Abstract
Although the mechanism(s) underlying mobilization of hematopoietic progenitor cells (HPCs) is unknown, detachment from the bone marrow (BM) microenvironment and motility are likely to play a role. This work analyzes the motile behavior of HPCs and the receptors involved. CD34+45lo/medScatterlo/med HPCs from granulocyte colony-stimulating factor (G-CSF)–mobilized blood and mobilized BM were compared with steady-state BM for their ability to bind hyaluronan (HA), their expression of the HA receptors RHAMM and CD44, and their motogenic behavior. Although RHAMM and CD44 are expressed by mobilized blood HPCs, function blocking monoclonal antibodies (MoAbs) identified RHAMM as a major HA binding receptor, with a less consistent participation by CD44. Permeabilization of mobilized blood HPCs showed a pool of intracellular (ic) RHAMM and a smaller pool of icCD44. In contrast, steady-state BM HPCs have significantly larger pools of icRHAMM and icCD44. Also, in contrast to mobilized blood HPCs, for steady-state BM HPCs, MoAbs to RHAMM and CD44 act as agonists to upregulate HA binding. The comparison between mobilized and steady-state BM HPCs suggests that G-CSF mobilization is associated with depletion of intracellular stores of HA receptors and modulates HA receptor usage. To confirm that mobilization alters the HA receptor distribution and usage by HPCs, samples of BM were collected at the peak of G-CSF mobilization in parallel with mobilized blood samples. HA receptor distribution of mobilized BM HPCs was closely matched with mobilized blood HPCs and different from steady-state BM HPCs. Mobilized BM HPCs had lower pools of icHA receptors, similar to those of mobilized blood HPCs. Treatment of mobilized BM HPCs with anti-RHAMM MoAb decreased HA binding, in contrast to steady-state BM HPCs. Thus, G-CSF mobilization may stimulate an autocrine stimulatory loop for HPCs in which HA interacts with basal levels of RHAMM and/or CD44 to stimulate receptor recycling. Consistent with this, treatment of HPCs with azide, nystatin, or cytochalasin B increased HA binding, implicating an energy-dependent process involving lipid rafts and the cytoskeleton. Of the sorted HPCs, 66% were adherent and 27% were motile on fibronectin plus HA. HPC adherence was inhibited by MoAbs to β1 integrin and CD44, but not to RHAMM, whereas HPC motility was inhibited by MoAb to RHAMM and β1 integrin, but not to CD44. This finding suggests that RHAMM and CD44 play reciprocal roles in adhesion and motility by HPCs. The G-CSF–associated alterations in RHAMM distribution and the RHAMM-dependent motility of HPCs suggest a potential role for HA and RHAMM in trafficking of HPCs and the possible use of HA as a mobilizing agent in vivo.
THE CELLS THAT COMPRISE the hematopoietic system are derived from multipotential stem cells present in the bone marrow (BM). These cells are defined by their ability to repopulate the hematopoietic system of BM transplant recipients and by their ability to give rise to colonies of hematopoietic cells in vitro. Because hematopoietic progenitor cells (HPCs) are most easily harvested from the blood, a variety of clinical tools, including chemotherapeutic drugs and stimulatory cytokines,1 2 are used to mobilize this subset to the circulation. Although the properties of HPCs have been extensively characterized, the mechanisms whereby they are induced to exit the BM and circulate in the blood are largely unknown. Clinically, this process, termed mobilization, is initiated through the administration of cytokines thought to stimulate their growth properties and, in an undefined way, their migratory properties.
Mobilization is almost certainly an active process. It likely involves an initial step in which HPCs modulate adhesion receptors to permit detachment from the BM microenvironment and a second step in which motile behavior is stimulated to permit migration and intravasation. Engraftment of an infused HPC requires implementation of a reverse process in which cells traffic to a BM site, extravasate, and anchor in a supportive and regulatory microenvironmental niche.3,4Although granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) are likely participants in these events, the mechanistic role of chemotherapy and cytokine remains unclear. In G-CSF receptor knock-out mice, the numbers of circulating HPCs did not increase after cyclophosphamide or interleukin-8 (IL-8) treatment,5 suggesting that G-CSF plays an important role in HPC migration. IL-6 appears to synergize with G-CSF in murine HPC mobilization.6 Cytokines have been shown to activate adhesion receptors on HPC,7-10 suggesting that they may induce behavioral properties that lead to mobilization.
Hyaluronan (HA), a glycosaminoglycan that plays a key role in structuring tissue architecture, is an important component in motility of normal and malignant hematopoietic cells, including T cells, B cells, monocytes, and thymocytes.11-15 CD44, a receptor for HA, has been shown to participate in the adhesion of normal and malignant stem cells to extracellular matrix components and to stromal elements,8,16-20 in cooperation with β1 integrins.16-18,21 CD44 is expressed by CD34+HPCs22,23 at similar24-26 or higher levels on GM-CSF–mobilized as compared with BM-localized HPCs.7 CD44 expression correlates with platelet recovery after transplantation27 and appears to be involved in lodging by murine colony-forming cells in spleen and BM.28 In CD44 knock-out mice, progenitor egress from the BM appears to be defective.29 RHAMM, a receptor for HA-mediated motility, regulates cell cycling, transduces signals, and dissolves focal adhesions.30-33 In contrast to CD44, RHAMM mediates motility, or deadhesion, of all hematopoietic cells tested to date.11-15
Operationally, HPC mobilization is the inverse of HPC adhesion, suggesting that RHAMM interactions with HA may facilitate migratory behavior, whereas CD44 interactions with HA may facilitate anchoring. The predominant role of RHAMM and HA in the motility of normal and malignant leukocytes11-15 also suggests that RHAMM may play a key role in the events underlying stem cell mobilization. To determine the expression of RHAMM, the extent of HA binding, and the ability of HPCs to undergo RHAMM and HA-mediated motile behavior, mobilized and BM-localized HPCs were analyzed ex vivo. The results show that G-CSF mobilization is accompanied by a decrease in intracellular RHAMM and CD44. Before G-CSF mobilization, monoclonal antibodies (MoAbs) to RHAMM and CD44 upregulate HA binding, but after G-CSF mobilization, MoAbs to RHAMM and CD44 are inhibitory. HPCs appear to recycle HA receptors, exhibit CD44-dependent adhesion, and undergo RHAMM-dependent motile behavior, implicating RHAMM and HA in stem cell mobilization and trafficking.
MATERIALS AND METHODS
Collection of HPCs.
HPCs were obtained from either harvested BM or from apheresis of G-CSF– or cyclophosphamide/G-CSF–mobilized peripheral blood. Mobilized blood was obtained from 66 patients with non-Hodgkin’s lymphoma, breast cancer, Hodgkin’s lymphoma, or multiple myeloma. Steady-state BM was obtained from 17 patients, 2 of whom had nonmalignant disease and 15 of whom had newly diagnosed lymphoma with no BM involvement. For 4 lymphoma patients, a BM sample was obtained at the peak G-CSF mobilization, at the time of the mobilized blood harvest; these mobilized BM harvests were of small volume and thus unlikely to be contaminated with peripheral blood cells. This study was approved by the Human Ethics Committee of the Cross Cancer Institute and of the Tom Baker Cancer Centre. All samples were obtained after informed consent was received. Samples were purified over Ficoll Paque (Pharmacia, Dorval, Quebec, Canada).
Fluorescent conjugates, antibodies, and reagents.
MoAb to CD34 (8G12; from Dr Peter Lansdorp, University of British Columbia, Vancouver, British Columbia, Canada) was custom conjugated to phycoerythrin (PE) or purchased (HPCA-2-PE; Becton Dickinson, San Jose, CA). MoAb to CD45 (17G10; Dr John Wilkins, University of Manitoba, Winnipeg, Manitoba, Canada) was custom conjugated to fluorescein isothiocyanate (FITC). CD45-PERCP was from Becton Dickinson and CD45-QR was from Sigma (St Louis, MO). MoAbs to RHAMM (3T3.5 and 3T3.7; from Dr Eva Turley, University of Toronto, Toronto, Ontario, Canada) and MoAb 50B4 (CD44; from Dr Michelle Letarte, University of Toronto) were either used in indirect immunofluorescence assays or were directly conjugated to FITC; the same pattern of results was obtained with both methods. MoAbs JB1A and 3S3 (β1 integrin) were from Dr J. Wilkins and were used in both FITC-conjugated or unconjugated forms. Goat antimouse Ig-FITC and IgG1/IgG2 isotype control MoAbs were from Southern Biotech (Birmingham, AL). HA-FITC was prepared as previously described15 using HA from Pharmacia. Where indicated, sodium azide (BDH Inc, Toronto, Ontario, Canada) at 0.02% to 0.2%, nystatin dihydrate (Sigma-Aldrich Canada Ltd, Oakville, Ontario, Canada) at 25 μg/mL, and cytochalasin B (Sigma-Aldrich Canada Ltd) at 20 μg/mL were added for 30 minutes at 37°C, before incubation with HA-FITC.
Three-color immunofluorescence (IF).
HPCs were defined based on their expression of CD34, CD45, and light scatter, as described elsewhere.34,35 Blood or BM cells were stained in three-color IF with CD34-PE, CD45-QR, or CD45-PERCP and either HA-FITC, MoAb to RHAMM, or CD44, followed by a second-stage goat antimouse Ig-FITC or a direct MoAb-FITC conjugate, as previously described.36 To detect HA binding, cells were incubated with 10 μg of HA-FITC for 30 minutes at room temperature, followed by washing and addition of the CD34 and CD45 MoAbs. Files of 50,000 to 100,000 cells were collected on a FACSort (Becton Dickinson) and analyzed using Cell Quest software. Sequential gating was used to select for CD45+ cells, then for CD34+ cells with low side scatter, and then backgated to ensure CD45lo/med and light scatterlow/med.34 35 A gate was then set to exclude any cells outside these regions, yielding a population of HPCs expressing CD34+45loSScloFSclo/med. The HA-FITC, CD44-FITC, or RHAMM-FITC staining was then plotted as a histogram. For experiments to block HA binding, cells were pretreated with the indicated unlabeled MoAb for 30 minutes at room temperature, followed by incubation with HA-FITC and the addition of CD34 and CD45 MoAbs. The values for RHAMM, CD44, and HA binding were reproducible in each of several aliquots of cells from the same sample. The staining pattern for RHAMM, CD44, and HA binding was a discrete peak of positive cells permitting the use of the mean fluorescence intensity (MFI) to indicate the degree of staining. In experiments involving cell permeabilization, cells were either treated or not treated with Intraprep permeabilization reagent (Coulter, Hialeah, FL) according to the manufacturer’s instructions, followed by three-color IF as described above. Myeloma plasma cells from BM were sorted (CD38hi, Ig+ cells) and used as a positive control for RHAMM reverse transcriptase-polymerase chain reaction (RT-PCR).
Analysis of cell adhesion and motility.
Blood or BM were sorted to obtain HPCs using an ELITE flow cytometer (Coulter). Purity of sorted populations (>97%) was confirmed by reanalysis. Sorted HPCs were washed, concentrated, and distributed into wells. Wells of chamber slides or Terasaki wells were coated with fibronectin (Fn; Sigma) at 10 μg/mL for at least 2 hours at 37°C, followed by removal of unbound Fn. A total of 2 × 105cells/well were added to chamber slides and 104 cells per well to Terasaki plates together with 20 μg/well of HA (Pharmacia) and were centrifuged to settle cells on the bottom surface of the well. Cells were rested for 30 minutes at 37°C, followed by time-lapse microscopy using an Olympus inverted microscope (Carson Group, Mississauga, Ontario, Canada) and Northern Eclipse Image analysis software (Empix, Toronto, Ontario, Canada). Cells were monitored for 20 minutes, collecting images every 15 seconds. Motile cells were defined as those migrating at least one cell diameter over the period of observation. Adherent cells were those remaining stationary during the observation period.
Statistical analysis.
Statistics were performed using SigmaStat 2.0 or SigmaPlot 4.0 (SPSS Inc, San Raphael, CA), as indicated in the table and figure legends.
RESULTS
HPCs express the HA receptors RHAMM and CD44 and bind HA.
HPCs from G-CSF–mobilized blood and from steady-state BM were identified using CD34 and CD45 as described in Materials and Methods. On average, 2.5% ± 0.4% HPCs were detected (0.1% to 7.4%) for a series of 49 mobilized blood collections. Steady-state BM (17 patients) contained 0.25% to 0.92% HPCs (mean, 0.51% ± 0.17%).
Table 1 shows the expression of RHAMM and CD44 on HPCs from mobilized blood or from steady-state BM. Expression of surface (s) RHAMM by HPCs from mobilized blood (M-BL) was variable (mean, 43%) and was significantly lower than sRHAMM on HPCs from steady-state BM (76%). The majority of HPCs from mobilized blood also expressed sCD44 (74% of HPCs), which is significantly less than the sCD44 expression by steady-state BM HPCs (100%). On average, 67% of mobilized blood HPCs and 70% of steady-state BM HPCs bound HA (Table 1). Although not significantly different, the overall intensity of HA binding was twofold higher on mobilized blood HPCs than on steady-state BM HPCs (Table 1, line 3, MFI). Regression analysis indicated a lack of correlation between surface RHAMM or CD44 and HA binding for HPCs from mobilized blood or from steady-state BM (r2 = −.1 to .07; not shown).
Cell Surface Expression of RHAMM, CD44, and HA Binding by CD34+45lo HPCs From Mobilized Blood and Steady-State BM
| . | Source of HPCs . | |||
|---|---|---|---|---|
| Mobilized Blood . | Steady-State BM . | |||
| % of HPC . | MFI . | % of HPC . | MFI . | |
| RHAMM | 43 ± 6* | 14 ± 2 | 76 ± 6 | 14 ± 2 |
| CD44 | 74 ± 6* | 85 ± 6 | 100 ± 0 | 75 ± 9 |
| HA binding | 67 ± 7† | 14 ± 2 | 70 ± 13 | 7 ± 1 |
| . | Source of HPCs . | |||
|---|---|---|---|---|
| Mobilized Blood . | Steady-State BM . | |||
| % of HPC . | MFI . | % of HPC . | MFI . | |
| RHAMM | 43 ± 6* | 14 ± 2 | 76 ± 6 | 14 ± 2 |
| CD44 | 74 ± 6* | 85 ± 6 | 100 ± 0 | 75 ± 9 |
| HA binding | 67 ± 7† | 14 ± 2 | 70 ± 13 | 7 ± 1 |
Values are the mean ± SE for HPCs from 33 mobilized blood samples and 10 SS-BM samples. Staining was by three-color IF using CD34-PE, CD45-PerCP, and either IgG1-FITC, RHAMM-FITC, CD44-FITC, or HA-FITC in comparison to identically gated isotype-matched controls.
P ≤ .008 as compared with steady-state BM HPCs using the Student’s t-test for samples with a normal distribution and the Mann Whitney rank sum test for samples failing normality or equal variance.
Not significant.
HA binding by mobilized blood HPCs occurs via RHAMM and to a lesser extent via CD44.
To determine which HA receptors mediated HA binding, mobilized blood HPCs were pretreated with function blocking antibodies to RHAMM and CD44 (Fig 1A). An MoAb to β1 integrin (JB1A) served as a negative control, because β1 integrin does not bind HA, but is expressed by HPCs (not shown).22,37 38 As expected, HPCs treated with anti-β1 integrin had HA binding that was equivalent to that of untreated HPCs. For all 7 randomly selected mobilized BL samples, the number of HPCs binding HA was strongly inhibited by treatment with anti-RHAMM. MoAb to CD44 significantly inhibited HA binding in 4 of 7 samples.
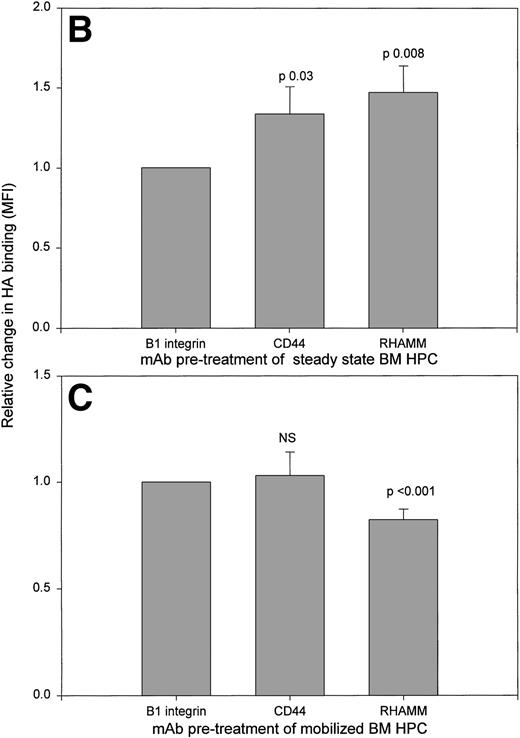
HA binding by HPCs from mobilized blood, steady-state BM, or mobilized BM exhibit different HA receptor usage. HA binding was measured using three-color IF in the presence or absence of the indicated MoAbs as inhibitors of binding. Files were then gated for the HPCs and their HA binding was plotted as a histogram. (A) Treatment of mobilized blood HPCs. Anti-β1 integrin (JB1A) gave no detectable inhibition in 7 of 7 samples. Values are the mean percentage of HPCs binding HA ± SE of all 7 samples. For the 4 samples in which inhibition by MoAb CD44 was observed, the mean was 13% ± 6% of HPCs. For samples inhibited by anti-RHAMM, the mean was 10% ± 4% of HPCs. NS, mean inhibition was not significantly different from that of untreated samples. ***P = .007 as compared with untreated or anti-β1 integrin-treated samples. (B) Treatment of steady-state BM HPCs. Treatment was as for (A) of 3 different steady-state BM HPC samples. Relative increase was calculated as the MFI of HA binding after pretreatment with anti-RHAMM or anti-CD44 divided by the MFI after anti-β1 integrin. The value of anti-β1 integrin-treated cells was set as 1.0 for each sample; anti-β1 integrin-treated and untreated cells had a similar intensity of HA binding. For all three samples, the pattern of MoAb modulation was the same. (C) Treatment of mobilized BM HPCs. HPCs from 3 different samples were treated as for (A). Relative decrease was calculated as the MFI of HA binding after pretreatment with anti-RHAMM or anti-CD44 divided by the MFI after pretreatment with anti-β1 integrin. The value of anti-β1 integrin-treated cells was set as 1.0 for each sample; anti-β1 integrin-treated and untreated cells had a similar intensity of HA binding. For all 3 samples the pattern of MoAb modulation was the same.
HA binding by HPCs from mobilized blood, steady-state BM, or mobilized BM exhibit different HA receptor usage. HA binding was measured using three-color IF in the presence or absence of the indicated MoAbs as inhibitors of binding. Files were then gated for the HPCs and their HA binding was plotted as a histogram. (A) Treatment of mobilized blood HPCs. Anti-β1 integrin (JB1A) gave no detectable inhibition in 7 of 7 samples. Values are the mean percentage of HPCs binding HA ± SE of all 7 samples. For the 4 samples in which inhibition by MoAb CD44 was observed, the mean was 13% ± 6% of HPCs. For samples inhibited by anti-RHAMM, the mean was 10% ± 4% of HPCs. NS, mean inhibition was not significantly different from that of untreated samples. ***P = .007 as compared with untreated or anti-β1 integrin-treated samples. (B) Treatment of steady-state BM HPCs. Treatment was as for (A) of 3 different steady-state BM HPC samples. Relative increase was calculated as the MFI of HA binding after pretreatment with anti-RHAMM or anti-CD44 divided by the MFI after anti-β1 integrin. The value of anti-β1 integrin-treated cells was set as 1.0 for each sample; anti-β1 integrin-treated and untreated cells had a similar intensity of HA binding. For all three samples, the pattern of MoAb modulation was the same. (C) Treatment of mobilized BM HPCs. HPCs from 3 different samples were treated as for (A). Relative decrease was calculated as the MFI of HA binding after pretreatment with anti-RHAMM or anti-CD44 divided by the MFI after pretreatment with anti-β1 integrin. The value of anti-β1 integrin-treated cells was set as 1.0 for each sample; anti-β1 integrin-treated and untreated cells had a similar intensity of HA binding. For all 3 samples the pattern of MoAb modulation was the same.
For steady-state BM HPCs, neither anti-RHAMM nor anti-CD44 decreased the number of HPCs able to bind HA (not shown). Thus, we assessed the ability of MoAb to RHAMM or CD44 to modulate the intensity of HA binding (Fig 1B). In contrast to mobilized blood HPCs, MoAb to both RHAMM and CD44, but not to β1 integrin, significantly increased HA binding by steady-state BM HPCs, as previously shown for human thymocytes.38a
Intracellular pools of RHAMM are greater in HPCs from steady-state BM than from mobilized blood HPCs.
The discordance between the number of mobilized blood HPCs expressing detectable RHAMM and the number of HPCs with RHAMM-dependent HA binding suggest that HA may trigger RHAMM redistribution and/or receptor recycling, as has been observed for malignant B and plasma cells in multiple myeloma15 and for human thymocytes.38aTo detect intracellular receptors, MoAb staining and HA binding were measured after permeabilization of HPCs. Permeabilization of mobilized blood HPCs showed a 12-fold increase in detectable RHAMM (Table 2, row 1, 22 of 22 patients), indicating a large intracellular (ic) pool. A smaller pool of icCD44 was detected (Table 2, row 2). The amount of icCD44 was significantly less than that of icRHAMM or icHA binding (Table 2, column 3), suggesting that the majority of CD44 is surface-localized, as expected.39 For mobilized blood HPCs, detectable icCD44 was found for 19 of 22 patients, and no detectable icCD44 was found in 3 of 22 patients. Consistent with the presence of icRHAMM, after permeabilization, HA binding was increased by 9.3-fold (Table 2, row 3, 22 of 22 patients), indicating that icHA receptors bind HA.
Mobilized Blood HPCs Have High Levels of Cytoplasmic HA Binding and of RHAMM, But Not of CD44
| . | MFI Treatment of HPCs Before Staining . | Ratio of Staining . | |
|---|---|---|---|
| Untreated . | Permeabilized . | Untreated:Permeabilized . | |
| RHAMM | 14 ± 2 | 121 ± 17 | 12.5 ± 1.7* |
| CD44 | 85 ± 6 | 212 ± 28 | 2.4 ± 0.3 |
| HA binding | 14 ± 2 | 107 ± 64 | 9.3 ± 2* |
| . | MFI Treatment of HPCs Before Staining . | Ratio of Staining . | |
|---|---|---|---|
| Untreated . | Permeabilized . | Untreated:Permeabilized . | |
| RHAMM | 14 ± 2 | 121 ± 17 | 12.5 ± 1.7* |
| CD44 | 85 ± 6 | 212 ± 28 | 2.4 ± 0.3 |
| HA binding | 14 ± 2 | 107 ± 64 | 9.3 ± 2* |
Cells were treated with phosphate-buffered saline or permeabilized followed by staining in three-color IF with CD34-PE, CD45-QR, and HA-FITC or MoAb to RHAMM-FITC (3T3.5), CD44-FITC (50B4), or isotype-matched controls. Files were gated for HPCs, and the staining for HA-binding or MoAb to RHAMM or CD44 was plotted as a histogram and compared with that of the isotype controls. Values are the mean ± SE of 24 samples. Ratios were calculated individually for each sample before calculating the mean values. MFI is the mean channel of staining for those cells with an intensity above that of identically gated isotype-matched control MoAbs. Statistics were as for Table 1.
P < .001 as compared with the ratio for CD44.
icHA receptors were also detected in permeabilized steady-state BM HPCs (Table 3). For steady-state BM HPCs, although on the cell surface the intensity of sCD44 exceeded that of sRHAMM (Table 3, column 1), inside the cell the levels of icRHAMM and of icHA binding were on average fourfold greater than that of icCD44 (Table 3, column 3). The levels of icRHAMM, icCD44, and icHA binding were all significantly greater in steady-state BM HPCs as compared with mobilized blood HPCs, indicating that G-CSF mobilization is associated with decreased intracellular pools of RHAMM and CD44. The patterns of surface and icHA receptors and HA binding were not significantly different between the CD38+ and CD38−subsets of HPCs from mobilized blood or steady-state BM (not shown).
Levels of icRHAMM, icCD44, and icHA Binding Are Significantly Greater in Steady-State BM HPCs Than in Mobilized Blood HPCs, and Levels of icRHAMM Are Significantly Greater in Steady-State BM HPCs as Compared With Mobilized BM HPCs
| . | MFI Treatment of Cells Before Staining . | Ratio of Staining . | |
|---|---|---|---|
| Untreated . | Permeabilized . | Intact:Permeabilized . | |
| Steady-state BM HPCs (11) | |||
| RHAMM | 14 ± 2 | 311 ± 463-150 | 21.3 ± 1.83-150,3-151 |
| CD44 | 74 ± 8 | 416 ± 50 | 5.5 ± 0.73-152 |
| HA binding | 7 ± 1 | 125 ± 21 | 22.7 ± 4.83-153,3-151 |
| Mobilized BM HPCs (4) | |||
| RHAMM | 15 ± 2 | 141 ± 323-155 | 9.3 ± 1.93-154 |
| CD44 | 81 ± 8 | 251 ± 66 | 3.9 ± 1.1 |
| HA binding | 14 ± 6 | 226 ± 112 | 13.7 ± 3.1 |
| . | MFI Treatment of Cells Before Staining . | Ratio of Staining . | |
|---|---|---|---|
| Untreated . | Permeabilized . | Intact:Permeabilized . | |
| Steady-state BM HPCs (11) | |||
| RHAMM | 14 ± 2 | 311 ± 463-150 | 21.3 ± 1.83-150,3-151 |
| CD44 | 74 ± 8 | 416 ± 50 | 5.5 ± 0.73-152 |
| HA binding | 7 ± 1 | 125 ± 21 | 22.7 ± 4.83-153,3-151 |
| Mobilized BM HPCs (4) | |||
| RHAMM | 15 ± 2 | 141 ± 323-155 | 9.3 ± 1.93-154 |
| CD44 | 81 ± 8 | 251 ± 66 | 3.9 ± 1.1 |
| HA binding | 14 ± 6 | 226 ± 112 | 13.7 ± 3.1 |
Values are the mean ± SE. The number in brackets is the number of individual patient samples. Staining, permeabilization, and analysis was as for Table 2. Mobilized BM HPC values were not significantly different from those of paired mobilized blood HPC samples.
P < .001 as compared with the values obtained for mobilized blood HPCs as shown in Table 2.
P = .004 as compared with ratios for CD44 in SS-BM HPCs.
P < .004 as compared with the values obtained for mobilized blood HPCs as shown in Table 2.
P < .01 as compared with the values obtained for mobilized blood HPCs as shown in Table 2.
P = .04 as compared with steady-state BM HPCs.
P = .002 as compared with steady-state BM.
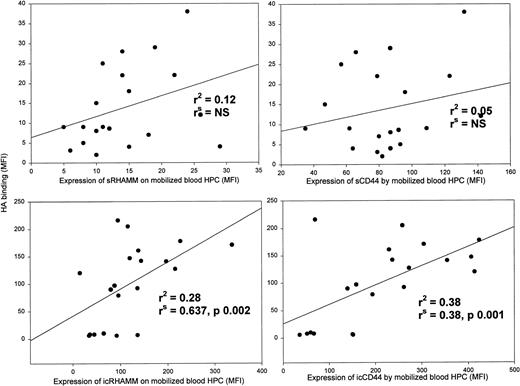
Spearman’s rank order correlation, which does not require a normal distribution, and linear regression analysis were used to evaluate correlations between HA receptors and surface or intracellular HA binding for mobilized blood HPC from each patient (Fig 2). Although both RHAMM and CD44 participate in HA binding (Fig 1A), there was no correlation between the levels of sRHAMM or sCD44 and sHA binding (r2< .15), as might be predicted if HA-dependent receptor redistribution were occurring. However, there was a significant correlation between icRHAMM or icCD44 and icHA binding (Fig 2, bottom panels), a situation in which redistribution may not be a factor. For steady-state BM HPCs, there were no significant correlations between sRHAMM/CD44 or icRHAMM/CD44 and HA binding (not shown).
For HPCs from mobilized blood, intracellular HA receptors correlate with HA binding, but surface HA receptors do not.r2 is the regression coefficient when HA binding is set as the dependent variable. rsis the Spearman rank order correlation coefficient that does not assume normal distribution and does not require assigning a dependent variable. Data points indicate individual patient samples assayed for both variables. Aliquots of the same samples were analyzed for all parameters in this figure.
For HPCs from mobilized blood, intracellular HA receptors correlate with HA binding, but surface HA receptors do not.r2 is the regression coefficient when HA binding is set as the dependent variable. rsis the Spearman rank order correlation coefficient that does not assume normal distribution and does not require assigning a dependent variable. Data points indicate individual patient samples assayed for both variables. Aliquots of the same samples were analyzed for all parameters in this figure.
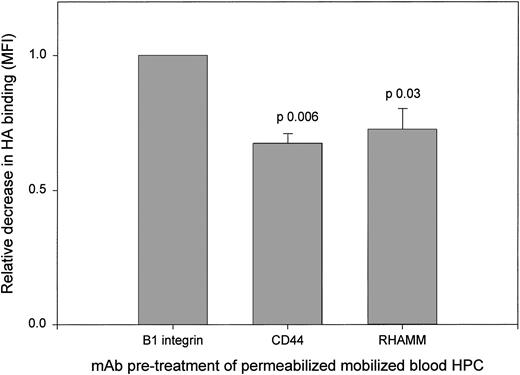
All HPCs exhibit strong icHA binding. To determine the receptors mediating icHA binding by permeabilized mobilized blood HPCs, we analyzed the ability of MoAbs to RHAMM and to CD44 to inhibit icHA binding (Fig 3). MoAbs to RHAMM and to CD44 mediated significant inhibition of HA binding as compared with the control anti-β1 MoAb, confirming that icRHAMM and icCD44 in mobilized blood HPCs are functional HA binding receptors. In contrast, the intensity of icHA binding by HPCs from steady-state BM was increased by MoAbs to CD44 and RHAMM (not shown), as was observed for sHA binding (Fig 1B), consistent with the lack of correlation between icHA receptors and icHA binding. This may suggest the need for a conformational change to acquire icHA binding function in steady-state BM HPCs.
HA binding by permeabilized mobilized blood HPCs is inhibited by anti-RHAMM and by anti-CD44. Mobilized blood HPCs were treated with MoAb before HA binding as in Fig 1. The relative decrease was calculated as the MFI of HA binding after pretreatment with anti-RHAMM or anti-CD44 divided by the MFI after anti-β1 integrin. For all 3 samples, the same pattern of MoAb inhibition was observed.
HA binding by permeabilized mobilized blood HPCs is inhibited by anti-RHAMM and by anti-CD44. Mobilized blood HPCs were treated with MoAb before HA binding as in Fig 1. The relative decrease was calculated as the MFI of HA binding after pretreatment with anti-RHAMM or anti-CD44 divided by the MFI after anti-β1 integrin. For all 3 samples, the same pattern of MoAb inhibition was observed.
HPCs from BM taken at the time of G-CSF mobilization resemble mobilized blood HPCs rather than steady-state BM HPCs.
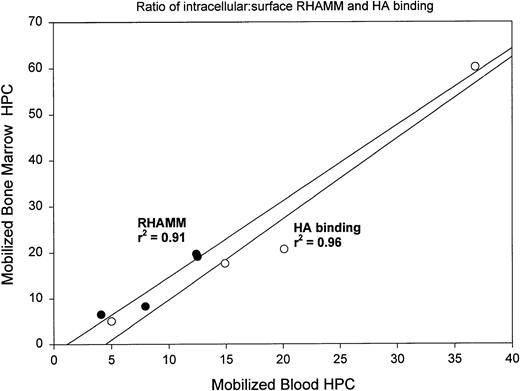
For 4 patients we obtained BM at the peak of G-CSF mobilization to determine whether G-CSF mobilization modulates the HA receptor distribution of BM HPCs. Mobilized BM samples were taken on the same day as mobilized blood samples and analyzed in parallel. After mobilization, BMC contained 0.02% to 0.18% HPCs (mean, 0.09% ± 0.07%; P < .001 as compared with steady-state BM). Analysis of sRHAMM, icRHAMM, and HA binding by HPCs from mobilized BM shows that these values strongly correlate with those of the paired mobilized blood HPCs taken at the same time (Fig 4). A similar pattern was seen for CD44 (not shown). There was a significant difference between the intensity of icRHAMM and in the ratio of s:icRHAMM for mobilized BM HPCs as compared with steady-state BM HPCs (Table 3). Thus, G-CSF mobilization is associated with altered distribution of RHAMM on/in HPCs for both blood and BM. In contrast to steady-state BM HPCs, which exhibited an increase in HA binding on treatment with both anti-RHAMM and anti-CD44 (Fig 1B), but similar to their mobilized blood counterparts, HA binding by mobilized BM HPCs was significantly decreased by anti-RHAMM, but was not affected by anti-CD44 or anti-β1 integrin (Fig 1C).
Ratios of ic:sRHAMM and ic:sHA binding for mobilized BM HPCs correlate with those of paired mobilized blood HPCs collected at the same point in time. For each of 4 mobilized BL/BM pairs, the ratio of icRHAMM MFI:sRHAMM MFI or of icHA binding MFI:sHA binding MFI was analyzed by linear regression with sRHAMM or sHA binding as the dependent variable. (•) Ratios for RHAMM; (○) ratios for HA binding.
Ratios of ic:sRHAMM and ic:sHA binding for mobilized BM HPCs correlate with those of paired mobilized blood HPCs collected at the same point in time. For each of 4 mobilized BL/BM pairs, the ratio of icRHAMM MFI:sRHAMM MFI or of icHA binding MFI:sHA binding MFI was analyzed by linear regression with sRHAMM or sHA binding as the dependent variable. (•) Ratios for RHAMM; (○) ratios for HA binding.
Intracellular RHAMM correlates significantly with levels of surface RHAMM for HPCs from steady-state BM but not from mobilized blood.
If sRHAMM derives from intracellular pools of RHAMM, we reasoned that the levels of icRHAMM should correlate with sRHAMM for steady-state BM, where ic pools have not been depleted by the mobilization process. However, for HPCs from mobilized blood, we predicted that pools of icRHAMM have been redistributed to the surface and thus may be depleted. As predicted, linear regression analysis and Spearman rank order correlation confirmed a significant positive relationship between icRHAMM and sRHAMM in HPCs from steady-state BM, but the surface and intracellular forms were not correlated for HPCs from mobilized blood (Fig 5).
In steady-state BM HPCs, but not in mobilized blood HPCs, the MFI of sRHAMM is strongly correlated with the MFI of icRHAMM. The MFI of sRHAMM and icRHAMM for mobilized blood HPCs (top panel) or steady-state BM HPCs (bottom panel) were analyzed by linear regression analysis and Spearman rank order correlation. The bottom panel shows the 95% confidence limits.
In steady-state BM HPCs, but not in mobilized blood HPCs, the MFI of sRHAMM is strongly correlated with the MFI of icRHAMM. The MFI of sRHAMM and icRHAMM for mobilized blood HPCs (top panel) or steady-state BM HPCs (bottom panel) were analyzed by linear regression analysis and Spearman rank order correlation. The bottom panel shows the 95% confidence limits.
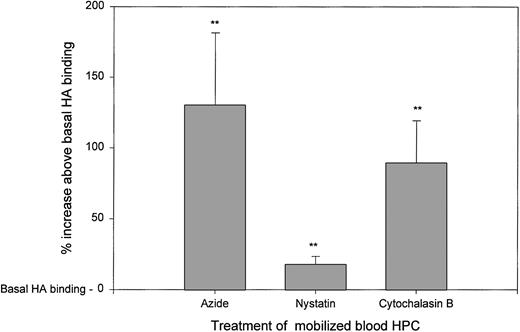
Metabolic inhibitors cause an increase in HA binding by intact HPCs.
Although pools of icRHAMM appear depleted in HPCs from mobilized blood, the levels of sRHAMM are also low. This may indicate receptor shedding or recycling after HA binding. Inhibitors of cell metabolism, caveolar-like lipid rafts, and cytoskeletal assembly were tested for their effects on HA binding and thus HA receptor function (Fig 6). Treatment with azide, which inhibits cell metabolism, resulted in a significant increase in HA receptor function (130% above basal levels of HA binding). RHAMM is gpi-linked,38a suggesting that on HA binding it may move into caveolar-like lipid rafts where signaling takes place.40 Treatment with nystatin, which disrupts lipid rafts,40 yielded a modest, but significant increase in HA receptor function (18% above basal levels of HA binding). Receptor recycling may involve the cellular cytoskeleton. Treatment with cytochalasin B, which inhibits cytoskeletal assembly and completely blocks motility,14 also significantly increased HA receptor function (89% above basal levels of HA binding). Thus, the relatively low sRHAMM in mobilized blood HPCs appears to reflect receptor redistribution and/or recycling after HA binding. When putative recycling is blocked by the inhibitors used in Fig 6, HA receptors accumulate on the cell surface. HA binding by HPCs from steady-state BM was less consistently increased, but was otherwise similar to that of mobilized blood HPCs (not shown).
HA binding by mobilized blood HPCs is increased when cell metabolism, lipid rafts, or cytoskeletal assembly are inhibited. Mobilized blood HPCs from 6 different individuals were treated with sodium azide, nystatin, or cytochalasin B as indicated in Materials and Methods, followed by the addition of HA-FITC and then MoAbs to stain HPCs. The same pattern was observed for all 6 samples. The percentage of increase above basal HA binding (set as 100%) in the absence of these inhibitors was calculated as the MFI of HA binding in the treated cultures divided by that of the control cultures × 100 − 100%. For azide, the mean was 130% ± 25%; for nystatin, the mean was 18% ± 3%; and for cytochalasin B, the mean was 89% ± 15% increase above basal HA binding.**P < .01 as compared with the basal HA binding of untreated HPCs. The pattern of inhibition for all three agents was the same for all 6 patient samples analyzed.
HA binding by mobilized blood HPCs is increased when cell metabolism, lipid rafts, or cytoskeletal assembly are inhibited. Mobilized blood HPCs from 6 different individuals were treated with sodium azide, nystatin, or cytochalasin B as indicated in Materials and Methods, followed by the addition of HA-FITC and then MoAbs to stain HPCs. The same pattern was observed for all 6 samples. The percentage of increase above basal HA binding (set as 100%) in the absence of these inhibitors was calculated as the MFI of HA binding in the treated cultures divided by that of the control cultures × 100 − 100%. For azide, the mean was 130% ± 25%; for nystatin, the mean was 18% ± 3%; and for cytochalasin B, the mean was 89% ± 15% increase above basal HA binding.**P < .01 as compared with the basal HA binding of untreated HPCs. The pattern of inhibition for all three agents was the same for all 6 patient samples analyzed.
CD44 and β1 integrin mediate HPC adhesion, whereas RHAMM and β1 integrin mediate motility.
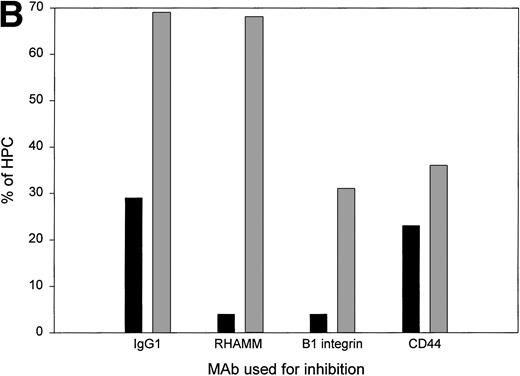
The expression of RHAMM suggested that HPCs may have migratory potential. To assess motogenic behavior, HPCs were sorted and placed into wells coated with Fn together with soluble HA to stimulate RHAMM redistribution, and their behavior was analyzed by time lapse image analysis (Fig 7A). For 15 different patients, 66% of HPCs were stationary. The majority of these stationary cells were actively deforming but firmly attached to the substrate and easily distinguished from nonadherent floating cells. For the same set of patients, 27% of HPCs were actively motile, which was defined as displacement of the cell body by at least one cell diameter.
For HPCs, CD44/β1 integrin mediates adhesion and RHAMM/β1 integrin mediates motility. (A) Sorted CD34+45loSSClo cells were deposited into chambers coated with fibronectin, together with soluble HA, and their behavior was monitored by time lapse microscopy. Cell adhesion was defined as the number of cells remaining stationary (not floating) throughout the period of analysis. Most were deforming cells. Cell motility was defined as the number of cells that migrated a distance of at least one cell diameter during the period of analysis (13 mobilized blood and 2 steady-state BM). The values for steady-state BM were 23% to 28% motile and 36% to 61% adherent HPCs. For each sample of sorted HPCs, we analyzed the cells in at least three fields (100 to 150 total cells) to permit analysis of at least 60 adherent cells and at least 20 motile cells. The data points represent the aggregate values of all fields for each individual HPC sample. (B) For this representative sample, HPCs were pretreated with either IgG1 isotype-matched control, anti-RHAMM, anti-β1 integrin, or anti-CD44 before the time lapse microscopy and image collection. RHAMM and CD44 MoAbs serve as reciprocal internal specificity controls for inhibition of adhesion ( ) and motility (▪), respectively. Similar experimental results for each MoAb were obtained for 3 to 6 individual HPC populations (8 mobilized blood and 1 steady-state BM). All experiments included an aliquot of sorted cells treated with an isotype-matched control MoAb and 2 to 3 of the test MoAbs. For 2 mobilized blood samples, sufficient cells were available to test the isotype control and all 3 test MoAbs in the same experiment.
) and motility (▪), respectively. Similar experimental results for each MoAb were obtained for 3 to 6 individual HPC populations (8 mobilized blood and 1 steady-state BM). All experiments included an aliquot of sorted cells treated with an isotype-matched control MoAb and 2 to 3 of the test MoAbs. For 2 mobilized blood samples, sufficient cells were available to test the isotype control and all 3 test MoAbs in the same experiment.
For HPCs, CD44/β1 integrin mediates adhesion and RHAMM/β1 integrin mediates motility. (A) Sorted CD34+45loSSClo cells were deposited into chambers coated with fibronectin, together with soluble HA, and their behavior was monitored by time lapse microscopy. Cell adhesion was defined as the number of cells remaining stationary (not floating) throughout the period of analysis. Most were deforming cells. Cell motility was defined as the number of cells that migrated a distance of at least one cell diameter during the period of analysis (13 mobilized blood and 2 steady-state BM). The values for steady-state BM were 23% to 28% motile and 36% to 61% adherent HPCs. For each sample of sorted HPCs, we analyzed the cells in at least three fields (100 to 150 total cells) to permit analysis of at least 60 adherent cells and at least 20 motile cells. The data points represent the aggregate values of all fields for each individual HPC sample. (B) For this representative sample, HPCs were pretreated with either IgG1 isotype-matched control, anti-RHAMM, anti-β1 integrin, or anti-CD44 before the time lapse microscopy and image collection. RHAMM and CD44 MoAbs serve as reciprocal internal specificity controls for inhibition of adhesion ( ) and motility (▪), respectively. Similar experimental results for each MoAb were obtained for 3 to 6 individual HPC populations (8 mobilized blood and 1 steady-state BM). All experiments included an aliquot of sorted cells treated with an isotype-matched control MoAb and 2 to 3 of the test MoAbs. For 2 mobilized blood samples, sufficient cells were available to test the isotype control and all 3 test MoAbs in the same experiment.
) and motility (▪), respectively. Similar experimental results for each MoAb were obtained for 3 to 6 individual HPC populations (8 mobilized blood and 1 steady-state BM). All experiments included an aliquot of sorted cells treated with an isotype-matched control MoAb and 2 to 3 of the test MoAbs. For 2 mobilized blood samples, sufficient cells were available to test the isotype control and all 3 test MoAbs in the same experiment.
MoAbs to RHAMM, CD44, and β1 integrin were added to mobilized blood HPCs to determine their role in adhesion and motility. The number of adherent cells was reduced twofold by anti-CD44 or anti-β1 integrin, as predicted from the work of Verfaillie et al,16-18 but was unaffected by anti-RHAMM MoAb. In contrast, the number of motile HPCs was reduced sevenfold by anti-RHAMM or anti-β1 integrin but was essentially unaffected by anti-CD44 (Fig 7B). The participation of β1 integrin in both adhesion and motility, together with the alternate use of CD44 or RHAMM, suggest that receptor cooperation may be important in determining HPC behavior.
DISCUSSION
The ability of HPCs to migrate to and from the BM via the blood is fundamental to their ability to populate distant BM sites and to repopulate their host after transplantation. Understanding their behavioral properties is thus highly relevant to understanding mobilization and engraftment. This work shows that G-CSF mobilization depletes intracellular stores of RHAMM and CD44 and modulates HA receptor usage. Furthermore, it indirectly implicates HA binding in RHAMM redistribution, providing a potential mechanism for the effects of G-CSF during mobilization. HA-dependent redistribution of RHAMM has been observed for human thymocytes,2 which, like HPCs, must undergo mobilization within a solid organ, in this case the thymus, to sites of cell death or into the peripheral circulation. This study demonstrates that HPCs have the HA receptor expression, HA binding capability, and RHAMM-dependent motogenic behavior required for migration through the body and suggests a role for HA and RHAMM in the events underlying stem cell mobilization and trafficking.
Consistent with an involvement of RHAMM and HA in mobilization, significant differences were observed between HPCs from steady-state BM and HPCs in mobilized blood. HPCs from M-BL have less sRHAMM, but a trend towards greater HA binding, than do HPC from steady state bone marrow (SS-BM). For M-BL HPCs, RHAMM and CD44 participate in HA binding, as measured using function-blocking MoAbs to inhibit HA binding. In contrast, for steady-state BM HPCs, MoAbs to RHAMM and CD44 mediate increased HA binding. Unexpectedly, although RHAMM appears to be the major HA binding receptor for mobilized blood HPCs, there was no correlation between sRHAMM or sCD44 and sHA binding. This was resolved by the observation that mobilized blood HPCs have substantial pools of icRHAMM and smaller pools of icCD44, suggesting that interactions with HA may stimulate HA receptor redistribution. icCD44 was significantly lower than icRHAMM or icHA binding. However, both RHAMM and CD44 participate in and significantly correlate with icHA binding, indicating that they are functional HA binding receptors in mobilized blood HPCs.
Detection of HA binding by intact cells involves a preincubation of live cells with HA. During this incubation period, RHAMM becomes detectable on the cell surface through its ability to bind HA, as evidenced by the inhibitory effects of anti-RHAMM on HA binding. The discordance between sRHAMM expression and RHAMM-dependent HA binding after incubation with HA-FITC appears to reflect recycling of RHAMM. For mobilized blood HPCs, HA binding was increased by agents that inhibit cell metabolism (azide) or disrupt lipid rafts (nystatin)40 and cytoskeletal assembly (cytochalasin B),14 40 suggesting that HA receptor recycling may be involved in HA binding and consequent migratory behavior. When putative HA receptor recycling is abrogated, HA receptors accumulate on the cell surface. These observations imply that the expression of RHAMM by mobilized blood HPCs, and their consequent motogenic behavior, may be regulated by an autocrine stimulatory loop. HA binding to basal levels of surface RHAMM appears to trigger deadhesion from the external microenvironment, followed by stimulation of RHAMM redistribution, RHAMM-dependent migratory behavior, and apparent recycling of HA receptors. This sequence of events is consistent with the functional prerequisites for cell motility in vitro and for stem cell mobilization and trafficking in vivo.
In contrast to the properties of mobilized blood HPCs, steady-state BM HPCs have a significantly higher intensity of intracellular RHAMM and CD44, as well as of HA binding by permeabilized HPCs. This suggests that intracellular stores of HA receptors are high until depleted during the events associated with G-CSF mobilization. Unlike the icHA receptors in mobilized blood HPCs, a subset of HA receptors in steady-state BM HPCs appeared to lack HA binding function. Consistent with this, treatment with anti-RHAMM or CD44 increased icHA binding, suggesting the acquisition of a functionally active conformation after interaction with these receptor agonists. There was a strong correlation between sRHAMM and icRHAMM for steady-state BM HPCs, as expected if the intracellular stores provide a reservoir of RHAMM for redistribution to the cell surface. Mobilized blood HPCs, in contrast, exhibited no correlation between sRHAMM and icRHAMM, as predicted if substantial redistribution and receptor engagement during migration from the BM had already taken place in vivo.
To identify the effects of G-CSF on HA receptors in mobilized BM HPCs, BM was obtained at the peak of G-CSF mobilization and analyzed in parallel with mobilized blood HPCs taken at the same point in time. Consistent with other work,41,42 immediately after cytokine treatment, HPCs were less frequent in mobilized BM than in steady-state BM, as predicted if most migrate to the periphery. However, Prosper et al43 have found that colony-forming units or long-term culture-initiating cells in BM did not change after mobilization. α4 and α5 integrin levels are reduced in mobilized blood HPCs as compared with mobilized or steady-state BM HPCs,7,25,43 as are β2 integrins,7,44 indicating that multiple changes in adhesion receptors accompany G-CSF mobilization. For the HA receptors analyzed here, HPCs remaining in the BM during mobilization had properties that closely matched their blood counterparts, making them distinct from HPCs in steady-state BM. This is in contrast to the patterns observed for VLA-4 integrin, where the expression pattern remained tissue-specific in steady state and after mobilization.43 Most steady-state BM analyzed here were from untreated cancer patients, whereas the mobilized BM were taken about 6 weeks after the last cycle of chemotherapy. It is possible that previous exposure to chemotherapy influences the pattern of HA receptors, and perhaps thus the propensity to mobilization, for HPCs in both blood and BM. The ratios of sRHAMM or CD44 to icRHAMM or CD44 in mobilized BM HPCs correlated strongly with those of mobilized blood HPCs. Unlike steady-state BM HPCs, which exhibited an increase in HA binding when treated with anti-RHAMM or anti-CD44, HA binding by mobilized BM HPCs was significantly inhibited by anti-RHAMM but was unaffected by anti-CD44. The inhibition of HA binding by anti-RHAMM was similar to that observed for malignant cells from untreated myeloma patients15 and thus is unlikely to reflect previous exposure to chemotherapy. Thus, like mobilized blood HPCs, during G-CSF mobilization, BM-localized HPCs use RHAMM as the predominant HA receptor. For anchored steady-state BM HPCs, but not for mobilized BM HPCs, both RHAMM and CD44 can be functionally upregulated by anti-RHAMM or anti-CD44 acting as agonists on large intracellular stores of HA receptors awaiting redistribution. This provides a potential mechanism for the deadhesion that is likely required for mobilization. By analogy, during mobilization with G-CSF and perhaps during naturally induced HPC migration, exposure to HA may trigger HA receptor redistribution and a predominant use of RHAMM for migratory behavior.
The role of CD44 in HA binding and in motogenic events underlying stem cell mobilization remains unclear. CD44 is expressed by BM and blood HPCs7,8,22 and is lower on cord blood HPCs as compared with adult BM HPCs,45 but appears to be higher after mobilization (Watanabe et al7 and this study). CD44 has been shown to play an important role in HPC adhesion to stroma and to ECM,8,16-19 behavior that is required after mobilization and trafficking to new BM sites. Thus, strong HA binding by CD44 may be downregulated until high-affinity integrin-mediated interactions with Fn and stroma are initiated.16,17 For human thymocytes, low-affinity interactions between β1 integrins and Fn appear to facilitate the rapid and repeated adhesion and deadhesion required for RHAMM-mediated motility.14 Alternatively, for malignant stem cells, high-affinity β1/Fn interactions facilitate CD44-mediated stable adhesion to HA.16 Integrins appear to play an important role in HPC anchoring to stroma or endothelium10,46 and in mobilization.47,48Adhesion, which requires strong stable binding, and motility, which requires dynamic weak adhesion/deadhesion, are probably mutually exclusive events in vivo. Thus, the events in which CD44 participates may occur at different stages in the life cycle of HPCs from those requiring RHAMM. MoAbs to CD44 inhibit stem cell engraftment19 and modulate hemopoiesis in long-term cultures.19,20 CD44 has also been shown to regulate adhesion and proliferation of HPCs.16-18 The pool of intracytoplasmic CD44 receptors in HPCs is small, suggesting that, among HA receptors, the major redistribution from intracellular regions to the surface involves RHAMM. Overall, these observations suggest that CD44 plays a regulatory role in hemopoiesis and/or mobilization that is implemented at a different time from that of RHAMM. The work here confirms that CD44 and RHAMM play reciprocal roles in mediating HPC behavior, with CD44 participating in adhesion and RHAMM mediating motility. These patterns are consistent with the HA receptor localization and usage detected for steady-state and mobilized HPCs, respectively. However, both adhesion and motility are dependent on interactions via β1 integrins that may be regulated by integrin avidity modulation. High-avidity β1 integrin cooperates with CD44 in adhesion,16 whereas low-avidity β1 integrin cooperates with RHAMM in motility.14 This suggests that, on HPCs, the binding conformation of β1 integrin may regulate its associations with HA receptors to determine the behavioral outcome of Fn and HA engagement.
The experiments described here model events that may underlie G-CSF mobilization of HPCs from the BM to the circulation. In the BM, engagement of HA receptors appears to increase RHAMM-dependent HA binding, and RHAMM/HA interactions are then likely to mediate deadhesive motile behavior. The results shown here predict that in vivo infusion of HA, analogous to exposure to HA in vitro, may cause hematopoietic cells to initiate migratory behavior culminating in exit from the BM via intravasation. Infused HA might be expected to cause redistribution of icHA receptors in hematopoietic cells of many types (HPCs and cells at all differentiation stages within hematopoietic lineages), thus increasing the surface density of HA receptors. These receptors would then interact with HA to cause deadhesion and initiation of the motile behavior required for migration to the blood. The redistribution of RHAMM to the cell surface may play a role in the G-CSF–associated reduced apoptosis and increased survival of mobilized HPCs,49 possibly by counteracting anoikis.50The ability to rapidly recycle RHAMM may also facilitate re-engraftment and a return to adherent behavior of HPCs after transplantation. In conclusion, this work suggests a novel means of stem cell mobilization using HA, perhaps together with the cytokines and chemotherapy, that may optimize the kinds and numbers of HPCs mobilized to the blood and ultimately the quality of the transplant.
ACKNOWLEDGMENT
The authors thank Sheryl Gares for her thorough and critical reading of this manuscript. We thank the Red Cross Blood Transfusion Service and the staff at the Tom Baker Cancer Center for their help in obtaining apheresis samples from peripheral blood stem cell collections.
Supported by the Cancer Research Society Inc of Canada. The University of Alberta flow cytometry facility is funded by grants from the Medical Research Council of Canada and the Alberta Cancer Board Research Infrastructure Maintenance program.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Linda M. Pilarski, PhD, Cross Cancer Institute, 11560 University Ave, Edmonton, AB T6G1Z2, Canada; e-mail: lpilarsk@gpu.srv.ualberta.ca.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal