Erythropoietin (EPO) is required for the survival and expansion of red blood cell progenitor cells and supports continued differentiation of these committed progenitors to mature red blood cells. After binding to its cognate receptor, EPO promotes receptor homodimerization, activation of receptor-associated JAK2, subsequent receptor tyrosine phosphorylation, and transduction of signal. EPO is also internalized and degraded in lysosomes. The contribution of EPO-induced receptor internalization to modulation of EPO signals has not been determined. To examine this question, we generated a panel of hematopoietic cell lines containing progressively truncated isoforms of the erythropoietin receptor (EPO-R) and determined the rate and extent of EPO internalization and receptor downregulation. We demonstrated that a membrane-proximal domain of the cytoplasmic tail of the EPO-R was the minimal region required for EPO-induced receptor internalization. This cytoplasmic domain is also the minimal domain required for activation of JAK2, a cytosolic tyrosine kinase essential for the function of the EPO-R. However, neither EPO activation of cytosolic JAK2 tyrosine kinase activity nor tyrosine phosphorylation of the EPO-R cytoplasmic tail was required for EPO-induced receptor downregulation. Both functional and nonfunctional cell surface receptor isoforms were internalized equally. These results suggest that, for downregulation of cell surface ligand occupied EPO-R and possibly for signaling receptors of the cytokine receptor superfamily in general, internalization of cell surface ligand occupied receptors may follow a pathway distinct from signaling receptors of the receptor tyrosine kinase (RTK) family.
ERYTHROPOIETIN (EPO) is a primary cytokine or growth factor regulating red blood cell development.1 EPO promotes survival of committed erythroid progenitors, leading to an expansion in their numbers, and supports the continued differentiation of these cells to mature red blood cells. Its action is mediated by a specific high-affinity cell surface receptor (EPO-R), which is expressed predominantly in mature committed erythroid progenitors cells present in the adult bone marrow.
The EPO-R is a member of the hematopoietic cytokine receptor superfamily. These signaling receptors are related structurally to one another and are encoded by genes that are members of an evolutionary conserved superfamily. All are type I transmembrane glycoproteins; the N-terminal extracellular domain of each is responsible for ligand recognition and binding. The C-terminal cytoplasmic domains of these receptors are largely divergent in primary sequence; however, 2 characteristics are shared among most. Most contain partially conserved proline-rich peptide sequences known as box1 and box2 adjacent to the transmembrane segment.2 Second, the cytoplasmic tails contain multiple tyrosine residues; some are phosphorylated in response to ligand binding and, thus, serve as potential docking sites for intracellular proteins containing phosphotyrosine-binding domains of the SH2-type.3-7
Ligand binding promotes multimerization of cytokine receptor subunits as either homodimers (eg, erythropoietin receptor and growth hormone receptor [GH-R]) or as heteromultimers (eg, the receptors for interleukin-6 [IL-6]: IL-6R and gp130). EPO-induced EPO-R dimerization leads to juxtaposition of their cytoplasmic domains to which a latent intracellular tyrosine kinase, JAK2, is tethered and hence leads to initiation of signaling through activation of the kinase activity of JAK2.8 Biochemical and genetic evidence indicates that JAK2 activation by the EPO-R is essential for the development of red blood cells.8,9 Tyrosine phosphorylation of individual tyrosine containing sequences within the receptor cytoplasmic tail, and JAK2 itself (presumably directly via JAK2 and possibly other cytosolic kinases) generates new docking sites for cytosolic signaling proteins or adapter proteins, latent cytosolic transcription factors (eg, STAT proteins), and negative regulatory proteins.3-7 Although the sites of tyrosine phosphorylation in the EPO-R cyto- plasmic tail, in response to EPO, have not been identified, recent evidence indicates that tyrosine phosphorylation of the cytoplasmic tail is required for optimal receptor function in vivo.10 11
After binding to its receptor, EPO has been shown to be internalized and degraded in lysosomes12; however, the contribution of receptor internalization and degradation to the modulation of EPO signals has not been well studied. Other classes of growth factor receptors (eg, the receptor tyrosine kinase [RTK] family), which are also important in the regulation of blood cell development, undergo regulated cellular trafficking after ligand binding. Mutations that inactivate the signaling capacity of these receptors block ligand-induced receptor downregulation, altering their functional capacity.13 Whether hematopoietic cytokine receptors, such as the EPO-R, also require activation of associated cytoplasmic tyrosine kinases or other signaling intermediates for receptor downregulation and signal attenuation is not known.
To determine whether EPO-induced EPO-R internalization and downregulation contribute to the modulation of receptor activity, we generated a series of hematopoietic cell lines containing progressively truncated cytoplasmic tail isoforms of the EPO-R. We demonstrate that a membrane proximal domain of the cytoplasmic tail of the EPO-R is the minimal cytoplasmic domain required for internalization of bound EPO. This domain is also the minimal cytoplasmic domain of the EPO-R required to activate JAK2 kinase activity. However, in contrast to RTK growth factor receptors, activation of JAK2 kinase and tyrosine phosphorylation of the EPO-R were not required for EPO-induced receptor downregulation. In addition, mitogenically inactive full-length isoforms of the EPO-R were internalized similar to wild-type receptors after EPO binding, indicating that receptor activation is not a strict requirement for receptor internalization. Thus, in contrast to other classes of growth factor receptors, internalization of cell surface receptors appears to be regulated independently from EPO signaling.
MATERIALS AND METHODS
Cell lines, antiserum, and reagents.
IL-3–dependent myeloid 32D cells and pro-B–cell BaF3 cells were maintained in RPMI, 10% fetal calf serum (FCS), and 15% WEHI-3B culture supernatant (as a source of IL-3). Polyclonal rabbit antimouse EPO-R antiserum has been described.14 Polyclonal rabbit antimouse JAK2 antiserum was obtained from Santa Cruz Labs (Santa Cruz, CA). Antiphosphotyrosine antiserum was obtained from Transduction Labs (Lexington, KY). Purified carrier-free human EPO (hEPO) was supplied by Abbott Laboratories (Abbott Park, IL). Vibrio cholerae neuraminidase was from Calbiochem,Diplococcus pneumoniae β-galactosidase was from Sigma (St Louis, MO), and bovine milk galactosyltransferase was from Fluka (Turku, Finland). UDP-(3H)galactose and Na 125I were from Amersham (Arlington Heights, IL).
Generation of 32D cells containing EPO-R constructs.
Construction of EPO-R cytoplasmic tail deletion mutants was accomplished by insertion of premature termination codons (via polymerase chain reaction [PCR]) into the EPO-R coding sequence. Mutant receptors were subcloned into the eukaryotic expression plasmid pMEX and sequenced. pMEX.EPO-R constructs were transfected into 32D cells by electroporation using a Bio-Rad gene pulser (Bio-Rad, Hercules, CA). After transfection, individual clones were selected in IL-3 and G418. Expression of the EPO-R constructs in 32D cell clones was determined by 125I-EPO binding and immunoblotting of cell extracts using an antibody to the N-terminus of the EPO-R.
Proliferation analysis.
The proliferation of 32D transfectants in response to IL-3 or EPO was determined by methylthiazol-tetrazolium (MTT) analysis. Cells were plated in a 96-well microtiter plate at 1 × 103 cells/well containing the indicated concentrations of EPO or Wehi-conditioned media (IL-3 source) for 72 to 86 hours at 37°C/5% CO2. The number of cells was then measured by the addition of 0.04 mL of 5 mg/mL MTT (dimethylthiazol-w-yl-2,5-diphenyltetrazolium; Sigma) for 4 to 8 hours at 37°C/5% CO2. The cells were then lysed by the addition of 0.1 mL of 0.4 N HCl in 2-propanol. The optical density was measured by an enzyme-linked immunosorbent assay (ELISA) plate reader at a wavelength of 600 nm. Cell proliferation in response to EPO was normalized by dividing the optical density (OD) at a given EPO concentration by the OD obtained in 15% Wehi conditioned medium (ie, in IL-3).
Immunoprecipitations and immunoblotting.
Cells were starved for 6 hours in serum-free OPTI-MEM (GIBCO-BRL, Grand Island, NY) at 37°C. The cells were then stimulated with EPO (20 to 50 U/mL) or left unstimulated for 7 minutes at 37°C. The cells were then added to 5 vol of ice-cold phosphate-buffered saline (PBS), pelleted, and immediately lysed in a lysis buffer containing 1% Triton X-100, 150 mmol/L NaCl, 10 mmol/L TrisCl, pH 7.4, 1 mmol/L EDTA, 1 mmol/L EGTA, 0.5% NP40, 0.2 mmol/L sodium vanadate, 1 mmol/L phenylmethylsulfonyl fluoride, and 0.5 TIU/mL aprotinin. Immunoprecipitations were performed as previously described.15 Immunoprecipitates or a fraction of the cell lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and transferred to a Hybond nitrocellulose membrane (Amersham), and immunoblotting with specific antiserum was performed. Detection was by the enhanced chemiluminescence method (ECL) system (Amersham).
Iodination of EPO.
EPO was iodinated using IODOGEN (Pierce, Rockford, IL). IODOGEN (10 mg) was incubated with 1 mCi of Na 125I for 7 minutes at 4°C in a final volume of 40 μL of water. EPO (1.75 μg) in 0.25 mol/L sodium phosphate buffer (pH 7.1) was then added to the reaction vial and incubated for an additional 10 minutes at 4°C. The reaction was stopped by transferring the liquid into a centrifuge tube containing 1 mg tyrosine powder. Free iodine was separated from the radiolabeled ligand by passing it through a Sephadex G-10 column (Pharmacia Biotech, Uppsala, Sweden) equilibrated first by PBS/0.02% Tween-20. The specific activity of 125I-labeled EPO was generally between 6 and 9 × 106 cpm/pmol.
EPO-binding studies.
Cells were washed in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), resuspended at a concentration of 6 × 107 cells/mL, incubated at room temperature for 30 minutes, and then pelleted. Aliquots of 3 × 106 cells in triplicate were incubated with a range of concentrations of iodinated EPO at 4°C overnight in binding buffer (RPMI/10% FCS/50 mmol/L HEPES, pH 7.2) in the absence or presence of 100-fold excess unlabeled EPO. After overnight incubation, cells were determined to be greater than 90% viable by trypan blue exclusion analysis. Free EPO was separated from cell-bound EPO by centrifugation through a 100% FBS cushion, and the cell pellets were counted in a gamma counter. The data were plotted according to the method of Scatchard. Dissociation constant (kd) and cell surface receptor numbers were then determined through Scatchard analyses.
EPO internalization studies.
32D cell lines expressing either wild-type EPO-R or mutant EPO-R were maintained in RPMI, 10% FCS, 15% Wehi 3B supernatant, and G418 (400 mg/mL) at a concentration of 1 × 106 cells/mL. Cells are harvested, washed twice in RPMI, and starved in binding buffer (RPMI, 25 mmol/L HEPES, 10% FCS) for 30 minutes at 37°C/5% CO2. After harvesting, cells were then aliquoted at 3 × 106 cells/1.5 mL into microcentrifuge tubes in triplicate. The cells were incubated on ice for 2 hours with 5 nmol/L125I-EPO in the presence or absence of 100-fold unlabeled EPO. After incubation, cells were washed twice in RPMI to remove unbound EPO, and then the cells were incubated at 37°C for varying amounts of time, generally from 0 to 80 minutes. The reaction was stopped by the addition of 9× vol of ice-cold binding buffer. The pelleted cells were resuspended in citrate-phosphate buffer (0.1 mol/L citrate-phosphate, 150 nmol/L NaCl, pH 2.6) and were incubated for 3 minutes on ice to release the remaining surface-bound EPO. The cells were then centrifuged for 30 seconds. The supernatant that contained the 125I-EPO removed from the surface was separated from the pellet that contained the 125I-EPO that was internalized. The radioactivity in each fraction was measured in a gamma-counter (Coulter, Hialeah, FL). Specific binding was determined by subtracting the total cell-associated radioactivity from the cell-associated radioactivity bound in the presence of excess unlabeled EPO. The initial rate of internalization was measured by calculating the slope of the linear portion of the curve, between 0 and 10 minutes. Each cell line was tested a minimum of 3 times.
Determination of cell surface erythropoietin receptor internalization relative to total cell surface glycoproteins nonspecific pinocytosis.
Cell surface glycoproteins were labeled with tritiated galactose as described.16 Briefly, cells were washed and treated with neuraminidase (0.02 U/mL) and β-galactosidase (0.03 U/mL) at 37°C for 1 hour. Cells were washed and surface glycoproteins were labeled by adding UDP-(3H)galactose at 150 μCi/mL and galactosyltransferase at 0.3 U/mL to the cells and incubating for 30 minutes on ice. Cells were then rapidly washed and resuspended in binding buffer and incubated at 37°C for various times. The cells were then chilled to 4°C. Half were lysed in lysis buffer; the other half was incubated at 4°C for 12 to 16 hours with β-galactosidase at 0.3 U/mL. These cells were then washed and lysed. After clarification of the lysates, each was precleared with normal rabbit serum and protein A-agarose. EPO-R polyclonal antiserum, directed against the N-terminal end of the EPO-R, was then added, followed by protein A-agarose. The immunoprecipitate was washed 4× in lysis buffer, and the amount of bound counts was determined. The proportion of cell surface EPO-R internalized was calculated by dividing the radioactivity immunoprecipitated from cells treated with β-galactosidase by the radioactivity in immunoprecipitates from cells not treated with β-galactosidase. Total cell surface glycoproteins internalization was determined from anti–EPO-R immunoprecipitates of lysates from (3H)galactose-labeled parental 32D cells that do not contain an EPO-R.
RESULTS
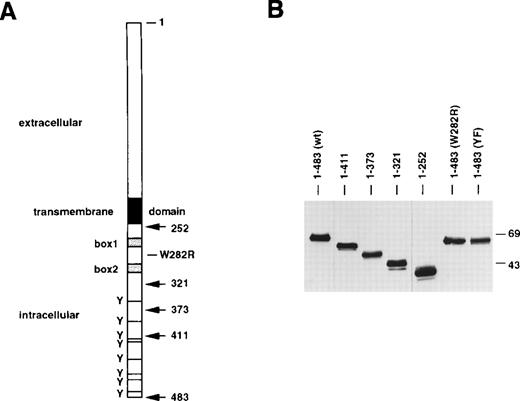
To determine if the cytoplasmic domain of the EPO-R regulates EPO internalization, a series of cytoplasmic tail truncated EPO-R variants were constructed (Fig 1A). Plasmids encoding the wild-type and truncated forms of the EPO-R were transfected into the IL-3–dependent myeloid cell line 32D. The transfected cells were selected for growth in IL-3 and G418. Individual clones were isolated and were maintained in culture under these conditions. Expression of wild-type EPO-R in these cells allows them to proliferate in response to EPO; yet, cells were exposed to EPO only for experimental manipulation. EPO binding studies were performed on multiple clones containing each construct. Parental 32D cells transfected with the parental pMEX plasmid (no EPO-R) and selected for growth in G418 and IL-3 (ie, 32D.Neo cells) did not specifically bind EPO, and EPO added to culture did not support proliferation of these cells. The number of EPO-binding sites (ie, cell surface EPO-Rs) and the dissociation constant for EPO binding are presented in Table 1. EPO binding curves were plotted, and saturation of all EPO-binding sites was achieved for all cell lines over the concentrations of EPO used. The kds for EPO binding to cells expressing truncated EPO-Rs were equivalent to that obtained for the wild-type receptor, except for cells containing EPO-R(1-252), in which the affinity for EPO was 2- to 3-fold lower. Despite this difference, the relative off rates for bound EPO did not significantly differ between clones (data not shown). The numbers of cell surface receptors were also roughly equivalent, except when the complete cytoplasmic tail was removed. Cells containing EPO-R(1-252) expressed 5-fold greater cell surface receptors than cells expressing wild-type receptors. Immunoblot analysis of detergent soluble extracts from the cell lines demonstrated that each expressed an EPO-R protein of the expected size and that the level of total cell expression did not greatly differ (Fig1B). The capacity of each cell line to proliferate in response to added EPO was determined. In contrast to other reports using Ba/F3 cells,17 none of the 32D clones expressing truncated forms of the EPO-R were hypersensitive to EPO (not shown). Indeed, EPO-hypersensitivity of various truncated EPO-R isoforms expressed in Ba/F3 cells has been suggested to result from residual IGF-1 in serum.18 32D.EPO-R(1-321) cells, a variant of the EPO-R that contains only Box1 and Box2, survive and proliferated weakly only in supraphysiologic concentrations of EPO (>10 U/mL; not shown), whereas 32D.EPO-R(1-252) cells did not proliferate or survive at concentrations of EPO up to 50 U/mL.
Schematic diagram of EPO-R variants and their level of expression in 32D cells. (A) Stick figure of the murine EPO-R depicting the various truncated EPO-R isoforms generated (arrows). 1-483 represents the wild-type EPO-R (lacking the signal peptide). The dark box represents the transmembrane domain. The gray boxes represent Box1 and Box2 domains. Specific point mutations constructed are designated. EPO-R(YF) is a full-length EPO-R in which all 8 intracellular (cytoplasmic) tyrosine residues (Y) were changed to phenylalanines. The positions of the tyrosine residues (horizontal line) relative to the sites of truncation are shown. TM, transmembrane domain. (B) Immunoblot analysis of detergent soluble extracts from 32D clones containing the various EPO-R isoforms. All lanes were loaded with a detergent soluble extract from 7.5 × 105 cells. A polyclonal rabbit antisera against the extracellular N-terminal peptide of the murine EPO-R was used. Molecular mass standards (in kilodaltons) are depicted on the right.
Schematic diagram of EPO-R variants and their level of expression in 32D cells. (A) Stick figure of the murine EPO-R depicting the various truncated EPO-R isoforms generated (arrows). 1-483 represents the wild-type EPO-R (lacking the signal peptide). The dark box represents the transmembrane domain. The gray boxes represent Box1 and Box2 domains. Specific point mutations constructed are designated. EPO-R(YF) is a full-length EPO-R in which all 8 intracellular (cytoplasmic) tyrosine residues (Y) were changed to phenylalanines. The positions of the tyrosine residues (horizontal line) relative to the sites of truncation are shown. TM, transmembrane domain. (B) Immunoblot analysis of detergent soluble extracts from 32D clones containing the various EPO-R isoforms. All lanes were loaded with a detergent soluble extract from 7.5 × 105 cells. A polyclonal rabbit antisera against the extracellular N-terminal peptide of the murine EPO-R was used. Molecular mass standards (in kilodaltons) are depicted on the right.
EPO Equilibrium Binding Studies on Selected Clones of 32D Cells Containing Variant Forms of the EPO-R
| Cell Line . | kd (pmol/L) . | Receptor No./Cell . | R2 Value . |
|---|---|---|---|
| 32D.EPO-R:1-483 (wild-type) | 220 260 | 834 1280 | .95 .93 |
| 345 | 1650 | .95 | |
| 32D.EPO-R:1-411 | 267 | 1413 | .94 |
| 325 | 1256 | .93 | |
| 383 | 1554 | .95 | |
| 32D.EPO-R:1-373 | 224 | 1300 | .96 |
| 258 | 1158 | .93 | |
| 373 | 1447 | .94 | |
| 32D.EPO-R:1-321 | 285 | 675 | .94 |
| 382 | 1055 | .95 | |
| 315 | 1252 | .94 | |
| 32D.EPO-R:1-252 | 650 | 6550 | .95 |
| 576 | 6882 | .93 | |
| 555 | 5897 | .94 | |
| 32D.EPO-R:1-483(W282R) | 402 | 1718 | .95 |
| 32D.EPO-R:1-483(YF) | 209 | 734 | .93 |
| Cell Line . | kd (pmol/L) . | Receptor No./Cell . | R2 Value . |
|---|---|---|---|
| 32D.EPO-R:1-483 (wild-type) | 220 260 | 834 1280 | .95 .93 |
| 345 | 1650 | .95 | |
| 32D.EPO-R:1-411 | 267 | 1413 | .94 |
| 325 | 1256 | .93 | |
| 383 | 1554 | .95 | |
| 32D.EPO-R:1-373 | 224 | 1300 | .96 |
| 258 | 1158 | .93 | |
| 373 | 1447 | .94 | |
| 32D.EPO-R:1-321 | 285 | 675 | .94 |
| 382 | 1055 | .95 | |
| 315 | 1252 | .94 | |
| 32D.EPO-R:1-252 | 650 | 6550 | .95 |
| 576 | 6882 | .93 | |
| 555 | 5897 | .94 | |
| 32D.EPO-R:1-483(W282R) | 402 | 1718 | .95 |
| 32D.EPO-R:1-483(YF) | 209 | 734 | .93 |
EPO binding studies were performed as described in Materials and Methods. EPO binding curves were plotted and the saturation of all EPO-binding sites was achieved for all cell lines over the concentrations of EPO used. For each cell line, at least 2 experiments were performed. Dissociation constant (kd) and cell surface receptor numbers were then determined through Scatchard analyses. R2values for all plots were greater than .93.
The membrane proximal domain of the EPO-R cytoplasmic tail was the minimal domain necessary for internalization of surface-bound EPO.
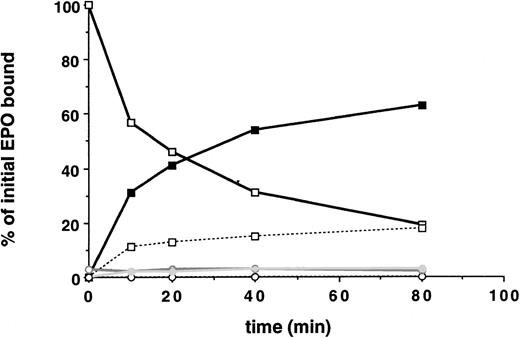
EPO internalization and EPO-R downregulation was first studied in 32D cells containing wild-type EPO-R and in 32D.Neo cells not containing an EPO-R. A saturating amount (5 nmol/L) of radio-iodinated EPO was added to the cells at 4°C. Cells were then washed and warmed to 37°C for various periods of time. At various time points, the radioactivity that was internalized, released into the medium, or remaining on the cell surface was measured. Multiple clones were examined. The initial rate of EPO internalization was determined from the slope of the linear portion of the uptake curve. As shown in Fig 2, 32D.EPO-R(wt or 1-483) internalized EPO efficiently during the 80-minute time course. After 40 minutes, 54% of bound EPO was internalized (solid squares), and the amount of cell surface bound EPO was downregulated by 69% (open squares). After 20 minutes, tricarboxylic acid-soluble counts were detectable in the medium, indicating that internalized EPO had been degraded and that 125I was being released from cells into the medium. In the presence of Na Azide, which prevents receptor internalization at 37°C, we did not observe any degradation of bound EPO (ie, all surface counts released after acid wash were precipitated by the addition of TCA), indicating that internalization was required for EPO degradation (not shown). These cells internalized EPO at an initial rate of 3.2% per minute (Table 2). Parental 32D cells transfected with the control vector pMEX showed neither binding nor internalization of EPO (Fig 2, open circles). This result demonstrated that a functional EPO-R complex capable of internalizing bound EPO was reconstituted in transfected 32D cells.
32D cells containing the EPO-R reconstitute EPO internalization and receptor downregulation. 125I-EPO internalization studies. Solid symbols represent the percentage of total bound counts at time 0 that were internalized. Open symbols represent the percentage of total bound counts at time 0 that remained surface bound. Open symbols and a broken line represent the percentage of total bound counts at time 0 that were released into the medium. Standard deviations for each point were within 10% of the value plotted and are not shown. At each time point, the summation of internalized EPO, surface-bound EPO, and counts in the medium equaled the amount of 125I-EPO bound at time 0. Internalization studies were performed on 3 different clones and were performed 2 times for each clone. Data presented are from a single representative clone of each. 32D.Wild-type EPO-R(1-483) cells, squares; 32D.Neo cells (no EPO-R), circles.
32D cells containing the EPO-R reconstitute EPO internalization and receptor downregulation. 125I-EPO internalization studies. Solid symbols represent the percentage of total bound counts at time 0 that were internalized. Open symbols represent the percentage of total bound counts at time 0 that remained surface bound. Open symbols and a broken line represent the percentage of total bound counts at time 0 that were released into the medium. Standard deviations for each point were within 10% of the value plotted and are not shown. At each time point, the summation of internalized EPO, surface-bound EPO, and counts in the medium equaled the amount of 125I-EPO bound at time 0. Internalization studies were performed on 3 different clones and were performed 2 times for each clone. Data presented are from a single representative clone of each. 32D.Wild-type EPO-R(1-483) cells, squares; 32D.Neo cells (no EPO-R), circles.
The Initial Rate of EPO Internalization and Extent of Bound EPO Internalized for 32D Cell Lines Expressing EPO-R Variants
| EPO-R Variant . | Internalization Rate (%/min) . | EPO Internalized at 40 Minutes (% initially bound) . |
|---|---|---|
| 1-483 (wild-type) | 3.2 | 54 |
| 1-411 | 3.6 | 50 |
| 1-373 | 4.2 | 58 |
| 1-321 | 3.8 | 55 |
| 1-252 | 1.0 | 20 |
| 1-483(W282R) | 3.0 | 52 |
| 1-483(YF) | 2.9 | 45 |
| EPO-R Variant . | Internalization Rate (%/min) . | EPO Internalized at 40 Minutes (% initially bound) . |
|---|---|---|
| 1-483 (wild-type) | 3.2 | 54 |
| 1-411 | 3.6 | 50 |
| 1-373 | 4.2 | 58 |
| 1-321 | 3.8 | 55 |
| 1-252 | 1.0 | 20 |
| 1-483(W282R) | 3.0 | 52 |
| 1-483(YF) | 2.9 | 45 |
The initial rate of internalization was determined from the slope of the linear portion of the curve between 0 and 10 minutes.
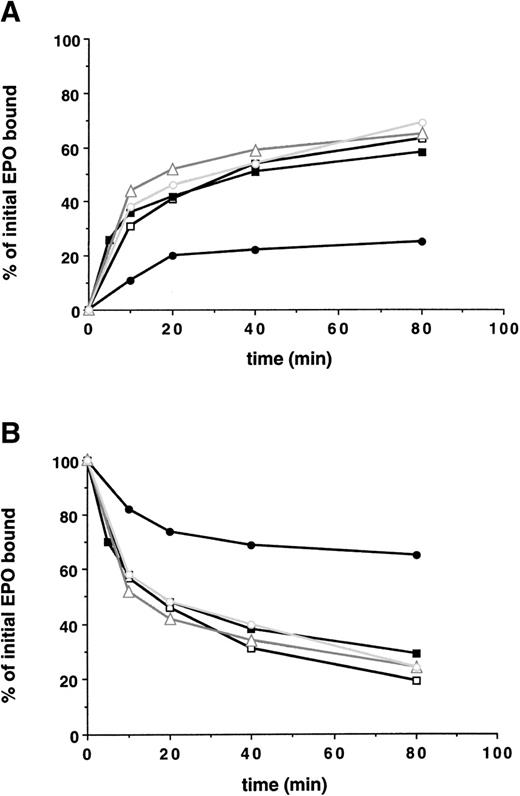
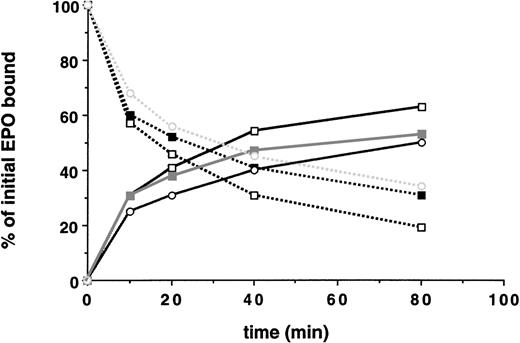
Next, the ability of the various cell clones containing truncated EPO-Rs to internalize bound EPO was studied, as shown in Fig 3. Receptors with up to 162 amino acids deleted from the cytoplasmic tail exhibited no significant impairment in the initial rate of EPO internalization, the extent of EPO internalization, and the degree of receptor downregulation. However, removal of an additional 69 amino acids (EPO-R:1-252) decreased the initial rate of internalization of bound EPO, reduced the extent of EPO internalized 3-fold, and decreased the extent of downregulation of surface bound EPO (Fig 3 and Table 2).
A membrane-proximal 69 amino acid domain of the EPO-R cytoplasmic tail is the minimal domain required for internalization of EPO and receptor downregulation. 125I-EPO internalization studies. (A) The percentage of total bound counts at time 0 that were internalized. (B) The percentage of total bound counts at time 0 that remained surface bound. Standard deviations for each point were within 10% of the value plotted and are not shown. At each time point, the summation of internalized EPO, surface-bound EPO, and counts in the medium equaled the amount of 125I-EPO bound at time 0. Internalization studies were performed on 2 clones for each EPO-R isoform and were performed 2 times for each clone. Data presented are from a single representative clone of each. (□) Wild-type EPO-R(1-483); (▪) EPO-R(1-411); (▵) EPO-R(1-373); (○) EPO-R(1-321); and (•) EPO-R(1-252).
A membrane-proximal 69 amino acid domain of the EPO-R cytoplasmic tail is the minimal domain required for internalization of EPO and receptor downregulation. 125I-EPO internalization studies. (A) The percentage of total bound counts at time 0 that were internalized. (B) The percentage of total bound counts at time 0 that remained surface bound. Standard deviations for each point were within 10% of the value plotted and are not shown. At each time point, the summation of internalized EPO, surface-bound EPO, and counts in the medium equaled the amount of 125I-EPO bound at time 0. Internalization studies were performed on 2 clones for each EPO-R isoform and were performed 2 times for each clone. Data presented are from a single representative clone of each. (□) Wild-type EPO-R(1-483); (▪) EPO-R(1-411); (▵) EPO-R(1-373); (○) EPO-R(1-321); and (•) EPO-R(1-252).
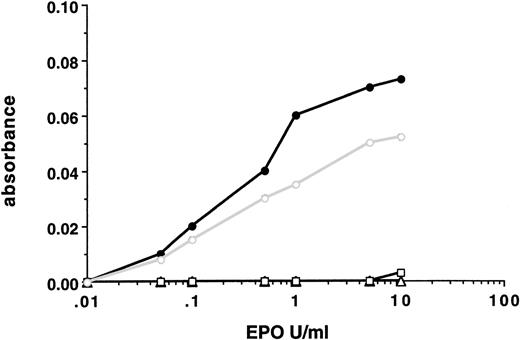
Despite the absence of a cytoplasmic tail, 32D cells containing EPO-R(1-252) internalized 20% of bound EPO over 40 minutes. To determine how much of this represented nonspecific pinocytosis complex oligosaccharide side chains present on the N-terminal, extracellular region of the cell surface, glycoproteins on 32D.Neo, 32D.EPO-R(wt), and 32D.EPO-R(1-252) cells were labeled with tritiated galactose. Cells were treated with neuraminidase and β-galactosidase to remove sialic acid and galactose residues and were then incubated with galactosyltransferase and UDP-(3H)galactose.16Under these conditions, the cell surface glycoproteins, including the EPO-R, are labeled with (3H)galactose. Labeled cells were warmed to 37°C to allow for receptor internalization to occur and were then rapidly chilled to 4°C to arrest receptor movement. Cells were then either lysed or treated with β-galactosidase to remove (3H)galactose that remained on the cell surface. Therefore, only receptors that were internalized would retain the (3H)galactose label. Before initiating the 37°C chase, the amount of (3H)galactose released by β-galactosidase treatment was shown to be greater than 95% for all cells tested.
In the absence of EPO, EPO-R(wt) and EPO-R(1-252) were internalized slowly, at the same rate as total cell surface glycoproteins are pinocytosed (Fig 4). When EPO was bound to the labeled surface receptors before the 37°C chase period, 40% of the wild-type receptors were internalized, whereas there was no change in the fraction of EPO-R(1-252) cell surface receptors internalized (Fig 4). Thus, the rate of internalization of the tailless receptor was indistinguishable from the rate at which total cell surface glycoproteins are internalized and likely represents nonspecific pinocytosis. Thus, the membrane proximal 69 amino acids of the EPO-R cytoplasmic tail was the minimal region required for internalization of bound EPO and receptor downregulation.
Effect of cytoplasmic domain deletion on receptor internalization. Cell surface glycoproteins were labeled with [3H]galactose, and receptor internalization was determined as described in Materials and Methods. The values shown are the average of 2 independent determinations. (□) The percentage of surface EPO-R internalized in 40 minutes at 37°C in the absence of EPO (−EPO); (▪) the percentage of surface EPO-R internalized in 40 minutes at 37°C in the presence of EPO (+EPO). Total cell surface glycoproteins internalization was determined from anti–EPO-R immunoprecipitates of lysates from (3H)galactose-labeled parental 32D cells that do not contain an EPO-R (total surface GP).
Effect of cytoplasmic domain deletion on receptor internalization. Cell surface glycoproteins were labeled with [3H]galactose, and receptor internalization was determined as described in Materials and Methods. The values shown are the average of 2 independent determinations. (□) The percentage of surface EPO-R internalized in 40 minutes at 37°C in the absence of EPO (−EPO); (▪) the percentage of surface EPO-R internalized in 40 minutes at 37°C in the presence of EPO (+EPO). Total cell surface glycoproteins internalization was determined from anti–EPO-R immunoprecipitates of lysates from (3H)galactose-labeled parental 32D cells that do not contain an EPO-R (total surface GP).
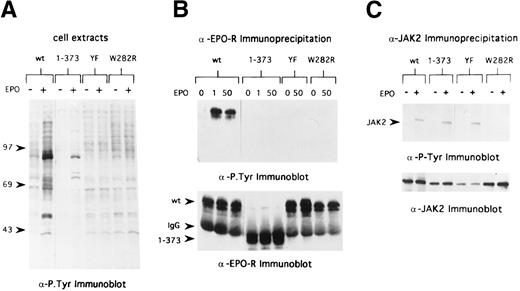
JAK2 tyrosine kinase activation was not required for internalization of EPO-R–bound EPO.
In the case of the EGF-R, receptor kinase activation and tyrosine phosphorylation of the receptor and cellular substrates can regulate endocytosis of the receptor.13,19 The membrane proximal region of the EPO-R required for internalization of bound EPO contains the Box1 and Box2 motifs, which are the minimal region of the EPO-R required for mitogenic responses and JAK2 binding site.20,21 To determine whether EPO-induced JAK2 tyrosine kinase activation is required for internalization of bound EPO, we expressed a mitogenically inactive form of the EPO-R, EPO-R(W282R), in 32D cells (Fig 1B). As shown in Fig 5, cells expressing this mutant receptor do not survive or proliferate in response to EPO. In addition, there was no change in the tyrosine-phosphorylated protein profile of 32D.EPO-R(W282R) cells upon addition of EPO (Fig 6A), and neither the EPO-R(W282R) (Fig 6B) nor JAK2 (Fig 6C) was tyrosine phosphorylated in response to EPO. Finally, we were unable to detect any activation of JAK2 kinase activity in 32D.EPO-R(W282R) cells in response to EPO, confirming the results of Witthuhn et al8 (not shown). Despite the absence of JAK2 tyrosine kinase activity in these cells, the initial rate of EPO internalization and extent of EPO internalization were similar to that of cells containing wild-type receptors (Fig 7 and Table 2). Thus, EPO-stimulated JAK2 kinase activity was not required for internalization of EPO-R–bound EPO.
32D cells containing EPO-R(W282R) or EPO-R(YF) do not proliferate in response to EPO. (•) MTT assay of 32D clones containing wild-type EPO-R; (○) EPO-R(1-373); (▵) EPO-R(W282R); and (□) EPO-R(YF). Data presented are from a single representative clone of each.
32D cells containing EPO-R(W282R) or EPO-R(YF) do not proliferate in response to EPO. (•) MTT assay of 32D clones containing wild-type EPO-R; (○) EPO-R(1-373); (▵) EPO-R(W282R); and (□) EPO-R(YF). Data presented are from a single representative clone of each.
EPO-R and JAK2 tyrosine phosphorylation in 32D cells containing variants of the EPO-R. (A) Cells were washed in RPMI and placed in OptiMEM (GIBCO) in the absence of serum and growth factors. After 6 hours, cells were stimulated with 50 U/mL EPO (+) for 7 minutes or were not stimulated (−). Cell extracts from 7.5 × 105 cells were separated by 8% SDS-PAGE under reducing conditions and transferred to Hybond membranes, and immunoblotting was performed with antiphosphotyrosine antibodies. Lanes 1 and 2, 32D.EPO-R(1-483), wild-type cells; lanes 3 and 4, 32D.EPO-R(1-373) cells; lanes 5 and 6, 32D.EPO-R(YF) cells; and lanes 7 and 8, 32D.EPO-R(W282R) cells. Molecular mass standards (in kilodaltons) are on the left. (B) Cell extracts from starved cells (0 EPO) or cells stimulated with 1 U/mL EPO or 50 U/mL EPO were immunoprecipitated with antisera against the N-terminal peptide of the murine EPO-R. Bound products were washed and separated by 8% SDS-PAGE under reducing conditions and transferred to Hybond membranes, and immunoblotting was performed with antiphosphotyrosine antibodies (upper panel). The blot was then stripped and reprobed with antisera against the EPO-R (lower panel); arrowheads on the left indicate the position of wild-type (wt) EPO-R, EPO-R(W282R) and EPO-R(YF), IgG, and EPO-R(1-373), respectively. Lanes 1 through 3, 32D.EPO-R(1-483) wt cells; lanes 4 through 6, 32D.EPO-R(1-373) cells; lanes 7 and 8, 32D.EPO-R(YF) cells; and lanes 9 and 10, 32D.EPO-R(W282R) cells. (C) Cell extracts from starved cells (−) or cells stimulated with 50 U/mL EPO (+) were immunoprecipitated with antisera against JAK2. Bound products separated by 8% SDS-PAGE under reducing conditions and transferred to Hybond membranes, and immunoblotting was performed with antiphosphotyrosine antibodies (upper panel); the arrowhead on the left identifies the position of JAK2. The blot was then stripped and reprobed with antisera against JAK2 (lower panel). Lanes 1 and 2, 32D.EPO-R(1-483) wt cells; lanes 3 and 4, 32D.EPO-R(1-373) cells; lanes 5 and 6, 32D.EPO-R(YF) cells; and lanes 7 and 8, 32D.EPO-R(W282R) cells.
EPO-R and JAK2 tyrosine phosphorylation in 32D cells containing variants of the EPO-R. (A) Cells were washed in RPMI and placed in OptiMEM (GIBCO) in the absence of serum and growth factors. After 6 hours, cells were stimulated with 50 U/mL EPO (+) for 7 minutes or were not stimulated (−). Cell extracts from 7.5 × 105 cells were separated by 8% SDS-PAGE under reducing conditions and transferred to Hybond membranes, and immunoblotting was performed with antiphosphotyrosine antibodies. Lanes 1 and 2, 32D.EPO-R(1-483), wild-type cells; lanes 3 and 4, 32D.EPO-R(1-373) cells; lanes 5 and 6, 32D.EPO-R(YF) cells; and lanes 7 and 8, 32D.EPO-R(W282R) cells. Molecular mass standards (in kilodaltons) are on the left. (B) Cell extracts from starved cells (0 EPO) or cells stimulated with 1 U/mL EPO or 50 U/mL EPO were immunoprecipitated with antisera against the N-terminal peptide of the murine EPO-R. Bound products were washed and separated by 8% SDS-PAGE under reducing conditions and transferred to Hybond membranes, and immunoblotting was performed with antiphosphotyrosine antibodies (upper panel). The blot was then stripped and reprobed with antisera against the EPO-R (lower panel); arrowheads on the left indicate the position of wild-type (wt) EPO-R, EPO-R(W282R) and EPO-R(YF), IgG, and EPO-R(1-373), respectively. Lanes 1 through 3, 32D.EPO-R(1-483) wt cells; lanes 4 through 6, 32D.EPO-R(1-373) cells; lanes 7 and 8, 32D.EPO-R(YF) cells; and lanes 9 and 10, 32D.EPO-R(W282R) cells. (C) Cell extracts from starved cells (−) or cells stimulated with 50 U/mL EPO (+) were immunoprecipitated with antisera against JAK2. Bound products separated by 8% SDS-PAGE under reducing conditions and transferred to Hybond membranes, and immunoblotting was performed with antiphosphotyrosine antibodies (upper panel); the arrowhead on the left identifies the position of JAK2. The blot was then stripped and reprobed with antisera against JAK2 (lower panel). Lanes 1 and 2, 32D.EPO-R(1-483) wt cells; lanes 3 and 4, 32D.EPO-R(1-373) cells; lanes 5 and 6, 32D.EPO-R(YF) cells; and lanes 7 and 8, 32D.EPO-R(W282R) cells.
Neither EPO-induced activation of JAK2 tyrosine kinase nor tyrosine phosphorylation of the EPO-R was required for EPO internalization and receptor downregulation. 125I-EPO internalization studies. Solid lines represent the percentage of total bound counts at time 0 that were internalized. Broken lines represent the percentage of total bound counts at time 0 that remained surface bound. Standard deviations for each point were within 10% of the value plotted and, thus, are not shown. Internalization studies were performed at least 3 times for each clone. Data presented are from a single representative clone of each. (□) Wild-type EPO-R(1-483); (▪) EPO-R(W282R); and (○) EPO-R(YF).
Neither EPO-induced activation of JAK2 tyrosine kinase nor tyrosine phosphorylation of the EPO-R was required for EPO internalization and receptor downregulation. 125I-EPO internalization studies. Solid lines represent the percentage of total bound counts at time 0 that were internalized. Broken lines represent the percentage of total bound counts at time 0 that remained surface bound. Standard deviations for each point were within 10% of the value plotted and, thus, are not shown. Internalization studies were performed at least 3 times for each clone. Data presented are from a single representative clone of each. (□) Wild-type EPO-R(1-483); (▪) EPO-R(W282R); and (○) EPO-R(YF).
Tyrosine phosphorylation of the EPO-R was not required for internalization of EPO-R–bound EPO.
To determine if tyrosine phosphorylation of the EPO-R was required for internalization of receptor-bound EPO, we generated 32D cells containing a full-length EPO-R in which all 8 cytoplasmic tyrosine residues were changed to phenylalanine residues (Fig 1). The 32D.EPO-R(YF) cells did not proliferate in response to EPO concentrations as high as 10 U/mL; however, they did survive in cultures containing 10 U/mL of EPO (Fig 5). Despite these altered growth parameters, JAK2 was tyrosine phosphorylated in response to EPO (Fig 6C) at a level comparable to cells expressing wt EPO-R, as has been previously reported.4 As expected, EPO-R(YF) was not tyrosine phosphorylated in response to EPO (Fig 6B). This mutant receptor bound EPO with a normal affinity (kd, 109 pmol/L) and internalized receptor-bound EPO as well as cells containing wild-type receptors (Fig 7 and Table 2). Similarly, EPO-R(W282R), which also was not tyrosine phosphorylated in response to EPO, internalized receptor-bound EPO as well as cells containing wild-type EPO-R (Fig 7and Table 2). Thus, tyrosine phosphorylation of the EPO-R was not required for internalization of EPO-R–bound EPO.
DISCUSSION
We have shown that a membrane proximal domain of the cytoplasmic tail of the EPO-R was the minimal domain required for internalization of receptor-bound EPO. This region contains the Box1 and Box2 motifs, which are conserved among most cytokine receptor family members and function as the site of receptor-JAK2 tyrosine kinase association.2,8,21 It is the minimal cytoplasmic region required for activation of JAK2 kinase and mitogenic signaling.8 In contrast to what has been found with other families of signal-transducing growth factor receptors (eg, EGF-R), activation of tyrosine kinase activity, specifically JAK2, and tyrosine phosphorylation of the EPO-R itself were not required for internalization of EPO.
A previous report suggested that the cytoplasmic tail of the EPO-R is not required for internalization of bound EPO.22 In that study, the internalization of surface-bound EPO was determined at a single 40-minute time point. In the current study, we determined the extent of EPO internalization at multiple early time points, allowing for the calculation of the initial rate of internalization. Cells containing EPO-R(1-252) internalized EPO at approximately one third the rate of cells expressing wild-type receptor, and the extent of EPO internalization was decreased 3-fold. For other type I cytokine receptors (eg, gp130 of the IL-6 receptor and GH-R), complete deletion of their cytoplasmic tails also reduced internalization of bound ligand to an extent similar to our results.23,24 In addition, a recent study of EPO internalization in BaF3 cells and COS cells, containing EPO-R isoforms, showed that a membrane-proximal cytoplasmic motif was required for internalization of receptor-bound EPO.25 The lower internalization rate of bound EPO in cells containing EPO-R(1-252) could be due to the higher kd values for EPO binding in these cells (ie, a lower affinity for EPO). If so, then one would expect significantly increased bound EPO off rates for these cells. However, relative to cells containing wild-type receptors, we did not observe a significant difference in EPO off-rates. Furthermore, we demonstrated that the rate of EPO-R internalization by 32D.EPO-R (1-252) cells approximated the rate of nonspecific pinocytosis. Thus, our results demonstrated that, when EPO-R(1-252) was expressed in the hematopoietic cell lines 32D, a cell line that reconstitutes EPO-R function, it failed to internalize bound EPO, indicating that the membrane-proximal region of the EPO-R cytoplasmic tail is important for internalizing bound EPO in hematopoietic cells. Furthermore, our results are in agreement with those obtained by Levin et al.25
Recently, it was shown that proteosomes may be involved in the downregulation of the EPO-R activation signals.26 CIS is an SH2 domain-containing cytosolic protein whose expression is induced by EPO signals, associates with the EPO-R via the second tyrosine residue of the cytoplasmic domain of the EPO-R, and decreases EPO-stimulated cell proliferation in some manner. CIS is ubiquitinated.26In the presence of proteosome inhibitors, EPO-induced STAT5 activity was prolonged. Therefore, do CIS, ubiquitination, or proteosomes regulate EPO internalization by the EPO-R? We show that 32D cells containing EPO-R isoforms lacking the CIS binding site [eg, EPO-R(1-321) and EPO-R(YF)] internalize EPO at the same rates and to the same extent as 32D cells containing wild-type receptors. Secondly, nonfunctional EPO-Rs [eg, EPO-R(YF) and EPO-R(W282R)] that should not induce expression of CIS internalize EPO as well as functional, wild-type EPO-R. Thus, the association of CIS with the EPO-R is unlikely to contribute to EPO internalization. When the EPO-R is expressed in a ts mutant CHO cell line unable to ubiquitinate cellular proteins and then placed at a nonpermissive temperature, internalization of bound EPO is not significantly altered, despite alterations in the cellular metabolism of the EPO-R (Beckman and Longmore, unpublished observation). Thus, proteosomes are unlikely to directly regulate EPO internalization by the EPO-R. However, it has not been shown whether the EPO-R is ubiquitinated.
Other signaling receptors containing intrinsic tyrosine kinase activity (eg, EGF-R and insulin receptors) are downregulated by internalization after ligand binding and targeting to the lysosome for degradation. Downregulation of activated EGF-R is thought to be important in modulating cellular responses to ligand.27 Whether the intrinsic tyrosine kinase activity of the activated RTKs and subsequent tyrosine phosphorylation of sites in the cytoplasmic tail of RTKs are directly required for their internalization remains controversial.13 Recent results suggest that EGF-R kinase activity as well as tyrosine phosphorylation of the receptor cytoplasmic tail and other cytosolic substrates are indeed required for sequestration of the EGF-R into clathrin-coated pits and downmodulation of its signals.28,29 Other studies suggest that receptor tyrosine kinase activity is not required for targeting the EGF-R to lysosomes, but rather that tyrosine kinase activity amplifies the conformation changes induced by ligand binding by exposing or generating other internalization and lysosomal targeting sequences.30 We have shown that mitogenically inactive forms of the EPO-R [EPO-R(W282R)] do not affect the internalization of bound EPO or of receptor downregulation. Thus, EPO-induced JAK2 tyrosine kinase activity is not required for EPO-R endocytosis. In addition, tyrosine phosphorylation of the EPO-R cytoplasmic tail was also not required for efficient endocytosis. Similar to our results, some mitogenically inactive forms of the GH-R internalize bound GH quite well24; however, in that study, activation of cytosolic tyrosine kinase activity and receptor phosphorylation was not directly determined.
Wang and Moran29 recently demonstrated that association of the adapter protein Grb2 with activated EGF-R was required for receptor endocytosis. Grb2 also associates with the cytoplasmic tail of the EPO-R after activation of the EPO-R, leading to activation of the Ras/MAPK signaling pathway.31 The region of the EPO-R required for Grb2 association maps to the distal cytoplasmic tail (aa373-483).31,32 32D.EPO-R(1-373) lack this domain, do not activate MAPK31 (not shown), and do not associate with Grb2, yet they proliferate as well as cells containing wild-type EPO-R (Fig 4). We found that these cells internalize bound EPO and downregulate surface EPO-Rs as well as cells containing wild-type EPO-R. Thus, in addition to the dispensability of JAK2 activity and receptor tyrosine phosphorylation, recruitment of Grb2 to the receptor and activation of the MAPK pathway is also not required for EPO-R downregulation. Taken together, these results suggest that, for signaling receptors of the cytokine receptor superfamily (specifically the EPO-R), downregulation of cell surface ligand occupied receptors follows a pathway distinct from signaling receptors of the RTK family. Precisely how cytokine receptors, such as the EPO-R, are internalized is the focus of future experiments.
Several families with dominantly inherited primary erythrocytosis have been shown to harbor mutations in one allele encoding the EPO-R (reviewed in Gregg and Prchal33).34 Most result in truncation (59 to 83 amino acids) of an intracellular C-terminal domain of the EPO-R thought to exert a negative effect upon receptor function. We found here that 32D cells containing an EPO-R truncated by 72 amino acids did not exhibit any alteration in EPO internalization compared with cells containing wild-type EPO-Rs; however, we did not isolate any clones containing EPO-R(1-411) that exhibited growth hypersensitivity to EPO. This suggests that defects in receptor internalization may not contribute to the EPO hypersensitivity observed in these patients.
We have shown that mitogenically inactive or attenuated EPO-Rs (eg, W282R, EPO-R:YF, and 1-321) do not exhibit any alteration in the rate or extent of receptor-bound EPO internalization as compared with cells containing functional EPO-Rs (eg, wt, 1-411, and 1-373). This suggests that, aside from degradation of internalized EPO, cell surface EPO-R internalization after EPO engagement may not contribute to the downregulation of known EPO signaling pathways. However, we have not excluded the possibility that once-internalized intracellular sorting fates of functional EPO-R isoforms differ from those of inactive receptor isoforms.
ACKNOWLEDGMENT
The authors thank Drs S. Watowich and M. Goldsmith for providing EPO-R(W282R) and EPO-R(YF) plasmids, respectively; Abbott Laboratories for purified EPO; Dr Sally York for helpful discussions; and Drs S. Kornfeld and L. Traub for review of the manuscript and helpful suggestions.
Supported in part by American Cancer Society Grant No. ACS-IRG 36-37 (G.D.L.) and by National Institutes of Health Grant No. CA75315 (G.D.L). G.D.L. was a Scholar of the James S. McDonnell Foundation. D.L.B. was supported as a Lucille P. Markey Pathway postdoctoral fellow. Support for L.L.L. was provided by a grant to Washington University from the Howard Hughes Medical Institute through the Undergraduate Biological Sciences Education Program.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Gregory D. Longmore, MD, Division of Hematology, Washington University School of Medicine, Campus Box 8125, 660 S Euclid Ave, St Louis MO 63110; e-mail: longmorg@medicine.wustl.edu.

![Fig. 4. Effect of cytoplasmic domain deletion on receptor internalization. Cell surface glycoproteins were labeled with [3H]galactose, and receptor internalization was determined as described in Materials and Methods. The values shown are the average of 2 independent determinations. (□) The percentage of surface EPO-R internalized in 40 minutes at 37°C in the absence of EPO (−EPO); (▪) the percentage of surface EPO-R internalized in 40 minutes at 37°C in the presence of EPO (+EPO). Total cell surface glycoproteins internalization was determined from anti–EPO-R immunoprecipitates of lysates from (3H)galactose-labeled parental 32D cells that do not contain an EPO-R (total surface GP).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/8/10.1182_blood.v94.8.2667.420k27_2667_2675/7/m_blod42027004x.jpeg?Expires=1767775321&Signature=XxYEThAUUWOmwFVpRWb3asEJxtflmVioGZxT-SikNY8odhtNq1d3rF-ektbr5fdK2WlZpNu23A1tEtMxWD8kATgOcvoDSBTnn7v5odntSj4H6n~gpGjvsw3vHh0SeBWn84XbCSD5CpGK7I1YYgP7KZsuuivGZJTMNo1QXQvFn8ecGTnkOFSP72DHQP9P-TmYc1nH6xfpfictSd2I0hKTOBulActiVrdV5McEd7-VfH5B6-0PN8glYGr2Zgghx9lRtlKbu5nA1lxCNpuAlb2p7oc3Mhest6jIsNis6pxxI2EDwI~sa3eARt0tWGgtZrpF1LxTfs~ZPqwA~r383kOvMg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal