The labile iron pool (LIP) of animal cells has been implicated in cell iron regulation and as a key component of the oxidative-stress response. A major mechanism commonly implied in the downregulation of LIP has been the induced expression of ferritin (FT), particularly the heavy subunits (H-FT) that display ferroxidase activity. The effects of H-FT on LIP and other physiological parameters were studied in murine erythroleukemia (MEL) cells stably transfected with H-FT subunits. Clones expressing different levels of H-FT displayed similar concentrations of total cell iron (0.3 ± 0.1 mmol/L) and of reduced/total glutathione. However, with increasing H-FT levels the cells expressed lower levels of LIP and reactive oxygen species (ROS) and ensuing cell death after iron loads and oxidative challenges. These results provide direct experimental support for the alleged roles of H-FT as a regulator of labile cell iron and as a possible attenuator of the oxidative cell response. H-FT overexpression was of no apparent consequence to the cellular proliferative capacity. However, concomitant with the acquisition of iron and redox regulatory capacities, the H-FT–transfectant cells commensurately acquired multidrug resistance (MDR) properties. These properties were identified as increased expression of MDR1 mRNA (by reverse transcription polymerase chain reaction [RT-PCR]), P-glycoprotein (Western immunoblotting), drug transport activity (verapamil-sensitive drug efflux), and drug cytotoxicity associated with increased MDR1 or PgP. Although enhanced MDR expression per se evoked no significant changes in either LIP levels or ROS production, it might be essential for the survival of H-FT transfectants, possibly by expediting the export of cell-generated metabolites.
A VARIETY OF PHYSIOLOGICAL and pathological conditions are attributed to metabolically active forms of cell iron associated with the labile iron pool (LIP).1 Elevated levels of LIP are commonly assumed to compromise cell integrity by metal-catalyzed formation of reactive oxygen species (ROS).1-5 Regulatory mechanisms have therefore been invoked in maintaining LIP at relatively low levels.6,7 The major one has been attributed to iron-responsive proteins (IRP) that purportedly sense cytosolic LIP levels and translationally control the expression of the major cell iron supplier transferrin receptor (TfR) and the iron withdrawing protein ferritin (FT).6,7 This apparently passive mode of LIP controlling its own levels is allegedly complemented by an “active” mode in which LIP is transcriptionally modulated, primarily via expression of FT.8 Of the two FT subunits, expression of the ferroxidase-carrying heavy subunits (H-FT) has been shown to be the most responsive to various chemical challenges and inducers.9,14 However, because only recently has a method for assessing LIP become available,15-17 it has not been possible to ascertain whether induced H-FT expression reflected rather than affected the cell LIP levels. That pertains equally to experimental and pathological conditions,18,19including the proposed H-FT modulation of LIP induced by myc oncogene.12 13
In this work we attempted to assess whether induced H-FT expression, by downregulating LIP levels, can in fact confer upon cells a first line of defense against chemically induced oxidative stress. Stable transfectants expressing H-FT in a manner independent of labile cell iron or IRP and at levels higher than untransfected cells were difficult to obtain with a variety of cell lines. This was overcome with murine erythroleukemia (MEL),20 which provided various clones with demonstrably higher H-FT levels and a commensurate capacity for withdrawing iron from the LIP, both under steady-state conditions and after acute iron loads.20,21 Whereas the proliferative capacity of the clones remained unchanged, the capacity to withstand oxidative damage when challenged with pro-oxidants increased with H-FT expression. However, with H-FT overexpression the cells concomitantly acquired multidrug resistance (MDR) properties. The latter were discovered in the clones during the assessment of LIP levels using the hydrophobic precursor of the fluorescent metalosensor calcein, namely the nonfluorescent acetomethoxy derivative calcein-AM. That probe,22,23 as well as many other hydrophobic probes, has been identified previously as a possible substrate of ATP-dependent MDR pumps.24-26 The MDR character associated with H-FT overexpression was reflected both functionally, as resistance to drugs and verapamil-sensitive drug pumping activity, and structurally, as increased MDR1a mRNA levels and membrane P-glycoprotein (PgP). Possible linkages between H-FT, LIP, and MDR functions are explored and discussed.
MATERIALS AND METHODS
Materials.
Calcein, calcein-AM, and Rhodamine 123 were procured from Molecular Probes (Eugene, OR). [3H]-thymidine was from the Radiochemical Centre (Amersham Life Science Ltd, Little Chalfont, UK). Iron nitrate (Spectrosol grade) standard was from BDH Chemicals Ltd (Pode, Dorset, UK). Unless specified otherwise, all other chemicals were from Sigma Chemical Co (St Louis, MO) or best available grade. SIH (salicylaldehyde hydrazone) was a kind gift of Prof Prem Ponka (McGill University, Montreal, Canada).
Cells.
The cell lines used in this study were of murine erythroid leukemia (MEL) origin stably transfected with the mouse H-FT gene, as described earlier.20,21 The properties of additional H-FT transfectants are described. All the MEL cells were grown in minimum essential medium, Earle’s salts base, (MEM-Eagle) supplemented with 10% fetal calf serum (FCS) (both from Biological Industries, Beth Haemek, Israel). They were periodically reselected for neomycin-resistance in the presence of G418 (800 μg/mL) and assayed for mycoplasma by a kit procured from Biological Industries. The number of cells were estimated in a Coulter Cell Counter (Coulter Electronics Ltd, Harpenden, Herts, UK). Cell volume was determined as described elsewhere.15 The human erythroleukemia K562 cells used were wild type and stable transfected with a retroviral construct pLMDR1L6 carrying the human MDR gene (kindly provided by Dr Igor Roninson, Chicago, IL).27 The cells were grown as described for MEL cells except in Dulbecco-MEM medium (DMEM).
Cell proliferation.
Cells were plated in octaplicate in 96-well culture plates (Nunc, Roskilde, Denmark) at a density of 25,000 cells/mL growth medium. The plates were analyzed daily for cell count as described above and for metabolic activity in the presence of Alamar Blue (5% final) (Almog Diagnostic, Rishon Letzion, Israel)28 and reading the fluorescence (after 4-hour incubation in culture conditions) in a BMG Fluorostar plate reader (BMG, Offenburg, Germany) at 540 nm exc and 590 nm emi.
Immunodetection of proteins.
Aliquots of 1 to 2 × 106 cells washed with isotonic buffer were extracted with 150 μL of Tris-HCl 10 mmol/L, NaCl 150 mmol/L, Triton X-100 0.5% (Boehringer Mannheim, Mannheim, Germany), NaN3 0.25 pH 7.4, and a cocktail of antiproteases (P-8340 Sigma, St Louis, MO) and phenylmethyl sulfonyl fluoride (PMSF) 250 μmol/L. The extract was centrifuged at 8,500g for 5 minutes at 5°C and the supernate frozen at −20°C after sampling for protein determination (BCA method; Sigma). Samples of 30 to 40 μg protein were loaded per lane.
Ferritin (H and L), P-glycoprotein, and actin were detected on immunoblots of samples run on Laemmli sodium dodecyl sulfate-polyacrylamide gel electrophoresis, (SDS-PAGE: 6% for PgP and 12% for H and L ferritin and actin). The primary antibodies used were monoclonal mouse antihuman PgP (C-219; Signet Laboratories Inc, Dedham, MA), monoclonal antiactin (IgM) (Amersham), and rabbit antimouse H and L ferritin (kindly provided by Dr Paolo Santambrogio, DIBIT, Milano, Italy). The secondary antibodies were goat antimouse or goat antirabbit gamma globulin conjugated to horseradish peroxidase (Jackson Immunoresearch, West Grove, PA) and they were used in conjunction with enhanced chemiluminescence (ECL) detection (Radiochemical Centre, Amersham) and densitometry tracing.
Reverse transcription polymerase chain reaction (RT-PCR) of H-FT and MDR1a (modified from Orly et al29).
RNA was isolated from exponentially growing MEL cells using Ultraspec RNA isolation reagent (Biotec Laboratories, Houston, TX). Total RNA (0.4 μg) was reverse transcribed (75 minutes, 42°C) using 500 ng polydeoxy thymidine [(pd(T)]12-18 primers (Pharmacia, Uppsala, Sweden) and 0.25 U avian myeloblast virus (AMV) reverse transcriptase (RT) (Promega) in a 0.02 mL reaction containing 1 × PCR buffer and 20 U RNAsin ribonuclease inhibitor (both from Promega). The RT reaction was terminated by heating for 5 minutes at 95°C and 0.08 mL of a mixture containing 1 × PCR buffer, 2.5 mmol/L MgCl2, and 1.5 U Taq DNA polymerase (TaqZol; Tal-Ron Ltd, Rehovot, Israel) was added to the RT RNA. The appropriate oligonucleotide primers were added last, and PCR was performed for up to 30 cycles (Mastercycler; Eppendorf, Hamburg, Germany) using the following temperatures: denaturing, 94°C (1 minute), annealing 59°C (2 minutes), extension, 72°C (3 minutes). Samples (0.01 mL) were removed from the PCR reactions after cycles 15 to 20 for H-FT, and after cycles 25 to 30 for MDR and L7. The primers used were: Mouse H-FT1 (access. # X12812): sense, 5′-GGAGTTGTATGCCTCCTAC-3′; antisense, 5′-GAGATATTCTGCCATGCC-3′); expected product 430 bp, spanning sequence 147-576. Mouse MDR1A (access. # M33581): sense, 5′-GGATCAGTGTTTCTAGAT-3′; antisense, 5′-CTGTATCCAGAGCTGATG-3′): expected product 352 bp, spanning sequence 3398-3749. Mouse ribosomal L7 protein, which served as the reference control (access. # M29016): sense, 5′-CAGATCTTCAATGGCACC-3′; antisense, 5′-GCAGATGATGCCAAACTTAC-3′); expected product 213 bp, spanning sequence 436-648. Tracking buffer containing Ficoll 15%, 100 mmol/L EDTA, 5 × TAE buffer, and Xylene Cyanol Blue dye, was added to the PCR products (1/5 volume), and the products were separated on a 1.6% agarose gel containing 1 × TAE buffer. The gel was stained for 30 minutes in SYBR-gold nucleic acid stain as per manufacturer’s instructions (Molecular Probes) and photographed under ultraviolet (UV) illumination. The relative band intensities were quantified by scanning-densitometry. The PCR cycle number that generated product quantities representing the up-slope of the curve was used for comparisons.
Cell iron.
Total cell iron was measured in triplicate samples of 5 × 106 cells suspended in 1 mL HBS buffer (150 mmol/L NaCl, 20 mmol/L HEPES). In method 1,30 the cells were mixed with an equal volume of an acid mixture (3 N HCl, 10% trichloroacetic acid, and 3% thioglycolic acid) and incubated for 2 hours at 37°C, cooled, centrifuged at 3,000 rpm for 30 minutes and mixed with 0.5 mL batophenan troline sulconate (BPS) (0.045% in 4.5 N Na-acetate, 0.2% thioglycolic acid), and the light absorption of the pink solution was read at 535 nm in a Spectronic 3000 UV-VIS Photodiode Spectrophotometer (Milton Roy, Oostende, Belgium). In method 2, 1 mL of the cell suspension was supplemented with 2 mL concentrated nitric acid, incubated for 30 minutes at 37°C, and the supernates used for assessing iron in a Zeeman Atomic Absorption Spectrometer (Spectra AA-300; Varian Instruments, Victoria, Australia). Calibrations in the range 5 to 20 ng Fe/mL were performed automatically by the instrument.
Cell LIP.15
A suspension of 1 × 106 cells/mL in HBS was incubated alone (control) or with ferrous ammonium sulfate (FAS) 20 μmol/L for 10 minutes at 37°C, washed, resuspended again in HBS, and reincubated alone or with H2O2 (5 μmol/L for 20 minutes at 37°C). After washing and resuspension in DMEM medium supplemented with 20 mmol/L HEPES and 1 mg/mL bovine serum albumin (BSA), the cells were loaded with CA-AM (0.125 μmol/L) for 5 minutes at 37°C, washed twice with medium, and processed for LIP measurements as described in detail previously.15
Cell glutathione (GSH). Total levels.31
Samples of 3 × 106 cells were pelleted by centrifugation and resuspended in 0.5 mL cold 0.6% sulfosalicylic acid made up in distilled water containing 0.5 mmol/L Na-EDTA. After 1 hour in ice, the cell extract was spun down at 14,000 rpm for 15 minutes at 4°C, the supernate transferred to an Eppendorf tube and a 50 μL aliquot added to a cuvette containing 60 μg DTNB, 200 μg NADPH, and 1 unit of GSH reductase in 1 mL phosphate-buffered saline (PBS) at room temperature. The reaction rate that is proportional to the concentration of total cell GSH was monitored by absorption at 412 nm in a Spectronic 3000 UV-VIS spectrophotometer.
GSH.32 33
Cell GSH was monitored with monochlorobimane (MCB). The reaction of MCB with cell GSH is catalyzed by GSH-S-transferase leading to the in situ quantitative formation of a fluorescence adduct that is retained within the cells.33 34 Aliquots of the various cell clones (3 × 105 cells into 200 μL of HBS) were supplemented with 20 μmol/L MCB (final concentration) and fluorescence was assessed for approximately 1.5 hours at 37°C using a Fluorostar Fluorescence plate reader (BMG, Offenburg, Germany) (Ex395 and Em 470 nm). The maximum fluorescence represented the amount of GSH per sample.
Cell ROS production.35
Method A. Samples of cells (1 × 106/mL) suspended in DMEM (no phenol red) and supplemented with 20 mmol/L Na-HEPES and 1% BSA were exposed to 10 μmol/L CDCF-DA (2′-7′-carboxy-dichlorofluorescin diacetate) for 20 minutes at 37°C. The reaction was stopped by rapid centrifugation and resuspension in HBS with or without FAS 20 μmol/L for 10 minutes, at 37°C. The basal- and H2O2- (0 to 20 μmol/L) induced conversion of the nonfluorescent 2′-7′-carboxy-dichlorofluorescein to 2′-7′-carboxy-dichlorofluorescein was followed with time by analyzing the fluorescence of 10,000 living cells by fluorescence-activated cell sorting (FACS) (Becton Dickinson FACS caliber, Immunocytometry systems [BDIS]; Becton Dickinson, Mountain View, CA). Values of median fluorescence intensity were used. Experiments were run on triplicate samples.
Method B. Cells, as in A, were preincubated with or without FAS (20 μmol/L) for 10 minutes at 37°C, washed and subsequently exposed to CDCF-DA in the FACS tubes submerged in a 37°C water bath. The fluorescence intensity profiles were followed by FACS at 10 minute intervals (while keeping the cells at 37°C). At 30 minutes, the cells were supplemented with H2O2 (5 to 250 μmol/L).
ROS and cell death.
Samples of different clones (1 × 106 cells/mL) suspended in HBS were incubated in the presence of different concentrations of H2O2 (0 to 1 mmol/L) for 30 minutes at 37°C. The reaction was stopped by rapid centrifugation and resuspension of the cells in growth medium. After 24 to 48 hours in full culture medium conditions, the cells were centrifuged and resuspended in PBS containing 2% FCS and propidium iodide (10 μg/mL per 1 × 106 cells). Samples of cells were analyzed by flow cytometry ( FACS) (Becton Dickinson) and the values of the median fluorescence intensity were taken for assessing the percent of dead cells in a given cell population (10,000 cells).36 37
MDR properties drug cytotoxicity.
Drug susceptibility to colchicine and vinblastine was followed by incubating cells (50 to 100,000 cells/mL) in culture medium with the indicated concentrations of drug for 48 to 72 hours (all assays done in triplicate in either 24- or 96-well culture plates; Nunc). Cell viability was assessed by incubating the cells for an additional 4- to 5-hour period in growth medium with the respective additive. For [3H]-thymidine (1 μCi/mL) incorporation into nucleic acids,38 cell samples were harvested and processed with a Matrix Cell Harvester (Packard Instruments, Meriden, CT). For metabolic activity, cells exposed to 5% Alamar Blue were analyzed as described above.
Drug transport (CA-AM and Rhodamine 123).
CA-AM (100 nmol/L) uptake and conversion into fluorescent CA were followed on line and in parallel in four stirred, thermostated (37°C) cuvettes placed in a four-cell turret of a PTI fluorescence station (PTI, Offenburg, Germany). Fluorescence measurements (485 nm excitation and 517 nm emission) were obtained sequentially for each cuvette at 2-second intervals and computer-recorded. Aliquots of 1 × 106 cells/mL were suspended in DMEM medium (no phenol red) or PBS supplemented with 5 mmol/L glucose at 37°C and, at the indicated time (approximately 10 minutes), either verapamil (5 to 10 μmol/L) or cyclosporin (8 μmol/L) was added. Cell-associated calcein fluorescence was also followed by FACS (fluorescein settings) by reading 10,000 cells at different time intervals of exposure of cells to CA-AM (± verapamil or cyclosporin). Rhodamine 123 uptake from a 5 μmol/L solution was followed in a similar manner except that aliquots of cells were washed with ice-cold PBS medium and the fluorescence of 10,000 cells estimated by FACS at room temperature.
Transport of probes was also assessed by FACS. For that, 1 mL aliquots containing 106 cells were rapidly centrifuged and resuspended in the same volume of HBS containing 5 mmol/L glucose either alone or with cyclosporin 10 μmol/L at 37°C. Aliquots of 100 μL were transferred into 900 μL of ice-cold HBS for zero fluorescence readings. To the remaining suspension R123 or CA-AM was added to final concentrations of 5 μmol/L or 125 nmol/L respectively. At the indicated times, further 100 μL samples were transferred rapidly into 900 μL of ice-cold HBS to stop probe uptake. For each timed sample 10,000 cells were analyzed.
RESULTS
Basic properties of the H-FT–expressing clones.
In an earlier characterization of MEL cells transfected with the mouse H-FT gene mutated in the iron-responsive element (IRE) we determined that the steady-state LIP levels were reduced in a manner commensurate with the levels of expressed H-FT protein.21 Concomitant with the downmodulation of LIP there was an activation of IRP20 and an increase in transferrin-receptor–mediated iron uptake (Glickstein and Cabantchik, unpublished observations). In this work we performed a more complete characterization of the clones with the aim of assessing the functional consequences of increased H-FT expression and reduction in LIP. These properties were initially studied in four clones expressing various levels of H-FT and LIP and cultured in equivalent conditions. We assessed the short-term and long-term consequences of H-FT overexpression, with particular emphasis on the capacity of the cells to respond to oxidative stress. The various parameters associated with labile and total cell iron and the reductive capacity of the cells in resting conditions are depicted in Table 1. The total cell iron concentration was determined on the basis of amount of acid-extracted cell iron measured either by atomic absorption or colorimetrically in conjunction with volume measurements of the cells. The latter was taken as the small solute accessible space of the cell.15 Although the LIP levels of the various clones grown in equivalent conditions were significantly different, their total iron content was apparently similar. Importantly, the steady-state LIP levels in all the clones represented only a minor fraction (less than 1%) of the total cell iron.
Basic Properties of MEL Clones Transfected With the Heavy Subunit of the Ferritin Gene (H-FT)
| . | WT . | Cl-6 . | Cl-12 . | Cl-16 . |
|---|---|---|---|---|
| H-FT (μg/mg protein)* | 0.20 ± 0.07 | 1.10 ± 0.33‡ | 0.90 ± 0.24‡ | 0.31 ± 0.07 |
| LIP (μmol/L)† | 1.30 ± 0.17 | 0.56 ± 0.14‡ | 0.65 ± 0.10‡ | 1.14 ± 0.13 |
| Total Iron μmol/L (BPS)1-153 | 397 ± 150 | 490 ± 88 | 470 ± 94 | 395 ± 140 |
| Total Iron μmol/L (AtAbs)1-155 | 340 ± 10 | 297 ± 23 | 410 ± 14 | 350 ± 20 |
| GSH + GSSG (mmol/L)1-154 | 25 ± 1 | 20 ± 7 | 17 ± 3 | 21 ± 1 |
| GSH (r.f.u.)# | 23 ± 7 | 21 ± 8 | 24 ± 3 | 22 ± 2 |
| . | WT . | Cl-6 . | Cl-12 . | Cl-16 . |
|---|---|---|---|---|
| H-FT (μg/mg protein)* | 0.20 ± 0.07 | 1.10 ± 0.33‡ | 0.90 ± 0.24‡ | 0.31 ± 0.07 |
| LIP (μmol/L)† | 1.30 ± 0.17 | 0.56 ± 0.14‡ | 0.65 ± 0.10‡ | 1.14 ± 0.13 |
| Total Iron μmol/L (BPS)1-153 | 397 ± 150 | 490 ± 88 | 470 ± 94 | 395 ± 140 |
| Total Iron μmol/L (AtAbs)1-155 | 340 ± 10 | 297 ± 23 | 410 ± 14 | 350 ± 20 |
| GSH + GSSG (mmol/L)1-154 | 25 ± 1 | 20 ± 7 | 17 ± 3 | 21 ± 1 |
| GSH (r.f.u.)# | 23 ± 7 | 21 ± 8 | 24 ± 3 | 22 ± 2 |
The various assays used for assessing the indicated properties of the wild type (WT) and the H-FT transfected are depicted in the Methods. Data of
and
were taken from Picard et al.20
Symbols indicate figures statistically significantly different from those of the WT by paired t-test at P < .05.
Assay based on the colorimetric bathophenanthroline sulfonate (BPS) test done on acid cell extracts in triplicates for n = 2 cell preparations.
Based on atomic absorption (AtAbs) measurements done on acid cell extracts in triplicates for n = 3 cell preparations.
Total cell glutathione (GSH + GSSG) was measured in acid cell extracts by the NADPH-GSHR-DTNB enzymatic test (n = 6 cell preparations of duplicate samples).
#GSH was measured in intact cells in triplicates with the MCB reagent (n = 6 cell preparations).
Under resting conditions, namely when unchallenged with pro-oxidants or exogenously added inducers of ROS formation, the various clones displayed similar levels of reductive power, as reflected in their GSH and GSH/GSSG contents (Table 1). The total GSH (GSH + GSSG) levels could be reproducibly determined in cell extracts by the DTNB-GSSG Reductase Recycling Assay.31,32 However, determination of either GSH or GSSG in cell extracts might give inconsistent results, apparently because of partial oxidation of GSH during or after cell disruption. Therefore, we determined the in situ GSH cell levels with the fluorogenic reagent monochlorobimane (MCB), that reacts with cell SH-containing substances in a manner that is specific and quantitative for GSH, as it is catalyzed by GSH-S-transferase.33 34
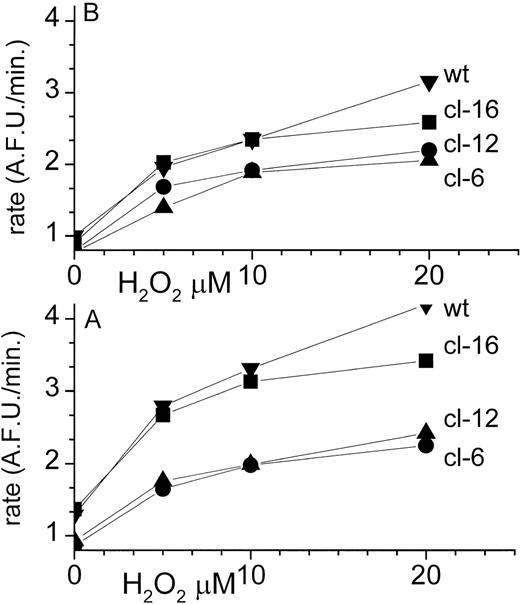
To assess whether the forms of iron found in the LIP were in fact redox active, we imposed on the cells a minor oxidative stress in the form of H2O2 (0 to 20 μmol/L for 20 minutes) and followed several properties in control cells and cells acutely loaded with Fe(II). The acute iron load was imposed to assess the alleged H-FT capacity to prevent iron in the LIP from engaging in ROS formation. The latter, as well as LIP, was measured during or immediately after the chemical treatments. The long-term effects of the acute chemical treatments were assessed in terms of cell death 24 to 48 hours later, because the acute treatments did not lead to immediate cell death. ROS levels were followed by FACS analysis of CDCF generated in cells via oxidation of a permeant nonfluorescent precursor.35 We ascertained that the loading of the precursor was not rate limiting for the assay. Under the experimental conditions used, less than 1% of the precursor was oxidized by the cells. The results depicted in Fig1A show that ROS production was significantly lower in H-FT–overexpressing clones (6 and 12) at all concentrations of H2O2 used. This was reflected in both the rates of CDCF oxidation and the levels of product attained. However, the differences among the clones were more accentuated after preloading the cells with Fe(II), as manifested primarily in the low H-FT–expressing clone 16 and nontransfected wild type. This is particularly evident in the contribution of the preloaded Fe(II) to the ROS forming capacity of the various clones, as given in the legend to Fig 1. This is also evident in the levels of basal ROS formation (no H2O2 added), which was relatively lower in the high H-FT–expressing clones. As a major fraction of the ROS formation could be inhibited by pretreating cells with iron chelators,1 3 it is implied that LIP plays a major role in catalyzing ROS formation.
In situ tracing of ROS formation in MEL cells. The various MEL clones were treated with the indicated concentration of H2O2 after loading with the nonfluorescent carboxy-2′,7′-di-Cl-fluorescein (CDCF) permeant analog (CDCFDA). The latter is converted intracellularly into the fluorescent analog by reacting with ROS in a metal-dependent fashion. Cells were preincubated for 10 minutes with FAS (20 μmol/L) (A, bottom) or buffered saline (B, top) and washed. Cell fluorescence was analyzed by FACS at different times after addition of H2O2. Data (n = 4 experiments run in triplicate samples) are given in terms of mean rates of fluorescence change with time (AFU/min = arbitrary fluorescence units/min) with SEM of less than 8% the indicated points in the graph. The increment in the fluorescence intensity ▵ attributable to FAS (▵ = A to B) (in AFU/min) was at 0, 10, and 20 μmol/L H2O2 respectively for wt: 0.31, 0.85, and 1.1; for cl-16: 0.45, 0.78, and 0.83; for cl-12: 0.18, 0.12, and 0.32; and for cl-6: 0.11, 0.10, and 0.10. ANOVA paired analysis of the n = 4 experiments showed statistically significant differences (P < .05) between data points at given H2O2 concentrations when cl-6 or cl-12 were compared with either cl-16 or wt but not when they were compared with each other.
In situ tracing of ROS formation in MEL cells. The various MEL clones were treated with the indicated concentration of H2O2 after loading with the nonfluorescent carboxy-2′,7′-di-Cl-fluorescein (CDCF) permeant analog (CDCFDA). The latter is converted intracellularly into the fluorescent analog by reacting with ROS in a metal-dependent fashion. Cells were preincubated for 10 minutes with FAS (20 μmol/L) (A, bottom) or buffered saline (B, top) and washed. Cell fluorescence was analyzed by FACS at different times after addition of H2O2. Data (n = 4 experiments run in triplicate samples) are given in terms of mean rates of fluorescence change with time (AFU/min = arbitrary fluorescence units/min) with SEM of less than 8% the indicated points in the graph. The increment in the fluorescence intensity ▵ attributable to FAS (▵ = A to B) (in AFU/min) was at 0, 10, and 20 μmol/L H2O2 respectively for wt: 0.31, 0.85, and 1.1; for cl-16: 0.45, 0.78, and 0.83; for cl-12: 0.18, 0.12, and 0.32; and for cl-6: 0.11, 0.10, and 0.10. ANOVA paired analysis of the n = 4 experiments showed statistically significant differences (P < .05) between data points at given H2O2 concentrations when cl-6 or cl-12 were compared with either cl-16 or wt but not when they were compared with each other.
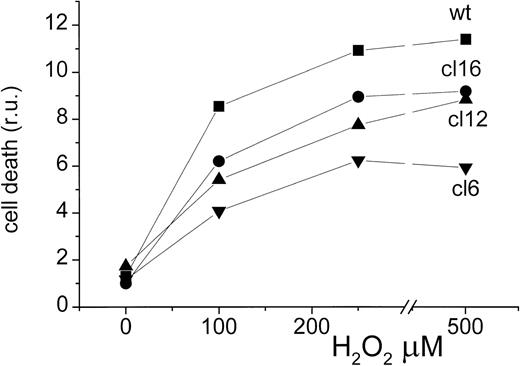
The increased ROS production in various clones was also assessed in terms of breakdown of the cell permeability barrier to the fluorogenic propidium iodide, after cell exposure to a wide range of H2O2 concentrations (Fig2). When fluorescence is monitored by flow-cell cytometry, it provides a measure for the number of damaged or dead cells in a given cell population.36 37 No attempts were made in this study to distinguish between necrosis and apoptosis. In general, no apparent cell damage was detected immediately after the chemical treatments were applied to cells. Cell damage or death was detectable only 24 hours later and it was highly dependent on the degree of the imposed oxidative stress, as given by the concentration of H2O2. The technique was not sufficiently sensitive for detecting significant changes after short cell treatments with ≤ 20 μmol/L H2O2. For stronger treatments, cell damage was consistently lower the higher the H-FT–expression capacity of the clone. Even with the highest concentration of H2O2 used the apparent plateau level attained was significantly lower in the H-FT–overexpressing clones.
ROS-induced cell death. The various MEL clones were treated with H2O2 for 20 minutes at room temperature and analyzed by FACS for viability (by propidium iodide) 48 hours later. Data are given in terms of units of fluorescence (= % dead cells) per total number of cells (live + dead) for one out of four independent experiments. ANOVA paired analysis for n = 4 set of data points at each H2O2 concentration were significantly different at P < .01 for all data analyzed.
ROS-induced cell death. The various MEL clones were treated with H2O2 for 20 minutes at room temperature and analyzed by FACS for viability (by propidium iodide) 48 hours later. Data are given in terms of units of fluorescence (= % dead cells) per total number of cells (live + dead) for one out of four independent experiments. ANOVA paired analysis for n = 4 set of data points at each H2O2 concentration were significantly different at P < .01 for all data analyzed.
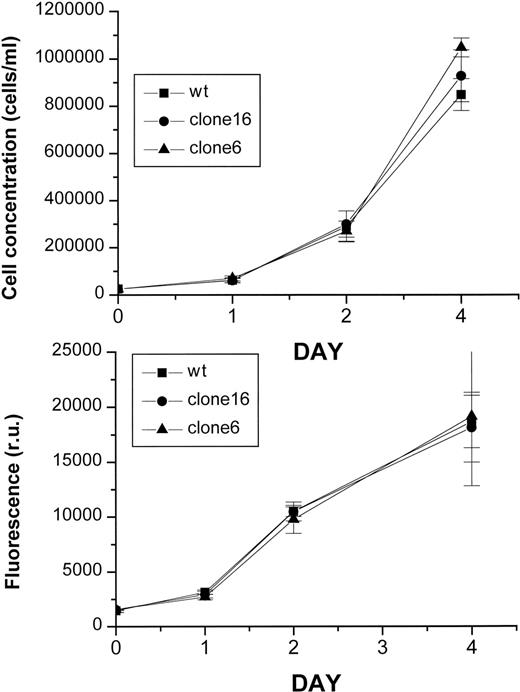
Because changes in LIP were implicated in myc-induced H-FT expression and cell growth,12 13 we assessed the possibility that H-FT overexpressers might display restrictive growth capacity. As observed in Fig 3, the growth rates of the various clones displaying the largest differences in H-FT levels were not significantly different than the untransfected (wild type) parent line. The same result was obtained whether proliferation was measured in terms of cell number or cell metabolic activity.
Growth rates of H-FT–overexpressing clones. The various MEL clones (wt, cl-6 and cl-16) were seeded at the same density (25,000 cells/mL) and their growth rate followed daily for 4 days either by cell count (top) or with Alamar Blue (4-hour development). Data are given as mean of octaplicates ± SE.
Growth rates of H-FT–overexpressing clones. The various MEL clones (wt, cl-6 and cl-16) were seeded at the same density (25,000 cells/mL) and their growth rate followed daily for 4 days either by cell count (top) or with Alamar Blue (4-hour development). Data are given as mean of octaplicates ± SE.
LIP, ROS, and MDR.
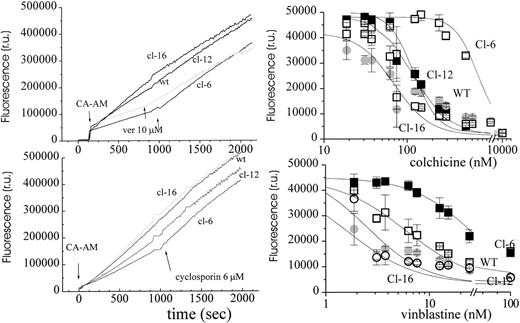
In the course of assessing LIP with calcein we consistently encountered relatively lower fluorescence levels in H-FT–expressing clones, as observed in Fig 4 (left). The fluorescent calcein is intracellularly generated from its nonfluorescent acetomethoxy precursor calcein-AM. The latter has been used previously as a hydrophobic substrate of various drug pumps that are known to confer MDR properties upon cells.22,23 This fortuitous finding led us to explore the possibility that H-FT–transfected cells might have also acquired an MDR phenotype, independent of the neo resistance gene acquired, which differs from MDR. As depicted in Fig 4(left), the time-dependent rise in cell fluorescence reflected both CA-AM net uptake into cells (ingress and egress) and intracellular de-esterification into the carboxylated (impermeant) fluorescent calcein. Addition of either cyclosporin or verapamil, two well-known blockers of the PgP pump and reversers of MDR,39,40 fully normalized the uptake to levels found in the wild type or the low H-FT–expressing clone 16 (Fig 4, left). These results led us to imply the involvement of an MDR pump. The ratios between the rates of fluorescence changes before and after addition of the MDR reversers were in the range of 3 to 10 for high H-FT expressers as compared with 1 to 2 for low expressers. The wide range of changes in ratio values between experiments was apparently associated with the age or metabolic state of the culture. However, the reversal was consistently three- to fourfold higher in the high as compared with low H-FT expressers. Because probenecid,23 a known blocker of the MRP-type of drug pump26 hardly affected the uptake profiles of calcein-AM (not shown) when used up to 10 mmol/L, we deduced that the MDR character displayed by the clones was likely to be associated with a PgP-type pump. As drug pumps have been implicated in some modes of multidrug resistance,25,26 we compared the clones in terms of susceptibility to two classes of hydrophobic cytotoxic drugs, the neutral colchicine, and the cationic vinblastine (Fig 4, right). Using Alamar Blue (AB) fluorescence for assessing cell metabolic activity and viability,38 we found the H-FT–overexpressing clones to be three- to sevenfold more resistant to the drugs in a 48-hour cytotoxicity test. Similar results were obtained using (3H)thymidine incorporation into nucleic acids (Glickstein and Cabantchik, unpublished observations).
MDR properties of H-FT–transfected MEL. Left: CAAM uptake into MEL cells and its reversal by verapamil (VP) and cyclosporin (CS). Cells (1 × 106/mL) were suspended in MEM medium at 37°C, supplemented with CA-AM (125 nmol/L) and fluorescence recorded in parallel with time. Verapamil 15 μmol/L (ver) or cyclosporin 6 μmol/L addition is indicated by the arrow. The relative rates of CA-AM entry into cells after and before addition of verapamil were: wt: 1.2; cl-6: 2.8; cl-12: 1.8; cl-16: 1.6. Cl-6 displayed ×3 and ×5 higher IC50 towards vinblastine and colchicine, respectively. Right: viability profiles. The plots depict the fluorescence intensity (540 to greater than 590 nm) of Alamar Blue (AB = 5%) for each cell system at the indicated concentration of the drug (total exposure time to drug: 48 hours and to Alamar Blue: 4 hours; 7 × 103 cells/well; n = 3). The average of n = 3 ± SE was plotted against the respective inhibitor concentration and analyzed by NLSQ (best fits depicted over the experimental points) yielding the following IC50 (μmol/L) for colchicine and vinblastine, respectively: wt (150 ± 47 and 8 ± 1.5); cl-6 (700 ± 23 and 23 ± 1); cl-12 (230 ± 14 and 12 ± 1); and cl-16 (100 ± 23 and 8 ± 1).
MDR properties of H-FT–transfected MEL. Left: CAAM uptake into MEL cells and its reversal by verapamil (VP) and cyclosporin (CS). Cells (1 × 106/mL) were suspended in MEM medium at 37°C, supplemented with CA-AM (125 nmol/L) and fluorescence recorded in parallel with time. Verapamil 15 μmol/L (ver) or cyclosporin 6 μmol/L addition is indicated by the arrow. The relative rates of CA-AM entry into cells after and before addition of verapamil were: wt: 1.2; cl-6: 2.8; cl-12: 1.8; cl-16: 1.6. Cl-6 displayed ×3 and ×5 higher IC50 towards vinblastine and colchicine, respectively. Right: viability profiles. The plots depict the fluorescence intensity (540 to greater than 590 nm) of Alamar Blue (AB = 5%) for each cell system at the indicated concentration of the drug (total exposure time to drug: 48 hours and to Alamar Blue: 4 hours; 7 × 103 cells/well; n = 3). The average of n = 3 ± SE was plotted against the respective inhibitor concentration and analyzed by NLSQ (best fits depicted over the experimental points) yielding the following IC50 (μmol/L) for colchicine and vinblastine, respectively: wt (150 ± 47 and 8 ± 1.5); cl-6 (700 ± 23 and 23 ± 1); cl-12 (230 ± 14 and 12 ± 1); and cl-16 (100 ± 23 and 8 ± 1).
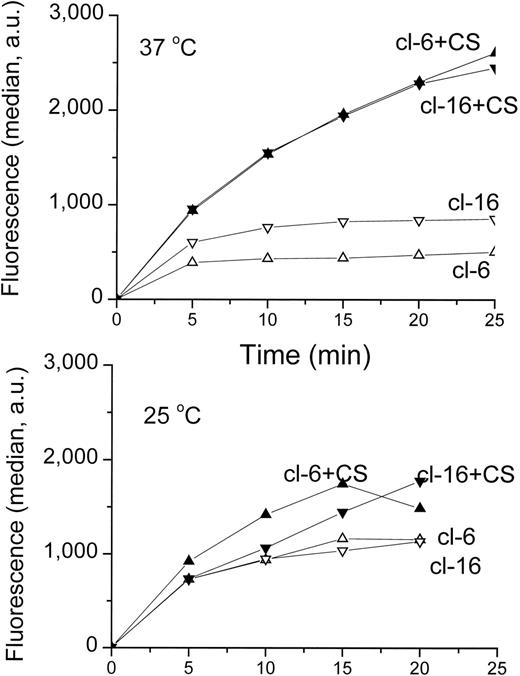
The characterization of the MDR phenotype of the two transfectants carrying extreme levels of H-FT expression, cl-6 and cl-16, were also expanded to other potential substrates and to temperatures in which ATP-driven pumps are inoperative (Fig 5). Using the cationic-hydrophobic substrate Rhodamine 123,41we found that the uptake of the dye at 25°C was in fact similar and virtually unaffected by cyclosporin in either clone. However, at 37°C, the uptake was apparently lower in both clones than at the lower temperature, due to the operation of a highly temperature-dependent MDR efflux pump. The effect of the putative pump on drug uptake and the reversal by cyclosporin were more pronounced in the high H-FT expresser cl-6 than in the cl-16 low H-FT expresser.
Uptake of Rhodamine 123 in H-FT–transfected clones. Role of temperature. Uptake of Rhodamine 123 (5 μmol/L in a buffered salt solution) into 106/mL cells was performed at the two indicated temperatures. At different times aliquots were transferred to an ice-cold buffered salt solution, and samples were aliquoted for FACS analysis (104 cells per sample). The median fluorescence values (given as arbitrary units, au) are plotted as a function of time (of n = 3 representative experiments).
Uptake of Rhodamine 123 in H-FT–transfected clones. Role of temperature. Uptake of Rhodamine 123 (5 μmol/L in a buffered salt solution) into 106/mL cells was performed at the two indicated temperatures. At different times aliquots were transferred to an ice-cold buffered salt solution, and samples were aliquoted for FACS analysis (104 cells per sample). The median fluorescence values (given as arbitrary units, au) are plotted as a function of time (of n = 3 representative experiments).
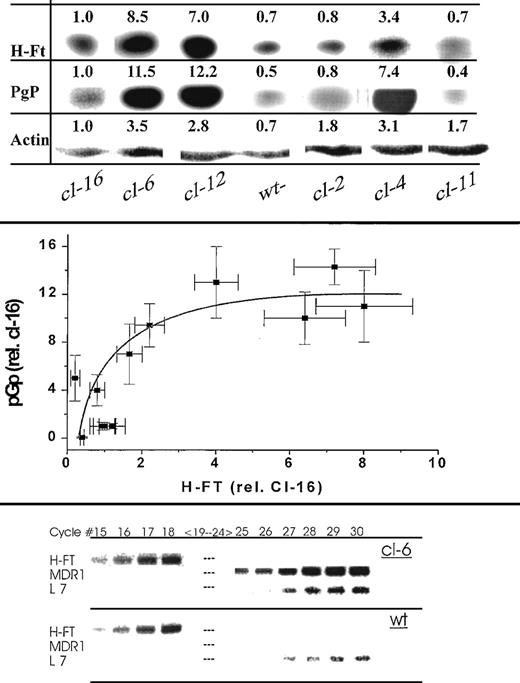
Because MDR pumping activity is highly dependent on factors affecting the metabolic status of cells24 and those might differ in the various transgene H-FT clones, we assessed directly the level of implicated PgP pump in the nuclei-free fraction of the cells. We included in this study additional H-FT transfectants, so as to establish possible correlations between H-FT and PgP levels. The H-FT and PgP levels were compared with those of actin, which was taken as a constitutive and, presumably, housekeeping marker of cell constituents. As observed in Fig 6, PgP was definitely expressed in all the H-FT transfectants. The three lowest H-FT–expressing clones cl-16, cl-11, and cl-2, were in fact also the lowest expressers of PgP. Analysis of the correspondence between the relative levels of PgP and H-FT in all the transfectants and the wild-type clones showed no linear relationship, but an apparent hyperbolic, possibly saturative trend (Fig 6, center). To ascertain that the higher levels of PgP protein resulted from increased expression of the MDR1a gene, we assessed the mRNA levels of H-FT, MDR1a, and L7 ribosomal protein (as a putative housekeeping marker) by RT-PCR, using specific primers to the respective mRNA sequences. As depicted in Fig 6 (bottom) for cl-6 and wild-type MEL cells, the mRNA levels of H-FT, and in particular MDR1a, were clearly higher in cl-6.
(top) Western immunoblots of cell lysates isolated from H-FT–transfected clones. Depicted are the ECL exposures of the trans-blots of SDS-PAGE of the respective samples (same protein load on gels) derived from the different clones using the following antibodies: anti-PgP mouse monoclonal, rabbit antimouse H-FT and anti–L-FT antibodies, rabbit antiactin and the respective goat antimouse or antirabbit IgG conjugated to HRP. The samples were run in parallel on separate gels, but the exposure times differed for the different antibodies used. The numbers above the bands represent the values of the densitometry tracings using the intensity of cl-16 for normalization (center). Correlation between H-FT and PgP levels of expression in MEL clones. The densitometry tracings of the immunoblots shown on the top and others (not shown) were normalized to the values obtained in cl-16 for both H-FT and MDR. The denstity values of each pair (n = 3) were from parallel SDS-PAGE runs originating from the same cell samples normalized to that of actin. The mean OD values are given as symbols and the ± SE as bars both PgP and H-FT, respectively (bottom). RT-PCR of H-FT, MDR1, and L7 ribosomal mRNAs of a high H-FT expresser and the wild-type untransfected clone. The propidium iodide stains are of the various mRNA samples analyzed by RT-PCR as described in Methods. For the relatively abundant H-FT message the system saturated at cycle 18, whereas for MDR1a and L7 it had to be run at higher cycle number. MDR1a was essentially undetected in the wild type (wt).
(top) Western immunoblots of cell lysates isolated from H-FT–transfected clones. Depicted are the ECL exposures of the trans-blots of SDS-PAGE of the respective samples (same protein load on gels) derived from the different clones using the following antibodies: anti-PgP mouse monoclonal, rabbit antimouse H-FT and anti–L-FT antibodies, rabbit antiactin and the respective goat antimouse or antirabbit IgG conjugated to HRP. The samples were run in parallel on separate gels, but the exposure times differed for the different antibodies used. The numbers above the bands represent the values of the densitometry tracings using the intensity of cl-16 for normalization (center). Correlation between H-FT and PgP levels of expression in MEL clones. The densitometry tracings of the immunoblots shown on the top and others (not shown) were normalized to the values obtained in cl-16 for both H-FT and MDR. The denstity values of each pair (n = 3) were from parallel SDS-PAGE runs originating from the same cell samples normalized to that of actin. The mean OD values are given as symbols and the ± SE as bars both PgP and H-FT, respectively (bottom). RT-PCR of H-FT, MDR1, and L7 ribosomal mRNAs of a high H-FT expresser and the wild-type untransfected clone. The propidium iodide stains are of the various mRNA samples analyzed by RT-PCR as described in Methods. For the relatively abundant H-FT message the system saturated at cycle 18, whereas for MDR1a and L7 it had to be run at higher cycle number. MDR1a was essentially undetected in the wild type (wt).
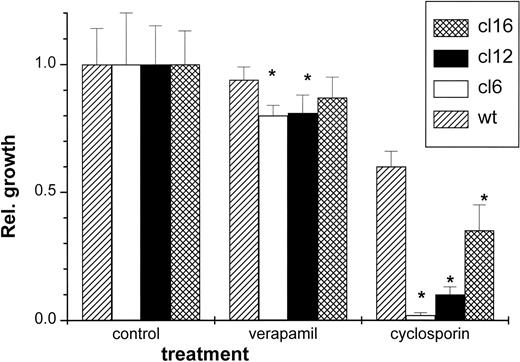
The possibility that PgP activity might be essential or favorable for the survival of H-FT overexpressers was given preliminary consideration. As an initial attempt we opted for inhibiting the PgP pump activity with agents that compromise cell viability only in a limited fashion. Cell viability was assessed after 24-hour exposure of cells to either 20 μmol/L verapamil or cyclosporin. The results shown in Fig 7 indicate that cell growth was significantly, but not substantially, inhibited by verapamil in all clones except wild type. However, more significant and substantial differences were obtained with cyclosporin, which differentially reduced cell growth in the H-FT transfectants in the rank order of H-FT expression.
Differential susceptibility of H-FT–transfected MEL clones to blockers of the PgP (MDR1) transporter. The various MEL clones were exposed to 20 μmol/L of verapamil or 5 μmol/L cyclosporin and assayed for metabolic activity 24 hours later by the Alamar Blue method, as described in Fig 3. The fluorescence intensity relative to the untreated control of each clone is depicted as a function of the treatment (± SE, n = 3), with * denoting statistically significant differences from control.
Differential susceptibility of H-FT–transfected MEL clones to blockers of the PgP (MDR1) transporter. The various MEL clones were exposed to 20 μmol/L of verapamil or 5 μmol/L cyclosporin and assayed for metabolic activity 24 hours later by the Alamar Blue method, as described in Fig 3. The fluorescence intensity relative to the untreated control of each clone is depicted as a function of the treatment (± SE, n = 3), with * denoting statistically significant differences from control.
The reciprocal phenomenon, namely a modification of LIP or ROS production by MDR itself, was explored in clones of K562 transfected with the human MDR1 gene. When compared with wild-type K562 cells by the verapamil reversal test (as shown in Fig 4 for MEL cells), functional PgP was 4- to 5-fold higher in the MDR-expressing clone. The steady-state LIP levels were not significantly altered by the transfected MDR1, as the analyzed free Fe values in the respective clones were 80 ± 9 nmol/L (n = 6) and 84 ± 8 (n = 4). Moreover, ROS production assessed by CDCF after hydroperoxide was not modified by MDR presence (not shown).
DISCUSSION
The present study addressed the possible roles of FT as a modulator of the cell LIP and the cell response to oxidative stress. We based the study on MEL clones that expressed a relatively wide range of H-FT levels, up to fivefold higher than the wild-type control. The fact that H-FT overexpression led to a demonstrably lower cell LIP (Table 1), to higher IRP activation,20 and to an increased Tf-TfR–mediated iron uptake (Glickstein and Cabantchik, unpublished information), indicated that at least some of the expressed H-FT was functional. This is also supported by measurements of iron levels found associated with H-FT in the various clones acutely exposed to permeant iron salts.20 However, the fact that the clones showed no differences in total cell iron accumulation might indicate that factors other than H-FT per se contributed to the cell iron maintenance. One such factor might be L-FT, whose reduced expression in H-FT–overexpressing clones,20 could slow down stable iron core formation in FT polymers.13,14 That is in line with our previous finding that some of the labeled iron taken up by H-FT in the transfected cells was chelatable, implying that a fraction of the H-FT–associated iron might have a labile character.21However, an additional and equally plausible factor might be associated with an increased H-FT turnover in overexpressing clones, a phenomenon we observed in cells with reduced or depleted LIP levels (Glickstein and Cabantchik, unpublished information).
The downmodulation of LIP by H-FT overexpression also had no apparent impact on the cell-reductive capacity, as measured by the GSH/GSSG levels (Table 1). However, the steady-state LIP levels largely dictated the response of the cells to H2O2 challenges, as shown by various parameters. First, clones with higher H-FT and lower LIP levels showed proportionally lower ROS production in the face of the pro-oxidant challenges (Fig 1 and Fig 3). Second, although the oxidative cell response was augmented by an acute rise in LIP (Fig 1), the response was more attenuated in the H-FT–expressing clones. Third, ROS formation could be blocked by membrane-permeating iron chelators, as shown previously for other cells1,3 and also with MEL cells (Epsztejn and Cabantchik, unpublished observations). Finally, the long-term cell damage or death induced by H2O2was significantly lower in the H-FT–overexpressing clones (Fig 3). Taken together, the studies indicate that the levels of ROS production and cell damage caused by H2O2 were correlated with the LIP levels in the H-FT–overexpressing clones. The possibility that factors other than LIP might have also compensated for the changes in ROS production, such as protective antioxidant enzymes, cannot be dismissed. However, the similar levels of GSH/GSSG among the clones would indicate otherwise. Moreover, because the proliferative capacity of the various clones was essentially similar (Fig 2), it appears that twofold differences in LIP levels might not be sufficient for limiting the rate of growth of cells. Thus, the results of this study might be relevant for understanding the implied protective role of H-FT in various cell stress-adaptive responses42-44 and cytokine14 and oncogene action.12-14 However, the role of H-FT in those phenomena awaits direct assessment of the LIP and its correlation to H-FT and ROS production.
An unexpected feature detected in the H-FT–overexpressing clones was an increase in MDR properties. Those were reflected both functionally and structurally as associated with increased PgP or MDR expression in all the H-FT–transfected clones (Fig 4 to 7). Although the quantitative correlation between the levels of expression of the H-FT and MDR genes was hyperbolic, possibly saturative (Fig 6, center), it was striking when compared on the basis of the highest and lowest H-FT expressers, cl-6 and cl-16 (Figs 5 and 6). It can therefore be surmised that the acquisition of the MDR character was not associated with the transfection per se but with the transfected gene. This raises the possibility that PgP might confer upon the H-FT overexpressers some essential cell property that H-FT per se might compromise. In support of this hypothesis, we found that classical blockers of PgP such as verapamil or cyclosporin differentially affected the survival of the H-FT/MDR overexpressers (Fig 7). Particularly interesting was the correlation found between levels of H-FT overexpressed in the clones and their susceptibility to cyclosporin. Clearly, that phenomenon will have to be assessed with more specific reverses of the MDR pump.
Although various mechanisms of induced expression of MDR genes have been described,25,26,45-51 including those invoking stress and metals,48,52-54 it is still unclear if and how H-FT per se might lead to induction of MDR. A pertinent property linking MDR and FT might be associated with a factor causing similar lens defects leading to cataract formation in both transgenic mice expressing MDR155 and humans displaying increased L-FT expression as a result of a mutation in IRP.56 PgP and possibly other MDR pumps have been regarded as physiological membrane cleansing mechanisms,25,26 although the natural substrates have not been identified. Previous studies have also suggested putative associations of FT with membranes. Recent work has indicated that PgP localizes in unique lipidic patches in the membrane,57,58possibly associated with cholesterol export from cells.59-62 In this context, it is conceivable that, in some overexpressing clones, H-FT–associated ferroxidase activity may cause direct or indirect chemical changes to the membrane. MDR could then be involved in the removal of the products of these changes. However, all these facts provide only circumstantial evidence for the alleged association between two distinct functions whose individual, let alone combined, physiological roles have yet to be determined.
ACKNOWLEDGMENT
We thank Drs Paolo Arosio and Paolo Santambrogio from DIBIT, San Raffaele Scientific Institute, Milano, Italy, for their assistance with the FT measurements and for provisions of invaluable antibodies and Dr Igor Roninson for kindly supplying the K562 MDR cell line.
Supported in part by the Israel Research Fund (ZIC), an EMBO Short term fellowship (VP), the Biomed II Program (ZIC and CB), a collaborative INSERM/CNR agreement (CB), and the Mirsky Fund for Cancer Research (INS).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal