Abstract
Hematopoietic progenitor cells (HPC) can be mobilized from the bone marrow into the peripheral circulation in response to a number of stimuli including hematopoietic growth factors, cytotoxic agents, and certain chemokines. Despite significant differences in their biological activities, these stimuli result in the mobilization of HPC with a similar phenotype, suggesting that a common mechanism for mobilization may exist. In this study, the role of granulocyte colony-stimulating factor (G-CSF) in progenitor mobilization was examined using G-CSF receptor (G-CSFR)–deficient mice. In contrast to wild-type mice, no increase in circulating colony-forming cells (CFU-C), CD34+ lineage− progenitors, or day 12 colony-forming unit-spleen progenitors (CFU-S) was detected in G-CSFR–deficient mice after cyclophosphamide administration. This defect was not due to a failure to regenerate HPC following cyclophosphamide administration as the number of CFU-C in the bone marrow of G-CSFR–deficient mice was increased relative to wild-type mice. Likewise, no increase in circulating CFU-C was detected in G-CSFR–deficient mice following interleukin-8 (IL-8) administration. In contrast, mobilization of HPC in response to flt-3 ligand was nearly normal. These results show that the G-CSFR is required for mobilization in response to cyclophosphamide or IL-8 but not flt-3 ligand and suggest that the G-CSFR may play an important and previously unexpected role in HPC migration.
THE USE OF hematopoietic progenitor cells to reconstitute hematopoiesis following myeloablative therapy has significantly improved the clinical outcome in patients with a variety of cancers. Recently, peripheral blood progenitor cells instead of bone marrow (BM)-derived progenitor cells have been used because of their reduced engraftment times and relative ease of collection. Hematopoietic progenitor cells (HPC) can be mobilized from the BM by diverse stimuli including chemotherapy, hematopoietic cytokines, and certain chemokines.1 Despite intensive study, the mechanisms that control the movement of HPC between BM and blood are incompletely understood.
Hematopoietic growth factors, along with chemotherapy, are the most commonly used agents to mobilize HPC. A partial list of the hematopoietic growth factors capable of mobilizing HPC include granulocyte colony-stimulating factor (G-CSF),2-4 granulocyte-macrophage colony-stimulating factor (GM-CSF),4,5 interleukin-12 (IL-12),6 IL-7,7 stem cell factor (SCF),8,9 and flt-3 ligand.10 Despite clear differences in biologic activities, several common features are observed during mobilization with these agents. First, a broad spectrum of HPC are mobilized including primitive pluripotent as well as committed myeloid, megakaryocytic, and erythroid progenitors.6-11 Second, relative to HPC resident in the BM, mobilized HPC have decreased expression of c-kit12 13 and the β-1-integrin VLA-4,14 the latter a potentially important finding given the recent reports that anti–VLA-4 antibodies can mobilize HPC.15,16 Finally, a high percentage of mobilized HPC appear to be quiescent17-19; in one recent report, 7% of mobilized HPC versus 47% of BM HPC were observed to be in S-phase.17 The observation that hematopoietic growth factors with distinct cellular targets and biologic activities result in the mobilization of HPC with a similar phenotype suggests that a common mechanism for mobilization may exist.
G-CSF is the most commonly used agent to mobilize HPC because of its potency and lack of serious toxicity. In addition, G-CSF recently has been shown to act synergistically with cytotoxic agents,20,21 SCF,22,23 or flt-3 ligand24,25 to induce HPC mobilization. To explore the mechanisms of G-CSF–induced mobilization we examined HPC mobilization in mice genetically deficient for the G-CSF receptor (G-CSFR). We previously have shown that G-CSFR–deficient mice have a quantitative defect in granulopoiesis, with the residual neutrophils appearing to be phenotypically normal.26 The defect in hematopoiesis in G-CSFR–deficient mice appears to be limited to granulopoiesis because the number and cytokine responsiveness of myeloid progenitors in the BM and spleen of these mice were near normal.26 Further, the number and function of primitive multipotent progenitors, as measured by day 12 CFU-S assays, were normal.27 In this study, mobilization of murine HPC in response to the three major types of mobilizing stimuli, cytotoxic agents (cyclophosphamide), chemokines (IL-8), and hematopoietic cytokines (flt-3 ligand) was examined. We show that HPC mobilization in G-CSFR–deficient mice by cyclophosphamide or IL-8 is markedly impaired whereas mobilization by flt-3 ligand is essentially intact.
MATERIALS AND METHODS
Mice
The G-CSFR–deficient mice (outbred C57BL/6 × 129 SvJ) were generated in our laboratory as described previously.26 The inbred 129 SvJ G-CSFR–deficient mice were generated as follows. The production of chimeric mice using a single RW4 ES clone containing a targeted null mutation of the G-CSFR has been described previously.26 The chimeric mice were intercrossed with 129 SvJ mice and their progeny genotyped to identify heterozygous G-CSFR mutant mice; these mice were then interbred to generate homozygous G-CSFR mutant mice on a 129 SvJ genetic background. All mice were housed in a specific-pathogen free environment and examined daily by veterinary staff for signs of illness.
Peripheral Blood, Spleen, and BM Analysis
Blood was obtained by retro-orbital venous plexus sampling in polypropylene tubes containing EDTA. Complete blood counts were determined using a Baker-9000 automated cell counter (Serono-Baker, Allentown, PA). BM was obtained by flushing both femoral bones with 3 mL of α-Minimum Essential Medium (α-MEM) containing 2% fetal bovine serum (FBS). Manual leukocyte differentials were performed on Wright-stained blood smears or cytospin preparations of BM mononuclear cells.
Colony-Forming Cell Assay (CFU-C)
BM and spleen mononuclear cells were enumerated using a hemacytometer. 2.5 × 104 BM, 1 × 105 spleen mononuclear cells, or 40 μL of EDTA-anticoagulated whole blood were plated in 1.25 mL of methylcellulose media supplemented with erythropoietin and pokeweed mitogen–stimulated murine spleen cell conditioned medium (MethoCult M3430; Stem Cell Technologies, Vancouver, BC, Canada) and placed at 37°C in a humidified chamber with 5% CO2 . Colonies containing at least 50 cells were scored on day 7-8.
Flow Cytometry
CD34+ lineage− cells were enumerated as described.28 Red blood cell–depleted peripheral blood mononuclear cells were incubated with biotin-conjugated rat-antimouse CD34 (RAM34, IgG2a ) and the following cocktail of lineage-restricted fluorescien isothiocyanate (FITC)-conjugated rat monoclonal antibodies: antimouse B220 (M1/70, IgG2b ), antimouse CD3 (M1/70, IgG2b ), and antimouse CDllb (M1/70, IgG2b ). After this incubation, cells were incubated with phycoerythrin (PE)-conjugated streptavidin (GIBCO-BRL, Gaithersburg, MD). FITC-conjugated rat IgG2b (R35-38) and biotin-conjugated rat IgG2a (R35-95) were used as isotype controls. All antibodies were purchased from Pharmingen (San Diego, CA). All cells were analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
CFU-Spleen (CFU-S) Assay
Day 12 CFU-S numbers were determined as described.29 Peripheral blood was obtained from donor mice by retro-orbital venous plexus sampling and approximately 200 μL injected into each of five lethally irradiated (900 cGy, single dose) wild-type recipient mice. Mice were killed after 12 days, their spleens harvested, and macroscopic colonies counted after overnight fixation in Tellesniczky's solution. No colonies were observed in saline-injected controls (data not shown).
Progenitor Mobilization in Mice
G-CSF.Recombinant human G-CSF (Amgen, Thousand Oaks, CA) was administered by daily subcutaneous injection at a dose of 250 μg/kg/d for 5 days. Peripheral blood was obtained before the first G-CSF dose and 4 to 6 hours after the final G-CSF dose.
Cyclophosphamide.Cyclophosphamide (Sigma, St Louis, MO) was reconstituted in sterile water and (200 mg/kg) administered as a single intraperitoneal injection. Mice were analyzed at the indicated times.
IL-8.Recombinant human IL-8 was a generous gift from Dainippon Pharmaceutical Co, Ltd (Osaka, Japan). rhIL-8 (30 μg/mouse) was administered by a single intraperitoneal injection and peripheral blood obtained at the indicated times. To minimize the effect of repeated phlebotomies, no mouse was subjected to more than three blood draws within 1 day.
Flt-3 ligand.Recombinant human flt-3 ligand was a generous gift from Immunex (Seattle, WA). rhFlt-3 ligand (10 μg/mouse/d) was given by subcutaneous injection for 10 days. Mice were analyzed in the morning following the final injection.
Statistical Analysis
Data are presented as mean ± SEM. Statistical significance was assessed by two-sided Student's t-test.
RESULTS
G-CSF is a potent stimulus for HPC mobilization in mice.2,4 5 To examine G-CSF–induced mobilization in G-CSFR–deficient mice, we stimulated mice (n = 6) with 250 μg/kg/d of human G-CSF for 5 days and measured their mobilization response. Wild-type mice had the expected increase in blood neutrophils (18.2 ± 4.3-fold increase over baseline) and CFU-C (48.3 ± 21.4-fold increase over baseline). In contrast, no significant increase in circulating neutrophils (0.6 ± 0.1-fold increase over baseline) or CFU-C (1.3 ± 1.3-fold increase over baseline) was detected after G-CSF stimulation of G-CSFR–deficient mice. These data show that G-CSF–induced HPC mobilization requires the G-CSFR.
Mobilization of HPC in Response to Cyclophosphamide Is Markedly Impaired in G-CSFR–Deficient Mice
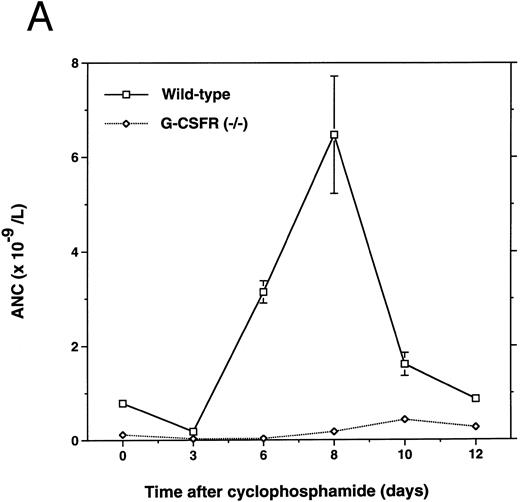
Cyclophosphamide treatment is another potent stimulus for HPC mobilization in mice.20,30,31 To determine whether cyclophosphamide-induced mobilization requires the G-CSFR, we challenged G-CSFR–deficient mice with this agent (Fig 1). In comparison with wild-type mice, neutrophil recovery was delayed and blunted in G-CSFR–deficient mice. Wild-type mice had the expected mobilization response with a 40-fold increase in blood CFU-C observed 8 days after cyclophosphamide.31 In contrast, no increase in CFU-C was detected in the blood of G-CSFR–deficient mice at any time during this study. Likewise, a significant increase in circulating CD34+ lineage− HPC was detected in wild-type but not G-CSFR–deficient mice (Fig 1C). To determine whether the defect in HPC mobilization in G-CSFR–deficient mice extended to more primitive HPC, we measured the level of CFU-S (d12) progenitors in peripheral blood on day 8 after cyclophosphamide administration (Fig 2). As reported previously,31 a significant increase in peripheral blood CFU-S (d12) progenitors was detected in wild-type mice. In contrast, no increase in CFU-S (d12) was detected in the blood of G-CSFR–deficient mice.
Mobilization of hematopoietic cells into peripheral blood in response to cyclophosphamide. Peripheral blood was obtained at the indicated times after a single intraperitoneal injection of 200 mg/kg of cyclophosphamide and analyzed for (A) absolute neutrophil count (ANC), (B) colony-forming cells (CFU-C), and (C) CD34+ lineage− progenitors as described in Materials and Methods. Four to six age- and sex-matched mice were analyzed at each time point. Data represent the mean ± SEM.
Mobilization of hematopoietic cells into peripheral blood in response to cyclophosphamide. Peripheral blood was obtained at the indicated times after a single intraperitoneal injection of 200 mg/kg of cyclophosphamide and analyzed for (A) absolute neutrophil count (ANC), (B) colony-forming cells (CFU-C), and (C) CD34+ lineage− progenitors as described in Materials and Methods. Four to six age- and sex-matched mice were analyzed at each time point. Data represent the mean ± SEM.
Mobilization of day 12 CFU-S into the peripheral blood after cyclophosphamide. Peripheral blood from donor mice was obtained 8 days after cyclophosphamide and 200 μL injected into each of five lethally irradiated recipient mice. Recipient mice were killed 12 days later and macroscopic spleen colonies enumerated (CFU-S [d12]). Two age- and sex-matched donor mice of each genotype were analyzed. The horizontal bars represent the mean of the data.
Mobilization of day 12 CFU-S into the peripheral blood after cyclophosphamide. Peripheral blood from donor mice was obtained 8 days after cyclophosphamide and 200 μL injected into each of five lethally irradiated recipient mice. Recipient mice were killed 12 days later and macroscopic spleen colonies enumerated (CFU-S [d12]). Two age- and sex-matched donor mice of each genotype were analyzed. The horizontal bars represent the mean of the data.
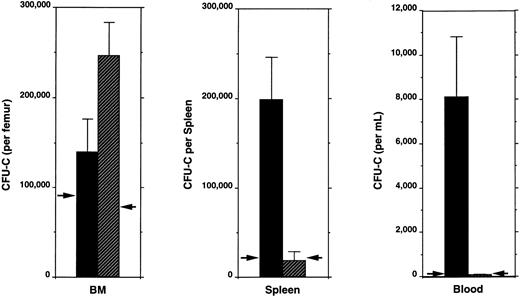
To exclude the possibility that the lack of an increase in peripheral HPC was due to an inability of G-CSFR–deficient mice to regenerate HPC after cyclophosphamide administration, we quantitated CFU-C in the BM, spleen, and blood of these mice (Fig 3 and Table 1). A similar increase from baseline of total body CFU-C was observed in wild-type and G-CSFR–deficient mice (Table 1) and, in fact, the absolute number of CFU-C present in the BM of G-CSFR–deficient mice on day 8 after cyclophosphamide administration was significantly increased relative to wild-type mice. However, despite the increase in total body CFU-C, no redistribution of these CFU-C from the BM to peripheral blood or spleen was observed. Interestingly, mature neutrophils (PMN) showed a similar pattern; the number of PMN in the BM was increased without a concomitant increase in circulating PMN (Table 1). Similar analyses were performed on day 12 after cyclophosphamide administration and showed that the number of CFU-C in the BM, spleen, and blood of both wild-type and G-CSFR–deficient mice had returned to near baseline levels (data not shown).
Tissue distribution of hematopoietic progenitors after cyclophosphamide administration. Bone marrow (BM), spleen, and peripheral blood were obtained 8 days after cyclophosphamide and assayed for colony-forming cell (CFU-C) content. The baseline level of CFU-C present in each tissue is indicated by arrowheads. Four to six age- and sex-matched mice were analyzed. Data represent the mean ± SEM. (▪), Wild type; (▨), G-CSFR (−/−).
Tissue distribution of hematopoietic progenitors after cyclophosphamide administration. Bone marrow (BM), spleen, and peripheral blood were obtained 8 days after cyclophosphamide and assayed for colony-forming cell (CFU-C) content. The baseline level of CFU-C present in each tissue is indicated by arrowheads. Four to six age- and sex-matched mice were analyzed. Data represent the mean ± SEM. (▪), Wild type; (▨), G-CSFR (−/−).
Tissue Distribution of Neutrophils and Hematopoietic Progenitors at Baseline or Following Cyclophosphamide or flt-3 Ligand Treatment
| Tissue . | G-CSFR Genotype . | Baseline . | Cyclophosphamide . | Flt-3 Ligand . | |||
|---|---|---|---|---|---|---|---|
| . | . | PMN (×10−6) . | CFU-C . | PMN (×10E-6) . | CFU-C . | PMN (×10−6) . | CFU-C . |
| Blood (per mL) | (+/+) | 0.79 ± 0.10 | 131 ± 28 | 6.47 ± 1.24 | 8,100 ± 2,730 | 7.12 ± 2.21 | 38,000 ± 6,100 |
| (−/−) | 0.12 ± 0.01* | 178 ± 24 | 0.18 ± 0.04* | 67 ± 28‡ | 1.72 ± 0.32 | 21,000 ± 4,200 | |
| BM (per femur) | (+/+) | 5.09 ± 0.58 | 96,262 ± 3,989 | 18.68 ± 1.94 | 132,525 ± 24,350 | 8.23 ± 0.48 | 254,338 ± 49,058 |
| (−/−) | 2.33 ± 0.44† | 79,525 ± 8,569 | 5.46 ± 1.12† | 222,502 ± 27,983ρ | 3.72 ± 2.45 | 127,188 ± 9,643 | |
| Spleen (total) | (+/+) | ND | 22,240 ± 3,705 | ND | 208,592 ± 32,000 | ND | 945,000 ± 81,624 |
| (−/−) | ND | 22,124 ± 5,587 | ND | 11,029 ± 2,846* | ND | 474,525 ± 90,302ρ | |
| Total (per mouse) | (+/+) | ND | 1,626,638 ± 67,975 | ND | 2,431,916 ± 400,241 | ND | 4,912,715 ± 772,881 |
| (−/−) | ND | 1,348,890 ± 146,111 | ND | 3,719,515 ± 467,286 | ND | 2,559,328 ± 216,829ρ | |
| Tissue . | G-CSFR Genotype . | Baseline . | Cyclophosphamide . | Flt-3 Ligand . | |||
|---|---|---|---|---|---|---|---|
| . | . | PMN (×10−6) . | CFU-C . | PMN (×10E-6) . | CFU-C . | PMN (×10−6) . | CFU-C . |
| Blood (per mL) | (+/+) | 0.79 ± 0.10 | 131 ± 28 | 6.47 ± 1.24 | 8,100 ± 2,730 | 7.12 ± 2.21 | 38,000 ± 6,100 |
| (−/−) | 0.12 ± 0.01* | 178 ± 24 | 0.18 ± 0.04* | 67 ± 28‡ | 1.72 ± 0.32 | 21,000 ± 4,200 | |
| BM (per femur) | (+/+) | 5.09 ± 0.58 | 96,262 ± 3,989 | 18.68 ± 1.94 | 132,525 ± 24,350 | 8.23 ± 0.48 | 254,338 ± 49,058 |
| (−/−) | 2.33 ± 0.44† | 79,525 ± 8,569 | 5.46 ± 1.12† | 222,502 ± 27,983ρ | 3.72 ± 2.45 | 127,188 ± 9,643 | |
| Spleen (total) | (+/+) | ND | 22,240 ± 3,705 | ND | 208,592 ± 32,000 | ND | 945,000 ± 81,624 |
| (−/−) | ND | 22,124 ± 5,587 | ND | 11,029 ± 2,846* | ND | 474,525 ± 90,302ρ | |
| Total (per mouse) | (+/+) | ND | 1,626,638 ± 67,975 | ND | 2,431,916 ± 400,241 | ND | 4,912,715 ± 772,881 |
| (−/−) | ND | 1,348,890 ± 146,111 | ND | 3,719,515 ± 467,286 | ND | 2,559,328 ± 216,829ρ | |
Band, ring, and polymorphonuclear neutrophils were scored as mature neutrophils (PMN). Total body CFU-C were calculated by assuming a blood volume of 1.8 mL and a whole femur equivalent to 6% of the total BM. Analyses were performed at baseline, 8 days after a single intraperitoneal injection of cyclophosphamide, or after 10 days of flt-3 ligand administration. Five to 6 age- and sex-matched mice were analyzed for each data entry. Data represent the mean ± SEM.
Abbreviation: ND, not done.
Statistical comparison to wild type: * P < .001; † P < .01; ‡ P < .02; ρ P < .05.
A recent report suggested that the magnitude of HPC mobilization after G-CSF varied significantly between mouse strains.32 We therefore examined the HPC mobilization response to cyclophosphamide in inbred 129 SvJ G-CSFR deficient mice. No increase from baseline in peripheral blood or spleen CFU-C was detected on day 8 after cyclophosphamide administration in 129 SvJ G-CSFR (−/−) mice despite a significant increase (albeit less than wild-type C57BL/6 × 129 SvJ outbred mice) in circulating CFU-C in wild-type 129 SvJ mice (data not shown). Collectively, these results clearly show that the G-CSFR is required for HPC mobilization in response to cyclophosphamide treatment in mice.
The Mobilization of CFU-C in Response to IL-8 Is Impaired in G-CSFR–Deficient Mice
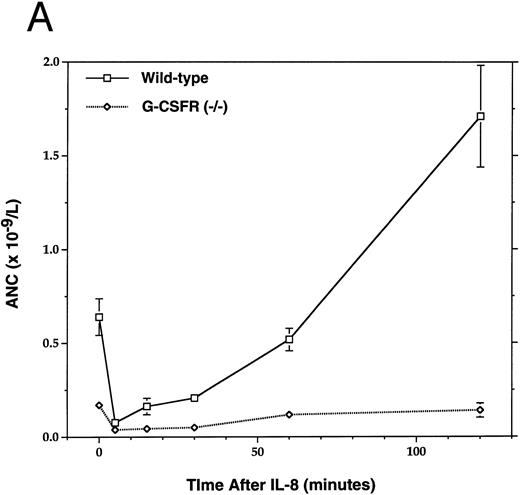
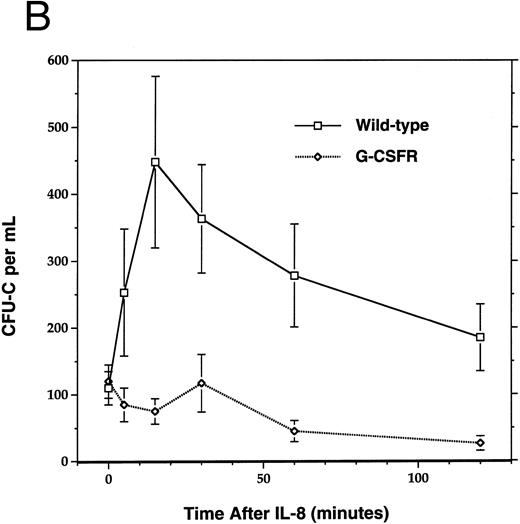
The chemokine IL-8 mobilizes HPC with kinetics distinct from cytotoxic therapy or hematopoietic growth factors; IL-8 administration in mice33 or rhesus monkeys34 induces a much more rapid increase (within 15 to 30 minutes) in circulating HPC raising the possibility that distinct mechanisms of HPC mobilization are being used. To determine whether IL-8–induced mobilization also requires the G-CSFR, we challenged G-CSFR–deficient mice with IL-8 (Fig 4). Although both wild-type and G-CSFR–deficient mice had the expected transient neutropenia after IL-8 administration, only the wild-type mice had the expected rebound neutrophilia (Fig 4A). As reported previously,33 IL-8 administration in wild-type mice induced a rapid (peak response at 15 minutes) fourfold increase in circulating CFU-C. In contrast, no increase in circulating CFU-C was detected at any time after IL-8 administration in G-CSFR–deficient mice. Similar results were obtained with 129/SvJ G-CSFR–deficient mice (data not shown). These results indicate that the G-CSFR also is required for IL-8–induced HPC mobilization in mice.
Mobilization of hematopoietic cells into peripheral blood after IL-8 administration. Peripheral blood was obtained at the indicated times after a single intraperitoneal injection of 30 μg of human recombinant IL-8 and analyzed for (A) absolute neutrophil count (ANC) and (B) colony-forming cells (CFU-C). Four to six age- and sex-matched mice were analyzed at each time point. Data represent the mean ± SEM.
Mobilization of hematopoietic cells into peripheral blood after IL-8 administration. Peripheral blood was obtained at the indicated times after a single intraperitoneal injection of 30 μg of human recombinant IL-8 and analyzed for (A) absolute neutrophil count (ANC) and (B) colony-forming cells (CFU-C). Four to six age- and sex-matched mice were analyzed at each time point. Data represent the mean ± SEM.
The Mobilization of HPC in Response to flt-3 Ligand Is Near Normal in G-CSFR–Deficient Mice
The hematopoietic cytokine flt-3 ligand is a potent stimulator of HPC mobilization in mice.10 However, unlike G-CSF, the administration of flt-3 ligand in mice leads to an absolute increase in total body HPC as well as a redistribution of HPC from the BM to the periphery.10 Therefore, we examined the ability of flt-3 ligand to induce HPC mobilization in G-CSFR–deficient mice (Fig 5 and Table 1). Although reduced relative to wild-type mice, flt-3 ligand administration for 10 days resulted in a significant expansion of total body CFU-C in G-CSFR–deficient mice. Further, in sharp contrast to cyclophosphamide treatment, treatment with flt-3 ligand clearly resulted in the redistribution of HPC from the BM to the spleen and blood (Table 1). Interestingly, flt-3 ligand treatment also resulted in the mobilization of PMN to the peripheral circulation. These data show that the G-CSFR is not required for flt-3 ligand-induced HPC mobilization.
Tissue distribution of hematopoietic progenitors after flt-3 ligand administration. Bone marrow (BM), spleen, and peripheral blood were obtained after 10 days of treatment with flt-3 ligand and assayed for colony-forming cell (CFU-C) content. The baseline level of CFU-C present in each tissue is indicated by arrowheads. Five age- and sex-matched mice were analyzed. Data represent the mean ± SEM. (▪), Wild type; (▨), G-CSFR (−/−).
Tissue distribution of hematopoietic progenitors after flt-3 ligand administration. Bone marrow (BM), spleen, and peripheral blood were obtained after 10 days of treatment with flt-3 ligand and assayed for colony-forming cell (CFU-C) content. The baseline level of CFU-C present in each tissue is indicated by arrowheads. Five age- and sex-matched mice were analyzed. Data represent the mean ± SEM. (▪), Wild type; (▨), G-CSFR (−/−).
DISCUSSION
Hematopoietic progenitors can be mobilized from the BM into the peripheral circulation in response to a number of stimuli including hematopoietic growth factors, cytotoxic agents, and certain chemokines. Despite significant differences in their biologic activities, these stimuli result in the mobilization of HPC with a similar phenotype, suggesting that a common mechanism for mobilization may exist. In this study we have examined the contribution of the G-CSFR to HPC mobilization and show that, in mice, the G-CSFR is required for mobilization by cyclophosphamide or IL-8 but not flt-3 ligand.
Myeloablative therapy with or without hematopoietic growth factors is used extensively to mobilize HPC in patients.1 The mechanisms that mediate this response are unknown; however, a role for hematopoietic growth factors can be postulated because the level of certain hematopoietic growth factors is markedly elevated following myeloablative therapy.35 36 In this study, we show that G-CSFR–deficient mice have a marked defect in HPC mobilization in response to cyclophosphamide (as measured by CFU-C and CFU-S [d12] assays and by flow cytometry for CD34+ lineage− cells). Although none of these progenitor assays directly measure hematopoietic stem cell activity, it seems unlikely that mobilization of hematopoietic stem cells is normal in G-CSFR–deficient mice because the mobilization of hematopoietic stem cells is closely associated with mobilization of more committed HPC.
The defect in HPC mobilization after cyclophosphamide in G-CSFR–deficient mice appears to be secondary to a failure to release HPC from the BM rather than to a failure to regenerate HPC since the total number of CFU-C present in these mice (at a time when peak HPC mobilization should have occurred) is actually increased relative to wild-type mice. These results may provide an explanation for the observation that G-CSF and cyclophosphamide act synergistically to mobilize HPC in mice20; cyclophosphamide may provide the major stimulus for HPC proliferation with G-CSF providing the stimulus for HPC migration from the BM.
The chemokine IL-8 can induce HPC mobilization in mice33 and rhesus monkeys.34 Several observations have lead to the hypothesis that IL-8 activation of neutrophils may be critical for IL-8–induced HPC mobilization. First, the rapid kinetics of IL-8–induced mobilization (initial response within 5 minutes after parenteral administration) suggests a direct mechanism for IL-8.33,34 Second, neutrophils are the major known target for IL-8.37 Third, IL-8 is a potent activator and chemoattractant for neutrophils.37 Although controversial, receptors for IL-8 may be present on endothelial cells, suggesting that alterations in endothelial cell function also may be important for IL-8–induced mobilization.38 39
In the present study, we show that IL-8–induced HPC mobilization in G-CSFR–deficient mice is impaired. In isolated cases, we have observed G-CSFR–deficient mice that have normal levels of circulating neutrophils; these mice still fail to mobilize HPC in response to IL-8, suggesting that neutropenia per se is not solely responsible for the mobilization defect. Although the residual neutrophils in G-CSFR–deficient mice appear phenotypically normal as assessed by morphology, expression of myeloperoxidase, Gr-1, and CDllb, and by their ability to emigrate in response to intra-peritoneal thioglycollate,26 these results suggest that an unidentified functional defect in G-CSFR–deficient neutrophils may exist. Experiments are underway to examine the in vitro IL-8 responses of neutrophils isolated from G-CSFR–deficient mice.
Flt-3 ligand is a potent stimulus for HPC mobilization in mice and, unlike G-CSF, results in an increase in total body HPC.10 Recently, G-CSF has been shown to act synergistically with flt-3 ligand to mobilize HPC.24,25 In this study, we show that flt-3 ligand–induced mobilization is essentially intact; although twofold fewer total body number of CFU-C were detected in G-CSFR–deficient mice relative to wild-type mice, the distribution of these progenitors into peripheral blood, spleen, and BM compartments was similar to wild-type mice. These observations show that the G-CSFR is not required for the efficient flt-3 ligand–induced migration of HPC from the BM to periphery. In this respect, it is interesting to note that flt-3 ligand treatment is not associated with the defect in neutrophil release from the BM noted after cyclophosphamide treatment. The submaximal increase in total body CFU-C in G-CSFR–deficient mice following flt-3 ligand treatment suggests two possibilities: that G-CSFR signals contribute significantly to flt-3 ligand–induced HPC proliferation in vivo, or that fewer flt-3 ligand responsive progenitors exist in G-CSFR–deficient mice. In support of the first possibility, a synergistic effect of G-CSF and flt-3 ligand on HPC proliferation has been detected in vitro.40
In summary, this study provides evidence that the G-CSFR plays an important and previously unexpected role in HPC mobilization. The G-CSFR is primarily expressed on hematopoietic cells including pluripotent and myeloid-committed progenitors and neutrophils.41 In addition, the G-CSFR is expressed on endothelial cells and can induce their proliferation in vitro.42 The current results suggests that G-CSFR signals generated in progenitor cells, neutrophils, or BM stromal endothelial cells are critical for mobilization. Studies are in progress to test this hypothesis and to further define the mobilization defect in G-CSFR–deficient mice.
ACKNOWLEDGMENT
We thank Nancy Link for her expert technical assistance. We thank Dr Joost Oppenheim for his assistance in obtaining the recombinant human IL-8.
Supported by the James S. McDonnell Foundation.
Address reprint requests to Daniel C. Link, MD, Washington University Medical School, Division of Bone Marrow Transplantation and Stem Cell Biology, Box 8007, 660 S Euclid Ave, St Louis, MO 63110-1093.



![Fig. 2. Mobilization of day 12 CFU-S into the peripheral blood after cyclophosphamide. Peripheral blood from donor mice was obtained 8 days after cyclophosphamide and 200 μL injected into each of five lethally irradiated recipient mice. Recipient mice were killed 12 days later and macroscopic spleen colonies enumerated (CFU-S [d12]). Two age- and sex-matched donor mice of each genotype were analyzed. The horizontal bars represent the mean of the data.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/7/10.1182_blood.v90.7.2522/3/m_bl_0036f2.jpeg?Expires=1769145264&Signature=MQRlN2w2LntWJQ7xVvj~mov0fK3kGU8CfibKfQA~EAVs~bRjlVdQ1FYutIadAPzSrTxRrmdxew-8SPHxnpI8xJ~zsKEAxkZ8bEMCWywTb96JUdO5VmiA3sUPPXSUmhCBeYg1gmQvEYIImThYH4e4YM6SN2-bbYQ8nWaOTzkBh0lxGsE3o-Sx968Bb8nHQWj4kfAd2~puz-zOFTZ6iU4O~YovgoFME1oV76-wC~1C07rej49Q4kmKrd-pYtlTt~sIdF1zgpc3KUrcKXXfryUWFudSiScd5168Zq730WPTPsi8X3C2kVCmxQaG3yg9pszqJPxnsH1ot7g8PsFDfo8c9g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal