Abstract
We have previously shown that nitric oxide (NO) stimulates apoptosis in different human neoplastic lymphoid cell lines through activation of caspases not only via CD95/CD95L interaction, but also independently of such death receptors. Here we investigated mitochondria-dependent mechanisms of NO-induced apoptosis in Jurkat leukemic cells. NO donor glycerol trinitrate (at the concentration, which induces apoptotic cell death) caused (1) a significant decrease in the concentration of cardiolipin, a major mitochondrial lipid; (2) a downregulation in respiratory chain complex activities; (3) a release of the mitochondrial protein cytochrome c into the cytosol; and (4) an activation of caspase-9 and caspase-3. These changes were accompanied by an increase in the number of cells with low mitochondrial transmembrane potential and with a high level of reactive oxygen species production. Higher resistance of the CD95-resistant Jurkat subclone (APO-R) cells to NO-mediated apoptosis correlated with the absence of cytochrome c release and with less alterations in other mitochondrial parameters. An inhibitor of lipid peroxidation, trolox, significantly suppressed NO-mediated apoptosis in APO-S Jurkat cells, whereas bongkrekic acid (BA), which blocks mitochondrial permeability transition, provided only a moderate antiapoptotic effect. Transfection of Jurkat cells with bcl-2 led to a complete block of apoptosis due to the prevention of changes in mitochondrial functions. We suggest that the mitochondrial damage (in particular, cardiolipin degradation and cytochrome c release) induced by NO in human leukemia cells plays a crucial role in the subsequent activation of caspase and apoptosis.
THE SMALL MOLECULE nitric oxide (NO) is generated from a guanido nitrogen of L-arginine by at least three distinct isoforms of NO synthase (NOS) encoded by three distinct genes.1 A low level of NO synthesized by constitutive NOS for short periods of time acts as a neurotransmitter and as a regulator of blood pressure and platelet aggregation.2 In contrast, a high level of NO, produced for long periods of time by inducible NOS after lipopolysaccharide (LPS) and cytokine challenge, is cytotoxic for pathogenes and tumor cells.3 It was shown that NO-mediated cytotoxicity involved the inhibition of mitochondrial respiration and DNA synthesis in tumor targets.4,5Moreover, this cytotoxic effect was recently found to be associated with apoptosis (programmed cell death) in normal6,7 and tumor cells.8-11
Apoptosis results from the action of a genetically encoded suicide program with characteristic biochemical and ultrastructural changes, which can be induced by different stimuli such as tumor necrosis factor (TNF), CD95 (FAS/APO-1) ligand, TNF-related apoptosis-inducing ligand (TRAIL) (APO-2 ligand), shortage of growth factors, oxygen, or certain metabolites.12 It is currently assumed that the activation of a cascade of cytoplasmic cystein proteases (caspases) is essential for apoptosis regardless of the initial death signal.13 Recently, it has been found that alterations in mitochondrial functions play a key role in the effector phase of apoptosis induced by different agents.14-16 These alterations include the disruption of mitochondrial transmembrane potential (Δψm), the generation of reactive oxygen species (ROS) and the opening of permeability transition (PT) pores.14,17 In addition, the release of the 15-kD mitochondrial protein cytochrome c, identified as apoptotic protease activating factor 2 (Apaf 2), induces the formation of the complex between Apaf 1 and caspase-9 (Apaf 3). The latter becomes activated under such conditions and in turn activates caspase-3, which leads to DNA fragmentation and apoptosis.18-20 Cytochromec is normally present on the outer surface of the inner mitochondrial membrane and shuttles electrons between complexes III and IV of the respiratory chain.18
Bcl-2 belongs to a growing family of proteins that can either block (Bcl-2, Bcl-xL, etc) or promote (Bax, Bad, Bak, etc) apoptosis. Bcl-2–related proteins are integrated in the outer mitochondrial, outer nuclear, and endoplasmic reticular membranes with the help of a carboxy-terminal membrane anchor.21,22 It was demonstrated that Bcl-2 overexpression specifically prevents cells from initiating apoptosis in response to a number of stimuli, including NO,23-25 and that the antiapoptotic effect of Bcl-2 involved the normalization of mitochondrial functions. Thus, Bcl-2 blocked mitochondrial PT and prevented the release of caspase activators (in particular, cytochrome c) from mitochondria and the disruption of Δψm induced by glucorticoids, DNA damage, oxidants, or ceramide.21,23,26 Moreover, according to recent data, high levels of Bcl-2 can delay cell death, even when cytochrome c is already released into the cytosole.27
We have previously shown that NO triggers apoptosis in different human neoplastic lymphoid cell lines and freshly isolated leukemic lymphocytes through activation of caspases, including caspase-8, the most CD95 receptor-proximal caspase.11 Furthermore, NO was able to induce activation of caspase-8 not only via CD95/CD95L interaction, but also independently of death receptors via direct caspase activation. Here we studied mitochondria-dependent mechanisms of NO-mediated apoptosis in human leukemia cells. Our results suggest that NO (at the concentration which induces apoptotic death in Jurkat cells) causes catabolism of the major mitochondrial phospholipid, cardiolipin, and the release of cytochromec into the cytosole, downregulates the activity of oxidative phosphorylation complex I, III, and IV, induces significant changes in mitochondrial transmembrane potential, and stimulates ROS production. All of these biochemical alterations were blocked in bcl-2–transfected Jurkat T cells, which are completely resistant to the apoptotic effect of NO.
MATERIALS AND METHODS
Cell lines.
Human leukemic T-cell lines (Jurkat APO-S and APO-R) were maintained in 5% CO2 at 37°C in RPMI 1640 medium (GIBCO-BRL, Eggenstein, Germany) containing 5% fetal calf serum. CD95-resistant (APO-R) Jurkat cells were derived from the parental CD95-sensitive (APO-S) clone by long-term culture in the presence of a lethal dose of activating CD95 antibody.28 Northern and Western blot analyses showed that APO-R Jurkat cells expressed CD95 mRNA, but failed to express CD95 protein. Both cell clones (APO-S and APO-R) were equally sensitive to CD95-independent apoptosis and expressed the same cell surface markers.28 Jurkat cells transfected with empty vector or containing bcl-2 were kindly provided by Dr M.E. Peter (German Cancer Research Center, Heidelberg, Germany) and cultured as described elsewhere.29 To evaluate apoptotic effects of NO, all above-mentioned cells were treated with glycerol trinitrate (GTN; Merck, Darmstadt, Germany), which is known to constitutively produce NO in the incubation medium.
Antibodies and other reagents.
The monoclonal antibody (MoAb) against cytochrome c (clone 7H8.2C12) was purchased from PharMingen (Hamburg, Germany). The horseradish peroxidase-conjugated goat antimouse MoAbs were purchased from Dianova (Hamburg, Germany). The following reagents were from Molecular Probes, Inc (Eugene, OR): 10-N-nonyl-3,6-bis(dimethylamino)acridine (NAO), 3,3′-dihexyloxacarbocyanine iodide [DiOC6(3)], dihydroethidine (HE), and propidium iodide (PI). Bongkrekic acid (BA) was kindly provided by Dr J.A. Duine (Delft University, Delft, The Netherlands). Water-soluble analogue of vitamine E, 6-hydroxyl-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox) and a fluorogenic substrate for caspase 9 Ac-Leu-Glu-His-Asp-amido-trifluoromethyl-coumarin (LEHD-AFC) was from Calbiochem (Bad Soden, Germany). A fluorogenic substrate for caspase 3 Asp-Glu-Val-Asp-aminomethyl-coumarin (DEVD-AMC) was purchased from Bachem (Heidelberg, Germany). All other chemicals used were of analytical grade and purchased from Sigma (Munich, Germany).
Apoptosis and cytotoxicity assay.
Apoptosis was assessed by determining DNA fragmentation (DNA-release assay) after lysing the cells in a hypotonic solution (0.1% sodium citrate, 0.1% Triton X-100) containing 50 μg/mL PI as described30 and analyzed by flow cytometry using a FACScan analyzer with CELLQuest software (Becton Dickinson, Heidelberg, Germany). Cytotoxicity was evaluated after treatment of cells with 1 μg/mL PI for 5 minutes before fluorescence-activated cell sorting (FACS) analysis. A total of 10,000 cells per sample was analyzed by FACScan and CELLQuest software (Becton Dickinson).
Cytofluorometric analysis of mitochondrial transmembrane potential, reactive oxygen species, and cardiolipin.
To measure Δψm and ROS production, cells (7 × 105/mL) were incubated with DiOC6(3) (40 nmol/L in phosphate-buffered saline [PBS]) and 5 μmol/L HE, respectively, at 37°C for 30 minutes.17 For complete depletion of Δψm (positive control), a mitochondrial uncoupler carbonyl cyanide m-chlorophenyl-hydrazone (CCCP, 50 μmol/L) was used.17 The content of a main mitochondrial lipid cardiolipin was analyzed after incubation with specific dye NAO (100 nmol/L) for 30 minutes at 37°C.31 A total of 1 μg/mL PI was added to the samples for 5 minutes before FACS analysis and measured at red fluorescence (FL3). Recordings were made only on PI negative (viable) cells at green fluorescence (FL1) for DiOC6(3) and NAO and at red fluorescence (FL2) for HE. Typically, 10,000 cells per sample were measured using FACScan and CELLQuest software (Becton Dickinson).
Measurement of the oxidative phosphorylation (OXPHOS) activity.
The activity of OXPHOS complexes was measured according to Hofhaus et al32 with small modifications. Briefly, upon treatment with GTN for 8 hours, 3 × 107 Jurkat cells were permeabilized for 1 minute with 7.5 μg/mL digitonin in medium A (20 mmol/L HEPES, 250 mmol/L sucrose, 10 mmol/L MgCl2, pH 7.1), washed twice in medium A and kept on ice. Oxygen consumption by permeabilized cells resuspended in 0.75 mL of respiratory medium (medium A supplemented with 2 mmol/L adenosine diphosphate (ADP) and 2 mmol/L KH2PO4) was measured at 37°C using a Clark-type oxygen electrode fitted to 2 mL water-jacketed closed chamber. The activity of the OXPHOS complexes I-III was recorded after incubation with 5 mmol/L glutamate and 5 mmol/L malate. To evaluate complexes II-III activity, complex I was inhibited by 0.1 μmol/L rotenone, and respiration was initiated by 5 mmol/L glycerol-3-phosphate and 5 mmol/L succinate. After inhibition of complex III with 100 nmol/L antimycin, 10 mmol/L ascorbate and 0.2 mmol/L N,N,N’,N’-tetramethyl-p-phenylenediamine were added to drive electrons directly to cytochrome c (complex IV). The activity of complex IV was then blocked with 0.1 mmol/L potassium cyanide (KCN) to evaluate cyanide resistant oxygen consumption. Data were expressed in fmol of O2 per minute per cell.
Determination of cytochrome c release.
The release of cytochrome c into the cytosol of Jurkat cells treated with GTN for 8 hours was measured as described elsewhere.33 Briefly, 6 × 107 cells were washed in PBS and resuspended in 4 vol of ice-cold buffer containing 20 mmol/L HEPES, 250 mmol/L sucrose, 2 mmol/L EDTA, 20 μg/mL phenylmethylsulfonyl fluoride, 2 μg/mL leupeptin, and 10 μg/mL aprotinin, pH 7.1. Cells were disrupted on ice with 15 strokes of Dounce homogenizer and centrifuged for 3 minutes at 3,000g to remove nuclei and unbroken cells. The supernatants were then centrifuged for 1 minute at 12,000g to isolate mitochondrial fraction. Resulting supernatants were centrifuged for 1 hour at 100,000g to sediment cell membranes. For Western blot detection of cytochrome c, the supernatants from the last centrifugation (fraction S100) and mitochondrial fractions were subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were then tranferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA) using a semidry blotting apparatus (Bio-Rad, Munich, Germany). The membrane was blocked with a 5% solution of nonfat dry milk in TBST buffer (25 mmol/L Tris-HCl, 137 mmol/L NaCl, 5 mmol/L KCl, 0.7 mmol/L CaCl2, 0.1 mmol/L MgCl2, 0.05% (vol/vol) Tween 20, pH 7.4) and incubated overnight at 4°C with MoAb against cytochrome c. Blots were extensively washed with TBST buffer and developed with goat antimouse MoAb (dilution 1:2,500). After washing the membrane with TBST buffer, the immunoreactive bands were visualized by enhanced chemiluminescece (ECL) method following the manufacturer’s protocol (Amersham, Braunscweig, Germany).
Caspase activity assay.
A total of 106 APO-S Jurkat cells (treated with GTN or control) was washed with PBS and lysed in buffer A (100 mmol/L HEPES, 10% sucrose, 10 μmol/L leupeptin, 10 μmol/L aprotinin, 1 mmol/L EDTA, 5 mmol/L dithiothreitol, and 0.15% 3-cholamidopropyl-dimethylammonio-1-propansulfonate, pH 7.4). To determine caspase-3 activity, 10 μL of cell lysates were incubated with 20 μL (75 μmol/L, final concentration) of fluorogeneic peptide DEVD-AMC (Bachem, Heidelberg, Germany) as described.34 The product of reaction fluorescent AMC was measured after 1 hour of incubation at excitation 355 nm, emission 485 nm using a plate reader (Victor 1420, Wallac, Freiburg, Germany). For caspase-9, a fluorogenic substrate LEHD-AFC (75 μmol/L, final concentration) was used and the product of reaction AFC was measured with the same plate reader at excitation 405 nm and emission 535 nm.35
RESULTS
NO inhibits the activity of OXPHOS complexes and induces cytochrome c release.
We have previously shown that treatment of Jurkat cells with increasing concentrations of the NO donor, GTN, caused a cytotoxic effect that was due to apoptosis.11 The level of NO-induced apoptosis was much higher in APO-S Jurkat cells that express CD95 on the cell surface and are sensitive to CD95-mediated kill than in APO-R cells, which lack CD95 expression and are resistant to anti-CD95 MoAb. To study mitochondrial involvement, we measured the activity of complexes of the mitochondrial electron transport chain (OXPHOS complexes) and the presence or absence of the mitochondrial protein cytochrome c in the cytosol of both APO-S and APO-R cells treated with NO for 8 hours. We showed previously that incubation with the NO donor, GTN, for 8 hours and its withdrawal for the next 16 hours was sufficient to achieve a maximal level of apoptosis 24 hours after GTN treatment.11 Incubation with 0.2 mmol/L GTN for 24 hours caused 33% apoptosis in APO-S and 10% in APO-R cells. After 48 hours, the level of NO-induced apoptosis reached 52% in APO-S and 20% in APO-R cells.
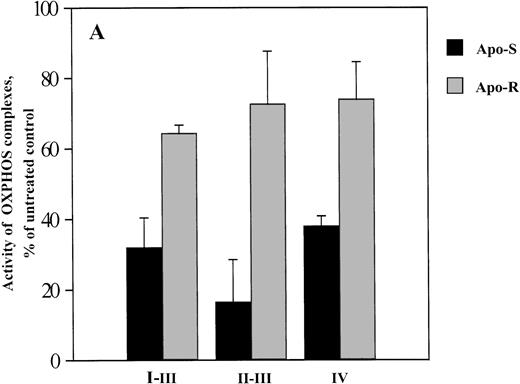
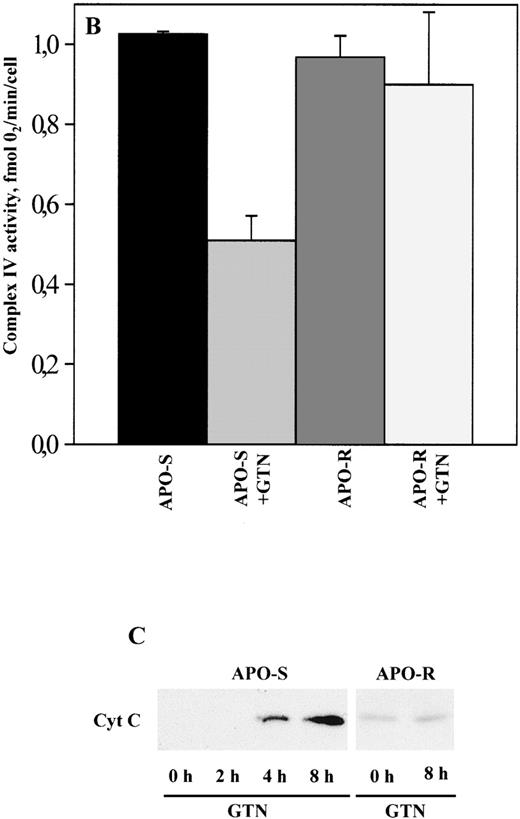
As shown in Fig 1A and B, the activity of OXPHOS complexes in APO-S Jurkat cells treated for 8 hours with 0.2 mmol/L GTN was significantly reduced (down to 17% of the level in untreated cells for complex III), whereas in APO-R cells, only a comparatively small decrease in mitochondrial respiratory function was seen. The substantial decrease in complex IV activity in APO-S cells (Fig 1B) was found to correlate with the detection of cytochromec in the cytosol fraction (S100) after 4 and 8 hours of incubation with GTN (Fig 1C) suggesting that the release of cytochromec is a possible reason for the suppression of complex IV activity. In contrast, NO treatment did not induce any migration of cytochrome c into the cytosol of APO-R cells (Fig 1C).
Effect of NO on the activity of OXPHOS complexes (A and B) and cytochrome c release (C) in APO-S and APO-R Jurkat cells. After treatment with the NO donor GTN (0.2 mmol/L) for 8 hours, 3 × 107 cells were permeabilized with digitonin. Oxygen consumption was measured at 37°C using a Clark type oxygen electrode as described in Materials and Methods. To evaluate cytochromec release, 6 × 107 cells were homogenized on ice and Western blot analysis with anticytochrome c MoAb was performed. (A) Downregulation of OXPHOS complex activity under NO treatment. O2 consumption is expressed as percentage of untreated control. (B) NO-mediated inhibition of complex IV activity. Data are expressed in fmol of consumed O2 per minute per cell. Each bar represents the mean ± standard deviation (SD) of three independent experiments. (C) Time-dependent release of cytochromec into the cytosol in APO-S, but not in APO-R cells. A representative experiment of three is shown.
Effect of NO on the activity of OXPHOS complexes (A and B) and cytochrome c release (C) in APO-S and APO-R Jurkat cells. After treatment with the NO donor GTN (0.2 mmol/L) for 8 hours, 3 × 107 cells were permeabilized with digitonin. Oxygen consumption was measured at 37°C using a Clark type oxygen electrode as described in Materials and Methods. To evaluate cytochromec release, 6 × 107 cells were homogenized on ice and Western blot analysis with anticytochrome c MoAb was performed. (A) Downregulation of OXPHOS complex activity under NO treatment. O2 consumption is expressed as percentage of untreated control. (B) NO-mediated inhibition of complex IV activity. Data are expressed in fmol of consumed O2 per minute per cell. Each bar represents the mean ± standard deviation (SD) of three independent experiments. (C) Time-dependent release of cytochromec into the cytosol in APO-S, but not in APO-R cells. A representative experiment of three is shown.
NO induces caspase activation.
Because the release of cytochrome c can initiate an apoptotic protease cascade,18-20 we tested NO-induced changes in the activity of caspase-9 and caspase-3, which are the members of this cascade that are the most proximal to cytochromec.19 20 It was found that NO treatment for 2 and 4 hours resulted in a very low level of activation of caspase-9 (Fig 2). However, after 8 hours, the activity of this enzyme was 4.7 times higher than in untreated cells (P < .001). A similar level of caspase-9 activation was observed after 24 hours. Caspase-3 activity was also significantly upregulated only 8 hours after addition of GTN and remained activated at 24 hours (Fig 2).
Activation of caspase-9 (⧫) and caspase-3 (▪) by NO in APO-S Jurkat cells. Cells were treated for different periods of time with 0.2 mmol/L GTN. Cell lysates were then prepared and incubated with fluorogeneic peptides: LEHD-AFC (for caspase-9) and DEVD-AMC (for caspase-3). The products of reaction AFC or AMC were measured after 1 hour of incubation using a plate reader. Each point represents the mean ± SD of three independent experiments.
Activation of caspase-9 (⧫) and caspase-3 (▪) by NO in APO-S Jurkat cells. Cells were treated for different periods of time with 0.2 mmol/L GTN. Cell lysates were then prepared and incubated with fluorogeneic peptides: LEHD-AFC (for caspase-9) and DEVD-AMC (for caspase-3). The products of reaction AFC or AMC were measured after 1 hour of incubation using a plate reader. Each point represents the mean ± SD of three independent experiments.
Therefore, NO-induced caspase-9 and caspase-3 activation in APO-S Jurkat cells, observed at 8 hours, follows detectable release of cytochrome c into the cytosole.
NO stimulates an increase in the number of Jurkat cells with low cardiolipin content, high ROS production, and low Δψm.
Next, we measured the content of the main mitochondrial lipid, cardiolipin (NAO staining), Δψm (DiOC6(3) staining) and ROS production (HE staining) in both APO-S and APO-R cells treated with NO. All of these mitochondrial parameters were tested in PI-negative live cells.
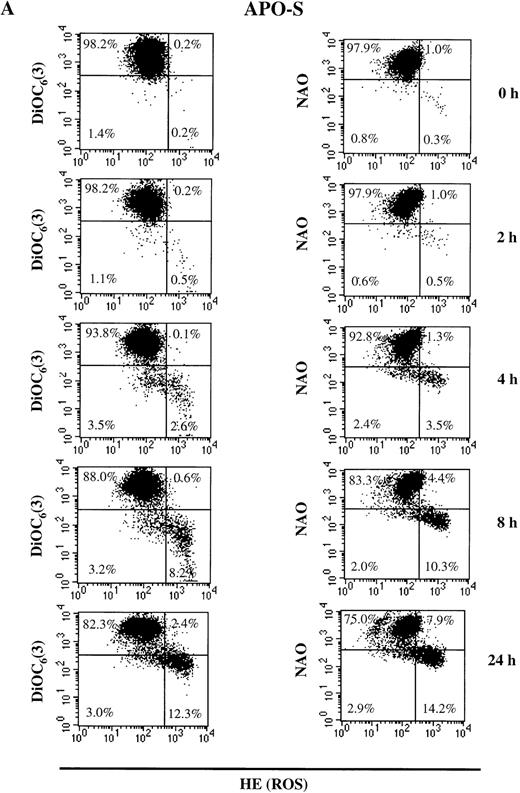
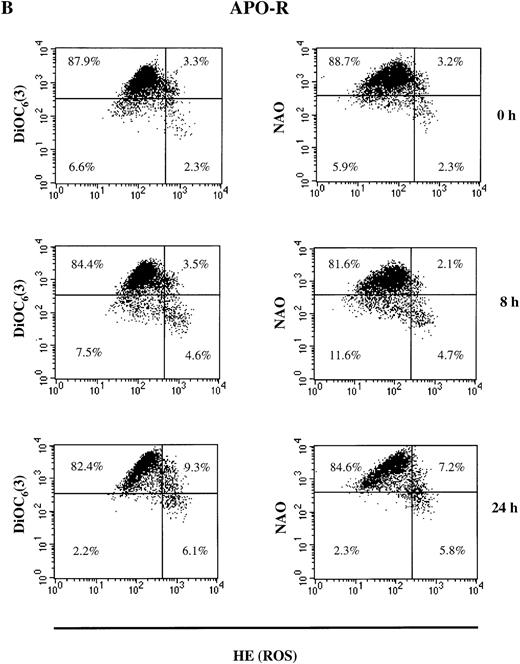
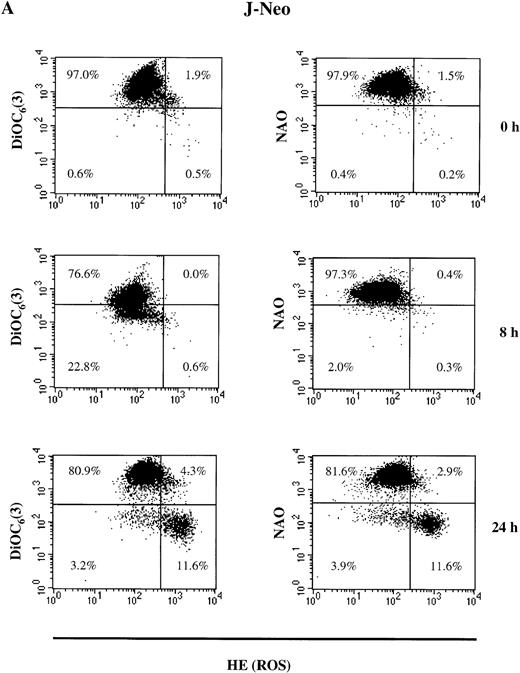
As shown in Fig 3A, the population of untreated live (PI-negative) APO-S cells was homogeneous. After incubation with the NO donor, GTN, live APO-S cells can be divided into two subpopulations: one with low Δψm(DiOC6(3)low) and high ROS production (HEhigh) and another with high Δψm(DiOC6(3)high) and low ROS production (HElow) (12.3% and 82.3%, respectively at 24 hours). After staining with NAO and HE, the live cells also formed two subpopulations under NO treatment: NAOlowHEhigh and NAOhigh HElow (14.2% and 75.0%, respectively at 24 hours). These two subpopulations were observed starting from 4 hours of GTN treatment. Interestingly, the kinetics of NAOlow HEhigh and DiOC6(3)low HEhigh cells were very similar at the time points tested. This suggests that in APO-S cells, NO stimulated the formation of a subpopulation with high ROS production, as well as decreased cardiolipin content and Δψm. When APO-R cells were stained, we found that the proportion of NAOlow HEhigh and DiOC6(3)low HEhigh cells was much lower than in APO-S cells (5.8% and 6.1%, respectively, at 24 hours; Fig 3B). In contrast to APO-S cells, we observed in APO-R cells an additional subpopulation with increased ROS production together with high levels of cardiolipin content and Δψm (7% to 9% after 24 hours).
NO-mediated changes in ▵ψm, ROS production, and cardiolipin content in APO-S (A) and APO-R (B) Jurkat cells. To measure ▵ψm and ROS production, cells were incubated with DiOC6(3) and HE, respectively, at 37°C for 30 minutes. The content of the main mitochondrial lipid, cardiolipin, was analyzed after incubation with NAO for 30 minutes at 37°C. Recordings were made only on PI negative (viable) cells at green fluorescence (FL1) for DiOC6(3) and NAO and at red fluorescence (FL2) for HE using FACScan and CELLQuest software. A representative experiment of four is shown.
NO-mediated changes in ▵ψm, ROS production, and cardiolipin content in APO-S (A) and APO-R (B) Jurkat cells. To measure ▵ψm and ROS production, cells were incubated with DiOC6(3) and HE, respectively, at 37°C for 30 minutes. The content of the main mitochondrial lipid, cardiolipin, was analyzed after incubation with NAO for 30 minutes at 37°C. Recordings were made only on PI negative (viable) cells at green fluorescence (FL1) for DiOC6(3) and NAO and at red fluorescence (FL2) for HE using FACScan and CELLQuest software. A representative experiment of four is shown.
APO-S Jurkat cells thus showed larger alterations in mitochondrial functions than did APO-R cells. This correlated with higher sensitivity of the APO-S cells to NO. There was a time-dependent increase in the APO-S cell subpopulation with low cardiolipin content, low Δψm and high ROS production.
Jurkat cells transfected with bcl-2 are completely resistant to apoptosis and show normal mitochondrial functions after NO treatment.
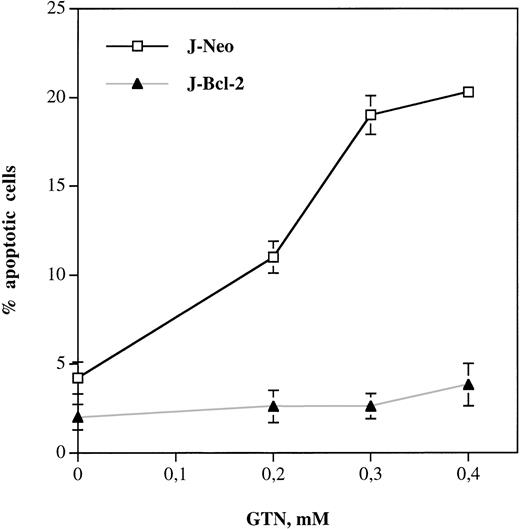
A growing number of studies suggested that overexpression of Bcl-2 could block apoptosis via mitochondrial effects.33,36,37Therefore, we used bcl-2–transfected Jurkat cells (J-Bcl-2)29 to investigate the effect of this protein on NO-mediated apoptosis. Figure 4 shows that Bcl-2 hyperexpression completely inhibited apoptosis in Jurkat cells on treatment with GTN even when excess concentrations were used (up to 0.4 mmol/L). Control Jurkat cells stably transfected only with the neomycin vector (J-Neo) showed a GTN concentration-dependent increase in the percentage of apoptotic cells.
Bcl-2 overexpression completely blocks NO-induced apoptosis in Jurkat leukemic cells. Cells transfected with neomycin vector only (J-Neo) or with bcl-2 expressing vector (J-Bcl-2) were incubated with NO donor GTN at different concentrations for 24 hours. Percentage of apoptotic cells was measured by the DNA-release assay using treatment with a hypotonic PI solution at 4°C overnight in the dark followed by flow cytometric analysis. Each bar represents the mean ± SD of three independent experiments.
Bcl-2 overexpression completely blocks NO-induced apoptosis in Jurkat leukemic cells. Cells transfected with neomycin vector only (J-Neo) or with bcl-2 expressing vector (J-Bcl-2) were incubated with NO donor GTN at different concentrations for 24 hours. Percentage of apoptotic cells was measured by the DNA-release assay using treatment with a hypotonic PI solution at 4°C overnight in the dark followed by flow cytometric analysis. Each bar represents the mean ± SD of three independent experiments.
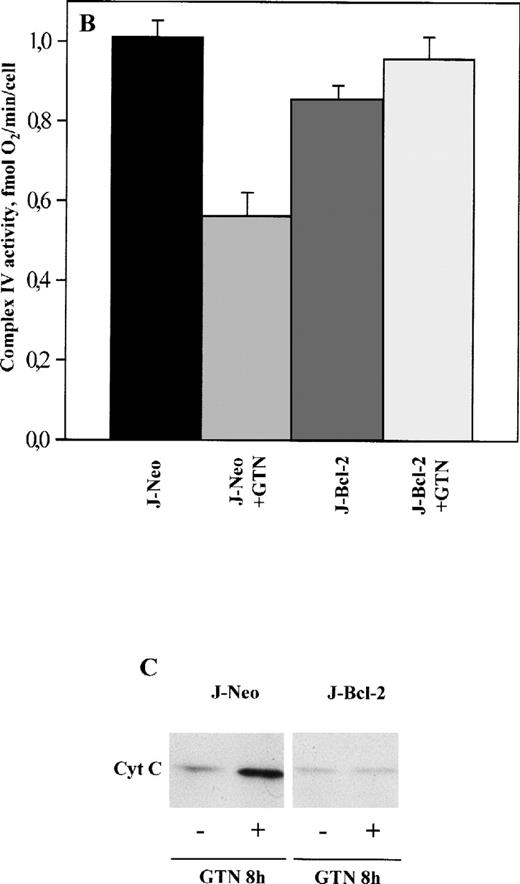
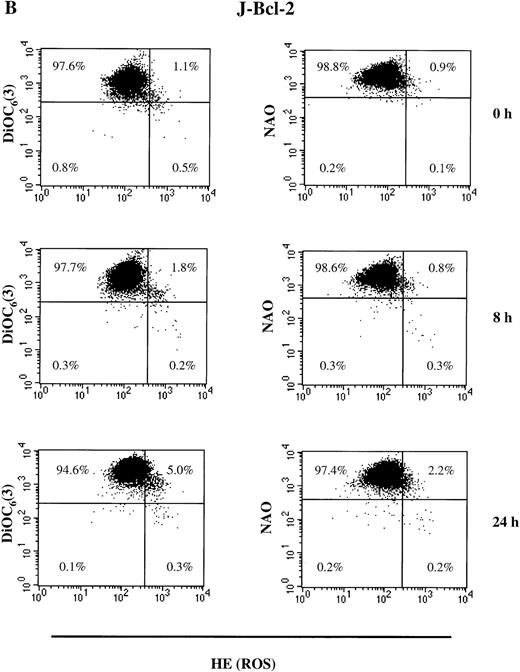
Next, we tested mitochondrial functions in J-Bcl-2 cells subjected to treatment with 0.3 mmol/L GTN. J-Neo cells (control) were found to be NO susceptible and similar to APO-S cells with regard to suppression in the activity of complexes I, II, and IV, as well as cytochromec release into the cytosol fraction (Fig 5A through C). In contrast, J-Bcl-2 cells were completely resistant to the above-mentioned mitochondrial changes. Interestingly, in the latter cells, NO treatment even stimulated to some extent the activity of mitochondrial respiratory chain complexes (up to 145% of the level in untreated cells in the case of complex I). Bcl-2 was also able to normalize the content of cardiolipin, Δψm and ROS production. As shown in Fig 6A and B, a time-dependent upregulation in the number of cells with low cardiolipin content, low Δψm, and high ROS production was found in J-Neo cells (Fig 6A), but not in J-Bcl-2 cells (Fig 6B). Live (PI-negative) control J-Neo cells had 11.6% of cells with the phenotype NAOlowHEhigh and DiOC6(3)lowHEhigh at 24 hours (Fig 6A), while less than 0.3% of J-Bcl-2 cells had this phenotype (Fig 6B). Bcl-2 overexpressing cells remained mostly homogenous after exposure to NO showing only a slight increase in the HEhigh cell subpopulation without any decrease in Δψm and cardiolipin concentration (Fig 6B).
Effect of NO on the activity of OXPHOS complexes (A and B) and cytochrome c release (C) in Jurkat cells transfected only with neomycin (J-Neo) and in Bcl-2 overexpessing Jurkat cells (J-Bcl-2). Evaluation of OXPHOS complex acitivity and cytochromec release was performed as described in the legend to Fig 1. (A) Decrease of OXPHOS complex activity under NO treatment. Data were expressed as a percentage to the untreated controls. (B) NO-mediated suppression of complex IV activity. Data were expressed in fmol of O2 per minute per cell. Each bar represents the mean ± SD of three independent experiments. (C) Release of cytochrome cinto the cytosole in Neo, but not in Bcl-2 overexpressing cells. A representative experiment of three is shown.
Effect of NO on the activity of OXPHOS complexes (A and B) and cytochrome c release (C) in Jurkat cells transfected only with neomycin (J-Neo) and in Bcl-2 overexpessing Jurkat cells (J-Bcl-2). Evaluation of OXPHOS complex acitivity and cytochromec release was performed as described in the legend to Fig 1. (A) Decrease of OXPHOS complex activity under NO treatment. Data were expressed as a percentage to the untreated controls. (B) NO-mediated suppression of complex IV activity. Data were expressed in fmol of O2 per minute per cell. Each bar represents the mean ± SD of three independent experiments. (C) Release of cytochrome cinto the cytosole in Neo, but not in Bcl-2 overexpressing cells. A representative experiment of three is shown.
NO-induced alterations in ▵ψm, ROS production, and cardiolipin content in Jurkat cells transfected only with neomycin (J-Neo) (A) and in Bcl-2 overexpessing Jurkat cells (J-Bcl-2) (B). To measure ▵ψm, ROS production, and cardiolipin content, cells were incubated with DiOC6(3), HE, and NAO, respectively at 37°C for 30 minutes. Recordings were made only on PI negative (viable) cells at green fluorescence (FL1) for DiOC6(3) and NAO and at red fluorescence (FL2) for HE. A representative experiment of four is shown.
NO-induced alterations in ▵ψm, ROS production, and cardiolipin content in Jurkat cells transfected only with neomycin (J-Neo) (A) and in Bcl-2 overexpessing Jurkat cells (J-Bcl-2) (B). To measure ▵ψm, ROS production, and cardiolipin content, cells were incubated with DiOC6(3), HE, and NAO, respectively at 37°C for 30 minutes. Recordings were made only on PI negative (viable) cells at green fluorescence (FL1) for DiOC6(3) and NAO and at red fluorescence (FL2) for HE. A representative experiment of four is shown.
Thus, Bcl-2 overexpression prevents the damage of mitochondrial functions induced by NO in human leukemia cells. This correlates with the acquisition of complete resistance to NO-mediated apoptosis.
Trolox and BA partially inhibit NO-induced apoptosis in APO-S cells.
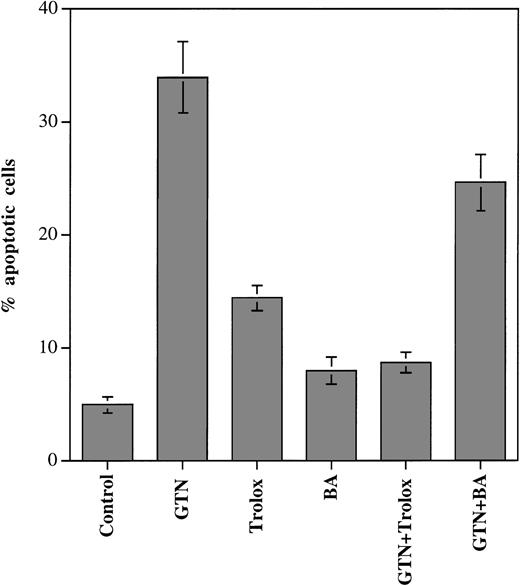
Although it seems established that the antiapoptotic effects of Bcl-2 are exerted at the mitochondrial level, the exact mechanism(s) of this effect are still unclear. Bcl-2 can block lipid peroxidation23,38 and the opening of mitochondrial PT pores26 that occurs during apoptotic cell death induced by different stimuli. To test the contribution of these Bcl-2–mediated protective mechanisms under treatment with NO at apoptosis-inducing concentrations, we used the lipid- and water-soluble vitamin E analogue trolox, a potent inhibitor of lipid peroxidation,39 and BA, which suppresses mitochondrial PT.40 We found that the addition of trolox to the incubation medium substantially downregulated the number of apoptotic APO-S cells induced by NO (from 34% to 9% dead cells; P < .001; Fig 7). Interestingly, incubation with trolox alone resulted in some increase in apoptotic cell death (to 14%). BA could reduce the apoptotic effects of GTN (from 34% to 25%; P < .05), although this reduction was much lower than that observed with trolox. The fact that the inhibitor of lipid peroxidation, trolox, can strongly downregulate NO-induced apoptosis suggests an important role of lipid peroxidation in this process in Jurkat cells. The protective effect of Bcl-2 may involve the suppression of lipid degradation in NO-treated Jurkat cells.
Influence of trolox and BA on NO-mediated apoptosis in APO-S Jurkat cells. Cells were incubated with NO donor GTN (0.2 mmol/L), trolox (5 mmol/L), and with BA (60 μmol/L) for 24 hours. Percentage of apoptotic cells was measured by the DNA-release assay using treatment with a hypotonic PI solution at 4°C overnight in the dark followed by flow cytometric analysis. Each point represents the mean ± SD of three independent experiments.
Influence of trolox and BA on NO-mediated apoptosis in APO-S Jurkat cells. Cells were incubated with NO donor GTN (0.2 mmol/L), trolox (5 mmol/L), and with BA (60 μmol/L) for 24 hours. Percentage of apoptotic cells was measured by the DNA-release assay using treatment with a hypotonic PI solution at 4°C overnight in the dark followed by flow cytometric analysis. Each point represents the mean ± SD of three independent experiments.
We next investigated whether trolox and BA can affect the changes in mitochondrial functions mediated by NO in Jurkat cells. The results of these experiments, recorded for live (PI negative) APO-S cells, are presented in Fig 8. After 24 hours of incubation together with GTN, trolox was found to significantly reduce the size of the subpopulation with low cardiolipin content (NAOlow) as compared with the cell cultures treated with GTN alone (from 30% to 10%; P < .01; Fig 8A). In contrast, the number of cells with low Δψm(DiOC6(3)low) was only slightly decreased and the proportion of HEhigh cells (high ROS production) at this time point remained at the same level as in the cells incubated with GTN alone. At an earlier time point (8 hours), trolox was able to downregulate the quantity of NAOlow cells as compared with the samples with GTN only (9% and 14%, respectively; P < .05), but it could induce almost no decrease in the proportion of DiOC6(3)low and HEhigh cells (Fig8A). When BA and GTN were added to APO-S cells for 24 hours, the number of DiOC6(3)low cells was lower than in cell cultures with GTN alone (22% and 28%, respectively; P < .05; Fig 8B). The proportion of NAOlow and HEhigh cells remained unchanged under these culture conditions.
Effect of trolox (A) and BA (B) on ▵ψm, ROS production, cardiolipin content, and of trolox on the activity of OXPHOS complex IV in NO-treated APO-S Jurkat cells. (A and B) Cells were incubated for 8 and 24 hours with NO donor GTN (0.2 mmol/L) alone, trolox (5 mmol/L) alone, BA (60 μmol/L) alone or with GTN together with either trolox or BA. Flow cytometry analysis for ▵ψm [DiOC6(3)], ROS production (HE), and for cardiolipin concentration (NAO) was performed as described in the legend to Fig 2. (□), DiOC6 (3)low; (░), HEhigh; (▪), NAOlow. (C) Cells were incubated with NO donor GTN (0.2 mmol/L) alone, trolox (5 mmol/L) alone, and with both agents for 8 hours. Activity of OXPHOS complex IV was measured as described in the legend to Fig 1. Each bar represents the mean ± SD of three independent experiments.
Effect of trolox (A) and BA (B) on ▵ψm, ROS production, cardiolipin content, and of trolox on the activity of OXPHOS complex IV in NO-treated APO-S Jurkat cells. (A and B) Cells were incubated for 8 and 24 hours with NO donor GTN (0.2 mmol/L) alone, trolox (5 mmol/L) alone, BA (60 μmol/L) alone or with GTN together with either trolox or BA. Flow cytometry analysis for ▵ψm [DiOC6(3)], ROS production (HE), and for cardiolipin concentration (NAO) was performed as described in the legend to Fig 2. (□), DiOC6 (3)low; (░), HEhigh; (▪), NAOlow. (C) Cells were incubated with NO donor GTN (0.2 mmol/L) alone, trolox (5 mmol/L) alone, and with both agents for 8 hours. Activity of OXPHOS complex IV was measured as described in the legend to Fig 1. Each bar represents the mean ± SD of three independent experiments.
As shown in Fig 8C, treatment of APO-S cells with the combination of GTN and trolox for 8 hours resulted in the attenuation of the GTN-mediated inhibitory effect on complex IV activity. Incubation with trolox alone for the same period of time caused a decrease of complex IV activity as compared with untreated control cells.
Thus, trolox, an inhibitor of lipid peroxidation, strongly suppressed the NO-mediated apoptosis in APO-S Jurkat cells that correlated with significant reduction of NO-induced changes in cardiolipin concentration and OXPHOS complex IV activity. In contrast, treatment with BA, an inhibitor of mitochondrial PT, caused only a moderate suppression of NO-induced apoptosis and some decrease in the number of APO-S cells with low Δψm.
DISCUSSION
Recent studies performed in different cellular systems, including cell-free extracts, clearly indicated a crucial role for mitochondrial damage in many forms of apoptosis in mammalian cells. An early disruption of Δψm due to the formation of PT pores14 and cytochrome c release from mitochondria15,18 are considered the most important changes in mitochondrial functions. Cytochrome c is normally located in mitochondrial membranes and shuttles electrons between complexes III and IV of the respiratory chain.18
It was already known that NO could inhibit mitochondrial respiration in different types of cells.5,9,39 Moreover, NO has been recognized to induce cell death via apoptosis,6-11 although the precise mechanism of this process is still obscure. We described previously that NO can induce apoptosis in different human neoplastic lymphoid cells through activation of caspases. This occurred not only via CD95/CD95L interaction, but also independently of death receptors.11 In the present study, we addressed the question whether alterations in mitochondrial functions might mediate NO-induced caspase activation and subsequent apoptosis in human malignant lymphoid cells, and if so, which type of alteration was relevant. Using GTN (a donor of exogenous NO) at the concentrations inducing apoptosis in CD95-sensitive (APO-S) Jurkat cells, we show for the first time, a release of cytochrome c from the mitochondria into the cytosol fraction as early as 4 hours after onset of the experiment. These data are in agreement with our earlier observation that incubation with GTN for at least 8 hours and its subsequent withdrawal for 16 hours was sufficient to achieve maximal apoptotis, ie, the latter became irreversible at this time point.11
It has been recently reported that released cytochrome c is absolutely required (together with Apaf 1) for the activation of caspase-9, which in turn cleaves and activates caspase-3.18-20 Both caspases are considered the most upstream members of the apoptotic protease cascade that is triggered by cytochrome c.19,20 Here, we compared the kinetics of both cytochrome c accumulation in the cytosol and the activation of the above-mentioned caspases in NO-treated APO-S Jurkat cells and found that the first process happened earlier (at 4 hours) than the latter (at 8 hours). This suggests that NO-mediated release of cytochrome c causes the activation of the caspase cascade followed by apoptosis, as has already been shown for CD95 and other apoptotic death signals.18 33
To elucidate the mechanism(s) of cytochrome c release upon NO treatment of APO-S Jurkat cells, we studied the function of respiratory chain complexes and the content of the major mitochondrial lipid, cardiolipin, which plays a crucial role in cytochrome cattachment to the inner mitochondrial membrane.21,41,42 In addition, we measured Δψm and ROS production. It was found that exposure to NO for 8 hours significantly inhibited the activity of all complexes of the mitochondrial electron transport chain. This correlated with cytochrome c release and could be one of the reasons for the observed suppression of complex IV activity (Fig 1B and C). Our data are in agreement with other publications reporting on NO-mediated inhibition of cytochrome c oxidase (complex IV) in different cell types via binding to its heme moiety in a reversible manner.43,44 This downregulation of complex IV activity resulted in the upregulation of ROS synthesis45 followed by the formation of the strong oxidant peroxynitrite anion (ONOO-), which can induce irreversible inhibition of complexes I and III, but not complex IV.46 The alternative mechanism of a direct effect of NO on these complexes is also possible in our system, as long-term exposure to NO in vitro has been recently shown to block complex I activity in murine macrophages due to S-nitrosylation of this enzyme.44
The inhibition of the activity of complexes I, II, and III (Fig 1A and B) implies the cessation of ROS release from the electron transport chain. However, we observed a steady increase of ROS production in APO-S Jurkat T cells at 8 and 24 hours of NO treatment (Fig 2A). An alternative source of ROS synthesis could be the peroxidation of mitochondrial lipids (especially cardiolipin).47 Indeed, Escames et al48 have recently reported on NO-mediated upregulation of this process in rat brain cells. Nevertheless, the role of mitochondrial lipid degradation in the apoptotic effect of NO has not yet been investigated. Here, we found a significant time-dependent decrease in the content of cardiolipin, a phospholipid located in the inner mitochondrial membrane (like cytochrome c). Cardiolipin is necessary for complex IV activity49 and plays a crucial role in the attachment of cytochrome c to the inner mitochondrial membrane.21 42 Thus, we suggest that the observed degradation of cardiolipin upon NO treatment may be responsible for cytochrome c release in APO-S Jurkat cells.
The critical role of lipid degradation in NO-mediated apoptosis in APO-S Jurkat cells was confirmed by experiments with the vitamin E analogue trolox. We found that this potent inhibitor of lipid peroxidation39 significantly inhibited NO-induced apoptotic cell death and restored complex IV activity. A protective effect of trolox on complex IV activity in NO-exposed rat astrocytes has also been reported by Heales et al.50 This is probably due to the prevention of cardiolipin oxidation.50 Indeed, trolox significantly reduced the number of NO-treated Jurkat cells with low cardiolipin content without any effect on the quantity of cells with low Δψm and high ROS production, indicating that lipid peroxidation was required for this type of apoptosis.
Another possibility for mitochondrial damage during NO-mediated apoptosis could be linked to the decrease of Δψm due to the opening of PT pores followed by the depolarization of the inner mitochondrial membrane and massive ROS production.51 This mechanism of NO-induced apoptosis was shown in thymocytes.52 We provide evidence for a time-dependent increase in the number of live (PI-negative) APO-S Jurkat cells with low Δψm upon GTN treatment. To elucidate the role of PT in the NO-induced apoptosis in our model, we used BA, which is known to be a specific inhibitor of PT affecting the molecular conformation of the adenine nucleotide translocator (a protein participating in the formation of PT pores).17 BA was shown to suppress dexamethasone-induced apoptosis in mouse thymocytes.17However, when testing BA in combination with GTN, we observed only a limited inhibitory effect on NO-induced apoptosis in APO-S Jurkat cells (Fig 6). Under these conditions, only a moderate decrease in the number of cells with low Δψm was observed, thereby suggesting that PT was not a major factor causing NO-induced reduction of Δψm and apoptosis in Jurkat cells.
We have earlier described a substantially lower NO-mediated apoptotic cell death in the CD95-resistant Jurkat subclone (APO-R) as compared with APO-S cells, which are sensitive to CD95-mediated kill.11 Interestingly, the basic level of mitochondrial parameters studied here (eg, cardiolipin content, Δψm, ROS production, and the activity of OXPHOS complexes) was similar in both cell lines. Nevertheless, NO failed to stimulate cytochromec release and decrease of cardiolipin concentration in APO-R cells. In these cells, it induced only weak alterations in respiratory complex activity, ROS production, and in Δψm as compared with the control values. This resistance correlated with a substantial protection from apoptosis (Figs 1 and 2).
Bcl-2, which is expressed in the outer mitochondrial membrane, has been shown to play a central role in the suppression of apoptosis in different cell systems.21-25 In target cells, Bcl-2 prevent not only the release of cytochrome c from mitochondria21,33 by inhibition of lipid peroxidation (in particular, cardiolipin),23,38 but also interfere with cytochrome c already released into the cytosol.27Other mechanisms of Bcl-2–related antiapoptotic effects at the mitochondrial level are linked with (1) inhibition of PT and stabilization of Δψm17; (2) prevention of caspase activation and cleavage of poly(ADP-ribose) polymerase cleavage53; (3) regulation of proton flux36; and (4) blocking of the proapoptotic effect of Bax protein.54 Although an inverse correlation between Bcl-2 expression and sensitivity to NO-mediated apoptosis has recently been shown in several cell lines,24 25 the mechanism of Bcl-2 protection from NO-mediated cell death is still elusive.
It has previously been reported that Jurkat cells express very low levels of the Bcl-2 protein.29 In our experiments, we used Jurkat cells transfected with bcl-2 and the same cell clone transfected only with the corresponding neomycin vector (as a control). We found that Bcl-2 overexpression resulted in a complete resistance to apoptosis in GTN-treated cells at all concentrations tested (up to 0.5 mmol/L), whereas the control cells were sensitive to NO. Furthermore, Bcl-2 was able to block the damage of mitochondrial functions observed after exposure to GTN. It normalized the content of cardiolipin and ROS production, Δψm and OXPHOS complex activities, prevented cytochrome c release and lipid peroxidation in mitochondria. From our data on the effect of BA, an inhibitor of mitochondrial PT, and of trolox, an inhibitor of lipid peroxidation (Fig 6), we suggest that Bcl-2–induced suppression of lipid peroxidation plays a more important role in protection against NO-mediated apoptosis than the inhibition of PT. However, the question of which mechanism(s) are crucial for the suppression of NO-mediated cell death via Bcl-2 needs further investigation.
Taken together, our results show that NO-induced caspase activation and subsequent apoptosis in human leukemia cells requires certain alterations in mitochondrial functions, which include (1) a significant decrease in the concentration of cardiolipin, a major mitochondrial lipid; (2) a release of the mitochondrial protein cytochrome cinto the cytosol; and (3) a downregulation in respiratory chain complex activities. Because NO-induced cell death was strongly suppressed by trolox (an inhibitor of lipid peroxidation), we suggest that the degradation of mitochondrial lipids can be an upstream step in this process. We also found an increase in the number of cells with low Δψm and high ROS production, which may be considered as secondary consequences of NO-mediated apoptosis. Bcl-2 overexpression completely blocked the apoptotic effect of NO due to the prevention of mitochondrial damage (including the normalization of cardiolipin content and block of cytochrome c release). This new insight into the mechanism of NO-induced cell death could be of importance for effective treatment of leukemia patients by using NO donors or other agents to induce apoptosis through damage of mitochondrial functions in leukemic cells.
ACKNOWLEDGMENT
We thank Dr M.E. Peter for providing the Jurkat cells transfected with empty vector or containing bcl-2, and Dr J.A. Duine for providing BA.
Supported in part by Grant No. 10-0980-Schi2, V.U. from the Dr. Mildred Scheel Stiftung.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Victor Umansky, PhD, Division of Cellular Immunology, Tumor Immunology Program, German Cancer Research Center, Abteilung 710, lm Neuenheimer Feld 280, D-69120 Heidelberg, Germany; e-mail: V.Umansky@dkfz-heidelberg.de.



![Fig. 8. Effect of trolox (A) and BA (B) on ▵ψm, ROS production, cardiolipin content, and of trolox on the activity of OXPHOS complex IV in NO-treated APO-S Jurkat cells. (A and B) Cells were incubated for 8 and 24 hours with NO donor GTN (0.2 mmol/L) alone, trolox (5 mmol/L) alone, BA (60 μmol/L) alone or with GTN together with either trolox or BA. Flow cytometry analysis for ▵ψm [DiOC6(3)], ROS production (HE), and for cardiolipin concentration (NAO) was performed as described in the legend to Fig 2. (□), DiOC6 (3)low; (░), HEhigh; (▪), NAOlow. (C) Cells were incubated with NO donor GTN (0.2 mmol/L) alone, trolox (5 mmol/L) alone, and with both agents for 8 hours. Activity of OXPHOS complex IV was measured as described in the legend to Fig 1. Each bar represents the mean ± SD of three independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/7/10.1182_blood.v93.7.2342/5/m_blod4070908abx.jpeg?Expires=1769238600&Signature=eZvsOoi8d0pD92Q3SgGFfPyX5loSeNIDcd0sKEIMeMWlr3aHRDXePtpc3UY0SSWrnFj-4DnSzOhzBak7LsIDRfTZc6DIdyUN00uRs2C3hLoXos0uGMQ5pTUjPoiz~lG5korVQoIZ-D52DPxJL~d21vvy6gW6zSa4Wf~c5DsMcq7BMZo2sIv4kUsDJcGz4POkzXqMKxlT7U1gl0XE1V9y0CReSvMkFxjynrCErh~JYrfcEdX3al56WlF4eK4IIQ7PsXG0hrUEAMneju9tuD~7XoEjwsYBPBWZ8TI5plv3oJqOvUkaeAQ9mMwxMsIMgtRH4O0GnjiekxIjqdqoBPHT7A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Effect of trolox (A) and BA (B) on ▵ψm, ROS production, cardiolipin content, and of trolox on the activity of OXPHOS complex IV in NO-treated APO-S Jurkat cells. (A and B) Cells were incubated for 8 and 24 hours with NO donor GTN (0.2 mmol/L) alone, trolox (5 mmol/L) alone, BA (60 μmol/L) alone or with GTN together with either trolox or BA. Flow cytometry analysis for ▵ψm [DiOC6(3)], ROS production (HE), and for cardiolipin concentration (NAO) was performed as described in the legend to Fig 2. (□), DiOC6 (3)low; (░), HEhigh; (▪), NAOlow. (C) Cells were incubated with NO donor GTN (0.2 mmol/L) alone, trolox (5 mmol/L) alone, and with both agents for 8 hours. Activity of OXPHOS complex IV was measured as described in the legend to Fig 1. Each bar represents the mean ± SD of three independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/7/10.1182_blood.v93.7.2342/5/m_blod40709008cx.jpeg?Expires=1769238600&Signature=Lbwg9qv5X8mb-XDTYLwkkw5rj6lHCdqYmSmFeV63WXaI4Vn4gr2piIugXpN9KOovCxVSVYyNgfEUNrCmbOHZxuRDywH0KGaWobn9~8Q1IxtfZxUBd2D~yq0~xNuhrvtPXUXxSsmYrfsraJJS2A1G2NWCP~TbxIhQP33MZOUjcztw5floVkKtMyPrbHv5gdEennVGB5L1VUFABkvLPHudD1Fyb~cyHBxwv0GjGmN3uKg0upXhf~kjhQWXL5kFwuwnv8ppowINhdP5HQIjc1fIOQxGunKpQtDrLLxXOu2QtfJhgyHwcf4oxQCzQfGmgu5JUA7aGheNacBpLPic5IBS5w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal