ELL-12, a liposome formulation of the ether-lipid 1-O-octadecyl-2-O-methyl-sn-glycero-3-phosphocholine (ET-18-OCH3), is a nonmyelosuppressive antiproliferative agent that is more effective and less toxic than the ether lipid itself in tumor model systems. We found that ELL-12 induced apoptosis in Jurkat, H9, and U-937 cells that was preceded by activation of executioner caspases. In addition, ELL-12 triggered release of cytochrome c from mitochondria to the cytoplasm before caspase-9 activation. Apoptosis, activation of caspases, and cytochromec release were blocked by Bcl-xL overexpression in Jurkat T cells, suggesting a critical role for mitochondria in ELL-12–triggered cell death. Furthermore, ELL-12 had no effect on expression of CD95 ligand, and inhibition of the Fas signaling pathway with antagonistic anti-CD95 antibody did not affect apoptosis induced by ELL-12. Hence, ELL-12 could be a promising adjunct for the treatment of tumors in addition to myelosuppressive chemotherapeutic drugs and/or those that use the CD95-ligand/receptor system to trigger apoptosis.
ALKYL-LYSOPHOSPHOLIPIDS, such as the ether lipid ET-18-OCH3(1-O-octadecyl-2-O-methyl-sn-glycero-3-phosphocholine), are antitumor agents that, unlike many other cancer therapeutic agents, do not directly target cellular DNA and are not myelosuppressive in animal models.1-3 Although ET-18-OCH3 has antitumor activity in several animal tumor models, its clinical use has been hampered by systemic toxic effects, including hemolysis.4-6 Several reports have documented the advantages of incorporating anticancer agents into liposomes to improve therapeutic efficiency while markedly reducing nonspecific toxicity in vivo.7-12 A stable, well-characterized liposome-based formulation of ET-18-OCH3, known as ELL-12, has been shown to be acutely less hemolytic both in vitro and in vivo13-15and is currently in clinical trials.16 In mice bearing P388 leukemia, Lewis lung carcinoma, or B16/F10 melanoma, ELL-12 had a therapeutic index at least 4-fold higher than ET-18-OCH3.13 By preventing the serious side effects of ET-18-OCH3, ELL-12 could potentially complement standard anticancer chemotherapeutic agents that target DNA.
The mechanism of the growth-inhibitory effects of ET-18-OCH3 has been extensively studied and appears to involve modulation of signal transduction events, including protein kinase C activity, interaction with cell membranes, inhibition of phospholipase C, influences on calcium flux, and modification of the cellular oxidative state.17-26 Recent work has established that the antineoplastic effect of ET-18-OCH3 could be due to its ability to promote apoptosis, both in human tumor cell lines and in primary tumor cell cultures from cancer patients.27-30Although the antiproliferative effect of ELL-12 has also been associated with induction of apoptosis,15 little is known of the mechanism of action of ELL-12.
Understanding of the biochemical events in apoptosis was significantly advanced by the identification of a family of aspartate-specific cysteine proteases, named caspases, which are involved in the initiation and amplification of the cell death machinery.31,32 Each caspase is synthesized as an inactive zymogen (30 to 50 kD) and is converted by proteolytic cleavage to yield an active enzyme composed of approximately 20-kD and approximately 10-kD subunits. The caspase family has been divided into initiator caspases (eg, caspases-8 and -10) that can activate downstream executioner caspases (eg, caspases-3, -7, and -6) responsible for the cleavage of a limited set of proteins resulting in the disassembly of the cell.31,32 Accumulating evidence also suggests that mitochondria have an essential role in the apoptotic program, and cytochrome c (cyt c) release from mitochondria is now emerging as an important step in the apoptotic pathway.33,34 Diverse apoptotic stimuli, including UVB, etoposide, staurosporine, ionizing radiation, cisplatin, ara-C, doxorubicin, betulinic acid, photodynamic therapy, and cytokines, induce cyt c release, which can be prevented by overexpression of Bcl-2 or Bcl-xL.33,34 Released cytc, in turn, binds to Apaf-1, a mammalian homologue of theCaenorhabditis elegans death-promoting protein CED-4,35 inducing it to associate with procaspase-9,36 thereby triggering its autoactivation into a mature form, which in turn can directly cleave procaspases-3 and -7.37
In the present study, we investigated the molecular mechanisms of apoptosis triggered by ELL-12, in particular its relationship with the caspase network and cyt c release. Our findings indicate that ELL-12 induces caspases activation through release of cyt c in a Bcl-xL–sensitive manner but independently of the CD95 (APO-1/Fas) ligand/receptor system.
MATERIALS AND METHODS
Cell culture.
Human T-cell leukemia Jurkat, human monocyte-like histiocytic lymphoma U-937 (ATCC, Rockville, MD), and H9 human T-cell lymphoma (gift of Dr Marcus Peter, German Cancer Research Center, Heidelberg, Germany) cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (20% for H9 cells). Bcl-xL–transfected and neomycin control vector (Neo)-transfected Jurkat (gift of Dr Charles Zacharchuk, National Institutes of Health, Bethesda, MD) were maintained under the same conditions in media supplemented with 1 mg/mL G418. For induction of apoptosis, logarithmically growing cells were washed twice and resuspended in serum-free RPMI 1640 medium (0.75 to 1 × 106 cells/mL), unless indicated otherwise.
Materials.
ET-18-OCH3, DOPC, and DOPE-GA were from Avanti Polar Lipids (Alabaster, AL). Anti-Fas IgM (clone CH-11) and neutralizing anti-Fas IgG (clone ZB4) were from Upstate Biotechnology (Lake Placid, NY). Ac-DEVD-CHO and Ac-DEVD-AMC were from Bachem (King of Prussia, PA).
Liposome preparation and characterization.
ET-18-OCH3 liposome formulation, ELL-12 [DOPC/Chol/DOPE-GA/ET-18-OCH3] (4:3:1:2, molar ratio), was prepared using the solvent evaporation method.38Briefly, DOPC, Chol, DOPE-GA, and ET-18-OCH3 were dissolved in chloroform/methanol (2:1, vol/vol) and mixed to give a final molar ratio of 4:3:1:2. After thorough mixing, the organic solvent was vacuum-evaporated at 45°C, and the thin dried film was hydrated with Dulbecco’s phosphate-buffered saline (PBS) without Ca2+ or Mg2+ for 1 hour at room temperature or above the transition temperature for the lipids. The resulting multilamellar preparations were extruded 10 times through 100-nm double-stacked Nucleopore filters using an extruder device (Lipex Biomembranes, Vancouver, British Columbia, Canada). The liposomes were examined by light microscopy to check morphology, and size was determined using a Nicomp Model 370 Submicron Particle Sizer system (Santa Barbara, CA). Liposome size was about 100 nm. ET-18-OCH3 and other lipid concentrations in the final preparations were determined using reverse-phase high-performance liquid chromatography with an evaporative light-scattering detector with 4% water in methanol as the mobile phase. Non–ET-18-OCH3–containing liposomes were prepared in the same way, except that the liposome composition was DOPC:Chol:DOPE-GA in a 6:3:1 mole ratio.
Staining of apoptotic nuclei.
Apoptosis was assessed as previously described39 by staining cells with bisbenzimide trihydrochloride (8 μg/mL in 30% [vol/vol] glycerol/PBS; Hoechst #33258; Calbiochem, San Diego, CA) for 10 minutes. Cells were counted using a Zeiss Photoscope II fluorescent microscope (Petersburg, VA). The percentage of apoptotic cells was calculated as number of apoptotic cells per the number of total cells counted.
Preparation of mitochondria and Western blot analysis for cyt c.
After treatment, cells were harvested by centrifugation at 1,000g for 5 minutes at 4°C. After washing twice with ice-cold PBS, mitochondrial and cytosolic fractions were prepared by resuspending cell pellets in 5 vol of ice-cold buffer A (20 mmol/L HEPES-KOH [pH 7.5], 10 mmol/L KCl, 1.5 mmol/L MgCl2, 1 mmol/L sodium EDTA, 1 mmol/L sodium EGTA, 1 mmol/L dithiothreitol, 0.1 mmol/L phenylmethylsulfonylfluoride, 20 μg/mL leupeptin, 10 μg/mL aprotinin, and 10 μg/mL pepstatin) containing 250 mmol/L sucrose. After swelling for 15 minutes on ice, the cells were homogenized by 15 to 20 passages through a 26-gauge needle, and the homogenates were centrifuged at 1,000g for 5 minutes at 4°C. The supernatants were centrifuged again at 12,000g for 15 minutes at 4°C, and the resulting mitochondria pellets were resuspended in cold buffer A and frozen in multiple aliquots at −80°C. The 12,000g supernatants were further centrifuged at 100,000g for 1 hour, and the resulting cytosolic fractions were aliquoted and frozen at −80°C. For Western blot analysis, equivalent amounts of mitochondrial and cytosolic fractions were loaded on 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Primary antibodies were the 7H8.2C12 cyt cmonoclonal antibody (MoAb; Research Diagnostics Inc, Flanders, NJ) and the 12C4-F12 cyt c oxidase subunit II MoAb (Molecular Probes, Eugene, OR).
Western blotting for caspases, Bid, PARP, Lamin B, CD95L, and Bcl-xL.
Cell lysates preparation and Western blotting were performed as previously described.39 Polyclonal rabbit anti–caspase-3 (gift of Dr Donald Nicholson, Merck-Frosst Centre for Therapeutic Research, Pointe Claire, Quebec, Canada), antimouse anti–caspase-8 antibody C15 (gift of Dr Marcus Peter), rabbit antisera specific for the p20 subunit of caspase-7 (gift of Dr Edward Gelmann, Lombardi Cancer Center, Washington, DC), monoclonal rat anti–caspase-7 (gift of Dr Junying Yuan, Harvard Medical School, Boston, MA), rabbit antisera for PARP (Boehringer Mannheim, Indianapolis, IN), rabbit anti–caspase-9 and rabbit anti–caspase-10 (Oncogene Research Products, Cambridge, MA), rabbit anti–caspase-2 (Santa Cruz Biotechnology, Santa Cruz, CA), polyclonal rabbit anti-Bid (gift of Dr Stanley Korsmeyer, Harvard Medical School), the anti-lamin B1 (Calbiochem, San Diego, CA), and anti-Bcl-x and the anti-CD95L (Transduction Laboratories, Lexington, KY) were used as primary antibodies. Binding was detected with antirat (Boehringer Mannheim), antirabbit, or antimouse (Bio-Rad, Hercules, CA) horseradish peroxidase-conjugated IgG and detected by enhanced chemiluminescence (SuperSignal or Ultra SuperSignal) as described by the manufacturer (Pierce, Rockford, IL).
Fluorogenic DEVD cleavage enzyme assays.
Enzyme reactions were performed in 96-well plates with 20 μg of cytosolic proteins and a final concentration of 20 μmol/L Ac-DEVD-AMC substrate as previously described.39 Fluorescent aminomethyl coumarin (AMC) product formation was measured over a 30-minute period at excitation and emission wavelengths of 360 nm and 460 nm using a Cytofluor II fluorometer plate reader (PerSeptive Biosystems, Framingham, MA).
RESULTS
ELL-12 induces apoptosis in a time- and concentration-dependent manner in Jurkat, H9, and U-937 cells.
Treatment of Jurkat T leukemia cells with ELL-12 for 5 hours in serum-free medium, or for longer times in the presence of serum, induced extensive cell death in a dose-dependent manner, as measured by the appearance of nuclear fragmentation (Fig 1A). In contrast, treatment of cells with liposomes lacking ET-18-OCH3 did not induce apoptosis (Fig 1A), whereas anti-Fas treatment induced extensive cell death (Fig1A). Because the presence of serum did not interfere with the ability of ELL-12 or Fas ligation to induce apoptosis (Fig 1A), but only delayed the onset of the apoptotic process, all further experiments were performed in serum-free media.
Induction of apoptosis and DEVDase activation by the liposomal ether-lipid ELL-12 in Jurkat, H9, and U-937 cells. (A) Jurkat T cells were treated in serum-free medium for 5 hours (solid squares and solid bars) or in the presence of serum for 24 hours (open squares and open bars) with the indicated concentrations of ELL-12 or with non–ET-18-OCH3 liposomes at a concentration equivalent to the highest concentration of ELL-12 (lp) or 50 ng/mL anti-Fas antibody. The percentage of apoptotic cells was evaluated by the DNA-specific fluorochrome Hoechst. Nuclei were visualized by fluorescence microscopy and a minimum of 1,000 cells were scored. Mean values ± SD from at least 3 different experiments are shown. (B) Jurkat T cells were treated with 10 μg/mL ELL-12 for the indicated times and the percentage of apoptotic nuclei (solid squares) was determined as described above. DEVDase activity (open squares) in extracts from duplicate cultures was measured with the fluorogenic substrate Ac-DEVD-AMC. Results are the means ± SD of at least 3 independent experiments. (C) H9 cells (solid squares) and U-937 cells (open squares) were treated in serum-free medium for 16 hours with the indicated doses of ELL-12 or with non–ET-18-OCH3 liposomes at a concentration equivalent to the highest dose of ELL-12. Cells were then stained with the DNA-specific fluorochrome Hoechst and nuclei were visualized by fluorescence microscopy. Apoptosis of cells incubated with non–ET-18-OCH3 liposomes was 11.5% in H9 cells and 5.6% in U-937 cells. Data shown are representative of 3 independent experiments. DEVDase activity in extracts from H9 (D) and U-937 cells (E) treated for the indicated times with 10 μg/mL (open squares) or 50 μg/mL ELL-12 (solid squares) was measured with the fluorogenic substrate Ac-DEVD-AMC. Results are the means ± SD of at least 3 independent experiments.
Induction of apoptosis and DEVDase activation by the liposomal ether-lipid ELL-12 in Jurkat, H9, and U-937 cells. (A) Jurkat T cells were treated in serum-free medium for 5 hours (solid squares and solid bars) or in the presence of serum for 24 hours (open squares and open bars) with the indicated concentrations of ELL-12 or with non–ET-18-OCH3 liposomes at a concentration equivalent to the highest concentration of ELL-12 (lp) or 50 ng/mL anti-Fas antibody. The percentage of apoptotic cells was evaluated by the DNA-specific fluorochrome Hoechst. Nuclei were visualized by fluorescence microscopy and a minimum of 1,000 cells were scored. Mean values ± SD from at least 3 different experiments are shown. (B) Jurkat T cells were treated with 10 μg/mL ELL-12 for the indicated times and the percentage of apoptotic nuclei (solid squares) was determined as described above. DEVDase activity (open squares) in extracts from duplicate cultures was measured with the fluorogenic substrate Ac-DEVD-AMC. Results are the means ± SD of at least 3 independent experiments. (C) H9 cells (solid squares) and U-937 cells (open squares) were treated in serum-free medium for 16 hours with the indicated doses of ELL-12 or with non–ET-18-OCH3 liposomes at a concentration equivalent to the highest dose of ELL-12. Cells were then stained with the DNA-specific fluorochrome Hoechst and nuclei were visualized by fluorescence microscopy. Apoptosis of cells incubated with non–ET-18-OCH3 liposomes was 11.5% in H9 cells and 5.6% in U-937 cells. Data shown are representative of 3 independent experiments. DEVDase activity in extracts from H9 (D) and U-937 cells (E) treated for the indicated times with 10 μg/mL (open squares) or 50 μg/mL ELL-12 (solid squares) was measured with the fluorogenic substrate Ac-DEVD-AMC. Results are the means ± SD of at least 3 independent experiments.
At 10 μg/mL of ELL-12, nuclear fragmentation became significant after 4 to 5 hours of treatment (Fig 1B). Blebbing of cell membranes was observed in almost all cells, which were fragmented into characteristic condensed nuclei and apoptotic bodies, whereas untreated cells did not exhibit any morphological changes (data not shown). Consistent with the results in Jurkat T cells, treatment of H9 T-cell lymphoma and U-937 monoblastic leukemia cells with ELL-12 displayed a time-dependent (data not shown) and dose-dependent nuclear apoptosis (Fig 1C). Liposomes lacking ET-18-OCH3 did not induce cell death (data not shown).
Executioner caspases mediate ELL-12–induced apoptosis in Jurkat, H9, and U-937 cells.
We used the fluorogenic substrate Ac-DEVD-AMC, which corresponds to the cleavage site found in numerous executioner caspases-3 and -7 targets, to measure the activity of these caspases in ELL-12–induced apoptosis. ELL-12 treatment of Jurkat cells resulted in a time-dependent increase in DEVDase proteolytic activity (Fig 1B), which correlated with the onset of apoptosis and preceded the appearance of fragmented nuclei by approximately 1 hour (Fig 1B). Likewise, a time-dependent DEVDase activity increase was found in H9 (Fig 1D) and U-937 cells (Fig 1E). In agreement with this finding, pretreatment of Jurkat, H9, and U-937 cells with the inhibitor of caspases-3 and -7 activity, Ac-DEVD-CHO, completely reversed apoptosis induced by ELL-12 (data not shown).
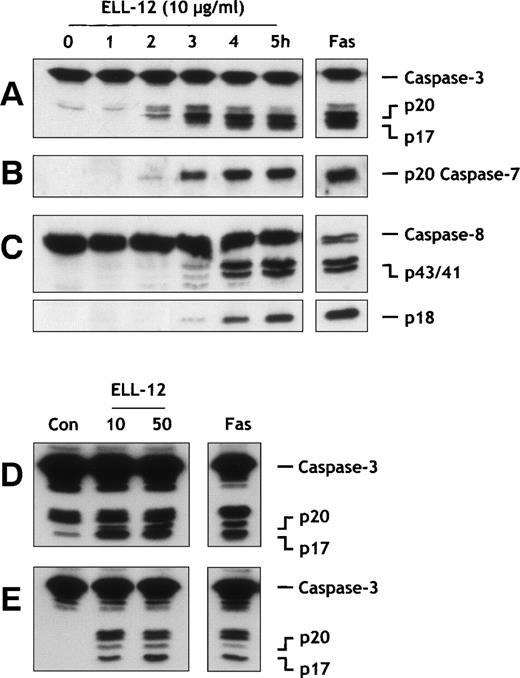
Proteolytic processing of procaspases-3 and -7 induced by ELL-12 treatment was examined using specific antibodies against their active subunits. Caspase-3 is synthesized as a 32-kD precursor that is cleaved to generate the mature form composed of 17-kD (p17) subunits through intermediary 20-kD (p20) and 12-kD (p12) subunits.40-42 As shown in Fig 2A, ELL-12 activated caspase-3 in Jurkat cells, as shown by the appearance of the p17 subunit within 2 to 3 hours after the addition of ELL-12. Its level increased progressively thereafter in a time-dependent fashion similar to the increase in DEVDase activity (Fig 1B), suggesting a correlation between the appearance of the active form of caspase-3 and the onset of apoptosis. Similarly, processing of caspase-3 was detected in H9 (Fig2D) and U-937 cells (Fig 2E). These data suggest that activation of caspase-3 plays an important role in apoptosis induced by ELL-12. Activation of caspase-7 requires cleavage of the 35-kD precursor into subunits of 20 and 12 kD.43 44 Similar to caspase-3, processing of caspase-7 into its active form was apparent after 2 to 3 hours of treatment with ELL-12 in Jurkat cells (Fig 2B). In H9 cells and U-937 cells, caspase-7 was also cleaved in response to ELL-12 (data not shown).
Caspases activation by ELL-12 treatment. Extracts from Jurkat T cells treated with 10 μg/mL ELL-12 for the indicated times were resolved by SDS-PAGE and probed with anti–caspase-3 (A), anti–caspase-7 (B), and anti–caspase-8 (C) antibodies. Cytosolic extracts from H9 cells (D) and U-937 cells (E) treated with 10 and 50 μg/mL ELL-12 or 50 ng/mL anti-Fas antibody for 16 hours were resolved by SDS-PAGE and probed with anti–caspase-3 antibody. The migration position of full-length caspase-3, the cleavage intermediate p20, the active subunit p17, the active caspase-7 subunit p20, full-length caspase-8, the cleavage intermediates p43 and p41, and the active subunit p18 are indicated.
Caspases activation by ELL-12 treatment. Extracts from Jurkat T cells treated with 10 μg/mL ELL-12 for the indicated times were resolved by SDS-PAGE and probed with anti–caspase-3 (A), anti–caspase-7 (B), and anti–caspase-8 (C) antibodies. Cytosolic extracts from H9 cells (D) and U-937 cells (E) treated with 10 and 50 μg/mL ELL-12 or 50 ng/mL anti-Fas antibody for 16 hours were resolved by SDS-PAGE and probed with anti–caspase-3 antibody. The migration position of full-length caspase-3, the cleavage intermediate p20, the active subunit p17, the active caspase-7 subunit p20, full-length caspase-8, the cleavage intermediates p43 and p41, and the active subunit p18 are indicated.
Bcl-xL completely inhibits apoptosis, DEVDase activity, and activation of executioner caspases-3, -6, and -7, PARP, and lamins in Jurkat cells.
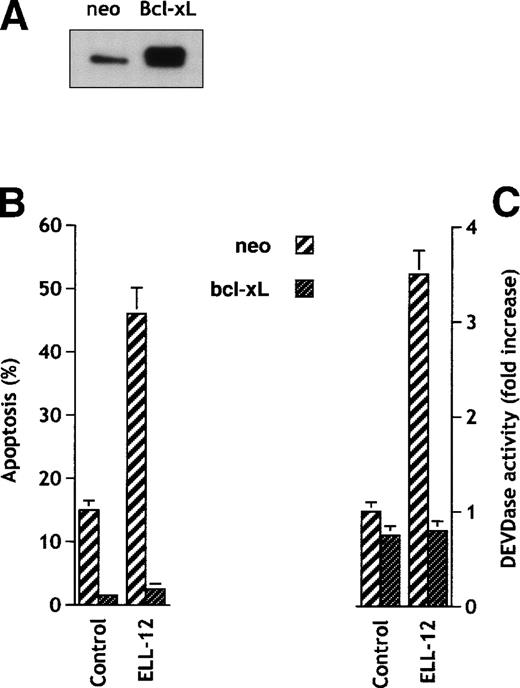
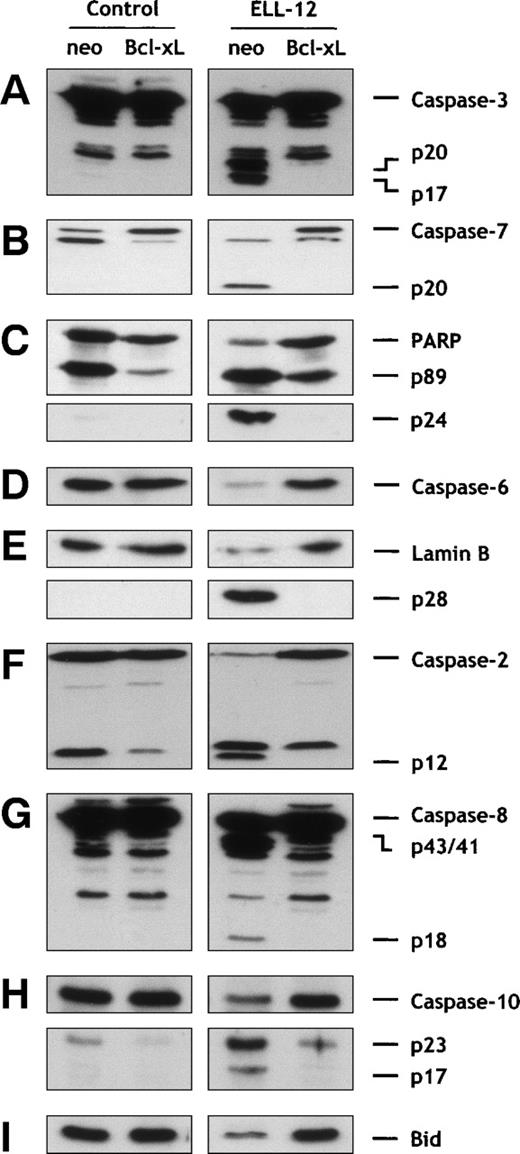
Cytoprotective Bcl-2 family members, Bcl-2 and Bcl-xL, protect against diverse apoptotic stimuli.45,46 It was thus of interest to determine whether apoptosis induced by ELL-12 was also under the control of these proteins. Jurkat cells stably transfected with bcl-xL expressed high levels of Bcl-xL protein (Fig 3A) and were found to be completely resistant to apoptosis induced by ELL-12 (Fig 3B). Similar results were obtained with the breast adenocarcinoma MCF-7 cell line overexpressing Bcl-xL where ELL-12–induced apoptosis was prevented (data not shown). Bcl-xL similarly prevented the ELL-12–induced increase in DEVDase activity in Jurkat T cells (Fig 3C). Correspondingly, processing of the executioner caspase-3 (Fig 4A) and caspase-7 (Fig 4B) into their mature forms was completely prevented in Jurkat/Bcl-xL cells. Cleavage of the DNA repair enzyme poly(ADP-ribose) polymerase (PARP), one of the known substrates for caspases-3 and -7,41 into apoptotic 89- and 24-kD fragments was also abrogated in Jurkat/Bcl-xL cells (Fig 4C). In vitro, caspase-3 can process caspase-6,47 which is responsible for proteolysis of lamins, intranuclear intermediate filament proteins forming a stratum interposed between the chromatin and the nuclear envelope, whose cleavage is required for fragmentation of the nucleus into multiple apoptotic bodies.48 ELL-12 induced activation of caspase-6 (Fig 4D) and subsequent degradation of lamins (Fig 4E) that were blocked in Jurkat/Bcl-xL cells. These results suggest that ELL-12 induces apoptosis and activation of caspases-3, -6, and -7 in a Bcl-xL–sensitive manner.
Bcl-xL blocks apoptosis and DEVDase activity in Jurkat cells. (A) Expression of Bcl-xL in transfected Jurkat cells. Cellular proteins from Jurkat stably transfected with empty vector (neo) or Bcl-xL expression vector were separated by SDS-PAGE and immunoblotted using anti–Bcl-x antibody. (B) Jurkat cells transfected with vector control or Bcl-xLexpression vector were treated without or with 10 μg/mL ELL-12 for 5 hours. Apoptosis was assessed by Hoechst staining. Results are the means ± SD of at least 3 independent experiments. (C) Activation of DEVD-specific caspases was measured by the cleavage of the fluorogenic substrate Ac-DEVD-AMC. Results are the means ± SD of at least 3 independent experiments.
Bcl-xL blocks apoptosis and DEVDase activity in Jurkat cells. (A) Expression of Bcl-xL in transfected Jurkat cells. Cellular proteins from Jurkat stably transfected with empty vector (neo) or Bcl-xL expression vector were separated by SDS-PAGE and immunoblotted using anti–Bcl-x antibody. (B) Jurkat cells transfected with vector control or Bcl-xLexpression vector were treated without or with 10 μg/mL ELL-12 for 5 hours. Apoptosis was assessed by Hoechst staining. Results are the means ± SD of at least 3 independent experiments. (C) Activation of DEVD-specific caspases was measured by the cleavage of the fluorogenic substrate Ac-DEVD-AMC. Results are the means ± SD of at least 3 independent experiments.
Bcl-xL overexpression inhibits caspases-2, -3, -6, -7, -8, and -10 and Bid activation induced by ELL-12 in Jurkat cells. Cytosolic extracts from Jurkat/neo or Jurkat/Bcl-xLcells treated for 5 hours without or with 10 μg/mL ELL-12 were subjected to SDS-PAGE and immunoblotted with anti–caspase-3 (A), anti–caspase-7 (B), anti-PARP (C), anti–caspase-6 (D), anti-lamin B (E), anti–caspase-2 (F), anti–caspase-8 (G), anti–caspase-10 (H), or anti-Bid (I). The migration position of full-length caspase-3, cleavage intermediate p20, active subunit p17, full-length caspase-7, active subunit p20, full-length PARP, cleaved forms p89 and p24, full-length caspase-6, full-length lamin B, cleaved form p28, full-length caspase-2, active form p12, full-length caspase-8, cleavage intermediates p43 and p41, active subunit p18, full-length caspase-10, cleavage intermediate p23, active subunit p17, and full-length Bid are indicated.
Bcl-xL overexpression inhibits caspases-2, -3, -6, -7, -8, and -10 and Bid activation induced by ELL-12 in Jurkat cells. Cytosolic extracts from Jurkat/neo or Jurkat/Bcl-xLcells treated for 5 hours without or with 10 μg/mL ELL-12 were subjected to SDS-PAGE and immunoblotted with anti–caspase-3 (A), anti–caspase-7 (B), anti-PARP (C), anti–caspase-6 (D), anti-lamin B (E), anti–caspase-2 (F), anti–caspase-8 (G), anti–caspase-10 (H), or anti-Bid (I). The migration position of full-length caspase-3, cleavage intermediate p20, active subunit p17, full-length caspase-7, active subunit p20, full-length PARP, cleaved forms p89 and p24, full-length caspase-6, full-length lamin B, cleaved form p28, full-length caspase-2, active form p12, full-length caspase-8, cleavage intermediates p43 and p41, active subunit p18, full-length caspase-10, cleavage intermediate p23, active subunit p17, and full-length Bid are indicated.
ELL-12 induces processing of caspases-2, -8, and -10 and Bid cleavage that are inhibited by Bcl-xL overexpression in Jurkat cells.
Caspases with long prodomains, such as caspases-2, -8, and -10, are commonly considered to be upstream caspases in apoptosis due to their ability to associate with the cell surface death receptor molecules, CD95 and TNFR1.31 Nevertheless, caspase-2 shares with executioner caspases-3 and -7 the optimal peptide recognition motif DExD.49 Moreover, processing of caspase-2 has been reported to be dependent of executioner caspase-3 in diverse cell lines,50,51 occurring later than activation of caspase-3–like proteases in Jurkat cells undergoing apoptosis.50 Here, ELL-12 induced activation of caspase-2 into its mature form (p12 subunit), which was totally prevented by Bcl-xL overexpression (Fig 4F).
Caspase-8 cleavage often represents the first detectable event in death receptor-mediated apoptosis.52 However, it has been recently demonstrated that, in type II cells, such as Jurkat, caspase-8 activation can also require involvement of mitochondria, inasmuch as Bcl-2 or Bcl-xL overexpression can inhibit its processing.53 Caspase-8 is expressed as a 55-kD precursor that is cleaved to generate the mature form composed of 18-kD (p18) subunits, through 2 intermediate cleavage products of 43 and 41 kD, and 10-kD (p10) subunits.54 As shown in Fig 4G, ELL-12 activated caspase-8. The appearance of 2 cleavage intermediates p43/41 and the p18 active subunit occurred within 3 hours after addition of ELL-12 (Fig 2C). In Jurkat cells overexpressing Bcl-xL, processing of caspase-8 induced by ELL-12 was totally inhibited (Fig4G). These results show that, in type II cells, caspase-8 can effectively be activated in a Bcl-xL–sensitive fashion and in a time-frame similar to activations of caspases-3 and -7 (Fig 2A through C).
Caspase-10 has been proposed to bind to Fas receptor-like caspase-8.55,56 Using an antibody specific for the p23/p17 processed forms, we demonstrated that the 55-kD precursor caspase-10 was activated in response to ELL-12 (Fig 4H). In agreement with previous data,54 caspase-10 activation occurs in a Bcl-xL–sensitive manner in Jurkat T cells (Fig 4H).
We next asked whether ELL-12 treatment could lead to cleavage of Bid, a pro-apoptotic Bcl-2 family member, which is cleaved by caspase-8 into a C-terminal fragment that can directly act on mitochondria to trigger cyt c release.57-60 In agreement with the Bcl-xL effect on ELL-12–induced activation of caspase-8, Bid activation by ELL-12 was impeded by Bcl-xL (Fig 4I). These results suggest that Bid activation may also be under the control of Bcl-xL.
ELL-12 induces translocation of mitochondrial cyt c into the cytosol and activation of caspase-9, effects that are blocked by Bcl-xL overexpression in Jurkat cells.
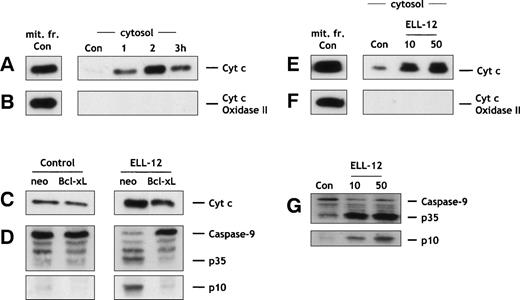
Because ELL-12–triggered apoptosis was tightly controlled by Bcl-xL, we therefore asked whether ELL-12 could also trigger mitochondrial cyt c release, because Bcl-2 and Bcl-xL interfere with cyt c release.61As shown in Fig 5A, cytosolic cyt clevels were markedly increased as early as 1 hour after treatment of Jurkat cells with ELL-12. These results demonstrate that cyt crelease is an early event in ELL-12–treated Jurkat cells, occurring before activation of caspase-3, -7, and -8 (Fig 2A through C). Cytc release induced by ELL-12 was inhibited in mitochondria from Bcl-xL–overexpressing cells (Fig 5C). Similarly, ELL-12 treatment triggered cyt c release in H9 cells (Fig 5E) and U-937 cells (data not shown).
ELL-12 triggers cytosolic accumulation of cyt c and caspase-9 activation that are inhibited by Bcl-xLoverexpression. Jurkat T cells treated with 10 μg/mL ELL-12 were harvested at the indicated times, and cytosolic and mitochondrial proteins were separated by 15% SDS-PAGE and analyzed by immunoblotting with anti-cyt c (A) or anti-cyt c oxidase (subunit II; B). Cyt c oxidase serves as a marker for mitochondrial contamination of cytosolic extracts. A mitochondrial extract from nontreated cells (mit. fr. Con) was used as a positive control for cytc and cyt c-oxidase (subunit II). Jurkat/neo and Jurkat/Bcl-xL cells, treated without or with 10 μg/mL ELL-12 for 2 hours, were harvested and cytosolic proteins were separated by 15% SDS-PAGE and analyzed by immunoblotting with anti-cytc (C). Cytosolic extracts from Jurkat/neo or Jurkat/Bcl-xL cells treated for 5 hours without or with 10 μg/mL of ELL-12 were subjected to SDS-PAGE and immunoblotted with anti–caspase-9 (D). Cytosolic and mitochondrial proteins from H9 cells treated with 10 and 50 μg/mL ELL-12 for 16 hours were separated by 15% SDS-PAGE and analyzed by immunoblotting with anti-cyt c(E) or anti-cyt c oxidase (subunit II; F). Cytosolic extracts from H9 cells treated for 16 hours without or with 10 and 50 μg/mL of ELL-12 were subjected to SDS-PAGE and immunoblotted with anti–caspase-9 (G). The migration positions of full-length caspase-9, cleavage intermediate p35, and active subunit p10 are indicated.
ELL-12 triggers cytosolic accumulation of cyt c and caspase-9 activation that are inhibited by Bcl-xLoverexpression. Jurkat T cells treated with 10 μg/mL ELL-12 were harvested at the indicated times, and cytosolic and mitochondrial proteins were separated by 15% SDS-PAGE and analyzed by immunoblotting with anti-cyt c (A) or anti-cyt c oxidase (subunit II; B). Cyt c oxidase serves as a marker for mitochondrial contamination of cytosolic extracts. A mitochondrial extract from nontreated cells (mit. fr. Con) was used as a positive control for cytc and cyt c-oxidase (subunit II). Jurkat/neo and Jurkat/Bcl-xL cells, treated without or with 10 μg/mL ELL-12 for 2 hours, were harvested and cytosolic proteins were separated by 15% SDS-PAGE and analyzed by immunoblotting with anti-cytc (C). Cytosolic extracts from Jurkat/neo or Jurkat/Bcl-xL cells treated for 5 hours without or with 10 μg/mL of ELL-12 were subjected to SDS-PAGE and immunoblotted with anti–caspase-9 (D). Cytosolic and mitochondrial proteins from H9 cells treated with 10 and 50 μg/mL ELL-12 for 16 hours were separated by 15% SDS-PAGE and analyzed by immunoblotting with anti-cyt c(E) or anti-cyt c oxidase (subunit II; F). Cytosolic extracts from H9 cells treated for 16 hours without or with 10 and 50 μg/mL of ELL-12 were subjected to SDS-PAGE and immunoblotted with anti–caspase-9 (G). The migration positions of full-length caspase-9, cleavage intermediate p35, and active subunit p10 are indicated.
Recent studies have shown that caspase-9 is activated by cyt cdue to clustering of caspase-9 by Apaf-1, leading to activation of caspases-3 and -7.35-37 Because ELL-12 induced cytc release and caspases-3 and -7 activation in a Bcl-xL–dependent pathway, we then examined whether caspase-9 was also activated by ELL-12. Caspase-9 exists as a pro-form of 46 kD that is processed into 35- and 10-kD forms. Caspase-9 was processed into its active forms in response to ELL-12 treatment in Jurkat (Fig 5D) and H9 cells (Fig 5G), and Bcl-xLoverexpression totally blocked this activation in Jurkat cells (Fig5D).
Induction of apoptosis by ELL-12 is not mediated through the CD95 receptor/ligand system in Jurkat and H9 cells.
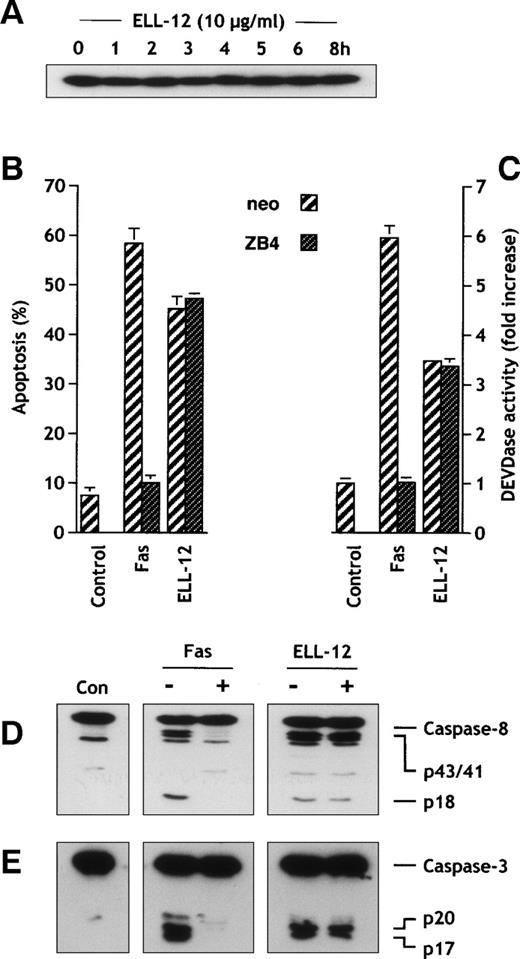
Involvement of the CD95 receptor/ligand system has been proposed to mediate apoptosis induced by several anticancer drugs in a variety of tumors cells.62 Because caspase-8 is activated in response to CD95 triggering through its recruitment to the CD95 receptor52 and because ELL-12 induced caspase-8 activation in Jurkat (Figs 2C and 4G) and also in H9 cells (see Fig 7A), we determined whether stimulation of the CD95 system could account for caspase-8 activation and apoptosis induced by ELL-12. ELL-12 did not induce upregulation of CD95 ligand protein (CD95L) in Jurkat (Fig 6A) or in H9 cells (Fig 7B). Moreover, blocking the CD95 receptor/ligand interaction by antagonist anti-Fas antibody clone ZB4 did not inhibit ELL-12–induced cell death, whereas apoptosis triggered by agonist anti-Fas antibody CH-11 was markedly reduced in Jurkat (Fig6B) and H9 cells (Fig 7C). Consistent with this result, we found that ZB4 antibody completely abrogated DEVDase activity induced by anti-Fas CH-11, but not that induced by ELL-12 in Jurkat cells (Fig 6C). Finally, Fas receptor blockade by ZB4 completely impeded caspases-8 and -3 processing induced by anti-Fas CH-11 but not that induced by ELL-12 (Fig 6D and E). Together, these findings negate a role for the CD95 ligand/receptor signaling in ELL-12–induced apoptosis in Jurkat and H9 cells.
ZB4 anti-Fas antibody antagonizes Fas-induced but not ELL-12–induced cell death, DEVDase activity, and caspases-8 and -3 activation in Jurkat cells. (A) Extracts from Jurkat T cells treated with 10 μg/mL ELL-12 for the indicated times were resolved by SDS-PAGE and probed with anti-CD95L antibody. (B) Jurkat T cells were pretreated with 300 ng/mL antagonist anti-Fas MoAb (clone ZB4) for 1 hour and then treated with 50 ng/mL anti-Fas MoAb or 10 μg/mL ELL-12 for an additional 5 hours. Apoptosis was assessed by Hoechst staining. Results are the means ± SD of 3 independent experiments. (C) Activation of DEVD-specific caspases was measured by the cleavage of the fluorogenic substrate Ac-DEVD-AMC. Results are the means ± SD of 3 independent experiments. Cytosolic extracts were subjected to 15% SDS-PAGE and immunoblotted with anti–caspase-8 (D) or anti–caspase-3 (E). (−) and (+) indicate cells treated without or with 300 ng/mL antagonist anti-Fas MoAb (clone ZB4). The migrations indicated are full-length caspase-8, the cleavage intermediates p43 and p41, the active subunit p18, full-length caspase-3, the cleavage intermediate p20, and the active subunit p17.
ZB4 anti-Fas antibody antagonizes Fas-induced but not ELL-12–induced cell death, DEVDase activity, and caspases-8 and -3 activation in Jurkat cells. (A) Extracts from Jurkat T cells treated with 10 μg/mL ELL-12 for the indicated times were resolved by SDS-PAGE and probed with anti-CD95L antibody. (B) Jurkat T cells were pretreated with 300 ng/mL antagonist anti-Fas MoAb (clone ZB4) for 1 hour and then treated with 50 ng/mL anti-Fas MoAb or 10 μg/mL ELL-12 for an additional 5 hours. Apoptosis was assessed by Hoechst staining. Results are the means ± SD of 3 independent experiments. (C) Activation of DEVD-specific caspases was measured by the cleavage of the fluorogenic substrate Ac-DEVD-AMC. Results are the means ± SD of 3 independent experiments. Cytosolic extracts were subjected to 15% SDS-PAGE and immunoblotted with anti–caspase-8 (D) or anti–caspase-3 (E). (−) and (+) indicate cells treated without or with 300 ng/mL antagonist anti-Fas MoAb (clone ZB4). The migrations indicated are full-length caspase-8, the cleavage intermediates p43 and p41, the active subunit p18, full-length caspase-3, the cleavage intermediate p20, and the active subunit p17.
ELL-12 induces caspase-8 activation and apoptosis independently of the CD95 receptor/ligand system in H9 lymphoma cells. Extracts from H9 cells treated with 10 and 50 μg/mL ELL-12 or 50 ng/mL anti-Fas antibody for 16 hours were resolved by SDS-PAGE and probed with anti–caspase-8 antibody (A) or anti-CD95L antibody (B). Migrations indicated are full-length caspase-8, the cleavage intermediates p43 and p41, the active subunits p18 and p16, and full-length CD95L. H9 cells were pretreated with 300 ng/mL antagonist anti-Fas MoAb (clone ZB4) for 1 hour and then treated with 50 ng/mL anti-Fas MoAb or 10 and 50 μg/mL ELL-12 for an additional 16 hours (C). Apoptosis was assessed by Hoechst staining. Results are the means ± SD of 3 independent experiments.
ELL-12 induces caspase-8 activation and apoptosis independently of the CD95 receptor/ligand system in H9 lymphoma cells. Extracts from H9 cells treated with 10 and 50 μg/mL ELL-12 or 50 ng/mL anti-Fas antibody for 16 hours were resolved by SDS-PAGE and probed with anti–caspase-8 antibody (A) or anti-CD95L antibody (B). Migrations indicated are full-length caspase-8, the cleavage intermediates p43 and p41, the active subunits p18 and p16, and full-length CD95L. H9 cells were pretreated with 300 ng/mL antagonist anti-Fas MoAb (clone ZB4) for 1 hour and then treated with 50 ng/mL anti-Fas MoAb or 10 and 50 μg/mL ELL-12 for an additional 16 hours (C). Apoptosis was assessed by Hoechst staining. Results are the means ± SD of 3 independent experiments.
CONCLUDING REMARKS
ET-18-OCH3 and related lipids have been administered intravenously and orally for treatment of leukemia, lymphomas, and solid human cancers and topically for treatment of skin metastases.6 Although beneficial responses were observed, especially by the topical route, intravenous and oral dosing were limited by serious gastrointestinal, hematological, and pulmonary side effects. Liposome-associated ET-18-OCH3 (ELL-12) was developed to reduce systemic side effects and therefore offers the potential of enhanced activity against various tumors concomitant with greatly reduced side effects.13-15
In this study, we report that ELL-12 triggers apoptosis via proteolytic processing of caspases that was blocked by caspase inhibitors. Treatment of Jurkat, H9, or U-937 cells with ELL-12 results in activation of executioner caspases, leading to cleavage of PARP or lamin B. Activation of the CD95 ligand/receptor system has been implicated in apoptosis triggered by anticancer drugs such as doxorubicin,63-66 bleomycin,67,68cisplatin,68 cytarabine,65etoposide,69 or methotrexate65 68 in a variety of tumor cells. In contrast, stimulation of the CD95 ligand/receptor system did not appear to account for activation of caspases induced by ELL-12 inasmuch as CD95L expression was unchanged and obstruction of CD95 ligand/receptor interaction had no effect on ELL-12–induced cell death in Jurkat and H9 cells.
A wealth of reports support the view that release of mitochondrial cytc may play an important role in the activation of the apoptotic machinery.33,34 Cyt c release was detected in all cell lines treated with ELL-12, and cyt c release clearly preceded activation of caspases and nuclear fragmentation. In addition, overexpression of Bcl-xL conferred protection against ELL-12–induced apoptosis by blocking cyt c release, caspase-9 activation, and subsequent cleavage of caspases. Although the molecular mechanism by which ELL-12 induces cyt c release is still unknown, the results presented here suggest that release of cytc is probably not mediated by Bid. This pro-apoptotic member of the Bcl-2 family has been shown to be activated by caspase-8 and then translocated from the cytosol to the mitochondria, where its truncated form (tBid) mediates the release of cyt c.57-59,70However, we found that processing of caspase-8 and subsequent Bid cleavage occurred downstream of mitochondrial events because Bcl-xL totally inhibited their processing. Moreover, cytc release distinctly preceded caspase-8 processing in Jurkat cells, negating a primary role for the caspase-8/Bid pathway in inducing cyt c release. It is noteworthy that several anticancer drugs, including hydroxamic acid, vinblastine, and dexamethasone, have been shown to induce apoptosis and caspase-3 activation without affecting cyt c levels,71 72indicating that triggering of apoptosis by chemotherapeutic agents can occur at multiple sites in the cell death pathway.
In summary, our findings suggest that ELL-12 induces caspases activation through release of cyt c in a Bcl-xL–sensitive manner, but independently of the CD95 (APO-1/Fas) ligand/receptor system. Therefore, ELL-12 could be useful as an adjunct for chemotherapy in cancer cells that have a defect in upstream apoptosis pathways acting by bypassing the requirement of the CD95 ligand/receptor system. Moreover, ELL-12 could also enhance the action of anticancer drugs that act by a cyt c-independent pathway by stimulation of cyt c-dependent apoptosis.
ACKNOWLEDGMENT
The authors thank Dr Donald Nicholson for caspase-3 antibody, Dr Edward Gelmann for caspase-7 antibody, Dr Marcus Peter for caspase-8 antibody and H9 cells, Drs Atan Gross and Stanley Korsmeyer for anti-Bid antibody, Dr Junying Yuan for caspase-7 antibody, and Dr Charles Zacharchuk for Jurkat/Bcl-xL cells.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Sarah Spiegel, PhD, Department of Biochemistry and Molecular Biology, Georgetown University Medical Center, 353 Basic Science Bldg, 3900 Reservoir Rd, NW, Washington, DC 20007; e-mail: spiegel@bc.georgetown.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal