Interaction of biomaterials with blood components including neutrophils is responsible for some of the clinical complications that have occurred in cardiopulmonary bypass, hemodialysis, and ventricular assist procedures. The possibility of inhibiting the initial adhesion of neutrophils to biomaterials has been studied extensively, but the problem remains unsolved. In this study, we investigated the effect of HK adsorption on polyurethane, a widely used component of extracorporeal and intracorporeal devices. HK and HKa were allowed to adsorb on 4 different charged polyurethanes: noncharged (PU), cationic (NR4), anionic (SO3), and zwitterionic (GPC) polyurethanes. The effect of kininogen adsorption on neutrophil adhesion, the surface density of the adsorbed kininogen, and the exposure of HK domains 3 and 5 (D3 and D5H), which are responsible for the binding of HK to the neutrophil integrin mβ2 or Mac-1, were examined. On PU, NR4, and SO3, kininogen adsorption reached 80% of monolayer coverage when 100 pmol/mL or higher concentration of protein solutions were used. The NR4 surface adsorbed the most kininogen along with a high exposure of D3 and D5H. The availability of D3 and D5Hallowed neutrophils to bind to the surface via the Mac-1 receptor; thus, on the NR4 surface, adsorbed kininogens lost their antiadhesive property, which resulted in a high degree of neutrophil adhesion. Increasing Mac-1 expression by exposure to fMLP increased the neutrophil adhesion on this surface. In contrast, exposure of D3 and D5H on SO3 was significantly less, because HK binds to anionic surfaces with similar protein sequences used for cell binding. This low binding site exposure preserved the antiadhesive property of HK. GPC was resistant to neutrophil adhesion even in the absence of adsorbed kininogens because of its phosphorylcholine moiety. Thus, both SO3 coupled with kininogen (or kininogen peptides) and GPC have the potential to markedly reduce neutrophil adhesion to biomaterial devices.
THE INTERACTION OF leukocytes with biomedical devices is an important event in the host response to biomaterials, because the inflammatory mediators released from adherent or activated leukocytes induces complications in the clinical use of dialyzers, oxygenators, vascular grafts, and hemofiltration units.1 2 After adhesion to a biomaterial, neutrophils undergo a number of processes, such as the secretion of neutral proteases and respiratory burst, which can result in the deterioration of the implant material and peripheral tissue damage.
Segmented polyurethane elastomers were introduced as biomaterials by Boretos and Pierce,3 because they possessed superior mechanical properties and exhibited good in vitro stability. Cooper et al have shown that the thromboresistance of PU can be increased by sulfonate incorporation.4,5 However, this modification in the absence of plasma proteins enhances neutrophil adhesion and neutrophil respiratory burst and could well induce complications in certain biomaterial applications.6,7 Recently, we reported reduced bacterial and neutrophil adhesion on polyurethanes containing the phosphorylcholine moiety.8,9 Other researchers have also demonstrated a decrease in plasma protein adsorption and in platelet adhesion on poly-alkyl-methacrylate–based polymers with pendant phosphorylcholine groups.10 11
Kininogens are plasma proteins that are natural substrates of plasma and tissue kallikrein and yield, on hydrolysis, the nonapeptide bradykinin. Bradykinin release has been implicated in hypotensive reactions during hemodialysis and cardiopulmonary bypass for patients receiving angiotensin converting enzyme (kininase 2) inhibitors. Two forms of purified human plasma kininogen (kinin precursors) were described by Jacobsen and Kriz12 in 1967: high molecular weight kininogen (HK) and low molecular weight kininogen (LK). HK consists of 6 domains, of which domains 3 and 5 (D3 and D5H) are responsible for the binding of HK to neutrophils.13 D5H contains 2 anionic surface binding subdomains: 1 rich in histidine and glycine14,15and the other rich in histidine, glycine, and lysine.15 LK is coded from the same single-copy gene as HK by differential splicing of the primary transcript, but LK does not contain regions resembling D5 and D6 and, instead, has a unique short light chain D5L. Plasma kallikrein in solution cleaves HK and eventually yields a stable product (HKa) composed of 2 disulfide-linked chains of molecular weight (Mr) 64 kD and 45 kD. Scott et al16 showed that the cleaved form, HKa, binds to a greater extent to an activating surface than HK, indicating that HK exists as a procofactor that can be activated via cleavage by kallikrein.
We postulated that the effects of adsorbed HK on neutrophil-surface interactions would vary with the charge of the surface and that binding of HK to anionic surfaces would prevent or reduce neutrophil adhesion and associated elastase release and superoxide production. We examined neutrophil adhesion to a series of polyurethanes containing different charges to which various forms of HK had been preadsorbed. The surface density of the adsorbed protein after adsorption was first quantified. To elucidate the role of the Mac-1 receptor on cell adhesion, the availability of Mac-1 binding sites on adsorbed HK and HKa was also studied, because D3 and D5H of HK are known to be responsible for mediating the binding of HK to neutrophils.
MATERIALS AND METHODS
Polymers
Four polyurethanes (PU, NR4, SO3, and GPC) were used in this study. The synthesis and the characterization of these polymers have been described previously.4,9 17 Briefly, the PU was a noncharged polyurethane made of 1/3/2 molar ratio of polytetramethylene oxide (PTMO), methylene diphenylene diisocyanate (MDI), and butane-1,4-diol (BD). The NR4 was a 100% quaternized (cationic) polyurethane using 3-trimethylamino-1,2-propanediol iodide as the chain extender. The SO3 was an anionic polyurethane derivatized from PU, with 20% of the urethane hydrogen replaced by propyl sulfonate groups via a bimolecular nucleophilic substitution. The GPC was a zwitterionic polyurethane chain extended with L-α-glycerophosphorylcholine.
Plasma Proteins
Plasma proteins used in this study were the procofactor (HK) and the activated (HKa) forms of high molecular weight kininogen. HK and HKa were purchased from Enzyme Research Laboratories (South Bend, IN) and tested using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to confirm their purity. HK was more than 90% a single band of 120 kD, and HKa on a reduced gel showed two bands of 62 and 45 kD, respectively.
Isolation of Human Neutrophils
Neutrophils were isolated from human whole blood anticoagulated with citrate phosphate dextrose using centrifugation with NIM (Cardinal Associates, Santa Fe, NM) as the density gradient isolation medium. The isolated neutrophils were suspended in 2 mL of Hank’s balanced salt solution (HBSS) containing 2 mg/mL bovine serum albumin (BSA) at 4°C. The purity, viability, and concentration of the cells were evaluated using a hemacytometer. The viability of the isolated cells in each experiment was always greater than 95%, as determined by trypan blue exclusion, and the concentration was always greater than 107 cells/mL. Untreated neutrophils were then diluted to a standard concentration of 105 cells/mL for the adhesion experiments. Fully activated neutrophils were obtained using the method previously described by Khan et al.18 In brief, isolated neutrophils were incubated with 5 μg/mL cytochalasin B for 3 minutes, followed by the addition of 10−7 M fMLP for 5 minutes at 37°C to fully activate the neutrophils and to maximally express Mac-1.18 After the activation, neutrophils were mixed 1:1 with HBSS at 4°C, followed by centrifugation at 250g for 5 minutes. Finally, they were diluted to 105 cells/mL for the adhesion experiments. This concentration was high enough to provide good visualization of cell adhesion, but dilute enough to minimize interactions between cells and to prevent the formation of cell aggregates.
Protein Adsorption
To analyze neutrophil interactions with protein adsorbed surfaces, the amount of plasma protein adsorbed was first determined with125I-labeled proteins at room temperature. HK and HKa were radiolabeled with Na 125I using the IODO-GEN method,19 such that the specific radioactivity of the proteins was greater than 2 mCi/mg. One and one-half inch glass tubings (ID, 12 mm) were coated with 1% solution of PU, NR4, SO3, or GPC in DMAc and dried with nitrogen at room temperature. This process was performed in triplicate and the uniformity of the polymer coating was checked under the optical microscope. Tubings with unevenly coated patterns were not used. Shunts composed of these polymer-coated tubings were used in the adsorption study. Each shunt was injected with HBSS for 2 hours, followed by the adsorption of different concentrations (10, 50, 100, 200, and 500 pmol/mL) of 125I-labeled HK or HKa to the inner surface of the shunt for 6 hours. Nonadsorbed protein was removed with HBSS rinsing at the end of the adsorption period. The radioactivity of the glass tubing was counted using a Beckman Gamma 5500 γ-counter (Beckman Coulter, Fullerton, CA). We verified that the radiolabeling did not modify the adsorption process by using mixtures of labeled and nonlabeled protein solutions.
Neutrophil Adhesion Assay
Radial flow chamber.
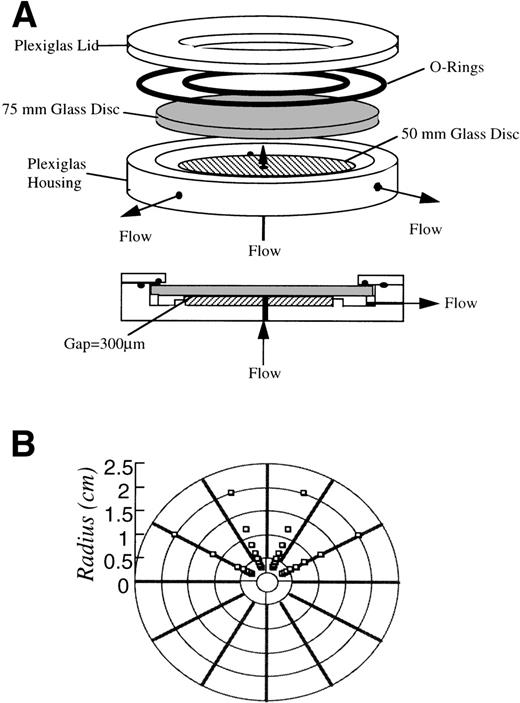
Neutrophil adhesion was performed using a radial flow chamber with automated video microscopy and image analyzing systems according to the design by Dickinson and Cooper.20 In brief, the radial flow geometry provides a well-defined laminar flow field with a continuous variation of shear rate for the study of neutrophil adhesion. A schematic of the radial flow chamber used in the experiments is shown in Fig 1. The flow cell was constructed according to a similar design by Cozens-Roberts et al.21The chamber was made of a Plexiglas housing, with 2 optically flat glass discs, one 75 mm and the other 50 mm in diameter. The 50-mm glass disc was permanently cemented to the housing and bored with a 0.125-inch diameter inlet port through its center. The 75-mm glass disc was removable and was pretreated under different conditions before the adhesion experiment. The neutrophil suspension entered at the center of the chamber and flowed radially outward between the 2 glass discs through a 300-μm gap.
(A) Schematic diagram of the radial flow chamber. The chamber was made of a Plexiglas housing with 2 optically flat glass discs, one 75 mm and the other 50 mm in diameter. The 50-mm glass disc was permanently cemented to the housing and bored with a 0.125-inch diameter inlet port through its center. The fluid flows in through the center of the cemented disc and flows out radially between 2 discs with a gap of 300 μm. (B) Schematic diagram of the scanned fields during experiments. A region containing 8 fields by 4 fields of the disc was scanned at increments proportional to the shear rate.
(A) Schematic diagram of the radial flow chamber. The chamber was made of a Plexiglas housing with 2 optically flat glass discs, one 75 mm and the other 50 mm in diameter. The 50-mm glass disc was permanently cemented to the housing and bored with a 0.125-inch diameter inlet port through its center. The fluid flows in through the center of the cemented disc and flows out radially between 2 discs with a gap of 300 μm. (B) Schematic diagram of the scanned fields during experiments. A region containing 8 fields by 4 fields of the disc was scanned at increments proportional to the shear rate.
Disc preparation.
The 75-mm glass discs were cleaned by soaking in concentrated chromium trioxide/sulfuric acid solution overnight, in deionized water twice for 1 hour, and were dried overnight in an oven at 60°C. The cleaned discs were spin-coated with 1 wt% polymer solution and dried. The coated discs were incubated in HBSS for approximately 24 hours before the cell adhesion experiments were performed. For experiments involving kininogen adsorption, discs were incubated with HK or HKa for 6 hours, followed by HBSS to remove nonadsorbed protein.
Adhesion experiments.
A background scan at every scanned flow field was performed with only buffer flowing through the chamber. The 105 cells/mL neutrophil suspension (activated or untreated) was then introduced into the chamber for the actual cell adhesion experiment with a continuous field scan over time. The volumetric flow rate was maintained at 2.5 mL/min. This produced a wall shear rate range of approximately 20 to 90 s−1. The duration of the adhesion experiment was approximately 20 minutes. At least 5 runs of adhesion experiments were performed for each material. At the end of this part of the experiment, the number of the adherent cells as well as the cell morphology were determined by image analysis methods applied to the videomicrographs. A degree of spreading was quantified by the circularity index (CI), which is defined as CI = (4π[observed area])/(observed perimeter)2. To calculate CI, the area and the perimeter of each adherent cell was obtained at the end of the adhesion experiment. CI ranges from 0 to 1 and equals unity for a perfect circle. At least 30 adherent cells in 20 s−1 shear rate region were used to calculate CI, and the experiment was performed in triplicate on every surface studied.
Purification of Monoclonal Antibodies (MoAbs)
Murine MoAb binding to HK were performed as described by Schmaier et al.22 The MoAb 2B5 reacts on immunoblotting with an epitope on the HK heavy chain (D1-D3) and prevents the inhibition of calpain by kininogen. The epitope of 2B5 has been further localized to D2 and D3 of HK.23 The MoAb C11C1 reacts with the light chain of HK on immunoblot and inhibits the coagulant activity.22 Dela Cadena and Colman14 have further shown that C11C1 reacts with D5, the histidine-glycine-lysine rich region of HK.
IgG from C11C1 culture supernatant and from 2B5 ascites fluid was purified using a Affi-Gel protein A affinity chromatography system (Bio-Rad, Richmond, CA). The purified IgG was dialyzed against HBSS buffer before use. The final protein concentrations of the purified IgG were determined by bicinchoninic acid (BCA) assay.24
Enzyme-Linked Immunosorbant Assay (ELISA)
The conformation of adsorbed HK and HKa, specifically the exposure of the D3 and D5H regions, was determined using an ELISA. Glass discs with 6.4-mm diameter (Richland Glass, Richland, NJ) were coated with polymers using the method described in the previous section. The polymer-coated glass discs were equilibrated with HBSS in a 96-well microtiter plate followed by 6 hours of HK or HKa (100 pmol/mL) adsorption. One percent BSA was then added for 2 hours to block nonspecific binding. Primary antibody at 20 μg/mL (2B5 or C11C1) was added for 2 hours, followed by 1 hour incubation of alkaline phosphatase-conjugated goat antimouse IgG (1:10,000 dilution).p-nitrophenyl phosphate (2 mg/mL) was then added for 0.5 hours for color development. The optical density of each well was detected using a Multiskan Bichromatic plate reader (ICN Biomedicals, Inc, Horsham, PA) at absorbance wavelength of 405 nm.
RESULTS
Kininogen Adsorption Study
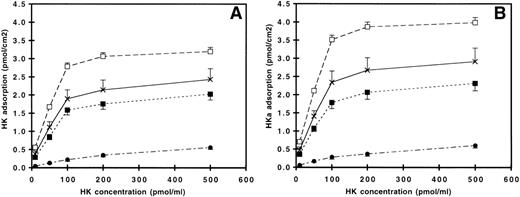
The surface density of HK and HKa on the polymer-coated surfaces (PU, NR4, SO3, and GPC) at different incubation concentration is shown in Fig 2A and B, respectively. For PU, NR4, and SO3, the plateau region between 200 and 500 pmol/mL concentration indicated the formation of a kininogen monolayer. Assuming the surface density of the adsorbed protein reaches monolayer coverage at 500 pmol/mL condition, the adsorption of both HK and HKa on these 3 surfaces covered more than 80% of surface when the 100 pmol/mL or more concentrated solution was used. The trends of HK and HKa adsorption on all polymer-coated surfaces were similar, with somewhat higher amounts of HKa adsorption seen on all surfaces. The cationic polyurethane-coated surface (NR4) adsorbed the highest amount of protein, followed by the underivatized polyurethane (PU)-, the anionic polyurethane (SO3)-, and the zwitterionic polyurethane (GPC)-coated surface. The amount of adsorbed protein on NR4 was significantly higher (P < .01) when compared with other surfaces, whereas the GPC surface had significantly less protein adsorption (P < .01).
Adsorption of (A) HK and (B) HKa on (×) PU, (□) NR4, (▪) SO3, and (•) GPC as a function of solution concentration. Error bars indicate the standard deviation of the mean.
Adsorption of (A) HK and (B) HKa on (×) PU, (□) NR4, (▪) SO3, and (•) GPC as a function of solution concentration. Error bars indicate the standard deviation of the mean.
Neutrophil Adhesion Study on HK and HKa Adsorbed Polymer Surfaces
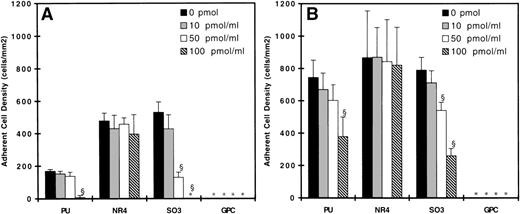
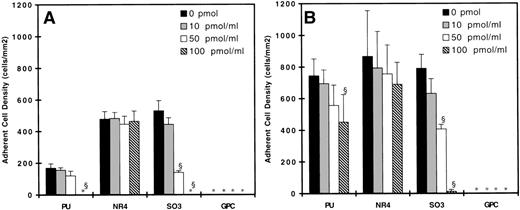
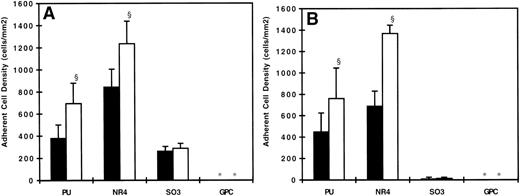
Figures 3 and 4 show the adherent cell density on all surfaces studied with untreated neutrophils as the adsorption concentration of kininogen increases from 0 to 100 pmol/mL. At 90 s−1, neutrophil adhesion was observed on the PU, NR4, and SO3 surfaces, but not on the GPC-coated surface (Figs 3A and 4A). Incubating surfaces with 100 pmol/mL HK (Fig 3) or HKa (Fig 4) virtually eliminated any adhesion on the PU- and SO3-coated surfaces. Incubating SO3 with 50 pmol/mL HK or HKa reduced cell adhesion substantially, but this effect was not observed on PU. On both SO3 and PU, incubating the surfaces with 10 pmol/mL kininogen did not result in any decrease in cell adhesion. On the NR4 surface, no difference in cell adhesion was observed with or without kininogen adsorption. At 20 s−1, neutrophil adhesion on the PU, NR4, and SO3surfaces without kininogen preadsorption was the highest, and again no adhesion was found on the GPC surface (Figs 3B and 4B). By increasing the kininogen adsorption, PU and SO3 showed a decrease in cell adhesion, and the decrease was most significant on the SO3 surface. In fact, almost all adhesion was eliminated on SO3 preadsorbed with 100 pmol/mL HKa. No decrease in the cell adhesion was again observed on NR4 after incubating with HK or HKa. The degree of cell adhesion on surfaces preadsorbed with 200 pmol/mL was similar to that preadsorbed with 100 pmol/mL and is not shown here.
Surface density of adherent neutrophils (untreated) after 20 minutes of adhesion experiments at (A) 90 s−1 and (B) 20 s−1 on HK preadsorbed surfaces. (▪) Bare polymer surfaces, (▩) adsorbed with 10 pmol/mL HK, (□) adsorbed with 50 pmol/mL HK, and () adsorbed with 100 pmol/mL HK (n = 3). (*) indicates no adherent cells found. Error bars indicate the standard deviation of the mean. §P < .01 versus bare polymer surface.
Surface density of adherent neutrophils (untreated) after 20 minutes of adhesion experiments at (A) 90 s−1 and (B) 20 s−1 on HK preadsorbed surfaces. (▪) Bare polymer surfaces, (▩) adsorbed with 10 pmol/mL HK, (□) adsorbed with 50 pmol/mL HK, and () adsorbed with 100 pmol/mL HK (n = 3). (*) indicates no adherent cells found. Error bars indicate the standard deviation of the mean. §P < .01 versus bare polymer surface.
Surface density of adherent neutrophils (untreated) after 20 minutes of adhesion experiments at (A) 90 s−1 and (B) 20 s−1 on HKa preadsorbed surfaces. (▪) Bare polymer surfaces, (▩) adsorbed with 10 pmol/mL HKa, (□) adsorbed with 50 pmol/mL HKa, and () adsorbed with 100 pmol/mL HKa (n = 3). (*) indicates no adherent cells found. Error bars indicate the standard deviation of the mean. §P < .01 versus bare polymer surface.
Surface density of adherent neutrophils (untreated) after 20 minutes of adhesion experiments at (A) 90 s−1 and (B) 20 s−1 on HKa preadsorbed surfaces. (▪) Bare polymer surfaces, (▩) adsorbed with 10 pmol/mL HKa, (□) adsorbed with 50 pmol/mL HKa, and () adsorbed with 100 pmol/mL HKa (n = 3). (*) indicates no adherent cells found. Error bars indicate the standard deviation of the mean. §P < .01 versus bare polymer surface.
Morphology of Adherent Neutrophils
The degree of spreading of adherent neutrophils was quantified using the CI shown in Table 1. In the absence of kininogens, the CI on SO3 was the lowest compared to PU and NR4 surfaces. CI increased on all 3 surfaces after the preadsorption of HK and HKa, and the increase was most significant on the SO3 surface. Lim and Cooper7 have shown a direct correlation between neutrophil respiratory burst and spreading on the bare SO3 surface. Other researchers have also shown that the increased adherence and spreading of neutrophil to nonphagocytosable surfaces lead to increased activation of oxygen metabolism and to defective bactericidal activity.25 26 We show here that, in the presence of adsorbed HK or HKa, cell adhesion and also the degree of spreading of the adherent cells is reduced significantly on the SO3 surface.
CI of Adherent Neutrophils
| Polymer . | CI . | ||
|---|---|---|---|
| No Kininogen . | With HK . | With HKa . | |
| PU | 0.72 ± 0.08 | 0.84 ± 0.04* | 0.89 ± 0.06* |
| NR4 | 0.79 ± 0.07 | 0.85 ± 0.05 | 0.88 ± 0.04 |
| SO3 | 0.54 ± 0.14 | 0.88 ± 0.06† | 0.86 ± 0.04† |
| Polymer . | CI . | ||
|---|---|---|---|
| No Kininogen . | With HK . | With HKa . | |
| PU | 0.72 ± 0.08 | 0.84 ± 0.04* | 0.89 ± 0.06* |
| NR4 | 0.79 ± 0.07 | 0.85 ± 0.05 | 0.88 ± 0.04 |
| SO3 | 0.54 ± 0.14 | 0.88 ± 0.06† | 0.86 ± 0.04† |
Data are expressed as mean ± standard deviation. GPC is not tabulated, because no adherent cell was found on this surface.
P < .01 v PU without kininogen.
P < .01 v SO3 without kininogen.
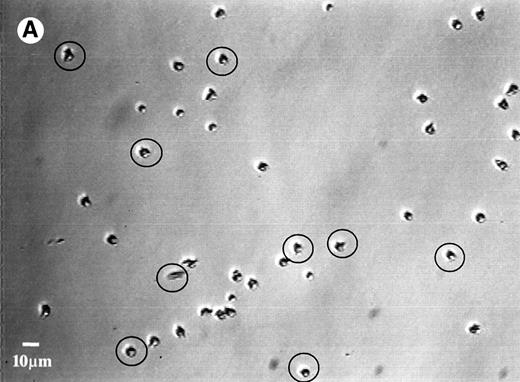
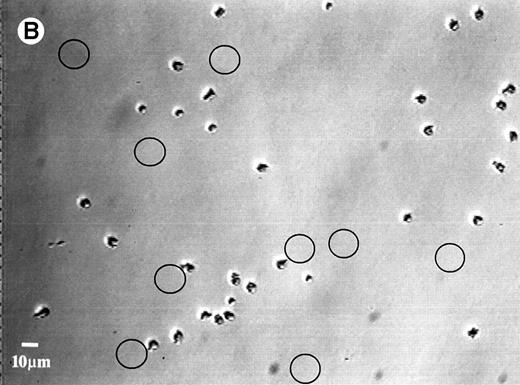
Upon increasing the surface shear rate by 12 times at the end of the adhesion study, no cell detachment was observed on any of the surfaces, except for the SO3 surface adsorbed with HK or HKa. Figure 5A shows the adherent cells on the HK-adsorbed SO3 surface before exposure to high shear, whereas Fig 5B shows the cells on the same surface after exposure to high shear. Locations where detachment occurred are circled. As previously reported, the degree of neutrophil detachment is generally inversely related to the extent of the cell adhesion on kininogen-preadsorbed glass.27 Low adhesion implies a weak interaction between cells and the kininogen-adsorbed surfaces, which allows the separation of adherent cells from the surface when they exposed to a high shear force.
Photomicrographs of the neutrophil distribution on the SO3 preadsorbed with HK (A) before and (B) after buffer flushing at a shear rate of 240 s−1. Circled areas indicate regions where cell detachment had taken place.
Photomicrographs of the neutrophil distribution on the SO3 preadsorbed with HK (A) before and (B) after buffer flushing at a shear rate of 240 s−1. Circled areas indicate regions where cell detachment had taken place.
The Adhesion of Activated Neutrophils on Surfaces Adsorbed With Kininogens
Figure 6A and B show the degree of adhesion after activating neutrophils with fMLP on surfaces preadsorbed with HK or HKa. The adhesion of activated neutrophils on both NR4and PU increased significantly (P < .01) when compared with those not activated with fMLP. This finding indicates that the adhesivity of activated neutrophils increased on these 2 surfaces. On the SO3 and GPC surfaces, no significant changes were observed on the adhesion when the neutrophils were activated.
Surface density of adherent neutrophils at 20 s−1 on polymer surfaces preadsorbed with 100 pmol/mL of (A) HK and (B) HKa. (□) Adhesion studies using untreated (non-fMLP activated) neutrophils and (▪) adhesion studies using fMLP-activated neutrophils (n = 3). (*) indicates no adherent cells found. Error bars indicate the standard deviation of the mean. §P < .01 versus untreated neutrophil adhesion.
Surface density of adherent neutrophils at 20 s−1 on polymer surfaces preadsorbed with 100 pmol/mL of (A) HK and (B) HKa. (□) Adhesion studies using untreated (non-fMLP activated) neutrophils and (▪) adhesion studies using fMLP-activated neutrophils (n = 3). (*) indicates no adherent cells found. Error bars indicate the standard deviation of the mean. §P < .01 versus untreated neutrophil adhesion.
Exposure of Mac-1 Binding Sites on Adsorbed Kininogen
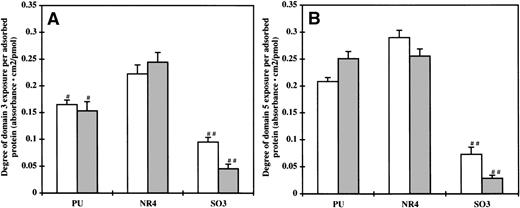
To investigate the availability of the Mac-1 binding sites on HK for neutrophil adhesion, a direct binding ELISA was performed using MoAbs 2B5 and C11C1, which targeted the binding regions D3 and D5H of HK, respectively. Figure7A and B show the results of the ELISA in arbitrary optical absorbance units per surface density of adsorbed protein. High absorbance indicates a high exposure of the binding sites. In general, there is a correlation between the exposure of the D3 and D5H. On the HK and HKa preadsorbed NR4surfaces, both D3 and D5H exposure was the highest. The exposure of D3, but not D5H, on the HK and HKa preadsorbed PU surfaces was less than that on the NR4 surface (P < .01). The exposure of both domains on the SO3 surface were significantly lower compared with the PU and NR4 surfaces (P < .01). The optical absorbance obtained on the protein preadsorbed GPC surfaces was negligible, most likely because of the small amount of absorbed protein present on this surface.
Degree of the (A) domain 3 and (B) domain 5 exposure on (□) HK and (▩) HKa preadsorbed surfaces (n = 5). Error bars indicate the standard deviation of the mean. #P < .01 versus NR4; ##P < .01 versus both PU and NR4.
Degree of the (A) domain 3 and (B) domain 5 exposure on (□) HK and (▩) HKa preadsorbed surfaces (n = 5). Error bars indicate the standard deviation of the mean. #P < .01 versus NR4; ##P < .01 versus both PU and NR4.
DISCUSSION
High molecular weight kininogen, especially the activated form (HKa), has a high affinity towards negatively charged surfaces and can displace fibrinogen from glass surfaces, a phenomenon that has been referred to as the Vroman effect.28 29
The surface density of adsorbed HK and HKa on the NR4 and PU surfaces was substantially higher than that on the SO3surface. The adsorption plateau found after 200 pmol/mL incubation (Fig2) suggests that a monolayer layer of adsorbed protein, instead of multilayers, was present on PU, NR4, and SO3surfaces. The higher surface density of adsorbed protein on NR4 and PU surfaces most likely results from a different conformation compared with proteins adsorbed on the SO3surface. We have previously shown that domain 5 (D5H) of HK contains anionic surface binding subdomains.14,15 Because neither PU nor NR4 is anionic, it is unlikely that HK and HKa adsorbed on these surfaces using the surface binding domain in D5H. Thus, the conformation of the adsorbed kininogens on these surfaces may be different from that on the SO3surface. Evidence for this is provided from different levels of antibody epitope exposure (see below). This change in conformation may explain why more protein adsorbed on the NR4 and PU surfaces. The GPC surface adsorbed submonolayer levels of the kininogen even at 500 pmol/mL adsorption concentration due to the presence of a zwitterionic phosphorylcholine group on the polymer. Other researchers have observed the similar phenomenon on polymers that contain the same phosphorylcholine groups.11 30
Both HK and HKa, after being adsorbed on glass, can inhibit neutrophil and bacterial adhesion,27,31 and when HKa is adsorbed on the glass surface, more than 90% of neutrophil adhesion is inhibited. SO3, which is an anionic polyurethane, contains negatively charged sulfonate groups. The ELISA results showed adsorbed HK and HKa exposed less D5H binding sites on the SO3surface. Thus, a surface coated with this polymer is likely to have both HK and HKa binding on the surface using the binding domain in D5H. We previously showed that HK binds to neutrophil Mac-1 receptor and the binding epitopes of HK have been located in D3 and D5H using MoAbs.13,32Recently, we have reported that HK binds directly to Mac-1 receptor both in transfected cells and in a purified system.33 When HK was bound to the SO3 surface using the surface binding domain in D5H, the Mac-1 receptor binding in the same domain was not available. This adsorbed protein layer then acted as a barrier preventing neutrophil adhering to the polymer surface and resulted in an antiadhesive phenomenon. Activating neutrophils with fMLP has been shown to increase Mac-1 expression;18however, because no specific binding sites were available on the SO3 surface, the adhesion of activated neutrophils did not increase. HKa has been shown to be more potent on displacing fibrinogen from glass, because it has a high affinity towards negatively charged surfaces.29 Here, the bound HKa on the SO3surface had not only the lowest exposure of D5H binding sites, the domain responsible for surface binding, but also the lowest exposure of D3 binding sites when compared with the HK-adsorbed surface. The low exposure of binding domains on kininogens due to its interaction with the anionic surface prevented both activated and untreated neutrophils from adhering to the SO3 surface.
No previous study has reported the presence of surface binding domains on HK to cationic (NR4) or nonionic (PU) polymer surfaces. Two glutamic acid-rich and aspartic acid-rich regions on HK34 (Cys310-Glu323 and Ser565-Asp576) may be responsible for binding to cationic surfaces, but this hypothesis requires further examination. In this study, the antiadhesive property of kininogen was partially observed on the PU surface, especially at higher shear rates (90 s−1), but it was not as marked as on the SO3 surface. Although the amount of kininogen adsorbed on the NR4 surface was the highest among all the surfaces studied, no decrease in cell adhesion was observed, because the degree of D3 and D5H binding site exposure was significantly higher than on the other surfaces studied. This binding site exposure allowed neutrophils to bind via their Mac-1 receptors, resulting in similar extents of cell adhesion as on the bare polymer surfaces. Activating neutrophils with fMLP increased the expression of Mac-1 receptor and thus increased the degree of cell adhesion, especially on NR4 preadsorbed with HK or HKa.
It should be pointed out that the cell adhesion and protein adsorption experiments were performed at room temperature. These results can be influenced by temperature, and different conclusions may be drawn if the experiments were performed at 37°C. Thus, it would be desirable to extend the investigations at the physiological relevant temperatures in future work.
We have reported that the GPC surface is resistant to both neutrophil and bacterial adhesion due to the presence of the phosphorylcholine moiety at its surface,8,9 and in this study, it also exhibited minimal HK and HKa adsorption compared with other surfaces studied. Phosphorylcholine is an important component of plasma membrane bilayer, and it has been proposed by Chapman35 and Ishihara et al30,36 that polymers containing this functionality mimic the plasma membrane of erythrocytes and endothelial cells and thus minimize interactions between the surface and the blood components. Alternatively, adsorption of HK on SO3 suggests that, if HK were coupled to an anionic surface, it could prevent neutrophil adhesion. The corresponding neutrophil activation leading to tissue injury by released elastase or reactive oxygen derivatives could be substantially diminished. Because the sequences of HK responsible for binding to neutrophils have been determined,18 coupling of selected peptides to SO3 might achieve the goal of producing a surface that will not allow adhesion of neutrophils. Both GPC and HK-bound SO3 are considered to be potential candidates for new materials with respect to their antiadhesive properties; however, more investigations are required to determine their practical applications.
ACKNOWLEDGMENT
The authors acknowledge the Blood Bank of Delaware for donating the whole blood used in the neutrophil adhesion study.
Supported by the National Institutes of Health through Grants No. HL-47179 and HL-56914 (Program Project).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Robert W. Colman, MD, Sol Sherry Thrombosis Research Center, Temple University School of Medicine, Philadelphia, PA 19140; e-mail: colmanr@astro.ocis.temple.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal