Abstract
Thrombopoietin (TPO) plays a critical role in megakaryocyte proliferation and differentiation. Using various cultured cell lines, several recent studies have implicated the mitogen-activated protein kinase (MAPK) pathway in megakaryocyte differentiation. In the study reported here, we examined the role played by thrombopoietin-induced MAPK activity in a cytokine-dependent cell line (BAF3/Mpl) and in primary murine megakaryocytes. In both systems, extracellular signal-regulated protein kinase (ERK) 1 and 2 MAPK phosphorylation was rapidly induced by TPO stimulation. To identify the Mpl domain responsible for MAPK activation, BAF3 cells expressing truncated forms of the Mpl receptor were studied. Phosphorylation of ERKs did not require elements of the cytoplasmic signaling domain distal to Box 2 and was not dependent on phosphorylation of the adapter protein Shc. ERK activation in murine megakaryocytes was maximal at 10 minutes and was markedly decreased over the subsequent 3 hours. Next, the physiologic consequences of MAPK inhibition were studied. Using the MAPK kinase (MEK) inhibitor, PD 98059, blockade of MAPK activity substantially reduced TPO-dependent proliferation in BAF3/Mpl cells and markedly decreased mean megakaryocyte ploidy in cultures. To exclude an indirect effect of MAPK inhibition on stromal cells in whole bone marrow, CD41+ cells were selected and then cultured in TPO. The number of polyploid megakaryocytes derived from the CD41-selected cells was also significantly reduced by MEK inhibition, as was their geometric mean ploidy. These studies show an important role for MAPK in TPO-induced endomitosis and underscore the value of primary cells when studying the physiologic effects of signaling pathways.
THROMBOPOIETIN (TPO), fulfills most, if not all, of the properties predicted for the primary regulator of platelet production. Both in vitro and in vivo experiments have demonstrated that TPO is sufficient for full megakaryocyte development.1-3 Binding of the hormone to its receptor, Mpl, results in activation of a variety of signaling molecules, including components of the JAK/STAT pathway4 and the Shc/Ras/MAPK pathway.5,6 Most of the studies to date have been conducted in cell lines that either normally express the Mpl receptor or have been engineered to express it on the cell surface. Activation of the JAK/STAT pathway has also been demonstrated both in normal megakaryocytes7 and in platelets.8-10However, the activation of MAPK pathway has never been reported in primary megakaryocytes.
Extrapolation of data derived from cell lines to normal physiologic conditions is difficult for several reasons. First, the expression of specific signaling molecules may differ between cell lines and primary cells. Second, signaling molecules in distinct cell types may be compartmentalized by both organelles and scaffolding proteins into different subcellular locations, potentially altering the cross-talk between pathways. Third, it is likely that transformed cell lines develop anomalous signaling pathways to promote their proliferation and survival, pathways that differ significantly from primary cells. Although much has been learned about the signaling pathways triggered by the binding of TPO to its receptor on cell lines, the physiologic role of each pathway in megakaryocyte proliferation and differentiation is still largely unknown. Truncated thrombopoietin receptors that fail to activate JAK2 are also unable to support proliferation of BAF3/Mpl11 and UT-712 cell lines in the presence of TPO. This suggests that the JAK/STAT pathway may contribute to TPO-induced proliferation. Distinct signaling pathways may control different cellular processes. For example, JAK/STAT may be responsible for proliferation and MAPK pathway may function for differentiation. Alternatively, various pathways may have complex interactions that lead to an integrated response or that may be redundant.
Mitogen-activated protein kinases are serine/threonine kinases that are highly conserved in all eukaryotic cells from yeast to humans. At least 6 signaling cascades classified as MAPK pathways have been identified13 and have been found to mediate cell proliferation, survival, apoptosis, and/or differentiation, depending on the cell line used. For instance, MAPK induces proliferation in NIH 3T3 cells, a murine fibroblastic cell line, but induces differentiation in PC12 cells, a neural pheochromocytoma cell line.14 Two of the classical MAPKs, extracellular signal-regulated protein kinase 1 (ERK1) and ERK2, have been shown to be involved in TPO signaling. A study in the erythroleukemia cell line, K562, has shown that constitutively active mutants of MEK or phorbol esters can cause megakaryocyte-like differentiation, including cell enlargement, inhibition of cell growth, and expression of platelet-specific glycoprotein IIb/IIIa on cell surfaces.15 In addition, a constitutively active form of ERK has been shown to induce the expression of megakaryocyte-specific surface markers and morphological changes in CMK cells, a human megakaryoblastic leukemia cell line. The MEK inhibitor, PD 98059, was found to block these effects.16 Although taken as evidence that MEK and MAPK are involved in normal megakaryocyte development, megakaryocytic differentiation was induced by stem cell factor in this system, which cannot promote differentiation in normal megakaryocyte progenitor cells. Recently, similar results were observed in UT7-Mpl cell line stimulated by thrombopoietin,17 although these cells fail to differentiate into fully mature megakaryocytes. Like all transformed or immortal cell lines, it is possible that UT-7 cells use aberrant pathways that are not present in normal cells. Alternatively, these pathways in cell lines may lead to differentiation by nonphysiologic mechanisms. For example, endomitosis in the HEL cell line is reportedly due to the absence of cdc2.18 In contrast, endomitosis in MegT cells is believed to result from the absence of cyclinB.19 Both of these proteins are present in primary endomitotic megakaryocytes.20 21
To define the physiologic role of the MAPK pathway in megakaryocytes, we used murine bone marrow cells cultured in TPO. We found that ERK1 and ERK2 were rapidly but only transiently activated in normal murine megakaryocytes after exposure to TPO, despite the continued presence of the hormone. Inhibition of the MAPK pathway significantly reduced megakaryocyte polyploidization in both whole bone marrow cultures and CD41-selected cell cultures. These results point to the importance of the MEK/ERKs pathway in a specific aspect of megakaryocyte development, endomitosis, and argue persuasively for the use of primary cells to dissect the role of various intracellular signals on cellular differentiation.
MATERIALS AND METHODS
Cell line and growth conditions.
BAF3/Mpl cells obtained from Zymogenetics, Inc (Seattle, WA)11,22 were maintained in RPMI 1640 (BioWhittaker, Walkersville, MD) with 10% heat-inactivated fetal calf serum (HyClone, Logan, UT), 2 mmol/L L-glutamine, 100 U/L penicillin, 100 mg/mL streptomycin, and 0.25 mg/mL amphotericin B (BioWhittaker) supplemented with murine interleukin-3 (mIL-3; 0.2% vol/vol conditioned culture medium from baby hamster kidneys cells engineered to constitutively secrete mIL-3). BAF3 cell lines expressing truncated forms of Mpl were previously described11 and were maintained in an identical fashion.
Purification of murine megakaryocytes.
BDF-1 mice (Jackson Labs, Bar Harbor, ME) were subcutaneously injected with pure, recombinant human TPO for 5 days (2 μg/d; Zymogenetics, Inc) and killed, and bone marrow cells were flushed from femurs and tibias into serum-free Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 1% Nutridoma (Boerhinger Mannheim, Indianapolis, IN) with penicillin, streptomycin, and L-glutamine. Cells were incubated (37°C, 5% CO2) with recombinant murine TPO (5% vol/vol conditioned medium, ∼37.5 ng/mL) for 72 hours. Mature megakaryocytes were purified over a discontinuous bovine serum albumin (BSA; Sigma, St Louis, MO) density gradient (0%/1.5%/3.0% BSA).7 Ninety percent of cells that settled to the bottom in 30 minutes were mature megakaryocytes by immunostaining. Additionally, some of mature megakaryocytes remained attached to the plastic syringe and could be recovered by gentle washing.
Stimulation of cells.
Cells were starved in serum-free, growth factor-free medium (16 hours for BAF3/Mpl cells and 7 hours for murine megakaryocytes). PD 98059 (New England Biolab, Beverly, MA) was dissolved in dimethyl sulfoxide (DMSO) and added to cells 20 minutes before TPO stimulation at final concentrations between 20 and 100 μmol/L. An equal volume of DMSO was used as a negative control. In some experiments, 100 nmol/L Wortmannin (Sigma), a PI3K inhibitor, was also used. After preincubation, cells were stimulated by TPO (2% vol/vol; conditioned medium, ∼14 ng/mL) for 10 minutes or for the indicated duration. In some experiments, fetal calf serum (10% vol/vol), recombinant murine stromal cell-derived factor 1 (SDF-1; 100 ng/mL; a gift from N. Yamamoto, Tokyo Medical and Dental University, Tokyo, Japan) or murine stem cell factor (SCF; 100 ng/mL; Kirin Pharmaceutical, Gunma, Japan) was added. The cells were then mixed with ice-cold phosphate-buffered saline (PBS) and a cell lysate was prepared by Triton-X 100 solubilization as previously described.4
A BAF3/Mpl cell proliferation assay was performed as previously described.11 Briefly, cells were washed twice with cytokine-free media and then grown in various concentrations of murine TPO-conditioned supernatant (0.01%, 0.1%, 1%, and 10% vol:vol; 1% is ∼7 ng/mL) or murine IL-3–conditioned medium at the maximal proliferation dose. After 36 hours of incubation, 3,4,5-dimethylthiazole-2-yl-2,5-diphenyl tetrazolium bromide (MTT; Sigma) was added (final concentration, 1 mg/mL) and incubation was continued for 5 hours. Cells were then lysed and absorbance at 570 to 630 nm was determined using an enzyme-linked immunosorbent assay (ELISA) plate reader. Each data point was performed in triplicate. Proliferation was expressed as a percentage of maximal mIL-3–induced growth.
Western blot analysis.
The protein concentrations of BAF3/Mpl cell lysate were measured using Protein/DC Assay (Bio-Rad, Hercules, CA) to assure equal loading between lanes. For megakaryocytes, equal numbers of purified cells were used to generate each sample. Lysates were denatured by boiling for 5 minutes in loading buffer as described by Laemmli23 and separated on 10% polyacrylamide gels. Proteins were then transferred to nitrocellulose membranes (Schleicher & Schuell, Keane, NH) and blocked for 16 hours in Tris-buffered saline with 0.05% Tween 20 (TBST) and 3% BSA. Rabbit polyclonal antibody specific for doubly phosphorylated MAPK was purchased from Promega (Madison, WI). Rabbit polyclonal ERK 2 antibody was obtained from Santa Cruz Biotech (Santa Cruz, CA). Phosphotyrosine antibody (4G10) and Shc antibody were purchased from Upstate Biotechnology, Inc (Lake Placid, NY). Each antibody was diluted in blocking buffer at the concentration recommended by each supplier and incubated with the blot at room temperature for 2 hours. After four 5-minute washes in TBST, the nitrocellulose blots were gently rocked (30 to 60 minutes at room temperature) in solution containing blocking buffer and the detecting antibody: goat antirabbit antibody (MAPKs and Shc) or goat antimouse antibody (4G10) coupled to horseradish peroxidase (Bio-Rad) at a final dilution of 1:5,000. The membrane was then washed 4 times in TBST (5 minutes each), incubated with chemiluminescent reagents (Santa Cruz Biotech), and exposed to film. In some cases, the antibodies were stripped off the blot by washing with 62.5 mmol/L Tris (pH 6.8), 2% sodium dodecyl sulfate, and 100 mmol/L β-mercaptoethanol at 50°C for 30 minutes. Subsequently, the blots were reblocked and reprobed with new primary and secondary antibodies.
Whole marrow culture.
BDF1 mice were killed, and the marrow was flushed from femurs and tibias. The cell suspension was then filtered through 2 layers of gauze, pelleted, and resuspended in red blood cell lysis buffer (140 mmol/L NH4Cl in 17 mmol/L Tris-HCl, pH 7.2). Cells were washed and incubated in IMDM/1% Nutridoma/penicillin/streptomycin/L-glutamine supplemented with 5% TPO-conditioned medium (approximate concentration, 35 ng/mL). PD 98059 at final concentrations of 20, 50, or 100 μmol/L was added. An equal volume of diluent (DMSO) was added to control cultures. Megakaryocyte cultures were then incubated for 72 hours at 37°C in a 5% CO2-containing fully humidified atmosphere. Cells were observed by inverted light microscopy, small aliquots were stained with trypan blue and counted for total viable cells by hemocytometer, and cytospins were prepared on BSA-coated slides. Slides were then Wright-stained to assess megakaryocytic morphology and stained for acetylcholinesterase activity as a marker of murine megakaryocyte differentiation. The acetylcholinesterase stain was performed according to the method of Jackson.24 The remaining cells were subjected to immunofluorescence staining and analyzed by flow cytometry.
Immunofluorescence and flow cytometric analysis.
Ten milliliters of megakaryocyte suspension culture was layered over 1 mL of 3% BSA in CATCH buffer (0.36% sodium citrate, 2 mmol/L theophylline, and 1 mmol/L adenosine in calcium-free, magnesium-free Hank’s Balanced Salt Solution), and the cells were pelleted at 200g. Cells were resuspended in 100 μL of 3% BSA in CATCH buffer. Antimouse Fc receptor antibody (Pharmingen, San Diego, CA) was added at a final concentration of 10 μg/mL to block nonspecific Fc binding and incubated on ice for 30 minutes. Fluorescein isothiocyanate (FITC)-conjugated antimurine CD41 or its isotype control (Pharmingen) was then added at 1:50 dilution and incubated on ice for 1 hour. One milliliter of 3% BSA in CATCH buffer was added, the suspension was layered over 5% BSA in CATCH, and the cells were pelleted at 200g. Cells were then resuspended in propidium iodide (PI) buffer containing 0.1% sodium citrate, 0.1% Triton X-100, 50 μg/mL PI, and 6% RNAse. Cells were then analyzed by two-colored flow cytometry. FITC-CD41+ cells (megakaryocytes) were gated to determined DNA content (ploidy) by PI stain and the distribution of cell size (forward scattering). Ploidy analysis is displayed semilogarithmically.
CD41-selected cell culture.
Bone marrow cells from BDF1 mice were separated by density gradient centrifugation. Five milliliters of cell suspension was layered on 3 mL of Optiprep (Nycomed, Oslo, Norway) at a specific gravity 1.080 and centrifuged at 400g for 20 minutes at room temperature. Low-density cells at the interface were then collected, washed twice, and resuspended in PBS with 5% fetal calf serum at 108 cells/mL. FITC-conjugated anti-CD41 antibody was then added at 0.35 μg/106 cells and incubated on ice for 45 minutes. Cells were then washed twice and incubated with 1:10 dilution of microbeads coated with anti-FITC antibody (Miltenyi Biotech, Auburn, CA) in PBS/0.5% BSA/2 mmol/L EDTA at 6°C to 12°C for 15 minutes and washed once. Labeled cells were then passed through a high gradient magnetic column, MidiMACS (Miltelyi Biotech). The positive fraction retained in the column after 3 washes was then eluted and cultured in 1% Nutridoma and 5% TPO-conditioned medium (approximate concentration, 35 ng/mL) with or without PD 98059. After 84 hours of culture, cells were then stained with anti–CD41-FITC and PI using the methods described above. Approximately 30% of cells after culture were CD41+megakaryocytes.
RESULTS
ERKs are activated by TPO in BAF3/Mpl cells and mediate TPO-induced proliferation.
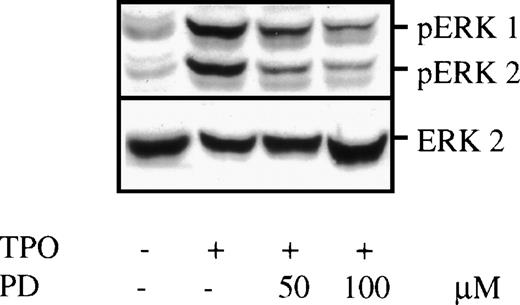
BAF3/Mpl cells were incubated in serum-free, growth factor-free medium for 16 hours to allow maximal dephosphorylation of cellular proteins. Cultures were then stimulated by TPO for 10 minutes in the presence or absence of the specific MEK inhibitor, PD 98059, and cell lysates were prepared and subjected to Western blot analysis. When probed with an antibody specific for the double-phosphorylated, activated form of ERKs (Fig 1), the TPO-stimulated cell lysate contained two prominent bands at 44 and 42 kD that were absent in unstimulated cells. These 44- and 42-kD bands were independently verified to represent activated ERK1 and ERK2, respectively, by blotting with an ERK1/2-specific antibody. Addition of the MEK inhibitor PD 98059 20 minutes before TPO stimulation substantially decreased ERK phosphorylation. The blot was stripped and reprobed with ERK2-specific antibody to demonstrate equal loading of each lane (see lower panel, Fig 1). It is noteworthy that concentrations of PD 98059 as high as 100 μmol/L could not completely inhibit TPO-induced MAPK activation in the BAF3/Mpl cell line, the maximal concentration achievable because of limited inhibitor solubility. These results suggest that an MEK-independent MAPK activation pathway may exist in BAF3 cells. The phosphoinositol-3-kinase (PI3K) pathway has been previously reported to induce MAPK phosphorylation without MEK activation.25 Consistent with this hypothesis, the combination of PD 98059 and Wortmannin to TPO-stimulated BAF3/Mpl cells at the concentration of 100 μmol/L and 100 nmol/L, respectively, resulted in more inhibition of MAPK phosphorylation than either inhibitor alone (data not shown). This result suggests that maximal TPO-induced ERK activation in this cell line may be dependent on both MEK- and PI3K-induced pathways.
TPO-induced MAPK phosphorylation in BAF3/Mpl cells. BAF3/Mpl cells were cultured in serum-free, cytokine-free media for 16 hours. Twenty minutes before stimulation, PD 98059 (PD) at a final concentration of either 50 or 100 μmol/L, was added. Cells were then stimulated by the addition of 14 ng/mL murine TPO for 10 minutes, lysed, and subjected to Western blot analysis. The blot was probed with anti–double-phosphorylated ERKs/MAPK antibody (upper panel), showing 2 specific bands corresponding to ERK1 (44 kD) and ERK2 (42 kD). ERK phosphorylation could be partially inhibited by PD 98059. The blot was then stripped and reprobed with anti-ERK2 antibody (lower panel) to assure equal loading in all lanes.
TPO-induced MAPK phosphorylation in BAF3/Mpl cells. BAF3/Mpl cells were cultured in serum-free, cytokine-free media for 16 hours. Twenty minutes before stimulation, PD 98059 (PD) at a final concentration of either 50 or 100 μmol/L, was added. Cells were then stimulated by the addition of 14 ng/mL murine TPO for 10 minutes, lysed, and subjected to Western blot analysis. The blot was probed with anti–double-phosphorylated ERKs/MAPK antibody (upper panel), showing 2 specific bands corresponding to ERK1 (44 kD) and ERK2 (42 kD). ERK phosphorylation could be partially inhibited by PD 98059. The blot was then stripped and reprobed with anti-ERK2 antibody (lower panel) to assure equal loading in all lanes.
Next, we determined the functional consequences of MAPK activation in BAF3/Mpl cells. Using an MTT reduction assay, we found that proliferation of BAF3/Mpl cells grown in TPO was decreased by PD 98059 in a dose-dependent manner (Fig 2). This suggests that ERK activation contributes to TPO-induced proliferation in BAF3/Mpl cells. In contrast, PD 98059 has no significant effect on IL-3–induced proliferation of BAF3/Mpl cells in 3 separate experiments. Correlated with these data, IL-3 induced only minor ERK phosphorylation in BAF3/Mpl cells compared with TPO (data not shown).
The effect of MEK inhibition on TPO-induced proliferation of BAF3/Mpl cells. BAF3/Mpl cells were cultured in the presence of various concentrations of recombinant murine TPO. The cultures also contained PD 98059 (dissolved in DMSO) at final concentrations of 50 μmol/L (▪) or 100 μmol/L (X), whereas the control cultures contained a similar volume of DMSO (○). The number of living cells was determined after 36-hour cultures using the MTT method described in Materials and Methods. The TPO-induced proliferation is presented as the percentage of maximal IL-3–induced proliferation. The abscissa is displayed in a logarithmic scale. The results represent the mean (±SD) of triplicate determination in a single representative experiment. This experiment has been performed 3 times with similar results.
The effect of MEK inhibition on TPO-induced proliferation of BAF3/Mpl cells. BAF3/Mpl cells were cultured in the presence of various concentrations of recombinant murine TPO. The cultures also contained PD 98059 (dissolved in DMSO) at final concentrations of 50 μmol/L (▪) or 100 μmol/L (X), whereas the control cultures contained a similar volume of DMSO (○). The number of living cells was determined after 36-hour cultures using the MTT method described in Materials and Methods. The TPO-induced proliferation is presented as the percentage of maximal IL-3–induced proliferation. The abscissa is displayed in a logarithmic scale. The results represent the mean (±SD) of triplicate determination in a single representative experiment. This experiment has been performed 3 times with similar results.
MAPK phosphorylation by TPO does not depend on Shc phosphorylation.
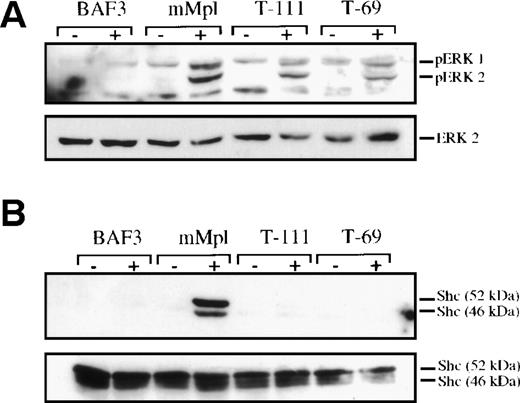
Previously, we used truncated forms of the Mpl cytoplasmic domain to identify subdomains involved in the activation of signaling proteins in response to TPO.11 A similar approach was taken to map the region of Mpl responsible for MAPK phosphorylation. We found that truncation of the 10 carboxy-terminal residues (T-111, missing the primary sites of Mpl tyrosine phosphorylation) resulted in a moderate reduction in detectable phospho-MAPK (Fig3A). Thrombopoietin stimulation in BaF3/Mpl cells with further truncation such that only 69 cytoplasmic amino acids of the cytoplasmic domain remain (T-69, including box1 and box2) still caused definite MAPK phosphorylation. In contrast, TPO does not induce significant tyrosine phosphorylation of the adapter protein Shc (Fig 3B) in cells expressing either of the truncated receptors. This difference is significant because Shc, which binds to the distal portion of the phosphorylated Mpl cytoplasmic domain, was believed to be a major route to Ras-Raf-MEK-MAPK activation. Therefore, the significant ERK phosphorylation in these cells (∼70% and 60% for T-111 and T-69, respectively, compared with full-length Mpl as measured by densitometry) suggests that a part of TPO-induced ERK activation is Shc independent.
MAPK phosphorylation is not fully dependent on Shc phosphorylation. Cell lysates were prepared either before (−) or after (+) stimulation with exogenous TPO (14 ng/mL for 10 minutes). Parental BAF3 cells were used as well as clones engineered to express the full-length murine Mpl receptor (mMpl) or mutated receptors, which were truncated after either 111 or 69 cytoplasmic amino acids (T-111 and T-69, respectively).11 (A) One hundred micrograms of each lysate was evaluated by Western blotting and probed to detect double-phosphorylated ERK1 and ERK2. The blot was stripped and reprobed with ERK2 antibody to confirm equal loading in all lanes. (B) Shc was immunoprecipitated from 1 mg of each cell lysate. The immunoprecipitated protein was evaluated by Western blotting and probed with a phosphotyrosine-specific antibody (4G10). The blot was stripped and reprobed to verify the presence of Shc in all lanes.
MAPK phosphorylation is not fully dependent on Shc phosphorylation. Cell lysates were prepared either before (−) or after (+) stimulation with exogenous TPO (14 ng/mL for 10 minutes). Parental BAF3 cells were used as well as clones engineered to express the full-length murine Mpl receptor (mMpl) or mutated receptors, which were truncated after either 111 or 69 cytoplasmic amino acids (T-111 and T-69, respectively).11 (A) One hundred micrograms of each lysate was evaluated by Western blotting and probed to detect double-phosphorylated ERK1 and ERK2. The blot was stripped and reprobed with ERK2 antibody to confirm equal loading in all lanes. (B) Shc was immunoprecipitated from 1 mg of each cell lysate. The immunoprecipitated protein was evaluated by Western blotting and probed with a phosphotyrosine-specific antibody (4G10). The blot was stripped and reprobed to verify the presence of Shc in all lanes.
ERK is activated by TPO in purified murine megakaryocytes.
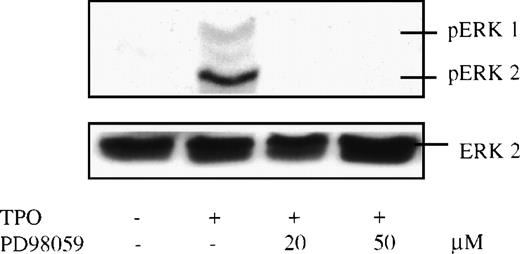
To determine whether the MAPKs are also activated by TPO in primary cells, mature murine megakaryocytes were expanded in vitro by incubation of whole bone marrow cells in serum-free media containing exogenous TPO. The large cells were purified using a unit gravity sedimentation column. The resulting cells (∼200,000 cells/mouse) contained approximately 90% mature megakaryocytes by immunostaining. Two hundred thousand megakaryocytes were starved in serum-free, cytokine-free conditions for 7 hours. The short period (compared with BAF3 cells) was chosen because primary megakaryocytes undergo apoptosis more rapidly than do transformed cells when deprived of serum and growth factors (data not shown). Megakaryocytes were then stimulated by TPO in the presence or absence of the MEK inhibitor (PD 98059), lysed, and subjected to Western blot analysis. Like the results in BAF3/Mpl cells, TPO induced marked ERK phosphorylation in normal murine megakaryocytes (Fig 4). But, in contrast to the transformed BAF3/Mpl cells, this effect was completely inhibited by the MEK inhibitor alone at a relatively low concentration of PD 98059 (20 μmol/L). Some investigators have proposed that the protein kinase C pathway plays a major role in MAPK activation. However, we found that ERK phosphorylation was only partially inhibited by Bis-indolylmalemide I, a specific protein kinase C (PKC) inhibitor (data not shown).
TPO-induced MAPK phosphorylation in purified murine megakaryocytes. Bone marrow cells were harvested from mice preinjected with 2 μg/d human TPO for 5 days, grown in serum-free medium with 35 ng/mL murine TPO for 3 days, and then purified by an albumin density-gradient column. Purified megakaryocytes were incubated in a serum-free, cytokine-free medium for 7 hours and stimulated with 14 ng/mL murine TPO for 10 minutes. Twenty minutes before stimulation, PD 98059 (PD) at 20 μmol/L or 50 μmol/L was added. The cell lysates were size-fractionated, transferred to nitrocellulose, and probed with anti–double-phosphorylated ERK antibody (upper panel). The blot was then stripped and reprobed with anti-ERK2 antibody (lower panel) to assure equal loading in all lanes.
TPO-induced MAPK phosphorylation in purified murine megakaryocytes. Bone marrow cells were harvested from mice preinjected with 2 μg/d human TPO for 5 days, grown in serum-free medium with 35 ng/mL murine TPO for 3 days, and then purified by an albumin density-gradient column. Purified megakaryocytes were incubated in a serum-free, cytokine-free medium for 7 hours and stimulated with 14 ng/mL murine TPO for 10 minutes. Twenty minutes before stimulation, PD 98059 (PD) at 20 μmol/L or 50 μmol/L was added. The cell lysates were size-fractionated, transferred to nitrocellulose, and probed with anti–double-phosphorylated ERK antibody (upper panel). The blot was then stripped and reprobed with anti-ERK2 antibody (lower panel) to assure equal loading in all lanes.
MAPK activation in megakaryocytes is transient.
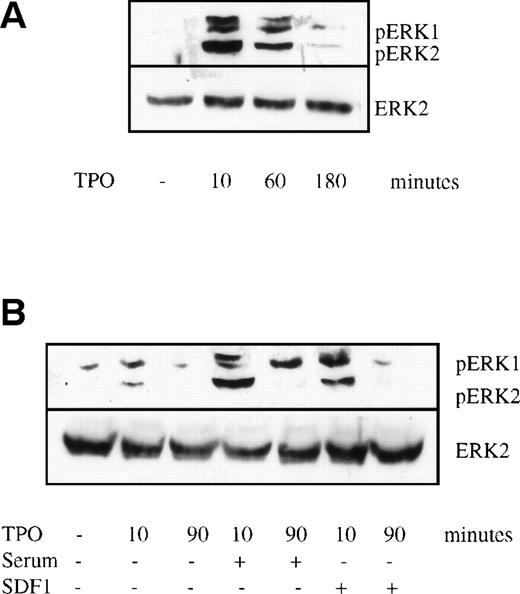
Evidence from other cell systems suggests that the duration of MAPK activation plays an important role in determining the cellular response; transient MAPK activation (minutes to hours) is typical of proliferation, whereas prolonged MAPK activation (lasting many hours or several days) is characteristic of differentiation.14 To evaluate the duration of ERK activation, purified murine megakaryocytes were starved for 7 hours and then stimulated by TPO for 10 minutes, 1 hour, and 3 hours. ERK phosphorylation was greatest at 10 minutes and then progressively decreased at the later time points (Fig 5A), becoming nearly undetectable by 3 hours. This result differs from a previous study in the UT-7 cell lines that suggested that sustained ERK activation was required for cellular differentiation.17
Time course of TPO-induced MAPK phosphorylation. Bone marrow cells were prepared and stimulated as described in the legend to Fig 4. Cell lysates were subjected to Western blot analysis and probed with anti–double-phosphorylated ERK antibody (upper panels) and then stripped and reprobed with anti-ERK2 antibody (lower panels). The cultures contained (A) 14 ng/mL TPO alone or (B) TPO plus 10% (vol:vol) fetal calf serum or TPO plus 100 ng/mL SDF-1. The TPO-inducible bands found just above ERK1 were sometimes seen. They were not cross-reactive with anti-ERK1 antibody as the blot was stripped and reprobed. The origin of these bands is still unclear.
Time course of TPO-induced MAPK phosphorylation. Bone marrow cells were prepared and stimulated as described in the legend to Fig 4. Cell lysates were subjected to Western blot analysis and probed with anti–double-phosphorylated ERK antibody (upper panels) and then stripped and reprobed with anti-ERK2 antibody (lower panels). The cultures contained (A) 14 ng/mL TPO alone or (B) TPO plus 10% (vol:vol) fetal calf serum or TPO plus 100 ng/mL SDF-1. The TPO-inducible bands found just above ERK1 were sometimes seen. They were not cross-reactive with anti-ERK1 antibody as the blot was stripped and reprobed. The origin of these bands is still unclear.
We hypothesized that other growth factors or cytokines might synergize with TPO to produce sustained ERK activation in primary megakaryocytes and thereby fall in line with the conclusions of others.14To evaluate this possibility, we studied the effect of fetal calf serum, SDF-1, and murine SCF on MAPK activation when used in combination with TPO. Each of these agents has been shown to act in synergy with TPO to augment megakaryocyte development.2,26 27 Murine megakaryocytes were starved and stimulated with TPO for 10 and 90 minutes with or without murine 100 ng/mL SCF, 10% fetal calf serum, or 100 ng/mL SDF-1. Neither fetal calf serum nor SDF-1 could prolong MAPK responses (Fig 5B), although the intensity of the double-phosphorylated ERK activation was significantly enhanced by both serum and SDF-1, demonstrating that converging signaling pathways from other receptors can enhance MAPK response to TPO, but do not prolong it. Murine SCF could neither enhance nor prolong MAPK phosphorylation in megakaryocytes (data not shown).
MAPK pathway contributes to megakaryocyte endomitosis.
To identify the functional effects of the MAPK pathway in megakaryocytes, murine marrow cells were cultured in serum-free media with TPO for 3 days. PD 98059 or diluent (DMSO) was added at the beginning of culture. The low amount of DMSO used in the culture had no adverse effects on bone marrow cells as compared with culture without DMSO. Nonadherent cells were stained by trypan blue and viable cells were counted using a hemocytometer. Total cell numbers were lower in the inhibitor group than in the control group (see table in Fig 7). This effect was most obvious on nonhematopoietic, adherent cells (bone marrow stromal cells). As seen by inverted microscopy, the numbers of adherent cells were greatly reduced at higher doses of PD 98059. At 50 μmol/L, the adherent cells were virtually absent, suggesting that MAPK pathway is essential for survival of these cells. The percentage of megakaryocytes in each culture were determined by staining cells with FITC-tagged antibody to CD41, a megakaryocyte-specific marker, and analyzing by flow cytometry. The total number of megakaryocytes was calculated as the total number of cells multiplied by the percentage of CD41+ cells. In multiple experiments, megakaryocyte number in whole bone marrow culture was not significantly affected by inhibition of MEK. We next studied the effect of blocking the MAPK pathway on several aspects of megakaryocyte differentiation. Cytospins were prepared and the slides were Wright-stained for morphologic analysis in 2 separate experiments. Megakaryocytes, with or without inhibitor, display similar morphology by light microscopy (Fig 6A and B). They could increase in size and nuclear lobulation, and some cells in both groups developed pink intracytoplasmic granules. Surface acetylcholinesterase activity, another marker of murine megakaryocyte differentiation, appeared similar in both the inhibitor and control groups in 2 separate experiments (Fig 6C and D). These results were observed at PD 98059 concentrations of 20 and 50 μmol/L. Therefore, we did not detect nonspecific toxicity of the MEK inhibitor on megakaryocytes in culture within this dose range.
MEK inhibition has no obvious effect on murine megakaryocyte morphology and acetylcholinesterase activity in whole marrow cultures. Murine bone marrow cells were harvested and split into 2 flasks. Both were grown in serum-free media with 35 ng/mL murine TPO for 3 days. One culture contained 50 μmol/L PD 98059 and the other contained an equal amount of DMSO. Cytospin preparations were then performed and stained with Giemsa ([A] DMSO; [B] PD 98059) or acetylcholinesterase activity (brown staining; [C] DMSO; [D] PD 98059).
MEK inhibition has no obvious effect on murine megakaryocyte morphology and acetylcholinesterase activity in whole marrow cultures. Murine bone marrow cells were harvested and split into 2 flasks. Both were grown in serum-free media with 35 ng/mL murine TPO for 3 days. One culture contained 50 μmol/L PD 98059 and the other contained an equal amount of DMSO. Cytospin preparations were then performed and stained with Giemsa ([A] DMSO; [B] PD 98059) or acetylcholinesterase activity (brown staining; [C] DMSO; [D] PD 98059).
As megakaryocytes mature, they undergo DNA replication without cytokinesis. The DNA content increases from 2 N to 4 N, 8 N, 16 N, up to 128 N in some cells. This process, termed endomitosis, is unique to normal megakaryocytic differentiation.28 To evaluate the DNA content of individual megakaryocytes, we used flow cytometry with CD41 to distinguish megakaryocytes from other lineages in liquid culture, and after cell permeabilization, with PI staining to evaluate DNA content. As shown in Fig 7, treatment of megakaryocyte cultures for 3 days with PD 98059 greatly reduced the proportion of cells developing 32 N and 64 N DNA content. In contrast, when murine marrow cells were grown in the same conditions, except with Bis-Indolylmalemide I (BIM), a specific PKC inhibitor, the cells developed their full polyploid potential (data not shown).
The effect of MEK inhibition on megakaryocyte endomitosis. Murine bone marrow cells were cultured as described in the legend to Fig 6. Cells were then stained with FITC-conjugated anti-CD41, solubilized, and stained with PI. In the left-hand panels, the abscissa represents log FITC intensity and the ordinate represents cell size, as determined by forward light scatter. Megakaryocytes, identified by CD41 positivity, were gated to analyze for DNA content by PI staining (right-hand panels). The dot plot of cells stained with the isotype control for anti CD41-FITC is also shown ([A] isotype). The log intensity of PI is on the abscissa and the numbers of cells are on the ordinate. The bars mark the DNA contents of 2N, 4N, 8N, 16N, 32N, 64N, and 128N, respectively. Cells grown in DMSO (A) are compared with cells grown in PD 98059 (B). These data are the representative results from 3 separate experiments. Cell numbers after 72-hour cultures are shown in the table (C). The total numbers of viable cells were determined using a hemocytometer. The percentage of CD41+cells in each population was determined by immunofluorescent staining and flow cytometry. Megakaryocyte numbers were calculated as follows: total viable cells multiplied by the percentage of CD41+cells. The results represent the mean of 3 to 4 experiments.
The effect of MEK inhibition on megakaryocyte endomitosis. Murine bone marrow cells were cultured as described in the legend to Fig 6. Cells were then stained with FITC-conjugated anti-CD41, solubilized, and stained with PI. In the left-hand panels, the abscissa represents log FITC intensity and the ordinate represents cell size, as determined by forward light scatter. Megakaryocytes, identified by CD41 positivity, were gated to analyze for DNA content by PI staining (right-hand panels). The dot plot of cells stained with the isotype control for anti CD41-FITC is also shown ([A] isotype). The log intensity of PI is on the abscissa and the numbers of cells are on the ordinate. The bars mark the DNA contents of 2N, 4N, 8N, 16N, 32N, 64N, and 128N, respectively. Cells grown in DMSO (A) are compared with cells grown in PD 98059 (B). These data are the representative results from 3 separate experiments. Cell numbers after 72-hour cultures are shown in the table (C). The total numbers of viable cells were determined using a hemocytometer. The percentage of CD41+cells in each population was determined by immunofluorescent staining and flow cytometry. Megakaryocyte numbers were calculated as follows: total viable cells multiplied by the percentage of CD41+cells. The results represent the mean of 3 to 4 experiments.
The interpretation of these data is still limited. Whole bone marrow cultures contain accessory cells that can produce growth factors and cytokines. Inhibition of MAPK decreased the numbers of these cells considerably and may have indirectly affected megakaryocyte maturation. To address this problem, bone marrow cells were selected for CD41 expression using a high gradient magnetic system (MiDiMACS) before culture in TPO. Consistent with the results in whole bone marrow cell cultures, the proportion of polyploid cells was substantially lower in the presence of PD 98059 (Fig 8). Data in these partially purified cells suggest that MEK inhibition directly affects megakaryocyte development. However, in contrast to the result in whole bone marrow cultures, the total number of cells and megakaryocytes decreased significantly in purified cell cultures containing PD 98059. Moreover, cells became smaller than the control culture as assessed by Giemsa staining.
The effect of MEK inhibition on megakaryocyte endomitosis in CD41-selected cell culture: Analysis of polyploid cells. Murine bone marrow cells were harvested and CD41+ cells were selected using a high gradient magnetic system (MiDiMACS). Purified cells were grown in serum-free media containing 35 ng/mL murine TPO for 84 hours. In 1 culture, PD 98059 at final concentration of 25 μmol/L was added initially and a similar dose was added 24 hours later (B). An equal volume of DMSO was added in the control culture. Cells were then stained with FITC-conjugated anti-CD41, solubilized, and stained with PI before being analyzed using flow cytometry. Only viable cells, defined by forward and side light scattering characteristics, were analyzed for fluorescent intensity. After culture, there was an 80% reduction in the total number of CD41+ cells in the presence of the inhibitor compared with control. However, equal number of cells were counted in each group. The histograms show the ploidy distribution of cells with DNA contents of 4N or higher. The consecutive peaks represent the frequencies of cells with DNA contents, as indicated by bars. These data are the representative results from 3 separate experiments.
The effect of MEK inhibition on megakaryocyte endomitosis in CD41-selected cell culture: Analysis of polyploid cells. Murine bone marrow cells were harvested and CD41+ cells were selected using a high gradient magnetic system (MiDiMACS). Purified cells were grown in serum-free media containing 35 ng/mL murine TPO for 84 hours. In 1 culture, PD 98059 at final concentration of 25 μmol/L was added initially and a similar dose was added 24 hours later (B). An equal volume of DMSO was added in the control culture. Cells were then stained with FITC-conjugated anti-CD41, solubilized, and stained with PI before being analyzed using flow cytometry. Only viable cells, defined by forward and side light scattering characteristics, were analyzed for fluorescent intensity. After culture, there was an 80% reduction in the total number of CD41+ cells in the presence of the inhibitor compared with control. However, equal number of cells were counted in each group. The histograms show the ploidy distribution of cells with DNA contents of 4N or higher. The consecutive peaks represent the frequencies of cells with DNA contents, as indicated by bars. These data are the representative results from 3 separate experiments.
DISCUSSION
The cloning of TPO has opened a new era of research into the molecular mechanisms of megakaryocyte development. Intracellular changes after ligand-receptor binding have been extensively studied in the past several years. Various immortalized cell lines expressing the TPO receptor have provided models for signal transduction experiments, because the large number of cells necessary for biochemical studies can be obtained. We have demonstrated in this study that MAPKs (ERK1 and ERK2) are activated in the BAF3/Mpl cell lines. Like other studies of the effects of MAPKs, our results with the MEK inhibitor, PD 98059, demonstrated an inhibitory effect on BAF3/Mpl cell proliferation. Therefore, the MAPK kinase pathway contributes to proliferation of BAF3/Mpl cells, but the effects on cellular maturation could not be evaluated, because these cells do not differentiate in response to TPO. Interestingly, PD 98059 could not totally block MAPK activation in this cell line. It is possible that there may be other MEK-independent pathways that are able to directly activate ERK, such as the PI3K pathway. The inhibitor, PD 98059, selectively inhibits MEK1 relative to MEK2.29 Therefore, an alternative explanation is that MEK2 may be more critical for ERK phosphorylation in BAF3 cells. Consequently, MEK1 inhibition alone by this compound cannot completely block ERK phosphorylation. In 1 series of experiments, the PI3K inhibitor, Wortmannin, when added to PD 98059, completely blocked ERK1 and ERK2 phosphorylation. However, additional experiments will be required to confirm the role of PI3K in ERK activation in BAF3 cells. A concern about this type of experiment is the specificity of a chemical inhibitor. PD 98059 may be able to interfere with biochemical reactions in cells other than the MAPK pathway. We have transfected BAF3/Mpl cells with a hyperactive ERK2 construct (a generous gift from Dr Terry Vik [Wells Center for Pediatric Research, Indianapolis, IN] and colleagues16) and found that these cells had higher proliferative response to TPO compared with cells transfected with a control vector. This result confirms that the ERK pathway is involved in TPO-induced proliferation. Although the use of cell lines is convenient, extrapolation to signaling in normal cells is not always reliable, because BAF3/Mpl may have acquired an aberrant pathway for unlimited growth.
As a result of these limitations, we expanded our studies of MAPK signaling to primary cell cultures, a physiologically relevant model for studying hematopoiesis. In the present study, 2 of the MAPKs, ERK1 and especially ERK2, have been found to be reproducibly phosphorylated after 10 minutes of TPO stimulation in murine megakaryocytes. We also found that MAPK phosphorylation wanes over a 3-hour time course, despite the continued presence of TPO, suggesting a role for an as-yet unidentified phosphatase. Addition of either fetal calf serum or SDF-1 increased the intensity of MAPK phosphorylation but could not prolong the response. Studies in other cell systems suggest that the duration of ERK activation affects the cellular response. A study in a pheochromocytoma cell line, PC12, has shown that transient ERK activation by epidermal growth factor (EGF) resulted in cell proliferation, but sustained ERK activation by nerve growth factor (NGF) resulted in differentiation.14 In PC12 cells engineered to express the platelet-derived growth factor (PDGF) receptor, PDGF induced sustained activation of ERK and differentiation.30 This may be the result of different rates of receptor downregulation, because the EGF receptor is downregulated more rapidly than the NGF receptor14 or differential activation of cellular phosphatases. Interestingly, PC12 overexpressing the EGF receptor can also cause differentiation, suggesting that the number of surface receptors determines the duration of responses.31 Nevertheless, this differential response may be dependent on some unknown property of this transformed cell line and may not represent the actual phenotype of primary neuronal cells. A recent study has shown that thrombopoietin induces sustained ERK activation in UT7/Mpl cells and results in cellular differentiation.17 This cell line was engineered to overexpress the Mpl receptor (possibly greater than Mpl expression in normal megakaryocytes that express ∼1,900 Mpl receptors per cell32). Therefore, sustained MAPK response in cell lines might be an artifact attributable to the overexpression of the Mpl receptor. Alternatively, mature and immature megakaryocytes may respond to TPO differently. Our density gradient purification system enriched primarily mature megakaryocytes that may no longer require or may no longer generate a prolonged MAPK signal. Therefore, the kinetics of the ERK response in immature megakaryocytes or their progenitors may require further investigation. Unfortunately, at present, primary megakaryocyte progenitors cannot be obtained in sufficient quantities for biochemical analysis.
The pathway from the Mpl receptor to MAPK phosphorylation/activation has yet to be fully defined. Much evidence has been gathered suggesting that the Shc adapter protein interacts with cytokine receptors via its phosphotyrosine binding domain, and upon phosphorylation, Shc can form a molecular bridge leading to Ras activation (association of GRB2/SOS complex). Previous reports suggested that the loss of Shc activity blocked TPO-induced partial differentiation of WEHI-3B and M1 cell lines.12 However, Porteu et al33 reported that deletion of Mpl cytoplasmic residues 71-94 prevented MAPK activation despite retaining the Shc recruitment and activation site. Our results clearly demonstrate that neither Shc phosphorylation nor residues 71-94 are absolutely required for TPO-induced MAPK phosphorylation in BAF3/Mpl cells (Fig 3). Such discrepant results further point out concerns over too great a reliance on conclusions derived from transformed cell lines. The Shc-independent pathway of ERK activation has to be further determined, but in our view, should be studied in primary cells.
In our culture system, bone marrow cells were grown in TPO-containing serum-free media for 3 to 4 days before the numbers and various aspects of differentiation of megakaryocytes were evaluated. Because of the short duration of culture, the roles of MAPK in very primitive cells cannot be evaluated. The stages of development we studied are the steps from committed megakaryocyte precursors to fully mature megakaryocytes. As shown in Fig 7C, the number of CD41+ megakaryocytes at the end of whole marrow cultures was not significantly affected by MAPK inhibition, whereas total cell numbers decreased. This indicates that MAPK is involved in proliferation of other murine marrow cells but is less critical for megakaryocytes, at least within the complex cellular environment of a whole marrow culture. Nevertheless, at 20 μmol/L PD 98059, there was clear inhibition of megakaryocyte endomitosis. At this dose, MAPK phosphorylation was markedly inhibited in megakaryocytes (Fig 3). Megakaryocyte morphologies, as observed by Giemsa staining and acetylcholinesterase activity, were all comparable between control cells and those grown in PD 98059, suggesting that the effect on endomitosis is not a nonspecific toxicity of PD 98059. However, whole marrow cultures also contain stromal cells that could possibly produce cytokines affecting megakaryocyte development. The inhibitor PD 98059 eliminated these stromal cells from the cultures. Thus, it was possible that the effects of the inhibitor on megakaryocytes were indirect. To address this issue, we purified CD41+ marrow cells that were highly enriched in megakaryocyte progenitors. The addition of PD 98059 to these cultures again led to substantial blockade of megakaryocyte endomitosis. However, unlike the whole bone marrow cells, culture of CD41-selected cells in PD 98059 significantly reduced the number and size of CD41+ cells present after 84 hours of culture. Although we cannot fully explain the difference between the 2 culture systems, it is possible that stromal cells in whole bone marrow can elaborate factors to support megakaryocyte growth compensating for the MEK inhibition. However, these factors in whole marrow could not compensate for the inhibition of the endomitotic process, suggesting that MAPK is critical for full polyploidization.
Polyploidy is widely believed to be essential for full megakaryocyte development, especially for maximal platelet production. An in vivo experiment has demonstrated that TPO is essential for full ploidy development.34 We propose that this action of TPO is mediated, at least in part, via the ERK-MAPK pathway. However, the mechanisms of MAPK-mediated megakaryocyte endomitosis remain unclear. MAPK has been shown to increase cyclin D1 expression in fibroblasts.35 Moreover, overexpression of cyclin D1 in megakaryocytic cell lines has been shown to enhance phorbol ester-induced differentiation.36 However, recent studies showed that cyclin D3 was prominently expressed in megakaryocytes and necessary for megakaryocyte differentiation,37,38 because antisense oligonucleotides to cyclin D3 inhibit megakaryocyte endomitosis in murine bone marrow culture system.37 In addition, cyclin D3 overexpression in vivo by a transgenic mouse model resulted in an increase in megakaryocyte number, size, and ploidy.38 TPO has been shown to increase cyclin D3 expression in megakaryocytes in vivo.38 Whether this increase is influenced by MAPK activation remains to be determined.
In conclusion, we found that the MAPK pathway is transiently activated by TPO in normal murine megakaryocytes, reflecting the effects in an Mpl bearing leukemic cell line. Although they have been shown to affect the proliferation of Mpl-expressing cell lines or to stimulate various aspects of cellular differentiation in other cell lines, we have found that ERK1 and ERK2 play a very important role in megakaryocyte development, supporting endomitosis. The target of MAPK responsible for coupling to the cell cycle changes necessary for the uncoupling of DNA synthesis and mitosis, the precise molecular pathways coupling the Mpl receptor and MAPK activation, and the extent to which other members of the MAPK family or other signaling pathways contribute to megakaryocyte differentiation remain active areas for further investigation.
ACKNOWLEDGMENT
The authors thank Norma Fox and Colleen O’Rork for technical assistance; Chong Kim and Kathryn Allen for assistance with flow cytometry; and Catherine Carow and Yoshitaka Miyakawa for their thoughtful discussions. Furthermore, we thank Zymogenetics (BAF3/Mpl cells), Kirin Pharmaceuticals (purified TPO and SCF), and Naoki Yamamoto (SDF-1) for generously providing important reagents.
P.R. and J.G.D. contributed equally to this work.
Supported by National Institutes of Health (NIH) Grant No. R01DK49855 (to K.K.); by Chulalongkorn University, Thailand (to P.R.); and by NIH Grant No. K08HL03498 and an ASH Fellow Scholar Award (to J.G.D.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kenneth Kaushansky, MD, Division of Hematology, University of Washington Medical Center, Seattle, WA 98195.


![Fig. 6. MEK inhibition has no obvious effect on murine megakaryocyte morphology and acetylcholinesterase activity in whole marrow cultures. Murine bone marrow cells were harvested and split into 2 flasks. Both were grown in serum-free media with 35 ng/mL murine TPO for 3 days. One culture contained 50 μmol/L PD 98059 and the other contained an equal amount of DMSO. Cytospin preparations were then performed and stained with Giemsa ([A] DMSO; [B] PD 98059) or acetylcholinesterase activity (brown staining; [C] DMSO; [D] PD 98059).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/4/10.1182_blood.v94.4.1273/4/m_blod41604006ay.jpeg?Expires=1767732860&Signature=FzmyasnuHdWV0Y8gCra1UOE5BSLvYo8r5~AKsflOa08AVGXyF-x30LrKWAXU9GCkXx0mfFuV8LPOCVzovWSokVmo76X-PUwkeSGndOv0qYIIFSt-ciZUgLwnDOCqAHHF5Wm2wbnFjuk~Mn2ngB-knqf2ZoTco~WwJSSFR6IdUhPIuXW3s69HNDjBxdSpGFSC4YsNu-z82GTjZX1gW~Em7OE1OX-CL6l1E2OqeR~DZ8Wgjsg~piWQrrXnui4Wtf0jsOQCPf6FaJ92xBxV-kZvHoPTL-qPxQJ8mM4A22TDuGAUF6okdOY4YWRAeQIJQUTzmdN1m7orP2PBk1UEcPb97w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. MEK inhibition has no obvious effect on murine megakaryocyte morphology and acetylcholinesterase activity in whole marrow cultures. Murine bone marrow cells were harvested and split into 2 flasks. Both were grown in serum-free media with 35 ng/mL murine TPO for 3 days. One culture contained 50 μmol/L PD 98059 and the other contained an equal amount of DMSO. Cytospin preparations were then performed and stained with Giemsa ([A] DMSO; [B] PD 98059) or acetylcholinesterase activity (brown staining; [C] DMSO; [D] PD 98059).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/4/10.1182_blood.v94.4.1273/4/m_blod41604006by.jpeg?Expires=1767732860&Signature=d~AXdI79ZD1KF1YMj2qRZR3VF2s5LsyXgt~nH8t9CJ9GPmFyifr0K9GvtDIWSUQON-NS~GYM-1ih~prlantspK3wyZtXsoo4rnBrC4uHXP4Psgub~uEzEtCEO4Y5BsJNqjBH6zlLvjqJAcHtaoUOVgxRucfskpr-FNqAzo0ADkPQKXJlMF2ol4--a53CYnLglxiVOJspZ6T2Mg5lq1d1MyQNbYJlcI1mP0ZGdqr50l99f-OavVkRZkqS1q-Ht1lfs9OyU0AHXrHViFES5WxAjpIDMRMS7uRuvTlt9mmmWEilnUOhZUNoVLHulhz62DCJuKWvkRM837WfywTnU5kJ8g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. MEK inhibition has no obvious effect on murine megakaryocyte morphology and acetylcholinesterase activity in whole marrow cultures. Murine bone marrow cells were harvested and split into 2 flasks. Both were grown in serum-free media with 35 ng/mL murine TPO for 3 days. One culture contained 50 μmol/L PD 98059 and the other contained an equal amount of DMSO. Cytospin preparations were then performed and stained with Giemsa ([A] DMSO; [B] PD 98059) or acetylcholinesterase activity (brown staining; [C] DMSO; [D] PD 98059).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/4/10.1182_blood.v94.4.1273/4/m_blod41604006cy.jpeg?Expires=1767732860&Signature=nZ7us8P3rMGGJ8-oIeRIh9qSQS8FiLiEpqWDRMkIP3b~9vu3G59TlbXc0VTgf3zJEDaOpj5JWeucpB2tyIYJwEwVgRJMBhhMfMWeKi8YHGOKPQ~HOnNRA6mZJ8qW3dVFxgaxVUlJArKb6QWyA4CoEegAr9E-wa899ydzEWT1S1cNCH0fLrXU67cRXIJITmDJXM79b-wXvH2LnRqSK3W0JsG0apT~kdj6kyilM~gevPoKg7qCd22p-jvwivjftt0wtI8DxzfbjMpZeaW8sej7qKph2OtGLVhjLQ9BIqOxlUccws6AIUEBynfQzsozbnZyWY~rXlvLY-hhZk3e0f0lYw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. MEK inhibition has no obvious effect on murine megakaryocyte morphology and acetylcholinesterase activity in whole marrow cultures. Murine bone marrow cells were harvested and split into 2 flasks. Both were grown in serum-free media with 35 ng/mL murine TPO for 3 days. One culture contained 50 μmol/L PD 98059 and the other contained an equal amount of DMSO. Cytospin preparations were then performed and stained with Giemsa ([A] DMSO; [B] PD 98059) or acetylcholinesterase activity (brown staining; [C] DMSO; [D] PD 98059).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/4/10.1182_blood.v94.4.1273/4/m_blod41604006dy.jpeg?Expires=1767732860&Signature=rpvuiajxsXaelzU~QQGENkSiD0MA0JlX4typn8vuBRsXrlNN~vIJ6dJAxpBMkk-G16MuPF6p4Ffh~CVB-dEdvNBxApoEhaH4esUt7AfuaSEBAm4nsI5Ob0PLH-S2y-uehe-Ie0--fIJEeBXBSTknpOq6YsMCd8TYVn7m56GNtzt5Mkp8K07P2fPHvgOFF2~Kexc9GSBeLAX8PCV-UOWKUEcFu1rdZnQhA1ggcGNPDuS94KQFmGnOi7v9lFYeWX3xfNbd9mguNZxRczwDGy-ktCopuHxn2FkWG4F6TtuJ0PnGD-XPIDOcm03up-SBVlTnKciukWOnB3MgQJJh1zhWbw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. The effect of MEK inhibition on megakaryocyte endomitosis. Murine bone marrow cells were cultured as described in the legend to Fig 6. Cells were then stained with FITC-conjugated anti-CD41, solubilized, and stained with PI. In the left-hand panels, the abscissa represents log FITC intensity and the ordinate represents cell size, as determined by forward light scatter. Megakaryocytes, identified by CD41 positivity, were gated to analyze for DNA content by PI staining (right-hand panels). The dot plot of cells stained with the isotype control for anti CD41-FITC is also shown ([A] isotype). The log intensity of PI is on the abscissa and the numbers of cells are on the ordinate. The bars mark the DNA contents of 2N, 4N, 8N, 16N, 32N, 64N, and 128N, respectively. Cells grown in DMSO (A) are compared with cells grown in PD 98059 (B). These data are the representative results from 3 separate experiments. Cell numbers after 72-hour cultures are shown in the table (C). The total numbers of viable cells were determined using a hemocytometer. The percentage of CD41+cells in each population was determined by immunofluorescent staining and flow cytometry. Megakaryocyte numbers were calculated as follows: total viable cells multiplied by the percentage of CD41+cells. The results represent the mean of 3 to 4 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/4/10.1182_blood.v94.4.1273/4/m_blod41604007x.jpeg?Expires=1767732860&Signature=v0oq9ZXp5nt6rSJqQ6IWqubKSbsZU8ydixN6QWD~D~xYOPvKyhrWNcXh4pZf2H5L5IzbgSJwAcYNS14c61nyn2vwbvd9ChTrLY9W96nSOEeFXZ5EM5GvnUlzwUhHdqkSlcAcxXOBBVBCW9dU9kFKPr-BnXH953aTjSS57vACAyFPRUyDWEe-8~hzw57puu9e7qZv0BXM4H0u2NpjQ23FxRwQ8Gsd5CJVyXOfQjfJv15G4OydUjXSi0X1lkmdH2L4HBjOZlR31s0Se2-TyRr2eZ9nwZRMpsj6rhm4awvIbok79GjswP5Iv~yjm~1pnnC8ew2D2dzQXd2wbjXo4nhhKA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal