Abstract
Recently, several reports of lineage-negative (lin−) CD34− cells with in vivo hematopoietic activity have focused interest on the properties and growth factor response characteristics of these cells. We have now identified a combination of 5 growth factors that are necessary and sufficient to stimulate a marked mitogenic and differentiation response by a subset of human lin−CD34−CD38− cells present in normal adult human marrow and granulocyte colony-stimulating factor (G-CSF)–mobilized blood. Less than 0.1% of the cells in highly purified (including doubly sorted) lin−CD34−CD38− cells from these 2 sources formed colonies directly in semisolid medium or generated such cells after 6 weeks in long-term culture. Nevertheless, approximately 1% of the same lin−CD34−CD38− cells were able to proliferate rapidly in serum-free liquid suspension cultures containing human flt-3 ligand, Steel factor, thrombopoietin, interleukin-3 (IL-3), and hyper–IL-6 to produce a net 28- ± 8-fold increase in total cells within 10 days. Of the cells present in these 10-day cultures, 5% ± 2% were CD34+ and 2.5% ± 0.9% were erythroid, granulopoietic, megakaryocytopoietic, or multilineage colony-forming cells (CFC) (13 ± 7 CFC per lin−CD34−CD38− pre-CFC). In contrast to lin−CD34+CD38−cells, this response of lin−CD34−CD38− cells required exposure to all of the 5 growth factors used. Up to 1.7 × 105 lin−CD34− adult marrow cells failed to engraft sublethally irradiated NOD/SCID-β2M−/− mice. These studies demonstrate unique properties of a rare subset of lin−CD34−CD38− cells present in both adult human marrow and mobilized blood samples that allow their rapid proliferation and differentiation in vitro within an overall period of 3 to 4 weeks. The rapidity of this response challenges current concepts about the normal duration and coordinated control of these processes in adults.
PRESENT DAY understanding of hematopoietic cell differentiation in the normal adult is based on evidence of an orderly sequence of changes in gene expression occurring over many cell generations. These are thought to lead first to lineage restriction and ultimately allow the production of large numbers of mature blood cells from a relatively small pool of stem cells. Such a hierarchical model is supported by an observed association of changes in the phenotype, proliferative potential, and differentiation ability of progenitors defined by various in vitro assays.1 Of particular interest to investigations of the molecular basis of this model has been the characterization of cells capable of sustaining the output of all blood cell lineages for long periods of time. Such cells are also of considerable practical relevance, because they include cells responsible for permanent engraftment in transplant recipients and are also the desired targets for many gene therapy strategies. In humans, expression of the CD34 antigen has been shown to identify a small population of hematopoietic cells that include most classes of multipotent as well as early committed lymphoid and myeloid progenitors.2 Purified human CD34+ cells have also been shown to reconstitute both lymphoid and myeloid compartments in autologous3 as well as allogeneic4 and xenogeneic5-7 recipients. Thus, expression of CD34 has come to be accepted as a hallmark of transplantable human hematopoietic stem cells. However, in mice, CD34−/lo hematopoietic stem cells also exist,8-10 and recently evidence of analogous cells in other species, including humans, has been reported.11-13 Interestingly, in large animals, very few lineage-negative (lin−) CD34− cells could be detected directly as colony-forming cells (CFC) in semisolid media or as their stromal cell-responsive precursors in long-term culture assays (referred to as long-term culture-initiating cells [LTC-IC]).11 13
In previous studies, we and others have shown that the most primitive CD34+ cells of human origin have different growth factor requirements from those able to stimulate their more differentiated progeny. These include differences both in the types and concentrations of the factors to which the cells are exposed.14-19 These investigations identified flt-3 ligand (FL), Steel factor (SF), interleukin-3 (IL-3), thrombopoietin (TPO), and factors that activate gp130 as potential contributors to the stimulation of very early human hematopoietic cells. In the present study, we used hyper–IL-6 (H–IL-6) to activate gp130. H–IL-6 is a recombinant growth factor fusion protein in which human IL-6 and its soluble receptor (sIL-6R) are linked by a flexible linker peptide.20 On the basis of preliminary results suggesting that adult human sources of highly purified lin−CD34− cells might proliferate and differentiate in response to a combination of these five growth factors, additional studies were designed to confirm and further characterize the cell types produced. The present report describes the results of these studies.
MATERIALS AND METHODS
Cells.
Normal adult human bone marrow cells were obtained either from donors of allogeneic marrow transplants at our center or cadaveric donors (Northwest Tissue Center, Seattle WA). Granulocyte colony-stimulating factor (G-CSF)–mobilized blood cells were from patients undergoing leukapheresis at our center for autologous transplantation. All cells were obtained with informed consent according to institutional guidelines. Low-density cells (<1.077 g/mL) were isolated using Ficoll-Paque (Pharmacia, Uppsala, Sweden) and resuspended in Hank’s HEPES-buffered salt solution containing 2% fetal calf serum (HFN; StemCell Technologies, Vancouver, British Columbia, Canada).
Flow cytometry.
Low-density marrow or blood cells were incubated for 30 minutes at 4°C with a cocktail of monoclonal antibodies against the following lin markers: CD2, CD3, CD14, CD16, CD19, CD24, CD56, CD66b, and glycophorin A. These markers were previously conjugated to antidextran antibodies (StemCell) and then with magnetic dextran-iron particles for another 30 minutes at 4°C. Labeled cells were removed using a StemSep column and magnet (StemCell) and the lin− cells collected in the flow through according to the manufacturer’s directions. These cells were then incubated for 30 minutes with monoclonal antibodies specific for CD34 (8G12) conjugated with fluorescein isothiocyanate (FITC; kindly provided by Dr P. Lansdorp, Terry Fox Laboratory, Vancouver, British Columbia, Canada) and CD38 (Leu-17) conjugated with R phycoerythrin (PE; Becton Dickinson, San Jose, CA) at 10 and 2.5 μg/mL, respectively. Cells were finally washed once in HFN and once again in HFN with 2 μg/mL propidium iodide (PI; Sigma Chemical Co, St Louis, MO) before being sorted on a FACStar Plus (Becton Dickinson) equipped with a 5-W argon laser and a 30-mW helium laser. PI− cells with low to medium forward and low side light scattering characteristics and a CD34−CD38− phenotype were collected in Iscove’s medium supplemented with a serum substitute for immediate culture (see below) or in HFN if they were first to be resorted, as indicated. CD34+ cells were collected simultaneously and the CD34+CD38− subpopulation was subsequently isolated. For assessment of in vivo repopulating activity, lin− cells were stained with anti-CD34-PE (Becton Dickinson) and anti-CD7-FITC (M-T701; Pharmingen, Mississauga, Ontario, Canada), and CD34−CD7+ and CD34−CD7− populations were separately collected (<5% and >95% of all lin−CD34− cells, respectively). Data acquisition and analysis was performed with PC-lysis software (Becton Dickinson). Gates defining negative populations were set using FITC- and PE-conjugated isotype controls and included 99.9% of cells reactive with these antibodies.
Progenitor assays.
Cells were plated at suitable frequencies in methylcellulose medium with 30% fetal calf serum (FCS) or a serum substitute (MethoCult H4230 and H4236, respectively; StemCell) supplemented with 40 μg/mL low-density human serum lipoproteins (Sigma; for H4236 only) and, unless otherwise indicated, with 3 U/mL human erythropoietin (StemCell), 50 ng/mL of SF (Terry Fox Laboratory), and 20 ng/mL each of IL-3 (Novartis, Basel, Switzerland), IL-6 (Cangene, Mississauga, ON), G-CSF (StemCell), and granulocyte-macrophage colony-stimulating factor (GM-CSF; Novartis) to assess their direct granulopoietic, erythropoietic, and multilineage CFC content, as described.14,15 Additional aliquots were assayed for cells able to generate colonies of CD41+ megakaryocytes in serum-free collagen cultures (MegaCult-C; StemCell) containing 50 ng/mL of TPO and 10 ng/mL each of IL-3 and IL-6 as recommended by the supplier, using a modified procedure originally developed for agarose cultures.21 For LTC-IC assays, cells were cocultured for 6 weeks at 37°C with pre-established, irradiated feeder layers of mouse fibroblasts engineered to produce human IL-3, G-CSF, and SF.22 These LTC were maintained in a medium consisting of MyeloCult (H5100; StemCell) supplemented with freshly dissolved 10−6 mol/L hydrocortisone sodium hemisuccinate (Sigma) with weekly half medium changes.22 At the end of 6 weeks, a single cell suspension was prepared of the whole culture and the cells were then assayed for CFC.
Serum-free liquid cultures.
Cells were cultured in 100 μL of phenol red-free Iscove’s medium supplemented with 10 mg/mL of bovine serum albumin, 10 μg/mL of bovine insulin, and 200 μg/mL of human transferrin (BIT 9500; StemCell) plus 40 μg/mL of human low-density lipoprotein (Sigma), 2 mmol/L L-glutamine (Sigma), 10−4 mol/L 2-mercaptoethanol (Sigma), and growth factors, as indicated, at the following final concentrations: SF at 100 ng/mL, FL (Immunex Corp, Seattle, WA) at 100 ng/mL, TPO (Genentech, San Francisco, CA) at 50 ng/mL, IL-3 at 20 ng/mL, and H–IL-6 prepared and purified as described20 at 10 ng/mL. After 10 days of incubation, cells were assayed for CFC and/or CD34 expression after labeling with anti-CD34-FITC and analysis on a FACScan flow cytometer (Becton Dickinson) using the same controls and gating criteria as for cell sorting.
Limiting dilution assays (LDA).
Multiple serum-free suspension cultures containing all 5 cytokines were set up with varying numbers of doubly sorted (from 10 to 500) lin−CD34−CD38−cells in 20 μL of medium in the individual wells of a 60-well Terasaki microwell plate (6 to 12 replicates per cell dose). After 10 days (unless specified otherwise), each well was examined for the presence of viable (refractile) cells and then assayed individually for CFC. Wells were scored as positive for growth factor responsiveness when approximately 20 viable cells (or more) could be seen on day 10. All other wells were scored as negative and usually contained no detectable cells. Wells were scored as positive for pre-CFC whenever ≥1 CFC was detected. The frequencies of growth factor-responsive cells and of pre-CFC in the lin−CD34−CD38−cells used to initiate the cultures were calculated from the proportion of negative wells obtained with different input cell numbers based on Poisson statistics and the method of maximum likelihood23using the L-Calc software program (StemCell).
RESULTS
Frequency of CD34−CD38− cells in normal adult marrow and mobilized blood.
Lin− low-density cells isolated from 5 normal adult marrow and 8 G-CSF–mobilized blood samples were sorted into CD34−CD38−, CD34+, and CD34+CD38− populations as described in Materials and Methods and shown in Fig 1. From such analyses, the size of each population was obtained and the corresponding number of cells present in the original low-density fraction calculated (assuming 100% recovery of each population in the lin− fraction). As summarized in Table 1, CD34−CD38− cells were 6- to 9-fold less numerous than CD34+ cells, similar in frequency to the CD34+CD38− cells, and constituted approximately 1 in 300 cells of the low-density population obtained from either marrow or mobilized blood. However, because the yield of all cells after removal of the lin+ cells was much higher for marrow than for the mobilized blood cell populations (5% ± 2%v 0.5% ± 0.4%), the yield of lin−CD34−CD38−marrow cells was also higher. Comparison of the frequencies of CD34+CD38− and CD34−CD38− cells in individual samples showed that the relative sizes of these 2 populations were not significantly correlated (R = .2, P > .05).
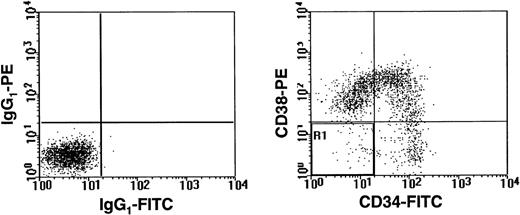
A representative FACS profile of normal adult human lin− bone marrow cells after staining with CD34-FITC and CD38-PE. Lineage marker-positive cells were removed using a StemSep column and the flow through (lin−) cells were then labeled. Dead (PI+) cells were excluded from the analysis. The left panel shows the level of nonspecific staining obtained with isotype control antibodies. The right panel shows the result obtained after staining with anti-CD34-FITC and anti-CD38-PE.
A representative FACS profile of normal adult human lin− bone marrow cells after staining with CD34-FITC and CD38-PE. Lineage marker-positive cells were removed using a StemSep column and the flow through (lin−) cells were then labeled. Dead (PI+) cells were excluded from the analysis. The left panel shows the level of nonspecific staining obtained with isotype control antibodies. The right panel shows the result obtained after staining with anti-CD34-FITC and anti-CD38-PE.
Proportions of Light Density and Lin−Marrow and Mobilized Blood Cells That Are Lin−CD34+, Lin−CD34+CD38−, or Lin−CD34−CD38−
| Tissue . | Fraction . | % Lin−CD34+ Cells . | % Lin− CD34+CD38− Cells . | % Lin− CD34−CD38− Cells . |
|---|---|---|---|---|
| Adult marrow (n = 5) | Low density | 2.6 ± 0.6 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| Lin− | 28 ± 5 | 2.4 ± 0.8 | 3.6 ± 1.3 | |
| Mobilized blood (n = 8) | Low density | 1.9 ± 0.8 | 0.12 ± 0.04 | 0.3 ± 0.1 |
| Lin− | 41 ± 8 | 3.2 ± 1.3 | 6 ± 2 |
| Tissue . | Fraction . | % Lin−CD34+ Cells . | % Lin− CD34+CD38− Cells . | % Lin− CD34−CD38− Cells . |
|---|---|---|---|---|
| Adult marrow (n = 5) | Low density | 2.6 ± 0.6 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| Lin− | 28 ± 5 | 2.4 ± 0.8 | 3.6 ± 1.3 | |
| Mobilized blood (n = 8) | Low density | 1.9 ± 0.8 | 0.12 ± 0.04 | 0.3 ± 0.1 |
| Lin− | 41 ± 8 | 3.2 ± 1.3 | 6 ± 2 |
The frequency of lin−CD34+, lin−CD34+CD38−, and lin−CD34−CD38− cells in the lin− population was determined by FACS analysis. These values were then used to calculate the frequency of these 2 cell types in the original low-density fraction, assuming equivalent nonspecific losses of both cell types during the StemSep separation procedure used to isolate the lin− preparation. Values shown are the mean ± SEM.
Progenitor content of lin−CD34−CD38−cells.
CFC and LTC-IC (6-week) assays were performed on the lin−CD34−CD38−cells isolated from 2 of the mobilized blood samples and 5 of the normal marrow samples (Table 2). The results showed these to be undetectable (<0.1%) in most instances. Based on the results obtained with the initial samples studied, the total number of cells assessed from the later samples analyzed was increased from 1 or 2 × 103 cells to 104cells. Although a few granulocyte-macrophage colonies were then obtained in direct CFC assays, the frequency of CFC in the lin−CD34−CD38−fraction was still less than 0.1%. This value is more than 10× lower than the frequency of CFC detectable in the lin−CD34+CD38−population present in normal adult human bone marrow24 and is also somewhat lower than that reported by Bhatia et al13for human cord blood lin−CD34−CD38− cells, which we have confirmed (unpublished findings). Because we found that the same lin−CD34−CD38−marrow cells could proliferate in liquid suspension cultures containing a serum substitute instead of FCS and a different combination of growth factors (than what had been added to the methylcellulose cultures used to detect CFC; see below), cells from 2 of the marrow samples were also assayed in methylcellulose medium of identical composition to the medium used in the liquid cultures. However, even under these conditions, no proliferation by up to 2 × 103lin−CD34−CD38−cells in semisolid medium was seen. In LTC-IC assays, 6 of 7 samples of lin−CD34−CD38−cells (2 blood samples and 4 marrow samples) were also negative. However, in 1 case (BM4 in Table 2), several hundred CFC were generated from 5,000 lin−CD34−CD38−cells. Note that the frequency of LTC-IC in the matching lin−CD34+CD38− cells isolated from this sample was less than 1%, in contrast to BM3, in which 10% of the lin−CD34+CD38− cells were LTC-IC (see Table 5, below). The large disparity in LTC-IC content of the CD34+ and CD34− subsets of lin−CD38− cells (compare results shown in Tables 2 and 5) argues strongly against the likelihood of cross-contamination as an explanation for a low but measurable frequency of CD34−CD38− LTC-IC and emphasizes the functional differences between the lin−CD34+CD38− and lin−CD34−CD38+populations in adult marrow.
Progenitor Content of Lin−CD34−CD38−Populations
| Assay Conditions . | Sample . | No.of Sorts* . | Cells/ Dish . | Colonies/ MC Dish . | CFC/103 Lin−CD34−CD38− Cells . |
|---|---|---|---|---|---|
| Serum-containing MC (standard CFC) assays† | mPB 1 mPB 2 BM 1 BM 2 BM 3 BM 4 BM 8 | 1 1 1 1 1 2 2 | 500 5,000 500 1,000 500 5,000 1,000 | 0, 0 3, 5 0, 0 0, 0 0, 0 6, 0 0, 0 | <1 <1 <1 <1 <1 <1 <1 |
| Serum-free MC assays‡ | BM 2 BM 3 | 1 1 | 1,000 500 | 0, 0 0, 0 | <1 <1 |
| Serum-containing MC assays of cells from 6-wk-old LTC2-153 | mPB 1 mPB 2 BM 1 BM 2 BM 3 BM 4 BM 8 | 1 1 1 1 1 2 2 | 500 5,000 500 1,000 500 5,000 1,000 | 0, 0 0, 0 0, 0 0, 0 0, 0 90, 68 0, 0 | <1 <0.1 <1 <0.5 <1 160 <0.5 |
| Assay Conditions . | Sample . | No.of Sorts* . | Cells/ Dish . | Colonies/ MC Dish . | CFC/103 Lin−CD34−CD38− Cells . |
|---|---|---|---|---|---|
| Serum-containing MC (standard CFC) assays† | mPB 1 mPB 2 BM 1 BM 2 BM 3 BM 4 BM 8 | 1 1 1 1 1 2 2 | 500 5,000 500 1,000 500 5,000 1,000 | 0, 0 3, 5 0, 0 0, 0 0, 0 6, 0 0, 0 | <1 <1 <1 <1 <1 <1 <1 |
| Serum-free MC assays‡ | BM 2 BM 3 | 1 1 | 1,000 500 | 0, 0 0, 0 | <1 <1 |
| Serum-containing MC assays of cells from 6-wk-old LTC2-153 | mPB 1 mPB 2 BM 1 BM 2 BM 3 BM 4 BM 8 | 1 1 1 1 1 2 2 | 500 5,000 500 1,000 500 5,000 1,000 | 0, 0 0, 0 0, 0 0, 0 0, 0 90, 68 0, 0 | <1 <0.1 <1 <0.5 <1 160 <0.5 |
Abbreviations: mPB, mobilized peripheral blood; BM, bone marrow (numbers specify sample numbers).
No. of sorts distinguishes experiments in which cells were plated directly after being sorted once (1) or after the lin−CD34−CD38− fraction collected from the first sort was resorted (2) before culture in methylcellulose (MC) or LTC.
Cells plated in 2 replicate methylcellulose cultures containing 30% FCS and 50 ng/mL SF; 20 ng/mL GM-CSF, G-CSF, IL-3, and IL-6; and 3 U/mL Epo as described in Materials and Methods.
These methylcellulose cultures contained a serum substitute instead of FCS and 100 ng/mL FL, 100 ng/mL SF, 50 ng/mL TPO, 20 ng/mL IL-3, and 10 ng/mL H–IL-6. They were thus identical in composition to the medium used for the 10-day liquid suspension cultures described in Materials and Methods except for the addition of an equivalent concentration of methylcellulose to that used in the standard CFC assays.
Cells/dish shows the number of cells placed into each LTC-IC assay culture. Six weeks later, the entire LTC was harvested and 10% of the cells assayed per CFC assay dish. Colonies/dish indicates the number of colonies seen in each of 2 replicate CFC assays.
Production of CD34+ cells and CFC in 10-day liquid cultures.
In serum-free liquid cultures containing FL, SF, TPO, IL-3, and H–IL-6, lin−CD34−CD38−cells proliferated extensively to yield (28 ± 8) × 103 PI− cells per 103 input cells (n = 4). When analyzed for CD34 expression, 5.4% ± 1.6% (n = 4) of these 10-day progeny were CD34+(Fig 2), resulting in a corresponding yield after 10 days of 1,700 ± 250 CD34+ cells per 103 initial lin−CD34−CD38−cells.
A representative FACS histogram showing the proportion of CD34+ cells in the population generated by stimulating lin−CD34−CD38− marrow cells for 10 days with FL, SF, TPO, IL-3, and H–IL-6.
A representative FACS histogram showing the proportion of CD34+ cells in the population generated by stimulating lin−CD34−CD38− marrow cells for 10 days with FL, SF, TPO, IL-3, and H–IL-6.
In 2 experiments in which the generation of cells detectable as CFC was assessed, parallel serum-free cultures were set up with either 103lin−CD34−CD38−cells or 103lin−CD34+CD38− cells isolated from the same samples. The effect of adding 3 different growth factor combinations on the progenitors obtained from each of these cultures 10 days later was then compared. As expected,14,15,18 19 FL + SF + IL-3, FL + TPO + H–IL-6, and the combination of all 5 of these growth factors stimulated the production of large numbers of CFC from the lin−CD34+CD38− cells in both experiments (Table 3). Readily detectable numbers of CFC were also present in 10-day cultures initiated with lin−CD34−CD38−cells (n = 9), but only when all 5 growth factors were present (n = 2). In an additional experiment (BM3 in Table 3), it was shown that omission of IL-3 reduced the number of CFC produced by adult lin−CD34−CD38−cells approximately 3-fold and that omission of any 1 of the other 4 factors eliminated CFC generation.
Production of CFC From Lin−CD34−CD38− Adult Human Marrow or Mobilized Peripheral Blood Cells in 10-Day Cultures Containing Various Cytokines
| Sample . | Phenotype . | No. of Sorts . | Cytokine Combination . | |||
|---|---|---|---|---|---|---|
| F + S + T + 3 + H6 . | F + S + 3 . | F + T + H6 . | F + S + T + H6 . | |||
| mPB1 | CD34−CD38− | 1 | 50 | 0 | 0 | ND |
| CD34+CD38− | 1 | 6,900 | 1,100 | 650 | ND | |
| BM2 | CD34−CD38− | 1 | 1,300 | 0 | 0 | ND |
| CD34+CD38− | 1 | 4,600 | 2,100 | 860 | ND | |
| BM1 | CD34−CD38− | 1 | 40 | ND | ND | ND |
| BM3 | CD34−CD38− | 1 | 2,100 | 0 | 0 | 650 |
| BM8 | CD34−CD38− | 1 | 140 | ND | ND | ND |
| BM4 | CD34−CD38− | 2 | 120 | ND | ND | ND |
| BM5 | CD34−CD38− | 2 | 440 | ND | ND | ND |
| BM6 | CD34−CD38− | 2 | 920 | ND | ND | ND |
| BM7 | CD34−CD38− | 2 | 500 | ND | ND | ND |
| Mean ± SEM | CD34−CD38− | 1 | 730 ± 420 | |||
| CD34−CD38− | 2 | 500 ± 160 | ||||
| Sample . | Phenotype . | No. of Sorts . | Cytokine Combination . | |||
|---|---|---|---|---|---|---|
| F + S + T + 3 + H6 . | F + S + 3 . | F + T + H6 . | F + S + T + H6 . | |||
| mPB1 | CD34−CD38− | 1 | 50 | 0 | 0 | ND |
| CD34+CD38− | 1 | 6,900 | 1,100 | 650 | ND | |
| BM2 | CD34−CD38− | 1 | 1,300 | 0 | 0 | ND |
| CD34+CD38− | 1 | 4,600 | 2,100 | 860 | ND | |
| BM1 | CD34−CD38− | 1 | 40 | ND | ND | ND |
| BM3 | CD34−CD38− | 1 | 2,100 | 0 | 0 | 650 |
| BM8 | CD34−CD38− | 1 | 140 | ND | ND | ND |
| BM4 | CD34−CD38− | 2 | 120 | ND | ND | ND |
| BM5 | CD34−CD38− | 2 | 440 | ND | ND | ND |
| BM6 | CD34−CD38− | 2 | 920 | ND | ND | ND |
| BM7 | CD34−CD38− | 2 | 500 | ND | ND | ND |
| Mean ± SEM | CD34−CD38− | 1 | 730 ± 420 | |||
| CD34−CD38− | 2 | 500 ± 160 | ||||
Cells (sorted once or twice, as indicated) were cultured (usually 500 cells per 100 μL × 2) for 10 days in serum-free medium with various cytokines (see text) and then CFC assays performed, usually by plating 10% of the harvested cells per 1 mL CFC assay. The data shown have been rounded off to 2 significant figures. More precise data on the distribution of the different types of CFC detected are shown in Table 4.
Abbreviations: ND, not done; mPB, mobilized peripheral blood; BM, bone marrow (number indicates sample number); F, FL; S, SF; 3, IL-3; H6, H–IL-6; T, TPO.
The colonies obtained in the CFC assay of cells produced in vitro by lin−CD34−CD38−cells that had been stimulated with the 5 growth factor combination contained either erythroblasts, granulocytes, macrophages, or megakaryocytes exclusively, or mixtures of these. In many cases, but not all, these colonies grew to a large size within the 2- to 3-week CFC assay period (Table 4). Thus, complete maturation along all of the major myeloid lineages could be reproducibly achieved within an overall period of 3 to 4 weeks of exposure of lin−CD34−CD38−cells and their progeny to an appropriate sequence of defined growth factors.
Distribution of Different Types of Progenitors Present in 10-Day Cultures Initiated With Lin−CD34−CD38− Adult Human Marrow or Mobilized Peripheral Blood Cells
| Sample . | CFU-E . | BFU-E . | CFU-GEMM . | CFU-GM . | CFU-Mk . |
|---|---|---|---|---|---|
| mPB1 | 0 | 2 | 0 | 48 | 0 |
| BM1 | 0 | 0 | 0 | 40 | ND |
| BM2 | 30 | 10 | 45 | 1,100 | 65 |
| BM3 | 15 | 90 | 50 | 1,800 | 120 |
| BM8 | 0 | 0 | 0 | 140 | ND |
| BM4 | 0 | 0 | 0 | 120 | 0 |
| BM5 | 24 | 36 | 0 | 380 | ND |
| BM6 | 12 | 72 | 12 | 820 | ND |
| BM7 | 84 | 0 | 0 | 420 | ND |
| Sample . | CFU-E . | BFU-E . | CFU-GEMM . | CFU-GM . | CFU-Mk . |
|---|---|---|---|---|---|
| mPB1 | 0 | 2 | 0 | 48 | 0 |
| BM1 | 0 | 0 | 0 | 40 | ND |
| BM2 | 30 | 10 | 45 | 1,100 | 65 |
| BM3 | 15 | 90 | 50 | 1,800 | 120 |
| BM8 | 0 | 0 | 0 | 140 | ND |
| BM4 | 0 | 0 | 0 | 120 | 0 |
| BM5 | 24 | 36 | 0 | 380 | ND |
| BM6 | 12 | 72 | 12 | 820 | ND |
| BM7 | 84 | 0 | 0 | 420 | ND |
Same experiments as described in Table 3. Values shown are the numbers of each type of CFC produced per 103lin−CD34−CD38− cells originally placed in culture, as determined by assessment of a fraction of each 10 day culture harvest. CFU-E, BFU-E, CFU-GEMM, and CFU-GM assayed in methylcellulose cultures. CFU-Mk assayed in collagen cultures (see also note to Table 3).
Abbreviations: ND, not done; mPB, mobilized peripheral blood; BM, bone marrow (number indicates sample number).
Resorting of the initial lin−CD34−CD38−cells isolated from 4 different marrows showed the purity of the first sort to be consistently greater than 98%. Moreover, the number (Table3) and types (Table 4) of CFC generated from resorted samples were similar to what had been obtained from cells isolated using a single sort. In 2 experiments, 1 of several replicate cultures of 103lin−CD34−CD38−cells each was harvested and assayed for CFC after 5 and 7 as well as 10 days of incubation. The results of these assays showed that no CFC could be detected after 5 days and that near maximum numbers were already detectable by 7 days.
The frequency of lin−CD34−CD38−cells able to proliferate in liquid cultures within 10 days in response to stimulation by FL, SF, TPO, IL-3, and H–IL-6, as well as the frequency of those with pre-CFC activity, was determined for 4 of the marrow samples by LDA (see Materials and Methods) of doubly sorted cells. As shown in Table 5, the frequencies of such cells varied over a 40-fold range between marrow samples (as did the frequency of LTC-IC in the lin−CD34+CD38− cells from the same samples), with an average frequency of growth factor-responsive lin−CD34−CD38−cells and pre-CFC of approximately 1 per 100 and approximately 1 per 200, respectively. (CFC were detected in only ∼50% of the wells and only in wells observed to contain viable cells). The results of a representative LDA experiment are shown in Fig 3. The average output of cells (after 10 days) per responsive lin−CD34−CD38−cell could thus be calculated to be approximately 104 and the corresponding average output of CFC per pre-CFC (after 10 days) to be 13 ± 7. Assuming 100% recovery of these pre-CFC in the lin−CD34−CD38−population means their frequency in normal adult human marrow would be less than 1 per 5 × 104 light-density cells.
Frequency of Growth Factor-Responsive Cells in Lin−CD34−CD38− Marrow Cells and LTC-IC in the Matching Lin−CD34+CD38−Populations
| Sample . | Lin−CD34−CD38− Clone-Forming Cells5-150 . | Lin−CD34−CD38− Pre-CFC5-150 . | Lin−CD34+CD38− LTC-IC5-151 . | ||
|---|---|---|---|---|---|
| Frequency . | ±SEM . | Frequency . | ±SEM . | Estimated Frequency . | |
| BM 3 | 1/68 | 1/48-1/96 | 1/85 | 1/61-1/120 | 1/10 |
| BM 4 | 1/470 | 1/280-1/780 | 1/1,100 | 1/560-1/2,250 | 1/113 |
| BM 5 | 1/360 | 1/220-1/580 | 1/890 | 1/500-1/1,600 | 1/23 |
| BM 6 | 1/28 | 1/17-1/45 | 1/28 | 1/17-1/45 | 1/3 |
| Mean | 1/130 | 1/70-1/260 | 1/220 | 1/90-1/540 | 1/17 (1/8-1/36) |
| Sample . | Lin−CD34−CD38− Clone-Forming Cells5-150 . | Lin−CD34−CD38− Pre-CFC5-150 . | Lin−CD34+CD38− LTC-IC5-151 . | ||
|---|---|---|---|---|---|
| Frequency . | ±SEM . | Frequency . | ±SEM . | Estimated Frequency . | |
| BM 3 | 1/68 | 1/48-1/96 | 1/85 | 1/61-1/120 | 1/10 |
| BM 4 | 1/470 | 1/280-1/780 | 1/1,100 | 1/560-1/2,250 | 1/113 |
| BM 5 | 1/360 | 1/220-1/580 | 1/890 | 1/500-1/1,600 | 1/23 |
| BM 6 | 1/28 | 1/17-1/45 | 1/28 | 1/17-1/45 | 1/3 |
| Mean | 1/130 | 1/70-1/260 | 1/220 | 1/90-1/540 | 1/17 (1/8-1/36) |
The frequency (and the range defined by ±SEM) of growth factor-responsive cells (cells able to make clones of viable progeny) and pre-CFC in the lin−CD34−CD38− was determined by LDA. For each marrow tested, 3 or 4 different numbers of lin−CD34−CD38− cells were stimulated with 5 growth factors for 10 days, and individual wells were scored for the presence of viable cells that were then harvested and assayed in methylcellulose cultures for the presence or absence of CFC. The overall mean values were calculated as geometric means, with the range shown as ±SEM for the intersample variation.
LTC-IC frequencies of lin−CD34+CD38− cells from the same samples were calculated by dividing bulk assay CFC outputs by 18, which is the mean 6-week CFC output per LTC-IC value previously established for adult marrow LTC-IC assayed under the conditions used here.22 The mean value is the geometric mean shown with the range defined by ±SEM.
Results of an LDA experiment to determine the frequency of growth factor-responsive cells (○) and pre-CFC (•) in the lin−CD34−CD38− population of a representative marrow sample.
Results of an LDA experiment to determine the frequency of growth factor-responsive cells (○) and pre-CFC (•) in the lin−CD34−CD38− population of a representative marrow sample.
LTC-IC were detected in 10-day cultures initiated with lin−CD34−CD38−cells from only 2 of 5 samples and, then, only when all 5 growth factors were also added to the LTC supernatants (yielding 10 and 60 CFC, respectively, after 6 weeks from 500 lin−CD34−CD38−cells initially placed in culture). Whether this reflects inadequate conditions in the primary liquid suspension cultures for the generation and amplification or maintenance of LTC-IC from a starting population of lin−CD34−CD38−cells has yet to be determined. Interestingly, in 2 separate experiments, no transplantable human hematopoietic cell activity could be detected in any of 4 sublethally irradiated (300 to 350 cGy) NOD/SCID-β2M−/−mice.25 These mice were injected immediately postirradiation with 106 irradiated unseparated human marrow (carrier) cells and either 2,000 or 4,000 doubly sorted lin−CD34−CD7+ cells (which represent a subset of the CD34−CD38− cells; Bhatia et al13 and our own findings) or 3 × 104 or 1.7 × 105 doubly sorted lin−CD34−CD7−cells per mouse. Two months later, all 4 mice were killed and their marrow cells were stained with antihuman CD34, CD45, CD71, CD19, CD20, CD15, and CD66b antibodies or isotype controls as described.26 Fluorescence-activated cell sorting (FACS) analysis of ≥ 20,000 PI− events failed to show any evidence of human cells in any of the 4 mice (<5 positive events outside the control gates), despite parallel data (Glimm and Eaves, unpublished observations) indicating that the NOD/SCID-β2M−/− mouse allows engraftment of 10× more transplantable lympho-myeloid stem cells from unseparated adult human marrow than the NOD/SCID mouse. In the present experiments, the lin−CD34−cells were split into CD7+ and CD7−subpopulations based on a recent report that lin−CD34−CD7+ cord blood cells may have in vivo lymphopoietic activity.27
DISCUSSION
In this study, we describe a growth factor combination that can initiate the rapid proliferation and differentiation in vitro of a subset (∼1%) of lin−CD34−CD38−cells that are present in normal adult human marrow. Determination by limiting dilution analysis of the frequency of those able to generate CFC in vitro (pre-CFC) showed that these represent a subset (∼50%) of the total growth factor-responsive lin−CD34−CD38−subpopulation. In confirmation of previous reports,11,13cells able to proliferate in semisolid media (CFC) or able to generate CFC for ≥6 weeks in stromal cell-containing cultures (LTC-IC) were not detected at equivalent frequencies among these cells. Preliminary studies have also not detected in vivo repopulating activity within the lin−CD34− adult marrow population. This latter finding contrasts with what has been described for transplants of adult marrow lin−CD34− cells using fetal sheep as recipients12 or for transplants of cord blood lin−CD34− cells using irradiated NOD/SCID mice as recipients.13 In the present experiments, a more sensitive host than the regular NOD/SCID mouse was used. Nevertheless, the number of cells transplanted and/or their homing efficiency28 may still have been limiting. Additional studies using other culture conditions to influence these parameters13 may thus be required to ultimately establish the relationship of the multipotent pre-CFC described here with other types of hematopoietic cells. Interestingly, however, lin−CD34−CD38−cells with hematopoietic progenitor activity were shown to be mobilized into the blood by in vivo G-CSF treatment. The distinct growth factor requirements exhibited by these adult lin−CD34−CD38−cells, their persistence at the same frequency in resorted, greater than 98% pure lin−CD34−CD38−cell populations, the failure to detect cells among the lin−CD34−CD38−cells that display the less restricted in vitro growth requirements of most CD34+ cells (including CD34+CD38− cells), and the fact that CD34+CD38− cells are not more prevalent in the lin− population all argue strongly against the possibility that the functional properties here assigned to lin−CD34−CD38−cells reflect contaminating CD34+ cells.
Although the frequencies of growth factor-responsive cells and pre-CFC within the lin−CD34−CD38−population isolated from different marrow samples were variable over a 40-fold range, the same variability was seen in the LTC-IC content of the matching lin−CD34+CD38−populations. This degree of variation may reflect in part the fact that some samples were from allogeneic transplant harvests, whereas others were from cadaveric vertebral body harvests. Importantly, despite this variation between samples, cells able to proliferate in liquid suspension culture were always more numerous than those able to proliferate directly in semisolid media, even when exposed to otherwise identical culture conditions. This latter finding focuses attention on an intriguing feature of the differentiation process that these cells undergo in liquid cultures containing FL, SL, TPO, IL-3, and H–IL-6; ie, the acquisition of an ability to proliferate in semisolid media. Although the molecular mechanisms underlying this latter property are not presently understood, it may be speculated that growth factor-induced changes in the structure of the cytoskeleton are involved. Interestingly, a similar discrepancy in ability to proliferate in liquid versus semisolid media has been demonstrated for freshly isolated CD34+CD38− human marrow cells.24
A second important characteristic of the cells generated within 7 to 10 days from lin−CD34−CD38−cells stimulated by FL, SF, TPO, IL-3, and H–IL-6 is their acquisition of an ability to rapidly enter the terminal phases of multiple blood cell differentiation programs when subsequently stimulated by appropriate late-acting growth factors. Moreover, this appears to occur without passage through a step in which the cells display LTC-IC activity. Such behavior contrasts markedly with that expected from a prolonged multistep process in which changes in responses to soluble or stromal-bound growth factors, proliferative potential, and differentiation status are coordinately regulated. Current acceptance of such a linkage is based on an observed hierarchy of progenitor subtypes detected during both steady-state and regenerating hematopoiesis in the adult. However, in vitro, wide variations in the rate of initiation of terminal differentiation have been noted previously.16 The rapidity of differentiation of lin−CD34−CD38−cells into terminal blood cells observed here provides further support for the concept that the speed at which hematopoietic cells differentiate may be subject to regulation by exogenous growth factor stimulation.15 29-31
ACKNOWLEDGMENT
The authors thank their many colleagues in the Division of Hematology of the University of British Columbia and the Stem Cell Assay Laboratory of the BC Cancer Agency for assistance in procuring and initial processing of patient samples. The technical assistance of Gayle Thornbury, Richard Zapf, and Giovanna Cameron in operating the FACS and the assistance of Tara Palmater in preparing the manuscript is also acknowledged. Thanks is also due to Dr P. Lansdorp, Cangene, Genentech, Novartis, and StemCell for generous gifts of reagents.
Supported by grants from the National Cancer Institute of Canada (NCIC) with funds from the Terry Fox Run, the National Institutes of Health (NHLBI-HL55435), and Novartis, Canada. M.G.B. was supported by a grant from the Association pour la Recherche Contre le Cancer (France), S.R.-J. was supported by grants from the Deutsche Forschungsgemeinschaft (Bonn, Germany) and the Stiftung Rheinland-Pfalz für Innovation (Mainz, Germany), and C.J.E. is a Terry Fox Cancer Research Scientist of the NCIC.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Connie J. Eaves, PhD, Terry Fox Laboratory, 601 W 10th Ave, Vancouver, British Columbia, Canada V5Z 1L3; e-mail:connie@terryfox.ubc.ca.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal