Abstract
Ligation of the Fas receptor induces death-inducing signaling complex (DISC) formation, caspase activation, and subsequent apoptotic death of several cell types. Epstein-Barr virus (EBV)-positive group III Burkitt’s lymphoma (BL) cell lines have a marked resistance to Fas-mediated apoptosis, although expressing each of the DISC components, Fas/ APO-1–associated death domain protein (FADD), and caspase-8 (FLICE/MACH/Mch5). The apoptotic pathway distal to the DISC is intact because ceramide analogs, staurosporine, and granzyme B activate caspase-3 and induce apoptosis. Fas resistance was not explained by the putative death-attenuating caspase-8 isoforms. However, while Fas-activated cytosolic extracts from sensitive cells were capable of processing both procaspase-8 and procaspase-3 into active subunit forms, resistant cell extracts did not possess either of these activities. Accordingly, reverse transcriptase-polymerase chain reaction (RT-PCR) analysis showed higher transcript levels for the FLICE-inhibitory protein (FLIPL) in resistant cells and the ratio of caspase-8 to FLIPLmeasured by competition RT-PCR analysis directly correlated with susceptibility to Fas-mediated apoptosis of all cell lines. In addition, modification of the caspase-8/FLIPL ratio by caspase-8 or FLIPL overexpression was able to alter the susceptibility status of the cell lines tested. Our results imply that the relative levels of caspase-8 and FLIPL are an important determinant of susceptibility to Fas-mediated apoptosis.
FAS (APO-1/CD95) RECEPTOR-mediated apoptosis is an effector of activation-induced T-lymphocyte death, peripheral T- and B-cell tolerance, and its physiologic importance is underscored by defects that result in autoimmunity with aberrant antibody production and lymphadenopathy in both mouse and humans.1-10 Ligation of the Fas receptor initiates a death signal by the formation of a death-inducing signaling complex (DISC).11 Interactions between the homologous death domains (DD) of Fas and the Fas/APO-1–associated death domain protein (FADD) carboxyl-terminus promote the recruitment of procaspase-8 (FLICE/MACH/Mch5) by homophilic interactions between death effector domains (DED) contained in the amino-terminus of FADD and the prodomain of caspase-8.12,13 The resultant conformational change in procaspase-8 leads to its activation and subsequent processing resulting in the cleavage of the FADD-homology domain from the ICE/CED-3–caspase-homology region.14 The former remains bound to the DISC, while liberated active caspase-8 initiates the apoptotic proteolytic cascade. Dimerization of caspase-8 is required for activation and yields an active tetrameric (p10/p18)2complex.15 Major substrates for caspase-8 include the zymogen forms of other caspases, such as caspase-6 (Mch2) and caspase-3 (Yama/CPP32β/Apopain), and the death substrate poly(adenosine diphosphate [ADP])-ribose polymerase (PARP).13,16 The conversion of caspases to their active forms and subsequent substrate cleavage are the quintessential events comprising the “execution phase” of the apoptotic pathway.17 18
The efficient triggering of Fas-mediated apoptosis suggests the requirement for strict controls within this signaling pathway. Although many cell types express the Fas antigen, few are constitutively susceptible to cell death triggered through it. Modulation of Fas susceptibility appears to be a prominent feature of the immune system. Resting T cells are initially resistant to Fas-mediated apoptosis, but can be rendered sensitive by activation with sequential phytohemagglutinin and interleukin-2 treatments.19 The initial resistance has been associated with a failure of caspase-8 to be recruited to the DISC by FADD.19 The downregulation of Fas sensitivity through alteration in FADD and caspase-8 association has also been demonstrated in a Fas-resistant pre-B–cell line.11 Furthermore, resistance can be induced in B lymphocytes by antigen receptor cross-linking and is a characteristic of the memory B-cell phenotype.20 21
A number of Epstein-Barr virus (EBV)-positive Burkitt’s lymphoma (BL) cell lines also develop resistance to Fas receptor cross-linking after having acquired a lymphoblastoid/group III, or activated, phenotype.22 In contrast, even though EBV-transformed primary B lymphocytes typically possess a classic lymphoblastoid group III phenotype, they are acutely sensitive to Fas-mediated apoptosis showing that EBV infection does not independently lead to Fas resistance.22 Furthermore, the sensitivity of Fas/APO-1–transfected EBV-negative BL cell lines to Fas ligation indicates that Fas resistance is not a general characteristic of the malignant B-cell phenotype.11 22 Because Fas resistance may be critical to the persistence of Burkitt’s lymphomas, we were interested in elucidating the basis for this phenomenon.
Recently, viral inhibitors of Fas- and tumor necrosis factor (TNF) receptor-induced cell death have been identified in several poxviruses and γ-herpes viruses, but not in EBV.23-25 These DED-containing proteins behave as decoy molecules blocking the interaction between FADD and caspase-8 essential for DISC assembly and caspase-8 activation. These inhibitory proteins have been collectively referred to as v-FLIPs, for viral FLICE-inhibitory proteins, and are thought to participate in viral pathogenesis by attenuation of the host immune responses mediated through death receptors.25 Unlike cowpox crmA, baculovirus p35, and vaccinia SPI-2 (B13R) gene products, but consistent with an inhibitory action proximal to caspase-8 activation, v-FLIPs cannot block the apoptotic activity of activated caspase-8.23-31
A cellular homologue of viral FLICE-inhibitory proteins (FLIP, also referred to as I-FLICE/FLAME-1/CASH/CLARP/CASPER/MRIT/Usurpin) has subsequently been identified by several groups.32-39 Stable transfection of cells with FLIP expression vectors protects them to varying degrees from apoptosis mediated by the Fas, TNF, and TNF-related apoptosis-inducing ligand (TRAIL) receptors.24,32,36,38 Alternative splicing leads to long (FLIPL, 55 kD) or short (FLIPS, 28 kD) form variants.36 FLIPL has a very similar structure to that of caspase-8 in that it contains two DEDs and a caspase homology domain. However, it lacks the caspase active site consensus pentapeptide and a tyrosine residue is substituted for the highly conserved cysteine, rendering this a catalytically-inert caspase. FLIPS, on the other hand, is truncated shortly after the 2 DEDs and completely lacks the caspase homology domain. Both FLIPs can interact with FADD and caspase-8 via the DED motifs and can be recruited to the Fas DISC.32,36,38 The caspase domain further contributes to the interaction with caspase-8. In addition, similarly to procaspase-8, FLIP can serve as a substrate for activated caspase-8, resulting in the generation of a C-terminally truncated 43-kD protein that possibly binds more tightly to caspase-8, preventing further activation.36 FLIP has been implicated in the regulation of the Fas pathway during T-lymphocyte activation and probably contributes to blocking caspase-8 recruitment to the DISC during the initial resistant phases.19,36 FLIP expression has also been detected in melanoma specimens and has been associated with the generation of Fas-resistance of several melanoma and chronic myelogenous leukemia (ie, K562) cell lines.34 36 Because FLIP was not detectable in nonmalignant melanocytes, its upregulation may be an event critical to tumorigenesis.
In this report, we examined several potential mechanisms used by EBV-positive BL cell lines for the establishment of Fas-resistance. To this end, 3 classes of cell lines were used: (1) EBV-transformed primary B lymphocytes (ie, SKW6.4), (2) EBV-negative BL cells, and (3) EBV-positive BL lines (groups I and III). This permitted examination of two aspects of this model system without genetic manipulation, namely the consequence of EBV infection on apoptosis and the influence host cell type has on the outcome of infection. We report a strong correlation between heightened FLIPL expression relative to that of caspase-8 and the generation of the resistant phenotype. This was strongly supported by our ability to reverse this phenotype by overexpression of caspase-8. Furthermore, this is a tumor-specific phenomenon, as lymphoblastoid cell lines (LCL) established from EBV-transformed primary B cells did not display the same pattern of FLIP and caspase-8 modulation.
MATERIALS AND METHODS
Cell lines and culture.
The cell lines used in this study include: (1) EBV-negative Burkitt’s lymphomas BJAB, Ramos, and ST486, (2) EBV-positive Burkitt’s lymphomas Akata, Daudi, Mutu-BL (groups I and III), Raji, Jijoye, and P3HR-1, and (3) EBV-positive B lymphoblastoid SKW6.4. The susceptibility/resistance of all cell lines to Fas-induced apoptosis and EBV status are indicated in Fig 1. All cultures were maintained in RPMI 1640 medium (GIBCO-BRL, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS), 2 mmol/L L-glutamine, and 100 U/mL penicillin-100 μg/mL streptomycin at 37°C in a humidified environment of 5% CO2 in air. Experiments using anti-Fas IgM and staurosporine were also performed in this medium. For treatment with C2-ceramide, the serum content was reduced to 1%.
Fas susceptibility and EBV status of cell lines used in this study. Fas-mediated apoptosis was assayed with MTT. Triplicate cultures of 100 μL (3 × 104 cells) were incubated with anti-Fas IgM (250 ng/mL) for 24 hours at 37°C. MTT was then added for an additional 2 hours followed by solubilization of the formazan crystals. Absorbance was read at 570 nmol/L and percentage apoptosis was calculated as previously described.40 Results are from 3 separate experiments and are expressed as mean percentage apoptosis ± standard deviation. Cell-surface expression of the Fas receptor was determined by flow cytometry and the results indicate Fas-positive (▪) and Fas-negative (□) cell lines. The EBV status (negative or positive) of each cell line was obtained from the literature and later confirmed in appropriate cell lines by LMP1 immunoblot analysis.61 62
Fas susceptibility and EBV status of cell lines used in this study. Fas-mediated apoptosis was assayed with MTT. Triplicate cultures of 100 μL (3 × 104 cells) were incubated with anti-Fas IgM (250 ng/mL) for 24 hours at 37°C. MTT was then added for an additional 2 hours followed by solubilization of the formazan crystals. Absorbance was read at 570 nmol/L and percentage apoptosis was calculated as previously described.40 Results are from 3 separate experiments and are expressed as mean percentage apoptosis ± standard deviation. Cell-surface expression of the Fas receptor was determined by flow cytometry and the results indicate Fas-positive (▪) and Fas-negative (□) cell lines. The EBV status (negative or positive) of each cell line was obtained from the literature and later confirmed in appropriate cell lines by LMP1 immunoblot analysis.61 62
Reagents.
The cytotoxic mouse anti-human Fas monoclonal antibody (anti-Fas; IgM, clone CH-11), mouse anti–caspase-8 (clone 5F7, IgG2b), and rabbit polyclonal anti-FLIP–CT antibodies were obtained from Upstate Biotechnology, Inc (Lake Placid, NY). Mouse monoclonal anti-FADD (clone 1, IgG1) and anti-CPP32 (clone 19, IgG2a) antibodies were both purchased from Transduction Laboratories (Lexington, KY). Polyclonal goat anti-FLICE/Mch5 p20 (C-20) was obtained from Santa Cruz Biotechnology, Inc (Santa Cruz, CA) and anti-ICE–LAP3 p20 (rabbit polyclonal) was the kind gift of V.M. Dixit (Genentech, Inc, South San Francisco, CA). MTT was purchased from Sigma (St Louis, MO), dissolved in phosphate-buffered saline (PBS), and filter-sterilized. C2-ceramide and staurosporine were purchased from Calbiochem (La Jolla, CA). Stock solutions were made in dimethyl sulfoxide (DMSO) and stored at −20°C until use. Purified granzyme B was supplied by Enzyme Systems Products (Livermore, CA). All polymerase chain reaction (PCR) primers were custom synthesized by GIBCO-BRL.
Flow cytometry.
Cell-surface expression of Fas antigen was determined by FACScan flow cytometric analysis as previously described.40 Briefly, cells were sequentially incubated with anti-Fas IgM (10 μg/mL) and flouroscein isothiocyanate-conjugated goat anti-mouse immunoglobulins (Dako, Glostrup, Denmark) followed by fixation with formaldehyde and analysis. Nonspecific binding of antibodies was controlled for with each cell line by using a nonimmune monoclonal antibody (clone P3, IgG) (generous gift of Dr Barton F. Haynes, Duke University, Durham, NC).
Measurement of cell death and DNA fragmentation analysis.
Cell death was determined using an MTT-based assay as previously described.40 Briefly, cell cultures in 96-well plates (3 × 104 cells/well in 100 μL) were exposed to anti-Fas IgM (250 ng/mL) for 24 hours. MTT was then added to each well and incubation was continued for another 2 hours followed by solubilization of the resulting formazan crystals overnight and measurement of absorbance at 570 nm. Apoptotic cell death was further confirmed by the appearance of morphologic changes such as plasma membrane blebbing, increased cell refractility, and nuclear condensation. For DNA fragmentation analysis, DNA was isolated from 1 × 106 cells, purified by organic extraction, ethanol-precipitated, and finally dissolved in 100 μL of Tris-EDTA (pH 8.0). Twenty microliters of each sample was treated with DNase-free RNase (35 μg/mL) for 30 minutes followed by electrophoresis through 1.2% ethidium bromide-agarose gels at constant current (20 mA) for 16 hours.
Immunoblot analysis.
Cell lysates were made in NP-40 lysis buffer (30 mmol/L Tris-Cl, pH 7.5, 1 mmol/L EDTA, 150 mmol/L NaCl, 1% NP-40) and protein content was quantitated with a detergent-compatible assay kit (Bio-Rad, Hercules, CA). Proteins (50 μg of lysate) were separated by denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose (Bio-Blot, Costar, Cambridge, MA). Membranes were blocked for at least 1 hour with 5% nonfat dry milk in Tris-buffered saline containing 0.05% Tween-20 (TBST). Primary antibodies were diluted in blocking buffer according to the manufacturers’ protocols and subsequently incubated with the blots for 2 hours at room temperature or overnight at 4°C. The membranes were washed three times with TBST and incubated with a 1:5,000 dilution of the appropriate horseradish peroxidase-conjugated anti-IgG in blocking buffer for 1 hour. After washing, the blots were developed with enhanced chemiluminescence (Amersham, Piscataway, NJ) and exposed to BioMax x-ray film (Eastman-Kodak, Rochester, NY).
Caspase activity assays.
Caspase-3–related protease activity in cell lysates was measured using the ApoAlert CPP32 assay kit (Clontech, Palo Alto, CA). Briefly, 2 × 106 cells were treated with anti-Fas IgM (250 ng/mL, 3 hours), C2-ceramide (10 μmol/L, 16 hours), and staurosporine (5 μmol/L, 16 hours), washed once with PBS, and lysed in 50 μL of cell lysis buffer on ice. Nuclei were removed from the lysates by centrifugation in a microcentrifuge for 30 seconds at 4°C. The pellet was discarded and the nuclei-free detergent extract was used in the caspase assays. An equal volume of 2X reaction buffer (containing 10 mmol/L dithiothreitol [DTT]) was then added and reactions were initiated by the addition of the colorimetric substrate DEVD-pNA (50 μmol/L final concentration) followed by incubation at 37°C for 1 hour. The samples were then transferred to 96-well plates and absorbance read with a plate reader at 405 nm.
Preparation of cytosolic extracts.
Untreated or anti-Fas–treated (1 and 2 hours) cells were harvested and washed once in chilled PBS. The cell pellets were resuspended in modified cytosolic extract preparation buffer (10 mmol/L HEPES, pH 7.5/10 mmol/L KCl/1 mmol/L DTT) and allowed to swell on ice for 15 minutes.41 The cells were lysed by aspiration through a 22-gauge needle 10 to 20 times and then centrifuged at 16,000gfor 15 minutes at 4°C. The 16K supernatant was used for caspase processing assays.
In vitro caspase processing assays.
[35S]methionine-labeled procaspase-8 and procaspase-3 (Yama) were generated from pcDNA3-FLICE-HA and pcDNA3-Yama-AU1 expression vectors by coupled in vitro transcription/translation with the TNT reticulocyte lysate system (Promega, Madison, WI) driven by the T7 promoter. The mixture was desalted by centrifugation through Bio-Spin columns (Bio-Rad). Caspase processing activity contained in cytosolic extracts was examined by combining 2 μL of the translated protein with 25 μg of extract in a total volume of 10 μL followed by incubation for 2 hours at 37°C. Reactions were terminated by the addition of Laemmli sample buffer containing β-mercaptoethanol. The samples were placed in a boiling water bath and subjected to SDS-PAGE through 15% gels. The gels were then fixed, dried, and exposed to BioMax x-ray film (Eastman-Kodak) according to standard protocol.
RNA isolation and first-strand cDNA synthesis.
Total cellular RNA was isolated from 1 × 107 cells using the TRIZOL reagent (GIBCO-BRL) according to the manufacturer’s protocol. First-strand cDNA was then synthesized from 5 μg of RNA with the cDNA preamplification system (GIBCO-BRL) using SuperScript II reverse transcriptase (RT) and an oligo(dT) primer.
PCR analyses.
PCR reactions (50 μL) were performed according to standard protocol with 2 μL of first-strand cDNA template, 10 μmol/L primers, 0.8 mmol/L dNTPs, and 0.5 U Taq polymerase. The amplification program was as follows: 94°C for 2 minutes (1 cycle), 94°C for 30 seconds, 58°C for 30 seconds, 72°C for 90 seconds (30 cycles). The following primer sequences were used for the detection of caspase-8/FLICE/MACH α and β isoforms12: MACHβ1(188).S (5′-TTG GAT CCA GAT GGA CTT CAG CAG AAA TCT T-3′), MACHβ1(521).S (5′-AAG TGA GCA GAT CAG AAT TGA G-3′), MACHβ1(575).S (5′-GAG GAT CCC CAA ATG CAA ACT GGA TGA TGA C-3′), MACHβ1(914).AS (5′-ATT CTC AAA CCC TGC ATC CAA GTG-3′), MACHβ1(930).AS (5′-GCC ACC AGCT AAA AAC ATT CTC AA-3′). Amplification products were subsequently separated on 1.2% Tris-acetate-EDTA (TAE) agarose gels. The sequences of the primers used for amplification of the FLICE caspase homology domain were CASP8(649).S (5′-TTG TGG CAT ATG AGT GAA TCA CAG ACT TTG GAC AAA G-3′) and CASP8(1440).AS (5′-CAG CCG GAT CCT CAA TCA GAA GGG AAG ACA AGT TT-3′). Full-length FLIPlong was amplified with the FLIPL (383).S (5′-GTA TAC ATA TGT CTG CTG AAG TCA TCC ATC AGG TTG-3′) and FLIPL (1822).AS (5′-GTG ACT CGA GTG TGT AGG AGA GGA TAA GTT TCT TTC TC-3′) primers.
CASP8/FLIP competition PCR (CFCP) analysis was performed by conducting amplifications in the presence of primer pairs for both caspase-8 (caspase domain) and FLIPL using the same conditions described above. The amplified products were separated by agarose gel electrophoresis, stained with ethidium bromide, and then analyzed by scanning densitometry. The caspase-8/FLIPL ratio for each sample was then obtained from the values for the relative band intensities.
Construction of caspase-8, FLIPL, and enhanced green fluorescent protein (EGFP) mammalian expression constructs.
Full-length caspase-8 (CASP8[FL]) and FLIPL were generated by high fidelity PCR amplification (Clontech, Palo Alto, CA) from SKW6.4 and Raji cDNA templates, respectively, using the CASP8(1)Kpn.S primer (5′-GAA CGG GGT ACC GCC ATG GAC TTC AGC AGA AAT CTT TAT GAT-3′) paired with the CASP8(1440).AS primer and the FLIPL(383)SmtAKpn.S primer (5′-CGG GGT ACC GCC ATG GCT GCT GAA GTC ATC CAT CAG GTT G-3′) paired with the FLIPL(1825)Xho.AS primer (5′-GTG ACT CGA GTT ATG TGT AGG AGA GGA TAA GTT TCT TTC TC-3′). To maximize FLIPLprotein expression, the forward primer was designed to mutate the thymidine in the +4 position of the sequence to a guanine (ie, resulting in conversion of Ser to Ala) in agreement with Kozak’s consensus sequence for optimal translation initiation. The resulting amplification product was therefore designated FLIPL(SmtA). Gel-purified CASP8(FL) and FLIPL(SmtA) products were TA-cloned into pCR3.1 (Invitrogen, Carlsbad, CA). After determination of both sequence integrity and orientation, the desired clones were excised by sequential NotI and KpnI digestions and ligated into KpnI+NotI-digested pCEP4 (Invitrogen). These expression constructs were designated pCEP4-CASP8(FL) and pCEP4-FLIPL(SmtA). The EGFP reporter construct pCEP4-EGFP was generated by ligation of theNotI/KpnI EGFP fragment from pEGFP-N1 (Clontech) into the same restriction sites of pCEP4. The vector pCEP4 was used for our constructs because it contains an EBV origin of replication (ori P) as well as the strong cytomegalovirus (CMV) promoter. We found the presence of ori P to be essential to obtaining easily detectable protein expression in EBV-infected cell lines (data not shown).
Transient transfections and measurement of apoptosis.
Before transfection, BJAB, SKW6.4, Mutu-BL III, and Raji cells were harvested, resuspended in twice the original volume of fresh complete medium, and incubated for 18 hours. Cells were then harvested and resuspended in fresh medium at a concentration of 2 × 107 cells/mL and placed on ice. Cotransfections with 1.5 μg of pCEP4-EGFP and 7.5 μg of either pCEP4-CASP8(FL) or pCEP4-FLIPL(SmtA) were performed by electroporation (1,700 μF, 72 Ω, 110 V) of 0.2 mL cells in 2-mm gap width cuvettes using a BTX ECM-600 electroporator (Genetronics, San Diego, CA). The samples were then diluted in 15 mL of prewarmed medium and incubated for 72 hours to permit recovery and maximum expression (ie, assessed by the visualization of EGFP). For assessment of apoptosis in response to Fas triggering, cultures were transferred into fresh medium containing 1% FBS, rested at 37°C for 4 hours, and then left untreated or challenged with anti-Fas IgM (500 ng/mL, clone CH-11) for 8 hours. Percentage apoptosis of EGFP-positive cells was determined using standard morphologic criteria (cytoplasmic condensation, plasma membrane blebbing, increased refractility) by combined fluorescence and light microscopy with a Leica inverted phase/fluorescence microscope (Leica Microsystems Inc, Deerfield, IL).
RESULTS
Apoptosis pathways distal to caspase-3 activation are intact in Fas-resistant group III BL cell lines.
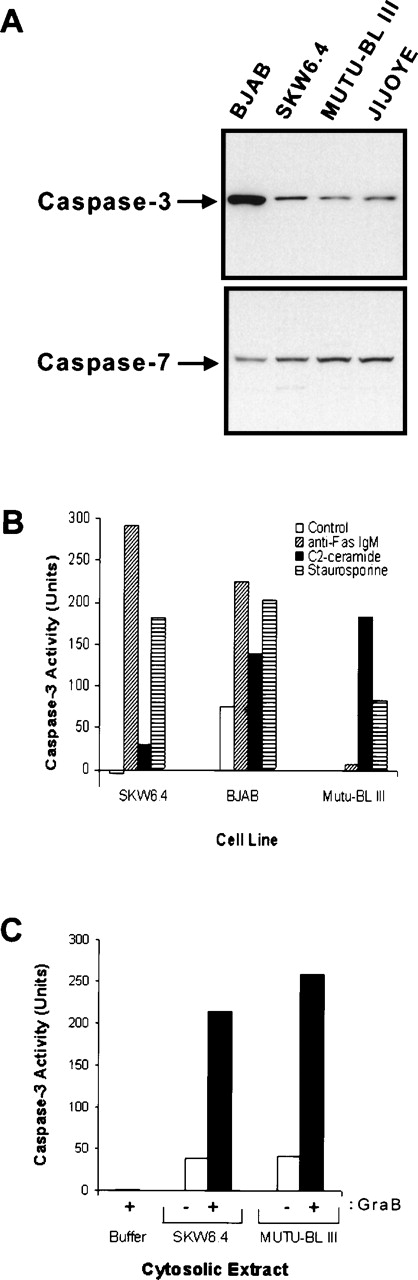
Previous studies by our group have shown that the synthetic ceramide analog C2-ceramide effectively induced apoptosis of Fas-resistant group III Mutu-BL cells. These results implied that even though early events of Fas signaling were disrupted in this BL cell line, components of this apoptotic pathway distal to caspase-3 activation were intact.40,42 To further characterize the defect in the Fas pathway in resistant BL cells, they were exposed to micromolar concentrations of the protein kinase inhibitor staurosporine, also known to trigger caspase-3 activation.16 43 Staurosporine induced apoptosis to different degrees in all cell cultures as shown by high-molecular-weight DNA degradation and/or the generation of the hallmark nucleosomal laddering pattern, which was very prominent in the Ramos EBV-negative group I BL cell line (Fig 2). In addition, immunoblotting of whole-cell lysates confirmed the presence of comparable levels of caspase-3 (Yama/CPP32/Apopain) and caspase-7 (ICE-LAP3/Mch3/CMH-1) in sensitive and resistant cell lines (Fig 3A).
Induction of apoptosis in Fas-resistant BL cell lines by staurosporine. Cells (1 × 106) were incubated with DMSO vehicle alone (0 μmol/L) or exposed to 2.5 and 5 μmol/L staurosporine for 16 hours and subsequently harvested for analysis of DNA fragmentation as described in Materials and Methods.
Induction of apoptosis in Fas-resistant BL cell lines by staurosporine. Cells (1 × 106) were incubated with DMSO vehicle alone (0 μmol/L) or exposed to 2.5 and 5 μmol/L staurosporine for 16 hours and subsequently harvested for analysis of DNA fragmentation as described in Materials and Methods.
Downstream caspases are present and functional in BL cell lines. (A) Immunoblot analysis for caspase-3 (Yama/CPP32) and caspase-7 (ICE-LAP3). Whole-cell lysates (50 μg) were separated on 15% SDS-PAGE gels under reducing conditions and immobilized on nitrocellulose membranes. Caspase-3 and caspase-7 were detected with anti-CPP32 and anti-ICE-LAP3 p20 antibodies, respectively, followed by development with chemiluminescence. (B) Caspase-3 can be activated in Fas-resistant BL cells. Cells (2 × 106; 5 × 105/mL) were left untreated (control, □) or treated with anti-Fas IgM (▨), C2-ceramide (▪), and staurosporine (▤). Nuclei-free lysates were made from each sample and used in DEVD-pNA cleavage assays. Controls for nonspecific protease activity were obtained by preincubating one set of staurosporine-induced cell lysates with the caspase-3 inhibitor DEVD-fmk (5 μmol/L) for 30 minutes at 37°C before addition of the substrate. Absorbance was read at 405 nm. Caspase activity is expressed in units with 1 unit being the amount of enzyme activity liberating 1 pmol of pNA per minute. (C) Induction of caspase-3 activity in resistant cell lysates by granzyme B. Cleavage assays were performed as in (B) with naı̈ve cell lysates of SKW6.4 and Mutu-BL III cultures. Before performing the cleavage assay, lysates were preincubated for 30 minutes at 37°C in the absence (−, □) or presence (+, ▪) of 5 U of granzyme B (GraB). Reactions performed without cytosolic extract (buffer) showed the failure of granzyme B to independently use DEVD-pNA as a substrate.
Downstream caspases are present and functional in BL cell lines. (A) Immunoblot analysis for caspase-3 (Yama/CPP32) and caspase-7 (ICE-LAP3). Whole-cell lysates (50 μg) were separated on 15% SDS-PAGE gels under reducing conditions and immobilized on nitrocellulose membranes. Caspase-3 and caspase-7 were detected with anti-CPP32 and anti-ICE-LAP3 p20 antibodies, respectively, followed by development with chemiluminescence. (B) Caspase-3 can be activated in Fas-resistant BL cells. Cells (2 × 106; 5 × 105/mL) were left untreated (control, □) or treated with anti-Fas IgM (▨), C2-ceramide (▪), and staurosporine (▤). Nuclei-free lysates were made from each sample and used in DEVD-pNA cleavage assays. Controls for nonspecific protease activity were obtained by preincubating one set of staurosporine-induced cell lysates with the caspase-3 inhibitor DEVD-fmk (5 μmol/L) for 30 minutes at 37°C before addition of the substrate. Absorbance was read at 405 nm. Caspase activity is expressed in units with 1 unit being the amount of enzyme activity liberating 1 pmol of pNA per minute. (C) Induction of caspase-3 activity in resistant cell lysates by granzyme B. Cleavage assays were performed as in (B) with naı̈ve cell lysates of SKW6.4 and Mutu-BL III cultures. Before performing the cleavage assay, lysates were preincubated for 30 minutes at 37°C in the absence (−, □) or presence (+, ▪) of 5 U of granzyme B (GraB). Reactions performed without cytosolic extract (buffer) showed the failure of granzyme B to independently use DEVD-pNA as a substrate.
These results implied that the apoptotic proteolytic cascade was intact. To more directly confirm that caspase-3 and other proteases with similar substrate specificity were processed and catalytically active in the BL cytosol, we evaluated the ability of various apoptotic cytosolic extracts to cleave the tetrapeptide substrate DEVD-pNA. The highest caspase-3 activity was consistently observed in the detergent extracts from Fas-induced SKW6.4 and BJAB cells, which contained 291 and 225 units total activity, respectively (Fig 3B). As expected, Fas stimulation failed to generate appreciable caspase-3 activity in Mutu-BL III cells. However, substantial caspase-3 activity could be generated in this cell line on exposure to C2-ceramide (10 μmol/L) for 16 hours and approached that present in Fas-stimulated BJAB cells. In fact, Mutu-BL III was more responsive to this ceramide analog than both Fas-susceptible cell lines tested. Although C2-ceramide did not activate caspase-3 as effectively as anti-Fas IgM did in SKW6.4 and BJAB cells, this result is consistent with our previous data showing Fas ligation to be the more efficient inducer of apoptosis (Fig 3B).40 44 In accordance with the DNA fragmentation data, caspase-3 activity could also be demonstrated in cytosolic extracts from staurosporine-treated Mutu-BL III cells (Figs 2 and 3B).
Because both of these agents involve as yet undefined events leading up to caspase-3 activation, we also wanted to determine if cleavage activity in resistant cell extracts could be generated by direct proteolytic processing of caspase-3. To this end, naı̈ve detergent extracts were preincubated with purified granzyme B followed by DEVD-pNA cleavage analysis. Granzyme B was able to induce approximately 214 and 258 units of caspase-3–like protease activities in both susceptible and resistant cytosolic extracts, respectively (Fig3C). Matched extracts preincubated in the absence of granzyme B displayed a baseline, but minimal, activity. In the absence of cytosolic extract, granzyme B was unable to cleave the substrate eliminating the possibility that granzyme B was responsible for the increased activity and consistent with DEVD not being a preferred sequence motif for this enzyme (Fig 3C).45 Taken together, these data clearly show that caspase-3 and related proteases can be activated and functional in Fas-resistant BL cells and suggest that the inhibition to the Fas pathway occurs proximal to caspase-3 activation.
DISC formation.
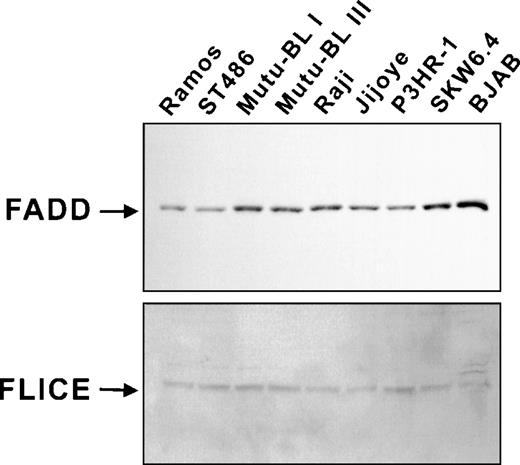
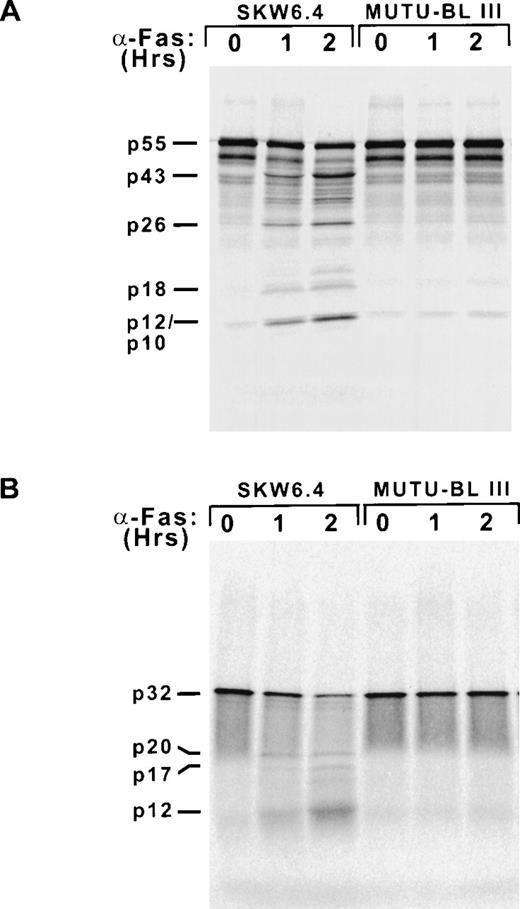
The fact that Fas-resistant group III BL cell lines could be induced to undergo apoptosis by agents activating effector caspases implied the presence of an inhibitory mechanism specific for the Fas pathway in this system, specifically acting upstream of the activation of caspase-3 and caspase-7. Therefore, it was critical to determine the integrity of the Fas death-inducing signaling complex in the BL milieu. Whole-cell lysates were examined by immunoblot analysis and demonstrated that the adapter protein FADD (26 kD) and caspase-8 (55 kD) were both present in all cell lines examined (Fig 4). We next wanted to determine if cross-linking Fas on BL cells leads to the formation of a functional DISC. This was examined indirectly with an in vitro caspase processing assay. The pro-forms of caspase-8 and caspase-3 were in vitro transcribed and translated in the presence of [35S]methionine, incubated with 16K cytosolic extracts of Fas-treated SKW6.4 and Mutu-BL III cells, and analyzed by SDS-PAGE and autoradiography (Fig 5). This approach was used rather than immunoblotting due to its higher sensitivity. As expected, Fas-activated SKW6.4 extracts had the capacity to process procaspase-8 (p55) into its active subunits (p18 and p10) as well as the prodomain (p26) (Fig 5A). The p43 intermediate results from the release of the p12 subunit after the initial caspase-8 cleavage event occurring at Asp374. In addition, these extracts were able to convert procaspase-3 (p32) into the p20 processing intermediate (representing the prodomain and p17 subunit after cleavage of p12) and the active p17 and p12 subunits (Fig 5B). This ability clearly illustrated the presence of endogenous active caspase-8 (p18/p10)2 in these extracts. In addition, these extracts probably contained appreciable amounts of the Fas DISC by virtue of their use of35S-labeled caspase-8 as a substrate.14 In contrast to the results above, extracts of anti-Fas–treated Mutu-BL cells were unable to convert caspase-8 or caspase-3 into fully active subunit forms or generate cleavage intermediates. This not only indicates the absence of active caspase-8 subunits, but also the failure of a functional DISC to have formed in response to Fas receptor cross-linking on BL cells. Furthermore, the failure to generate the p43 caspase-8 processing intermediate argues against the presence of a cellular counterpart of the cowpox crmA serpin/caspase inhibitor, as p43 can be generated in crmA-overexpressing cells.14
DISC components are present in Fas-resistant cell lines. Expression of FADD and caspase-8 was assessed by immunoblot analysis as described in Materials and Methods.
DISC components are present in Fas-resistant cell lines. Expression of FADD and caspase-8 was assessed by immunoblot analysis as described in Materials and Methods.
Caspase-8 and caspase-3 are not activated in Fas-stimulated BL cells. Cytosolic extracts made from untreated (0) and Fas-induced (1 and 2 hours) SKW6.4 and Mutu-BL III cells.35S-labeled caspases were generated by coupled in vitro transcription/translation driven by the T7 promoter of the pcDNA3 expression construct. Cleavage reactions were initiated by combining labeled (A) caspase-8 or (B) caspase-3 with the cytosolic extracts (25 μg) and incubated at 37°C for 2 hours. Reactions were terminated by the addition of Laemmli sample buffer and subjected to 15% SDS-PAGE and autoradiography.
Caspase-8 and caspase-3 are not activated in Fas-stimulated BL cells. Cytosolic extracts made from untreated (0) and Fas-induced (1 and 2 hours) SKW6.4 and Mutu-BL III cells.35S-labeled caspases were generated by coupled in vitro transcription/translation driven by the T7 promoter of the pcDNA3 expression construct. Cleavage reactions were initiated by combining labeled (A) caspase-8 or (B) caspase-3 with the cytosolic extracts (25 μg) and incubated at 37°C for 2 hours. Reactions were terminated by the addition of Laemmli sample buffer and subjected to 15% SDS-PAGE and autoradiography.
Fas-susceptible and -resistant BL cells have similar caspase-8/FLICE/MACH isoform profiles.
The absence of caspase-8 processing activity in Fas-resistant Mutu-BL III cells could have been due to the presence of death-attenuating caspase-8/FLICE/MACH isoforms associating with the DISC. This was a particularly attractive hypothesis because these were originally cloned from Raji BL cells and inhibited Fas-induced apoptosis after transfection.12 An RT-PCR approach similar to that used for their cloning was used to compare the various isoform transcripts present in Fas-sensitive and -resistant cell lines. In general, the transcript encoding the full-length α1 isoform could be detected in all cell lines analyzed and was represented by the 1,939-, 1,606-, and 1,556-bp products generated with the 188, 521, and 575 sense primers, respectively (Fig 6). However, under the PCR conditions used, only the third α-specific primer combination (ie, 1,556-bp product) was able to yield a prominent amplification product in the Fas-resistant cell line Mutu-BL III. The other 2 caspase-8/FLICE/MACH α isoforms, notably the α3 isoform, which could protect against Fas-mediated apoptosis, were not present (Fig6).12 This would have appeared as a 1,300-bp amplification product using the third pair of PCR primers (ie, 575.S and 930.AS). Furthermore, this assay indicated that caspase-8/FLICE/MACH α1 expression in Fas-susceptible SKW6.4 cells was somewhat higher than in resistant Mutu-BL. In addition, FLICE/MACH β3 and β4 isoforms lacking most of the ICE/CED-3 caspase homology domain were not only detected in Fas-susceptible cell lines, but at higher levels than in resistant cells. In conclusion, an inhibitory caspase-8 isoform (ie, α3) is not responsible for the observed lack of DISC activity and consequential acquisition of Fas resistance in BL cells.
and β Caspase-8 (FLICE/MACH) isoforms are expressed in both Fas-susceptible and -resistant cell lines. First-strand cDNA was prepared from total RNA isolated from the indicated cell lines. Amplification reactions were performed with the indicated sense primers complimentary to sequences shared by both and β isoforms and isoform-specific antisense primers. Products were then separated on 1.2% TAE-ethidium bromide agarose gels and photographed.
and β Caspase-8 (FLICE/MACH) isoforms are expressed in both Fas-susceptible and -resistant cell lines. First-strand cDNA was prepared from total RNA isolated from the indicated cell lines. Amplification reactions were performed with the indicated sense primers complimentary to sequences shared by both and β isoforms and isoform-specific antisense primers. Products were then separated on 1.2% TAE-ethidium bromide agarose gels and photographed.
The equilibrium between caspase-8 and FLIPL levels regulates susceptibility to Fas-mediated apoptosis in BL cells.
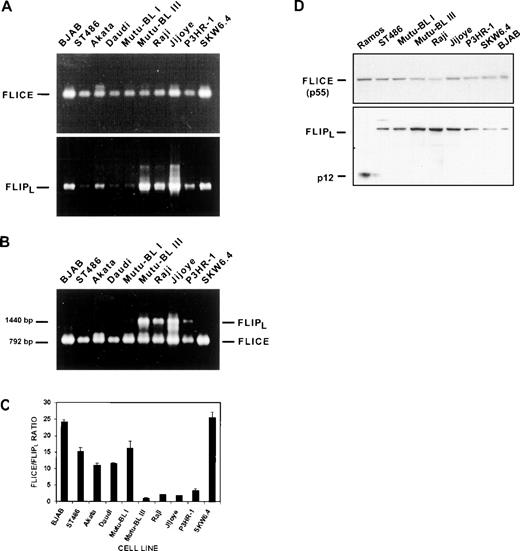
The results presented above suggested that cellular FLIP was a strong candidate for mediating Fas resistance in this system: (1) Fas-crosslinked BL cells lacked DISC activity despite the presence of all its components and (2) the confirmation of the integrity of apoptosis pathways distal to caspase-3 activation. Ten B-cell lines were screened by RT-PCR for FLIPL expression (Fig 7A). All of the resistant EBV-positive BL cell lines (ie, Mutu-BL III, Raji, Jijoye, P3HR-1) expressed the mature transcript encoding the long form of FLIP (1,440 bp). The larger amplification products, especially visible in the Jijoye cDNA sample, were cloned, sequenced, and determined to be unprocessed FLIPL transcripts (data not shown). However, FLIPL was easily detectable in the two most Fas-sensitive lines (ie, SKW6.4 and BJAB) as well. This result suggested that the presence of FLIPL might not be the sole factor responsible for conferring resistance on these cells. Moreover, it has been demonstrated that FLIPL overexpression can lead to apoptosis via caspase-8 activation, while only moderate expression was necessary for inhibition.33,35 36 RT-PCR analysis of the same samples performed with primer pairs specific for the caspase-8 caspase-homology domain (792 bp) showed higher caspase-8 expression in the BJAB and SKW6.4 cell lines compared with all of the BL lines (Fig7A). This implied that the equilibrium between caspase-8 and FLIPL levels might regulate Fas-induced apoptosis. This was tested directly by performing caspase-8/FLIPL competitive PCR (CFCP) analysis. We chose this method because it could simultaneously compare the levels of transcripts for each protein in each cDNA sample. A built-in endpoint for the reaction was provided by limiting concentrations of dNTPs. If primers were the limiting reagent, cDNAs from both transcripts could theoretically be amplified equally regardless of their starting concentrations. All samples were subjected to this analysis using the primer pairs for both caspase-8 and FLIPL described above. Caspase-8 was the primary amplification product in this assay system using cDNA samples from the 3 Fas-susceptible cell lines BJAB, ST486, and SKW6.4 (Fig 7B). This was the case with the EBV-positive, but Fas-negative, group I BL cell lines as well. In contrast, amplification of cDNA templates from all 4 resistant group III cell lines yielded products for both caspase-8 and FLIPL (Fig 7B). To provide a quantitative characterization of this apparent distinction between sensitive and resistant cell lines, we analyzed the ethidium-stained PCR products by scanning densitometry and calculated the caspase-8/FLIPL ratio from the relative values obtained (Fig 7C). Considering only the Fas-positive cell lines, the resulting graph was identical in appearance to that in Fig 1 depicting percentage apoptosis in response to Fas ligation. The results of the caspase-8/FLIPLcompetitive PCR analysis indicate a strong correlation between a low caspase-8/FLIPL ratio (ie, ≤5) and Fas resistance in group III BL cells. Immunoblot analysis supported these results by showing heightened FLIPL protein expression in group III BL cell extracts compared with those of EBV-negative BL and nonmalignant SKW6.4 lymphoblastoid cell lines (Fig 7D). Although the Ramos cell line was negative for expresssion of full-length FLIPL in these experiments, a 12-kD species (p12) was observed. Presumably, this represents the processed FLIPL carboxyl-terminal p12 subunit, as the antibody used in this analysis was specific for this domain. However, the mechanism of its generation is unclear.
FLIPL mediates Fas-resistance in EBV-positive Burkitt’s lymphoma cell lines. (A) FLIPL transcripts were detected by RT-PCR analysis of total RNA samples. The amplification product corresponding to the mature 1,440-bp coding sequence is indicated. Higher-molecular-weight products representing unprocessed transcripts were also detected and can be seen in the Mutu-BL III and Jijoye lanes. FLICE/caspase-8 transcript levels were determined with primers specific for the caspase-homology domain (792-bp). (B) Caspase-8/FLIPL competitive PCR analysis. FLIPLand FLICE/caspase-8 transcript levels were directly compared in the same sample by performing RT-PCR reactions containing primer pairs for both genes under conditions of limiting dNTPs. Gene-specific products and their sizes are indicated on the right and left, respectively. (C) The FLICE/FLIPL ratios were calculated from arbitrary values obtained by scanning densitometry of the gel from (B) and are graphically represented. Data were obtained from scans using 3 different exposures. Results are expressed as mean ± standard deviation and are representative of at least 3 separate experiments. (D) Direct comparison of FLICE/caspase-8 and FLIPL protein expression. Immunoblot analysis was performed on a panel of EBV-negative and EBV-positive BL cell lysates. The same membranes were used for analysis of both FLICE/caspase-8 and FLIPL by sequential antibody hybridizations. The 55-kD forms of both proteins are indicated, as well as the FLIPL p12 cleavage product predominant in the Ramos cell line.
FLIPL mediates Fas-resistance in EBV-positive Burkitt’s lymphoma cell lines. (A) FLIPL transcripts were detected by RT-PCR analysis of total RNA samples. The amplification product corresponding to the mature 1,440-bp coding sequence is indicated. Higher-molecular-weight products representing unprocessed transcripts were also detected and can be seen in the Mutu-BL III and Jijoye lanes. FLICE/caspase-8 transcript levels were determined with primers specific for the caspase-homology domain (792-bp). (B) Caspase-8/FLIPL competitive PCR analysis. FLIPLand FLICE/caspase-8 transcript levels were directly compared in the same sample by performing RT-PCR reactions containing primer pairs for both genes under conditions of limiting dNTPs. Gene-specific products and their sizes are indicated on the right and left, respectively. (C) The FLICE/FLIPL ratios were calculated from arbitrary values obtained by scanning densitometry of the gel from (B) and are graphically represented. Data were obtained from scans using 3 different exposures. Results are expressed as mean ± standard deviation and are representative of at least 3 separate experiments. (D) Direct comparison of FLICE/caspase-8 and FLIPL protein expression. Immunoblot analysis was performed on a panel of EBV-negative and EBV-positive BL cell lysates. The same membranes were used for analysis of both FLICE/caspase-8 and FLIPL by sequential antibody hybridizations. The 55-kD forms of both proteins are indicated, as well as the FLIPL p12 cleavage product predominant in the Ramos cell line.
Overexpression of caspase-8 renders group III BL cells susceptible to Fas-mediated apoptosis.
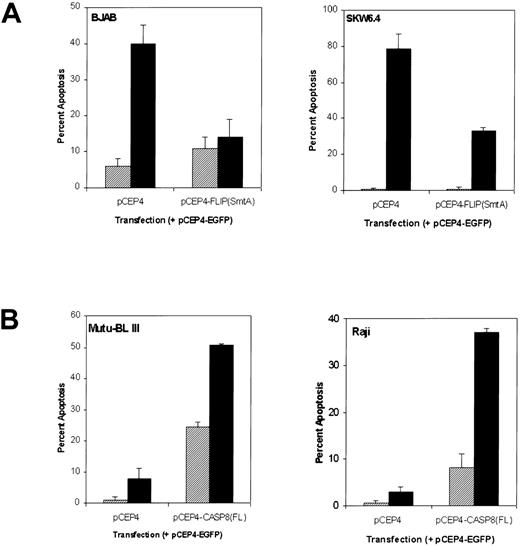
To further validate our hypothesis that the equilibrium between caspase-8 and FLIPL regulates Fas-mediated apoptosis, particularly Fas-resistance in group III BL cell lines, we manipulated the caspase-8/ FLIPL ratio by cotransfecting resistant and susceptible cells with either caspase-8 or FLIPL expression constructs, respectively, in combination with an EGFP construct. Transient overexpression of FLIPL protected both BJAB and EBV-positive SKW6.4 cells from Fas-mediated cell death as shown by a 65% and 58% reduction in apoptosis compared with that of vector-transfected controls, respectively (Fig 8A). Importantly, overexpression of caspase-8 in Fas-resistant Mutu-BL III and Raji cells resulted in heightened susceptibility to Fas-mediated apoptosis, 25% and 29%, respectively (Fig 8B). In addition, caspase-8 overexpression induced spontaneous apoptosis of Mutu-BL III cultures, accounting for an additional 25% cell death. These results support a FLIPL-mediated mechanism of Fas-resistance in EBV-positive BL cell lines, which can be overcome by increasing the expression of caspase-8 and in turn the caspase-8/ FLIPL ratio.
Susceptibility of BL cell lines to Fas-mediated apoptosis can be regulated by caspase-8 and FLIPL. (A) Percent apoptosis in Fas-sensitive BJAB and SKW6.4 cell lines cotransfected with pCEP4-FLIPL (SmtA) plus the pCEP4-EGFP reporter construct. After incubation for 72 hours, the transfected cultures were either left untreated (▨) or treated with anti-Fas IgM (500 ng/mL, ▪) for an additional 8 hours. Percentage apoptosis was measured in 300 EGFP-positive cells using a combination of fluorescence and phase microscopy as described in Materials and Methods. (B) The Fas-resistant BL cell lines Mutu-BL III and Raji were cotransfected with pCEP4-CASP8(FL) and pCEP4-EGFP expression constructs. Transient transfection and evaluation of Fas-mediated apoptosis was performed as described in (A).
Susceptibility of BL cell lines to Fas-mediated apoptosis can be regulated by caspase-8 and FLIPL. (A) Percent apoptosis in Fas-sensitive BJAB and SKW6.4 cell lines cotransfected with pCEP4-FLIPL (SmtA) plus the pCEP4-EGFP reporter construct. After incubation for 72 hours, the transfected cultures were either left untreated (▨) or treated with anti-Fas IgM (500 ng/mL, ▪) for an additional 8 hours. Percentage apoptosis was measured in 300 EGFP-positive cells using a combination of fluorescence and phase microscopy as described in Materials and Methods. (B) The Fas-resistant BL cell lines Mutu-BL III and Raji were cotransfected with pCEP4-CASP8(FL) and pCEP4-EGFP expression constructs. Transient transfection and evaluation of Fas-mediated apoptosis was performed as described in (A).
DISCUSSION
The results presented implicate the FLICE-inhibitory protein as a causative factor in the resistance to Fas-mediated apoptosis displayed by EBV-positive Burkitt’s lymphoma cell lines. The ability of FLIP to inhibit Fas signal transduction is apparently influenced by the level of caspase-8 expression in that the magnitude of the caspase-8/FLIPL expression ratio correlates directly with Fas susceptibility. Furthermore, the decrease in the caspase-8/FLIPL ratio and the acquisition of Fas resistance is not a general feature of all Burkitt’s lymphomas, but only induced upon EBV infection and subsequent progression from group I to the lymphoblastoid/group III phenotype (Figs 1 and 7C). This is a response specific to BL cells, as LCLs (eg, SKW6.4) established from EBV transformation of nonmalignant B lymphocytes exhibit a high caspase-8/FLIPL ratio and profound sensitivity to the cytotoxic effects of Fas triggering. Because FLIPL can block signals emanating from several death receptors, the EBV-induced shift of the caspase-8/FLIPL ratio in favor of BL apoptosis resistance represents an added survival advantage and novel mechanism of viral tumorigenesis.
EBV also engages and initiates a variety of antiapoptotic mechanisms as a result of latency gene expression in lymphoblastoid cells. LMP1 is known to be critical to growth transformation and mimics proliferative signals by the binding of p80 TNF receptor-associated factors/proteins (TRAFs) and subsequent activation of NF-kappa B.46-48 As a result, LMP1 can also induce resistance to TNF-α– and p53-mediated apoptosis by NF-kappa B-mediated transcriptional activation of the gene for the A20 zinc finger protein.47,49 However, A20 does not inhibit Fas-induced apoptosis.50 LMP1 also induces upregulation of Bcl-2 by an NF-kappa B-independent mechanism providing an additional survival advantage to LCLs and group III BL cell lines under suboptimal growth conditions. It has previously been shown that high LMP1 expression can also be toxic, suggesting a role for Bcl-2 in antagonizing LMP1-mediated apoptosis.51,52 EBV also encodes its own Bcl-2 homologue, BHRF1, which functions during the lytic cycle to delay cell death induced by viral replication.53However, Bcl-2- or BHRF1-transfected BJAB B cells were not protected from Fas-mediated apoptosis.54
Although EBV gene expression demonstrably contributes to immortalization, transformation, and/or oncogenicity, it is not clear how the virus imparts Fas resistance on BL cells. A search of the protein and nucleic acid databases failed to identify homologues to known viral or cellular inhibitors of receptor-mediated apoptosis encoded by the EBV genome. Instead, we propose EBV might arrest Fas signal transduction by cooperating with a BL-specific protein to transcriptionally modulate caspase-8 and FLIPL levels to yield a low caspase-8/FLIPL ratio in a manner similar to EBNA-3C augmentation of c-Ha-ras transformation of rat fibroblasts.55 In this system, this is potentially the primary mechanism of Fas signal attenuation because a potentialcrmA-like inhibitor was ruled out by the ability of granzyme B to activate caspase-3 activity in Fas-resistant cell extracts (Fig 3C). Furthermore, the complete lack of caspase-8 activity in Fas-stimulated resistant cells (Fig 5A) is consistent with the functioning of FLIPL proximal to caspase-8 activation. In the presence of a crmA-like protein, the initial cleavage at Asp374 generating the p43 intermediate still would have occurred.14
Our data provide insight into a novel tumorigenic mechanism of EBV involving the development of resistance to Fas-mediated apoptosis through the antagonistic regulation of caspase-8 and FLIP expression. This might be yet another example of how EBV functionally integrates with its host machinery to ensure its survival. While EBV infection of primary B lymphocytes consistently leads to a stable lymphoblastoid phenotype, the result with Burkitt’s lymphomas is not as predictable.56 EBV-positive BL cells can be phenotypically unstable during long-term passage due to their ability to repress the expression of viral proteins (eg, LMP1), adhesion molecules, and class I major histocompatibility antigens. Restriction of EBV gene expression in BL is a common occurrence and may represent a mechanism of evasion from immune system recognition, as well as contributing to resistance to cytotoxic T lymphocyte (CTL)-mediated killing.57-59 The activation of FLIPL and downregulation of caspase-8 might contribute to this resistance by inhibition of the Fas component of CTL-mediated killing. This may also be of critical importance in EBV-associated B lymphomas that do not display a restricted pattern of EBV gene expression occurring in immunocompromised patients.60 In conclusion, we propose that death receptor-induced cell death of EBV-positive group III BL cell lines is inhibited via a novel mechanism, which uses FLIPL. Future studies will be aimed at confirming the validity of this putative mechanism and elucidating the mechanism by which the EBV-malignant B-cell interaction modulates caspase-8 and FLIPLexpression.
ACKNOWLEDGMENT
The authors thank Dr Maria Mudryj (UC Davis School of Medicine) for careful reading of the manuscript and Dr Andrew P. Spicer (UC Davis School of Medicine) for helpful advice regarding transfection. We are also grateful to Dr Vishva M. Dixit (Genentech, Inc, South San Francisco, CA) for the gifts of the BJAB cell line, ICE-LAP3 antibody, and caspase-8 and Yama expression constructs. We also thank Drs Alan B. Rickinson (University of Birmingham, Birmingham, UK) and Jeffrey Sample (St Jude’s Children’s Hospital, Memphis, TN) for supplying the Mutu-BL cell lines.
Supported by Grant No. AR41053 (to M.F.S.) from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Michael F. Seldin, MD, PhD, Rowe Program in Genetics, Department of Biological Chemistry, University of California-Davis, 4303 Tupper Hall, One Shields Ave, Davis, CA 95616; e-mail: mfseldin@ucdavis.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal