Abstract
Bone marrow (BM) transplantation still must overcome multiple difficulties and should benefit from better understanding of stem-cell homing and mobilization. Here, we analyzed the involvement of several adhesion molecules in the two processes by treating mice with monoclonal antibodies against these molecules. Treatment of lethally irradiated mice grafted with isogeneic BM cells showed that at least two migration pathways are important for stem-cell homing to the BM, whereas only one of them is involved in lodging of colony-forming unit–spleen (CFU-S) in the spleen. We confirm that the VLA-4/VCAM-1 adhesion pathway is important for stem-cell homing to the BM only and show that CD44 is involved in CFU-S lodging in both BM and spleen. These results show that entry of CFU-S into the spleen is regulated. The observation that when one migration pathway is altered, CFU-S do not enter the BM via the other pathway may indicate that the two mechanisms involved in CFU-S homing into the BM are linked. The adhesion molecules VLA-4 and CD44 are also implied in the mobilization of stem cells into the blood stream of mice injected once with anti–VLA-4 or anti-CD44. Anti–VLA-4 administration led to a significant increase in circulating stem cells as early as 8 hours after treatment. Stem cells mobilized by anti–VLA-4 comprise cells with high self-renewal potential and thus may be used for long-term reconstitution of the hematopoietic tissue.
© 1998 by The American Society of Hematology.
CELLULAR ADHESION and its involvement in hematopoiesis have been analyzed using a number of different approaches. From these studies, the notion has emerged that interactions between the bone marrow (BM) microenvironment and hematopoietic stem and progenitor cells (HSPC) are essential for the regulation of commitment, proliferation, and differentiation of HSPC that is for the regulation of blood cell formation. Most stromal-hematopoietic cell interactions are mediated by mutual recognition of adhesive receptors/ligands located at the surface of both HSPC and stromal cells as well as in the surrounding extracellular matrix (ECM). The adhesion molecules involved in such processes not only have a prominent role in the regulation of hematopoiesis, but also in the regulation of stem- and progenitor-cell trafficking between hemolymphopoietic tissues and the blood stream. In mice, during development stem cells migrate from yolk sac to fetal liver and spleen to BM. After birth, although stem and progenitor cells are almost exclusively located in the BM, and to a lesser extent in the spleen, they still exhibit migratory properties. Under physiological conditions, stem cells migrate within the BM cavity and intravasate into the peripheral circulation. This process can be amplified by various treatments, in particular injection of cytokines such as granulocyte colony-stimulating factor (G-CSF), and is in this case referred to as stem-cell mobilization. The reverse process, namely the homing of stem cells to the extravascular compartment of the BM, occurs in irradiated recipients after transplantation of hematopoietic cells. The mechanisms underlying the movement of cells from and toward the marrow are not well understood. One possibility is the modification of the expression and/or affinity of adhesion molecules by stem and progenitor cells.1-6
HSPC in both the human and the mouse express a number of cell adhesion molecules. These include in particular the integrins α4β1 (VLA-4 or CD49d/CD29), a receptor for VCAM-1 (CD106) and fibronectin, and αLβ2 (LFA-1 or CD11a/CD18), a coreceptor for ICAM-1 (CD54) that is expressed on both hematopoietic and stromal cells; L-selectin (CD62-L), a ligand for the CD34 form found on endothelial cells; and the glycoprotein CD44, which binds to hyaluronate and other ECM proteins.7-13 There is evidence that a number of these molecules are involved in the migratory pathways used during HSPC homing or mobilization. The potential role of α4β1/VCAM-1 as mediators of HSPC migration is supported by in vitro as well as in vivo experiments. Antibodies against these molecules prevent binding of stem cells to fibronectin coated dishes7and to stromal layers,14-17 inhibit the entry of stem cells into the BM of irradiated mice,17 and mobilize colony forming unit–culture (CFU-C) progenitor cells into the blood of unmanipulated animals.17,18 In contrast, the function of CD11a/CD18 and CD54 at the progenitor level is unknown. Reports on L-selectin function in stem cells are few and concern human cells, and a role for L-selectin in CD34+ cells is suggested.10,19,20 Antibodies to CD44, which is highly expressed on both HSPC and stromal cells, were shown in vitro to inhibit21,22 or to enhance23,24 hematopoiesis. In addition, a galactose/mannose-specific lectin has been shown to be specific for stem-cell homing to the BM.25However, the functional significance of these molecules in HSPC trafficking is far from being well characterized.
The present study was aimed at determining the involvement of various adhesion molecules shown to be expressed by hematopoietic and stromal cells in the homing and mobilization of mouse stem cells.
MATERIALS AND METHODS
Animals.
BALB/c female mice aged 6 to 8 weeks were purchased from CERJ (Le Genest-Saint-Isle, France) and maintained under specific pathogen-free conditions. Primary and secondary recipients were lethally irradiated with 7.75 Gy (60Co source). The radiation was delivered 24 hours before reconstitution to allow clearance of the majority of the killed cells.
Preparation of cell suspensions.
Single-cell suspensions of spleen were prepared in minimum essential medium (MEM) using a homogenizer. BM cells were flushed from the tibia and femur with medium. Mice were bled retroorbitally. Citrated blood was pooled from 15 mice. In some experiments, erythrocytes were lysed during a 5-minute incubation in ammonium chloride (0.85%), and leukocytes were centrifuged through a 1-cm fetal calf serum (FCS) cushion to remove cell debris. In other experiments blood leukocytes were collected after centrifugation on a Ficoll discontinuous density gradient.
Cell viability was determined by the Trypan blue exclusion test.
Antibodies.
The following antibodies were used: anti–α4 integrin (anti–VLA-4:PS/2), anti–L-selectin (Mel-14), anti–VCAM-1 (M/K-2.7), anti-CD11a (anti–LFA-1:35-89.9), anti-CD44 (IM7), anti-Ly6A/E (anti–Sca-1:E13 161-7), anti-Ly6G (anti-GR1:RA6-8C5), and anti–MAdCAM-1 (MECA-367 and MECA-89). Antibodies were purified on protein G (Pharmacia Fine Chemical, Orsay, France) and injected into mice in this form. For immunofluorescence staining some of these reagents were conjugated to biotin.
Biotinylated anti–α4 integrin (LPAM-1) and phycoerythrin (PE) anti-rat kappa chain (MARK-1) were purchased from Pharmingen (Clinisciences, France). PE-streptavidin was from Caltag (Tebu, Le Perray en Yvelines, France). Purified control rat Ig were obtained from Sigma (L'Isle d'Abeau, France)
Flow cytometry.
Cells from spleen and blood of donors from mobilization experiments were incubated in microtiter plates (106 cells/well) with saturating concentrations of biotinylated antibodies, followed by incubation with PE-streptavidin. Cells were also incubated with PE–MARK-1 to reveal the injected antibody bound to the cell surface.
Progenitor cell assays.
Colony-forming unit–granulocyte-macrophage (CFU-GM) and burst-forming unit–erythroid (BFU-E) were assayed in quadruplicate according to the technique of Worton26 and Iscove and Sieber,27respectively, and slightly modified. CFU-GM cultures were stimulated with 20% standard colony-stimulating factor (CSF) prepared according to Horiuchi et al.28 For cultivation of BFU-E, 2 U/mL recombinant human erythropoietin (a gift from Cilag, Levallois-Perret, France) and 10% WEHI-3B–conditioned medium were used.
After a culture period of 7 days for CFU-GM and 8 days for BFU-E at 37°C, 5% CO2 colonies (>50 cells) were counted.
The number of cells plated was 8.104 cells for BM, 106 for spleen, and 1 to 2 × 106 cells for blood.
Antibody coating of BM cells.
10 × 106 BM cells from normal mice were incubated with saturating concentrations of monoclonal antibody (MoAb; 20 μg in 200 μL medium) for 30 minutes on ice. After one washing cells were counted and tested in colony-forming unit–spleen (CFU-S) and progenitor-cell assays.
Homing of CFU-S into bone marrow and spleen.
To prevent interference with proliferation as well as recirculation effects, short-term experiments are required for homing studies; hence, CFU-S seeding was analyzed 3 hours after BM cell grafting.
To measure the number of day-12 CFU-S that can home into the BM and the spleen during a 3-hour interval, we modified the assay developed by Siminovitch et al,29 which allows one to determine the fraction (f) of the injected CFU-S that lodge in the spleen and there form colonies. A cell suspension was prepared from the BM of 3 to 4 donor mice. Aliquots of cells were incubated on ice for 30 minutes in RPMI 1640 medium with 200 μg/107 cells of purified antibodies directed against leukocyte surface antigens. At the end of the incubation the cell and antibody mixture was injected into four primary recipients (107 cells/mouse). Antibodies specific for endothelial cell markers were injected intraperitoneally (300 μg anti–VCAM-1 or 500 μg anti–MAdCAM-1/mouse) into recipients 2 to 3 hours before transplantation of 107 unlabeled cells in medium. Three hours after BM cell grafting the recipients were killed, BM and spleen cell suspensions were prepared, and the cellularity of both tissues was determined using Trypan blue exclusion. Eight lethally irradiated recipients received intravenously a known number (usually around 106) of BM or spleen cells each. Twelve days later mice were killed and their spleens excised and fixed in Bouin's solution. Macroscopic splenic colonies were counted 24 hours later.30
Mobilization of CFU-S and progenitors.
To test the effect of various antibodies on the mobilization of stem and progenitor cells in the circulation, normal mice received intraperitoneally 300 μg/mouse MoAb, control rat Ig, or phosphate-buffered saline (PBS; 15 mice/experimental group). Peripheral blood was collected 8, 24, and 48 hours after anti–VLA-4 injection and 48 hours after administration of anti-CD44 and anti-CD11a antibodies. After elimination of erythrocytes 5 × 105 nucleated blood cells from anti–VLA-4 treated mice and 106 nucleated blood cells from all other groups were injected to irradiated recipient mice. One million nucleated blood cells were plated in CFU-GM and BFU-E assays. The concentration of leukocytes, determined before erythrocyte removal, was used to calculate the number of CFU-S and progenitor cells per milliliter of blood.
Proliferative potential of mobilized CFU-S.
To examine the ability of mobilized stem cells to reconstitute the hematopoietic tissue, the pre–CFU-S assay described by Spangrude et al was performed. This assay detects very early stage cells in hematopoiesis that have a much higher self-renewal potential than CFU-S.31 Mice injected with 300 μg of anti–VLA-4 antibody, with irrelevant rat IgG2b (RA6-8C5) or PBS were bled 48 hours later, and 106 nucleated blood cells were grafted to primary irradiated recipient mice. Thirteen days later, the recipients were killed, and BM cells from tibias and femurs were collected. Secondary irradiated recipients were injected with an appropriate number of cells (cells present in 1/4 to 2 legs, according to the group) to assess day-12 CFU-S.
Statistical analysis.
For CFU-S assay logarithmic transformation of colony number was used to approximate a gaussian distribution. For each replicate experiment, the antibody-treated group was compared with its control by estimating within-experiment mean and standard error of their ratio (treated/control), taking into account the number of cells injected into recipients and the cellularity of each organ. From replicate experiments, between-experiment mean and standard error of treated to control ratio were estimated. Student's t-test was applied to assess the effect of antibody treatment by using the between-experiment mean and the largest standard error (between or within) with its degree of freedom. Confidence interval of this ratio was calculated by using the inverse transformation of logarithm.
For progenitor assays, Student's t-test was used.
RESULTS
Homing of day-12 CFU-S to BM and spleen in recipients treated with anti-adhesion molecule antibodies.
To analyze the receptors involved in the homing of CFU-S, donor BM cells were allowed to circulate for 3 hours in primary lethally irradiated recipients in the presence of antibodies directed to these receptors. The number of CFU-S that entered the BM and spleen of these animals during this interval was determined in secondary lethally irradiated recipients. Because of the short turnover of some surface receptors, donor BM cells were coated with an antibody and injected into recipients together with 200 μg/mouse of that antibody. Thus, cells remained optimally labeled during the 3-hour interval.
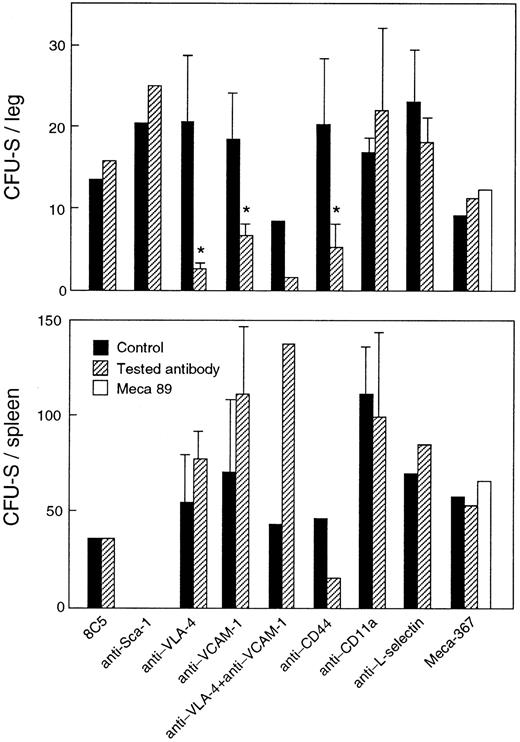
Antibodies to both hematopoietic and stromal cell surface were tested (Fig 1). Among the former, anti–VLA-4 and anti-CD44 antibodies induced 86% and 77% inhibition of CFU-S entry into BM, respectively, as compared to control rat Ig (P < .05). Anti–VCAM-1 antibody, a counter-receptor for VLA-4, also prevented CFU-S homing to BM, but somewhat less efficiently (62% inhibition, P < .05). When recipients were treated with both anti–VCAM-1 and anti–VLA-4 antibodies, the inhibition of CFU-S entry into the BM was similar to that induced by anti–VLA-4 alone. Antibodies to CD11a and L-selectin as well as control antibodies RA6-8C5, which do not recognize stem cells, and anti–Sca-1, which do, had no effect on CFU-S homing.
Homing of CFU-S in BM and spleen. BM and spleen cells of primary irradiated recipients, injected with 107 BM cells in PBS (▪) or mixed with 200 mg the listed antibodies (▨) or Meca-89 (□) 3 hours before killing, were administered to secondary irradiated recipients. The spleen of the secondary recipients were obtained 12 days later for spleen colony counting. The number of CFU-S found in one leg or the spleen of primary recipients was calculated and represents the mean ± SEM of two to four experiments. * P < .05.
Homing of CFU-S in BM and spleen. BM and spleen cells of primary irradiated recipients, injected with 107 BM cells in PBS (▪) or mixed with 200 mg the listed antibodies (▨) or Meca-89 (□) 3 hours before killing, were administered to secondary irradiated recipients. The spleen of the secondary recipients were obtained 12 days later for spleen colony counting. The number of CFU-S found in one leg or the spleen of primary recipients was calculated and represents the mean ± SEM of two to four experiments. * P < .05.
Migration of CFU-S to spleen was inhibited by anti-CD44 only (66% inhibition; P < .05 for each of 2 independent experiments). Coating of donor BM cells and treatment of recipients with antibodies to VLA-4 and VCAM-1 induced, on the contrary, a nonsignificant but reproducible increase in stem-cell trafficking to the spleen. The inhibition induced by anti-CD44 was not the consequence of a cytotoxic effect. Indeed BM cells coated with anti-CD44 generated similar numbers of colonies derived from CFU-S in vivo and CFU-GM and BFU-E in vitro, as described thereafter. In addition, the cell content of the BM, spleen, and blood was not modified in mice treated with 200 μg /mouse of anti-CD44 for the next 48 hours (not shown).
The expression of VCAM-1 by BM sinusoid endothelial cells is constitutive in normal mice.32 In the spleen, whereas normal vascular endothelium does not express VCAM-1, the marginal sinus expresses the vascular addressin MAdCAM-1,33 but its function there remains obscure. The prime ligand for MAdCAM-1 is the α4β7 integrin, whose presence on hematopoietic stem cells has not been analyzed. As members of the Ig superfamily, VCAM-1 and MAdCAM-1 share primary aminoacid sequence homology. To test the possible involvement of MadCAM-1 in stem-cell homing to the spleen, primary recipients were injected with 500 μg of MECA-367 or MECA-89. Both antibodies recognize MAdCAM-1, but only MECA-367 blocks binding of MAdCAM-1 ligands. This treatment did not affect CFU-S homing to the spleen. Not surprisingly, MECA-367 had no effect on CFU-S entry into BM where MAdCAM-1 is not expressed.
Mobilization of day-12 CFU-S and progenitor cells by anti-adhesion molecule antibodies.
To study the role of some adhesive receptors in the maintenance of stem and progenitor cells within the BM, we tested the capacity of antibodies to such receptors to induce the mobilization of these cells in the blood circulation of normal mice.
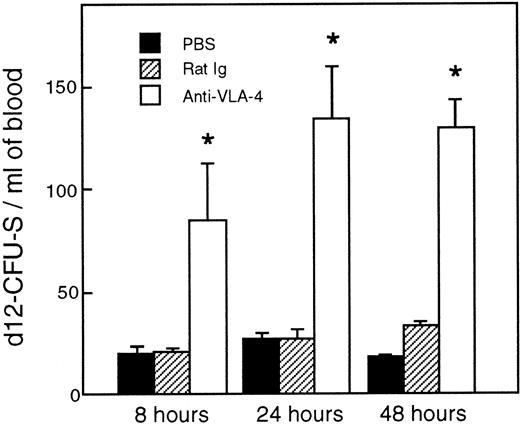
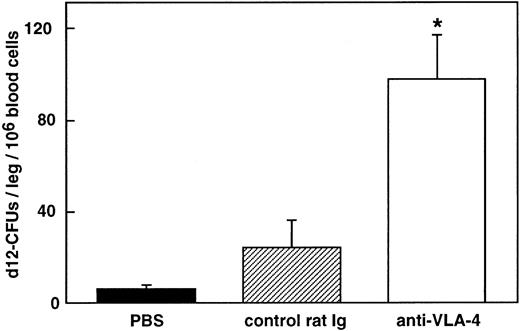
As shown in Fig 2, a single injection of 300 μg/mouse anti–VLA-4 induced an increase in day-12 CFU-S in the blood stream. Relative to control mice, fourfold more day-12 CFU-S were present in the circulation of anti–VLA-4–treated mice as early as 8 hours after antibody administration. The number of day-12 CFU-S kept increasing until 24 hours (P < .02) and remained at this level at least until 48 hours after treatment (P < .002).
Increase in CFU-S in the blood after anti–VLA-4 administration (□). Control mice were injected with PBS (▪) and control rat Ig (▨). Values are mean ± SEM of three experiments. *P < .05; P values compare experimental data with blood from mice injected with rat Ig.
Increase in CFU-S in the blood after anti–VLA-4 administration (□). Control mice were injected with PBS (▪) and control rat Ig (▨). Values are mean ± SEM of three experiments. *P < .05; P values compare experimental data with blood from mice injected with rat Ig.
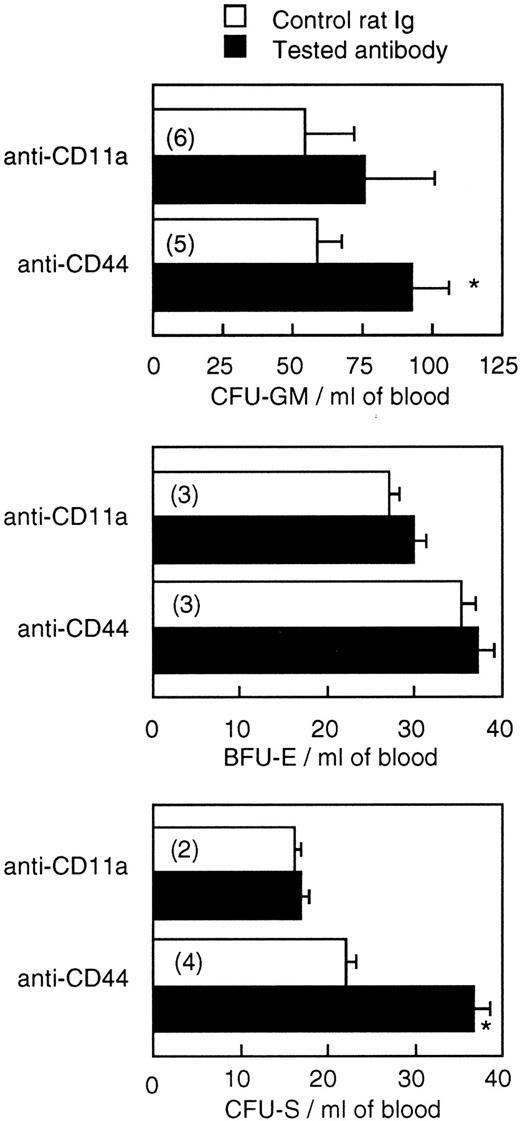
On the basis of these results, the effects of anti-CD44 and anti-CD11a were tested 48 hours after their administration. Figure 3 shows that one injection of 300 μg/mouse of anti-CD44, 2 days before blood harvesting, induced an increase in blood-borne CFU-S content (P < .03) as well as CFU-GM number (P < .01) but had no effect on BFU-E number. Anti-CD11a had an effect on neither CFU-S nor progenitor cell numbers.
Effect of administration of a single dose (300 mg/mouse) of anti-CD11a and anti-CD44 on the number of CFU-GM, BFU-E, and CFU-S in the blood of BALB/c mice. Values are mean ± SEM of two to six experiments (the number of experiments is shown in parentheses). *P < .05.
Effect of administration of a single dose (300 mg/mouse) of anti-CD11a and anti-CD44 on the number of CFU-GM, BFU-E, and CFU-S in the blood of BALB/c mice. Values are mean ± SEM of two to six experiments (the number of experiments is shown in parentheses). *P < .05.
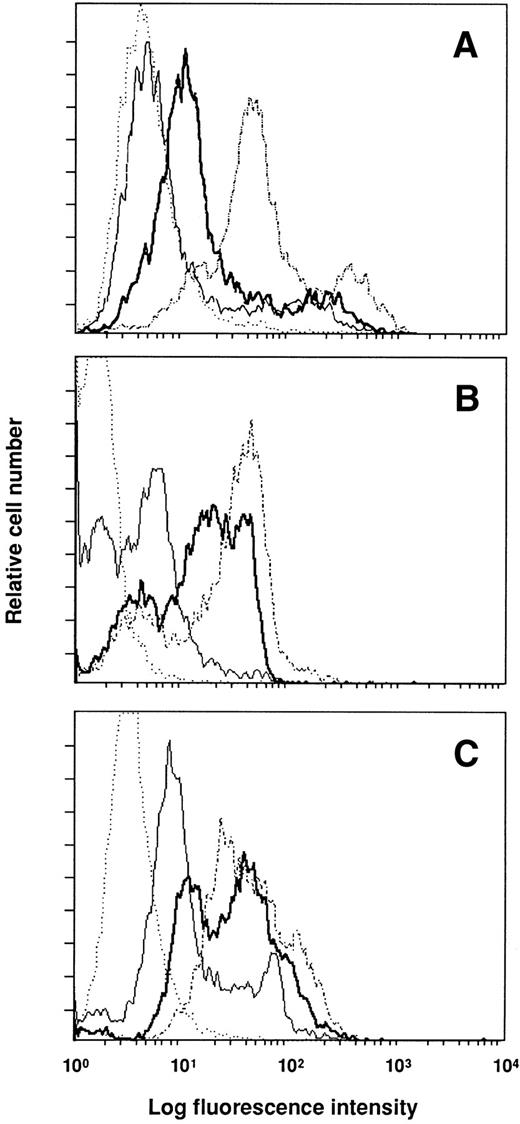
To determine whether the injected antibodies were bound to their targets and their effects on antigen expression, cells of donor mice were analyzed by flow cytometry. Figure 4shows the results for blood mononuclear cells, which were similar to those obtained with spleen cells. Injection of anti–VLA-4 and anti-CD11a into normal mice resulted 2 days later in the labeling of a large proportion of blood leukocytes, whereas anti-CD44 was bound to fewer cells. The comparison of the mean fluorescent channel of the positive peak between mice injected with control rat Ig and test antibody shows that the three antibodies induced downmodulation of expression of the antigen recognized. The decrease in specific antigen expression induced by anti-CD44 was greater (114 for control micev 60 after anti-CD44) than that observed after anti–VLA-4 and anti-CD11a treatment (35 v 20 for anti–VLA-4, and 63 v49 for anti-CD11a).
Expression of surface markers on blood mononuclear cells 48 hours after the administration of antibodies. Mice were injected with 300 mg of anti-CD44 (A), anti–VLA-4 (B), and anti-CD11a (C). Control mice received 300 mg of rat Ig. Cells were stained with the injected antibody and FITC–MARK-1 (control mice, dashed line; treated mice, thick line) to test the global expression of the surface antigen; or with FITC–MARK-1 alone (control mice, dotted line; treated mice, thin line) to detect the injected antibody bound to the cell surface.
Expression of surface markers on blood mononuclear cells 48 hours after the administration of antibodies. Mice were injected with 300 mg of anti-CD44 (A), anti–VLA-4 (B), and anti-CD11a (C). Control mice received 300 mg of rat Ig. Cells were stained with the injected antibody and FITC–MARK-1 (control mice, dashed line; treated mice, thick line) to test the global expression of the surface antigen; or with FITC–MARK-1 alone (control mice, dotted line; treated mice, thin line) to detect the injected antibody bound to the cell surface.
Anti–VLA-4 treatment mobilizes marrow repopulating activity.
To determine whether long-term repopulating cells were included among the cells mobilized by anti–VLA-4, blood cells were tested using the pre–CFU-S assay. The number of pre–CFU-S was significantly increased in the blood of mice treated with one dose of antibody 48 hours before killing (98 ± 19 and 24 ±12 CFU-S/leg of primary recipients/106 blood cells of mice treated with anti–VLA-4 and control rat antibody, respectively; P < .05; Fig 5).
Hematopoietic reconstituting activity of cells mobilized in the blood after anti–VLA-4 administration. Lethally primary irradiated recipients received an injection of 106 blood cells collected in mice injected with anti–VLA-4 (□), control rat Ig (▨), and PBS (▪). Thirteen days later, BM was obtained for CFU-S determination in secondary irradiated recipients. Values represent the mean ± SEM of three experiments. * P < .05.
Hematopoietic reconstituting activity of cells mobilized in the blood after anti–VLA-4 administration. Lethally primary irradiated recipients received an injection of 106 blood cells collected in mice injected with anti–VLA-4 (□), control rat Ig (▨), and PBS (▪). Thirteen days later, BM was obtained for CFU-S determination in secondary irradiated recipients. Values represent the mean ± SEM of three experiments. * P < .05.
Antibody coating does not alter colony formation in vivo and in vitro.
Incubation of BM cells (homing experiments) and injection of donors (mobilization experiments) with antibodies results in the labeling of the cells expressing the recognized antigens, including CFU-S and progenitors. Thus, the number of spleen colonies counted in the secondary recipients could have been underestimated if coating interferes for several days in CFU-S migration to the spleen. Similarly, it was important to test whether coating of progenitors modifies their growth in vitro.
Therefore, we tested the influence of cell coating with antibodies that were found to modify stem-cell homing to and maintenance in the hematopoietic tissue, that is anti–VLA-4 and anti-CD44. Control antibodies used were anti-CD11a and purified rat Ig. Table 1 shows that incubation of BM cells with anti-CD44, anti–VLA-4, and anti-CD11a followed by one washing before intravenous injection into irradiated recipients does not modify the development of spleen colonies by day-12 CFU-S compared with cells incubated with control rat Ig. Likewise, coating of cells did not affect colony formation by CFU-GM and BFU-E.
Effect of BM-Cell Coating With Anti-Adhesion Molecule MoAbs on Colony Growth In Vitro and In Vivo
| BM-Cell Treatment-150 . | CFU-GM . | . | BFU-E . | . | Day-12 CFU-S . | . |
|---|---|---|---|---|---|---|
| Control Ig | 120 ± 31-151 | 9 | 31 ± 4 | |||
| (n = 4)-152 | (n = 2) | (n = 3) | ||||
| Anti-CD44 | 111 ± 27 | 10 | 26 ± 5 | |||
| Control Ig | 126 ± 32 | 30 ± 20 | 31 ± 3 | |||
| (n = 4) | (n = 3) | (n = 4) | ||||
| Anti–VLA-4 | 107 ± 27 | 28 ± 17 | 30 ± 7 | |||
| Control Ig | 126 ± 7 | 23 ± 14 | 27 ± 1 | |||
| (n = 3) | (n = 3) | (n = 3) | ||||
| Anti-CD11a | 90 ± 18 | 21 ± 10 | 21 ± 5 |
| BM-Cell Treatment-150 . | CFU-GM . | . | BFU-E . | . | Day-12 CFU-S . | . |
|---|---|---|---|---|---|---|
| Control Ig | 120 ± 31-151 | 9 | 31 ± 4 | |||
| (n = 4)-152 | (n = 2) | (n = 3) | ||||
| Anti-CD44 | 111 ± 27 | 10 | 26 ± 5 | |||
| Control Ig | 126 ± 32 | 30 ± 20 | 31 ± 3 | |||
| (n = 4) | (n = 3) | (n = 4) | ||||
| Anti–VLA-4 | 107 ± 27 | 28 ± 17 | 30 ± 7 | |||
| Control Ig | 126 ± 7 | 23 ± 14 | 27 ± 1 | |||
| (n = 3) | (n = 3) | (n = 3) | ||||
| Anti-CD11a | 90 ± 18 | 21 ± 10 | 21 ± 5 |
Comparison of the number of colonies generated by BM cells coated with control rat Ig versus tested antibody was statistically nonsignificant in all cases.
107 cells were incubated with 20 μg antibody or rat Ig for 30 minutes on ice and washed.
Mean ± SEM of the number of colonies/105 BM cells.
n = number of experiments.
DISCUSSION
The aim of this study was to examine the role of various adhesion molecules in the trafficking of hematopoietic stem and progenitor cells between the BM, the spleen, and the blood circulation. BM transplantation still must overcome multiple difficulties and should benefit from a better understanding of stem-cell homing to and egress from the BM.
In a first step we showed that homing of day-12 CFU-S to the BM involves the migration pathway VLA-4/VCAM-1 and the molecule CD44, while seeding of the spleen is independent of VLA-4 and VCAM-1 but implies CD44. Anti-CD44 antibody impeded CFU-S homing in the BM as efficiently as anti–VLA-4 antibody. Antibodies against L-selectin, LFA-1, and MAdCAM-1 had no effect on CFU-S homing.
The present results show that homing of CFU-S is mediated by different adhesive pathways, one of which (VLA-4/VCAM-1) is restricted to BM and another of which (CD44) is common to both BM and spleen. Differences in the molecular mechanisms involved in the seeding of early progenitors in BM and spleen have already been reported. Konno et al described a galactosyl- and mannosyl-specific recognition mechanism for CFU-S homing to BM only.34 The VLA-4/VCAM-1 adhesion pathway was shown to distinguish the processes involved in CFU-S and progenitor homing to BM and spleen by Papayannopoulou et al.17 From these works emerged the question of whether the entry of CFU-S in the spleen was passive. That CD44 mediates CFU-S homing to the spleen indicates that it is controlled. In general, the mechanisms mediating lymphohematopoietic cell trafficking to the spleen are still poorly understood, but they are undoubtedly selective.
Homing of HSCP, like that of differentiated lymphohematopoietic cells, is most likely a multistep process. To precisely define the level of action of the antibodies used is difficult. They could hinder the interaction of progenitors with the endothelium, the transmigration, or the association with the stroma. In vivo, VCAM-1 is found on both marrow sinus endothelial cells and reticular cells.32Therefore, VCAM-1 may be involved in the migration to and the retention in the marrow of VLA-4–expressing stem cells and progenitors.
VLA-4 (α4β1 integrin) was first implicated in the in vitro adhesive interactions between hematopoietic progenitor cells and the BM microenvironment where VCAM-1 and fibronectin, its two predominant ligands, are expressed. Inhibition of these interactions by antibodies to VLA-4 or VCAM-1 led to the suppression of B lymphopoiesis in Whitlock and Witte-type cultures, but had little or no effect on myelopoiesis in Dexter-type cultures14,15 or in cocultures of BM cells with hematopoiesis-supporting stromal cells MS-5 (our unpublished data). Conversely, addition of anti-CD44 in long-term BM cultures inhibited both B lymphopoiesis and myelopoiesis.21More recently, mice chimeric for the expression of α4integrins brought evidence that VLA-4 seems not to play an essential role in hematopoietic fetal migration and differentiation pathways but is required in adults for lymphopoiesis only.35 Likewise, VCAM-1 was shown not to be essential for hematopoietic development in mice deficient for VCAM-1.36
Overall it appears that in vitro VLA-4 and VCAM-1 molecules are essentially involved in lymphopoiesis, whereas CD44 is required for both lymphopoiesis and myelopoiesis. Thus, it is interesting to note that homing of stem cells to BM that supports both lymphoid and myeloid cell formation is VLA-4–, VCAM-1–, and CD44-dependent, whereas entry of stem cells into the spleen that essentially supports myelopoiesis is mediated by CD44 only. Furthermore, in the spleen VCAM-1 is not constitutively expressed by endothelial cells and is only faintly represented on scattered reticular and dendritic cells.15Although irradiation might induce VCAM-1 expression on endothelial cells and increase it on reticular cells, as was observed in the BM,32 these contrasts in VCAM-1 expression in normal spleen and BM might explain the different mechanisms brought into play for CFU-S homing.
The inhibition of CFU-S homing to BM was always higher with anti–VLA-4 than with anti–VCAM-1. This may indicate that VCAM-1 is the predominant ligand for VLA-4 but not the only one. Indeed, Williams et al have shown that adhesion of CFU-S to stromal cells involves recognition of fibronectin by VLA-4.7 In the same vein, we observed that the adhesion of CFU-S, CFU-GM, and BFU-E to the stromal cells MS-5 was in all cases inhibited more efficiently with anti–VLA-4 than with anti–VCAM-1 (unpublished data). In addition, that anti–VLA-4 and anti–VCAM-1 antibodies did not inhibit completely CFU-S homing to the BM may indicate that some day-12 CFU-S can lodge in this tissue using other pathway(s) and are responsible for the almost normal hematopoiesis seen in VLA-4– and VCAM-1–deficient mice.35,36 The fact that anti-CD44 and anti–VLA-4 induce inhibition of CFU-S homing to the BM as high as 80% suggests that CFU-S do not follow independently either pathway; when one adhesive pathway is affected, CFU-S do not enter BM via the other pathway. This is reminiscent of the cooperation between VLA-4 and CD44 in establishing adhesion of committed human progenitors.21 37
Opposing effects of anti-CD44 antibodies have been observed in various experimental systems. However, they brought evidence for an important role of CD44 in the regulation of normal hematopoiesis by stromal cells. Ligands of CD44 include several extracellular matrix components (hyaluronate, fibronectin, collagen). Because the antibody used in this study does not prevent binding of CD44 to hyaluronate, the latter is not the ligand for CD44 expressed by murine stem cells, contrary to what was reported for human CFU-GM.38 We found that anti-CD44 in short-term experiments prevented homing of CFU-S to the spleen, whereas the number of spleen colonies generated by BM cells coated with anti-CD44 was not different from that obtained with unlabeled cells. This indicates that the turnover of the CD44 molecule is most likely short, and CFU-S could enter the spleen when surface CD44 molecules were renewed. These results are at variance with the findings of Khaldoyanidi et al39 that coating of BM cells with anti-CD44 followed by a 6-hour incubation at 37°C led to a greater than 50% decrease in the number of spleen colonies. They also showed that reconstitution of irradiated recipients by BM cells was compromised when recipients were treated by anti-CD44 at the time of BM grafting. Because this effect was observed 4 to 12 days after BM cell injection, it is not possible to distinguish between a role of CD44 in the homing or in the proliferation of CFU-S. Similarly, we observed that coating of cells with anti-CD44 did not prevent colony formation in vitro. This finding confirms that of Miyake et al.21
In a second step we tested whether CFU-S mobilization in the blood circulation could be achieved by treatment with antibodies to molecules involved in homing, as reported for CFU-GM and BFU-E after multiple injections of anti–VLA-4 and anti–VCAM-1.17 18 The present results show that as early as 8 hours after a single injection of anti–VLA-4 the number of day-12 CFU-S was already increased in the blood stream and that this mobilization lasted for at least 48 hours. The marrow repopulating ability of the cells mobilized in the circulation was increased, indicating that stem cells with a high self-renewal potential were recruited in the blood. Treatment of normal mice with anti-CD44 mobilized day-12 CFU-S and CFU-GM but not BFU-E. The differences in progenitor responses are not yet understood. Peripheralization of CFU-S and progenitors was much less efficient with anti-CD44 than with anti–VLA-4 antibody. This may indicate that retention of stem cells in the BM via the interactions of CD44 with its ligand(s) is not as essential as other adhesion pathways.
Collectively, we have shown that the same adhesion molecules (VLA-4, CD44) are implied in the homing to and release from BM of stem cells. At least two migration pathways are important for stem cells homing to BM; only one of them is involved in lodging of CFU-S in the spleen. We confirm that the VLA-4/VCAM-1 adhesion pathway is important for stem-cell homing to BM only, and we show that CD44 is involved in CFU-S lodging in both BM and spleen. These results show that entry of CFU-S into the spleen is regulated. It seems likely that the two mechanisms involved in CFU-S homing into BM are linked. Lastly, stem cells mobilized by anti–VLA-4 comprise cells with high self-renewal potential and thus may be used for long-term reconstitution of the hematopoietic tissue.
ACKNOWLEDGMENT
The authors thank Dr E.C. Butcher (Stanford University) for the generous gift of anti-addressin–secreting hybridoma cells, C. Slama for secretarial help, D. Broneer for reading the manuscript, and M. Netter for artwork.
Supported by institutional funds from CNRS and INSERM and by grants from ARC (No. 1804 to F.L. and No. 2016 to F.S.).
Address reprint requests to Françoise Lepault, PhD, CNRS URA 1461, Hôpital Necker, 161 rue de Sèvres, 75730 Paris, Cedex 15, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal