Abstract
Interleukin-12 (IL-12) is a potent proinflammatory and immunoregulatory cytokine skewing T lymphocytes to express a type 1 cytokine pattern. Optimal expression of IL-12 mRNA and bioactivity in vitro requires specific priming of monocytes by interferon-γ (IFN-γ) or granulocyte-macrophage colony-stimulating factor (GM-CSF) before lipopolysaccharide (LPS) stimulation. We show here for the first time that the production of IL-12 by IFN-γ– or GM-CSF–primed human monocytes can be completely suppressed by preincubation with LPS (fromEscherichia coli Serotype 055:B5) for 6 to 24 hours before the priming procedure. A dose-dependent suppression of IL-12p70 was measured on the levels of intracellular cytokine production and cytokine secretion. mRNA studies on the expression of p40 and p35 showed an LPS-induced downregulation of both subunits. The results of several different experimental approaches suggest that IL-12 downregulation was not due to endogenous IL-10, IL-4, prostaglandin E2 (PGE2), tumor necrosis factor- (TNF-), or nitric oxide (NO) production induced by LPS. Moreover, preincubation of monocytes with LPS did not lead to a downregulation of the CD14 antigen, which is an LPS receptor. LPS preincubation in this experimental setting did not result in a general hyporesponsiveness of the monocytes, as IL-6 production as well as IFN-γ–induced upregulation of CD54 did not decline. Downregulation of IL-12 was not due to changes in mRNA stability. These findings show that the immunoregulatory important cytokine, IL-12, underlies itself a complex regulation.
INTERLEUKIN-12 (IL-12) is a 75-kD heterodimeric cytokine that plays a crucial role in both the innate and the acquired immune response. The major cells producing IL-12 were found to be macrophages/monocytes, but other cell types have also been reported to produce IL-12. These include dendritic cells, neutrophils, keratinocytes, and murine mast cells.1
The two covalently disulfide-linked N-glycosylated polypeptide chains of approximately 40 kD (p40) and 35 kD (p35) are encoded by two separate genes2 located on different chromosomes and are independently regulated.1,3 Both subunits have to be produced within the same cell to obtain the biologically active dimer.2 In contrast to p40, p35 is only secreted as part of the p70 heterodimer. The secreted monomeric p40 has no biologic activity, whereas the p40 homodimer p(40)2 binds to the IL-12 receptor, but does not transduce a signal, thus acting as an IL-12 antagonist.4
Although initially described as a cytokine activating cytotoxic lymphocytes spontaneously, the major biological significance of IL-12 secretion lies in its effects on T-helper cells. IL-12 is known to drive Th1 reactions in physiological and pathological immune responses, mainly mediated by its capacity to stimulate growth and interferon-γ (IFN-γ) production in T cells and natural killer (NK) cells.5-7 Some investigators even consider this cytokine to be an obligatory factor for Th1 generation and proliferation.8 9
In addition to the promotion of Th1 cell development, IL-12 has a broad range of biologic activities including the regulation and proliferation of T and NK cells, the differentiation of CD8+ T cells, and hematopoiesis. Its involvement in autoimmunity has been shown in different diseases. When applied in vivo, IL-12 was shown to enhance the resistance to bacterial and parasitic infections, to promote antitumor immunity, and to influence antiviral responses including human immunodeficiency virus (HIV) in vivo or in vitro. Modulation of IL-12–dependent signaling may provide a therapeutic option for altering the Th1-Th2 balance in allergic and autoimmune diseases, as well as in other conditions.
Bacteria, bacterial products including lipopolysaccharide (LPS), lipoteichonic acid, protein extracts, heat shock proteins, and intracellular parasites have been described to induce production of IL-12 by macrophages/monocytes.1,10 11
The most potent inhibitor of IL-12 synthesis by macrophages/monocytes appears to be IL-10, which acts at the protein, as well as at the mRNA level. IL-10 negatively regulates IL-12p40 expression/accumulation.12,13 Similar to IL-10, addition of prostaglandin E2 (PGE2) to cultures of LPS-stimulated human peripheral blood mononuclear cell (PBMC) downregulates IL-12 production.14 IL-4, IL-13, and transforming growth factor-β (TGF-β) can also suppress the production of IL-12 when added simultaneously with bacterial inducers. In contrast, pretreatment with IL-4 or IL-13 followed by activation with LPS enhances IL-12 production.15
In this study, we show that the well-known induction of IL-12 production with LPS also underlies a complex regulation, with a sequence-dependent reaction to known stimuli. Preincubation with LPS can efficiently inhibit the production of bioactive IL-12 by monocytes stimulated with a combination of IFN-γ or granulocyte-macrophage colony-stimulating factor (GM-CSF) and LPS.
MATERIALS AND METHODS
Cytokines and reagents.
All cytokines were used as purified recombinant human preparations. Human IFN-γ was kindly provided by Dr Karl Thomae GmbH (Biberach an der Riss, Germany), LPS was derived from Escherichia coli(E coli) Serotype 055:B5 (Sigma, Deisenhofen, Germany), GM-CSF (Genzyme, Rüsselsheim, Germany) anti–IL-4 antibody useful for neutralization of human IL-4 bioactivity with a low endotoxin level (R&D Systems, Wiesbaden, Germany), neutralizing rat anti-human IL-10 monoclonal antibody (MoAb) with a low endotoxin level (PharMingen, Hamburg, Germany), neutralizing anti-human tumor necrosis factor-α (TNF-α) antibody with a low endotoxin level (R&D Systems), indomethacin (Serva, Braunschweig, Germany), N-methyl-L-arginine (NMLA) (Sigma); actinomycin D (Sigma), paclitaxel (PTXL; Taxol from Taxus barcarta, Bristol Arzneimittel GmbH, München, Germany). For the 0.82 mmol/L stock solution of PTXL, the endotoxin content was less than 25 pg/mL as determined by the Limulus amoebocyte lysate assay.
Isolation and culture of human monocytes.
PBMC from healthy donors were separated by Ficoll-Hypaque density gradient centrifugation and resuspended in Iscove medium supplemented with 4% AB serum (IAB medium). Monocytes were isolated by adherence on petri dishes (Heraeus, Hannover, Germany). After 3 hours (37°C, 5% CO2), the nonadherent cells were removed by several washes with phosphate-buffered saline (PBS) and the adherent cell population was detached. Detached cells were washed and resuspended in IAB medium and then incubated in round bottom 96-well microtiter plates for several hours. Monocytes were subsequently exposed to LPS or medium for 24 hours (here referred to as “preincubation”). After a change of culture medium, cells were primed with IFN-γ (300 U/mL) or GM-CSF (100 ng/mL) 1 to 2 hours before additon of LPS (50 ng/mL) (in the following referred to as “stimulation”) for various times as indicated in the Results. When indicated in the Results, monocytes were further purified after the adherent step using Dynabeads M-450 Sheep anti-mouse IgG (Dynal, Hamburg, Germany). Briefly, cells were incubated with a combination of CD2 and CD21 antibodies (mouse IgG1; Dako, Hamburg, Germany) for 2 hours at 4°. The protocol was then followed according to the suppliers’ instructions. The resulting cell preparations contained up to 90% monocytes, as assessed by CD14 staining and fluorescence-activated cell sorting (FACS) analysis.
mRNA isolation and reverse transcription (RT).
mRNA was isolated from 100,000 monocytes/well using a mRNA isolation kit (“mRNA direct isolation kit”; Dynal) according to the supplier’s instructions. Resulting Poly(A)+ RNA was stored at −80°C. RNA was then subjected to first-strand cDNA synthesis using Oligo(dT)15 for full-length cDNA synthesis. The RT reaction mixture contained final concentrations of 50 U Expand-RT (Boehringer-Mannheim, Mannheim, Germany), 20 U of RNase inhibitor (RNase out; Life Technologies, Eggenheim, Germany), 10 mmol/L dithiothreitol (DTT), 1× first-strand RT buffer for Expand-RT, 0.5 mmol/L of each deoxynucleoside triphosphate (dNTP) (Boehringer-Mannheim), and 80 pmol Oligo(dT)15 (Boehringer-Mannheim). To control for DNA contamination, cDNA synthesis was performed in the absence of RT. First-strand cDNA was stored at −20°C.
Semiquantitative polymerase chain reaction (PCR).
A method of immunochemical detection of DNA fragments with digoxigenin-labeled samples and biotinylated capture probes was used as previously described.16 For PCR amplification, the resulting cDNA was either diluted and labeled with dig-NTP (PCR-enzyme–linked immunosorbent assay [ELISA], Dig-Labeling kit; Boehringer-Mannheim) at a final concentration of 200 μmol/L or undiluted cDNA without further addition of NTPs was amplified. The PCR reaction mixture contained a final concentration of 1 mmol/L MgCl2, 0.1 μmol/L of the specific primers and 1.25 U Taq-Polymerase (Life Technologies). Primers specific for IL-12p40 and p35 were purchased from Biosource (Ratingen, Germany) and Stratagene (Heidelberg, Germany), respectively. For β-actin, the following primers were used (forward: 5′GAGCGGGAAATCGTGCGTGACATT, reverse: 5′GAAGGTAGTTTCGTG-GATGCC). PCR reactions were performed for 28 cycles with annealing temperatures of 52°C for p40, 62°C for p35, and 60°C for β-actin. To control for saturation effects of the PCR reaction, preliminary experiments were assayed at different cycle numbers by removing part of the reaction at appropriate times.
Detection.
An aliquot of each PCR reaction was subjected to electrophoresis on a 2% agarose gel (Qualex Gold, AGS, Heidelberg, Germany) stained with ethidium bromide, visualized, and photographed under ultraviolet (UV) illumination. The expected size of amplified fragments was 225 bp for β-actin, 290 bp for p40, and 414 bp for p35.
Semiquantitative detection of the signals was performed using the PCR-ELISA detection kit (Boehringer-Mannheim) according to the supplier’s instructions. Briefly, the diluted amplicon solution was subjected to a hybridization with 21 bp biotinylated capture probes that specifically bind at sites between the primer sequences (for β-actin: 5′BiotGTGGCCATC-TCTTGCTCGAA, for p35: 5′BiotCCGTGGCTGAGGTCTTGTCGG, for p40: 5′BiotGCT-GACGGCCCTCAGCAGGTT). The hybridization mixture was then incubated in streptavidin-coated tubes. Using antidigoxigenin-horseradish peroxidase (HRP) conjugate and the substrate (ABTS) as described in the protocol, the optical density was measured at 422 nm. Positive controls (the kit is provided with a control reaction consisting of human genomic DNA and PCR primers for the tissue plasminogen activator [tPA] gene [to control for the “dig-labeling” step] as well as with a digoxigenin-labeled PCR product and a biotin-labeled capture probe that is complementary to the control PCR fragment [to control for the “detection”-step and as quantitative control]) and negative controls (water and PCR without prior RT) were analyzed along with the probes.
Flow cytometric analysis of intracellular cytokines.
Intracellular staining and quantification of cytokines was performed with modifications as previously described.17 During the stimulation procedure, brefeldine (Sigma) was added at 3 μg/mL. Cells were harvested and washed twice in PBS and then fixed with 4% ice-cold phosphate-buffered paraformaldehyde for 15 minutes at 4°C and washed in PBS. To facilitate diffusion of Ab through the cell membranes, cells were permeabilized in PBS with 0.1% saponin (Riedel de Haen, Seelze, Germany) for 15 minutes. Thereafter, pretitrated cytokine-specific MoAb diluted in the permeabilization buffer (PBS-saponin) were added and incubated for 45 min at 4°. The PE-conjugated cytokine specific MoAb and IgG1 isotype control MoAb were used at final concentrations of 2 μg/mL (monoclonal mouse anti-human IL-12 [p40/p70]: this antibody reacts with human IL-12p40 monomer and with the p70 heterodimer, but not with the p35 monomer; monoclonal rat anti-human-IL-6; PharMingen). After subsequent washings in permeabilization buffer, cells were resuspended and measured in PBS by flow cytometric analysis. Expression of surface antigens on monocytes was assessed using labeled CD14 (Coulter-Immunotech, Hamburg, Germany) or CD54 (anti-intercellular adhesion molecule [ICAM]-1; Coulter-Immunotech) antibodies. Samples were analyzed on a FACScan flow cytometer (Becton Dickinson, Heidelberg, Germany). Results were analyzed using Cellquest software (Becton Dickinson).
Cytokine determinations.
IL-12p70 ELISA was performed using an IL-12p70 detection kit (R&D Systems), which recognizes only the IL-12p70 heterodimer. The same samples were analyzed in another ELISA specific for p40 (R&D Systems) to determine the amount of non-p70 bound-p40. IL-10 production was measured by ELISA (Laboserv, Giessen, Germany).
RESULTS
Dose-dependent suppression of IL-12 production by preincubation with LPS.
Stimulation of monocytes with 300 U/mL IFN-γ 2 hours before addition of 10 to 50 ng/mL of LPS (designated as “stimulation” in this report) resulted in a marked increase of IL-12 production measured on mRNA level, as well as on protein level. “Stimulation” for 24 hours resulted in 23% (mean value) of monocytes stained positive for intracellular IL-12 expression (v <0.5% unstimulated cells) and a mean IL-12p70 contents of 35 pg/mL in the supernatants. Similar results were obtained using GM-CSF instead of IFN-γ. In unstimulated cells, IL-12p70 was undetectable with the ELISA used. LPS alone (ie, without a priming dose of IFN-γ or GM-CSF) did not induce any detectable IL-12 response on the protein level, as has been shown by others.3
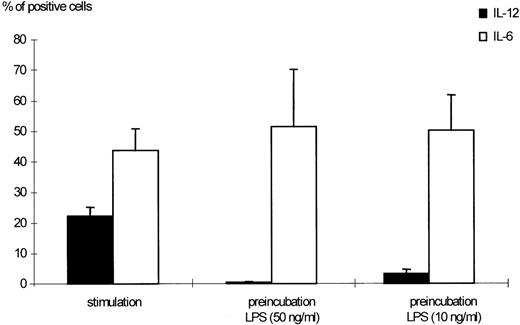
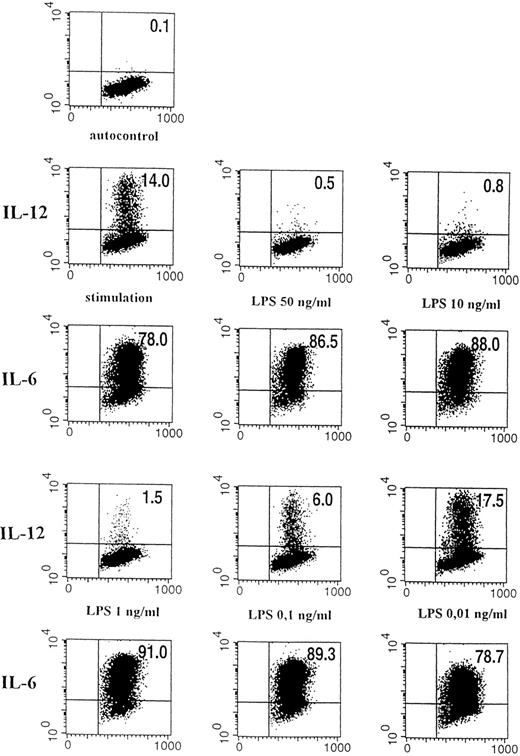
Preexposure of monocytes to LPS before the stimulation procedure (designated as “preincubation” in this report) significantly decreased the production of bioactive IL-12. As shown in Fig 1, preincubation with 50 ng/mL LPS suppressed intracellular IL-12 production to nearly undetectable levels. A clear dose dependency of this effect was evident (Figs 1 and2), with a marked suppression of intracellular IL-12 production using LPS doses down to 1 ng/mL. A total of 0.1 ng/mL LPS still caused a reduction of more than 50%, while 0.01 ng/mL LPS did not alter IL-12 production as compared with the positive control (ie, “stimulation” without “preincubation”).
Effects of LPS preincubation on intracellular IL-12 (p40/p70) production by human monocytes. Human monocytes were stimulated with 300 U/mL IFN-γ and 50 ng/mL LPS for 24 hours. Preincubation was performed as indicated in Materials and Methods using 50 and 10 ng/mL LPS, respectively. The percentages of monocytes positive for IL-12 (p40/p70) or IL-6, as detected by flow cytometric measurement, are shown. Results are given as mean ± standard error of mean (SEM) of 13 different donors. The downmodulation of IL-12 by preincubation with LPS was significant (P < .0001 when 50 ng/mL LPS were used, P < .005 for 10 ng/mL LPS).
Effects of LPS preincubation on intracellular IL-12 (p40/p70) production by human monocytes. Human monocytes were stimulated with 300 U/mL IFN-γ and 50 ng/mL LPS for 24 hours. Preincubation was performed as indicated in Materials and Methods using 50 and 10 ng/mL LPS, respectively. The percentages of monocytes positive for IL-12 (p40/p70) or IL-6, as detected by flow cytometric measurement, are shown. Results are given as mean ± standard error of mean (SEM) of 13 different donors. The downmodulation of IL-12 by preincubation with LPS was significant (P < .0001 when 50 ng/mL LPS were used, P < .005 for 10 ng/mL LPS).
Dose-dependent effect of LPS on intracellular IL-12 (p40/p70) expression. Flow cytometric analysis of stimulated human monocytes (IFN-γ 300 U/mL, LPS 50 ng/mL) pretreated for 24 hours with varying concentrations of LPS. Intracellular cytokine staining for IL-12 (p40/p70) and IL-6 is shown. The quadrant markers for the dot plot were set based on the negative staining controls using isotype-matched Ig antibodies. The percentages of cells in the corresponding quadrants are given. On the horizontal axis, forward scatter is depicted, and on the vertical axis, binding of PE-labeled anti–IL-6 or anti–IL-12 (p40/p70) antibody is shown. The corresponding results for IL-12 and IL-6 are given. (Top) Negative control (autofluorescence of monocytes); (first two lines left panel) stimulation with IFN-γ/LPS for 24 hours without LPS preincubation; (middle) preincubation with 50 ng/mL LPS before stimulation; (right) preincubation with 10 ng/mL LPS; (last two lines left panel) 1 ng/mL LPS; middle: 0.1 ng/mL LPS; right: 0.01 ng/mL LPS.
Dose-dependent effect of LPS on intracellular IL-12 (p40/p70) expression. Flow cytometric analysis of stimulated human monocytes (IFN-γ 300 U/mL, LPS 50 ng/mL) pretreated for 24 hours with varying concentrations of LPS. Intracellular cytokine staining for IL-12 (p40/p70) and IL-6 is shown. The quadrant markers for the dot plot were set based on the negative staining controls using isotype-matched Ig antibodies. The percentages of cells in the corresponding quadrants are given. On the horizontal axis, forward scatter is depicted, and on the vertical axis, binding of PE-labeled anti–IL-6 or anti–IL-12 (p40/p70) antibody is shown. The corresponding results for IL-12 and IL-6 are given. (Top) Negative control (autofluorescence of monocytes); (first two lines left panel) stimulation with IFN-γ/LPS for 24 hours without LPS preincubation; (middle) preincubation with 50 ng/mL LPS before stimulation; (right) preincubation with 10 ng/mL LPS; (last two lines left panel) 1 ng/mL LPS; middle: 0.1 ng/mL LPS; right: 0.01 ng/mL LPS.
Suppression was not due to downmodulation of the CD14 antigen as determined by flow cytometric analysis of receptor expression after LPS preincubation (81.6%; standard deviation [SD], 11.4%; n = 3, of gated cells stained positive) as compared with LPS-free medium (86.0%; SD, 4.42%; n = 3).
In this experimental setting, suppression was selective for IL-12, as IL-6 downregulation was not seen under the described experimental conditions. Figures 1 and 2 show the data obtained for IL-12 (p40/p70) in comparison to IL-6. A general state of hyporesponsiveness to the priming signal can be ruled out, as the upregulation of CD54 induced by IFN-γ was even further increased in cells preincubated with LPS (Fig 3).
Effect of LPS preincubation on ICAM-1 (CD54) expression by stimulated monocytes. The binding of fluorescein isothiocyanate (FITC)-labeled CD54 antibodies to monocytes is shown. An overlay histogram is given to compare membrane expression of CD54 on unstimulated cells (filled histogram), monocytes stimulated with IFN-γ (300 U/mL) (A) or IFN-γ/LPS (B) (open histogram, thin line) and IFN-γ (A) or IFN-γ/LPS (B) stimulated monocytes preincubated with LPS for 24 hours (open histogram, thick line). One representative experiment of 3 is depicted.
Effect of LPS preincubation on ICAM-1 (CD54) expression by stimulated monocytes. The binding of fluorescein isothiocyanate (FITC)-labeled CD54 antibodies to monocytes is shown. An overlay histogram is given to compare membrane expression of CD54 on unstimulated cells (filled histogram), monocytes stimulated with IFN-γ (300 U/mL) (A) or IFN-γ/LPS (B) (open histogram, thin line) and IFN-γ (A) or IFN-γ/LPS (B) stimulated monocytes preincubated with LPS for 24 hours (open histogram, thick line). One representative experiment of 3 is depicted.
LPS preincubation did not exclusively interact with IFN-γ–induced signal cascade. With GM-CSF as a priming signal, similar results were obtained: LPS preincubation-induced suppression of IL-12 (p40/p70) on monocytes primed with 100 ng/mL GM-CSF instead of IFN-γ was over 95% in 2 experiments.
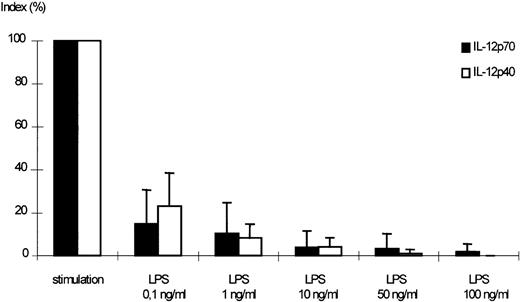
A dose-dependent reduction of IL-12p70 secretion was observed in supernatants of monocytes, which had been preincubated with LPS in varying concentrations before stimulation (Fig 4).
Dose-dependent effect of LPS on the secretion of IL-12p70 and p40 by stimulated monocytes. Monocytes were pretreated with medium alone or varying concentrations of LPS for 24 hours. After washing, cells were challenged with IFN-γ (300 U/mL) and LPS (50 ng/mL) for 24 hours, culture supernatants were harvested, and assayed for IL-12p70 and p40 accumulation by ELISA. A stimulation index was calculated using LPS/IFN-γ–stimulated cells without pretreatment as positive control (100%; mean of p70 positive control: 35.4 pg/mL, mean of p40 positive control: 1,331 pg/mL). Mean values of 5 independent experiments ± SEM are shown. The downmodulation of IL-12p40 and p70 was significant (P < .05) for all LPS concentrations.
Dose-dependent effect of LPS on the secretion of IL-12p70 and p40 by stimulated monocytes. Monocytes were pretreated with medium alone or varying concentrations of LPS for 24 hours. After washing, cells were challenged with IFN-γ (300 U/mL) and LPS (50 ng/mL) for 24 hours, culture supernatants were harvested, and assayed for IL-12p70 and p40 accumulation by ELISA. A stimulation index was calculated using LPS/IFN-γ–stimulated cells without pretreatment as positive control (100%; mean of p70 positive control: 35.4 pg/mL, mean of p40 positive control: 1,331 pg/mL). Mean values of 5 independent experiments ± SEM are shown. The downmodulation of IL-12p40 and p70 was significant (P < .05) for all LPS concentrations.
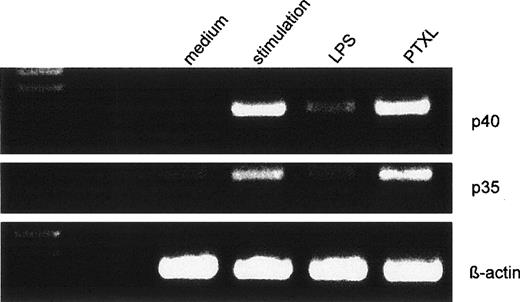
On the mRNA level, a dose-dependent suppression could be seen for IL-12p40 and p35 accumulation when 10 ng/mL to 100 ng/mL LPS were used (Fig 5 shows a representative experiment).
Dose-dependent suppression of p40 and p35 mRNA expression by LPS pretreatment. Human monocytes were preincubated with varying concentrations of LPS before stimulation with IFN-γ/LPS for 5 hours. Amplified cDNA fragments for β-actin, p40, and p35 were resolved on a 2% agarose gel and visualized by ethidium bromide. The amplified cDNA fragments had the expected lengths of 290 bp for p40, 414 bp for p35, and 225 bp for β-actin.
Dose-dependent suppression of p40 and p35 mRNA expression by LPS pretreatment. Human monocytes were preincubated with varying concentrations of LPS before stimulation with IFN-γ/LPS for 5 hours. Amplified cDNA fragments for β-actin, p40, and p35 were resolved on a 2% agarose gel and visualized by ethidium bromide. The amplified cDNA fragments had the expected lengths of 290 bp for p40, 414 bp for p35, and 225 bp for β-actin.
The LPS-induced IL-12 downregulation was already detectable after a “preincubation” period of only 2 hours and could still be observed when supernanants were collected 48 hours after the stimulation procedure, indicating that recovery is not fast. In some experiments, monocytes were preincubated for 24 hours with LPS, washed, and incubated for another 6 to 48 hours before stimulation with IFN-γ/LPS. In these experiments, the suppression was still the same, showing that LPS-induced IL-12 hyporesponsiveness is long-lasting (data not shown).
Both IL-12 subunits are suppressed by LPS preincubation.
Figures 4 and 6 show that p40 suppression was in parallel to p70. p40 production after stimulation without preincubation with LPS was 25-fold to 40-fold higher than p70 production (as has been described by others1,10 13).
Effects of blocking IL-10, IL-4, NO, or prostaglandins on LPS-induced IL-12p70 and IL-12p40 downregulation. Monocytes were incubated with LPS (50 ng/mL) or with LPS and neutralizing anti–IL-10 antibody (10 μg/mL), anti–IL-4 antibody (10 μg/mL), indomethacin (1 × 10−6 mol/L) or NMLA (10 μmol/L) for 24 hours. Supernatants were removed and cells in fresh medium were stimulated with IFN-γ (300 U/mL) and LPS (50 ng/mL) for another 24 hours. The mean values of p70 and p40 levels in the supernatants of at least 3 separate experiments are shown. Bars indicate SEM.
Effects of blocking IL-10, IL-4, NO, or prostaglandins on LPS-induced IL-12p70 and IL-12p40 downregulation. Monocytes were incubated with LPS (50 ng/mL) or with LPS and neutralizing anti–IL-10 antibody (10 μg/mL), anti–IL-4 antibody (10 μg/mL), indomethacin (1 × 10−6 mol/L) or NMLA (10 μmol/L) for 24 hours. Supernatants were removed and cells in fresh medium were stimulated with IFN-γ (300 U/mL) and LPS (50 ng/mL) for another 24 hours. The mean values of p70 and p40 levels in the supernatants of at least 3 separate experiments are shown. Bars indicate SEM.
As shown in Figs 5 and 7, a clear reduction of IL-12p40 and p35 mRNA accumulation was observed on stimulation with IFN-γ/LPS for 5 hours after LPS preincubation. A marked suppression of p40 and p35 mRNA was seen in LPS preincubated and GM-CSF/LPS–stimulated monocytes as compared with monocytes stimulated with GM-CSF/LPS alone (data not shown). Relative quantities of both subunits were determined by PCR-ELISA. A significant reduction 5 hours after stimulation is shown in Fig 8. A significant inhibition was still observed for both subunits 24 hours after stimulation (data not shown). Kinetic experiments showed a peak in IL-12p40 mRNA accumulation between 5 and 8 hours after stimulation with IFN-γ/LPS (Fig 8). LPS-pretreated monocytes reacted to IFN-γ/LPS stimulation with a maximal IL-12p40 mRNA accumulation between 5 and 8 hours as well, although on a lower absolute level (Fig8). Using actinomycin D (5 μg/mL), which blocks transcription, we could not detect any difference in RNA stability between LPS pretreated and nonpretreated monocytes: in both experimental settings, a marked decline of mRNA specific for IL-12p40 was observable 6 to 8 hours after addition of actinomycin D.
Effects of LPS or PTXL pretreatment on the mRNA expression for IL-12p40 and p35 in human monocytes. Human monocytes were preincubated with LPS (50 ng/mL) or PTXL (10 μmol/L) for 24 hours before stimulation with IFN-γ/LPS. Five hours after the stimulation procedure, mRNA was isolated and expression of p40, p35, and β-actin was determined by RT-PCR. The resulting cDNA fragments were resolved on a 2% agarose gel and visualized by ethidium bromide. The amplified cDNA fragments had the expected lengths of 290 bp for p40, 414 bp for p35, and 225 bp for β-actin. One representative of 5 independent experiments is shown.
Effects of LPS or PTXL pretreatment on the mRNA expression for IL-12p40 and p35 in human monocytes. Human monocytes were preincubated with LPS (50 ng/mL) or PTXL (10 μmol/L) for 24 hours before stimulation with IFN-γ/LPS. Five hours after the stimulation procedure, mRNA was isolated and expression of p40, p35, and β-actin was determined by RT-PCR. The resulting cDNA fragments were resolved on a 2% agarose gel and visualized by ethidium bromide. The amplified cDNA fragments had the expected lengths of 290 bp for p40, 414 bp for p35, and 225 bp for β-actin. One representative of 5 independent experiments is shown.
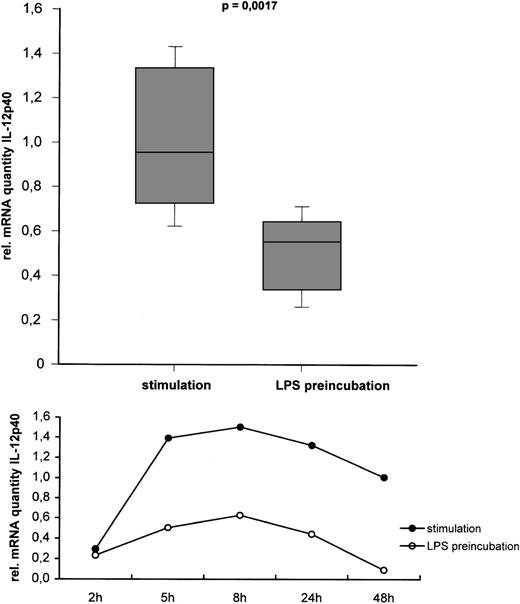
Effect of LPS pretreatment on IL-12p40 mRNA expression. RT-PCR analysis of IL-12p40 in monocytes stimulated with and without prior exposure to LPS for 24 hours. Cells were harvested 5 hours after the stimulation procedure. Relative quantities of RT-PCR signal for p40 as determined by PCR-ELISA followed by normalization to β-actin are shown. Results are given as box plots with vertical lines displaying the 10th and 90th percentile. Horizontal lines show the median of the results of 10 independent experiments. For statistical analysis, the Mann-Whitney Rank Sum Test was performed. A representative kinetic experiment shows IL-12p40 mRNA, which was harvested at different time points between 2 hours to 48 hours after the stimulation procedure.
Effect of LPS pretreatment on IL-12p40 mRNA expression. RT-PCR analysis of IL-12p40 in monocytes stimulated with and without prior exposure to LPS for 24 hours. Cells were harvested 5 hours after the stimulation procedure. Relative quantities of RT-PCR signal for p40 as determined by PCR-ELISA followed by normalization to β-actin are shown. Results are given as box plots with vertical lines displaying the 10th and 90th percentile. Horizontal lines show the median of the results of 10 independent experiments. For statistical analysis, the Mann-Whitney Rank Sum Test was performed. A representative kinetic experiment shows IL-12p40 mRNA, which was harvested at different time points between 2 hours to 48 hours after the stimulation procedure.
LPS-induced IL-12 suppression is not due to enhanced endogenous production of IL-10, IL-4, PGE2, TNF-α, or nitric oxide (NO).
IL-10, IL-4, and PGE2 have been shown to downregulate IL-12 production under defined experimental conditions. We therefore tested whether endogenous production of these mediators might be responsible for the LPS-induced IL-12 downregulation using indomethacin or neutralizing antibodies for IL-4 and IL-10, respectively. The downregulatory effect of LPS remained stable in the presence of neutralizing anti–IL-10, anti–IL-4 antibodies, and the prostaglandin synthesis inhibitor, indomethacin, respectively, during preincubation with a secretion of IL-12 diminished to nearly undetectable levels. Figure 6 summarizes the amounts of IL-12 in the supernatants as determined by an ELISA specific for p70 and p40, respectively. Figure 9 shows the results obtained for intracellular cytokine staining.
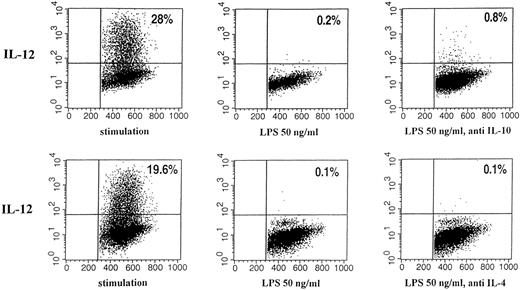
Effect of neutralizing anti–IL-10 and anti–IL-4 antibody on intracellular IL-12 (p40/p70) staining of human monocytes pretreated with LPS or medium. Immunofluorescence was performed with human monocytes stimulated as described for 24 hours. The binding of phycoerythrin (PE)-labeled mouse anti-human IL-12 (p40/p70) is shown (vertical; horizontal: forward scatter). Quadrants were set according to the isotype-matched controls. The percentages of cells in the corresponding quadrants are given. (Left) Stimulation; (middle) preincubation with 50 ng/mL LPS; (right) preincubation with 50 ng/mL LPS and neutralizing IL-10 antibody or neutralizing IL-4 antibody, respectively.
Effect of neutralizing anti–IL-10 and anti–IL-4 antibody on intracellular IL-12 (p40/p70) staining of human monocytes pretreated with LPS or medium. Immunofluorescence was performed with human monocytes stimulated as described for 24 hours. The binding of phycoerythrin (PE)-labeled mouse anti-human IL-12 (p40/p70) is shown (vertical; horizontal: forward scatter). Quadrants were set according to the isotype-matched controls. The percentages of cells in the corresponding quadrants are given. (Left) Stimulation; (middle) preincubation with 50 ng/mL LPS; (right) preincubation with 50 ng/mL LPS and neutralizing IL-10 antibody or neutralizing IL-4 antibody, respectively.
The LPS preincubation induced effect could be seen on monocytes obtained via adherent step, as well as on highly purified monocytes using Dynabeads.
Using an ELISA specific for IL-10, it could be shown that IL-10 production increased after LPS/IFN-γ stimulation. LPS preincubation did not, however, further induce IL-10 secretion (data not shown).
NO and TNF-α have both been implicated in IL-12 suppression in the murine system. The NO-synthase inhibitor NMLA (Fig 6), as well as a neutralizing anti-human TNF-α antibody, had no effect on the reduction of IL-12 on preincubation with LPS. The mean IL-12 (p40/p70) inhibition seen with LPS preincubation in the presence of 5 μg/mL anti-human TNF-α neutralizing antibody was 98.7% (±1.3% SD) as compared with 99.4% (±0.9% SD) inhibition without the antibody, as determined in 3 independent experiments by intracellular cytokine staining.
Suppression of IL-12 production is induced by PTXL preincubation on intracellular level, but not on mRNA level.
The ability of PTXL, known to stimulate monocytes via a similar intracellular transduction pathway as LPS, to downregulate IL-12 production was tested in some experiments in parallel to LPS. In contrast to LPS, we detected no downregulation of p40 and p35 mRNA neither 5 nor 24 hours after the stimulation procedure (Fig 7). On the intracellular level, however, we detected a marked downregulation of IL-12 (p40/p70) (Fig 10) using 5 to 20 μmol/L PTXL. IL-6 production remained stable (Fig 10) when monocytes were preincubated with the same doses of PTXL before IFN-γ/LPS stimulation. Cells were shown to survive the stimulation procedure in separate experiments (data not shown).
Effects of PTXL preincubation on the intracellular IL-12 (p40/p70) production of human monocytes. Monocytes were preincubated with medium, LPS (50 ng/mL), or PTXL (10 μmol/L) for 24 hours. Subsequently, cells were stimulated with 300 U/mL IFN-γ and 50 ng/mL LPS for another 24 hours. The percentage of monocytes positive for IL-12 (p40/p70) or IL-6 as detected by flow cytometric measurement are shown. Results are given as mean ± SEM of 5 independent experiments, performed with cells from different donors.
Effects of PTXL preincubation on the intracellular IL-12 (p40/p70) production of human monocytes. Monocytes were preincubated with medium, LPS (50 ng/mL), or PTXL (10 μmol/L) for 24 hours. Subsequently, cells were stimulated with 300 U/mL IFN-γ and 50 ng/mL LPS for another 24 hours. The percentage of monocytes positive for IL-12 (p40/p70) or IL-6 as detected by flow cytometric measurement are shown. Results are given as mean ± SEM of 5 independent experiments, performed with cells from different donors.
DISCUSSION
In the present study, we provide evidence that the important proinflammatory and immunoregulatory cytokine, IL-12, can be selectively downregulated by preincubation with LPS before stimulation with IFN-γ or with GM-CSF and LPS.
Endotoxic LPS, a major component of the outer membrane of gram-negative bacteria, is known to activate the immune system by inducing the release of inflammatory mediators. Monocytes/macrophages require a priming “signal” before LPS stimulation for the optimal production of proinflammatory mediators. Previously, it was described that IFN-γ efficiently primes monocytes for IL-12 production in response to LPS,3 18 which could be confirmed in this study.
LPS can induce a state of hyporesponsiveness to its own effects, a phenomenon mediated by monocytes/macrophages.19 An LPS-induced “tolerance” has been described for TNF, IL-6, IL-8, IL-10, NO, and other cytokines,20-27 characterized by a diminished synthesis of these mediators by monocytes. This phenomenon has been extensively studied in various animal species and cell types in vitro and in vivo, but the cellular and molecular changes that contribute to this kind of induced hyporesponsiveness are not fully understood. It has been demonstrated that the sequence, amount, and duration of stimuli given is crucial in this context. LPS-induced tolerance appears to be a complex process and may vary considerably in different experimental models. The ability of LPS to inhibit subsequent induction of IL-12p70 is a new finding. This may be of particular importance because of the crucial role of IL-12 in acute bacterial infections, in autoimmune disorders, and in the development of specific immunity against a number of intracellular pathogens. Moreover, our findings point to the necessity to deplete LPS from reagents in studies, which address the regulation of IL-12.
In further experiments, we examined whether endogenous production of known IL-12 antagonists play a causal role in LPS-mediated IL-12 suppression.
The most potent inhibitor of IL-12 synthesis by monocytes/macrophages appears to be IL-10, which acts at the protein as well as at the mRNA level.12,13 IL-10 itself can be affected by LPS-induced hyporesponsiveness.28 Randow et al28 have identified IL-10 and TGF-β as an endogenous mediator for LPS-mediated TNF downregulation. In contrast, we did not observe an enhancement of IL-12 production by neutralization of endogenously produced IL-10 in our experimental setting.
IL-4 is another antagonist of proinflammatory monokines, which has been shown to downregulate IL-12,13,15 as well as the expression of CD14 in normal human monocytes.29 Similar to IL-10, the addition of neutralizing anti–IL-4 antibody to monocyte cell cultures failed to overcome the LPS-mediated suppression of IL-12 release. Moreover, LPS-induced IL-12 downregulation was also observed in highly purified monocytes containing only small amounts of potentially IL-4 producing lymphocytes, which suggests that T-cell–derived IL-4 is not causal for the observed effects.
Recently, it was reported that PGE2 is a potent downregulator of IL-12p40 subunit production by LPS-stimulated monocytes in whole blood cultures.14 Both IL-12 and PGE2 are secreted by monocytes, macrophages, and other antigen presenting cells (APC) in response to a variety of compounds, including bacterial products.30 Indomethacin did not revert the suppressive effect of LPS, which excludes a role of endogenously produced PGE2 in our experiments. This is in accordance with the studies by Bodgan et al,20 who could not define a role of PGE2 in LPS-mediated suppression of IFN-γ–induced reactive nitrogen intermediates (RNI) release.
It is well known that LPS can induce the expression of the TNF receptor on murine macrophages, as well as the secretion of TNF protein,31 suggesting that LPS effects are in part mediated by the autocrine action of TNF-α.
IL-12p40 gene expression in murine macrophages has been shown to be also regulated by NO.32 It has been discussed that endogenous NO is involved in LPS-induced desensitization of mouse macrophages.33 In the murine system, both TNF-α34 and NO35 have been shown to be involved in IL-12 suppression in certain conditions. Using the NO-synthase inhibitor, NMLA, and a neutralizing anti-human TNF-α antibody, respectively, we were able to show that in our experimental setting, neither molecule is responsible for the LPS-mediated suppression of IL-12 production.
LPS-induced cytokine downregulation may function in at least 2 different ways in monocytes. LPS preincubation may either induce a general “hyporesponsiveness”/“desensitization” affecting a broad range of proinflammatory responses or it may deviate the monocyte in a specific way. Bogdan et al20 pointed to the difference between LPS hyporesponsiveness in which monocytes/macrophages exposed to a primary dose of LPS become refractory to a challenge dose of LPS, and the suppressive activity that LPS exerts on certain mediators, such as NO. The data presented in this report point to a restricted modulation of monocyte functions.
In our experimental setting, LPS pretreatment did not result in a complete desensitization of monocytes, as IL-6 was not affected by LPS-mediated downregulation. This is in accordance with an in vivo study of Mackensen et al,22 who found a marked downregulation of TNF, IL-8, G-CSF, M-CSF, but not of IL-6 after repeated injections of LPS. Mengozzi et al23 also demonstrated IL-6 to be unaffected by LPS tolerance in human monocytes. In different experimental settings, however, others found IL-6 to be affected by downregulation in vitro27 and in vivo.21
A general state of hyporesponsiveness to the priming signal can also be ruled out, as the upregulation of CD54 on IFN-γ priming was increased in monocytes preincubated with LPS.
Furthermore, we were able to show that LPS preincubation did not interfere exclusively with IFN-γ signal transduction pathways, as the same effect on IL-12 expression was observable when GM-CSF instead of IFN-γ was used as a priming signal.
Enhanced expression of IL-12p40 leads to formation of the p40 homodimer, which has been shown in a murine cell system to antagonize IL-12 activities.36 Experiments with recombinant (p40)2 indicate that an about 10-fold to 100-fold excess of IL-12(p40)2 over IL-12 is required to achieve a 50% to 90% inhibition of the effects of IL-12 on Th1 cells.1 In accordance with the findings of others,3,13 we found that both subunits of IL-12 are inducible by stimulation with IFN-γ/LPS and that both are downregulated on LPS preincubation. We found that p40 parallels the downregulation of p70 protein. On the mRNA level, a reduction of both p35 and p40 accumulation was observed, which confirms very recent data by Karp et al.37 With the methodical approach performed, there is no indication for this mRNA reduction to be regulated on the level of mRNA stability.
We used amplification by RT-PCR to facilitate detection of the mRNA species, arguing that although RT-PCR is a nonquantitative method, large differences in the intensity of the bands obtained with the same primer set should nonetheless reflect significant differences in the expression levels of the amplified mRNA. We have checked to be within the linear range with the cycle numbers performed. Using the PCR-ELISA, our main point was to depict differences between two samples within the same experiment. With this semiquantitative method, absolute mRNA amounts were not determined, as a competetive PCR was not performed.
LPS-induced IL-12 suppression was not due to downmodulation of the LPS receptor, CD14. This is in accordance with published data.21,26,38 Other investigators were able to detect an LPS-induced downmodulation of CD14 expression.23Differences may be due to different stimulation schemes and incubation times, as well as different sources of monocytes.
Furthermore Fahmi et al,39 who examined the LPS-binding sites in LPS-tolerant mouse macrophages, found no correlation between desensitization of macrophages to endotoxin effects and downregulation of LPS-binding sites. They concluded that downregulation of LPS receptors is not a prerequisite for the induction of LPS tolerance, and that different LPS substructures can downregulate differently the various responses of the cells to LPS, thus suggesting that different pathways are operative for induction of endotoxin tolerance.
LPS and the antitumor agent, PTXL, induce similar responses in murine macrophages, although there are no obvious structural similarities between the 2 agents. Like LPS, PTXL provides a “second” signal for macrophage activation to tumoricidal activity.40PTXL-induced macrophage stimulation requires an LPS-responsive genetic background41 (most probably the wild-type Tlr-4 [LPS] receptor42) and PTXL-induced macrophage stimulation could be blocked by well-characterized LPS antagonist.43 These data imply an intimate association of LPS and PTXL signaling mechanisms leading to macrophage activation.
PTXL interacts with the cytoskeleton by binding on β-tubulin and stabilizing microtubules. Probably other molecular targets and signaling mechanisms of PTXL are involved in the reaction by which PTXL mimics LPS effects in macrophages. In our study, we found a PTXL-induced IL-12 downregulation on the protein, but not on the mRNA level. This indicates that downregulation of IL-12 is a complex process.
The herein described suppression of IL-12 production by preincubation with LPS seems to be specific for LPS and cannot be completely mimicked by PTXL, despite similar signaling mechanisms. Because different substructures of LPS can have different downregulatory effects on macrophages (at least in murine models36), the identification of the responsible “active side” in LPS-induced IL-12 suppression should be addressed in further studies.
Sutterwala et al44 have recently demonstrated that ligation of phagocytic receptors like the scavenger and Fcγ receptor may be an important downmodulator of IL-12 mRNA synthesis and protein secretion. In addition, we could recently show that the anaphylatoxin C5a leads to a marked downregulation of IL-12 in IFN-γ–primed monocytes.45 In conclusion, the clearance of extracellular bacteria or opsonized particles, the contact of monocytes/macrophages to C5a, or a LPS-induced state of hyporesponsiveness may contribute to the cessation of IL-12 production. This downmodulation can potentially influence the development of immunity to both extracellular and intracellular pathogens.
LPS can act as a bifunctional modulator on IL-12 production, exerting either enhancing or suppressive effects, depending on its concentration, sequence, and duration of its addition with respect to costimulators. The complete elucidation of the cellular events induced by LPS will aid in understanding inflammatory processes and may potentially lead to novel therapeutic strategies in sepis, shock, allergic, and autoimmune diseases.
Supported by DFG Grants No. SFB 244, C13, and DFG We 1289/2-1.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Miriam Wittmann, MD, Hannover Medical University, Department of Dermatology and Allergology, Ricklinger Str. 5, D-30449 Hannover, Germany.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal