Thrombomodulin (TM) is a widely expressed glycoprotein receptor that plays a physiologically important role in maintaining normal hemostatic balance postnatally. Inactivation of the TM gene in mice results in embryonic lethality without thrombosis, suggesting that structures of TM not recognized to be involved in coagulation might be critical for normal fetal development. Therefore, the in vivo role of the cytoplasmic domain of TM was studied by using homologous recombination in ES cells to create mice that lack this region of TM (TMcyt/cyt). Cross-breeding of F1 TMwt/cyt mice (1 wild-type and 1 mutant allele) resulted in more than 300 healthy offspring with a normal Mendelian inheritance pattern of 25.7% TMwt/wt, 46.6% TMwt/cyt, and 27.7% TMcyt/cyt mice, indicating that the tail of TM is not necessary for normal fetal development. Phenotypic analyses showed that the TMcyt/cyt mice responded identically to their wild-type littermates after procoagulant, proinflammatory, and skin wound challenges. Plasma levels of plasminogen, plasminogen activator inhibitor 1 (PAI-1), and 2-antiplasmin were unaltered, but plasmin:2-antiplasmin (PAP) levels were significantly lower in TMcyt/cyt mice than in TMwt/wt mice (0.46 ± 0.2 and 1.99 ± 0.1 ng/mL, respectively; P < .001). Tissue levels of TM antigen were also unaffected. However, functional levels of plasma TM in the TMcyt/cyt mice, as measured by thrombin-dependent activation of protein C, were significantly increased (P < .001). This supported the hypothesis that suppression in PAP levels may be due to augmented activation of thrombin-activatable fibrinolysis inhibitor (TAFI), with resultant inhibition of plasmin generation. In conclusion, these studies exclude the cytoplasmic domain of TM from playing a role in the early embryonic lethality of TM-null mice and support its function in regulating plasmin generation in plasma.
THROMBOMODULIN (TM) is a transmembrane receptor for thrombin that is widely expressed in a variety of tissues in adults and during development.1-3 The function of TM is best characterized with respect to its role in hemostasis, in which it acts as a cofactor in the activation of protein C, thereby providing anticoagulant properties,4,5 the physiological importance of which is exemplified by the prethrombotic state of those who are functionally deficient in protein C6 or who have factor VLeiden.7 Mutations in TM have also been reported to be associated with a thrombotic tendency.8Recently, TM was found to be a cofactor in the activation of plasma procarboxypeptidase B (thrombin-activatable fibrinolysis inhibitor [TAFI]),9 which interferes in the transformation of plasminogen to plasmin by altering the cofactor, fibrin. Thus, TM is presumed to have both antifibrinolytic and anticoagulant properties.
A critical role of TM during development, unrelated to coagulation, is evidenced by the embryonic lethality before the development of a vascular tree in mice with TM gene inactivation.10Furthermore, mice expressing a TM variant with markedly reduced thrombin-dependent protein C activation survived to adulthood,11 12 additional evidence that TM has noncoagulation roles during critical stages of murine development.
The lethal phenotype of TM−/− embryos implies that normal development beyond 8.5 to 9.5 days post coitum (dpc) depends on expression of intact TM at a specific site at that developmental stage or, alternatively, that a structural domain of TM, not involved in coagulation, is essential for normal development. Although there is considerable information as to the role of epidermal growth factor-like repeats,3-6 little is known about the function of the other domains of TM.13-15 The N-terminal extracellular region with weak homology to lectins16-18 appears to be required for recycling of the molecule in vitro19 and may have a yet to be identified ligand. The juxtamembranous serine-threonine–rich region contains sites for N- and O-linked glycosylation and addition of a chondroitin sulfate moiety, the latter which enhances the anticoagulant function of TM. The cytoplasmic tail is not required for constitutive recycling of TM in vitro,20,21 although it may be important for multimerization. Although the cytoplasmic tail of TM contains serine, threonine, and tyrosine, consensus sequences for phosphorylation have not been identified. However, most intriguing is the recent observation that both the N-terminal lectin-like domain and the cytoplasmic domain of TM may be important in modulating the growth of tumor cells.22
To determine the physiologic role of the cytoplasmic tail of TM, we have used gene targeting in embryonic stem (ES) cells to generate mice lacking this domain. Mice expressing tail-less TM (ie, lacking the cytoplasmic domain) are both viable and fertile, with a normal response to skin wounds, and to procoagulant and proinflammatory stimuli. The cytoplasmic domain of TM regulates its functional level in plasma, in turn regulating the fibrinolytic pathway possibly via activation of TAFI.
MATERIALS AND METHODS
Isolation of the murine TM gene.
Oligonucleotide primers TM.3330s (5′-TCTCCGCACTAGCCAAGCTGCAG) and TM.3331as (5′-CTGCGGGAGCTGTAAACCGATCC), based on the published murine TM cDNA sequence,23 were used to screen a murine 129Sv genomic PAC library (Genome Systems, South Bend, IN). Three clones were provided, each demonstrated by Southern blotting to contain the murine TM gene. As previously reported, an intracisternal A-particle (IAP) provirus was found in the 5′ end of theTM gene in this strain of mice.24 A 12-kbKpn I fragment, containing the entire coding region, was subcloned into pBS (Stratagene Inc, Mississauga, Ontario, Canada), resulting in Kpn12/BS.
Construction of a targeting vector to delete the cytoplasmic domain of TM.
To replace the wild-type coding region of TM with one that encodes a tail-less TM (ie, lacking the cytoplasmic domain of TM), polymerase chain reaction (PCR)-based mutagenesis with complementary oligonucleotides TM.S1951i (sense 5′-CTCTGTCACCTGCGCAAGTGAGGGATTTGCTCCAGA) and TM.AS1850i (antisense 5′-TCTGGAGCAAATCCCTCACTTGCGCAGGTGACAGAG) was used. Two PCRs were performed. In the first, oligonucleotide primer TMS.1951i was paired with antisense primer TM.AS2613EO (5′-TGGACTAGTTAATTAAGATCTTCCTCGAGGCGCGCCGTTCAGCTGAAATATTTTAGC), resulting in a 643-bp fragment. In the second, antisense oligonucleotide primer TM.AS1850i was paired with sense primer TM.S-240 (5′-TTCTGTGGTGGCGCCTGCAGGCCACGCCCG), yielding a 2,050-bp fragment. These products were purified and used for recombinant PCR with oligonucleotide primers TM.S-240 and TM.AS2613EO. The recombined 2,740-bp amplicon was subcloned into the TA-cloning vector pCR2.1 (Invitrogen, San Diego, CA), and DNA sequencing confirmed the presence of the desired deletion. This DNA fragment extends from a Nar I restriction enzyme site 230 bp upstream of the transcriptional start site, through the coding region of the gene, and 643 bp into the 3′ untranslated region (3′-UTR). Oligonucleotide primer TM.AS2613EO resulted in the addition ofAsc I, Xho I, Bgl II, Pac I, andSpe I restriction sites at the 3′ end of the recombined product to be used for subcloning and ES cell DNA screening. The final translated protein product represents the intact TM protein, lacking the COOH-terminal 34 amino acid residues of the cytoplasmic tail (NH2-KQGAARAELEYKCASSAKEVVLQHVRTDRTLQKF), yet retaining the native in-frame stop codon.
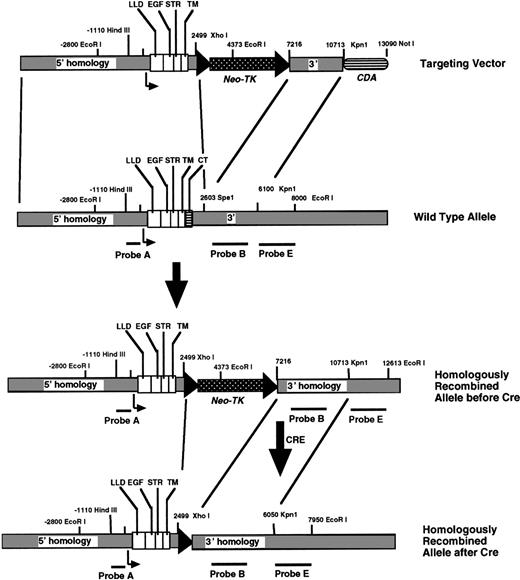
A targeting vector was constructed (Fig 1) by replacing the above mutated TM DNA into Nar I-SpeI–digested Kpn12/BS, generating Kpn12mut/BS. The 3.5-kb SpeI-Spe I fragment of 3′ homology was excised from Kpn12mut/BS, and the remaining vector was religated. A 1.5-kbKpn I/Bgl I fragment was removed from the most 5′ end of the gene and, after mung bean nuclease digestion, the ends of the vector were also religated, resulting in approximately 3.4 kb of 5′ homology. After digestion of the latter construct withXho I and Bgl II, a loxP-flanked neomycin phosphotransferase-thymidine kinase (neo-tk) gene cassette was subsequently inserted within the 3′-UTR. The resultant vector was cut with Pac I, and the previously purified 3.5-kbSpe I-Spe I fragment representing 3′-homology was inserted in the correct orientation. Finally, for negative selection, the gene encoding cytosine deaminase (cda) was inserted at the 3′ end of the targeting vector between the Sal I andNot I sites.
Strategy to introduce TM lacking the cytoplasmic domain into ES cells via homologous recombination. The wild-type allele for the TM gene, which is intronless, encodes a lectin-like domain (LLD), 6 epidermal growth-factor like repeats (EGF), a serine-threonine rich region (STR), a single transmembrane domain (TM), and a cytoplasmic tail (CT). DNA probes used for Southern blotting are shown. After homologous recombination of the targeting vector, the targeted ES cells were exposed to cre-recombinase for excision of theneo-TK genes.
Strategy to introduce TM lacking the cytoplasmic domain into ES cells via homologous recombination. The wild-type allele for the TM gene, which is intronless, encodes a lectin-like domain (LLD), 6 epidermal growth-factor like repeats (EGF), a serine-threonine rich region (STR), a single transmembrane domain (TM), and a cytoplasmic tail (CT). DNA probes used for Southern blotting are shown. After homologous recombination of the targeting vector, the targeted ES cells were exposed to cre-recombinase for excision of theneo-TK genes.
Targeting of mutated TM gene into ES cells.
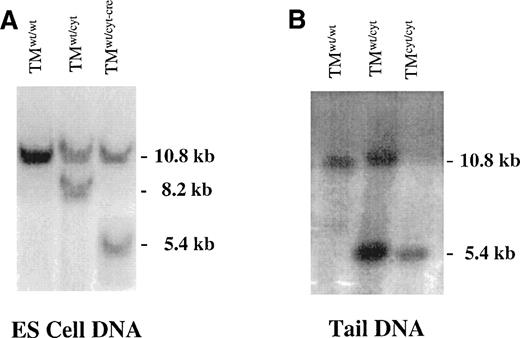
Targeting vector DNA (20 μg) was linearized with Not I and introduced into R1 ES cells by electroporation, after which the cells were plated onto confluent layers of neomycin-resistant embryonic fibroblasts in the presence of G418 and 5-fluorocytosine (5-FC). DNA from surviving colonies was screened for homologous recombination by Southern blotting using a 3′ external probe E (Fig 2A). Random integrations were excluded by Southern blotting with a neomycin DNA probe and internal probes A and B (Fig 1). Using DNA from the homologously recombined ES cell clones, the expected deletion was confirmed by PCR with primer pair TM.s1303 (5′-AGAGTGCGTGGAGCTTCTGGATC) and TM.as2250 (5′-TGCCTTCAAGCCTACAGATCAG), followed by DNA sequencing of the PCR product.
Southern blots of DNA derived from targeted ES cells and tails of gene-targeted mice. (A) Southern blot of genomic DNA from ES cells before electroporation (TMwt/wt), with homologous recombination of the targeting vector (TMwt/cyt)), and after successful cre-recombinase excision of the neo-tk genes from the targeted allele (TMwt/cyt-cre). (B) Southern blot of tail DNA from gene-targeted mice. A single 5.4-kb band is detected in the TMcyt/cyt mice, reflecting homologous recombination of both TM alleles with the mutant form. In (A) and (B), DNA was digested with EcoRI/Xho I and bands were detected with probe E (see Fig 1).
Southern blots of DNA derived from targeted ES cells and tails of gene-targeted mice. (A) Southern blot of genomic DNA from ES cells before electroporation (TMwt/wt), with homologous recombination of the targeting vector (TMwt/cyt)), and after successful cre-recombinase excision of the neo-tk genes from the targeted allele (TMwt/cyt-cre). (B) Southern blot of tail DNA from gene-targeted mice. A single 5.4-kb band is detected in the TMcyt/cyt mice, reflecting homologous recombination of both TM alleles with the mutant form. In (A) and (B), DNA was digested with EcoRI/Xho I and bands were detected with probe E (see Fig 1).
In vitro excision of lox-P flanked neo-TK from targeted ES cell clones.
Targeted ES cell clones were expanded and the cre-recombinasegene25 was transiently introduced by electroporation. Transfected ES cells were plated at low density on feeders and exposed to gancyclovir, and surviving colonies were selected. Excision of theneo-TK gene was confirmed by Southern blotting and PCR analysis.
Introduction of mutated TM into mice.
Targeted ES cells were aggregated26 with morula-stage embryos derived from C57Bl6/J mice and introduced into pseudopregnant female National Institutes of Health (NIH) Swiss white mice. Two chimeric male offspring resulted in the establishment of germline transmission of the mutant TM allele. Large numbers of F1 and F2 offspring were intercrossed, avoiding brother-sister matings. Genotyping was performed on tail DNA both by Southern blotting and by PCR. The chimeric males were also backcrossed with C57Bl/6 and 129sv/ev mouse pedigrees for comparative purposes.
Expression of recombinant mutated murine TM in mammalian cells.
The cDNA encoding wild-type and tail-less TM were subcloned into the expression vector pcDNA3.1 (Invitrogen) for transfection into COS cells. Serial dilution of the cells under continuous selection with G418 resulted in isolated clones of TM-expressing cells. A vector-alone control COS cell line was also generated. Expression of cell surface TM was confirmed by indirect immunofluorescence27 using highly specific rabbit antirat TM antisera (gift of Dr Robert Jackman, Boston, MA). The cofactor activity of cell-surface expressed recombinant TM was evaluated by activation of purified bovine protein C with exogenously added bovine thrombin.28
RNA isolation and reverse transcriptase-PCR (RT-PCR).
Total RNA was isolated from tissue by the method of Chomczynski and Sacchi.29 For RT-PCR, cDNA was synthesized from total RNA by reverse transcription using murine leukemia virus (M-MLV) reverse transcriptase and a cDNA synthesis kit (NV Life Technologies, Merelbeke, Belgium). First-strand synthesis was primed using random hexanucleotides. To confirm the deletion of the cytoplasmic domain of TM in the gene-targeted mice by RT-PCR, oligonucleotide primers that flank the deleted region, TM.s1303 and TM.as2250, were used, and the amplicon was sequenced.
Quantitation of tissue levels of TM.
Relative amounts of TM in lung tissue and plasma were quantitated using a previously reported sandwich radio-immunoassay30 with monoclonal antibodies 201B and 34A (kindly provided by Dr S. Kennel, Oak Ridge, TN).
Embryo sections and immunohistochemical staining.
For embryonic samples, mice were mated, conception was assessed by the presence of a coital plug (with the morning of coital plug being scored as 0.5 dpc), pregnant females were killed at specific developmental time points, and embryos were carefully removed by dissection. Whole embryos were fixed and embedded in paraffin, and 10-μm sagittal sections were made and either stained with hematoxylin and eosin or incubated with rabbit anti-TM antisera (1:1,000) for subsequent detection by immunoperoxidase staining.
Plasma levels of components of the fibrinolytic system.
Quantitation of plasma levels of fibrinopeptide A (FPA).
Antibodies directed against the carboxy-terminus of FPA were raised against a homologue of murine FPA that was synthesized by solid-phase methods33 (QCB, Inc, Hopkinton, MA). An amino-terminal cysteine residue of the FPA homologue was cross-linked to Keyhole Limpet hemocyanin (Sigma, St Louis, MO) with m-maleimidobenzoic acid N-hydroxysuccinimide ester (Pierce Chemical Co, Rockford, IL) according to the manufacturer’s instructions, and Ellman’s reagent (Pierce Chemical Co) was used to compare the sulfhydryl concentration of the conjugate with that of the starting material. Murine FPA (112 mol) was covalently coupled to each mole of Keyhole Limpet hemocyanin. Immunization of sheep by subcutaneous injection of the coupled murine FPA suspended in complete Freund’s adjuvant, followed by incomplete Freund’s for booster immunizations, was performed by Affinity Biologicals (Hamilton, Ontario, Canada). IgG fractions were isolated from the sheep sera, dialyzed against HEPES-buffered saline, and then dialyzed against an equal volume of glycerol for storage in aliquots at −20°C.
Murine blood from the inferior vena cava was collected into a plastic syringe preloaded with 1/10 vol of an anticoagulant solution consisting of 100 kallikrein inhibitor units (KIU)/mL bovine lung aprotinin (American Diagnostica, Montreal, Quebec, Canada), 1,300 U/mL porcine intestinal mucosa heparin (Sigma H9399), 10 mmol/L adenosine (Calbiochem, Mississauga, Ontario, Canada), 20 mmol/L theophylline, and 0.1 mmol/L MeO-Suc-Ala-Ala-Pro-ValCh2Cl (CK-20; Enzyme Systems Products, Livermore, CA) in HEPES-buffered saline, pH 7.4. Plasma fractions obtained by 5 minutes of centrifugation at 15,000g were stored at −70°C for subsequent assay. Murine FPA homologues, with or without an amino-terminal tyrosine residue, were synthesized (QCB, Inc). The tyrosinated peptide was radiolabeled with 125I-Na with a specific activity of at least 0.32 μCi/pmol. Known concentrations of murine FPA or plasma sample (500 μL) diluted in 50 mmol/L Tris-HCl, pH 8.5, 100 mmol/L NaCl, 0.1% ovalbumin, 0.02% sodium azide (TBS) were incubated overnight at 4°C with tracer (50 μL; ∼15,000 cpm) and sheep antimouse FPA IgG (100 μL; at a dilution to bind ∼35% of total cpm). Unbound tracer was precipitated by addition of 1 ml of 1.25% (wt/vol) suspension of activated charcoal in TBS and incubation for 20 minutes at 4°C, followed by centrifugation at 3,000g for 20 minutes at 4°C. Supernatants were decanted and counted for 1 minute (LKB Instruments, Gaithersburg, MD). Based on standard curves generated with the FPA homologue, the assay has a limit of detection of 0.01 nmol/L mouse FPA and interassay and intra-assay coefficients of variation of 4.7% and 2.8%, respectively.
Functional level of plasma TM.
After anesthesia of mice, blood was drawn via cardiac puncture into preloaded syringes containing 1/10 vol 3.8% sodium citrate. Ten microliters of bovine thrombin (15 nmol/L) was added to 120 μL HEPES-buffered saline, 5 μL plasma, and 20 μL bovine protein C (2,372 nmol/L), and the reaction was incubated at 37°C for 45 minutes, after which a molar excess of PPACK, with respect to thrombin, was added. The TM-dependent conversion of protein C to activated protein C was measured by adding the reaction mixture to 500 μL of 0.4 mmol/L chromogenic substrate S2238 (Helena Laboratories, Beaumont, TX) and quantitating the rate of change in absorbance at 405 nm. Preincubation of plasma with PPACK, hirudin, or specific polyclonal anti-TM antibodies known to interfere with TM cofactor activity resulted in the absence of conversion of protein C to its active form, as measured by the chromogenic assay.
Protein electrophoresis and immunoblot of plasma TM.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed using an 8% gel according to the method of Laemmli34 under nonreducing conditions and as previously described.35 Citrated plasma obtained from 8-week-old mice was loaded in each lane in Laemmli buffer, and after electrophoretic separation and transfer to nitrocellulose, the filter was incubated overnight with monoclonal anti-TM antibodies 201B and 34A (Dr S. Kennel) in Blotto at a concentration of 0.5 μg/mL. Detection was accomplished using the ECL method (Amersham, Gent, Belgium).
Thrombogenic stresses.
Mice were exposed to 5.5% oxygen for 16 to 18 hours in a normobaric chamber, as previously described,36 after which they were immediately anesthetized. The sternum was split for cardiac puncture to withdraw blood into appropriate anticoagulants for subsequent assays. The vasculature was perfused via the heart with phosphate-buffered saline (PBS). Tissues were quickly dissected and either fixed for histological analysis or placed into liquid nitrogen for protein or RNA studies. Tissue levels of fibrin were determined as reported.11 Transverse sections of the lungs were cut for immunoperoxidase staining (without counter-stain) with polyclonal goat antimouse fibrin/ogen antibody (1:400) (Nordic, Trilburg, The Netherlands).
Induction of disseminated intravascular coagulation in mice.
Lipopolysaccharide (LPS) from Escherichia coli serotype 0111:B4 (Sigma) was injected intraperitoneally into 10- to 12-week-old mice. Animals were closely monitored each day until either recovery or cessation of breathing.
Wound-healing in mice.
Under anesthesia, two linear, parallel, vertical 3-cm incisions were made on the back, through the skin to the depth of the dermis. Mice were then housed in separate cages to prevent scratching. Wounds were inspected daily. After 8 days, the animals were killed and the surrounding tissue including each wound was excised and fixed for histological analysis.
Animal care.
All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Leuven.
Statistical analyses.
Statistical analyses of data using standard methods were conducted with the StatView computer program (Abacus Concepts Inc, Berkeley, CA). The means are provided with associated standard errors (SD). Pvalues were determined using the unpaired t-test.
RESULTS
Deletion of the cytoplasmic domain of TM and expression in COS cells.
To evaluate the role of the cytoplasmic domain of murine TM, we deleted the entire domain using recombinant PCR while retaining the native in-frame stop codon. The two basic amino acid residues within the cytoplasmic domain and immediately adjacent to the membrane, arginine and lysine, were maintained to minimize the possibility of shedding of the mutated molecule from the cell surface. COS cells were transfected with murine TM cDNA encoding both wild-type and tail-less TM. Northern analysis of RNA derived from the TM-expressing cells and control cells transfected with the expression vector (pcDNA3.1) alone confirmed the specificity and expected TM mRNA processing (not shown). Indirect immunofluorescence using a specific rabbit anti-TM antibody showed that wild-type and tail-less TM could be transported through the cell for stable surface expression. Furthermore, protein C activation by thrombin was markedly accelerated on the surface of COS cells that expressed either wild-type or tail-less murine TM. The rate of change in absorbance of the chromogenic substrate S2238 at 405 nm used to determine TM-cofactor function in thrombin-dependent activation of protein C was 0.01 U/min in vector-alone transfected COS cells. However, it was 0.14 ± 0.02 (n = 3) U/min and 0.16 ± 0.03 (n = 3) U/min for those COS cells transfected with wild-type or tail-less TM, respectively, indicating that both forms of TM were functional, as would be expected given that the entire extracellular domains of the protein were intact.
Generation of mice lacking the cytoplasmic domain of TM.
A targeting vector was constructed (Fig 1) in which the wild-type coding region of the murine TM gene (TM is intronless) was replaced with one that encoded a truncated form of TM, ie, lacking the most COOH-terminal 34 amino acid residues of the cytoplasmic domain, while retaining an in-frame stop codon. After electroporation of R1 ES cells, more than 300 clones were picked, 2 of which were determined to have homologously recombined the replacement vector in a single copy, as evaluated by Southern blotting (Fig 2A). PCR and DNA sequencing were used to confirm that the entire coding region of the mutated allele with the appropriate deletion was intact. After expansion of the two positive ES cell clones, excision of the lox-Pflanked neo-TK cassette was accomplished by transient introduction of the cre-recombinase gene. Successful excision was demonstrated in numerous cre-recombinase exposed, gancyclovir-resistant ES cell clones. Several of these were aggregated for generation of chimeric mice, two of which transmitted to germline. The reported results are not likely to reflect a strain-specific artifact, because in limited studies, back-crossing onto 129sv/se and Bl6 backgrounds resulted in similar phenotypes.
Viability of gene-targeted mice.
Cross-breeding of F1, TMwt/cyt (1 mutant and 1 wild-type allele) mice has resulted in more than 300 offspring. Genotyping of tail DNA was performed by PCR analysis and occasionally confirmed by Southern blotting (Fig 2). The genotypes of F2 progeny were distributed in a Mendelian inheritance pattern of 25.7% (TMwt/wt), 46.6% (TMwt/cyt), and 27.7% (TMcyt/cyt) at birth, indicating that intrauterine death was not occurring. Furthermore, there was an equal distribution of male and female births, and there were no detectable differences in weight, appearance, or growth and development up to 10 months of age. Females and males were fertile, producing offspring of the different genotypes in the expected distribution.
Expression of TM by TMwt/wt, Tmwt/cyt, and TMcyt/cyt mice.
Deletion of the cytoplasmic domain of TM in vivo did not affect cellular distribution, quantitative synthesis, cell membrane expression, or protein C cofactor activity of the molecule. Immunoperoxidase staining of sagittal sections of 14.5 dpc embryos showed TM in all tissues as previously reported, including within the lung, brain, spleen, liver, kidneys, and heart. The total amount of TM in lung tissue was quantitated using a double antibody sandwich radioimmunoassay. From four mice of each genotype, 3 measurements were performed on each lung sample. TMwt/wt, TMwt/cyt, and TMcyt/cyt mice had similar TM lung tissue levels of 340 ± 21, 350 ± 10, and 280 ± 23 cpm/μg of total protein, respectively. Similarly, there were no differences in TM antigen levels in the brain and kidney of the same mice (not shown).
Plasma levels of fibrinolytic components and functional TM.
In view of the recently described role of TM in mediating an antifibrinolytic effect via TAFIa, PAP complex in plasma was measured by using an enzyme-linked immunosorbent assay (ELISA) in which one antibody detects murine plasmin/ogen while the second antibody detects α2-antiplasmin. In five separate experiments, performed with 3 to 5 mice from each genotype, there was a significantly lower plasma level of PAP in the mice expressing tail-less TM (P < .005; Table 1). Plasma levels of α2-antiplasmin and plasminogen were essentially identical (P > .1) in each group (TMwt/wtvTMcyt/cyt) by using sensitive and specific ELISAs (Table 2). Thus, mice expressing tail-less TM have significantly lower plasmin generation than their wild-type counterparts.
Antigenic Levels of Plasma PAP and Functional Levels of Plasma TM in the Gene-Targeted Mice
| . | PAP (ng/mL) (n = 6) . | Function of Plasma TM (ΔA405/min) (n = 4) . |
|---|---|---|
| TMwt/wt | 1.99 ± 0.1 | 0.12 ± .01 |
| TMwt/cyt | 0.79 ± 0.6 | 0.19 ± .02 |
| TMcyt/cyt | 0.46 ± 0.2 | 0.22 ± .03 |
| . | PAP (ng/mL) (n = 6) . | Function of Plasma TM (ΔA405/min) (n = 4) . |
|---|---|---|
| TMwt/wt | 1.99 ± 0.1 | 0.12 ± .01 |
| TMwt/cyt | 0.79 ± 0.6 | 0.19 ± .02 |
| TMcyt/cyt | 0.46 ± 0.2 | 0.22 ± .03 |
Values reflect the means with associated standard deviations.P values indicate highly significant differences in levels of functional TM and PAP in the plasma of TMwt/wt versus TMcyt/cyt mice.
Plasma Levels of Plasminogen, 2-Antiplasmin, and PAI-1 in the Gene-Targeted Mice
| . | Plasminogen (μg/mL) . | α2-Antiplasmin (μg/mL) . | PAI-1 (ng/mL) . |
|---|---|---|---|
| TMwt/wt | 156 ± 10 | 74 ± 9 | 1.2 ± 0.3 |
| TMwt/cyt | 139 ± 11 | 60 ± 6 | 2.6 ± 0.8 |
| TMcyt/cyt | 148 ± 17 | 61 ± 8 | 2.4 ± 0.6 |
| . | Plasminogen (μg/mL) . | α2-Antiplasmin (μg/mL) . | PAI-1 (ng/mL) . |
|---|---|---|---|
| TMwt/wt | 156 ± 10 | 74 ± 9 | 1.2 ± 0.3 |
| TMwt/cyt | 139 ± 11 | 60 ± 6 | 2.6 ± 0.8 |
| TMcyt/cyt | 148 ± 17 | 61 ± 8 | 2.4 ± 0.6 |
Values reflect the means (n = 5) with associated standard deviations and P values. For each measure, there was no significant difference between any group (P > .1).
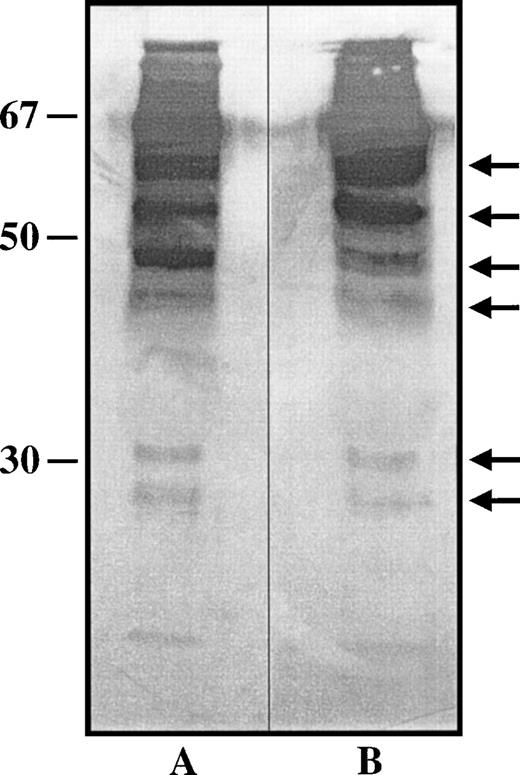
PAI-1 levels were not significantly different in the plasma of those mice expressing tail-less TM as compared with their wild-type littermates (Table 2) or the TMwt/cyt mice, indicating that the cytoplasmic domain of TM does not mediate regulation of release of PAI-1. An increase in soluble forms of TM in the plasma might enhance the activation of TAFI, thereby diminishing plasmin generation. Using a chromogenic substrate assay to measure thrombin-dependent protein C activation as a function of plasma TM,28 it was determined that mice expressing tail-less TM had significantly higher functional levels of soluble plasma TM (Table 1). Protein C activation was entirely abrogated by preincubation of plasma samples with anti-TM antisera or with PPACK, indicating the specificity of the protein C activation by the thrombin-TM complex and supporting our hypothesis that excess functional TM in the plasma might result in suppression of plasmin generation via the activation of TAFI. In 4 mice of each genotype, plasma TM antigen levels were not significantly different (P > .1; 58 ± 7 cpm/μg and 63 ± 9 cpm/μg in wild-type and mutant mice, respectively). Furthermore, Western immunoblotting showed that the pattern of plasma TM fragments was similar in the TMcyt/cyt mice and their wild-type littermates (Fig 3).
Western immunoblot detection of plasma TM. Citrated murine plasma from TMwt/wt and TMcyt/cyt mice (lanes A and B, respectively) in Laemmli buffer was separated electrophoretically by SDS-PAGE under nonreducing conditions, and the gel was transferred to a nitrocellulose filter for immuno-detection of TM with specific monoclonal anti-TM antibodies, as detailed in Materials and Methods. Molecular weight markers are on the left. Several molecular forms of TM were identified, the most prominent of which are noted with arrows on the right. No differences in patterns between the two lanes could be discerned.
Western immunoblot detection of plasma TM. Citrated murine plasma from TMwt/wt and TMcyt/cyt mice (lanes A and B, respectively) in Laemmli buffer was separated electrophoretically by SDS-PAGE under nonreducing conditions, and the gel was transferred to a nitrocellulose filter for immuno-detection of TM with specific monoclonal anti-TM antibodies, as detailed in Materials and Methods. Molecular weight markers are on the left. Several molecular forms of TM were identified, the most prominent of which are noted with arrows on the right. No differences in patterns between the two lanes could be discerned.
Thrombogenic stresses.
Lethal doses of LPS (40 μg/g of body weight) resulted in similar survival times of 33 ± 13 hours and 28 ± 11 hours (n = 7, P = .22) for TMwt/wt and TMcyt/cytmice, respectively. Human protein C has been shown in some animal models to protect against the lethality of Escherichia coliinfusions.37 Pretreatment of LPS-exposed mice with bovine protein C (10 μg) had no effect on survival. Sublethal doses of LPS (5 μg/g) resulted in blindness, altered hair appearance, and irregular behaviour within 20 to 30 hours. There were no obvious differences in time of onset, severity of symptoms, or time to full recovery (∼72 hours after LPS administration) between genotypes, indicating that the cytoplasmic domain does not play a direct role in inflammation or coagulation. Pretreatment with bovine protein C again had no effect on the response to sublethal doses ofLPS.
Exposure of mice to hypoxia for 16 to 18 hours has been shown to result in the deposition of fibrin and platelet thrombi within the lung vasculature, with increases in thrombogenicity observed in mice heterozygous for the TM gene12 and in mice expressing TM that has approximately 0.5% protein C cofactor activity.11 The thrombogenic response to hypoxia was studied by quantitation of lung tissue fibrin,11measurement of FPA, and computer-aided quantitation of fibrin/ogen immunostaining in lung tissue sections (Table 3). Baseline levels of these parameters in the TMwt/wt, TMwt/cyt, and TMcyt/cyt mice were similar, and hypoxia did not significantly affect these markers (P > .1). Previous in vitro and in vivo studies have indicated that TM mRNA levels may be augmented in response to stress.38 Quantitation of lung tissue TM antigen in mice expressing wild-type and tail-less TM before and after hypoxia exposure showed no significant response to the stress. Overall, the results of these experiments would suggest that the cytoplasmic domain of TM has no role in altering the coagulation system in response to this stress.
Lung Tissue TM, Fibrin/ogen and Fibrin, and Plasma FPA Levels in Gene-Targeted Mice Exposed to Hypoxia
| . | Lung TM (cpm/μg) . | Fibrin/ogen Staining (U/area) . | Fibrin (ng/4 mg) . | FPA (nmol/L) . |
|---|---|---|---|---|
| TMwt/wt | 340 ± 21 | 3.9 ± 2.7 | 57 ± 55 | 1.0 ± 0.1 |
| TMwt/wt + hypoxia | 290 ± 69 | 9.5 ± 6.2 | 408 ± 444 | 1.9 ± 1.8 |
| TMwt/cyt | 350 ± 10 | 8.5 ± 3.6 | 187 ± 242 | 5.8 ± 6.9 |
| TMwt/cyt + hypoxia | 4.8 ± 1.9 | 76 ± 162 | 1.9 ± 3.9 | |
| TMcyt/cyt | 280 ± 23 | 4.1 ± 2.6 | 30 ± 41 | 1.8 ± 1.2 |
| TMcyt/cyt + hypoxia | 270 ± 82 | 7.5 ± 2.5 | 150 ± 240 | 1.8 ± 2.6 |
| . | Lung TM (cpm/μg) . | Fibrin/ogen Staining (U/area) . | Fibrin (ng/4 mg) . | FPA (nmol/L) . |
|---|---|---|---|---|
| TMwt/wt | 340 ± 21 | 3.9 ± 2.7 | 57 ± 55 | 1.0 ± 0.1 |
| TMwt/wt + hypoxia | 290 ± 69 | 9.5 ± 6.2 | 408 ± 444 | 1.9 ± 1.8 |
| TMwt/cyt | 350 ± 10 | 8.5 ± 3.6 | 187 ± 242 | 5.8 ± 6.9 |
| TMwt/cyt + hypoxia | 4.8 ± 1.9 | 76 ± 162 | 1.9 ± 3.9 | |
| TMcyt/cyt | 280 ± 23 | 4.1 ± 2.6 | 30 ± 41 | 1.8 ± 1.2 |
| TMcyt/cyt + hypoxia | 270 ± 82 | 7.5 ± 2.5 | 150 ± 240 | 1.8 ± 2.6 |
Results reflect the means (n = 5) with associated standard deviations. For each measure, there was no significant difference between any group (P > .5).
Wound healing.
TM expression by suprabasal keratinocytes has been demonstrated to be upregulated both during epidermal differentiation and after injury, particularly at the migrating edge of a healing skin wound.39 However, the presence or absence of the cytoplasmic tail of TM had no effect on the rate of wound healing, the apparent quality of the wounds, or the histologic appearance of sections of the wounds (data not shown).
DISCUSSION
TM is known to be expressed by a wide variety of tissues, including several tumor cells, syncytiotrophoblasts, vascular endothelium, neutrophils, monocytes, synovial lining cells, platelets, smooth muscle cells, keratinocytes, and meningeal cells. Referred to as fetomodulin, TM was recognized as a marker protein of fetal development.2,3 The multidomain structure of this glycoprotein suggests that it may have several functions in addition to its role as a cofactor in activating protein C and TAFI. The observation that inactivation of the TM gene in mice leads to embryonic lethality without evidence of thrombosis,10warranted a search for alternative functions for this protein, as well as for the etiology of the intrauterine death of TM null embryos.
Recent in vitro studies have elucidated several of the structure-function correlates of TM. For example, EGF-like repeats 3 through 6 are critically involved in both the activation of protein C5 and TAFI.40,41 Attachment of the chondroitin sulfate moiety in the juxtamembranous serine-threonine region provides optimization of anticoagulant function.42 The NH2-terminal lectin-like domain may interact with other proteins and thereby mediate regulation of intracellular routing,19 whereas the cytoplasmic tail of TM is probably required for multimerization of the receptor and may contribute to clathrin-coated pit-mediated endocytosis.20 Finally, both the lectin-like and the cytoplasmic domains have been implicated in regulating a thrombin-independent antiproliferative effect.22
To directly evaluate the in vivo role of the cytoplasmic domain of TM, mice that are lacking this structure were generated by homologous recombination in ES cells. The targeting vector strategy was designed to maintain the integrity of the gene encoding TM while selectively deleting the DNA sequence encoding the cytoplasmic domain. TheCre-lox P system was used to excise the selection marker genes,neomycin phosphotransferase and thymidine kinase, from the 3′-untranslated region of the targeted gene, excluding the possibility of these affecting the phenotype of the resultant gene-targeted mice. Although a single loxP site was left within the 3′-UTR, this insertion did not appear to affect TM protein levels in the mutant mice, indicating that the effect of the mutant alleles was restricted to a structural defect and did not alter overall quantitative expression of TM.
TM has well-defined anticoagulant properties, mediating the activation of protein C, the latter which also possesses an anti-inflammatory role. In view of the link via TM between coagulation and inflammation, the response of mice expressing tail-less TM to both procoagulant and proinflammatory stimuli was evaluated. The effects of hypoxic injury to induce fibrin deposition in the lungs, and of lethal and sublethal injections of LPS, were identical irrespective of the integrity of the cytoplasmic domain of TM. These results support the biochemical and cell culture data indicating that the anticoagulant properties of TM reside within the EGF-like repeats and that regulation of expression of cell-surface functional TM does not depend on the cytoplasmic domain.
Preinfusion of the mice with bovine protein C before LPSexposure had no apparent anti-inflammatory effect. Although purified bovine protein C may be activated by human or bovine thrombin in the presence of murine TM as a cofactor, it is not known whether bovine activated protein C has anti-inflammatory activity in vivo in mice. Thus, these results should be cautiously interpreted until the experiments are repeated exclusively with murine-derived proteins.
Links between coagulation and fibrinolysis have been further documented by the observation that generation of the fibrinolysis inhibitor, TAFIa, by thrombin requires TM as a cofactor.43 It has long been recognized that keratinocytes can produce tissue type plasminogen activator (t-PA) and urokinase type plasminogen activator (u-PA) both in vitro and in vivo.44 Direct evidence for fibrinolysis playing a critical role in epithelialization was provided by studies in which the gene encoding plasminogen was inactivated in mice, resulting in profoundly impaired wound healing.45,46 TM also is believed to be involved in this process, but its specific role is unknown. TM expression by suprabasal keratinocytes is dramatically enhanced at the migrating edge of a healing wound. It was therefore reasonable to assume that TM may serve to regulate fibrin deposition, fibrin dissolution, and inflammation at the site of a wound, by virtue of its functions via protein C and TAFI. Raife et al47 have previously shown that overexpression of TM in transgenic mice does not affect wound healing, although collagen deposition was altered. Although not a quantitative study, we could detect no obvious difference in either the formation or healing of skin wounds in mice lacking the cytoplasmic domain of TM, suggesting that signals critical for normal wound healing do not appear to be mediated by this domain. To definitively identify the role of TM in keratinocytes and skin wound healing, cell-specific inactivation ofTM will be necessary. These studies are in progress.
In view of the activation of TAFI being dependent on TM, we quantitated several fibrinolytic parameters in the mutant mice. Those mice expressing tail-less TM had significantly lower PAP levels than their wild-type counterparts (P < .001). However, both plasminogen and α2-antiplasmin levels were similar in both wild-type and mutant mice, supporting the conclusion that plasmin generation was diminished in those mice with tail-less TM. We would not have expected, based on this observation, that the TMcyt/cyt mice would exhibit a predisposition to thrombosis, given the fact that inactivation of the genes for t-PA and u-PA in mice did not result in significant spontaneous fibrin deposition.48 Nonetheless, the possibility that the cytoplasmic domain of TM might modulate plasmin generation was intriguing in that new insights into fibrinolysis regulation may be provided. There are several potential explanations for the surprising decrease in PAP levels in the TMcyt/cyt mice. Plasmin generation is regulated by the interplay between plasminogen activators and inhibitors. Furthermore, thrombin has been shown in vitro to regulate the production of PAI-1 and t-PA in human umbilical vein endothelial cells (HUVEC), augmenting both, presumably via activation of protein kinase C.49 It has not been determined whether one of the thrombin receptors or TM mediated these effects. We therefore considered the possibility that the cytoplasmic domain of TM might mediate signals that regulate the release of functional t-PA, u-PA, or PAI-1, in which case suppression of plasminogen activators or increases in PAI-1 would be expected to decrease the generation of plasmin and thus explain the lower PAP levels in the TMcyt/cyt mice. We excluded the possibility that the cytoplasmic domain of TM mediates, either directly or indirectly, an increase in PAI-1 levels, but it remains to be established whether the tail of TM plays a role in regulating release of plasminogen activators. Detailed signaling, protein, and mRNA expression studies on cells isolated from the TMcyt/cyt mice will help to elucidate the role of the cytoplasmic domain of TM in the fibrinolytic pathway and the functional relationship(s) between thrombin receptors and TM.
PAP levels may also be diminished in the TMcyt/cyt mice as a result of TM-mediated activation of TAFI. Tail-less TM might be more effective than wild-type TM as a cofactor in activation of TAFI, resulting in reduced plasmin generation. Alternatively, the TMcyt/cyt mice may have increased shedding of TM, resulting in augmented activation of TAFI. However, the inclusion of two basic amino acids of the cytosolic tail of TM adjacent to the membrane was designed to minimize shedding of tail-less TM from the cell surface. TM antigen levels were indeed very similar in the lung, kidney, and brains of mice expressing wild-type and tail-less TM, and there was no evidence of increased antigenic TM in the plasma of the TMcyt/cyt mice. In contrast, functional levels of TM, measured by thrombin-dependent activation of protein C, were significantly elevated in the TMcyt/cyt mice. Subtle increases in TM shedded into the plasma might account for this increase. Alternatively, this apparent discrepancy suggests that the cytoplasmic domain of TM plays a role in regulating the pattern of proteolysis as it is released into plasma. Two monoclonal antibodies were used in the quantitative sandwich radioimmunoassay for TM, and because multiple proteolytic forms of TM are known to exist in plasma, all of the forms are not necessarily detected with equivalent sensitivity. Consequently, a relative increase in the TAFI-activatable forms of TM might not have been detected by the radioimmunoassay or discerned by Western immunoblot. In summary, increased plasma levels of functional TM probably contribute to suppression of plasma PAP levels via TAFI activation.
The most notable observation from these studies is that the cytoplasmic domain of TM is not required for normal fetal development. The pattern of expression of TM during development was unaltered by the absence of the cytoplasmic tail. Furthermore, postnatal growth and development up to 1 year of age was similar in mice expressing tail-less or full-length TM. In view of the suggestion by Zhang et al22that the cytoplasmic tail may modulate cell proliferation, mice expressing tail-less TM were examined for spontaneous development of tumors, none of which were evident during the period of observation up to 1 year. However, this does not necessarily exclude a role for the cytoplasmic domain in tumor/cell proliferation. Genetic crosses with mice predisposed to developing tumors are being set up to more directly evaluate the function of the cytoplasmic domain of TM in this process. Finally, to evaluate the role of other structural domains of TM during embryonic development, further in vivo gene targeting studies are underway.
Supported in part by the Heart and Stroke Foundation of Ontario. E.M.C. and J.I.W. are Career Investigators of the Heart and Stroke Foundation of Canada.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Edward M. Conway, MD, Center for Transgene Technology and Gene Therapy, KU Leuven, Gasthuisberg O&N, 9th Floor, Herestraat 49, B-3000 Leuven, Belgium; e-mail:ed.conway@med.kuleuven.ac.be.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal