Abstract
We have previously demonstrated that PU.1 is required for the production of lymphoid and myeloid, but not of erythroid progenitors in the fetal liver. In this study, competitive reconstitution assays show that E14.5 PU.1−/− hematopoietic progenitors (HPC) fail to sustain definitive/adult erythropoiesis or to contribute to the lymphoid and myeloid lineages. PU.1−/−HPC are unable to respond synergistically to erythropoietin plus stem cell factor and have reduced expression of c-kit, which may explain the erythroid defect. Fluorescently labeled,PU.1−/−, AA4.1+, fetal liver HPC were transferred into irradiated recipients, where they demonstrated a severely impaired ability to home to and colonize the bone marrow.PU.1−/− HPC were found to lack integrins 4 (VLA-4/CD49d), 5 (VLA-5/CD49e), and CD11b (M). Collectively, this study has shown that PU.1 plays an important role in controlling migration of hematopoietic progenitors to the bone marrow and the establishment of long-term multilineage hematopoiesis.
ALL BLOOD CELLS originate from a common progenitor termed the hematopoietic pluripotent stem cell (HSC). These cells have the capacity to self-renew, provide radioprotection, and long-term repopulating activity.1 Fetal hematopoiesis is a dynamic developmental process dominated by migrating populations of hematopoietic stem cells, progenitors, and precursors.2 The earliest detectable site of hematopoiesis occurs in the yolk sac at day 7. This stage of fetal hematopoiesis, referred to as embryonic or primitive hematopoiesis, is characterized by the generation of nucleated erythrocytes and a limited number of primitive macrophages. The adult/definitive hematopoietic system is thought to be generated from an intraembryonic site. At approximately day 8, hematopoietic activity can be detected in the para-aortic splanchopleura (PAS), which later develops into the aortic-gonad-mesonephros region (AGM).3,4 At day 9, the fetal liver (FL) becomes the active site of fetal hematopoiesis and remains the dominant site until the early neonatal period.5 The shift to the FL involves an initial wave of colonization by primitive erythroid and erythro-myeloid progenitors emanating from the yolk sac, followed by further waves of colonization consisting of circulating committed precursors and uncommitted multipotent progenitors from the PAS/AGM regions.6 Definitive HSC arise intraembyronically in the AGM region at day 10 and subsequently migrate to the yolk sac and FL.7-10 The frequency of HSC peaks in the FL at day 14.5 and then decreases as hematopoiesis gradually shifts to the bone marrow, the principal site of hematopoiesis in the adult.9 11
Regulation of HSC renewal, proliferation, and differentiation is still a poorly understood process. HSC are thought to be subjected to both stochastic and instructive elements within the hematopoietic microenvironment. The microenvironment consists of cellular contacts mediated by a myriad of different adhesion molecules and their cognate ligands together with locally produced cytokines. These factors act together to modulate stem cell differentiation, proliferation, and survival. Changes in the microenvironment that occur as HSC migrate from the FL to bone marrow may be responsible for a number of pronounced phenotypic and functional differences between fetal and adult HSC.1 The importance of the microenvironment has recently been highlighted by the demonstration that the developmental potential of HSC could be reprogrammed by changing their microenvironment.12 Little is known about the molecular mechanisms that enable different hematopoietic microenvironments to alter the functional properties of HSC during different stages of gestation. However, one key regulatory step modulating HSC function is transcription factor-dependent alterations in gene expression. Defining which transcription factors modulate gene expression in HSC is, therefore, fundamental to our understanding of hematopoiesis.
We and others have used gene targeting in mice to probe the function of transcription factors during hematopoiesis.13 The transcription factor PU.1, a member of the ets family of DNA binding proteins, is expressed only in the hematopoietic system.14 Targeted mutagenesis of the PU.1 gene causes a late gestational embryonic lethal phenotype and a profound defect in yolk sac and FL hematopoiesis.15-17 Extensive functional and phenotypic analyses have shown the total absence of lymphoid and myeloid lineages, but normal numbers of both erythroid and megakaryocytic progenitors in the mutants. These results suggest that PU.1 is required for the function of a multipotential lymphoid-myeloid progenitor population in the FL. Furthermore, normal levels of erythropoiesis and megakaryopoiesis in the FL and yolk sac indicate that hematopoiesis was properly initiated at both sites. Additional experimentation, consisting of FL adoptive transfer experiments and the generation of embryonic stem (ES) stem-cell–derived chimeric mice, established that the PU.1mutation is cell intrinsic and cannot be rescued by a wild-type (WT) microenvironment.17 The inability ofPU.1−/− FL cells to offer short-term multilineage reconstitution and radioprotection provided the first indication of a possible dysfunction in the FL HSC compartment. The short duration of the radioprotection assay prevented any further conclusions concerning the long-term repopulating potential ofPU.1−/− HSC. In addition, the ability of PU.1−/− ES cells to contribute to the erythroid lineage in fetal chimeras but not in adult chimeras suggests that the requirement for PU.1 may change during hematopoietic development.17
In this report, we have initiated a series of experiments to study the functional behavior of PU.1−/− fetal HSC and multipotent hematopoietic progenitor cells (HPC) in the adult microenivironment. Competitive repopulation assays (CRA) showed thatPU.1−/− HSC are incapable of establishing long-term repopulation of the erythroid, lymphoid, or myeloid lineages. The lack of contribution suggests thatPU.1−/− fetal HSC are at a competitive disadvantage compared with WT adult HSC in the bone marrow.PU.1−/− HPC failed to respond synergistically to erythropoietin (Epo) and stem cell factor (SCF), which play an important role during normal definitive erythropoiesis. The expression of c-kit onPU.1−/− HPC was severely reduced. This may explain the inability of PU.1−/−HPC to sustain erythropoiesis in the bone marrow. Homing and engraftment studies demonstrated thatPU.1−/− HPC are severely impaired in their ability to migrate to and colonize the bone marrow.PU.1−/− HPC do not express a number of adhesion molecules, including α-integrins VLA-4/CD49d and VLA-5/CD49e, which have previously been shown to be important for HPC function. The lack of adhesion molecule expression may explain the dysfunctional properties of PU.1−/− fetal HPC in vivo. Collectively, this study establishes that PU.1 is required for proper migration and engraftment of HPC in the adult bone marrow and suggests a new role for PU.1 in maintaining definitive erythropoiesis.
MATERIALS AND METHODS
Mouse strains.
PU.1 WT (PU.1+/+ or PU.1+/−) and mutant (PU.1−/−) embryos were generated and genotyped as previously described.15C57BL/6-Ly 5.1 mice were obtained from Jackson Laboratories (Bar Harbor, ME).
Antibodies/fluorescence-activated cell sorting (FACS) analysis.
Flow cytometric analysis was undertaken on single-cell suspensions prepared from FL and peripheral blood samples as previously described.15 Cell samples were stained with either fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies (MoAbs) as stipulated by the manufacturer (Pharmigen, San Diego, CA). PE-conjugated MoAbs included RA3-6B2 (B220), RM4-5 (CD4), and M1/70 (CD11b/Mac-1). FITC-conjugated MoAb included A20 (Ly5.1/CD45.1), 104 (Ly5.2/CD45.2), and 2B8 (c-kit/CD117).
Erythroid progenitor assay.
E16.5 WT or PU.1−/− FL single-cell suspensions (2 × 105) were plated in 1.3 mL of a serum-free methylcellulose medium as described by Ratajczak et al.18Briefly, a methylcellulose mixture was prepared containing a final concentration of 0.9% methylcellulose, 1% delipidized bovine serum albumin, 270 μg/mL saturated transferrin, 20 μg/mL insulin, 5.6 μg/mL cholesterol, 2 mmol/L L-glutamine, 0.01% penicillin/streptomycin, and 100 μmol/L monothioglycerol. Methylcellulose cultures were supplemented with Epo (4 U/mL; Amgen, Thousand Oaks, CA) or Epo plus SCF (50 ng/mL; R&D Systems, Minneapolis, MN) to stimulate the generation of erythroid progenitors.
Hemoglobin (Hbb) assays.
Cystamine-modified hemoglobin samples were analyzed by cellulose acetate electrophoresis as previously described.17 After electrophoresis, cellulose acetate plates were stained in a 1% Ponceau S solution (Sigma Laboratories, St Louis, MO) containing 5% trichloroacetic acid for 5 minutes and rinsed in 5% acetic acid. Quantification was performed by scanning densitometry image analysis to determine relative contributions of donor versus recipient hemoglobin isoforms.
In utero HPC transplants.
PU.1+/− (Ly5.2) and WT (Ly 5.1) pairs were crossed to generate timed pregnancies. E14.5, AA4.1+ WT (Ly 5.1) HPCs were enriched by magnetic bead selection to a cell density of 105/5 μL as described below. Anesthetized pregnantPU.1+/− females had their uterine horns surgically exposed, and each embryo (Ly 5.2) was injected with 105 cells intraperitoneally via 60-μm glass needles through the uterine wall. After replacement of the uterine horns and suturing, the manipulated embryos were carried to term. Pups were genotyped at 3 weeks of age and were subsequently analyzed at 6 weeks to determine HPC contribution.
CRA.
Recipient animals (C57BL/6-Ly 5.1) were irradiated with a single dose of 960 rads from a 137Cs source. Donor cells (Ly 5.2) were prepared as pooled single-cell suspension (106/mL) in phosphate-buffered saline (PBS) from E14.5, PU.1 WT (+/+ or +/−) or mutant (−/−) FLs. Syngeneic competitive bone marrow cells (Ly 5.1) were prepared as a single-cell suspension in PBS. A mixture of 70% (5 × 105), 80% (1 × 106), or 90% (2 × 106) Ly 5.2 FL cells along with a radioprotective dose of syngeneic Ly 5.1 bone marrow cells (2 × 105) were retro-orbitally injected into anesthesized, lethally irradiated Ly 5.1 animals. After transplantation, the animals were maintained on water containing 20 mL/L of Sulfamethoxazole and Trimethoprim. On a monthly basis, approximately 0.2 mL of peripheral blood was obtained from recipient mice by tail bleeds. The harvested blood was mixed with an equal volume of 2% dextran sulfate, incubated at 37°C to precipitate erythrocytes, and treated with ammonium chloride lysis buffer to lyse any remaining erythrocytes. Mononuclear cells in the resulting supernatant were washed twice with PBS and analyzed by flow cytometry to detect Ly 5.2-derived lymphoid and myeloid cells. Contribution to the erythroid lineage was assessed by analyzing the presence of hemoglobin isoforms as described above.
Enrichment and fluorescein labeling of FL AA4.1+ HPC.
Single-cell suspensions were prepared in Dulbecco’s modified Iscove’s medium (DMIM) from E14.5 FL and pooled as either WT orPU.1−/− samples as described above. The AA4.1+ fraction was isolated from pooled FL cells by magnetic bead positive selection using MidiMACS separation columns and goat anti-rat IgG MicroBeads as recommended by Miltenyi Biotec (Auburn, CA). Briefly, pooled FL cells were incubated at a cell density of 5 × 106 to 1 × 107 cells/mL with saturating amounts of AA4.1 MoAb/MicroBeads for 30 minutes at 4°C. The AA4.1 MoAb/MicroBeads were prepared by mixing an AA4.1 MoAb, obtained from a hybridoma culture supernatant as previously described,17 with goat antirat IgG MicroBeads for 15 minutes at 4°C. The samples were loaded onto the MidiMACS separation columns, washed, and eluted as specified by the manufacturer. This procedure routinely resulted in a 100-fold enrichment of AA4.1+ cells.
For homing experiments, pooled FL cells were fluorescently labeled with carboxyfluorescein diacetate succinimidyl ester (CFDASE; Molecular Probes, Inc, Eugene, OR), as described.19 Briefly, 1 × 107 cells that have been extensively washed in PBS were incubated with a 10 μmol/L CFDASE solution for 10 minutes at room temperature while slowly agitating. The labeling reaction was quenched by adding an equal volume of newborn calf serum (NCS) and washing extensively with PBS. Alternatively, FL cells were fluorescently labeled using FITC (Isomer I; Molecular Probes) according to Butcher and Weissman.20 Briefly, cells at a density of 3 to 4 × 107 were labeled with a 60 μg/mL solution of FITC for 20 minutes at room temperature in a 1:1 mixture of complete DMIM and 1× PBS (pH 7.4) with a final serum concentration of 5%. After FITC labeling, the cells were washed with complete DMIM and centrifuged through a serum gradient. Labeled cells were passed through a Nitex membrane before selection with AA4.1 MoAb/MicroBeads.
Short-term homing and engraftment of HPC.
Fluorescein-labeled AA4.1+ cells (1 to 2 × 105) in 0.1 mL of PBS were transferred retro-orbitally into lethally irradiated C57B/6 recipients as described above. Recipient animals were killed 5 to 48 hours posttransfer, and single-cell suspensions were prepared from the bone marrow, liver, spleen, and thymus. For the bone marrow, cells were harvested from both the femur and tibia and subsequently pooled. After transfer of the cell suspensions to flat-bottom multiwell tissue plates, the number of fluorescent cells in the entire sample was detected visually using a Nikon (Melville, NY) Diaphot 300 inverted microscope with an external fluorescent light source. Phase contrast visualization was used to confirm the viable nature of the cells counted. Nontransfered fluorescein-labeled cells provided a staining control. After quantification of total cell numbers per sample, results were normalized and reported as fluorescent cells per 107 total cells.
Reverse transcriptase polymerase chain reaction (RT-PCR) analysis of HPC.
Total RNA was isolated from E14.5 AA4.1+ pooled FL cells using RNAzol (Tel-Test, Woodlands, TX) according to the manufacturer’s specification. Random-primed cDNA was synthesized from 2.0 μg of total RNA using the First Strand Synthesis Kit (Pharmacia Biotech, Uppsala, Sweden) as stipulated by the manufacturer. All PCR reactions were performed using 30 cycles and the following annealing temperature and primer pairs: α4/VLA-4 (62°C; forward, 5′-CTGCACAGCCACGG GTCGAAG-3′; reverse, 5′-GAGAT CTGAGAAG CCATCTGC-3′), α5/VLA-5 (57°C; forward, 5′-GGCTTCTCCGTCCAGT TTTAC-3′; reverse, 5′-GATGTAGACCCTG CCCAC CTCC-3′), β1(62°C; forward, 5′-G TGAACAGTGAAGACAT GGACGC-3′; reverse, 5′-GGTGAGATTGAAGTGGGA GCACTC-3′), β7 (57°C; forward, 5′-CCATCTGGACAGCAATGGTGTC-3′; reverse, 5′-GGAGGCAGTGAGTAGCTTGAAGAG-3′), CD11b/αM (57°C; forward, 5′-GACCCA GGTTACCGTCTACTAC-3′; reverse, 5′-TTCAGCACTGGGGTCCTTTC AAGC-3′), β2/CD18 (62°C; forward, 5′-CTTCCTGGGATCT GCTGT GTC-3′; reverse, 5′-CCAGC TTCTTGACGTTGTTGAGG-3′), hypoxanthine phosphoribosyl transferase (HPRT; 62°C; forward, 5′-CACAGGACTAGAACAC CTGC-3′; reverse, 5′-GCTGGTGAAAAGGACCTCT-3′). PCR products were fractionated in 2% agarose and examined further by Southern blot analysis when necessary using cDNA fragments as hybridization probes.
RESULTS
In utero transplantation of WT HSC rescues PU.1−/− animals from a lethal hematopoietic defect.
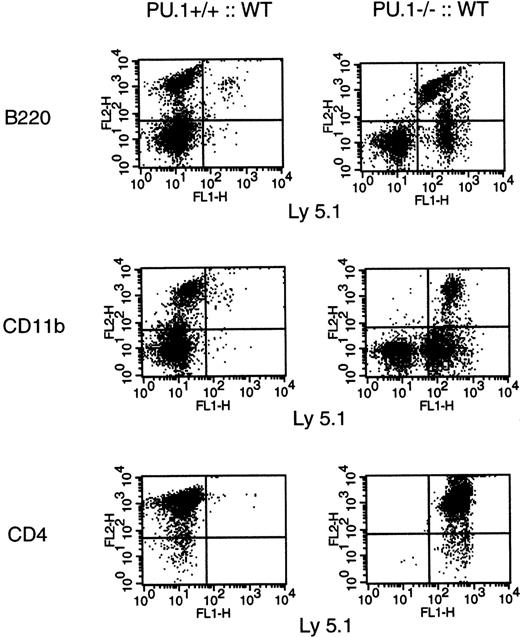
PU.1−/− embryos die by E17.5, apparently due to a severe hematopoietic defect.15 The mutant embryos are normal morphologically with unimpaired erythrocyte and platelet production. Therefore, it is unclear why the embryos die. To determine if the defects found inPU.1−/− embryos are purely hematopoietic in origin, WT (Ly 5.1) hematopoietic progenitors were transplanted in utero. Both donor WT × WT (Ly 5.1) and recipientPU.1+/− × PU.1+/−(Ly 5.2) crosses were performed to generate timed embryos. AA4.1+ HPC were enriched from E14.5 WT (Ly 5.1) pooled FL cells and injected intraperitoneally into recipient embryos (Ly 5.2). AA4.1 is a known surface marker expressed on HSC and multipotent progenitors in the FL.21 22 The manipulated embryos were brought to term, genotyped by Southern blot analysis, and analyzed for hematopoietic contributions at 6 weeks of age.PU.1−/− animals were fully rescued by the transplantation of WT HPC and were indistinguishable fromPU.1+/+ orPU.1+/− littermates. Flow cytometric analysis of B cells (B220+), monocytes (CD11b+), and T cells (CD4+) demonstrated that only donor-derived progenitors (Ly 5.1+) contributed to hematopoiesis in these animals (Fig 1). In contrast, donor cells rarely contributed to hematopoiesis inPU.1+/+ or PU.1+/−recipients. A total of 3 rescuedPU.1−/− and 6 transplanted WT animals were examined with equivalent results. The rescue experiments demonstrate that the lethal nature of thePU.1−/− defect is of hematopoietic origin. Furthermore, hematopoiesis can be supported by aPU.1−/− microenvironment.
In utero transplant of WT hematopoietic progenitors. E14.5 embryos (Ly 5.2) from PU.1+/− matings were injected with 105 WT AA4.1+ HPC (Ly 5.1). The resulting animals were then genotyped by Southern blot at 3 weeks of age. At 6 weeks of age, bone marrow underwent 2-color flow cytometric analysis for B-cell (B220+/Ly 5.1+) and monocyte (CD11b+/Ly 5.1+) origin, and the thymus was analyzed for T-cell (CD4+/Ly 5.1+) origin.
In utero transplant of WT hematopoietic progenitors. E14.5 embryos (Ly 5.2) from PU.1+/− matings were injected with 105 WT AA4.1+ HPC (Ly 5.1). The resulting animals were then genotyped by Southern blot at 3 weeks of age. At 6 weeks of age, bone marrow underwent 2-color flow cytometric analysis for B-cell (B220+/Ly 5.1+) and monocyte (CD11b+/Ly 5.1+) origin, and the thymus was analyzed for T-cell (CD4+/Ly 5.1+) origin.
PU.1−/− HSCs lack long-term repopulating activity in radioablated adult mice.
Previous adoptive transfer experiments determined thatPU.1−/− fetal HPC are unable to provide radioprotection to lethally irradiated adult recipients.17However, the recipient animals did have detectable levels of donor-derived erythrocytes, but no lymphoid or myeloid engraftment, before dying 2 to 3 weeks postirradiation. The early death of the recipient animals receiving PU.1−/− FL cells prevented any further conclusions being drawn concerning HSC and/or HPC function. Therefore, a competitive repopulation assay was used to examine the functional status ofPU.1−/− HSC in the adult bone marrow microenvironment. This assay system is designed to test the ability of HSC to offer multilineage reconstitution in vivo in the presence of a competing radioprotective dose of syngeneic bone marrow cells.23
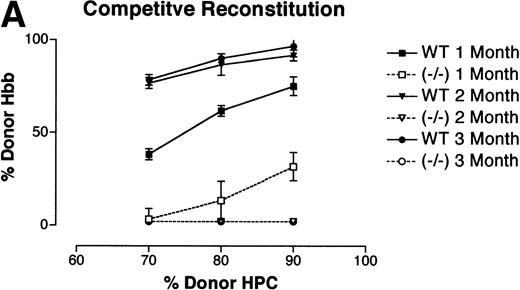
E14.5 FL contain the highest level of multilineage repopulating activity.9 Therefore, CRAs were performed with pooled WT (PU.1+/+ or PU.1+/−littermates) or PU.1−/− E14.5 FL samples (Ly 5.2) in competition with a radioprotective dose of syngeneic bone marrow (Ly 5.1). Two groups of 8 recipients received 70% (5 × 105) WT orPU.1−/− FL cells plus 30% (2 × 105) syngeneic bone marrow, 2 groups received 80% (1 × 106) FL cells with 20% (2 × 105) syngeneic marrow, and a final 2 groups received 90% (2 × 106) donor to 10% (2 × 105) syngeneic. After transplantation, the recipients were bled on a monthly basis and analyzed for donor-derived contribution to the erythroid, lymphoid, and myeloid lineages. Donor-derived erythrocytes were detected by differences in Hbb isoforms. Cellulose acetate electrophoresis was used to distinguish differences between the Hbbd (Ly 5.2, donor) and the Hbbs (Ly 5.1, syngeneic) isoforms. Relative contributions of the hemoglobin isoforms were quantified by scanning densitometry. The presence of donor-derived lymphoid and myeloid cells was ascertained by 2-color flow cytometry using lineage- and Ly 5.2-specific MoAbs. The CRA were repeated a total of 3 independent times.
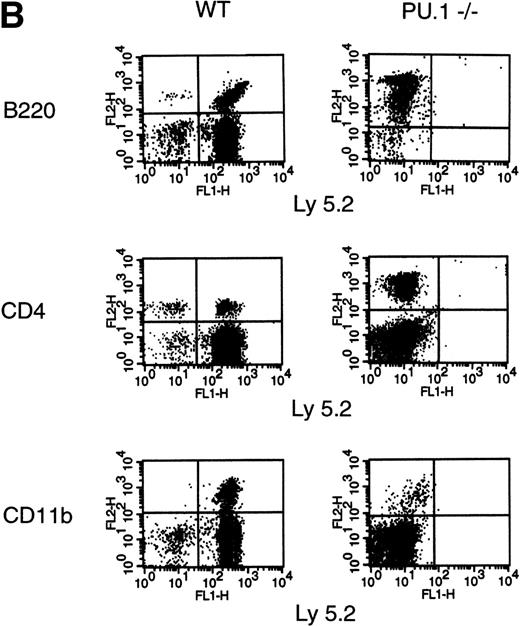
Reconstitution of the erythroid lineage by WT andPU.1−/− FL cells is shown in Fig 2A. Reconstitution by donor-derived erythrocytes is expressed as the percentage of donor-derived hemoglobin (Hbbd) in the peripheral blood. At 1 month posttransplant, WT FL cells were contributing to erythrocyte production at nearly the expected ratios based on input percentage. By 2 and 3 months posttransplant, the WT FL cells were contributing at equal to or better than the expected ratios. In contrast, FL cells isolated fromPU.1−/− littermates only produced erythrocytes for approximately 1 month and at greatly reduced levels. Results for reconstitution of the lymphoid and myeloid compartments are shown in Fig 2B. Unlike the contribution to the erythroid lineage byPU.1−/− HPC, there were no detectable PU.1−/− (Ly 5.2+)-derived B cells (B220+), T cells (CD4+), or monocytes (CD11b+) posttransplantation (Fig 2B). Reconstitution was monitored for a total of 6 months with no detectable contribution byPU.1−/− HSC. For WT FL cells, a substantial portion of mononuclear cells in the peripheral blood expressed Ly 5.2 and lineage markers (B220, CD4, and CD11b) for the full course of the experiment. These results demonstrate thatPU.1−/− FL HPC are incapable of contributing to long-term erythropoiesis, lymphopoiesis, or myelopoiesis in the bone marrow.
CRA of the hematopoietic lineages by WT andPU.1−/− E14.5 FL cells. Irradiated Ly 5.1 adult mice (Hbbs) were transplanted with either 70% (5 × 105), 80% (1 × 106), or 90% (2 × 106) E14.5 FL progenitors from donor WT (PU.1+/+,/−) orPU.1−/− embryonic littermates (Ly 5.2, Hbbd). A radioprotective dose of 2 × 105normal adult bone marrow cells was included as a source of competitive, syngeneic HPC. Peripheral blood was taken from recipient animals on a monthly basis for 6 months postengraftment. (A) Contribution of donor HPC to peripheral blood erythrocytes. Cellulose acetate electrophoreisis was used to separate donor from recipient hemoglobin isoforms. Relative contribution was determined by scanning densitometry and expressed as the percentage of donor contribution to total hemoglobin production. Data from 3 independent experiments are depicted on the graph. Solid lines represent animals that received WT donor HPC. Dashed lines represent animals that receivedPU.1−/− donor HPC. (B) Contribution of donor HPC to the lymphoid and myeloid lineages. Contribution to the lymphoid and myeloid lineages was determined by flow cytrometric analysis using an Ly 5.2-specific MoAb to identify donor-derived cells and lineage-specific MoAbs to characterize the B-cell (B220+), T-cell (CD4+), and monocyte (CD11b+) populations. Representative FACS profiles are shown for peripheral blood samples obtained at 2 months posttransplantation for lethally irradiated Ly 5.1+ adult mice receiving either pooled WT or PU.1−/−E14.5 FL HPC (Ly 5.2+).
CRA of the hematopoietic lineages by WT andPU.1−/− E14.5 FL cells. Irradiated Ly 5.1 adult mice (Hbbs) were transplanted with either 70% (5 × 105), 80% (1 × 106), or 90% (2 × 106) E14.5 FL progenitors from donor WT (PU.1+/+,/−) orPU.1−/− embryonic littermates (Ly 5.2, Hbbd). A radioprotective dose of 2 × 105normal adult bone marrow cells was included as a source of competitive, syngeneic HPC. Peripheral blood was taken from recipient animals on a monthly basis for 6 months postengraftment. (A) Contribution of donor HPC to peripheral blood erythrocytes. Cellulose acetate electrophoreisis was used to separate donor from recipient hemoglobin isoforms. Relative contribution was determined by scanning densitometry and expressed as the percentage of donor contribution to total hemoglobin production. Data from 3 independent experiments are depicted on the graph. Solid lines represent animals that received WT donor HPC. Dashed lines represent animals that receivedPU.1−/− donor HPC. (B) Contribution of donor HPC to the lymphoid and myeloid lineages. Contribution to the lymphoid and myeloid lineages was determined by flow cytrometric analysis using an Ly 5.2-specific MoAb to identify donor-derived cells and lineage-specific MoAbs to characterize the B-cell (B220+), T-cell (CD4+), and monocyte (CD11b+) populations. Representative FACS profiles are shown for peripheral blood samples obtained at 2 months posttransplantation for lethally irradiated Ly 5.1+ adult mice receiving either pooled WT or PU.1−/−E14.5 FL HPC (Ly 5.2+).
PU.1−/− FL erythroid progenitors fail to respond to SCF stimulation.
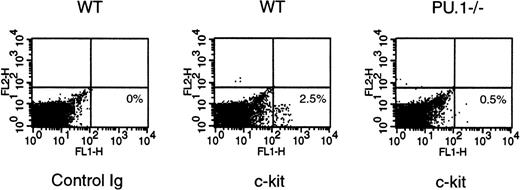
The inability of E14.5 PU.1−/− FL HPC to support long-term erythropoiesis contrasts with the normal number of FL or yolk sac erythroid progenitors inPU.1−/− embryos and with the capacity of PU.1−/− ES cells to establish erythropoiesis in fetal chimeras.16,17 The differing requirement for PU.1 alludes to a fundamental difference between fetal and adult erythropoiesis. One critical regulatory signal governing erythropoiesis that has been shown to change during development emanates from the stem cell factor receptor (c-kit).24,25 In vivo administration of anti–c-kit MoAbs showed that primitive erythropoiesis in the yolk sac and FL is independent of c-kit function, whereas definitive erythropoiesis depends on c-kit activity. To examine the ability of SCF to promote erythropoiesis, a serum-free progenitor assay system, was chosen to test the synergistic activity between Epo and SCF for the generation of erythroid progenitors (colony-forming unit-erythroid [CFU-E]).18 The assay was performed in duplicate on 6 different embryos of each genotype. E16.5 WT FL cells produced 9 ± 6 CFU-E per 106 cells in response to Epo alone. PU.1−/− cells produced an equivalent 7 ± 2 CFU-E with Epo alone. WT cells had a synergistic response to a combination of Epo and SCF to produce 551 ± 230 CFU-E per 106 cells. In contrast,PU.1−/− cells still only produce 13 ± 1 CFU-E in response to Epo plus SCF, indicating a lack of synergy. Day E16.5 was chosen for this analysis because our previous studies quantifying FL erythroid progenitors showed normal numbers ofPU.1−/− CFU-E when assayed in the presence of serum with a cocktail of growth factors.17 Flow cytrometry analysis was performed to ascertain the effect of thePU.1 mutation on c-kit expression. Very few E16.5PU.1−/− FL cells express detectable levels of c-kit on their surface (Fig 3). This analysis has shown that the lack of c-kit expression by E16.5 PU.1−/− FL cells may explain the inability ofPU.i−/− erythroid progenitors to synergistically respond to Epo plus SCF.
c-kit expression on WT andPU.1−/− E16.5 FL cells. c-kitexpression by WT and PU.1−/− E16.5 FL cells was examined by flow cytometry using a c-kit–specific MoAb. Representative data for staining by isotype-match control or an anti–c-kit MoAb for individual WT orPU.1−/− E16.5 FL cells are shown.
c-kit expression on WT andPU.1−/− E16.5 FL cells. c-kitexpression by WT and PU.1−/− E16.5 FL cells was examined by flow cytometry using a c-kit–specific MoAb. Representative data for staining by isotype-match control or an anti–c-kit MoAb for individual WT orPU.1−/− E16.5 FL cells are shown.
The effect of PU.1 on homing and engraftment of FL progenitors in the adult bone marrow.
One possible explanation for the lack of long-term hematopoiesis in recipient adult animals is that PU.1−/−HPC are more prone to programmed cell death. TUNEL assay of E14.5 whole FL cell suspensions or AA4.1+ enriched progenitor cell populations, containing a mixture of HSC and multipotent progenitor cells, showed no detectable apoptotic cells in either population (data not shown). These results suggest that an increased rate of apoptosis is an unlikely explanation for the aberrant behavior of the HPC compartment in PU.1−/− FL. An alternative possibility to explain the inability ofPU.1−/− HSC to establish long-term reconstitution in adult animals would be a defect in the ability of progenitor cells to home to and engraft in the adult bone marrow.
To monitor migration, pooled FLs from WT (PU.1+/+,+/−) andPU.1−/− E14.5 embryos were fluorescently labeled with either CFDASE or FITC. After fluorescent labeling, tagged cells were enriched for AA4.1+ progenitors by magnetic bead selection and injected retro-orbitally into lethally irradiated adult mice. Recipient animals were killed at 5, 16, or 48 hours posttransplant. Bone marrow, spleen, thymus, and liver were examined for the presence of transplanted fluorescent cells (Table 1). The results for the spleen and thymus were essentially constant for all time points, with approximately 2-fold fewer PU.1−/−AA4.1+ HPC migrating to these tissues. A striking difference was observed between the WT andPU.1−/− AA4.1+ FL progenitors in the bone marrow. At 5 hours posttransplant, there was a 2.5-fold reduction in PU.1−/− HPC migrating to the bone marrow. By 16 hours posttransplant, there were 8.5-fold fewer PU.1−/− HPC in the marrow. The gap widened to an 11-fold decrease in the marrow by 48 hours. In contrast, after 48 hours, there was a 9-fold increase inPU.1−/− AA4.1+ progenitors detected in the adult liver when compared with WT. The data presented in Table 1 represent a single homing assay that has been repeated 4 independent times with similar results. Therefore, HPC fromPU.1−/− FLs are severely impaired in their ability to home to and engraft the adult bone marrow. The altered migration of the mutant progenitor cells results in their abnormal accumulation in the liver after transplantation.
Quantification of Fluorescently Tagged E14.5 WT andPU.1−/− AA4.1+ FL Cells Migrating to the Bone Marrow, Spleen, Thymus, and Liver
| Genotype . | Bone Marrow . | Spleen . | Thymus . | Liver . |
|---|---|---|---|---|
| (A) 5 Hours posttransplant | ||||
| WT (n = 3) | 149 ± 14 | 151 ± 22 | 61 ± 11 | 117 ± 33 |
| PU.1−/− (n = 3) | 67 ± 17 | 82 ± 7 | 42 ± 8 | 201 ± 30 |
| (B) 16 Hours posttransplant | ||||
| WT (n = 3) | 184 ± 25 | 85 ± 7 | 160 ± 60 | 45 ± 8 |
| PU.1−/− (n = 3) | 22 ± 12 | 54 ± 13 | 72 ± 22 | 310 ± 60 |
| (C) 48 Hours posttransplant | ||||
| WT (n = 2) | 98 ± 20 | 265 ± 50 | 147 ± 3 | 39 ± 7 |
| PU.1−/− (n = 2) | 9 ± 4 | 138 ± 21 | 70 ± 15 | 349 ± 35 |
| Genotype . | Bone Marrow . | Spleen . | Thymus . | Liver . |
|---|---|---|---|---|
| (A) 5 Hours posttransplant | ||||
| WT (n = 3) | 149 ± 14 | 151 ± 22 | 61 ± 11 | 117 ± 33 |
| PU.1−/− (n = 3) | 67 ± 17 | 82 ± 7 | 42 ± 8 | 201 ± 30 |
| (B) 16 Hours posttransplant | ||||
| WT (n = 3) | 184 ± 25 | 85 ± 7 | 160 ± 60 | 45 ± 8 |
| PU.1−/− (n = 3) | 22 ± 12 | 54 ± 13 | 72 ± 22 | 310 ± 60 |
| (C) 48 Hours posttransplant | ||||
| WT (n = 2) | 98 ± 20 | 265 ± 50 | 147 ± 3 | 39 ± 7 |
| PU.1−/− (n = 2) | 9 ± 4 | 138 ± 21 | 70 ± 15 | 349 ± 35 |
Approximately 1 to 2 × 105 fluorescently labeled, AA4.1+ FL cells were transplanted into lethally irradiated adult recipients. At 5, 16, or 48 hours posttransplantation, recipient bone marrow, spleen, thymus, and liver were examined for the number of fluorescently tagged cells. Results are depicted as the mean number (±SD) of fluorescent cells per 107 cells counted.
Adhesion molecule profile of hematopoietic progenitors isolated from PU.1−/− E14.5 FL.
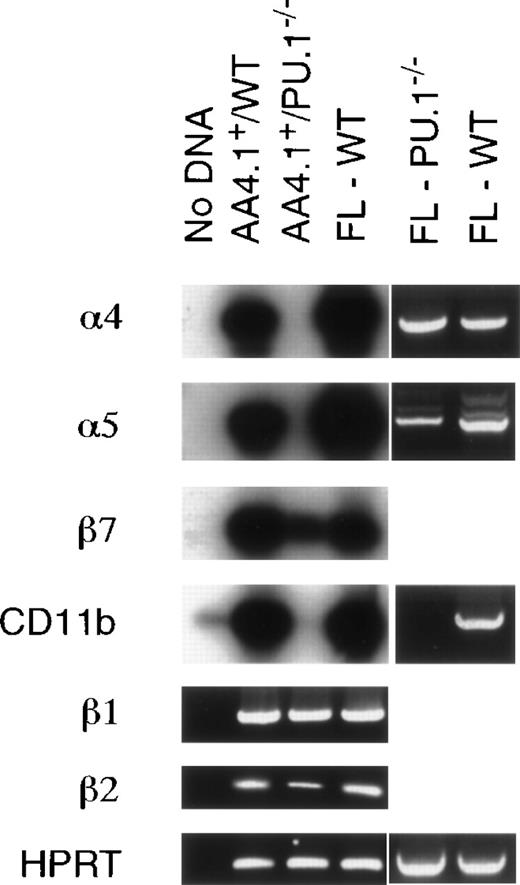
The observed defect in the ability ofPU.1−/− progenitors to home to the bone marrow prompted an evaluation of a number of adhesion molecules thought to mediate hematopoietic progenitor cell migration. Initial flow cytometry experiments demonstrated that the intergrin CD11b was missing and suggested that integrins α4 and α5 were affected on HPC, but not in the FL as a whole (data not shown). Integrins α4/VLA-4 and α5/VLA-5 have been strongly implicated in the attachment of hematopoeitic progenitors to bone marrow stroma and associated extracellular matrix.26-28 To more closely examine integrin α4 and α5 expression, RT-PCR analysis was undertaken on total cellular RNA isolated from either whole FLs or AA4.1+enriched cell populations from WT andPU.1−/− embryos. RT-PCR followed by Southern blotting was chosen as the most sensitive method to examine expression given the difficulty of obtaining large numbers ofPU.1−/− HPC. Figure 4 shows that the integrin CD11b/αM is not expressed by PU.1−/− AA4.1+ cells, whereas expression of its heterodimeric binding partner, intregin β2/CD18, is reduced. Both the α4 and α5 integrins were expressed in wholePU.1−/− FLs, but not in the AA4.1+PU.1−/− FL HPC, even after Southern blot analysis of the PCR reactions products (Fig 4). Expression of the integrin β1, which has been demonstrated to affect HSC migration to the FL,29 was unaffected by thePU.1 mutation. Another integrin, β7, possessing a role in lymphocyte homing,30 showed a minor reduction in expression. These results are consistent with the flow cytometric characterization of FL cells fromPU.1−/− embyros. Thus, a number of functionally important integrins are not properly expressed in the absence of PU.1, which may explain the aberrant homing and engraftment properties of PU.1−/− HPC.
Adhesion molecule profile of E14.5 AA4.1+FL cells. Total RNA was isolated from either whole FLs or AA4.1+ enriched cells from E14.5 WT andPU.1−/− embryos. RNA expression was examined using primers specific for integrins CD11b, 4, 5, β1, β2, and β7. Expression of the house keeping gene HPRT was used to normalize variation between RNA samples. Results are displayed as either ethidium bromide-stained PCR products or, to enhance sensensitivity of the expression analysis, the RT-PCR products were further analyzed by Southern blot hybridization using cDNA fragments as hybridization probes.
Adhesion molecule profile of E14.5 AA4.1+FL cells. Total RNA was isolated from either whole FLs or AA4.1+ enriched cells from E14.5 WT andPU.1−/− embryos. RNA expression was examined using primers specific for integrins CD11b, 4, 5, β1, β2, and β7. Expression of the house keeping gene HPRT was used to normalize variation between RNA samples. Results are displayed as either ethidium bromide-stained PCR products or, to enhance sensensitivity of the expression analysis, the RT-PCR products were further analyzed by Southern blot hybridization using cDNA fragments as hybridization probes.
DISCUSSION
Phenotypic and functional analysis ofPU.1−/− embryos demonstrated severe abnormalities in fetal hematopoiesis occurring in both the FL and yolk sac.15-17 This analysis showed a cell-intrinsic defect in lymphoid and myeloid development. In contrast, both primitive and definitive erythropoiesis, along with megakaryopoiesis, appeared normal in the yolk sac and FL. A second targeted mutation of the PU.1allele demonstrates a similar phenotype with respect to fetal hematopoiesis.31 However, mice containing the secondPU.1 mutation can be kept alive for 2 weeks by high-dose antibiotics. During those 2 weeks, T cells and abnormal neutrophils can develop. Recent work with our mutation demonstrates that similar neutrophils32 and T cells32a can be cultured. Therefore, with respect to hematopoietic development, the two PU.1 mutations appear almost identical. Strain differences may contribute to the variable onset of lethality. Rescue ofPU.1−/− animals in utero by WT HSC (Fig 1) demonstrates that mutant embryos die from hematopoietic deficiencies. Furthermore, PU.1−/−micoenvironments can foster proper development of WT hematopoietic progenitors. These data suggest a role for PU.1 in a multipotential lymphoid-myeloid progenitor population in the FL. Our prediction is that one or more PU.1-regulated genes are required for efficient commitment and/or differentiation of multipotent progenitors to either the B-lymphoid or myeloid lineage. This hypothesis has recently been supported by work that demonstrates that PU.1 can irreversible commit retroviral transformed avian multipotent progenitor cells to the myeloid lineage.33 The normal levels of erythroid and megakaryocytic progenitors found inPU.1−/− embyros suggests that PU.1−/− HSC form in the AGM region and are able to colonize the yolk sac and FL.15,16 However, PU.1−/− HSC do not provide radioprotection for lethally irradiated recipients. In addition,PU.1−/− ES cells contribute to fetal but not adult erythropoiesis in chimeric animals.17 These data suggest that PU.1−/− HSC may be functionally impaired and point to a possible role for PU.1 during adult erythropoiesis.
The competitive reconstitution experiments were designed to allow donor-derived hematopoiesis to be evaluated over the course of several months. In the presence of a radioprotective dose of competitor adult bone marrow cells, there were significant levels ofPU.1−/− derived erythrocytes detectable for 1 month (Fig 2A). These results show thatPU.1−/− FL HPC can migrate to the adult bone marrow and contribute to erythropoiesis, but they are quickly out-competed by syngeneic WT cells. This suggests that, on a per cell basis, PU.1−/− fetal HPC are much less efficient than WT bone marrow cells. The absence of donor-derivedPU.1−/− erythrocytes beyond 2 months in radiation chimeras is entirely consistent with the lack of contribution to the erythroid lineage by PU.1−/− ES cells in adult chimeras.17 When reconstitution of the lymphoid and myeloid lineages was examined, no contribution was seen from PU.1−/− HSC (Fig 2B). FL HSC should be highly favored in these competitive reconstitutions due to their 4- to 5-fold greater long-term repopulating capacity.9,22 34 Furthermore, the lack of any detectable Ly 5.2+ hematopoietic cells beyond 2 months in the recipients indicates the importance of PU.1 for the function of HSC in the adult bone marrow. It is still unclear exactly how far along the lymphoid and/or myeloid differentiation pathwaysPU.1−/− HSC can proceed in a competitive microenvironment.
In situ immunochemical expression analysis of the murine bone marrow has documented PU.1 protein expression in immature erthyroblasts. PU.1 expression is downregulated upon terminal differentiation into erythrocytes.35 Continued expression of PU.1 in erythroblasts leads to growth immortalization and subsequent conversion to an erythroleukemic state.14 One key regulatory cascade operative during definitive erythropoiesis requires synergy between the cytokines Epo and SCF.36 Both Epo and SCF have been shown to be necessary for erythroid differentiation, proliferation, and survival.37-40 Serum-free clonogenic assays can directly measure synergistic and/or additive effects of growth factors.18 It is important to examine growth factor responsiveness using a defined assay system due to wide degree of functional redundancies between cytokines and cytokine receptors. For example, thrombopoietin can rescue CFU-E colonies in EpoR knockout mice.39 Serum-free clonogenic assays of E16.5PU.1−/− FL erythroid progenitors showed their lack of synergy to Epo plus SCF. Flow cytometric analysis ofPU.1−/− E16.5 FL cells showed little or no expression of c-kit (Fig 3). The possibility of PU.1-dependent expression of c-kit in erythroid progenitors is supported by recent analysis that has shown an ets family member, in conjunction with c-myb, is involved in the regulation of thec-kit promoter.41 One postulate is that, during repopulation, PU.1−/− erythroid progenitors are at a competitive disadvantage in the bone marrow due to their inability to respond to differentiative, proliferative, and survival signals emanating from EpoR and c-kit.
We next examined the homing potential ofPU.1−/− HPC with a quantitative homing assay. PU.1−/− HPC were 11-fold less efficient at homing/colonizing to the adult bone marrow after 48 hours (Table 1). There was an accompanying 9-fold increase in the frequency of PU.1−/− HPC found in the adult liver. Cell migration through the bone marrow microenvironment has been hypothesized to be a 2-step process of initial cell binding followed by transenthothelial migration into the hematopoietic compartment.42 For both steps, the importance of cell-cell interactions is clear. This prompted an evaluation of adhesion molecule expression by HPC isolated from PU.1−/− E14.5 FLs (Fig 4). PU.1−/− HPC lack expression of a number of integrins, including CD11b, α4/VLA-4, and α5/VLA-5, but retain expression of β1, β2, and β7.
Of particular interest was the absence of α4/VLA-4. Considerable data have implicated α4/VLA-4 as a key regulatory surface molecule mediating HPC/stromal cell contact in vitro and controlling HPC homing in vivo.43-45 The lack of α4/VLA-4 expression in PU.1−/− HPC is supported by a previous study that identified a functional ets regulatory element in the α4/VLA-4 promoter.46 However, the investigators in this study failed to determine which ets family member bound this element. Flow cytrometric and RT-PCR analyses have shown that α4/VLA-4 is still expressed outside the AA4.1+ HPC population in PU.1−/− FL (Fig 4). Thus, these data suggest a direct role for PU.1 in regulating α4/VLA-4 expression in HPC. Another ets family member may be involved in α4/VLA-4 expression outside the HPC compartment. However, the lack of α4 is not sufficient to explain the PU.1−/−homing defect, because HSC migration to the bone marrow during fetal development appears to be normal in the absence of α4 expression.47 The ability of a recombinant fibronectin fragment containing binding sites for α4, α5, and CD44 to inhibit multilineage engraftment has indicated a potential role for these adhesion molecules in interactions with the bone marrow stroma.48 Therefore, the 11-fold decrease in bone marrow homing/colonization of PU.1−/− HPC is most likely the aggregate effect of several missing adhesion molecules. The decrease may also reflect a reduction in the absolute number of HPC in AA4.1+ population ofPU.1−/− animals that are capable of homing to any tissue. Accumulation ofPU.1−/− HPC in the adult liver raises the interesting possibility of an active tropic mechanism to explain this observation. Both PU.1−/− embryos and fetal chimeras made with PU.1−/− ES cells indicate that mutant HSC home to and function in the FL microenvironment. β1 integrin has been shown to be required for proper homing of HSC to the FL.29 Without β1, cells fail to enter the FL and remain in the circulation, suggesting that β1 may be directly involved in liver homing.PU.1−/− progenitors retain β1 expression, but lack expression of other integrins. Perhaps this absence of other integrins causesPU.1−/− progenitors to retain their liver tropism resulting in their abnormal accumulation in the adult liver after transplantation. The data presented in this study demonstrate that PU.1 is required for fetal HSC to establish and maintain long-term hematopoiesis in the bone marrow. In addition, we have shown that PU.1 is necessary for long-term definitive erythropoiesis. Therefore, the PU.1−/−mouse provides an ideal model system to study the mechanism of hematopoietic progenitor migration to and multilineage engraftment of the adult bone marrow. We are currently in the process of reintroducing missing integrin molecules into PU.1−/−HPC via retroviral transduction to ascertain their effect on progenitor cell function in the bone marrow. These future studies should provide insight into adhesion molecule-dependent events that control hematpoietic stem and progenitor cell migration and subsequent multilineage repopulation in the bone marrow microenvironment.
ACKNOWLEDGMENT
The authors thank Irving Weissman (Stanford University, Stanford, CA) for the α4 and β7integrin cDNA fragments and H. Scott Baldwin (University of Pennsylvania, Philadelphia, PA) for the α5 integrin cDNA fragment. We also thank Hamid Bassiri (University of Pennsylvania) for advice on detecting apoptosis, Andrew D. Wells (University of Pennsylvania) for the CFDASE labeling protocol, Mariusz Ratajczak (University of Pennsylvania) for assistance in setting up the serum-free progenitor assay system, and Debbie Knobleman for assistance with the in utero transplants.
Supported by National Institutes of Health Grants No. CA72769 and HL58716 to E.W.S. E.W.S. is a Leukemia Society of America Scholar.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Edward W. Scott, PhD, Institute for Human Gene Therapy, Stellar Chance Laboratories, Room 401a, 422 Curie Blvd, University of Pennsylvania, Philadelphia, PA 19104-6140; e-mail:scotte@mail.med.upenn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal