Abstract
Hodgkin’s disease is histopathologically characterized by the relative scarcity of neoplastic Hodgkin and Reed-Sternberg cells and for yet unknown reasons by an abundant reactive background of T lymphocytes and often eosinophils. Eotaxin is a CC-chemokine attracting eosinophils and T helper 2 (Th2) cells in allergic inflammation. We now report that eotaxin is strongly expressed in fibroblasts of Hodgkin’s disease tissues, whereas Hodgkin/Reed-Sternberg cells do not express this chemokine. In tissue culture, Hodgkin’s disease tumor cells induce eotaxin expression in cocultured dermal fibroblasts in a concentration leading to a specific chemotactic response of a Th2 cell clone. Production of tumor necrosis factor- (TNF-) by Hodgkin/Reed-Sternberg cells appears to be responsible for this induction, because blocking of TNF- by neutralizing antibodies prevented fibroblast eotaxin expression. Our data suggest that eotaxin is involved in the pathobiology of Hodgkin’s disease by contributing to eosinophil and T-lymphocyte recruitment.
CYTOKINES PLAY AN important role in the pathogenesis of Hodgkin’s disease (HD).1,2 Characteristic clinical symptoms such as B symptoms (fever, night sweats, and weight loss) and immunosuppression are mediated by neoplastic or reactive cell-derived cytokines such as interleukin-1 (IL-1), IL-6, transforming growth factor-β (TGF-β), and tumor necrosis factor-α (TNF-α).3,4 Proliferation and differentiation of neoplastic cells are affected by cytokines acting as autocrine or paracrine growth factors.1 The typical histopathologic feature of HD, that is, relative scarcity of Hodgkin/Reed-Sternberg cells surrounded by massive infiltration of reactive cells, is assumed to be caused by various cytokines such as IL-1, IL-5, IL-6, IL-7, IL-8, lymphotoxin-α (LT-α), TNF-α, TGF-β, and granulocyte-macrophage colony-stimulating factor (GM-CSF).1 5-7Mechanisms that lead to increased recruitment of eosinophils and T lymphocytes, which constitute the vast majority of cells in tumor tissues, are largely unknown.
Tissue eosinophilia is mainly found in nodular sclerosis and mixed cellularity subtypes, which represent greater than 80% of all HD cases.8,9 Production of IL-5 and GM-CSF by Hodgkin/Reed-Sternberg cells may account in part for the recruitment and functional activation of eosinophils.10-13 The pathobiologic significance of tissue eosinophilia became evident, because eosinophils provide ligands for TNF superfamily receptors (CD30 and CD40) expressed on Hodgkin/Reed-Sternberg cells, thereby functionally interacting with Hodgkin/Reed-Sternberg cells and contributing to tumor cell proliferation.14-17 Furthermore, eosinophils may be involved in connective tissue remodeling and collagen formation in HD tissues, because they produce TGF-β and stimulate fibroblast DNA synthesis.18,19 It is of interest that CD4+ T lymphocytes with a T helper 2 (Th2)-like immunophenotype are the most abundant cell type in Hodgkin’s lymphoma tissues.20 Th2 cells produce IL-5, which primes and activates eosinophils.21 Moreover, interaction of these T cells with neoplastic cells and eosinophils also involves ligands of TNF receptors (CD30L and CD40L).14 T lymphocytes as well as eosinophils transmit via these ligands proliferative and antiapoptotic signals to Hodgkin/Reed-Sternberg cells and thereby influence tumor biology.14 15
Recently, the human CC-chemokine eotaxin has been identified as a potent attractant for eosinophils and Th2 lymphocytes.21-27Chemokines are small proteins with a molecular weight in the range of 8 to 12 kD. There are 4 different groups designated as CXC, CC, C, and CX3C, depending on the presence of 4 cysteins in highly conserved positions.28-30 Eosinophils mainly express receptors of the CC group of chemokines (CCR1 and CCR3), whereas in T lymphocytes, a great variety of chemokine receptors is found (CCR1-3 and CXCR3-5).28 The receptor for eotaxin, CCR3, is expressed on hematopoietic cells involved in allergic responses: eosinophils, basophils, and a subset of T lymphocytes (Th2 cells).31-35
In this study, we show that the chemoattractant eotaxin is strongly expressed in fibroblasts of HD tissues. Our data indicate that HD-derived cell lines induce the expression of eotaxin in fibroblasts by TNF-α. Eotaxin is then able to attract via its receptor CCR3 eosinophils and Th2 lymphocytes in tumor tissues. We therefore suggest that eotaxin contributes to the characteristic histopathologic features of Hodgkin’s lymphoma.
MATERIALS AND METHODS
Cell culture.
Human cell lines analyzed in this study were as follows: the Hodgkin cell lines, L428, L1236, KM-H2, L591, HD-LM2, and HD-MyZ; the pre-B–cell line, Blin-1; the T-cell lines, Molt-4 and Jurkat; the mature B-cell line, BL-60, Daudi; the myelocytic-monocytic cell line, U937; the breast cancer cell line, R30C; adenocarcinoma cell line, HeLa; and normal human dermal fibroblasts (NHDF). All cell lines were maintained in RPMI 1640 (Seromed-Biochrom, Hamburg, Germany), 10% heat-inactivated fetal calf serum, 2 mmol/L L-glutamine, and penicillin-streptomycin except for NHDF maintained in Basal Medium Eagle (BME; GIBCO, Karlsruhe, Germany). The human Th2 cell clone PM18.8 (a generous gift from F. Sallusto, Basel, Switzerland26) was maintained in RPMI supplemented with 2 mmol/L L-glutamine, 1% nonessential amino acids, 1% pyruvate, 50 μg/mL kanamycin, 5 × 10−5mol/L 2-mercaptoethanol (GIBCO), and 5% human serum (Sigma, Deisenhofen, Germany). Phytohemagglutinin (PHA) was purchased from Boehringer Mannheim (Mannheim, Germany); human recombinant IL-2, TNF-α, and neutralizing antibody against TNF-α were purchased from Calbiochem (Bad Soden, Germany). For cocultivation experiments, confluent NHDF and cell lines (1 × 106 cells/mL medium) were incubated for 24 hours in serum-free RPMI before cocultivation. Subsequently, NHDF and cell lines were cocultured for 48 hours in 6-well plates separated by micropore membranes (Falcon, Heidelberg, Germany) before cells were harvested for mRNA extraction.
Northern blot analysis.
Total RNA preparations were performed using the guanidium isothiocyanate-phenol chloroform method as described previously.36 For Northern analysis, 10 to 30 μg of total RNA was subjected to gel electrophoresis on a 1.1% formaldehyde-1.2% agarose gel and transferred to a nylon membrane (Appligene, Heidelberg, Germany). After UV cross-linking, the membrane was prehybridized (ExpressHyb hybridization solution; Clontech, Heidelberg, Germany) at 68°C for 1 hour. The blots were hybridized with a32P-random prime-labeled DNA probe overnight at 68°C. Probes were human eotaxin (290 bp, entire coding region) and GAPDH (249 bp of coding region) cDNAs. The membranes were washed for 40 minutes at room temperature in 2× SSC, 0.1% sodium dodecyl sulfate (SDS) and then for 40 minutes at 50°C in 0.5% SSC, 0.1% SDS. All experiments were performed in triplicate.
Enzyme-linked immunosorbent assay (ELISA).
ELISA was performed by coating 100 μL of mouse antihuman eotaxin monoclonal antibody (MoAb; R&D Systems, Wiesbaden, Germany) onto 96-well immunoplates (Nunc, Wiesbaden, Germany) at a concentration of 5 μg/mL in carbonate buffer overnight at 4°C. Plates were washed 3 times with phosphate-buffered saline (PBS)/Tween 20, and 250 μL/well of blocking buffer (PBS containing 4% bovine serum albumin [BSA]) was added for 2 hours at 37°C. After washing the plates (PBS/Tween 20), 100 μL recombinant human eotaxin (R&D Systems) in various concentration (10 pg up to 1,000 ng) or supernatant of cultured cell lines was added and incubated for 1.5 hours at 37°C. Plates were washed 3 times, and 100 μL of a rabbit antihuman eotaxin polyclonal antibody 1:2,000 diluted in PBS/1% BSA was added to each well. After the wash, 0.5 μg/mL of a horseradish peroxidase-linked antirabbit IgG derived from goats (Sigma) was diluted 1:1,000 in PBS/1% BSA and added for 20 minutes at 37°C. After incubation and washing, plates were developed using the o-phenylenediamine dihydrochloride substrate method according to the instructions of the manufacturer (Sigma). Plates were read at 490 nm on an ELISA reader. Under these conditions, this assay was sensitive to 50 pg/mL.
Chemotaxis assay.
Chemotactic factors diluted in assay medium were added to the 12-well tissue culture plates (Costar, Cambridge, MA) in a final volume of 500 μL. Collagen-coated (mouse Typ IV collagen in 0.01 N HCl; Becton Dickinson, Heidelberg, Germany) transwells (5-μm pores) were inserted into each well and 1 to 5 × 105 cells of the human Th2 cell clone PM18.8 expressing the CCR3 receptor for eotaxin were added to the top chamber in a final volume of 100 μL. The plate was then incubated at 37°C for 2.5 to 4 hours. The number of migrated cells across the membrane was counted in a Neubauer chamber. Monoclonal anti-eotaxin antibody (R&D Systems) and isotype control antibody (mouse IgG1, κ; Becton Dickinson) were used in a concentration of 10 μg/mL for 30 minutes.
Immunohistology.
Five-micrometer sections of frozen tissue blocks were stained using the immunoalkaline phosphatase (APAAP) method.37 The primary antibody was monoclonal anti-eotaxin antibody (R&D Systems) used in a concentration of 1:500. Frozen sections of nodular sclerosing classical Hodgkin’s disease cases were subjected to double labelings. The sections were first incubated with anti-CD3 (clone UCHT1; Dako, Glostrup, Denmark) or alternatively with the anti–macrophage-associated antigen (Ber-MAC3; Dako). Bound antibodies were visualized with the streptavidin/biotin/peroxidase technique. This step was followed by incubation with the anti-eotaxin MoAb that was visualized with the APAAP technique using new fuchsin as chromogen.
In situ hybridization.
The cRNA probe was prepared by subcloning an eotaxin gene cDNA fragment (290 bp; kindly provided by J. Bartels, Kiel, Germany38) in the run-off transcription vector pGEM1 (Promega Biotec, Heidelberg, Germany). After linearization, run-off antisense transcripts with incorporation of 35S-labeled UTP and CTP were generated using T7 RNA polymerases (Promega-Biotech, Madison, Wi). In situ hybridization for the detection of eotaxin transcripts was performed as described previously using microwave irradiation before the hybridization.6 Slides were hybridized with 4 × 105 cpm of labeled probes overnight at 50°C.
RESULTS
Eotaxin is expressed in fibroblasts of HD tissues.
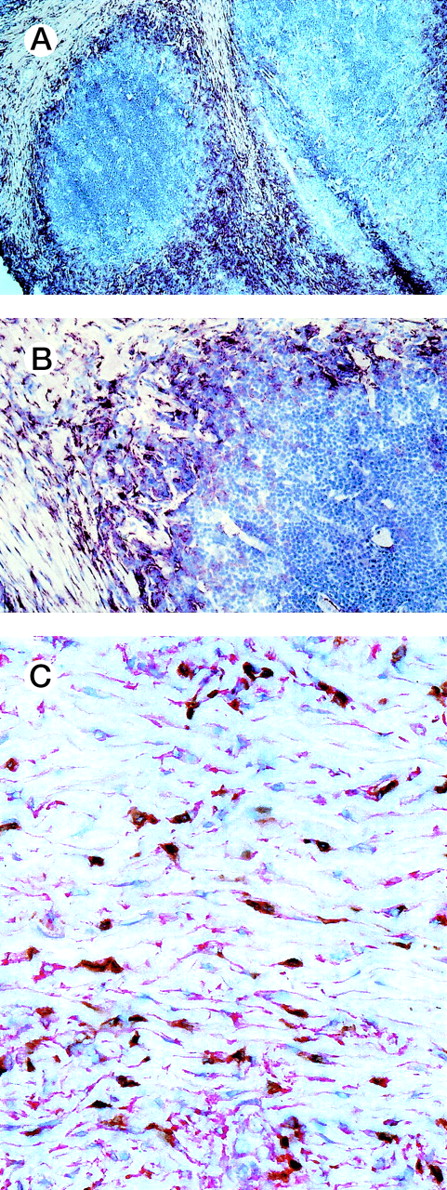
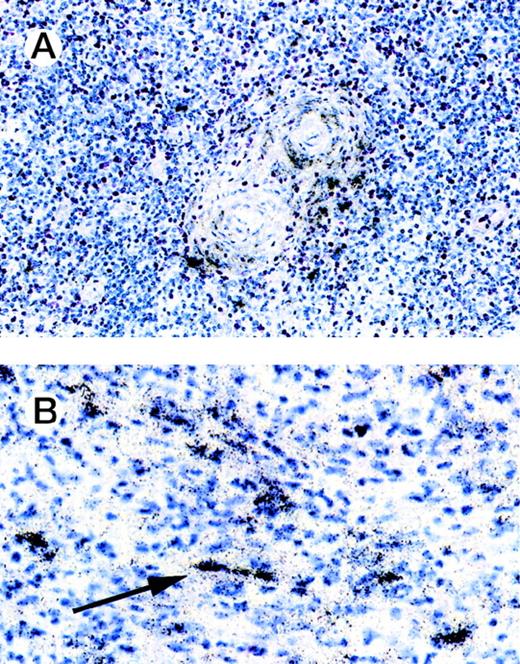
To evaluate the causes of tissue eosinophilia and T-lymphocyte infiltration in HD, we examined whether the eosinophil- and Th2 lymphocyte-specific chemoattractant eotaxin is expressed in HD tissues. We performed immunohistology of 9 cases of the nodular sclerosis and 1 case of the mixed cellularity subtypes by using a human anti-eotaxin MoAb alone or in conjunction with a T-cell or a macrophage-specific marker (Fig 1). Most of the spindle shaped eotaxin-expressing cells located within the collagen tissue bands in cases with nodular sclerosing Hodgkin’s disease did not show a coexpression of CD3 (Fig 1C). However, a number of cells expressing macrophage-associated antigen were found to coexpress eotaxin (data not shown). This implies that a proportion of macrophages in Hodgkin’s disease does express eotaxin but that the majority of the eotaxin-positive cells represent fibroblasts. Hodgkin/Reed-Sternberg cells and other reactive cells do not express the chemokine. Frozen sections from reactive lymphoid tissue and of cutaneous biopsies with unspecific chronic dermatitis served as negative controls. Eotaxin was not expressed in lymphatic or dermal connective tissues (data not shown). Using in situ hybridization, eotaxin transcripts were found in 10 of 16 cases of classical HD (6 of 8 nodular sclerosis, 4 of 7 mixed cellularity, and 1 unclassifiable). Labeled cells were found in the connective tissue of the fibroseptae (nodular sclerosis type), around blood vessels, and in capsula and subcapsula areas (Fig 2). In addition, eotaxin-positive cells were observed in the cellular infiltrates. By morphology these cells may represent fibroblasts or, in some cases, also macrophages.
Immunohistology of HD tissues. Immunohistology of frozen sections of nodular sclerosis subtype of HD stained for eotaxin using a monoclonal anti-eotaxin antibody (red reaction product-APAAP technique). (A and B) Eotaxin-expressing cells are located within the collagen tissue bands. Hodgkin/Reed-Sternberg cells and other reactive cells are eotaxin negative. (C) Double labeling for CD3 (brown reaction product-streptavidin/biotin method) and eotaxin in a case of nodular sclerosing classical HD shows that most of the spindle-shaped eotaxin-expressing cells do not coexpress CD3.
Immunohistology of HD tissues. Immunohistology of frozen sections of nodular sclerosis subtype of HD stained for eotaxin using a monoclonal anti-eotaxin antibody (red reaction product-APAAP technique). (A and B) Eotaxin-expressing cells are located within the collagen tissue bands. Hodgkin/Reed-Sternberg cells and other reactive cells are eotaxin negative. (C) Double labeling for CD3 (brown reaction product-streptavidin/biotin method) and eotaxin in a case of nodular sclerosing classical HD shows that most of the spindle-shaped eotaxin-expressing cells do not coexpress CD3.
In situ hybridization of HD lymph nodes. In situ hybridization with eotaxin antisense probe is shown. Eotaxin-specific signals are found around blood vessels. Labeled cells were also observed in the cellular infiltrates. Using morphology, these cells may represent fibroblasts or, in some cases, macrophages (solid arrow).
In situ hybridization of HD lymph nodes. In situ hybridization with eotaxin antisense probe is shown. Eotaxin-specific signals are found around blood vessels. Labeled cells were also observed in the cellular infiltrates. Using morphology, these cells may represent fibroblasts or, in some cases, macrophages (solid arrow).
Eotaxin is induced in fibroblasts after cocultivation with Hodgkin/Reed-Sternberg cells.
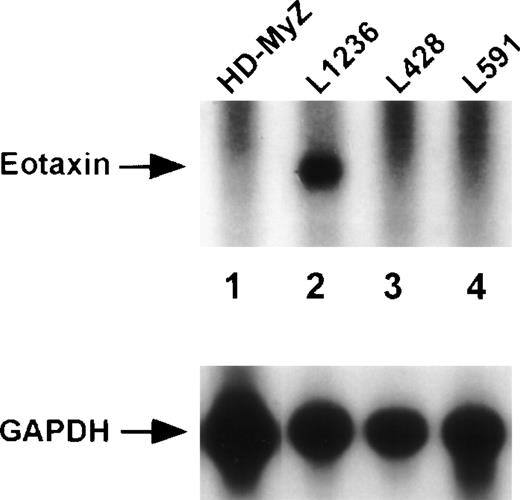
Next, we determined eotaxin mRNA and protein expression in HD-derived cell lines (Fig 3). In accordance with immunohistology, the majority of Hodgkin cell lines HD-MyZ, L428, L591 (Fig 3, lanes 1, 3, and 4), and KM-H2, HD-LM2 (data not shown) did not show eotaxin mRNA or protein expression. Only the Hodgkin cell line L1236 contained low levels of eotaxin mRNA (Fig 3, lane 2). However, protein expression could not be detected by ELISA in the culture medium of Hodgkin cell lines, which was conditioned for 48 hours (data not shown). Other hematopoietic cells, such as pre-B (Blin-1), T (Molt-4), and monocytic cells (U937), and nonhematopoietic breast cancer (R30C) and adenocarcinoma cells (HeLa) also did not show eotaxin mRNA expression (data not shown).
Expression of the chemoattractant eotaxin in Hodgkin cell lines. Northern blot analysis of total RNA (30 μg) isolated from the Hodgkin cell lines HD-MyZ (lane 1), L1236 (lane 2), L428 (lane 3), and L591 (lane 4). The blot was hybridized with eotaxin- and subsequently with GAPDH-specific cDNA probes.
Expression of the chemoattractant eotaxin in Hodgkin cell lines. Northern blot analysis of total RNA (30 μg) isolated from the Hodgkin cell lines HD-MyZ (lane 1), L1236 (lane 2), L428 (lane 3), and L591 (lane 4). The blot was hybridized with eotaxin- and subsequently with GAPDH-specific cDNA probes.
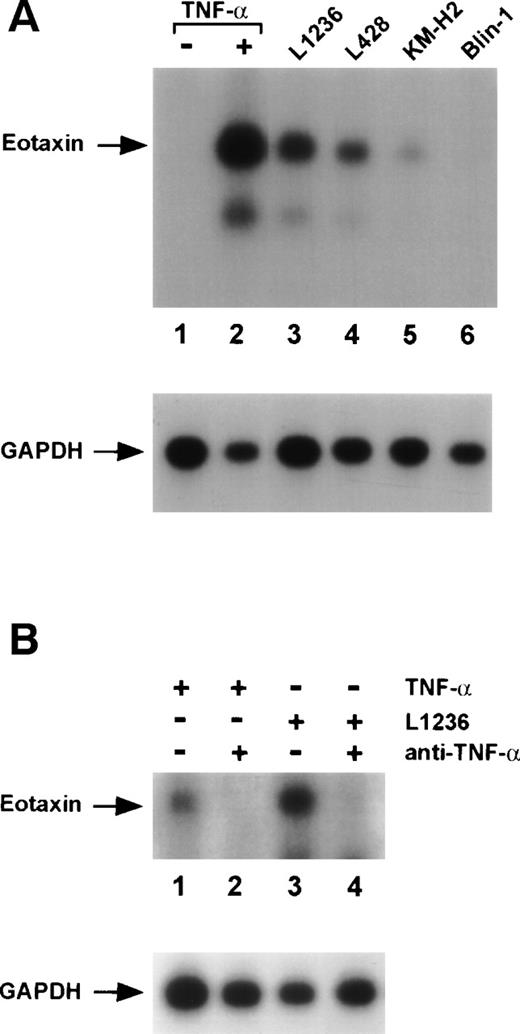
We hypothesized that Hodgkin/Reed-Sternberg cells that secrete high levels of various cytokines could induce eotaxin in surrounding connective tissue cells. Furthermore, recombinant TNF-α is known to strongly stimulate eotaxin expression in fibroblasts.38 Therefore, we cocultured Hodgkin cell lines with normal human dermal fibroblasts. All tested Hodgkin cell lines L1236, L428, and KM-H2 were able to induce eotaxin mRNA expression in fibroblasts after cocultivation (Fig4A, lanes 3 through 5). Cocultivation with non-Hodgkin lymphoma cell lines of pre-B–cell (Blin-1; Fig 4A, lane 6), B-cell (BL60, Daudi), and T-cell origin (Jurkat) did not induce eotaxin in fibroblasts (data not shown). The Hodgkin cell lines as well as the control cell lines did not express eotaxin after cocultivation with fibroblasts, indicating that factors produced by fibroblasts are not able to stimulate these cell lines (data not shown).
Induction of eotaxin mRNA expression in normal human dermal fibroblasts. Northern blot analysis of total RNA (10 μg) isolated from fibroblasts. Blots were hybridized with eotaxin- and subsequently with GAPDH-specific probes. (A) Untreated fibroblasts (lane 1), fibroblasts after stimulation with 30 ng/mL TNF- (lane 2), and fibroblasts after cocultivation with the Hodgkin cell lines L1236 (lane 3), L428 (lane 4), KM-H2 (lane 5), and the pre-B–cell line Blin-1 (lane 6). (B) Fibroblasts after stimulation with 10 ng/mL TNF- (lanes 1 and 2) and incubation with anti–TNF- antibodies (lane 2). Fibroblasts after cultivation in supernatant of the Hodgkin cell line L1236 (lanes 3 and 4) and incubation with anti–TNF- antibodies (lane 4).
Induction of eotaxin mRNA expression in normal human dermal fibroblasts. Northern blot analysis of total RNA (10 μg) isolated from fibroblasts. Blots were hybridized with eotaxin- and subsequently with GAPDH-specific probes. (A) Untreated fibroblasts (lane 1), fibroblasts after stimulation with 30 ng/mL TNF- (lane 2), and fibroblasts after cocultivation with the Hodgkin cell lines L1236 (lane 3), L428 (lane 4), KM-H2 (lane 5), and the pre-B–cell line Blin-1 (lane 6). (B) Fibroblasts after stimulation with 10 ng/mL TNF- (lanes 1 and 2) and incubation with anti–TNF- antibodies (lane 2). Fibroblasts after cultivation in supernatant of the Hodgkin cell line L1236 (lanes 3 and 4) and incubation with anti–TNF- antibodies (lane 4).
We next examined whether TNF-α secreted by Hodgkin cell lines might be responsible for the observed induction of eotaxin in fibroblasts. Therefore, we blocked TNF-α in the culture medium of the Hodgkin cell line L1236 by neutralizing antibodies. Supernatant of L1236 cells was produced for 24 hours and anti–TNF-α antibody was added 2 hours before cultivation of fibroblasts in this supernatant for 48 hours. Blocking of TNF-α almost completely inhibited eotaxin mRNA expression in fibroblasts (Fig 4B, lanes 2 and 4).
Eotaxin secreted by stimulated fibroblasts induces specific chemotactic responses of human Th2 cells.
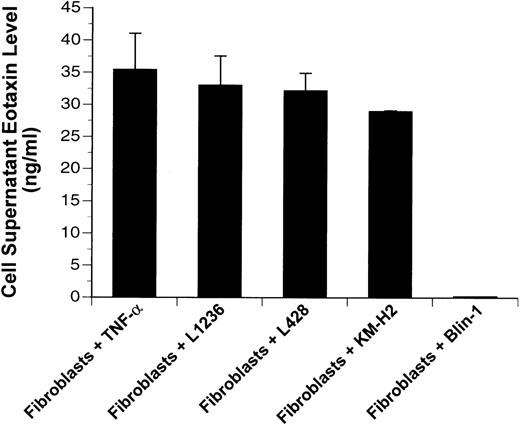
In supernatants of fibroblasts cocultured with Hodgkin cell lines (L1236, L428, and KM-H2) for 48 hours, we detected by ELISA high levels (∼30 to 45 ng/mL) of eotaxin comparable to levels after stimulation with TNF-α, which served as a positive control ( Fig5). In contrast, supernatants of fibroblasts cocultured with the B-lymphoma cell line Blin-1 did not contain eotaxin protein. Supernatants of all cell lines (L1236, L428, and KM-H2) or of unstimulated fibroblasts were also conditioned for 48 hours and showed undetectable levels of eotaxin (data not shown).
Eotaxin protein levels of cell supernatants. Eotaxin protein was measured by ELISA in supernatants of fibroblasts after stimulation with 30 ng/mL TNF- or cocultivation with the Hodgkin cell lines (L1236, L428, and KM-H2) and the pre-B–cell line (Blin-1). Errors are shown as the standard deviation.
Eotaxin protein levels of cell supernatants. Eotaxin protein was measured by ELISA in supernatants of fibroblasts after stimulation with 30 ng/mL TNF- or cocultivation with the Hodgkin cell lines (L1236, L428, and KM-H2) and the pre-B–cell line (Blin-1). Errors are shown as the standard deviation.
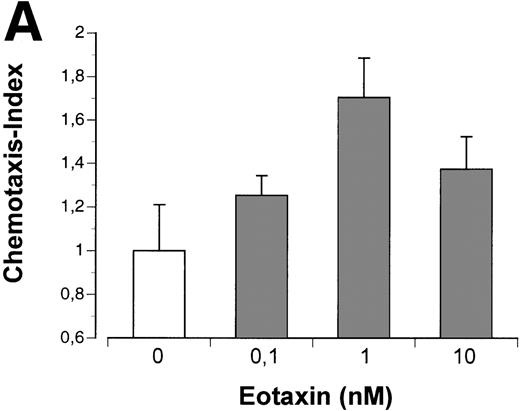
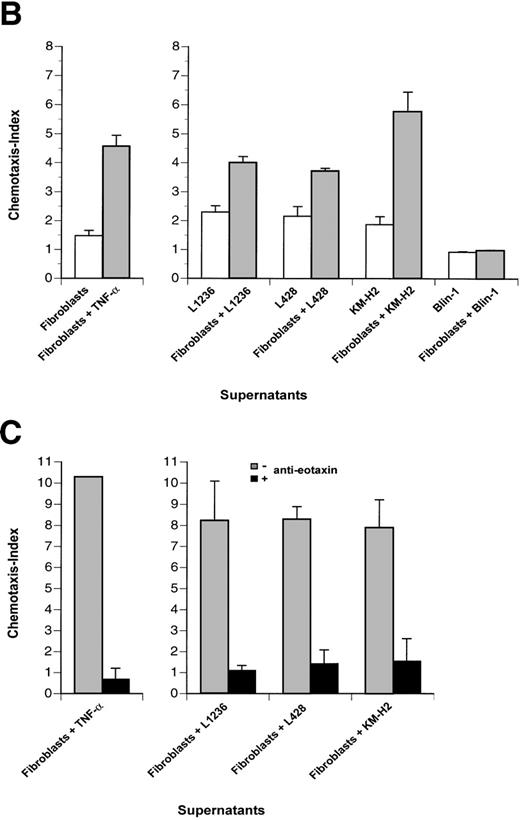
To analyze functional activity of eotaxin secreted by fibroblasts, we used a chemotaxis assay with the human T-helper 2 cell clone PM18.8 expressing the CCR3 receptor for eotaxin.21 This T-helper 2 cell clone exhibited a dose-dependent chemotactic response to recombinant human eotaxin with desensitization at high doses (Fig 6A). PM18.8 cells showed a significant migratory response only towards supernatants of stimulated fibroblasts containing eotaxin protein, whereas supernatants of all cell lines or unstimulated fibroblasts did not induce specific chemotaxis (Fig 6B). Specificity of chemotactic responses was controlled by blocking the chemoattractant eotaxin with a human antieotaxin MoAb (Fig 6C). Anti-eotaxin antibodies efficiently inhibited migratory responses of the Th2 cells in contrast to irrelevant antibodies of the same isotype (data not shown). These data showed that helper T lymphocytes bearing the CCR3 receptor were stimulated to chemotactic responses by eotaxin that was secreted by fibroblasts cocultured with Hodgkin cell lines.
Th2 lymphocyte chemotaxis in response to recombinant eotaxin and to supernatants of Hodgkin cell lines and fibroblasts. (A) The number of Th2 cells migrated at indicated concentrations of eotaxin (in nanomoles per liter) is given relative to the number of Th2 cells migrated at medium control (no chemokine), which was set arbitrarily at 1 (chemotaxis index). (B and C) (□) Th2 cells migrated in response to supernatants of cell lines indicated; (▩) Th2 cells migrated in response to supernatant of fibroblasts stimulated with TNF- (left panel) or to supernatants of fibroblasts cocultured with cell lines indicated (right panel); (▪) migration of Th2 cells after blocking the chemoattractant eotaxin with anti-eotaxin MoAb. Values of columns are given relative to values of Th2 cells migrated at medium control, which were set arbitrarily at 1 (chemotaxis index). Th2 cells migrated for 2.5 hours (A and B) and for 4 hours (C), respectively. Values of induction and inhibition of specific chemotactic responses are statistically significant for all supernatants (P < .005 using the Student’s t-test). Results are the mean values of 3 independent experiments and errors are shown as the standard deviation.
Th2 lymphocyte chemotaxis in response to recombinant eotaxin and to supernatants of Hodgkin cell lines and fibroblasts. (A) The number of Th2 cells migrated at indicated concentrations of eotaxin (in nanomoles per liter) is given relative to the number of Th2 cells migrated at medium control (no chemokine), which was set arbitrarily at 1 (chemotaxis index). (B and C) (□) Th2 cells migrated in response to supernatants of cell lines indicated; (▩) Th2 cells migrated in response to supernatant of fibroblasts stimulated with TNF- (left panel) or to supernatants of fibroblasts cocultured with cell lines indicated (right panel); (▪) migration of Th2 cells after blocking the chemoattractant eotaxin with anti-eotaxin MoAb. Values of columns are given relative to values of Th2 cells migrated at medium control, which were set arbitrarily at 1 (chemotaxis index). Th2 cells migrated for 2.5 hours (A and B) and for 4 hours (C), respectively. Values of induction and inhibition of specific chemotactic responses are statistically significant for all supernatants (P < .005 using the Student’s t-test). Results are the mean values of 3 independent experiments and errors are shown as the standard deviation.
DISCUSSION
Hodgkin’s disease is histopathologically characterized by the relative scarcity of Hodgkin and Reed-Sternberg cells, by the neoplastic cell clone, and for yet unknown reasons by an abundant infiltration of T lymphocytes and often eosinophils. In this study, we investigated whether the eosinophil- and Th2 lymphocyte-specific chemokine could play a role in the pathobiology of this disease. As a first step, we analyzed eotaxin expression in HD-derived cell lines. However, the majority of these cell lines did not express the chemokine, with one exception. Only the Hodgkin cell line L1236 contained small amounts of eotaxin mRNA that were not translated to protein.
In parallel, we analyzed lymph nodes of the nodular sclerosis and mixed cellularity subtypes of HD by in situ hybridization and immunohistology. We show here that eotaxin mRNA and protein are strongly expressed in fibroblasts and in a proportion of macrophages of HD tissues, whereas neoplastic cells are indeed devoid of eotaxin. This finding and the absence of eotaxin in control tissues such as normal lymphoid tissue and cutaneous biopsies indicate that expression of eotaxin in HD tissues may contribute to the recruitment of eosinophils and T lymphocytes.
We hypothesized that Hodgkin/Reed-Sternberg cells producing cytokines could thereby induce expression of eotaxin in fibroblasts. To verify this hypothesis, we cocultured Hodgkin cell lines with human dermal fibroblasts. Our data show that neoplastic cells strongly stimulate fibroblasts to express the chemoattractant eotaxin. We also provide evidence that production of TNF-α by Hodgkin/Reed-Sternberg cells is responsible for the induction of eotaxin in fibroblasts, because blocking of TNF-α by a neutralizing antibody prevented eotaxin mRNA expression. In dermal fibroblasts, expression of eotaxin is known to be stimulated by recombinant TNF-α, suggesting that eotaxin may serve as a potential agonist for eosinophil and T-lymphocyte infiltration in patients with inflammatory skin diseases.38
Next, we examined the functional consequences of eotaxin expression by fibroblasts testing eotaxin containing supernatants of Hodgkin cell lines cocultured with fibroblasts in chemotaxis assays. The human T-helper 2 cell clone PM18.8 that expresses the CCR3 receptor for eotaxin showed specific chemotactic responses to eotaxin secreted by stimulated fibroblasts. These findings indicate that binding of eotaxin to its receptor on Th2 cells and eosinophils may contribute to the recruitment of these cells in HD. Interestingly, the majority of cells composing the tumor tissue are CD4+ T lymphocytes with a T-helper 2 (Th2)-like immunophenotype.20
Are Th2 cells and eosinophils involved in the pathobiology of HD? It was reported that Th2 cells represent not a selective population that might recognize a common tumor antigen.39 Irrespective of their specificity, they are rather recruited by chemoattractants such as eotaxin and stimulate tumor growth via cytokines of the TNF family that lead to proliferation and cellular activation of Hodgkin/Reed-Sternberg cells.15 Eosinophils also contribute to tumor cell proliferation induced by ligands of the TNF family.14 15 Therefore, eosinophils and Th2 cells after recruitment by the chemoattractants are important elements in the development and phenotype of HD.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Franziska Jundt, MD, Max Delbrück Center for Molecular Medicine, Robert-Rössle-Str. 10, D-13122 Berlin, Germany; e-mail: fjundt@mdc-berlin.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal