown in Fig 6). The number of single platelets attached to the surface after 3 minutes of perfusion was measured as a function of the wall shear rate.
DISCUSSION
The nonbanded collagen structures seen with the substrate derived from pepsin-solubilized type I collagen (Fig 1B) may be a distinctive feature of fibrils generated by enzymatic cleavage of collagen precursors,14 because monomers obtained from pepsin-treated collagen have altered nonhelical extremities.13 Presumably, monomers prepared in this manner lack crucial domains required for the assembly of lateral aggregates present in native fibrillar type I collagen, but can generate spiraled structures by lateral packing of subfibrils. These structural differences had a profound effect on the mechanisms of platelet adhesion and thrombus formation under flow: platelet α2β1 function was essential for the permanent arrest of platelets on nonbanded collagen, whereas native banded type I collagen supported platelet adhesion and thrombus growth even when α2β1 function was compromised. Studies of patients with a deficiency of platelet α2subunit or with autoantibodies directed against this protein attest to the involvement of α2β1 in collagen-platelet interactions in vivo, because platelet adhesion to collagen and collagen-induced aggregation are both impaired.36-39 However, simple collagen-like peptides consisting essentially of a repeating Gly-Pro-Hyp sequence have been synthesized that are potent platelet agonists whose activity is totally α2β1-independent.34 The platelet reactivity of these peptides could be attributed solely to their tertiary (triple helical) and quaternary (polymeric, cross-linked) structures. Thus, platelet-collagen interactions in vivo are likely to involve the integration of at least 2 distinct receptor-collagen interactions acting in concert: one involving α2β1 as an adhesion/signaling receptor and the other involving the recognition of collagen structural features by a receptor other than α2β1. A likely candidate for the latter function is GP VI which appears to play a major role in collagen-induced platelet activation.8
Recent studies involving the adhesion of GP VI-deficient platelets to collagen under flow testify to the importance of GP VI as a signaling receptor required for the activation of platelet αIIbβ3.8 Moreover, GP VI-deficient platelets were unresponsive to the collagen-related peptides comprising a repeating Gly-Pro-Hyp motif that mimic the collagen tertiary and quaternary structure.40 GP VI therefore appears to act as a signaling receptor after recognition/coupling with distinct structural components of collagen. In this context, collagen-induced activation of the protein-tyrosine kinase Syk was severely compromised in GP VI-deficient platelets.41 In a recent report, the activation of αIIbβ3 induced by platelet adhesion to collagen was found to be mediated by both α2β1 and GP VI.42 In this study, platelet adhesion to insoluble fibrillar type I collagen under static conditions was mediated by both α2β1and GP VI, whereas platelet adhesion to monomeric type I collagen was exclusively mediated by α2β1. This report is entirely consistent with the present study in which we demonstrate that, under physiologically relevant flow conditions, platelet adhesion and subsequent thrombus formation on nonbanded collagen requires competent α2β1 function, whereas with banded collagen another receptor other than α2β1, possibly GP VI, is involved and can mediate platelet adhesion and thrombus formation even when α2β1 function is blocked. However, it should be noted that, although the anti-α2β1 antibody blocks adhesion to nonbanded collagen, this does not exclude the role for a second collagen receptor with this substrate, which may act in concert or synergistically with α2β1 such that participation of both receptors may be required for stable platelet attachment. Such a mechanism would be analogous to that previously described for the irreversible adhesion of platelets to immobilized vWF that requires the functional integration of 2 receptors, namely GP Ib α and αIIbβ3.29,43Furthermore, a conformational change triggered by collagen binding to α2β1 may induce an increased affinity of the receptor for its ligand.44
Reconstituted type I collagen showed distinct functional properties compared with the nonbanded collagen from which it was derived. First, platelet adhesion and thrombus growth under flow did not require competent α2β1 function, although when this receptor was blocked, there was a prolonged lag phase before the accrual of firmly attached platelets compared with that seen with native banded collagen (Fig 3A). The delayed response before the onset of α2β1-independent platelet attachment proved to be related to the time-dependent binding of plasma components, as evidenced by the significant increase in the total thrombus volume measured after 2.5 minutes of perfusion when reconstituted collagen was pre-exposed to plasma under flow or to purified vWF under stationary conditions (Fig 4A). Thus, the rate of surface coverage by platelets and the development of platelet thrombi on this substrate are processes that are greatly accelerated by the presence of collagen-bound vWF and/or other collagen-bound plasma components when α2β1 function is compromised. The present findings indicate that collagen structural characteristics may regulate the extent and/or affinity of the binding of components, including vWF or other αIIbβ3 ligands such as fibronectin, present in plasma. In this context, activated αIIbβ3 promotes platelet arrest and subsequent thrombus growth on surface-bound vWF by interacting with the Arg-Gly-Asp sequence in the C1 domain near the carboxyl terminus of the vWF subunit.29 The extent to which this process occurs when plasma vWF or other αIIbβ3 ligands are immobilized onto collagen may therefore be regulated by the morphology of the collagen fibrils. Despite differences with regard to the role of α2β1 in mediating the adhesion of platelets flowing over banded and nonbanded collagens, binding of plasma vWF was requisite at 1,500 s−1, but not at 100 s−1, for both substrates.
Although platelet α2β1 has generally been considered to be constitutively active,45 recent studies suggest that α2β1 on nonactivated platelets does not bind soluble type III collagen, whereas activation of platelets transforms it to a state with higher affinity for soluble type III collagen.35 It can be inferred from the present study that soluble type I collagen binds to α2β1 on resting platelets, because it induces platelet aggregation in an α2β1-dependent manner (Fig 5). Furthermore, when soluble type I collagen was immobilized on a surface, blocking platelet activation with prostaglandin E1(PGE1) did not prevent α2β1-dependent platelet attachment under flow (data not shown). It therefore appears that α2β1 on nonactivated platelets can recognize and ligate domains in soluble type I collagen when the latter is immobilized on a surface. We have also demonstrated the inhibitory efficacy of the anti-α2β1 antibody under conditions in which platelets are known to be activated (eg, under high shear flow when αIIbβ3 function is blocked to prevent platelet aggregation; Fig 3B). Thus, the anti-α2β1 antibody used in these studies blocks α2β1 function on both resting and activated platelets.
Because there are multiple potential collagen receptors on platelets, their relative contribution in adhesion/signaling induced by collagen is difficult to discern. As shown in the present study, multiple collagen receptors can participate in adhesion to banded (native or reconstituted) type I collagen under flow, whereas α2β1 is absolutely required for the initial attachment of platelets to nonbanded collagen fibrils. Furthermore, conditions can be obtained (by concurrent blocking of GP Ibα and αIIbβ3) where competent α2β1 function is requisite for platelet attachment to this substrate under flow. Such conditions could therefore be exploited to compare distinct signaling pathways associated with α2β1-dependent and α2β1-independent adhesion to immobilized collagen under flow.
Although at least 7 genetically distinct collagens (types I, III, IV, V, VI, VIII, and XIII) have been identified as constituents of the vessel wall,46 their relative contribution to the thrombogenicity of the extracellular matrix after vascular injury by tissue trauma or by inflammatory and degenerative processes has not been clearly defined. Structural differences in platelet binding to these different collagen types under flow have been reported.2,47 For example, pepsin-digested collagen type VI-coated surfaces were found to support platelet adhesion only at relatively low wall shear rates (100 s−1), although these surfaces were found to be more reactive than collagen type I surfaces under the same low shear conditions.47 Such studies imply that the relevance of different collagen types in hemostasis and thrombosis may depend on their relative distribution in the vascular extracelluar matrix as well as the prevailing flow conditions in different regions of the vasculature. However, more recent studies have demonstrated that intact tetrameric collagen type VI supports platelet adhesion and aggregation at high shear rates in a manner similar to that seen for collagen type I,48suggesting a stringent requirement for an intact conformational macromolecular structure. Whereas collagen types III, IV, and VI may be more relevant in normal blood vessels, collagen type I may be important in fibrous atherosclerotic plaques that contain a higher proportion of collagen type I compared with normal arterial vessel walls49 and in deep vascular injuries where it is abundant in the media and adventitia of blood vessel.50 Physiologic degradation of collagen fibrils by interstitial enzymes such as matrix metalloproteinases is known to occur at extracellular spaces; their assembly to form spiral, nonbanded collagen may represent a process that regulates platelet responses to vascular injury at sites of vascular wound repair during remodeling of the vasculature.
In conclusion, our results show that type I collagens with different structural characteristics elicit platelet adhesion and aggregation through distinct mechanisms as evidenced by the differential role of platelet α2β1 under various flow conditions. We also demonstrate that a collagen receptor(s) other than α2β1 can selectively engage domains in banded, but not nonbanded type I collagen when α2β1 function is blocked and that the state of collagen fibril assembly may regulate the extent and affinity of the binding of plasma vWF as well as other plasma components, crucial for a full thrombotic response under high shear flow.
ACKNOWLEDGMENT
The authors thank James R. Roberts and Benjamin Gutierrez for preparing monoclonal antibodies; Rolf Habermann, Judith Dent, and Dr Enrique Saldivar for help with videomicroscopy, computation, and image analysis; Dr Malcolm Wood and George Klier (deceased) for assistance with surface replication analysis; and Dr Virgil Woods for providing the nonfunction blocking anti-α2β1monoclonal antibody, 12F1.
Supported in part by Grants No. HL-31950, HL-42846, and HL-48728 from the National Institutes of Health. Additional support was provided by National Institutes of Health Grant No. RR0833 to the General Clinical Research Center of Scripps Clinic and Research Foundation and by the Stein Endowment Fund.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Brian Savage, PhD, Department of Molecular and Experimental Medicine, MEM-175, The Scripps Research Institute, 10550 N Torrey Pines Rd, La Jolla, CA 92037; e-mail:brian@scripps.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal