Comparative measurements of different types of hematopoietic progenitors present in human fetal liver, cord blood, and adult marrow showed a large (up to 250-fold), stage-specific, but lineage-unrestricted, amplification of the colony-forming cell (CFC) compartment in the fetal liver, with a higher ratio of all types of CFC to long-term culture-initiating cells (LTC-IC) and a lower ratio of total (mature) cells to CFC. Human fetal liver LTC-IC were also found to produce more CFC in LTC than cord blood or adult marrow LTC-IC, and more of the fetal liver LTC-IC–derived CFC were erythroid. Human fetal liver cells regenerated human multilineage hematopoiesis in NOD/SCID mice with the same kinetics as human cord blood and adult marrow cells, but sustained a high level of terminal erythropoiesis not seen in adult marrow-engrafted mice unless exogenous human erythropoietin (Epo) was injected. This may be due to a demonstrated 10-fold lower activity of murine versus human Epo on human cells, sufficient to distinguish between a differential Epo sensitivity of fetal and adult erythroid precursors. Examination of human LTC-IC, CFC, and erythroblasts generated either in NOD/SCID mice and/or in LTC showed the types of cells and hemoglobins produced also to reflect their ontological origin, regardless of the environment in which the erythroid precursors were generated. We suggest that ontogeny may affect the behavior of cells at many stages of hematopoietic cell differentiation through key changes in shared signaling pathways.
HEMATOPOIESIS IS FIRST detectable in the extravascular regions of the developing human fetal liver during week 6 of gestation, which then remains the major site of hematopoiesis until birth.1 In addition to morphologically recognizable hematopoietic cells, the human fetal liver has been shown to contain the full hierarchy of more primitive progenitors detectable by functional endpoints. These include erythroid, megakaryopoietic, granulopoietic, and multilineage colony-forming cells (CFC) detectable in semisolid culture assays,2-7 their more primitive precursors, referred to as long-term culture-initiating cells (LTC-IC) because they are able to proliferate and differentiate in vitro for more than 5 weeks on stromal feeder layers,7 and transplantable stem cells able to engraft xenogeneic8-11 as well as allogeneic recipients.12 Comparisons of analogous cell types in murine fetal tissues and adult marrow have shown differences in their rate of turnover,13 surface phenotype,14,15 rate of regeneration posttransplant,16-21 responsiveness to specific growth factors,22-27 and differentiation programs executed by their lineage-restricted progeny.28-30 Previous studies have provided some indication that similar differences between fetal and adult hematopoietic cells of human origin also exist.31-37
In vivo, the fetal liver is characterized by a predominance of terminally differentiating erythroid cells. This could reflect an intrinsically increased probability of pluripotent fetal liver stem cells to commit to this pathway or the acquisition by their erythroid-committed progenitors of features that favor expansion of their differentiating progeny in vivo. To investigate these possibilities, we examined the erythropoietic potential and types of hemoglobin produced by the erythroid progeny of very primitive (uncommitted) human fetal liver cells stimulated to differentiate under a variety of conditions either in vivo (in NOD/SCID mice) and/or in vitro (in CFC and LTC-IC assays). We then compared the results with parallel measurements for cells derived from newborn and adult sources of human hematopoietic cells (ie, cord blood and adult marrow).
MATERIALS AND METHODS
Human cells.
Human bone marrow cells were either aspirate samples obtained from normal individuals donating marrow for allogeneic marrow transplantation or were from cyropreserved cadaveric marrow samples obtained from the Northwest Tissue Center (Seattle, WA). Cord blood samples were obtained from mothers undergoing cesarean delivery of normal, full-term infants and livers were from 14- to 21-week-old aborted fetuses. The age of the embryo was determined by a foot length measurement. (There was no evidence of a consistent change in any parameter assessed in cells obtained from fetal livers over this range in gestational age and, accordingly, data from different fetal livers have been pooled.) For all human samples, approved institutional procedures for obtaining informed consent were followed. Single-cell suspensions were obtained from the human fetal livers by pushing the sample through a coarse sieve. The low-density (< 1.077 g/mL) cells were then isolated by density centrifugation on Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden). To obtain a population that was further enriched in CD34+ cells, cells bearing lineage markers expressed by different types of mature cells (lin+cells) were removed using a StemSep column according to the suppliers’ directions (StemCell Technologies, Vancouver, British Columbia, Canada).
Animals.
NOD/LtSz-scid/scid (NOD/SCID) mice38 were bred and maintained in microisolators under defined sterile conditions in the animal facility of the British Columbia Cancer Research Centre (Vancouver, British Columbia, Canada). Six- to 8-week-old mice were sublethally irradiated with 350 cGy from a 137Cs source the day before being intravenously injected with human cells. In some experiments, mice were injected intraperitoneally 3 times a week for 2 weeks from day 15 to day 30 posttransplant with the following combination of human growth factors: 10 μg per mouse of Steel factor (SF; Amgen, Thousand Oaks, CA), 6 μg per mouse of interleukin-3 (IL-3; Novartis, Basel, Switzerland), 6 μg per mouse of granulocyte-macrophage colony-stimulating factor (GM-CSF; Novartis), and 10 U per mouse of erythropoietin (Epo; StemCell) per injection. These mice were killed 2 hours after the last injection of growth factors. Cells were flushed from the shafts of the 4 hind leg long bones of all mice using a syringe and 21-g needle prefilled with cold Hanks’ balanced salt solution supplemented with 5% fetal calf serum (HF; StemCell) plus 5% pooled normal human serum (HF/5% HS), and a single-cell suspension was obtained by gentle aspiration.
Flow cytometry.
For immunophenotyping of human cells, suspensions were first incubated for 10 minutes at 4°C with HF/5% HS supplemented with 3 mg/mL of an antimouse Fc receptor antibody (2.4 G2) to block Fc receptors and prevent nonspecific antibody binding39 and were then labeled for 30 minutes at 4°C with antihuman CD34-fluorescein isothiocyanate (FITC; 8G12)40 and CD19-phycoerythrin (PE) and CD20-PE (Becton Dickinson [BD], San Jose, CA), or antihuman CD71-PE (OKT-9), CD45-PE, CD15-FITC, and CD66b-FITC (Pharmingen, Mississauga, Ontario, Canada), or glycophorin-A-FITC (10F7) and antimouse Ter-119-PE (Pharmingen), or CD41 (3H2) only, or CD34-FITC (8G12) and CD38-PE (BD), as described.41,42 Various phenotypes within the viable (propidium iodide-negative [PI−], Sigma Chemical Co, St Louis, MO) fraction were determined using a FACSort (BD) and LYSIS II software (BD). Assessment of CD34+ cells was further restricted to cells with medium-to-high forward light scattering (FSC) and low side light scattering (SSC) properties. Positivity in all cases was defined as fluorescence that exceeded 99.98% (ie, >5 such events in 20,000 analyzed) of that obtained with irrelevant isotype-matched control antibodies labeled with the corresponding fluorochromes. For in vitro assays of human progenitor activity, cells labeled with both antihuman CD45-PE and CD34-FITC were isolated by fluorescence-activated cell sorting (FACS) as described.41 42
For intracellular staining and analysis of human cells producing different hemoglobins (Hb), similar appearing, well-hemoglobinized, erythroblast-containing colonies generated in direct methylcellulose assays or in methylcellulose assays of 6-week LTC were harvested after 12 to 14 days of growth, pooled, and suspended in phosphate-buffered saline (PBS; StemCell). Because erythroid colonies from fetal liver and cord blood progenitors develop slightly faster, these were generally harvested on day 12, whereas those derived from adult marrow were usually harvested 2 days later. Cells obtained from engrafted NOD/SCID mice were washed 1 to 3 times in PBS and then incubated for 1 hour at room temperature in 1 mL of 4% formaldehyde in PBS (BDH Inc, Toronto, Ontario, Canada). Glutaraldehyde (EM grade) in PBS (Polyscience Inc, Warrington, PA) was then added (final concentration, 0.01%), and the cells were vortexed vigorously before centrifugation and resuspension in 250 μL of 5% nonfat dry milk in PBS for 10 minutes at room temperature. Cells were finally resuspended in 500 μL of 0.01% Triton X-100 (Bio-Rad, Richmond, CA) in PBS/0.1% bovine serum albumin (BSA; StemCell) and stained with FITC- and PE-labeled isotype control antibodies (or streptavidin-PE [SAv-PE; Pharmingen] alone in some cases) or antihuman Hb F-FITC (or HbF-biotin + SAv-PE in some cases; Isolab Inc, Akron, OH) plus anti-glycophorin A-PE (30 minutes at room temperature). The cells were then washed once, resuspended in PBS, and analyzed by FACS. To maximize specificity, gates were set first to exclude cell debris (very low FSC events) and then to select positive cells within the glycophorin A+ population.
In vitro progenitor assays.
Assays for colony-forming unit-erythroid (CFU-E), burst-forming unit-erythroid (BFU-E), colony-forming unit–granulocyte-macrophage (CFU-GM), and colony-forming unit granulocyte, erythroid, monocyte, megakaryocyte (CFU-GEMM) were performed in methylcellulose medium (H4330; StemCell) supplemented with 50 ng/mL of human SF and 20 ng/mL each of IL-3 and GM-CSF (Novartis), IL-6 (Cangene, Mississauga, Ontario, Canada), granulocyte colony-stimulating factor (G-CSF; StemCell), and 3 U/mL Epo as described.43
Colony-forming units-megakaryocyte (CFU-Mk) were assayed separately in a serum-free agarose culture medium supplemented with 50 ng/mL of human thrombopoietin (TPO; Genentech, San Francisco, CA) and 10 ng/mL each of IL-3 and IL-6 to allow specific identification by APAAP staining of pure Mk (≥3 CD41+ cells/colony) as well as mixed Mk colonies (containing other lineages as well as CD41+ Mk), as previously described.44
Cells were assayed for LTC-IC in 6-week cocultures that contained mixed feeders of M2-10B4 and Sl/Sl fibroblasts genetically engineered to produce human IL-3 (10 ng/mL), human G-CSF (130 ng/mL), and human SF (10 ng/mL), as previously described.43 LTC-IC frequencies were determined by limiting dilution analysis of the proportions of negative cultures (<1 CFC produced per culture) in groups of cultures seeded with different numbers of test cells (3 to 4 dilutions spanning a 30-fold range, 13 to 24 cultures per dilution). Otherwise, LTC-IC values were determined by dividing the total CFC output obtained from bulk culture assays by the CFC per LTC-IC value determined for the respective type of LTC-IC (see Table 3).
Proliferation bioassays of murine and human Epo.
Purified recombinant murine and human Epo were gifts from Dr E. Goldwasser (University of Chicago, Chicago, IL) and Stem Cell, respectively. Murine BAF/3 cells expressing the murine Epo receptor45 were obtained from Dr G. Krystal (Terry Fox Laboratory, Vancouver, British Columbia, Canada) and maintained in RPMI with 10% fetal calf serum (FCS; Stem Cell) supplemented with 0.5 ng/mL murine IL-3 (Terry Fox Laboratory). Human TF-1 cells46 were obtained from Dr R. Kay (Terry Fox Laboratory) and maintained in RPMI with 10% FCS and 2.5 ng/mL human GM-CSF. To assay the response of BAF/3 cells to murine and human Epo, 104 log phase cells were incubated for 24 hours at 37°C in 100 mL of RPMI with 5% FCS containing various concentrations of Epo. To assay the response of TF-1 cells, log phase cells were first incubated for 24 hours at 37°C in RPMI with 10% FCS and were then transferred at 104 per 100 mL RPMI plus 5% FCS plus Epo for another 48 hours. In both cases,3H-thymidine (8 μCi/mL, 2.0 Ci/mmol; NEN Life Science Product, Inc, Boston, MA) was added 4 hours before harvesting the cells for measurement of their radioactivity.
Statistical analysis.
All values shown are the mean ± SEM unless otherwise indicated. Significant differences (P < .05) were established using the Student’s t-test.
RESULTS
Frequencies of different types of CFC in human fetal liver by comparison with cord blood and adult marrow.
Table 1 shows a comparison of the frequencies of different types of CFC in the light density fraction of 9 independently assessed human fetal liver samples. For comparison, the same reagents and procedures were used to analyze the light density cells from 12 human cord bloods and 7 normal adult human marrow samples. The frequency of every progenitor type measured (relative to the total nucleated cell population) was found to be higher in fetal liver than in either cord blood or adult marrow, although none of the differences was statistically significant (P > .05) due to the extensive variability in CFC frequencies in individual samples. The increased frequency of fetal liver CFC was most pronounced for CFU-GEMM (60- and 250-fold higher, respectively, in fetal liver), intermediate for BFU-E/CFU-E and CFU-GM (∼50-fold and 100-fold higher, respectively, in fetal liver), and least for CFU Mk (∼3-fold higher in fetal liver).
Comparison of the Frequencies of Different Types of CFC Per 105 Light-Density Human Fetal Liver, Cord Blood, and Adult Marrow Cells and Their Representation in the Total CFC Compartment
| Type of CFC Assayed . | No. of CFC Per 105 Cells Assayed . | % of Total CFC . | ||||
|---|---|---|---|---|---|---|
| Fetal Liver . | Cord Blood . | Adult Marrow . | Fetal Liver . | Cord Blood . | Adult Marrow . | |
| CFU-GEMM | 490 ± 320 | 8 ± 4 | 2 ± 1 | 8 ± 5 | 3 ± 1 | 4 ± 5 |
| CFU-GM | 2,500 ± 1,400 | 120 ± 30 | 35 ± 6 | 35 ± 6 | 63 ± 3 | 53 ± 4 |
| BFU-E | 2,900 ± 800 | 58 ± 10 | 27 ± 5 | 55 ± 5 | 32 ± 3 | 39 ± 5 |
| CFU-E | 130 ± 50 | 4 ± 1 | 4 ± 1 | 2 ± 1 | 2 ± 1 | 4 ± 2 |
| CFU-Mk (>50 Mk/col) | 21 ± 20 | 15 ± 8 | 14 ± 7 | 0.1 ± 0.1 | 5 ± 2 | 4 ± 2 |
| CFU-Mk (3-50 Mk/col) | 120 ± 90 | 26 ± 11 | 35 ± 11 | 0.6 ± 0.5 | 11 ± 5 | 13 ± 3 |
| Total | 6,045 ± 2,100 | 190 ± 40 | 66 ± 25 | |||
| Type of CFC Assayed . | No. of CFC Per 105 Cells Assayed . | % of Total CFC . | ||||
|---|---|---|---|---|---|---|
| Fetal Liver . | Cord Blood . | Adult Marrow . | Fetal Liver . | Cord Blood . | Adult Marrow . | |
| CFU-GEMM | 490 ± 320 | 8 ± 4 | 2 ± 1 | 8 ± 5 | 3 ± 1 | 4 ± 5 |
| CFU-GM | 2,500 ± 1,400 | 120 ± 30 | 35 ± 6 | 35 ± 6 | 63 ± 3 | 53 ± 4 |
| BFU-E | 2,900 ± 800 | 58 ± 10 | 27 ± 5 | 55 ± 5 | 32 ± 3 | 39 ± 5 |
| CFU-E | 130 ± 50 | 4 ± 1 | 4 ± 1 | 2 ± 1 | 2 ± 1 | 4 ± 2 |
| CFU-Mk (>50 Mk/col) | 21 ± 20 | 15 ± 8 | 14 ± 7 | 0.1 ± 0.1 | 5 ± 2 | 4 ± 2 |
| CFU-Mk (3-50 Mk/col) | 120 ± 90 | 26 ± 11 | 35 ± 11 | 0.6 ± 0.5 | 11 ± 5 | 13 ± 3 |
| Total | 6,045 ± 2,100 | 190 ± 40 | 66 ± 25 | |||
Values for the first 4 types of CFC are from 9 fetal liver, 12 cord blood, and 7 adult marrow samples assessed individually. Values for the CFU-Mk are from 13 fetal liver, 6 cord blood, and 8 adult marrow samples. Total refers to CFU-GEMM plus CFU-GM + BFU-E + CFU-E only.
Assessment of the frequency and CFC output of human fetal liver LTC-IC.
Preliminary experiments demonstrated that LTC-IC assays could not be performed directly on low-density human fetal liver cells, because this resulted in the rapid production of large numbers of macrophages, which was usually followed within the first 2 weeks by a complete destruction of the feeder layer (data not shown). However, initial removal of lin+ cells from the input fetal liver population allowed the presence of LTC-IC to be shown. Limiting dilution assays were used to determine the frequency of LTC-IC in the lin−cells (62% ± 8% CD34+ cells) obtained from 4 different fetal livers and in the CD34+CD38− cells isolated by FACS from 2 of these (same samples as used to obtain CFC frequencies in Table 1). This showed that 1.0% ± 0.2% of the lin− fetal liver cells were LTC-IC. Calculation of the LTC-IC content of the original low-density fetal liver cell population (assuming all LTC-IC were recovered in the lin− fraction) gives a frequency (7.6 ± 1.7 LTC-IC per 104 light-density fetal liver cells) which is approximately twice that previously determined for low-density adult marrow cells.43 In the 2 experiments in which a direct comparison was made of the frequency of LTC-IC in the lin− (70% CD34+) and CD34+CD38− cells obtained from the same fetal liver samples, isolation of the CD34+CD38− cells gave a further approximately 4-fold enrichment of the LTC-IC, at a final purity of 4%, and showed that approximately 79% of the LTC-IC were recovered in the CD34+CD38− cell fraction.
Assessment in 1 of these experiments of the frequency of fetal liver LTC-IC using parental M2-10B4 cells (not producing human growth factors) as feeders showed that the number of LTC-IC detected was 2.5-fold lower than the value obtained with the human growth-factor–producing feeders. This difference is similar to what we have previously found for adult marrow and cord blood LTC-IC.43
The LTC-IC frequency data obtained from the limiting dilution analyses were also used to calculate the average 6-week output per LTC-IC of each type of CFC assessed. Table 2 shows the results obtained together with those we have previously reported for adult marrow and cord blood LTC-IC assayed using the same reagents and procedures.43 The results for lin−CD34+ and CD34+CD38− fetal liver LTC-IC were not different (data not shown) and have been combined. By comparison with cord blood and adult marrow, fetal liver LTC-IC were found to produce, on average, significantly more (P< .05) CFC of all types, as well as proportionately more CFU-E and BFU-E (P = .06 for fetal liver v cord blood LTC-IC andP = .05 for fetal liver v adult marrow LTC-IC). Examination of the CFC produced by single LTC-IC (cultures initiated with a cell number containing on average ≤1 LTC-IC) showed a wide range of values (from 0 to 464 CFC per well). A broad range of CFC outputs was previously also described for individual adult marrow LTC-IC detected using human marrow feeders.47 On average, 20% of the wells plated with a single fetal liver LTC-IC in the present experiments contained both CFU-GM and BFU-E (and/or CFU-GEMM) as compared with 11% and less than 4% for single cord blood and adult marrow LTC-IC, respectively, assayed under the same conditions.
Output of Different Types of CFC in LTC From LTC-IC Changes According to the Ontological Origin of the LTC-IC
| CFC Analyzed . | No. of CFC Per LTC-IC . | % of Total CFC Per LTC-IC . | ||||
|---|---|---|---|---|---|---|
| Fetal Liver . | Cord Blood . | Adult Marrow . | Fetal Liver . | Cord Blood . | Adult Marrow . | |
| CFU-E + BFU-E | 14 ± 7 | 0.6 ± 0.4 | 0.2 ± 0.1 | 17 ± 7 | 2 ± 2 | 1 ± 1 |
| CFU-GM | 57 ± 14 | 28 ± 1 | 18 ± 3 | 81 ± 9 | 98 ± 2 | 99 ± 1 |
| CFU-GEMM | 3 ± 1 | 0.03 ± 0.03 | 0.003 ± 0.003 | 2 ± 1 | 0.09 ± 0.09 | 0.03 ± 0.03 |
| Total | 72 ± 18 | 28 ± 1 | 18 ± 2 | 100 | 100 | 100 |
| CFC Analyzed . | No. of CFC Per LTC-IC . | % of Total CFC Per LTC-IC . | ||||
|---|---|---|---|---|---|---|
| Fetal Liver . | Cord Blood . | Adult Marrow . | Fetal Liver . | Cord Blood . | Adult Marrow . | |
| CFU-E + BFU-E | 14 ± 7 | 0.6 ± 0.4 | 0.2 ± 0.1 | 17 ± 7 | 2 ± 2 | 1 ± 1 |
| CFU-GM | 57 ± 14 | 28 ± 1 | 18 ± 3 | 81 ± 9 | 98 ± 2 | 99 ± 1 |
| CFU-GEMM | 3 ± 1 | 0.03 ± 0.03 | 0.003 ± 0.003 | 2 ± 1 | 0.09 ± 0.09 | 0.03 ± 0.03 |
| Total | 72 ± 18 | 28 ± 1 | 18 ± 2 | 100 | 100 | 100 |
Values for fetal liver LTC-IC were derived from limiting dilution experiments described in the text. In each experiment, the total number of CFC (of the type indicated) produced in all of the cultures was divided by the total number of LTC-IC placed into these cultures. Values for cord blood and adult marrow LTC-IC were similarly calculated from the limiting dilution experiments reported in Hogge et al.43
Analysis of the hematopoietic cell types regenerated in sublethally irradiated NOD/SCID mice transplanted with human fetal liver cells.
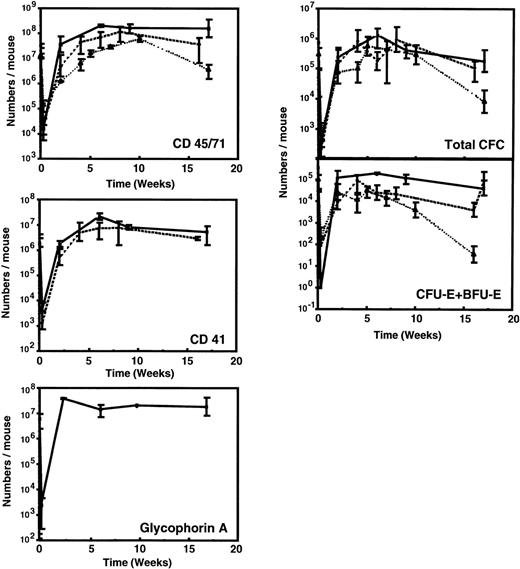
Figure 1 and Table 3 show the time course of changes in the numbers of various types of human hematopoietic cells detectable in the marrow of sublethally irradiated NOD/SCID mice assessed up to 16 weeks after the intravenous injection of 107 low-density human fetal liver cells. For comparison, comparable data (where available) from previously published time course studies of the patterns of engraftment seen with transplants of 107 low-density human cord blood42 or 2 × 107 low-density normal adult human marrow cells48 are shown. As found for other sources of human hematopoietic stem cells, less than 0.1% of any of the types of cells in human fetal liver that were injected could be found in the marrow of the mice 2 to 3 days later. After this, there was a rapid (within 2 to 4 weeks) increase in the marrow of the mice of many primitive and mature human hematopoietic cell types. All of these reached peak levels approximately 6 to 8 weeks posttransplant and were then sustained for the duration of the experiment, ie, up to 16 weeks posttransplant. Human populations detected included CD45/71+ cells, CD34+ cells, CD41+(Mk-lineage) cells, BFU-E, CFU-E, CFU-GM, and LTC-IC.
Kinetics of appearance of different types of human hematopoietic cells in NOD/SCID mice transplanted with 107low-density human fetal liver cells. Values shown represent the numbers calculated to be present in each entire mouse, assuming 2 femurs and 2 tibias comprise 25% of this value.60 Results from 2 independent experiments have been pooled (2 to 6 mice per time point). Previously published data for grafts of 107 light-density human cord blood cells (dashed line)42 or 2 × 107 light-density human adult marrow cells (dotted line)48 are shown for comparison.
Kinetics of appearance of different types of human hematopoietic cells in NOD/SCID mice transplanted with 107low-density human fetal liver cells. Values shown represent the numbers calculated to be present in each entire mouse, assuming 2 femurs and 2 tibias comprise 25% of this value.60 Results from 2 independent experiments have been pooled (2 to 6 mice per time point). Previously published data for grafts of 107 light-density human cord blood cells (dashed line)42 or 2 × 107 light-density human adult marrow cells (dotted line)48 are shown for comparison.
Kinetics of LTC-IC Production in NOD/SCID Mice Transplanted With 107 Light-Density Human Fetal Liver or Cord Blood or 2 × 107 Light-Density Adult Marrow Cells Are Similar
| Time Posttransplant (wks) . | Fetal Liver . | Cord Blood . | Adult Marrow . |
|---|---|---|---|
| Input | 11,500 ± 600 | 730 ± 360 | 2,500 ± 1,100 |
| 2 | 10,600 ± 9,300 | 110 ± 50 | 50 ± 20 |
| 6 | 80,600 ± 58,100 | 1,430 ± 250 | 1,030 ± 540 |
| 8-10 | 52,000 ± 11,700 | 3,400 ± 1,150 | 720 ± 630 |
| Time Posttransplant (wks) . | Fetal Liver . | Cord Blood . | Adult Marrow . |
|---|---|---|---|
| Input | 11,500 ± 600 | 730 ± 360 | 2,500 ± 1,100 |
| 2 | 10,600 ± 9,300 | 110 ± 50 | 50 ± 20 |
| 6 | 80,600 ± 58,100 | 1,430 ± 250 | 1,030 ± 540 |
| 8-10 | 52,000 ± 11,700 | 3,400 ± 1,150 | 720 ± 630 |
Values shown are the absolute LTC-IC numbers calculated per mouse body, assuming 2 femurs and 2 tibias represent 25% of the total60 and that the CFC output per LTC-IC is the same as the LTC-IC in the suspension originally transplanted (ie, 72 for fetal liver-derived LTC-IC, from Table 2; 28 for cord blood-derived LTC-IC and 18 for adult marrow-derived LTC-IC from Hogge et al43). The fetal liver data are from the same 2 experiments shown in Fig 1. The cord blood and adult marrow data are derived from Cashman et al.42 48
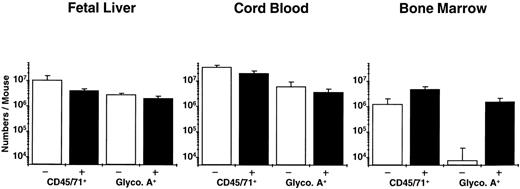
Analysis of the distribution of human CFC subtypes among those present at different times posttransplant (Table 4) indicates that there was a slight shift between week 2 and week 6 in favor of granulopoietic CFC, as seen previously in NOD/SCID mice engrafted with human cord blood and adult marrow cells. Also of note is the observation that fetal liver grafts regenerated relatively more progeny LTC-IC (8-fold over input) than was achieved previously with transplants of cord blood (5-fold over input) or adult marrow (decreased 2-fold below input). Assessment of the number of glycophorin A+ cells (erythroblasts) showed large numbers of these to be present in the fetal liver and cord blood-engrafted mice, but none was detected in the marrow-engrafted mice (Fig 2). A 2-week course of 6 injections of human Epo, SF, IL-3, and GM-CSF restored the ability of adult human erythroid progenitors to mature into erythroblasts in vivo (as previously reported for similar transplants in SCID mice49), but had no effect (P > .05) on terminal human erythropoiesis in the mice engrafted with fetal liver or cord blood cells (Fig 2; P = .4 and P = .2, respectively) or on any other type of human progenitor in any of the groups (data not shown).
Relative Frequencies of Different Types of CFC in Human Fetal Liver-Engrafted NOD/SCID Mice Assessed at Different Time Points Posttransplant
| Time Posttransplant (wks) . | % of Total CFC . | |||
|---|---|---|---|---|
| CFU-E . | BFU-E . | CFU-GEMM . | CFU-GM . | |
| Input | 2 ± 2 | 40 ± 4 | 3 ± 3 | 55 ± 10 |
| Week 2 | 8 ± 1 | 43 ± 5 | 9 ± 5 | 40 ± 8 |
| Week 6 | 2.5 ± 0.2 | 20 ± 2 | 3 ± 1 | 75 ± 1 |
| Week 9 | 1 ± 0.3 | 28 ± 2 | 4 ± 1 | 67 ± 2 |
| Time Posttransplant (wks) . | % of Total CFC . | |||
|---|---|---|---|---|
| CFU-E . | BFU-E . | CFU-GEMM . | CFU-GM . | |
| Input | 2 ± 2 | 40 ± 4 | 3 ± 3 | 55 ± 10 |
| Week 2 | 8 ± 1 | 43 ± 5 | 9 ± 5 | 40 ± 8 |
| Week 6 | 2.5 ± 0.2 | 20 ± 2 | 3 ± 1 | 75 ± 1 |
| Week 9 | 1 ± 0.3 | 28 ± 2 | 4 ± 1 | 67 ± 2 |
Values shown are from the same 2 experiments shown in Fig 1.
Total human (CD45/71+) and mature erythroid (glycophorin A+) cells in NOD/SCID mice transplanted with equivalent grafts of human fetal liver, cord blood, or adult marrow cells and then injected with recombinant human growth factors (▪) or not (□) as described in the text. The difference plus and minus growth factors for all pairs was not significant (P > .05) except for glycophorin A+ cells in the mice transplanted with adult marrow (P << .001).
Total human (CD45/71+) and mature erythroid (glycophorin A+) cells in NOD/SCID mice transplanted with equivalent grafts of human fetal liver, cord blood, or adult marrow cells and then injected with recombinant human growth factors (▪) or not (□) as described in the text. The difference plus and minus growth factors for all pairs was not significant (P > .05) except for glycophorin A+ cells in the mice transplanted with adult marrow (P << .001).
Because we had found that freshly isolated human fetal liver LTC-IC display an enhanced erythropoietic activity in vitro by comparison with those present in cord blood or adult marrow, it was of interest to also examine the types of CFC produced by the human LTC-IC regenerated in fetal liver-engrafted mice. As shown in Table 5, these were characterized by the same increased output of CFU-E and BFU-E as the LTC-IC present in freshly isolated fetal liver suspensions.
Comparison of Different Types of CFC Produced by the LTC-IC Regenerated in Human Fetal Liver-Engrafted NOD/SCID Mice at Different Time Points Posttransplant
| Time Posttransplant (wks) . | Relative Proportions of Total CFC Produced in LTC-IC Assays . | |||
|---|---|---|---|---|
| CFU-E . | BFU-E . | CFU-GM . | CFU-GEMM . | |
| Input | 4 ± 4 | 13 ± 7 | 83 ± 9 | 0.2 ± 0.2 |
| Week 2 | 3 ± 2 | 21 ± 9 | 75 ± 11 | 1 ± 1 |
| Week 6 | 2 ± 1 | 13 ± 2 | 84 ± 4 | 2 ± 1 |
| Week 9 | 2 ± 1 | 27 ± 6 | 70 ± 6 | 1 ± 1 |
| Time Posttransplant (wks) . | Relative Proportions of Total CFC Produced in LTC-IC Assays . | |||
|---|---|---|---|---|
| CFU-E . | BFU-E . | CFU-GM . | CFU-GEMM . | |
| Input | 4 ± 4 | 13 ± 7 | 83 ± 9 | 0.2 ± 0.2 |
| Week 2 | 3 ± 2 | 21 ± 9 | 75 ± 11 | 1 ± 1 |
| Week 6 | 2 ± 1 | 13 ± 2 | 84 ± 4 | 2 ± 1 |
| Week 9 | 2 ± 1 | 27 ± 6 | 70 ± 6 | 1 ± 1 |
Values shown are from the same 2 experiments shown in Fig 1.
Murine Epo has a reduced activity on human cells.
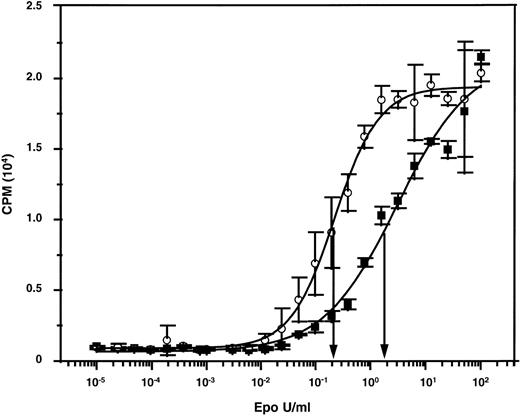
A reduced ability of adult versus fetal and neonatal human erythroid cells to undergo terminal maturation in NOD/SCID mice would be anticipated from a slight differential (decreased) responsiveness of adult cells to Epo if the level of murine Epo in the mice were limiting. We therefore compared the ability of murine and human Epo to stimulate murine and human Epo receptor-expressing cells. When murine targets were used (murine Epo receptor-expressing BAF/3 cells),45 both preparations gave superimposable dose-response curves (data not shown). The unitage of the human Epo preparation could thus be used to assign a unitage to the murine Epo preparation. The 2 preparations were then tested for their abilities to stimulate the proliferation of human TF-1 cells.46 As shown in Fig 3, concentrations of murine Epo that had an equivalent ability to human Epo to stimulate murine cells were approximately 10-fold less active on human cells. Thus, levels of murine Epo that are sufficient to sustain murine erythropoiesis might also be adequate to stimulate human erythropoietic cells of fetal (and neonatal) but not adult origin, which could thus explain the unique requirement of adult human erythroid precursors for additional (exogenously administered) human Epo to terminally differentiate in an in vivo murine environment.
Dose-response curves showing the differential mitogenic effects of murine and human Epo on human TF-1 cells. The specific activities of both preparations were standardized using murine Epo-expressing BAF/3 cells.
Dose-response curves showing the differential mitogenic effects of murine and human Epo on human TF-1 cells. The specific activities of both preparations were standardized using murine Epo-expressing BAF/3 cells.
Fidelity of globin gene expression in the human erythroid progeny of cells produced under different conditions from ontologically distinct sources.
Terminally differentiating human erythroblasts generated in vitro from progenitors produced in LTC or in NOD/SCID mice or in LTC initiated with cells from NOD/SCID mice were assessed for the type of Hb they contained by FACS analysis of intracytoplasmically stained fixed cell preparations (as described in Materials and Methods). For each sample, the preparation was counter-stained with antihuman glycophorin A antibody labeled with a different fluorochrome to enhance the specificity of the procedure. Representative profiles are shown in Fig 4. The data for all sources of erythroblasts are summarized in Table 6. Most of the erythroblasts within, or derived in vitro from progenitors from, suspensions of fetal liver or cord blood expressed high levels of HbA and HbF. As expected, most of the erythroblasts present in freshly obtained adult marrow contained HbA and very few contained HbF, although increased production of HbF+ cells was seen when marrow progenitors were stimulated to generate erythroblasts in vitro.
FACS profiles of glycophorin A+ cells obtained directly from fetal liver, cord blood, and adult marrow or from directly plated 12- to 14-day-old erythroid colonies generated in methylcellulose assays of human fetal liver, cord blood, or adult marrow cells or from human CFU-E/BFU-E produced in NOD/SCID mice engrafted for 6 weeks with human fetal liver, cord blood, or adult marrow cells. Harvested cells were stained with anti-glycophorin A and anti-Hb antibodies as described in the Materials and Methods. Note that the intensity of staining with the 2 different anti-Hb antibodies provide only a relative measure of the amount of HbA and HbF in each cell and therefore cannot be used to compare absolute HbA and HbF expression levels.
FACS profiles of glycophorin A+ cells obtained directly from fetal liver, cord blood, and adult marrow or from directly plated 12- to 14-day-old erythroid colonies generated in methylcellulose assays of human fetal liver, cord blood, or adult marrow cells or from human CFU-E/BFU-E produced in NOD/SCID mice engrafted for 6 weeks with human fetal liver, cord blood, or adult marrow cells. Harvested cells were stained with anti-glycophorin A and anti-Hb antibodies as described in the Materials and Methods. Note that the intensity of staining with the 2 different anti-Hb antibodies provide only a relative measure of the amount of HbA and HbF in each cell and therefore cannot be used to compare absolute HbA and HbF expression levels.
Proportion of Erythroid Cells Produced From Different Sources of Progenitors Containing Different Types of Hb
| Origin of Erythroblasts . | % HbA+ Erythroid Cells . | % HbF+Erythroid Cells . | ||||
|---|---|---|---|---|---|---|
| Fetal Liver . | Cord Blood . | Adult Marrow . | Fetal Liver . | Cord Blood . | Adult Marrow . | |
| Erythroblasts | 82 ± 14 | 97 ± 3 | 94 | 84 ± 8 | 96 ± 4 | 4 |
| BFU-E/CFU-E | 65 ± 12 | 62 ± 7 | 76 ± 5 | 96 ± 0.2 | 76 ± 13 | 36 ± 8 |
| LTC-IC–derived BFU-E/CFU-E | 84 ± 14 | 76 ± 19 | Not detectable | 92 ± 4 | 88 ± 2 | Not detectable |
| BFU-E/CFU-E from NOD/SCID mice | 63 ± 12 | 61 ± 11 | 55 ± 13 | 87 ± 6 | 38 ± 8 | 20 ± 1 |
| LTC-IC–derived BFU-E/CFU-E from NOD/SCID mice | 66 ± 6 | ND | Not detectable | 71 ± 4 | ND | Not detectable |
| Origin of Erythroblasts . | % HbA+ Erythroid Cells . | % HbF+Erythroid Cells . | ||||
|---|---|---|---|---|---|---|
| Fetal Liver . | Cord Blood . | Adult Marrow . | Fetal Liver . | Cord Blood . | Adult Marrow . | |
| Erythroblasts | 82 ± 14 | 97 ± 3 | 94 | 84 ± 8 | 96 ± 4 | 4 |
| BFU-E/CFU-E | 65 ± 12 | 62 ± 7 | 76 ± 5 | 96 ± 0.2 | 76 ± 13 | 36 ± 8 |
| LTC-IC–derived BFU-E/CFU-E | 84 ± 14 | 76 ± 19 | Not detectable | 92 ± 4 | 88 ± 2 | Not detectable |
| BFU-E/CFU-E from NOD/SCID mice | 63 ± 12 | 61 ± 11 | 55 ± 13 | 87 ± 6 | 38 ± 8 | 20 ± 1 |
| LTC-IC–derived BFU-E/CFU-E from NOD/SCID mice | 66 ± 6 | ND | Not detectable | 71 ± 4 | ND | Not detectable |
Values shown are the percent of the total number of human glycophorin A+ cells that stained positive for the type of Hb being analyzed. Human CFC and LTC-IC from engrafted NOD/SCID mice were assessed 6 weeks posttransplant.
Abbreviation: ND, not done.
Analysis of the erythroblast progeny of progenitors generated from human fetal liver cells within the microenvironment of the adult NOD/SCID mouse bone marrow, or in vitro from LTC-IC generated in human fetal liver-engrafted mice, showed most of these to continue to be HbF+. Similarly, the relatively low proportion of HbF+ erythroblasts produced in vitro from progenitors generated in adult human marrow-engrafted mice was the same as for those generated from freshly isolated adult human marrow progenitors. Interestingly, in the human cord blood-engrafted mice, the proportion of HbF+ erythroblasts obtained from progenitors produced 6 weeks posttransplant had declined to half the level seen in erythroblasts obtained from freshly isolated progenitors (P < .04).
To examine the dual expression of HbA and HbF in the same cells (which precluded the use of glycophorin A-staining), cells were harvested from maturing erythroid colonies generated in vitro from progenitors of different sources. These provide an enriched, although not pure, population of erythroblasts. As can be seen in Fig 5, most of the Hb+ cells obtained from colonies of fresh fetal liver origin contained both HbA and HbF. Similar results were obtained for colonies generated from human BFU-E isolated from fetal liver-engrafted mice. In contrast, many more of the Hb+ cells in colonies produced by adult marrow contained exclusively HbA.
Demonstration of cells containing both HbF and HbA in 12- to 14-day-old erythroid colonies generated in methylcellulose assays of human fetal liver or adult marrow CFC and CFC obtained from NOD/SCID mice transplanted 6 weeks previously with human fetal liver cells.
Demonstration of cells containing both HbF and HbA in 12- to 14-day-old erythroid colonies generated in methylcellulose assays of human fetal liver or adult marrow CFC and CFC obtained from NOD/SCID mice transplanted 6 weeks previously with human fetal liver cells.
DISCUSSION
The present study shows a number of important features of human fetal liver hematopoiesis that identify differences in how this process is regulated in the fetus and in the adult. At both stages of ontogeny, terminally differentiating erythroid and myeloid cells make up the majority of the light-density population in the hematopoietic tissues in which they are produced. However, erythroid cells predominate in the fetal liver, whereas myeloid cells predominate in the adult. In contrast, more primitive cells, detected in vitro as erythroid and granulopoietic CFC, although present at a higher overall frequency (relative to the total number of cells) in fetal liver, were also found to be present in both fetal liver and adult marrow at similar frequencies to each other (Table 1).5 33 The lack of a selective increase in the number of erythroid progenitors accumulated in vivo does not argue in favor of a model of increased commitment of fetal hematopoietic stem cells to the erythroid pathway to explain the predominance of erythroid cells seen in the terminally differentiating fetal liver populations. Nevertheless, a shift in that direction was suggested here by the increased output of erythroid progenitors from individual fetal liver (as compared with adult marrow) LTC-IC under LTC conditions. These conditions are nonpermissive for erythropoiesis and hence allow detection of the first progenitors to commit to that pathway.
We also found that the frequency of megakaryocyte progenitors was increased, although to a much smaller degree, whereas the frequency of multipotent CFC was increased to a greater extent than the granulopoietic and erythroid progenitors. As noted by others,7 50 we observed the maximum size of colonies produced in vitro by all types of CFC to be larger than those produced by their adult marrow counterparts. However, this increased CFC proliferative potential of fetal liver CFC is not realized in vivo, because the ratio of CFC to mature cells is decreased. This could occur either because the microenvironment of the fetal liver promotes the more rapid differentiation of fetal progenitors and/or because a higher rate of apoptosis occurs among their progeny. Because the composition of the mature cell compartment in adult marrow and fetal liver also differ, the mechanisms responsible for affecting these changes in cell output must be distinct for each lineage.
Given the large increases seen in the frequency of CFC in the human fetal liver (overall, ∼70-fold relative to adult marrow), the discovery that the frequency of LTC-IC is only 2-fold higher was unanticipated. This means that the ratio of CFC to LTC-IC in the fetal liver is much higher than in adult marrow. Interestingly, this feature was reproduced in the LTC system, in which we also noted an increased (4-fold higher) 6-week output of CFC by comparison with adult marrow LTC-IC (72 v 18 CFC per LTC-IC). A similarly enhanced output of CFC by fetal liver LTC-IC was noted by Roy et al7 using a shorter (5-week) assay on parental M2-10B4 cell feeders that may detect a less primitive LTC-IC subset.43 Similarly, a greater output of progeny from fetal versus adult sources of CD34+CD45RA−CD71−31 or CD34+CD38− cells51 in stroma-free cytokine-supplemented cultures has been reported. Such a result could be explained by a decreased self-renewal probability of primitive fetal cells resulting in an expanded yield of CFC or a reduced cell cycle time resulting in an accumulation of more CFC through more cell generations. It is also possible that the progeny of fetal LTC-IC may acquire an ability to form colonies in semisolid media without such a precipitous loss of proliferative potential, or they may be less susceptible to conditions that cause apoptosis of their adult counterparts. Current evidence argues against the first possibility, because fetal stem cells of both human52 and murine17,19 origin have been found to display increased rather than decreased self-renewal properties. In addition, evidence of a shorter cell cycle time and faster differentiation of fetal hematopoietic cells has been reported.3,16 53
The present studies also provide evidence of the intrinsic determination of a number of other properties of fetal human hematopoietic cells that are linked to their embryonic status. These include an increased output of erythroid CFC by fetal LTC-IC, an increased sensitivity of fetal CFU-E to factors that support their terminal differentiation, and an increased production of HbF by their erythroblast progeny. All of these characteristics were shown to be stably transmitted to the progeny of fetal stem cells stimulated to proliferate and differentiate in the marrow of NOD/SCID mice; in some cases, even those generated after transfer of in vivo-derived LTC-IC to cultures containing murine stromal feeder layers engineered to produce human G-CSF, IL-3, and SF. Less stability of HbF production was seen previously when fetal sheep cells were transplanted into adult sheep54 or when human fetal cells were cultured for prolonged periods in the absence of stroma.55 From such results it has been suggested that the type of hemoglobin produced can be influenced by the environment in which the cells are generated. On the other hand, a fetal human erythroid program was seen in the erythroid cells produced in both patients56,57 and sheep58 transplanted with fetal cells, consistent with the results reported here. The continued production in the presence of murine stroma of human progenitors that remain committed to a high level of HbF and low HbA synthesis appears to represent only one of several manifestations of a preserved fetal hematopoietic program that affects multiple stages of differentiation. It seems unlikely that the different behaviors of corresponding stages of differentiation of fetal and adult cells will reflect a single common underlying intrinsic molecular alteration. However, it is interesting to note that, even in adult erythroid precursors, HbF expression is enhanced by strong growth factor stimulation34 59 and that a feature of fetal erythroid precursors appears to be their enhanced sensitivity to growth factor stimulation. Such findings thus invite speculation as to whether some basic differences in the intracellular signaling machinery of fetal hematopoietic cells may account for their distinct biology. Investigations of this possibility are now underway.
ACKNOWLEDGMENT
The authors thank the staff of the Stem Cell Assay Service for their assistance in the initial preparation of primary human cell samples, Jessyca Maltman and Maya Sinclaire for their assistance in the animal studies, Gayle Thornbury and Giovanna Cameron for operating the FACS, and Tara Palmater for secretarial assistance. We are also grateful to E. Goldwasser (University of Chicago), P. Lansdorp, G. Krystal, and R. Kay (Terry Fox Laboratory), and Amgen, Cangene, Isolab, Novartis, and Stem Cell for valuable gifts of cells or reagents.
Supported by grants from the National Institutes of Health (PO1 HL55435), Novartis (Basel, Switzerland), and the National Cancer Institute of Canada (NCIC) with funds from the Terry Fox Run. T.L.H. holds a United Kingdom Leukemia Research Fund Senior Lectureship and P.P.Y.C. holds a Roman Babicki Graduate Studentship from the University of British Columbia. C.J.E. is a Terry Fox Cancer Research Scientist of the NCIC.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Connie J. Eaves, PhD, Terry Fox Laboratory, 601 W 10th Ave, Vancouver, BC, V5Z 1L3 Canada; e-mail:connie@terryfox.ubc.ca.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal