Thrombopoietin (TPO) stimulates proliferation and differentiation of cells of the megakaryocytic lineage. It exerts its function by binding and activating c-mpl, a member of the hematopoietic receptor superfamily. Upon binding of TPO to its receptor, numerous signaling events are triggered. These include activation of the Jak-STAT (signal transducers and activators of transcription) pathway, mitogen-activated protein kinase (MAPK), Tec, and phospatidylinositol (PI) 3-kinase and phosphorylation of Shc and Vav. The contribution of different signaling pathways to the induction of specific cellular processes such as proliferation and differentiation is incompletely understood. We have previously described a mutant of c-mpl that fails to activate the Jak-STAT pathway but nevertheless retains its ability to mediate proliferation and activation of most signaling events in the murine hematopoietic precursor cell lines BAF/3 and 32D. We confirm here the ability of this mutant to mediate proliferation in the absence of Jak-STAT activation in the human cell line UT-7 and further show that this mutant fails to mediate TPO-induced megakaryocytic differentiation. Comparison of the signaling capacity of this mutant in UT-7 and BAF/3 cells shows considerable cell-type–specific differences. Whereas in BAF/3 cells the mutant still mediates activation of Shc, MAPK, Vav, and PI 3-kinase at levels comparable to the wild-type receptor, these events are strongly diminished in UT-7 cells expressing the mutant. Furthermore, we show that the C-terminal 25 amino acid residues of the receptor mutant are crucial for the mitogenic response in UT-7 cells.
THROMBOPOIETIN (TPO) is the major regulator of megakaryopoiesis and platelet production.1-3It exerts its function through binding and activation of the TPO receptor c-mpl,4-6 a member of the hematopoietic receptor superfamily.7 Members of this family are type I transmembrane receptors that are characterized by conserved cysteine residues and a common amino acid motif (WSXWS) in the extracellular domain and the lack of intrinsic tyrosine kinase activity in the intracellular domain.7 Engagement of c-mpl by TPO leads to the stimulation of multiple intracellular signaling events. These events include the phosphorylation and activation of Jak-2, Tyk-2, STAT1, STAT3, and STAT5.8-12 Jak-2 and Tyk-2 are nonreceptor tyrosine kinases that, upon activation, phosphorylate their major target proteins, the STATs. Tyrosine phosphorylation of STATs leads to their translocation from the cytoplasm to the nucleus, where they bind to specific DNA motifs and function as transcriptional activators.7,13 Other intracellular targets of thrombopoietin receptor activation include Shc, MAPK, Jun N-terminal kinase (JNK), Raf-1, Cbl, Vav, and the phosphatases SHPTP-1 and SHPTP-2.8-11,14 More recently, activation of phosphatidylinositol (PI) 3-kinase, Tec kinase, and protein kinase C by TPO has been described.15-18
The exact role of cytokines in hematopoietic lineage development remains controversial.19 In many instances, cytokines appear to regulate the expansion of their target cells by influencing survival and proliferation rather than by promoting differentiation of precursor cells. For example, in models for erythropoiesis, the erythropoietin receptor can be successfully replaced by the prolactin receptor.20,21 Moreover, interleukin-7 (IL-7) receptor gene disruption in mice leads to an almost complete loss of the T-cell lineage, but production of T cells can be restored in the absence of the IL-7 receptor merely by expressing the apoptosis inhibitor bcl-2 to save the cells from programmed cell death.22,23 In contrast, the TPO receptor has been shown to signal megakaryocytic differentiation in several cell lines in which other cytokine receptors fail to do so.24,25 In both of 2 human leukemia-derived cell lines, UT-7 and F-36P, c-mpl expression followed by TPO stimulation induces morphological differentiation into megakaryocytes as well as upregulation of megakaryocytic surface markers and polyploidy, whereas erythropoietin (Epo), IL-3, and granulocyte-macrophage colony-stimulating factor (GM-CSF) are only able to promote growth and survival.24 25
The signaling events that are specifically activated by the TPO receptor to induce megakaryocytic differentiation are not well-characterized. Both Ras activation and MAPK have been implicated in signaling differentiation.24,25 The contribution of the Jak-Stat pathway has been difficult to analyze, because initial mutants of the TPO receptor that disrupted the Jak-Stat pathway rendered the receptor completely nonfunctional and incapable of signaling for proliferation and survival.26,27 We have recently discovered that a 10 aa intracellular deletion in the TPO receptor proximal to the transmembrane region completely abrogates the JAK/Stat signaling pathway but nevertheless permits proliferation and cell survival of the cell lines BAF/3 and 32D.15 We report here the effect of this deletion on signaling and megakaryocytic differentiation using UT-7 cells.
MATERIALS AND METHODS
Cytokines and Antibodies
Human recombinant TPO (Escherichia coli-derived) was kindly provided by Amgen (Thousand Oaks, CA). Where indicated, mammalian-derived human rTPO from R&D Systems (Minneapolis, MN) was used. Human IL-3 and granulocyte colony-stimulating factor (GM-CSF) were a gift from Dr Arthur Bank (Columbia University, New York, NY). Human Epo was purchased from R&D Systems. Phycoerythrin-conjugated monoclonal antibodies (MoAbs) to CD41a were obtained from PharMingen (San Diego, CA). Polyclonal rabbit antisera against Jak-2 and Shc were purchased from Upstate Biotechnology (Lake Placid, NY). Anti–Tyk-2 antibodies were kindly provided by Dr John Krolewski (Columbia University). Polyclonal antibodies against Vav, STAT5a, STAT5b, and Erk-1 and monoclonal anti-Erk2 and anti-myc (clone 9E10) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Horseradish peroxidase-conjugated antiphosphotyrosine MoAb RC20 (clone PY20) was purchased from Transduction Laboratories (Lexington, KY). Antibodies to active MAPK were obtained from Promega (Madison, WI). Antibodies that recognize specifically the phosphorylated form of Akt were purchased from New England Biolabs (Beverly, MA). Polyclonal antibodies against Tec17 were a generous gift from Dr Hiroyuki Mano (Jichi Medical School, Minamikawachi, Japan). Anti-STAT1 antibodies (29130) were kindly provided by Dr Christiau Schindler (Columbia University).
Cell Lines and Culture
UT-7 cells28 were cultured in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10% fetal calf serum (FCS), 2 mmol/L L-glutamine, antibiotics, and 5 ng/mL human recombinant GM-CSF. The amphotropic retroviral packaging line Phoenix-ampho29 was maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FCS, 2 mmol/L L-glutamine, and antibiotics.
Retroviral Expression Constructs
The generation of the receptor mutants c-mplΔ7 (deletion of the first 10 amino acids [aa] of the intracellular domain), c-mplΔ8 (deletion of the N-terminal half of box1), and c-mplΔ7ΔC (deletion of the last 25 aa in c-mplΔ7) has been described previously.15The wild-type receptor and the deletion mutants (fused to a C-terminal myc-epitope) were isolated from the vector MT21myc and subcloned into the EcoRI-Xho I sites of the retroviral expression vector MSCV-IRES-GFP (provided by Gary Nolan, Stanford University, Stanford, CA). An internal ribosomal entry site (IRES) separates the receptor cDNA from the green fluorescent protein (GFP) cDNA in this vector and enables the simultaneous expression of both proteins from the murine stem cell virus (MSCV) promoter.
Production of Recombinant Retrovirus and Infection of UT-7 Cells
The amphotropic packaging cell line Phoenix-ampho was transiently transfected by the calcium phosphate precipitation method29with MSCV-IRES-GFP or with MSCV-IRES-GFP constructs encoding wild-type or mutant c-mpl. Virus-containing supernatant was collected 48 hours after transfection and used to infect UT-7 cells. Cells (5 × 106) were incubated in 2 mL virus-containing supernatant (diluted 1:2 in UT-7 culture medium) in the presence of 8 μg polybrene/mL for 4 hours. Subsequently, 8 mL of UT-7 culture medium was added and the cells were cultured for 3 days. Infected cells were selected based on the expression of GFP (renders the cells green fluorescent) using a fluorescence activated cell sorter (FACScan; Becton Dickinson, Mountain View, CA). After 3 subsequent rounds of sorting, a pure population of GFP-positive cells was obtained. Expression of the receptor protein was confirmed by staining the GFP-positive cell population with a TPO-Fc fusion protein.
Expression of a TPO-Fc Fusion Protein and Detection of c-mpl Surface Expression
Full-length cDNA encoding TPO was amplified by polymerase chain reaction from mouse liver Marathon-Ready cDNA (Clontech Laboratories, Palo Alto, CA) using the following oligos: 5′-GTTAGAATTCTGGCCAGAATGGAGCTG-3′ and 5′-GTGGCCCGGGCCTGTTTCCTGAGACAAATTC-3′. The cDNA was cloned into the EcoRI and Srf I sites of plasmid MT2130 that contained the gene encoding the Fc part of hIgG1. In the resulting construct (MT21-TPO-Fc), the Fc part of hIgG1 is fused in frame to the C-terminus of TPO. COS cells were transiently transfected with 5 μg of the construct by the chloroquine diethyl aminoethyl (DEAE)-dextran method,15 and culture supernatant was collected after 48 hours. The culture supernatant was tested for the presence of the TPO-Fc fusion protein by Western blot analysis with antibodies to hIgG1 (Sigma, St Louis, MO). To study cell surface expression of c-mpl, 1 × 106 cells were washed twice in phosphate-buffered saline (PBS) containing 3% FCS followed by incubation with undiluted culture supernatant (control or TPO-Fc containing) for 1 hour at 4°C. Cells were then washed 3 times in PBS/3% FCS. Bound TPO-Fc was detected by incubating the cells with a phycoerythrin-conjugated antihuman IgG antiserum (Sigma). After 30 minutes at 4°C, cells were washed 3 times in PBS/3% FCS and analyzed on a FACScan.
Proliferation Assay
Cells were cultured at a density of 1 × 104 per 200 μL in a 96-well round-bottom microtiter plate with varying concentrations of recombinant cytokines in culture medium for 72 hours. During the last 6 hours of culture, cells were pulse-labeled with 0.5 μCi of [3H] thymidine (specific activity, 5 Ci/mmol; Amersham, Arlington Heights, IL), and [3H] thymidine incorporation was quantified by scintillation counting as described.15
Growth Factor Stimulation, Western Blot Analysis, and Immunoprecipitation
UT-7 cells were growth factor-starved for 8 to 12 hours in IMDM supplemented with 10% FCS. Stimulation was performed at a concentration of 5 × 107 cells/mL with 200 ng/mL TPO or 50 ng/mL GM-CSF, if not indicated otherwise. Stimulation was stopped and cell extracts were prepared with lysis buffer (20 mmol/L Tris-HCl, pH 8, 138 mmol/L NaCl, 10% glycerol, 1% NP-40, 0.025 mmol/L p-nitrophenyl guanidinobenzoate, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 1 mmol/L Na3VO4, 2 mmol/L EDTA, 10 mmol/L NaF) at 2 × 106 cells/250 μL, as described.8 Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Western blot analyses and immunoprecipitations were performed as described.8
Electrophoretic Mobility Shift Assay (EMSA)
PI 3-Kinase Assay
PI 3-kinase activity was measured as described.15
RESULTS
Expression of TPO Receptor Mutants in UT-7 Cells
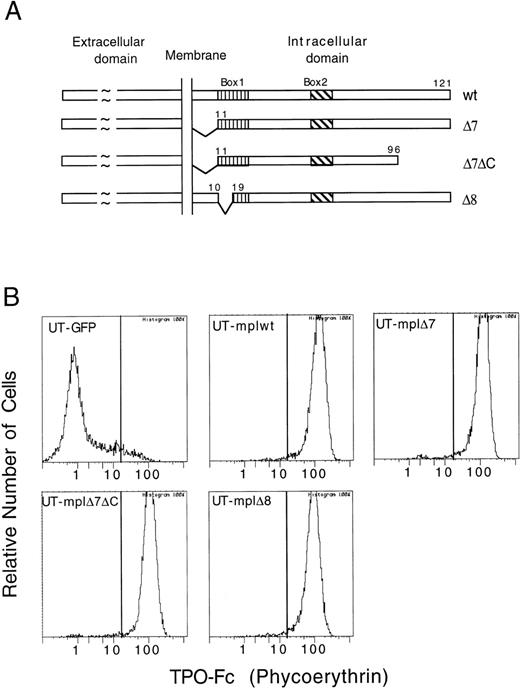
A panel of c-mpl deletion mutants was introduced into UT-7 cells. The receptor mutants analyzed in this study were described previously15 and are shown in Fig 1A. Briefly, c-mplΔ7 lacks the first 10 aa of the intracellular domain, c-mplΔ7ΔC has an additional deletion of 25 aa at the C-terminus of c-mplΔ7, and c-mplΔ8 lacks 8 aa in the N-terminal half of box1. UT-7 cells stably expressing wild-type or mutant c-mpl were established by retroviral infection. To this end, the receptor constructs were cloned into the retroviral vector MSCV-IRES-GFP and retrovirus-containing supernatant produced in a transient packaging system was used to infect UT-7 cells. MSCV-IRES-GFP enables the simultaneous expression of the receptor protein and GFP and allows for the selection of infected cells based on GFP expression using a fluorescence-activated cell sorter. Cell surface expression of the receptor protein was confirmed with a TPO-Fc fusion protein (Fig 1B). UT-7 cell populations expressing similar levels of c-mplwt (UT-mplwt), c-mplΔ7 (UT-mplΔ7), c-mplΔ7ΔC (UT-mplΔ7ΔC), or c-mplΔ8 (UT-mplΔ8) were selected for further analysis (Fig 1B).
Cell surface expression of wild-type and mutant c-mpl on UT-7. (A) Schematic representation of c-mplwt and deletion mutants. (B) UT-mplwt, UT-mpl▵7, UT-mpl▵7▵C, and UT-mpl▵8 cells express similar levels of receptor as detected by their ability to bind a TPO-Fc fusion protein (see Materials and Methods). No binding was detected on UT-7 cells infected with empty MCSV-pIRES-GFP virus (UT-GFP).
Cell surface expression of wild-type and mutant c-mpl on UT-7. (A) Schematic representation of c-mplwt and deletion mutants. (B) UT-mplwt, UT-mpl▵7, UT-mpl▵7▵C, and UT-mpl▵8 cells express similar levels of receptor as detected by their ability to bind a TPO-Fc fusion protein (see Materials and Methods). No binding was detected on UT-7 cells infected with empty MCSV-pIRES-GFP virus (UT-GFP).
Proliferative Response Mediated by TPO Receptor Mutants
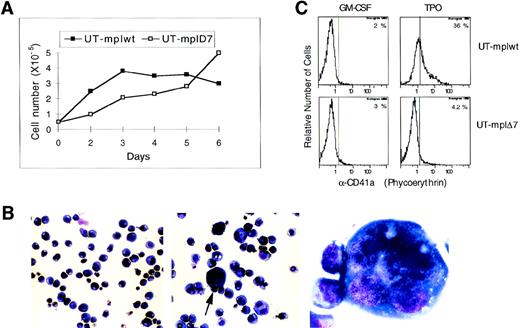
To determine whether our deletion mutants were able to confer TPO responsiveness to UT-7 cells, we analyzed the short-term mitogenic response (72 hours) and growth during prolonged culture (>3 months). Both UT-mplwt and UT-mplΔ7 responded strongly to TPO, although UT-mplΔ7 required higher TPO concentrations to reach the same maximal proliferation as cells expressing the wild-type receptor (Fig 2A). UT-mplΔ7 required 200- to 300-fold higher TPO concentration to reach half-maximal proliferation when compared with UT-mplwt (4 v 0.015 ng/mL; Fig2A). UT-mplΔ8 and parental UT-7 cells did not respond to TPO at all. (Experiments in Fig 2 were performed with E coli-derived TPO and were confirmed with mammalian-derived TPO.) UT-mplΔ7 could be maintained in TPO for a prolonged period of time (>3 months, not shown). Figure 2B shows that the magnitude of the short-term mitogenic response of UT-mplwt and UT-mplΔ7 to TPO was comparable to the one induced by GM-CSF or IL-3 and stronger than the one induced by Epo. Thus, c-mplΔ7 was competent to mediate proliferation of UT-7 cells, as was previously observed in the murine cell lines BAF/3 and 32D. Remarkably, the proliferative response mediated by c-mplΔ7 is completely abrogated by additional truncation of the 25 aa at the C-terminus of the receptor in UT-mplΔ7ΔC (Fig 2A).
Mitogenic response mediated by c-mpl mutants. The mitogenic response was measured by [3H] thymidine incorporation and is shown as a percentage of the maximal GM-CSF–induced response. The mean of triplicate counts for each data point is shown. Standard deviations are indicated by error bars. (A) The mitogenic response of UT-mplwt, UT-mpl▵7, UT-mpl▵7▵C, and UT-mpl▵8 cells and parental UT-7 cells was measured at different TPO concentrations. (B) Comparison of the maximal response of UT-mplwt (▪) and UT-mpl▵7 (□) to GM-CSF (1 ng/mL), IL-3 (2.5 ng/mL), Epo (2.5 U/mL), and TPO (12.5 ng/mL).
Mitogenic response mediated by c-mpl mutants. The mitogenic response was measured by [3H] thymidine incorporation and is shown as a percentage of the maximal GM-CSF–induced response. The mean of triplicate counts for each data point is shown. Standard deviations are indicated by error bars. (A) The mitogenic response of UT-mplwt, UT-mpl▵7, UT-mpl▵7▵C, and UT-mpl▵8 cells and parental UT-7 cells was measured at different TPO concentrations. (B) Comparison of the maximal response of UT-mplwt (▪) and UT-mpl▵7 (□) to GM-CSF (1 ng/mL), IL-3 (2.5 ng/mL), Epo (2.5 U/mL), and TPO (12.5 ng/mL).
Differentiation Mediated by TPO Receptor Mutants
To study induction of differentiation by TPO signaling, UT-7 cells expressing c-mplwt or c-mplΔ7 were cultured in TPO (25 ng/mL) and cell numbers and cell morphology were studied daily for 6 days. The number of UT-mplwt cells increased approximately 7-fold within 3 days and then stayed constant (Fig 3A). The growth rate of UT-mplΔ7 was lower but cells continued to divide up to and beyond day 6 (Fig 3A). In a 6-hour [3H] thymidine incorporation assay performed after 64 hours of TPO stimulation (Fig2A), both UT-mplwt and UT-mplΔ7 proliferate at the same rate, indicating that the initial more vigorous growth induced by the wild-type receptor is already slowing down at 72 hours.
Analysis of TPO-induced differentiation in UT-mplwt and UT-mpl▵7. (A) Cells (0.5 × 105) were cultured in TPO (25 ng/mL) for 6 days and the cell number was counted on the indicated days. (B) Cells cultured in TPO for 6 days were cytocentrifuged on glass slides and stained with May-Grunewald-Giemsa stain. UT-mplwt cells (middle panel: original magnification × 200, arrow indicates cells shown in right panel at original magnification × 600) show an increase in cell size and cell adherence and some cells become polyploid (right panel), whereas UT-mpl▵7 cells (left panel) look identical to cells cultured in GM-CSF (not shown). (C) Surface expression of CD41a was analyzed on UT-mplwt and UT-mpl▵7 cells after 6 days in TPO or GM-CSF by flow cytometry.
Analysis of TPO-induced differentiation in UT-mplwt and UT-mpl▵7. (A) Cells (0.5 × 105) were cultured in TPO (25 ng/mL) for 6 days and the cell number was counted on the indicated days. (B) Cells cultured in TPO for 6 days were cytocentrifuged on glass slides and stained with May-Grunewald-Giemsa stain. UT-mplwt cells (middle panel: original magnification × 200, arrow indicates cells shown in right panel at original magnification × 600) show an increase in cell size and cell adherence and some cells become polyploid (right panel), whereas UT-mpl▵7 cells (left panel) look identical to cells cultured in GM-CSF (not shown). (C) Surface expression of CD41a was analyzed on UT-mplwt and UT-mpl▵7 cells after 6 days in TPO or GM-CSF by flow cytometry.
UT-mplΔ7 cultured in TPO showed no changes in morphology and were indistinguishable from cells cultured in GM-CSF (Fig 3B), whereas cells expressing the wild-type receptor showed a striking increase in cell size and cell adherence (Fig 3B). Analysis of surface expression of the megakaryocytic marker CD41a by flow cytometry showed that only UT-mplwt and not UT-mplΔ7 showed an upregulation of CD41a when cultured in TPO as compared with GM-CSF for 6 days (Fig 3C) or up to 3 weeks (data not shown). The failure of UT-mplΔ7 to undergo differentiation was also observed at very high TPO concentrations (up to 1,000 ng/mL, data not shown). We analyzed whether the simultaneous stimulation of UT-mplΔ7 cells with TPO and one of the other cytokines (GM-CSF, Epo, or IL-3) might restore the differentiative response. UT-mplwt and UT-mplΔ7 cells were cultured for 7 days in each of the 3 other cytokines alone or in combination with TPO. Neither IL-3 nor GM-CSF nor Epo was able to complement the defect of c-mplΔ7 in mediating TPO-induced CD41a upregulation on UT-mplΔ7 cells. Moreover, these cytokines did not suppress or augment the differentiation induced by TPO and the wild-type receptor as determined by the level of CD41a expression (Table 1). Thus, whereas the c-mplΔ7 mutant was competent to mediate proliferative signaling, it was unable to induce changes in morphology or the expression of differentiation markers characteristic of megakaryocytes.
Growth Factor-Stimulated CD41a Expression
| . | % CD41a+ Cells* . | |
|---|---|---|
| UT-mplwt . | UT-mplΔ7 . | |
| TPO (25 ng/mL) | 34.8 | 4.9 |
| Epo (1 U/mL) | 4.7 | 4.6 |
| GM-CSF (5 ng/mL) | 1.9 | 2.58 |
| IL-3 (1 ng/mL) | 2.9 | 3 |
| TPO/Epo | 35.4 | 2.98 |
| TPO/GM-CSF | 35.1 | 2.99 |
| TPO/IL-3 | 35.2 | 3.14 |
| . | % CD41a+ Cells* . | |
|---|---|---|
| UT-mplwt . | UT-mplΔ7 . | |
| TPO (25 ng/mL) | 34.8 | 4.9 |
| Epo (1 U/mL) | 4.7 | 4.6 |
| GM-CSF (5 ng/mL) | 1.9 | 2.58 |
| IL-3 (1 ng/mL) | 2.9 | 3 |
| TPO/Epo | 35.4 | 2.98 |
| TPO/GM-CSF | 35.1 | 2.99 |
| TPO/IL-3 | 35.2 | 3.14 |
Cells were cultured at 0.5 × 105 cells/mL in medium containing the indicated cytokines. After 6 days, expression of CD41a was analyzed.
Characterization of Signaling Events
Jak-STAT pathway.
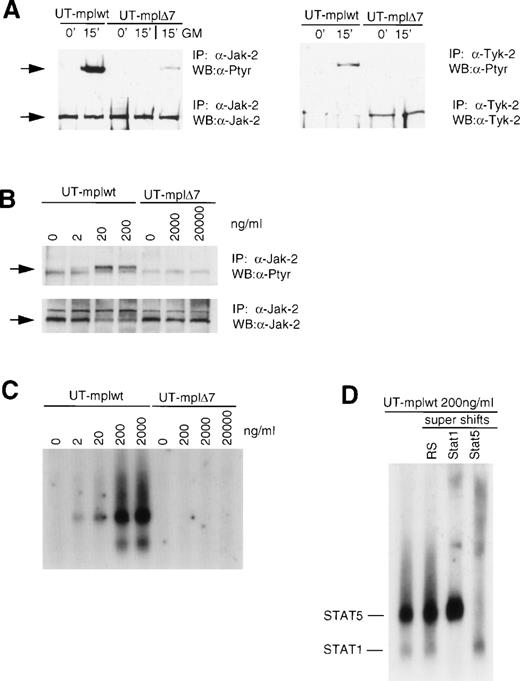
We have previously shown that c-mplΔ7 does not activate the Jak-STAT pathway in BAF/3 and 32D cells.15 To confirm this observation in UT-7 cells, we analyzed tyrosine phosphorylation of Jak-2 and Tyk-2 in TPO-stimulated UT-mplwt and c-mplΔ7 cells (Fig 4A and Table 2). TPO (200 ng/mL) induced strong tyrosine phosphorylation of Jak-2 and Tyk-2 after 15 minutes in cells expressing the wild-type receptor but not in cells expressing c-mpl Δ7. In contrast, GM-CSF (50 ng/mL) rapidly induced tyrosine phosphorylation of Jak-2 in UT-cmplΔ7 cells, indicating that the Jak kinases were functional in these cells. Identical results were observed after 1, 5, and 30 minutes (data not shown). To exclude the possibility that the lack of signal observed with c-mplΔ7 merely reflected a shift in the dose-response curve similar to that seen in the proliferation assay in Fig 2A, we compared Jak-2 tyrosine phosphorylation in response to escalating TPO concentrations (Fig 4B). In UT-mplwt cells, Jak-2 phosphorylation could be clearly detected after stimulation with 2 ng/mL and the maximal response was detected at 20 ng/mL. Stimulation with 200 ng/mL did not lead to a further increase of the response. In contrast, in UT-cmplΔ7 cells, Jak-2 phosphorylation was undetectable even after stimulation with TPO concentrations of 2,000 or 20,000 ng/mL. TPO also failed to induce tyrosine phosphorylation of Jak-2 and Tyk-2 in UT-mplΔ7ΔC and UT-mplΔ8 (data not shown). The major downstream targets of Jak kinases are the STAT proteins. Tyrosine phosphorylation of STATs results in their translocation to the nucleus and binding to specific promoter elements. Using a DNA probe (GAS-element) that binds STAT proteins activated by TPO, we detected STAT binding activity in UT-mplwt but not UT-mplΔ7 stimulated with a range of TPO concentrations (Fig 4C and Table 2). In UT-mplwt cells, maximal complex formation was observed at 200 ng/mL, but a clear response could already be detected at 2 ng/mL, whereas no response was detectable in UT-mplΔ7 cells, even after stimulation with 20,000 ng/mL. Supershift assays performed with UT-mplwt stimulated with 200 ng/mL showed that the complex with the higher mobility was shifted with antibodies to STAT1 and the complex with the lower mobility with antibodies to STAT5 (Fig 4D). In summary, the results confirm that c-mpl Δ7 fails to mediate Jak-STAT activation in UT-7 cells. Thus, the mutant receptor failed to generate a signal even with a 10,000-fold excess in TPO concentration compared with the amount necessary to detect a signal with the wild-type receptor.
C-mpl▵7 does not mediate Jak-STAT activation. (A) TPO stimulates tyrosine phosphorylation of Jak-2 and Tyk-2 in UT-mplwt but not in UT-mpl▵7 cells. Growth factor-deprived cells were left untreated or were stimulated with TPO (200 ng/mL) or GM-CSF (50 ng/mL) for the indicated times and lysates were prepared. Jak-2 and Tyk-2 were immunoprecipitated with anti–Jak-2 or anti–Tyk-2 antiserum, respectively, and subsequently immunoblotted with antiphosphotyrosine antibodies. Membranes were stripped and reprobed with anti-Jak2 or anti-Tyk-2 antiserum to confirm equal loading of protein in all lanes. (B) Tyrosine phosphorylation of Jak-2 in response to increasing TPO concentrations. Whereas phosphorylation of Jak-2 in UT-mplwt cells was detectable after stimulation with 2 ng/mL, Jak-2 phosphorylation in UT-mpl▵7 cells was undetectable even at 20 μg/mL. (C) GAS-binding activity was detected in UT-mplwt but not in UT-mpl▵7 cells. Growth factor-deprived cells were left untreated or were stimulated with the indicated TPO concentrations for 15 minutes. Cell extracts were prepared and analyzed by EMSA using the IRF-1 GAS probe. (D) The identity of the GAS-binding complexes in UT-mplwt cells (200 ng/mL) was examined by supershift assays with antibodies specific for STAT1 and STAT5 (STAT5a and 5b antibodies were pooled). IP, immunoprecipitation; WB, Western blot; RS, control rabbit serum.
C-mpl▵7 does not mediate Jak-STAT activation. (A) TPO stimulates tyrosine phosphorylation of Jak-2 and Tyk-2 in UT-mplwt but not in UT-mpl▵7 cells. Growth factor-deprived cells were left untreated or were stimulated with TPO (200 ng/mL) or GM-CSF (50 ng/mL) for the indicated times and lysates were prepared. Jak-2 and Tyk-2 were immunoprecipitated with anti–Jak-2 or anti–Tyk-2 antiserum, respectively, and subsequently immunoblotted with antiphosphotyrosine antibodies. Membranes were stripped and reprobed with anti-Jak2 or anti-Tyk-2 antiserum to confirm equal loading of protein in all lanes. (B) Tyrosine phosphorylation of Jak-2 in response to increasing TPO concentrations. Whereas phosphorylation of Jak-2 in UT-mplwt cells was detectable after stimulation with 2 ng/mL, Jak-2 phosphorylation in UT-mpl▵7 cells was undetectable even at 20 μg/mL. (C) GAS-binding activity was detected in UT-mplwt but not in UT-mpl▵7 cells. Growth factor-deprived cells were left untreated or were stimulated with the indicated TPO concentrations for 15 minutes. Cell extracts were prepared and analyzed by EMSA using the IRF-1 GAS probe. (D) The identity of the GAS-binding complexes in UT-mplwt cells (200 ng/mL) was examined by supershift assays with antibodies specific for STAT1 and STAT5 (STAT5a and 5b antibodies were pooled). IP, immunoprecipitation; WB, Western blot; RS, control rabbit serum.
Summary of Events Mediated by c-mpl and Its Mutants in UT-7
| . | TPO . | GM-CSF . | |||
|---|---|---|---|---|---|
| UT- mplwt . | UT- mplΔ7 . | UT- mplΔ7ΔC . | UT- mplΔ8 . | UT- mplΔ7* . | |
| Proliferation | ++++ | +++ | − | − | ++++ |
| Differentiation | ++++ | − | − | − | − |
| Jak-2 | ++++ | − | − | − | ++ |
| Tyk-2 | ++++ | − | − | − | ND |
| STATs | ++++ | − | ND | ND | ND |
| Tec | ++ | ++ | ND | ND | ND |
| Shc | ++++ | + | − | − | +++ |
| Vav | ++++ | + | − | − | ++ |
| c-mpl | ++++ | − | − | − | ND |
| MAPK | ++++ | + | − | − | ++ |
| Akt | ++++ | +/− | − | − | ++ |
| PI 3-kinase | ++++ | + | ND | ND | ++++ |
| . | TPO . | GM-CSF . | |||
|---|---|---|---|---|---|
| UT- mplwt . | UT- mplΔ7 . | UT- mplΔ7ΔC . | UT- mplΔ8 . | UT- mplΔ7* . | |
| Proliferation | ++++ | +++ | − | − | ++++ |
| Differentiation | ++++ | − | − | − | − |
| Jak-2 | ++++ | − | − | − | ++ |
| Tyk-2 | ++++ | − | − | − | ND |
| STATs | ++++ | − | ND | ND | ND |
| Tec | ++ | ++ | ND | ND | ND |
| Shc | ++++ | + | − | − | +++ |
| Vav | ++++ | + | − | − | ++ |
| c-mpl | ++++ | − | − | − | ND |
| MAPK | ++++ | + | − | − | ++ |
| Akt | ++++ | +/− | − | − | ++ |
| PI 3-kinase | ++++ | + | ND | ND | ++++ |
Abbreviation: ND, not determined.
Identical results were obtained when UT-mplwt cells were stimulated with GM-CSF. The GM-CSF–stimulated phosphorylation of Jak-2, Vav, MAPK, and Akt in both UT-mplwt and UT-mplΔ7 cells was weaker than the TPO-stimulated phosphorylation in UT-mplwt.
Tyrosine kinase Tec.
Tec phosphorylation was detected 5 and 15 minutes after TPO stimulation in UT-mplwt and UT-mplΔ7 (Fig 5A and Table 2). The relatively weak increase in tyrosine phosphorylation of Tec after stimulation is consistent with previous observations by others.17 There was no difference in the level of phosphorylation between the wild-type receptor and the mutant, indicating that Tec activation is not impaired by the 10 aa membrane-proximal deletion.
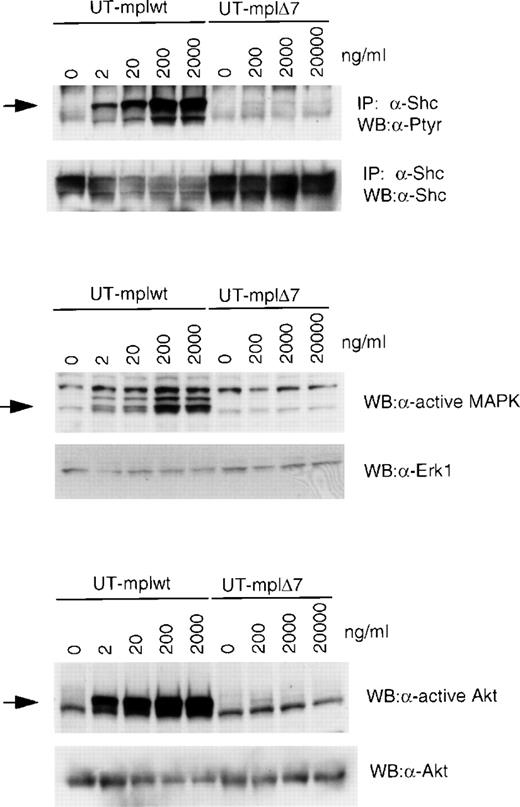
Effect of TPO stimulation on Tec, Shc, Vav, the receptor itself, MAPK, Akt, and PI 3-K. Growth factor-deprived UT-mplwt and UT-mpl▵7 cells were either left untreated or were stimulated with TPO (200 ng/mL) or GM-CSF (50 ng/mL) for the indicated times and cell extracts were prepared. Immunoprecipitations were performed with antibodies to Tec (A), Shc (B), Vav (C), and myc (D), and the immunoprecipitates were blotted with antiphosphotyrosine antibodies (A through D). To confirm equal loading of protein, membranes were stripped and reprobed with the antibodies used for immunoprecipitations. (E and F) Cell lysates were immunoblotted with anti-active MAPK that recognizes the active forms of Erk-1 and Erk-2 (E) or anti-active Akt antibodies that recognize the phosphorylated form of Akt (F). Membranes were stripped and reprobed with anti-Erk2 and anti-Akt antibodies, respectively, to confirm equal protein loading. (G) PI 3-K activity was measured in antiphosphotyrosine immunoprecipitates. PI-3 P formation was visualized by TLC and subsequent autoradiography.
Effect of TPO stimulation on Tec, Shc, Vav, the receptor itself, MAPK, Akt, and PI 3-K. Growth factor-deprived UT-mplwt and UT-mpl▵7 cells were either left untreated or were stimulated with TPO (200 ng/mL) or GM-CSF (50 ng/mL) for the indicated times and cell extracts were prepared. Immunoprecipitations were performed with antibodies to Tec (A), Shc (B), Vav (C), and myc (D), and the immunoprecipitates were blotted with antiphosphotyrosine antibodies (A through D). To confirm equal loading of protein, membranes were stripped and reprobed with the antibodies used for immunoprecipitations. (E and F) Cell lysates were immunoblotted with anti-active MAPK that recognizes the active forms of Erk-1 and Erk-2 (E) or anti-active Akt antibodies that recognize the phosphorylated form of Akt (F). Membranes were stripped and reprobed with anti-Erk2 and anti-Akt antibodies, respectively, to confirm equal protein loading. (G) PI 3-K activity was measured in antiphosphotyrosine immunoprecipitates. PI-3 P formation was visualized by TLC and subsequent autoradiography.
Targets of tyrosine phosphorylation: Shc, Vav, and c-mpl.
Tyrosine phosphorylation of Shc, Vav, and c-mpl itself was readily detected in TPO-stimulated UT-mplwt cells after 15 minutes. Only very weak phosphorylation of Vav and Shc and no phosphorylation of c-mplΔ7 was detected in UT-mplΔ7 cells after 5 and 15 minutes of stimulation with TPO, whereas GM-CSF induced strong phosphorylation of Shc and Vav in the same cells (Fig 5B through D and Table 2). In UT-mplΔ7ΔC and UT-mplΔ8, none of these molecules was tyrosine phosphorylated upon TPO stimulation (not shown; Table 2). We also performed a dose-response study of Shc tyrosine phosphorylation in cells expressing c-mplwt and c-mplΔ7 (Fig 6). At a TPO concentration of 2 ng/mL, Shc phosphorylation was clearly detectable in UT-mplwt, and at 200 ng/mL the response was maximal. However, in UT-mplΔ7, only a very slight signal was detected at 200 ng/mL, and stimulation with increasing concentrations (up to 20,000 ng/mL) did not enhance the response.
Analysis of Shc, MAPK, and Akt phosphorylation in response to increasing TPO concentrations. UT-mplwt and UT-mpl▵7 cells were stimulated for 15 minutes (Shc) or 5 minutes (MAPK and Akt) with the indicated TPO conentrations, and phosphorylation of Shc, MAPK, and Akt was analyzed as described in the legend of Fig 5.
Analysis of Shc, MAPK, and Akt phosphorylation in response to increasing TPO concentrations. UT-mplwt and UT-mpl▵7 cells were stimulated for 15 minutes (Shc) or 5 minutes (MAPK and Akt) with the indicated TPO conentrations, and phosphorylation of Shc, MAPK, and Akt was analyzed as described in the legend of Fig 5.
Serine/threonine kinases: MAPK and Akt.
Activation of MAPK and Akt was analyzed with antibodies that specifically recognize the phosphorylated and activated forms of these serine/threonine kinases. Both kinases were activated in TPO-stimulated UT-mplwt after 5, 15, and 30 minutes, but their activation was nearly undetectable in UT-mplΔ7 cells at any of these time points (Fig 5E and F and Table 2). GM-CSF readily induced activation of MAPK and AKT in the same cells (Fig 5E and F). No activation of these kinases was detected in TPO-stimulated UT-mplΔ7ΔC and UT-mplΔ8 (not shown; Table 2). Analysis of MAPK and Akt phosphorylation in response to a wide range of TPO concentrations (Fig 6) showed that, in UT-mplwt cells, phosphorylation was clearly detectable after stimulation with 2 ng/mL and that a plateau was reached at 200 ng/mL. In UT-mplΔ7 cells, no significant signal was detected even after stimulation with concentrations as high as 20,000 ng/mL.
PI-3 kinase.
PI-3 kinase activity was measured in antiphosphotyrosine immunoprecipitates from stimulated cells and was shown to be strongly activated in UT-mplwt cells after 1 and 5 minutes of stimulation with TPO. In UT-mplΔ7 cells, similarly strong activation of PI-3 kinase was observed after GM-CSF stimulation, but activation by TPO was much weaker and only detected after 5 minutes of stimulation (Fig 5G and Table 2).
DISCUSSION
The data reported here confirm and extend our previous finding that the Jak-STAT pathway is not required for TPO-induced proliferation.15 The receptor mutant c-mplΔ7 lacking the 10 membrane-proximal aa does not mediate phosphorylation of Jaks and activation of their target proteins, the STATs, in UT-7 cells, but nevertheless mediates proliferation in response to TPO. This closely resembles our finding in BAF/3 and 32D cells.15 However, in UT-7 cells, signaling by the mutant is more severely impaired than previously observed in the murine cell lines. This might reflect a lesser degree of receptor cross-talk, possibly due to expression of a murine receptor in a human cell line. In UT-mplΔ7 cells, phosphorylation of the receptor itself does not occur, showing that it is similarly not required for proliferation. Likewise, activation of PI3-kinase and phosphorylation of Shc, Vav, MAPK, and Akt were strongly reduced, and yet vigorous proliferation could be induced (Table 2). Because the TPO concentration required to induce half-maximal proliferation in UT-mplΔ7 is 200- to 300-fold higher than for UT-mplwt (Fig 2A), we performed dose-escalation studies for the signaling assays to exclude the possibility that the failure to detect signals was due to insufficient TPO concentrations. TPO concentrations used to stimulate UT-mplΔ7 were 10,000-fold higher than the concentrations resulting in a clear signal in UT-mplwt, but activation of the Jak-STAT pathway did not occur.
However, despite significant impairments in signal transduction, the c-mplΔ7 mutant is able to mediate a strong mitogenic response, raising the possibility that as yet unidentified events contribute to the proliferative response mediated by this receptor mutant. We note that the proliferative response mediated by the mutant receptor is comparable to the one induced by IL-3, GM-CSF, and Epo in the same cells (Fig 2B) and gives rise to a 10-fold increase in cell number within 6 days (Fig 3A). The preserved proliferative response is critically dependent on the 25 C-terminal aa, because their additional deletion abrogated the proliferative response induced by the receptor mutant c-mplΔ7. In the context of BAF/3 cells, this additional truncation only reduced proliferation, likely reflecting the less pronounced impairment of signaling by c-mplΔ7 in those cells.15 Thus, the involvement of this region in proliferation may have been less obvious because of multiple redundant pathways that are involved in the mitogenic response. In agreement with this conjecture, the proliferation mediated by the wild-type receptor is not significantly impaired by truncation of the C-terminal 25 aa in BAF/3 cells (M.D. and S.G, unpublished observations; and previous studies26,27). Phosphorylation of Shc has been mapped to this region,26,27 and we cannot exclude the possibility that this explains the abrogation of proliferation in UT-mplΔ7ΔC cells. However, we do not consider this possibility particularly likely, because Shc is only slightly phosphorylated even with an intact C-terminus in UT-mplΔ7 cells (Table 2). Screening for new molecular interactions in this region to further elucidate its involvement in proliferation may yield new partners of the receptor. The existence of a Jak-independent mitogenic pathway has also been suggested by another study.33 It was shown that inhibition of Ras abolishes the mitogenic response mediated by a constitutive active tyk-2 but not by the IL-3 receptor in BAF/3 cells, indicating that as yet unidentified mitogenic signals emanate from the IL-3 receptor.33
When stimulated with TPO, UT-7 cells expressing the wild-type receptor undergo differentiation resembling maturation of the megakaryocytic lineage. This differentiation is signified by an increase in CD41 expression and morphological changes (Fig 3), but ploidy increases in only approximately 5% of the cells.24 In contrast to the preserved ability of c-mplΔ7 to mediate proliferation, its ability to mediate differentiation in UT-7 cells is completely ablated. This demonstrates that the signals required for proliferation and megakaryotic differentiation are different. Many mutations of c-mpl that have been examined for their effect on megakaryocytic differentiation either abolish or preserve both proliferation and differentiation.34 However, an internal deletion of 23 aa in the C-terminal half of the receptor likewise preserves proliferation but not differentiation in UT-7.24 In that case, the differentiation was attributed to MAPK activation, because simultaneous activation of the Epo receptor or a constitutive-active MAPK mutant could restore the ability of the mutant to mediate differentiation.24,35 In the case of our mutant c-mplΔ7, differentiation cannot be restored by complementation through simultaneous stimulation with Epo, GM-CSF, or IL-3. This is remarkable, because many of the signaling events by these cytokine receptors are overlapping and, in the case of Epo, almost identical.13,36We are unable to attribute the ablation of differentiation in c-mplΔ7 to one signaling cascade alone, because multiple pathways are impaired. Both Tyk-2 and Jak-2 are candidates for playing an important role in this process, but their wide distribution and involvement in the signaling of many different receptors exclude the possibility that they are the sole mediators of megakaryocytic differentiation.13Recently, Jak-2 was implicated in megakaryopoiesis by targeted disruption of Jak-2 in mice, but so far it remains unresolved whether its role is in lineage commitment or clonal expansion.37,38The absence of Jak-2 expression results in embryonic lethality at day 12.5 due to the absence of definitive erythropoiesis. Jak-2–deficient fetal liver cells do not respond to Epo, TPO, IL-3, or GM-CSF in colony-forming assays.37 Committed erythroid progenitor cells appear to form in the absence of Jak-2, because the colony-forming potential can be restored by gene transfer of Jak-2. However, mature enucleated erythroid cells are never observed in fetal livers of Jak-2–deficient embryos,38 and it is impossible to conclude whether this is due to a failure in differentiation or a failure in expansion of differentiating cells or both. The analysis of the presence or absence of megakaryocytic precursors in Jak-2–deficient mice has not been reported.37 38
A better understanding of the signaling requirements for megakaryocytic differentiation will necessitate the identification of target molecules that transmit the differentiation program to the transcriptional machinery. The cell lines described here will aid the search for the molecular targets of TPO signaling that switch the program of UT-7 cells from proliferation to differentiation.
ACKNOWLEDGMENT
The authors thank Dr Gary Nolan for the plasmid MSCV-IRES-GFP and the Phoenix cell line, Dr Hiroyuki Mano for anti-tec antibodies, Dr N. Komatsu for UT-7 cells, and Amgen for a generous supply of TPO. We also thank Pang-Dian Fan for critically reviewing the manuscript and Juntao Liu for his assistance with the fluorescence activated cell sorter.
Supported by grants of the National Institute of Health and the Cancer Research Institute awarded to P.B.R. S.P.G. is a Howard Hughes Medical Institute investigator. M.D. is an associate of Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Stephen P. Goff, PhD, Howard Hughes Medical Institute, Columbia University College of Physicians and Surgeons, 701 W 168th St, New York, NY 10032; e-mail: goff@cuccfa.ccc.columbia.edu.

![Fig. 2. Mitogenic response mediated by c-mpl mutants. The mitogenic response was measured by [3H] thymidine incorporation and is shown as a percentage of the maximal GM-CSF–induced response. The mean of triplicate counts for each data point is shown. Standard deviations are indicated by error bars. (A) The mitogenic response of UT-mplwt, UT-mpl▵7, UT-mpl▵7▵C, and UT-mpl▵8 cells and parental UT-7 cells was measured at different TPO concentrations. (B) Comparison of the maximal response of UT-mplwt (▪) and UT-mpl▵7 (□) to GM-CSF (1 ng/mL), IL-3 (2.5 ng/mL), Epo (2.5 U/mL), and TPO (12.5 ng/mL).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/8/10.1182_blood.v94.8.2676.420k28_2676_2685/7/m_blod42028002x.jpeg?Expires=1770030882&Signature=uPzOYIc1w0RXKaxjKe7FChnyrW2Q84USZ7oVRUUpSm4TkN2xH7LgTOEjSmFiktPGwC9c-gpt8064nmH9E4irkf9DbxdlpRG8xUYrp4KhcV0PxlsYjuJUhlydw21hR7qfIrSZxWsYeb85Y9-n5wnR7aCgngzerVc330NCWzf8xi4mkrrh5U3ehFwlEKfKwz-hX3N-ShcmvF0k~Ev-7~6bSPASif~Eg9QqKiYFJWswy16uBEpzZebcCgCeUPT5YT-7nE5cl4UZKCCs2OmEeOVyAItYcV2O2ViVF0ufTVWEldBb20yGlI3itxRS1H2Cbl8PChmGdn~xbCoNK4fzT220fw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal