Abstract
Cytokine receptors have been shown in cell culture systems to use phosphotyrosine residues as docking sites for certain signal transduction intermediates. Studies using various cellular backgrounds have yielded conflicting information about the importance of such residues. The present studies were undertaken to determine whether or not tyrosine residues within the erythropoietin receptor (EPOR) are essential for biologic activity during hematopoiesis in vivo. A variant of the EPOR was constructed that contains both a substitution (R129C) causing constitutive receptor activation as well as replacement of all eight cytoplasmic tyrosines by phenylalanines (cEPORYF). A comparison between animals exposed to recombinant retroviruses expressing cEPOR and cEPORYF showed that efficient red blood cell (RBC) development in vivo is dependent on the presence of tyrosine residues in the cytoplasmic domain of the EPOR. In addition, an inefficient EPOR tyrosine independent pathway supporting RBC development was detected. Tyrosine add-back mutants showed that multiple individual tyrosines have the capacity to restore full erythropoietic potential to the EPOR as determined in whole animals. The analysis of primary erythroid progenitors transduced with the various cEPOR tyrosine mutants and tyrosine add-backs showed that only tyrosine 343 (Y1) and tyrosine 479 (Y8) were capable of supporting immature burst-forming unit–erythroid progenitor development. Thus, this receptor is characterized by striking functional redundancy of tyrosines in a biologically relevant context. However, selective tyrosine residues may be uniquely important for early signals supporting erythroid development.
MAMMALIAN BLOOD CELL development depends on exquisite timing, critical cell fate decisions, and remarkable renewal capacity.1 These features are coordinated in vivo to produce an orderly sequence of steps by which stem cell populations differentiate into mature erythroid, myeloid, and lymphoid cell types. Hematopoietic cytokines are critical for the development and expansion of blood cells. However, whether or not cell fate decisions during blood cell development are directly affected by hematopoietic cytokines is not entirely clear.2 If so, the specific signaling pathways controlling the distinct biological outcomes resulting from cytokine actions are not known.
A prototypical hematopoietic cytokine is erythropoietin (EPO), a 34-kD glycoprotein that is a major determinant of erythroid cell development.3 Extensive cell culture and genetic analyses have shown that EPO exerts survival, proliferative, and differentiative effects on red blood cell (RBC) progenitors.3-8 These actions are mediated by the erythropoietin receptor (EPOR),9 which is believed to operate as a noncovalent complex containing two identical 64-kD transmembrane proteins. Engagement by EPO apparently promotes dimerization of these receptor subunits, thereby initiating transmembrane signaling that leads to cellular responses.10Indeed, a mutant EPOR (EPOR(R129C) or cEPOR) that homodimerizes constitutively in the absence of ligand also causes ligand-independent growth of cell lines containing this form of the EPOR.11Like other cytokine receptors, the native EPOR has been the subject of extensive molecular characterization in immortalized cell lines to define its functional domains and the signal transduction circuitry linked to these domains.
An important scientific goal is to define the molecular mechanisms by which specificity is maintained in signaling responses mediated by hematopoietic cytokine receptors such as the EPOR, and to relate these mechanisms to blood cell development within the whole animal context. Studies of structure-function relationships within the EPOR in immortalized cell culture models collectively implicate phosphorylation of specific tyrosine residues within the cytoplasmic tail of the EPOR as a key determinant of growth signaling and/or selective activation of various signaling intermediates.12-15Although such studies have been quite instructive, the information derived is confounded somewhat by limitations inherent to studies using immortalized cell culture systems, such as variability caused by differences in cell context, and the inability of cell lines to recapitulate all cellular responses ascribed to EPO. For example, one major signaling pathway engaged by the EPOR is the JAK-STAT system. The tyrosine kinase JAK2 becomes activated rapidly upon receptor engagement16 followed by induction of the transcription factor STAT-5.12,13,17-21 Studies with selective EPOR cytoplasmic tyrosine mutants in cell culture systems have suggested a surprising degree of redundancy in specific tyrosine use in the activation of STAT-5.12,13 17-21 More importantly, these results offer little information about how these and other early signaling events are linked to downstream cellular consequences (eg, expansion of erythroid progenitor pools, maturation of RBCs, and survival effects) that are important for normal RBC physiology in vivo.
The present studies employed a novel strategy to investigate structure-function relationships of the tyrosine residues in the cytoplasmic tail of the EPOR as they relate to complete erythropoiesis in vivo. This approach exploits the previous report that the constitutively active mutant cEPOR11 causes dramatic expansion of the erythroid cell compartment in mice upon introduction via recombinant spleen focus-forming viruses.22 We have used this “dominant” genetic system to test the signal transduction and biological activities of site-directed mutants of the EPOR in vivo. The results showed both the tyrosine-dependence of these processes for efficient RBC development, and a remarkable degree of functional redundancy in tyrosine use.
MATERIALS AND METHODS
Constructs and viruses.
A synthetic DNA fragment encoding a mutant cytoplasmic tail of the murine EPOR in which all eight tyrosine residues were replaced by phenylalanines was constructed by oligonucleotide annealing, and this DNA fragment was subcloned into the corresponding region of the cEPOR-cDNA to generate cEPORYF. Tyrosine add-back mutants (Fig 1A) were prepared by polymerase chain reaction–based amplification of short segments using primers containing the desired tyrosine replacement substitutions, and subsequent insertion of these segments into the corresponding region of the cEPORYF-cDNA backbone. All constructs were verified by automated DNA sequencing. For studies in cell lines, these cDNAs were inserted into the pCMV4Neo expression vector. For studies in vivo, these cDNAs were subcloned into the previously described proviral plasmid pSFF. Retroviruses were generated from these plasmids and characterized as previously described22 23 and as described below, and adult NIH/Swiss mice subsequently were inoculated as described.
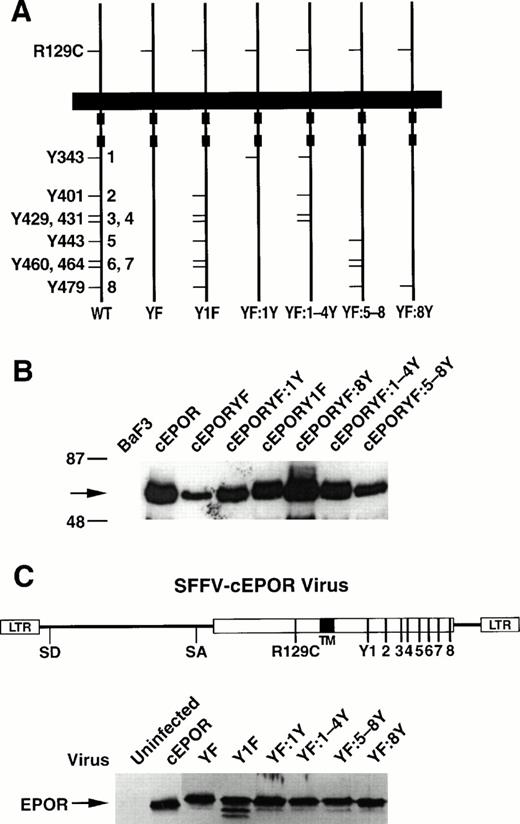
Tyrosine mutants of the EPOR and effects in cell culture. (A) Schematic of the parental cEPOR containing an intact cytoplasmic tail and the R129C substitution in the extracellular domain (WT), the cEPORYF mutant in which all eight cytoplasmic tyrosines have been replaced by phenylalanines (YF), and various mutants retaining selected tyrosine residues as shown (Y1F, YF:1Y, YF:1-4Y, YF:5-8Y, and YF:8Y). For convenience, specific tyrosine positions in the cytoplasmic segment have been numbered 1-8 as indicated. (B) Immunoblot analysis showing comparable levels of the cEPOR variants in transfected BaF3 cell lines. Arrow, EPOR. (C) Titers of SFFV-cEPOR viruses containing the tyrosine variants described above, as determined in infection analyses with fibroblasts. The schematic represents a genetic map of these recombinant retroviruses. The immunoblot displays detection of the cEPOR proteins from the indicated viruses.
Tyrosine mutants of the EPOR and effects in cell culture. (A) Schematic of the parental cEPOR containing an intact cytoplasmic tail and the R129C substitution in the extracellular domain (WT), the cEPORYF mutant in which all eight cytoplasmic tyrosines have been replaced by phenylalanines (YF), and various mutants retaining selected tyrosine residues as shown (Y1F, YF:1Y, YF:1-4Y, YF:5-8Y, and YF:8Y). For convenience, specific tyrosine positions in the cytoplasmic segment have been numbered 1-8 as indicated. (B) Immunoblot analysis showing comparable levels of the cEPOR variants in transfected BaF3 cell lines. Arrow, EPOR. (C) Titers of SFFV-cEPOR viruses containing the tyrosine variants described above, as determined in infection analyses with fibroblasts. The schematic represents a genetic map of these recombinant retroviruses. The immunoblot displays detection of the cEPOR proteins from the indicated viruses.
Cell lines and growth factors.
The interleukin-3 (IL-3)–dependent pro-B cell line BaF3 has been described previously.24 Growth factor independence was assessed by transfecting cell cultures with the indicated plasmids by electroporation as previously described, culturing the cells in IL-3 and G418 for 1 week, and then changing the medium to exclude IL-3. After 10 days it was readily apparent by visual inspection and trypan blue staining which cultures were viable and expanding (designated herein as factor-independent) compared with those that had expired. The cEPORYF cell line required the continuous presence of IL-3. Hematopoietic cell lines were established from spleens of infected mice (see below) as described previously.22 23 Briefly, splenic cells from infected mice exhibiting splenomegaly were dispersed and placed in culture medium (Iscove's modified Dulbecco's medium/20% fetal calf serum, containing β-mercaptoethanol) lacking any added growth factors. Such cell lines from animals infected with each of the viruses were maintained indefinitely in culture, except those from mice infected with SFFV-cEPORYF (see text). Culture supernatants from WEHI-3B cells (American Type Culture Collection) were used as a source of IL-3. Recombinant human EPO was provided as a gift by Ortho Biotech (Raritan, NJ).
Expression analysis and immunoblotting.
Whole cell lysates prepared from transfectants or from the murine cell lines described above were analyzed by immunoblotting with an antisera directed against the C-terminal segments of the EPOR as described previously.25 26 For analysis of viral titers, approximately 5 × 105 NIH 3T3 fibroblast cells in a P100 plate were infected with 4 mL of culture supernatent from retroviral producer cells. After infection, cells grown to confluence were harvested and lysed. From the detergent soluble extract 150 μg of protein was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 8% gels), transferred to nitrocellulose, and immunoblotted with antisera raised against the C-terminal tail of the EPOR (see above). For splenic extracts, single cell suspensions of virally infected or uninfected spleen cells were prepared. The cells were washed once with phosphate-buffered saline (PBS) and lysed in 1% Triton X-100; 150 mmol/L NaCl; 20 mmol/L Tris.Cl, pH 7.4; 1 mmol/L EDTA; 0.5% NP-40, containing aprotinin; and phenylmethylsulfonyl fluoride (PMSF) at 4°C for 15 minutes. Samples were clarified by centrifugation at 10,000g for 15 minutes. Soluble protein concentration was determined using the Pierce Chemical BCA protein determination kit (Pierce Chemical Corp, Rockford, IL). From each extract 150 μg of protein was applied to an 8% SDS-PAGE and products were resolved under reducing conditions, transfered to nitrocellulose, and immunoblotted with antisera against the EPOR.
Signal transduction assays.
JAK2 phosphorylation was measured by preparing whole cell lysates from resting or stimulated cells and conducting immunoprecipitation with an anti-JAK2 antiserum followed by immunoblot analysis as described previously.27 Electrophoretic mobility shift assays (EMSAs) were performed as described previously.28 Mitogen-activated kinase (MAPK) assays were performed using a nonradioactive MAP kinase assay kit (New England Biolabs, Beverly, MA) according to the manufacturer's instructions. In brief, 30 to 50 × 106 cells per sample were washed twice in calcium-magnesium–free PBS and stripped of growth factors by incubation in 150 mmol/L NaCl and 10 mmol/L Na citrate, pH 4, for 1 minute at room temperature. Cells were starved in RPMI 1430 supplemented with 1% bovine serum albumin (BSA; Sigma Chemical Co, St Louis, MO) for 6 hours at 37°C. Cells were stimulated with the indicated cytokines for 10 minutes and lysed in 220 to 300 ml of lysis buffer. Immunoprecipitations were performed with the anti-phosphoMAPK antibody. In vitro kinase assays were performed using GST-Elk1 as an exogenous substrate; these kinase assays were subjected to SDS-PAGE, followed by immunoblotting with an anti-phosphoElk1 antibody.
Infection and culture of hematopoietic progenitor cells.
Single cell suspensions of fetal liver were prepared from day 13 pregnant DBA-2 mice (Charles River Laboratories, Bar Harbor, ME). Cells were washed three times in α medium. Cells (106) were resuspended in medium containing fresh virus, and 4 μg/mL polybrene was added; cells were then incubated at 37°C for 3 hours. After infection, samples were washed in α medium and replated in α medium containing 30% fetal bovine serum (Sterile Systems, Logan, UT), 1% crystallized BSA, 1.2% 1,500 centipoise methylcellulose (1 poise = 0.1 Pa.sec; Fischer Scientific Corp, Pittsburgh, PA), recombinant murine stem cell factor (20 ng/mL; Genzyme Corp, Boston, MA) and 50 μmol/L 2-mercaptoethanol (Sigma) at a cell concentration of 2 × 104/mL. Partially purified human urinary EPO (specific activity = 250 U/mg), a generous gift from M. Kawakita (Kumamoto University, Japan), was used where indicated.
RESULTS
Tyrosine-dependent signaling by the EPOR.
Studies of mutants of the EPOR expressed in various cellular backgrounds have yielded somewhat conflicting information about the importance of tyrosine residues for receptor function.12,13,15,29 In addition, the actual sites of EPO-dependent tyrosine phosphorylation of the EPOR in either erythroid progenitors cells in vivo or established erythroid cell lines have not been identified. Therefore, we tested the capacity of cytoplasmic tyrosine mutants of the EPOR to support RBC development in vivo by using a receptor backbone containing the dominant EPO-independent mutation (R129C) of the EPOR (referred to here as cEPOR).11
To establish the feasibility of such an approach, we constructed an EPOR variant that contains the R129C mutation as well as replacement of all eight cytoplasmic tyrosines by phenylalanines (cEPORYF) (Fig1A). Stable transfectants of the IL-3–dependent cell line BaF3 were prepared that express either cEPOR or cEPORYF, and expression of each receptor was verified by immunoblotting (Fig 1B). Cells expressing cEPOR grew continuously in culture even on complete withdrawal of cytokines (IL-3 and EPO), whereas cells expressing cEPORYF ceased to grow in the absence of cytokines; these cells did exhibit modest growth with the addition of exogenous EPO levels (10 U/mL). These observations suggested that cEPORYF was not entirely devoid of growth-signaling potential but rather was much less efficient compared with wild-type cEPOR. These findings are consistent with earlier reports of a shifted dose response to EPO by tyrosine-negative mutants of the EPOR.12
To evaluate this function further in a more biologically relevant cellular background, genes encoding cEPOR or cEPORYF were transferred into primary hematopoietic progenitor cells via recombinant erythroleukemic spleen focus-forming virus (SFFV; Fig 1C). Both viruses were of comparable titer, as they infected and expressed their cEPOR transgene products in fibroblasts with equal efficiency (Fig 1C, lanes 2 and 3). These viruses were then used to infect day 13 murine fetal liver cells, and the development of erythroid colonies was measured. To negate any contribution of endogenous wild-type EPOR to the outcome, cultures were performed in the absence of added EPO (Table1). As previously reported,30when transduced into erythroid progenitors, the parental cEPOR potently stimulated EPO-independent mature colony-forming unit–erythroid (CFU-E) progenitor development and immature burst-forming unit–erythroid (BFU-E) progenitor development (Table 1). Interestingly, expression of the cEPORYF gene in erythroid progenitors supported EPO-independent CFU-E development at a level above background but significantly less than that of cEPOR (Table 1). Furthermore, in contrast to cEPOR, cEPORYF failed to support any detectable EPO-independent immature BFU-E progenitor development (Table 1). This distinction between BFU-E and CFU-E responses implies somewhat different requirements for tyrosines at different stages of erythroid maturation (see below).
Progenitor Cell Assay Employing Primary Fetal Liver Cells
| Virus . | CFU-E . | CFU-E Background . | BFU-E . | BFU-E Background . |
|---|---|---|---|---|
| Uninfected | 30 ± 9 | 0 | 4 ± 2 | 0 |
| Uninfected + EPO | 172 ± 21 | 142 | 13 ± 2 | 9 |
| cEPOR | 244 ± 18 | 214 | 51 ± 6 | 47 |
| cEPORYF | 107 ± 6 | 77 | 1 ± 1 | 0 |
| cEPOR: Y1F,2-8Y | 149 ± 19 | 119 | 53 ± 4 | 49 |
| cEPORYF: 1Y | 183 ± 35 | 153 | 34 ± 4 | 30 |
| cEPORYF: 2Y | 178 ± 9 | 148 | 6 ± 1 | 2 |
| cEPORYF: 3Y | 174 ± 13 | 144 | 3 ± 2 | 0 |
| cEPORYF: 4Y | 143 ± 24 | 113 | 9 ± 1 | 5 |
| cEPORYF: 1-4Y | 179 ± 30 | 149 | ND | ND |
| cEPORYF: 5-8Y | 217 ± 42 | 187 | ND | ND |
| cEPORYF: 8Y | 172 ± 36 | 142 | 46 ± 7 | 42 |
| Virus . | CFU-E . | CFU-E Background . | BFU-E . | BFU-E Background . |
|---|---|---|---|---|
| Uninfected | 30 ± 9 | 0 | 4 ± 2 | 0 |
| Uninfected + EPO | 172 ± 21 | 142 | 13 ± 2 | 9 |
| cEPOR | 244 ± 18 | 214 | 51 ± 6 | 47 |
| cEPORYF | 107 ± 6 | 77 | 1 ± 1 | 0 |
| cEPOR: Y1F,2-8Y | 149 ± 19 | 119 | 53 ± 4 | 49 |
| cEPORYF: 1Y | 183 ± 35 | 153 | 34 ± 4 | 30 |
| cEPORYF: 2Y | 178 ± 9 | 148 | 6 ± 1 | 2 |
| cEPORYF: 3Y | 174 ± 13 | 144 | 3 ± 2 | 0 |
| cEPORYF: 4Y | 143 ± 24 | 113 | 9 ± 1 | 5 |
| cEPORYF: 1-4Y | 179 ± 30 | 149 | ND | ND |
| cEPORYF: 5-8Y | 217 ± 42 | 187 | ND | ND |
| cEPORYF: 8Y | 172 ± 36 | 142 | 46 ± 7 | 42 |
Day 13 fetal liver cells were infected with virus as described in Materials and Methods, washed, and cultured in methylcellulose at 2 × 104 cells/mL in the presence of stem cell factor but no other added growth factors. CFU-E were scored on day 2 and BFU-E were scored on day 7. For samples containing added EPO, the concentration was 2 U/mL. Results shown are means of triplicates ± standard deviation. Background was defined as the average colony count in uninfected cells without the addition of EPO.
Abbreviation: ND, not determined.
To assist in delineating signaling processes linked to the receptor that may contribute to these cellular responses, the BaF3 transfectants were evaluated for several recognized signal transduction pathways. Immunoprecipitation of whole cell lysates with anti-JAK2 antibody followed by immunoblotting with anti-phosphotyrosine antibody showed comparable levels of constitutive tyrosine phosphorylation of JAK2 in both transfectants with none detectable in the untransfected parental line (Fig 2A); these constitutive levels of phosphorylation in the transfectants were modestly increased with the addition of low-dose (1 U/mL) or high-dose (10 U/mL) EPO to the cultures. Thus, despite quantitative differences in the overall expression of cEPOR and cEPORYF protein in the transfectants, such differences were not reflected in important distinctions at the level of this early marker of signal transduction.
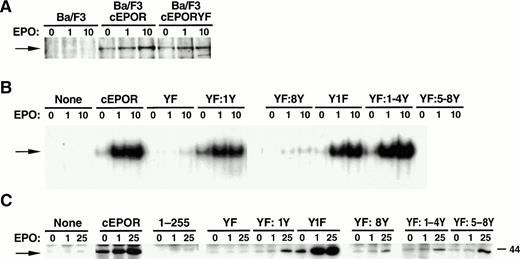
Signal transduction characteristics of cEPOR mutants in BaF3 cells. (A) Whole cell lysates of the BaF3 transfectants described in Fig 1 were subjected to immunoprecipitation with anti-JAK2 antiserum followed by separation by polyacrylamide gel electrophoresis and immunoblotting analysis with anti-phosphotyrosine antibody. Cells were either resting (0) or stimulated with EPO at low-dose (1, 1 U/mL) or high-dose (10, 10 U/mL) EPO for 15 minutes before lysis. The blots were reprobed with anti-JAK2 antibody to verify the identity of the phosphoprotein band and equivalent gel loading (not shown). (B) Nuclear extracts prepared from resting or EPO-stimulated BaF3 cell lines were prepared and subjected to EMSA with an oligonucleotide probe containing a STAT-binding sequence (FcγRI). Supershift analysis with anti-STAT5 antisera was used to confirm the identity of the STAT within the retarded nucleoprotein complex (not shown). (C) Whole cell extracts prepared from resting (0) or EPO-stimulated (1 U/mL or 25 U/mL, as indicated) BaF3 cell lines were prepared and subjected to in vitro kinase assays as described in Materials and Methods.
Signal transduction characteristics of cEPOR mutants in BaF3 cells. (A) Whole cell lysates of the BaF3 transfectants described in Fig 1 were subjected to immunoprecipitation with anti-JAK2 antiserum followed by separation by polyacrylamide gel electrophoresis and immunoblotting analysis with anti-phosphotyrosine antibody. Cells were either resting (0) or stimulated with EPO at low-dose (1, 1 U/mL) or high-dose (10, 10 U/mL) EPO for 15 minutes before lysis. The blots were reprobed with anti-JAK2 antibody to verify the identity of the phosphoprotein band and equivalent gel loading (not shown). (B) Nuclear extracts prepared from resting or EPO-stimulated BaF3 cell lines were prepared and subjected to EMSA with an oligonucleotide probe containing a STAT-binding sequence (FcγRI). Supershift analysis with anti-STAT5 antisera was used to confirm the identity of the STAT within the retarded nucleoprotein complex (not shown). (C) Whole cell extracts prepared from resting (0) or EPO-stimulated (1 U/mL or 25 U/mL, as indicated) BaF3 cell lines were prepared and subjected to in vitro kinase assays as described in Materials and Methods.
Despite comparable levels of JAK2 phosphorylation, EMSAs showed a marked difference in the induction of STAT5 by these receptors. Constitutive STAT5 DNA-binding activity was very faintly detectable in cEPOR cells in the absence of exogenous EPO, and strongly induced by low or high levels of exogenous EPO (Fig 2B). STAT DNA-binding activity was nominal in cEPORYF cells even at high levels of EPO (Fig 2B), as has been reported previously by others using EPORYF constructs without the R129C backbone.12,15,29 The fact that some markers of signaling via the cEPOR can be detected in transfected cells only after the addition of EPO has been reported previously,31 and may simply reflect limitations in the sensitivity among various assays.
Because the activation of MAPKs has been linked to cytokine-dependent regulation of cell cycle progression, the cEPOR and cEPORYF transfectants were compared for their abilities to couple to MAPK activation as measured by in vitro kinase assays. As observed in the STAT analyses, activation of MAPK by the cEPOR was detectable with the addition of low- or high-dose EPO, but was negligible with cEPORYF (Fig2C). Therefore, by multiple signal transduction parameters, cEPORYF was found to be largely uncoupled from downstream signaling responses.
Tyrosine-dependent erythropoiesis mediated by the EPOR in vivo.
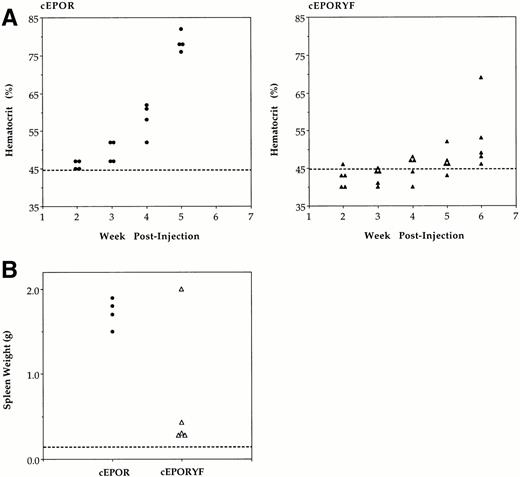
To evaluate the relative capacity of these receptors to support hematopoiesis in vivo, adult mice were infected with a mixture of the defective SFFV-cEPOR virus and helper Rauscher (R)-MuLV.25R-MuLV does not affect hematopoiesis of infected mice within the time course of these experiments; rather, it simply supplies the capacity for defective virus replication within the host.23 Weekly hematocrits were determined (Fig 3A), and when severe elevations in hematocrit were persistent, or at 6 weeks following infection, mice were killed and spleen weight determined (Fig3B). As expected, by 5 weeks after infection with SFFV-cEPOR, all animals exhibited a marked expansion of mature RBCs (elevation in hematocrit), indicative of stimulated erythroid differentiation.22 In addition, massive splenomegaly developed during this period, indicative of marked expansion of erythroid progenitor populations (erythroid proliferation).23 25 In contrast, SFFV-cEPORYF virus was inefficient at stimulating either phase of erythropoiesis, as indicated by only slight elevation in hematocrit or spleen size (Fig 3). Infected diseased spleens were found to express cEPOR or cEPORYF proteins at comparable levels when the differences in cellularity of diseased and normal spleens were considered. Cell lysates prepared from spleen cell suspensions from these animals were tested by immunoblotting with the anti-EPOR antiserum, which showed strong receptor expression in mice infected with either construct (Fig 4B). As determined in spleens from uninfected control animals, endogenous EPOR protein was expressed at levels below the detection limits of the assay. It is important to note that in animals infected with SFFV-cEPOR virus the enlarged spleens have been previously shown to be markedly and specifically enriched for erythroid cells compared with normal spleens from animals not manifesting disease. Therefore, the modest apparent differences in expression of cEPOR compared with cEPORYF in infected spleens (with equivalent loading of extracts on a total protein basis) most likely reflects differences in the degree of replacement of splenic tissue with erythroid progenitors rather than differences in receptor expression on a per-erythroid cell basis.
Hematopoietic consequences of SFFV-cEPOR viruses in infected mice. (A) Hematocrits of mice infected with SFFV-cEPOR (•, left) or SFFV-cEPORYF (▴, right) measured serially following inoculation. Each symbol represents a value from an individual animal, and the dashed line indicates the normal hematocrit of an adult mouse. (B) Spleen weights of mice infected with SFFV-cEPOR or SFFV-cEPORYF determined after euthanizing the mice at the end of the experiment. Each symbol represents a value from an individual animal, and the dashed line indicates the normal spleen weight of an adult mouse.
Hematopoietic consequences of SFFV-cEPOR viruses in infected mice. (A) Hematocrits of mice infected with SFFV-cEPOR (•, left) or SFFV-cEPORYF (▴, right) measured serially following inoculation. Each symbol represents a value from an individual animal, and the dashed line indicates the normal hematocrit of an adult mouse. (B) Spleen weights of mice infected with SFFV-cEPOR or SFFV-cEPORYF determined after euthanizing the mice at the end of the experiment. Each symbol represents a value from an individual animal, and the dashed line indicates the normal spleen weight of an adult mouse.
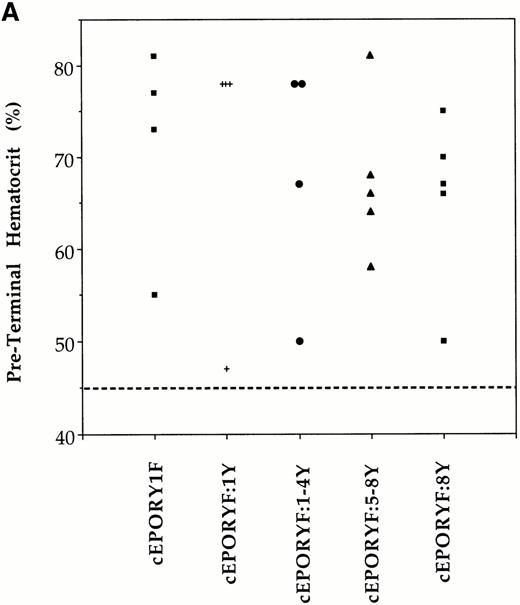
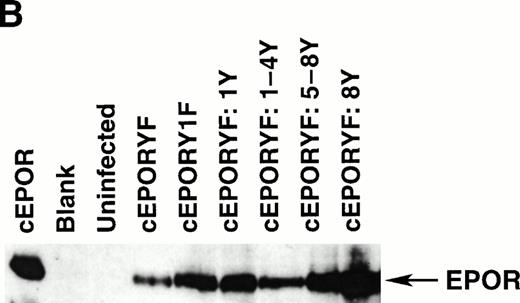
Stimulated erythropoiesis in mice infected with viruses encoding tyrosine add-back mutants of the EPOR. (A) Immediately before termination, hematocrits were determined for each animal infected with viruses encoding the indicated cEPOR variants. Each symbol represents a value from an individual animal, and the dashed line indicates the normal hematocrit of an adult mouse. (B) Immunoblot analysis for expression of the transduced EPOR protein in primary spleen cells isolated from infected mice.
Stimulated erythropoiesis in mice infected with viruses encoding tyrosine add-back mutants of the EPOR. (A) Immediately before termination, hematocrits were determined for each animal infected with viruses encoding the indicated cEPOR variants. Each symbol represents a value from an individual animal, and the dashed line indicates the normal hematocrit of an adult mouse. (B) Immunoblot analysis for expression of the transduced EPOR protein in primary spleen cells isolated from infected mice.
One of the five mice injected with SFFV-cEPORYF developed a late, acute rise in hematocrit and splenomegaly. However, in contrast to mice infected with SFFV-cEPOR, no immunologically detectable EPOR was present in the enlarged spleen or in primary splenic cells isolated from this animal and grown in culture for 7 days (data not shown). Furthermore, it was not possible to establish permanent growth factor–independent cell lines from this diseased spleen, whereas such lines were readily established from animals infected with cEPOR and other constructs (see below). All other mice infected with SFFV-cEPORYF developed a slight increase in hematocrit, and a small increase in splenic weight after 6 weeks. Detectable cEPORYF protein was present in splenic extracts from all these mice (data not shown). Therefore, the mechanism of disease in this single animal remains unclear and appears to be distinct from that produced by the cEPOR and other related variants (see Discussion). Nonetheless, a comparison between animals exposed to viruses expressing cEPOR and cEPORYF shows that efficient RBC development in vivo is dependent on the presence of tyrosine residues in the cytoplasmic domain of the EPOR. In addition, the results suggest that an inefficient EPOR tyrosine-independent pathway supporting RBC development is active.
Reconstitution of EPOR-mediated signaling by multiple tyrosines.
This inefficiency of cEPORYF in stimulating erythropoiesis allowed us to begin to determine the contribution of specific tyrosine residues in the EPOR cytoplasmic tail to RBC development in vivo. Selected tyrosine add-back mutants were constructed in the cEPOR backbone (Fig 1A) and expressed in BaF3 cells to assess cellular responses. Unlike the cEPORYF construct, each of the add-back constructs was capable of supporting continuous growth factor-independent growth of transfected BaF3 cells, indicating that they had more pronounced potential for stimulating proliferation compared with cEPORYF.
The selective functioning of cEPORYF in CFU-E development but not in BFU-E development suggested a differential role for tyrosines at specific stages of erythroid maturation. For further analyses in primary erythroid progenitor cell assays and in vivo, recombinant SFFV viruses containing these EPOR tyrosine add-back mutants were generated and found to exhibit equivalent titers as compared with SFFV-cEPOR and SFFV-cEPORYF viruses (Fig 1C). When expressed in primary CFU-E progenitors, each of these tyrosine add-back mutants strongly supported mature CFU-E development on introduction into day 13 fetal liver cells (Table 1), indicating that multiple individual tyrosine residues or combinations of tyrosines had the capacity to enhance the biological signaling potential of the cEPORYF. Interestingly, of the tyrosine add-backs tested, only Y1 and Y8 restored the capacity of cEPORYF to support immature BFU-E development when expressed in primary progenitor cells (Table 1).
Various aspects of signaling function were evaluated in the cytokine-independent transfectants expressing cEPORYF tyrosine add-back mutants. With regard to the JAK-STAT pathway, cEPORY1F, cEPORYF:1Y, and cEPORYF:1-4Y all exhibited constitutive or readily inducible STAT DNA-binding activity, whereas cEPORYF:5-8Y and cEPORYF:8Y failed to show detectable activity even with the addition of high-dose EPO (Fig2B); these findings are consistent with those reported by others using EPOR variants lacking R129C in BaF3.12,14 15 In contrast, only cEPORY1F was a robust inducer of MAPK, whereas cEPORYF:1Y, cEPORYF:1-4Y, cEPORYF:5-8Y, and cEPORYF:8Y showed MAPK induction only with the addition of high-dose EPO (Fig 2C). Therefore, some degree of selectivity was evident in the pattern of coupling to STAT5 and MAPK by these tyrosines, but in each case functional redundancy was evident among two or more tyrosine positions.
Reconstitution of EPOR-mediated erythropoiesis by multiple tyrosines.
To examine these properties in vivo, adult mice were subsequently infected with selected viruses and the hematopoietic compartment was evaluated. As in the progenitor cell assay, multiple independent tyrosines were observed to be capable of restoring RBC development signals to cEPORYF in whole animals. Preterminal hematocrits, indicative of effective RBC differentiation, were elevated in at least 75% of mice infected with each of these add-back constructs (Fig 4A). Spleen weight, indicative of expansion of RBC progenitor populations, was also increased in all diseased mice (not shown). Moreover, from the enlarged spleens we were readily able to establish permanent growth factor–independent cell lines ex vivo representing each of the add-back mutants. Immunoblot studies of cellular lysates from infected spleens showed that all expressed proteins derived from the transduced cEPOR variant, although at somewhat varying levels (Fig 4B). Therefore, multiple tyrosine residues of the EPOR cytoplasmic tail individually and in combinations appear competent to mediate the biological signals regulating both expansion of erythroid progenitors and formation of mature RBCs in the whole animal.
DISCUSSION
Extensive information has been developed regarding the structure-function relationships that govern signal transduction by cytokine receptors such as the EPOR in cell culture models. Likewise, gene deletion methods have facilitated delineation of the major functions of cytokine/cytokine receptor systems such as the EPO/EPOR complex in animals.32 33 The present studies were undertaken as a first effort to integrate these scientific areas by relating molecular features of the EPOR to biologic functions to which it is coupled in vivo.
The experimental strategy was to employ a “dominant” system in which a constitutively active mutant of the EPOR (cEPOR)11was used as a receptor backbone on which site-directed mutations selectively affecting signaling pathways were constructed. Based on prior experience with the parental cEPOR construct,22 it was anticipated that delivery of such constructs to hematopoietic progenitor cells via recombinant spleen focus-forming viruses would permit these receptors to engage their signal transduction programs (and associated biological outcomes) without the necessity of silencing the endogenous EPO/EPOR system by gene disruption. Because animals infected with the parental cEPOR virus reliably develop marked expansion of the hematopoietic compartment with predictable kinetics, this biologic response served as a useful readout of receptor activity. In this setting, proliferation of erythropoietic precursors was manifested as marked splenomegaly, whereas RBC maturation was represented by striking increases in peripheral hematocrits. While these conditions per se are certainly pathological, they represent predictable exaggerations of normal EPOR-dependent processes. Therefore, they serve as useful experimental markers of receptor-dependent function in this animal model.
Based on our prior observations of redundancy within other cytokine receptors,34 we initiated the present studies with a mutant EPOR in which all eight cytoplasmic tyrosines had been silenced by substitution with phenylalanine (cEPORYF). Consistent with the findings of others,12,15,29 this mutant showed marked impairment of its capacity to cause growth factor–independent proliferation of cell lines compared with the parental cEPOR. Likewise, it appeared to be substantially uncoupled from signal transduction pathways typically linked to the EPOR. Importantly, when introduced into mice via the SFFV system, this receptor variant likewise exhibited drastically reduced capacity to expand erythroid progenitor cells or to promote differentiation leading to mature RBCs in the periphery. These findings provide strong evidence that these biological processes in vivo depend substantially upon tyrosine residues, and indicate that tyrosines within such a receptor type represent relevant functional sites in the whole organism context. It is nonetheless notable that, as has been observed in prior studies in various cell line systems12,15,29 and in erythroid progenitors from EPOR(−/−) mice,35 the present studies confirm that such tyrosines are not absolutely essential for receptor activity; both the cellular studies (with transfectants and transduced primary progenitors) and the animal infections (see below) revealed measurable but clearly diminished erythopoietic responses to cEPORYF. These results suggest that less efficient tyrosine-independent pathways of signaling are also represented in the biological program driven by the EPOR.
The observation that one animal in five underwent hematopoietic expansion on introduction of the cEPORYF variant is intriguing and indicates the complexity of undertaking such physiological studies. Three lines of evidence suggest that the process manifested by this animal may not be identical to that of the reference cEPOR animals. First, the disease was delayed significantly in this animal compared with the wild-type cEPOR and cEPORYF add-back animals, suggesting that the mechanism underlying it may differ. Second, we were unable to establish permanent cell lines from the spleen of this animal, despite the ease of sustaining lines from the other infected animals. Third, immunoblotting of primary splenocyte cultures or whole splenic tissue from this animal showed no detectable cEPORYF expression, whereas strong expression was readily detected in spleen and splenic cell lines from animals infected with viruses carrying the parental cEPOR gene or other spleens from other animals infected with SFFV-cEPORYF that did not exhibit disease. Further investigation is warranted to determine whether this delayed disease represents the inefficient tyrosine-independent signaling function.
Further insights were derived by analyzing add-back mutants in which various tyrosine residues were reconstituted individually or in combinations in the cEPORYF backbone. Interestingly, growth signaling as measured by induction of growth factor–independent proliferation of transfected cell lines was restored by multiple and diverse tyrosines within the cytoplasmic segment of the EPOR. Additionally, several tyrosines apparently restored receptor coupling to STAT5 or MAPK in specific patterns characterized by a degree of redundancy. Various tyrosines also restored the erythropoietic response to cEPORYF when these add-back mutants were expressed in primary mature CFU-E progenitors. These results indicate that this process is driven by functionally redundant tyrosine-containing elements within the cytoplasmic tail of the EPOR, a general phenomenon which has been reported previously in the interleukin-2 receptor system36and which has been observed recently in the EPOR system in studies employing transfected cell lines15 or in hematopoietic progenitor cells derived from EPOR(−/−) mice.35Although numerous individual tyrosines were capable of supporting mature CFU-E development in the progenitor cell assay, only tyrosine-343 (Y1) and tyrosine-479 (Y8) supported complete erythropoiesis as measured by CFU-E and more immature BFU-E progenitor development. Because gene disruption experiments have indicated that the EPOR is not essential for definitive BFU-E formation, the physiological importance of forced expression of cEPOR or various mutants within developing BFU-E is questionable. Nonetheless, using this approach we have identified specific EPOR cytoplasmic tyrosine residues that exhibit differential functional capacities; this observation would not have been possible in studies with nondifferentiating, nonerythroid cell lines in culture. However, it is not possible to conclude definitively which signaling pathways are responsible for the cellular response because there was no clear association between the erythropoietic or growth response in cell culture and the apparent efficiency of linkage to STAT5 or MAPK per se. That the functional difference observed between CFU-E and BFU-E regarding specific tyrosine-dependence was not manifest in the whole animal context is not surprising because expansion of the CFU-E pool alone would be expected to result in erythrocytosis and splenomegaly, which, as has been observed in Friend virus disease, is a retrovirus that affects predominantly CFU-E development.37
The possibility that endogenous EPO may have contributed to the effects observed in the present system in vivo must also be considered. Indeed, it has been established that progenitor cells transduced with cEPOR exhibit an enhanced response with the addition of exogenous EPO despite the fact that they develop in an EPO-independent manner in methylcellulose culture.38 In any event, the effect of cEPORYF is markedly lower than that of cEPOR in vivo. Furthermore, the ex vivo progenitor assays were performed in the absence of added EPO. Finally, each of the tyrosine add-back receptors promoted massive eythroid expansion—an outcome not observed when mice were infected with SFFV viruses expressing wild-type EPOR lacking the critical R129C mutation.23 Therefore, this system appears to be useful as a first step toward dissecting these receptor-dependent processes in animals.
The present studies employed both primary hematopoietic progenitor cells and whole animals as a first step toward defining the structure-function relationships of the EPOR as they relate to erythropoiesis. In view of the variability often seen among various established cell line systems, these strategies were employed to evaluate these features in the biological context in which they normally are manifested. The central findings generally correspond well with those observed in prior cell culture systems; however, some specificity of EPOR cytoplasmic tyrosine residue requirement for supporting immature erythroid progenitor development was detected. Further studies using the present system and other strategies should reveal more about the molecular mechanisms of specificity by these receptors, the role of cellular context in determining signal transduction processes and outcomes, and the connections of biological outcomes to various intracellular signaling pathways.
ACKNOWLEDGMENT
The authors acknowledge the excellent assistance of Jessica Diamond, John Carroll, and Amy Corder in the preparation of this manuscript. Recombinant human EPO was the generous gift of Ortho Biotech.
Supported by the James S. McDonnell Foundation and The American Cancer Society (I.R.G.-36-37); the office of Research and Development, Medical Research Service Department of the Veterans Administration; and the J. David Gladstone Institutes and the National Institutes of Health (GM54531 and CA75315).
Address reprint requests to Mark A. Goldsmith, MD, PhD, Gladstone Institute of Virology and Immunology, PO Box 419100, San Francisco, CA 94141-9100.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal