Paroxysmal nocturnal hemoglobinuria (PNH), frequently occurring during suppressed hematopoiesis including aplastic anemia (AA), is a clonal disorder associated with an increased incidence of thrombotic events. Complement-mediated hemolysis, impairment of the fibrinolytic system, or platelet activation are thought to be responsible for the associated thrombotic risk. We investigated here the elevation of membrane-derived procoagulant microparticles in the blood flow of such patients. Elevated levels of circulating microparticles were in fact detected in both de novo PNH patients and AA subjects with a PNH clone, but not in those with AA without a PNH clone. The cellular origin of the microparticles was determined in PNH samples; most stemmed from platelets. Glycophorin A+ particles were rarely detected. Therefore, platelet activation, resulting in the dissemination of procoagulant phospholipids in the blood flow, could be one of the main causes for the elevated thrombotic risk associated with PNH. These observations suggest that shed membrane particles can be considered a valuable biological parameter for the assessment of possible thrombotic complications in patients with PNH.
PAROXYSMAL nocturnal hemoglobinuria (PNH) is an acquired clonal disorder characterized by the presence of abnormal hematopoietic cells deficient in glycosylphosphatidylinositol (GPI)-anchored proteins.1The association with suppressed hematopoiesis, including aplastic anemia (AA), occurs frequently.2 The main clinical manifestation is hemolytic anemia and the most common complications are thrombosis, pancytopenia, and myelodysplastic syndrome or acute leukemia.3 Although intravascular hemolysis can easily be explained by a deficiency of GPI-anchored complement regulatory proteins such as CD59 and CD55 on the membrane of red blood cells (RBC),4 the mechanism responsible for the increased incidence of thrombotic events in PNH remains unclear. Apart from hemolysis itself, several failures of the fibrinolytic system have been highlighted, including a deficiency of urokinase-type plasminogen activator receptor on leukocytes presenting the PNH phenotype5 and increased plasma levels of soluble urokinase-type plasminogen activator receptor.6Furthermore, one patient with thrombotic complications in the course of AA and/or PNH syndrome was shown to have dysplasminogenemia.7 Other studies did not identify any fibrinolytic defects but rather pointed to varying degrees of platelet activation in PNH individuals.8 Beyond the increase of expression of activation-dependent proteins, platelet stimulation is accompanied by the loss of membrane phospholipid asymmetry. This results in phosphatidylserine externalization and microvesicle generation, as shown in an in vitro study.9 Furthermore, transverse redistribution of plasma membrane phosphatidylserine and cell fragmentation into phosphatidylserine-bearing microparticles is a hallmark of cells undergoing apoptosis,10,11 and phosphatidylserine becomes a determinant for phagocyte recognition of senescent or apoptotic cells to be cleared.12,13 AA is precisely a clinical situation in which programmed cell death is thought to occur to a high degree.14
Membrane-derived microparticles have been shown to provide the catalytic surface necessary for the assembly of the procoagulant enzyme complexes, prothrombinase15 and tenase.16 In the blood flow, the presence of high levels of procoagulant microparticles, stemming from lysed RBC, apoptotic cells, or activated platelets, could therefore be responsible for the dissemination of prothrombotic seats. This prompted us to assess the increase of circulating microparticles in the peripheral blood of AA and PNH patients using insolubilized annexin V (AV), a protein showing a strong affinity for phosphatidylserine, through which capture was feasible.11 Such circulating particles carry membrane antigens specific for the cells they stem from and through which capture was also achieved to determine their cellular origin.
PATIENTS AND METHODS
Patients.
Details of the patient clinical courses, treatment, and evolution have been previously reported in detail.2,17 In brief, patients were considered to have PNH if the Ham-Dacie’s test was positive at diagnosis. Patients with a previous history of AA who later developed a positive Ham-Dacie’s test and/or had evidence of defective expression of GPI-linked proteins by flow cytometry were considered to have an AA/PNH syndrome. Of the 29 patients with either PNH (n = 12) or an AA/PNH syndrome, 2 patients (P1 and P4) had a history of thrombotic complications. Patient P1 had PNH and developed a Budd-Chiari syndrome.17 Patient P4, with an AA/PNH syndrome, had a positive Ham-Dacie’s test 4 years after diagnosis and evidence of a deficiency in GPI-anchored proteins. Almost 10 years after being treated by immunosuppressive therapy, she had thrombosis of the lower limb.
Materials.
The monoclonal antibody (MoAb) against glycophorin A was from Immunotech S.A. (Marseilles, France). The MoAbs to human platelet glycoprotein Ibα (GPIbα) and glycoprotein IIIa (GPIIIa) were kind gifts from Dr F. Lanza (Unité 311 INSERM, Strasbourg, France). The irrelevant biotinylated Ig (IgG1Bi) was from Leinco Technologies (Ballwin, MO). Purified human blood coagulation factors were the same as those used in a recent study reported by our group.18 Factor V was a product from Diagnostica Stago (Asnières, France). Recombinant human annexin V was purchased from Euromedex (Souffelweyersheim, France) and conjugated with fluorescein isothiocyanate (FITC; annexin VFITC) following the procedure described by Dachary-Prigent et al.19 High binding capacity streptavidin-coated microtitration plates, 1-O-n-octyl-β-D-glucopyranoside, biotin-X-OSu, and Chromozym TH were from Boehringer Mannheim (Mannheim, Germany). Human serum albumin (HSA), the streptavidin-R-phycoerythrin conjugate, phosphatidylcholine, and phosphatidylserine from bovine brain were products from Sigma Chemical Co (St Louis, MO). Calcium ionophore A23187, D-phenylalanyl-prolyl-arginyl chloromethyl ketone (FPR.CK), and 1,5-dansyl-glutamyl-glycyl-arginyl chloromethyl ketone (Dns-EGR.CK) were obtained from Calbiochem (San Diego, CA). All other reagents were of the highest available purity grade.
Methods.
The preparation of platelet-free plasma samples, the biotinylation of annexin V and MoAbs, the capture of microparticles by immobilized annexin V or MoAbs, and the prothrombinase assay for the estimation of the amount of captured microparticles are detailed in Aupeix et al.11 It has to be mentioned that different incubation times were used for microparticle capture by annexin V (30 minutes) and MoAbs (2 hours). However, to exclude that complement attack of cells might occur during the blood drawing procedure and the separation of plasma from cells by centrifugation, some PNH and control blood samples were drawn into both EDTA and citrated anticoagulants. The two successive centrifugation steps, requiring, respectively, 10 minutes and 1 minute, were performed immediately, and plasma separated from cells was processed for the determination of its microparticle content.11 No difference was noticed between EDTA, commonly used for complement assays, and citrated anticoagulant, normally used for routine hemostasis assays, allowing us to perform the study with citrated samples that were also used for the hemostasis follow-up of the patients.
Flow cytometry.
RBC were analyzed by flow cytometry using a FACScan flow cytometer (Becton Dickinson, San Jose, CA). The sheath fluid was Isoton II balanced electrolyte solution (Coulter, Krefeld, Germany). Data acquisition and analysis were conducted with the CellQuest software (Becton Dickinson, San Jose, CA). Analysis of the ability of RBC to undergo membrane vesiculation after stimulation by ionophore was performed on 10,000 events per sample. Annexin VFITC was used as a probe of phosphatidylserine exposure simultaneously for RBC and derived microparticles.18 Glycophorin A labeling was performed using the biotinylated antibody and the streptavidin-R-phycoerythrin conjugate.
Functional detection of procoagulant phospholipid exposure in stimulated cells and derived microparticles.
Procoagulant phospholipid exposure in stimulated RBC and derived microparticles was investigated using a human prothrombinase assay in which phosphatidylserine promotes the activation of prothrombin by factor Xa in the presence of factor Va.20 Thrombin generated by functional prothrombinase complex was measured using a chromogenic assay as already described elsewhere.18 The ability of RBC to expose phosphatidylserine and to release procoagulant microparticles was examined after stimulation by 5 μmol/L calcium ionophore A23187 for 90 minutes at 37°C in the presence of 2 mmol/L external CaCl2. RBC were separated from derived microparticles by centrifugation at 12,000g for 30 seconds before measurement. In each case, results of PNH samples were compared with the prothrombinase activities developed in counterparts from healthy volunteers.
Statistical analysis.
Data are represented as the mean ± standard deviation (SD). Statistical analysis was performed using the Student’s two-tailedt-test or a variance comparison (according to the ratio method).
RESULTS
Capture and antigenic characterization of circulating particles in blood samples from AA and PNH patients.
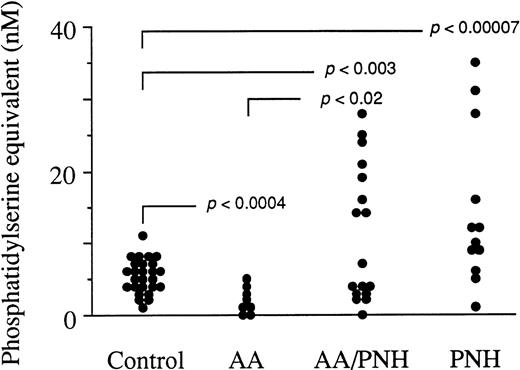
To assess one of the possible origins of thrombosis that frequently occurs as a complication of PNH, we measured the levels of circulating particles in peripheral blood of PNH individuals and control subjects (Fig 1). AA blood samples were also assayed to explore the possible biological link between this disorder and PNH. High to very high levels of circulating particles were indeed detected in some PNH samples when compared with the control group (P < .00007). The mean ± SD for the control group was 5.3 ± 2.2 nmol/L phosphatidylserine equivalent, that of the aplastic anemia group without a PNH clone (AA) was 1.9 ± 1.6 nmol/L, that of the aplastic anemia with a PNH clone group (AA/PNH) was 11.1 ± 9.1 nmol/L, and that of the PNH group 14.4 ± 10.5 nmol/L phosphatidylserine equivalent. Interestingly, a clear difference was established between the two AA groups (P < .02). Some AA/PNH samples contained high microparticle levels, comparable with those of the PNH group, whereas AA samples were measured at lower values than the control group (P < .0004).
Amount of circulating microparticles in peripheral blood samples from 26 control subjects, 8 AA individuals without a PNH clone (AA), 17 AA individuals with a PNH clone (AA/PNH), and 12 PNH patients. The particle capture procedure involving insolubilized AV as well as the assay of their phosphatidylserine content based on the ability of this phospholipid to promote the assembly of the clotting prothrombinase enzyme complex are detailed in Aupeix et al.11 The mean ± SD for the control group is 5.3 ± 2.2 nmol/L phosphatidylserine equivalent, that of the AA group is 1.9 ± 1.6 nmol/L, that of the AA/PNH group is 11.1 ± 9.1 nmol/L, and that of the PNH group is 14.4 ± 10.5 nmol/L phosphatidylserine equivalent. P values reflect the significance of the patients’ circulating particle levels compared with healthy controls or patients from other indicated groups. Statistical analysis was performed using the Student’s two-tailed t-test.
Amount of circulating microparticles in peripheral blood samples from 26 control subjects, 8 AA individuals without a PNH clone (AA), 17 AA individuals with a PNH clone (AA/PNH), and 12 PNH patients. The particle capture procedure involving insolubilized AV as well as the assay of their phosphatidylserine content based on the ability of this phospholipid to promote the assembly of the clotting prothrombinase enzyme complex are detailed in Aupeix et al.11 The mean ± SD for the control group is 5.3 ± 2.2 nmol/L phosphatidylserine equivalent, that of the AA group is 1.9 ± 1.6 nmol/L, that of the AA/PNH group is 11.1 ± 9.1 nmol/L, and that of the PNH group is 14.4 ± 10.5 nmol/L phosphatidylserine equivalent. P values reflect the significance of the patients’ circulating particle levels compared with healthy controls or patients from other indicated groups. Statistical analysis was performed using the Student’s two-tailed t-test.
Therefore, we searched for a possible link between the level of the PNH clone expression and the proportion of circulating particles. This was performed taking into account the proportions of GPI-deficient polynuclear cells, monocytes, platelets, or erythrocytes. Correlation coefficients never exceeded 0.7. No clear correlation could therefore be evidenced.
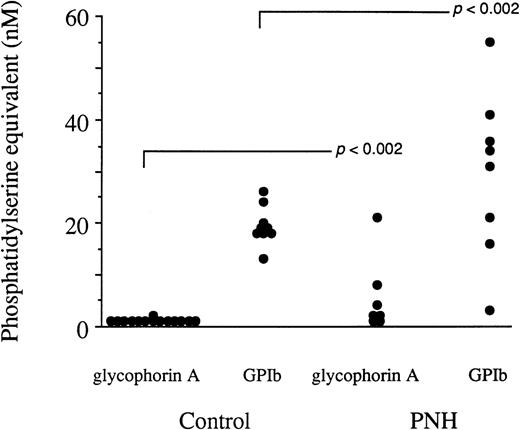
The cellular origin of these circulating particles was determined. No significant amount of glycophorin A+ particles was captured in most PNH samples (individuals having received recent blood transfusions were, of course, excluded from the study), although PNH RBC are believed to be one of the targets of the complement membrane attack complex leading to hemolysis (Fig2). However, the presence of glycophorin A at the surface of PNH RBC was verified by flow cytometry analysis. The expression of glycophorin A in PNH RBC was not impaired when compared with that of normal erythrocytes, without or after stimulation by calcium ionophore (data not shown). In contrast, very high levels of platelet-derived particles bearing the GPIbα-specific marker were captured in several PNH samples. The antibody directed to GPIIIa yielded basically identical results (data not shown). The means of the amount of GPIbα+ particles were not significantly different between PNH and control group at the .05 level, but the variances comparison (using the ratio method) showed a clear difference in the distribution of the values at the .002 level. This is consistent with a situation in which some patients are in the range of the control group, whereas others present very high levels of circulating GPIbα+particles. It has to be emphasized that no direct comparison between capture by annexin V and antibodies could be performed because preincubation times and affinities for the respective ligands are different.11
The cellular origin of circulating microparticles in peripheral blood samples from PNH individuals and control subjects. The capture procedure involved insolubilized MoAbs to human glycophorin A, human GPIb, and irrelevant IgG1, the latter yielding control values that never exceeded 3 nmol/L phosphatidylserine equivalent and were subtracted from those reported in this figure. The assay of the phosphatidylserine content of captured particles is based on the ability of this phospholipid to promote the assembly of the prothrombinase enzyme complex. Each value is the mean of triplicate determinations. The means corresponding to the PNH samples were not significantly different from their control counterparts at the .05 level using the Student’s two-tailed t-test. Variances were also compared (using the ratio method) and the distribution of the values was shown to be different between the PNH and the control samples at the .002 level.
The cellular origin of circulating microparticles in peripheral blood samples from PNH individuals and control subjects. The capture procedure involved insolubilized MoAbs to human glycophorin A, human GPIb, and irrelevant IgG1, the latter yielding control values that never exceeded 3 nmol/L phosphatidylserine equivalent and were subtracted from those reported in this figure. The assay of the phosphatidylserine content of captured particles is based on the ability of this phospholipid to promote the assembly of the prothrombinase enzyme complex. Each value is the mean of triplicate determinations. The means corresponding to the PNH samples were not significantly different from their control counterparts at the .05 level using the Student’s two-tailed t-test. Variances were also compared (using the ratio method) and the distribution of the values was shown to be different between the PNH and the control samples at the .002 level.
Assessment of phosphatidylserine exposure in individual PNH samples.
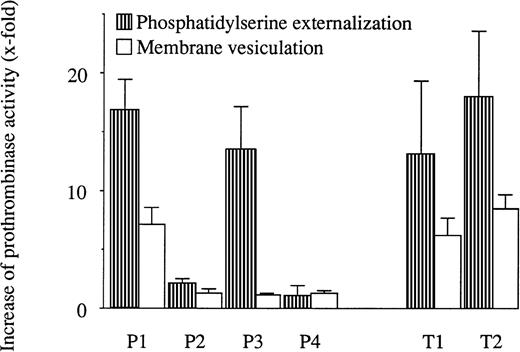
The ability of PNH RBC to externalize phosphatidylserine and to vesiculate was examined in a functional prothrombinase assay with 4 PNH samples: P1, P2, P3 (already used for the control of glycophorin A expression), and P4. PNH RBC showed impaired procoagulant phospholipid externalization in 2 cases (P2 and P4) among the 4 samples tested (Fig 3). Vesiculation, assessed in the supernatant of stimulated RBC, was almost undetectable in 3 (P2, P3, and P4) among the 4 PNH RBC samples. Finally, in 1 PNH RBC sample (P1), procoagulant phospholipid externalization and membrane vesiculation appeared normal. It is of interest to notice that the absence of activability of P2 RBC is probably related to its high basal stimulation state (see legend of Fig 3). This was not the case for P4 and P3 samples.
Procoagulant phospholipid externalization and membrane vesiculation in calcium ionophore-stimulated RBC from 4 PNH patients (P) and 2 healthy subjects (T) measured by prothrombinase assay. Cells were stimulated by 5 μmol/L calcium ionophore A23187 in the presence of 2 mmol/L external CaCl2 for 90 minutes at 37°C. Stimulated cells were centrifuged for 30 seconds at 12,000g. Microparticle release was measured in the supernatant, whereas phosphatidylserine externalization was measured on the pelleted cells. Data (n = 3) represent the increase of prothrombinase activity after stimulation and are expressed as the ratio between the activity before and after ionophore stimulation. The basal activity of the RBC before stimulation was 2.2, 4.5, 0.6, 0.2, 1.0, and 0.3 nmol/L phosphatidylserine equivalent for P1, P2, P3, P4, T1, and T2, respectively. In the supernatant, corresponding basal activities were measured at 1.2, 1.3, 0.4, 0.2, 0.4, and 0.4 nmol/L phosphatidylserine equivalent.
Procoagulant phospholipid externalization and membrane vesiculation in calcium ionophore-stimulated RBC from 4 PNH patients (P) and 2 healthy subjects (T) measured by prothrombinase assay. Cells were stimulated by 5 μmol/L calcium ionophore A23187 in the presence of 2 mmol/L external CaCl2 for 90 minutes at 37°C. Stimulated cells were centrifuged for 30 seconds at 12,000g. Microparticle release was measured in the supernatant, whereas phosphatidylserine externalization was measured on the pelleted cells. Data (n = 3) represent the increase of prothrombinase activity after stimulation and are expressed as the ratio between the activity before and after ionophore stimulation. The basal activity of the RBC before stimulation was 2.2, 4.5, 0.6, 0.2, 1.0, and 0.3 nmol/L phosphatidylserine equivalent for P1, P2, P3, P4, T1, and T2, respectively. In the supernatant, corresponding basal activities were measured at 1.2, 1.3, 0.4, 0.2, 0.4, and 0.4 nmol/L phosphatidylserine equivalent.
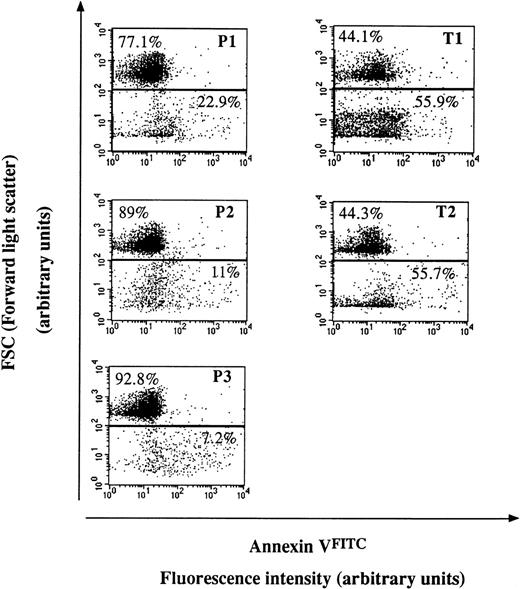
To confirm the prothrombinase results, procoagulant phospholipids were probed with AVFITC on stimulated PNH and control RBC and on derived microparticles from the same individuals (Fig 4). The flow cytometry analysis showed lower to moderate levels of procoagulant microparticles in the supernatant of ionophore-stimulated PNH RBC compared with control RBC.
Flow cytometry analysis of the shedding of membrane microparticles from PNH (P) and control (T) RBC after calcium ionophore treatment. Dot plot representations of AVFITC-labeled cell and particle suspensions. Cells have the highest forward scatter signal (FSC), whereas derived microparticles have a lower one. The proportion of events in each gate is indicated. Stimulation was achieved by 5 μmol/L calcium ionophore A23187 in the presence of 2 mmol/L external CaCl2 for 90 minutes at 37°C. Fluorescence intensity reflects the extent of AVFITC labeling of the population of interest, testifying to the degree of phosphatidylserine externalization. Each dot plot corresponds to 10,000 events and is representative of three experiments performed likewise.
Flow cytometry analysis of the shedding of membrane microparticles from PNH (P) and control (T) RBC after calcium ionophore treatment. Dot plot representations of AVFITC-labeled cell and particle suspensions. Cells have the highest forward scatter signal (FSC), whereas derived microparticles have a lower one. The proportion of events in each gate is indicated. Stimulation was achieved by 5 μmol/L calcium ionophore A23187 in the presence of 2 mmol/L external CaCl2 for 90 minutes at 37°C. Fluorescence intensity reflects the extent of AVFITC labeling of the population of interest, testifying to the degree of phosphatidylserine externalization. Each dot plot corresponds to 10,000 events and is representative of three experiments performed likewise.
Finally, 3 PNH RBC samples of 4 (P2, P3, and P4) showed an impaired vesiculation in a functional prothrombinase assay (Fig 3) and 3 PNH RBC samples of 3 (P1, P2, and P3) were unable to shed normal levels of particles as deduced from flow cytometry analysis (Fig 4). P1 sample led to apparent contradictory results. The proportion of shed membrane microparticles after stimulation was lower than in controls when measured by flow cytometry, but the functional prothrombinase assay did not show any vesiculation impairment.
DISCUSSION
The present study clearly demonstrates the presence of elevated levels of phosphatidylserine-bearing microparticles in the peripheral circulation of several PNH patients or subjects with AA with a PNH clone. The procoagulant potential disseminated by these particles in the blood flow could be responsible, at least in part, for the high incidence of associated thrombotic complications. The basal level of circulating particles detected in control subjects probably reflects a balance between cell proliferation, stimulation, and death and concerns microparticles that transiently escape destruction by phagocytosis,13 phospholipases,21 or confinement by specific adhesion.22 In pathological situations in which apoptosis or cell stimulation is known to occur at a high degree, the elimination systems could be saturated, giving rise to increased levels of circulating shed microparticles. Interestingly, samples from AA patients who did not develop a PNH clone were measured under the control level. Therefore, the susceptibility of RBC and platelets lacking GPI-linked complement inhibitors of the membrane attack complex may account for the high levels of shed microparticles. But again, the proportion of circulating microparticles is probably dependent on the relative efficiency of clearance systems. Hence, individual variability of the elimination response may account for the lack of clear correlation between the PNH clone level and the particle proportion.
Very low amounts of particles bearing the RBC marker glycophorin A were captured in PNH plasmas, whereas hemolysis often occurs during the course of PNH. The normal expression of glycophorin A on PNH RBC and the specificity of the corresponding antibody were controlled because of the qualitative abnormality of glycophorin A reported by Parker et al.23 The circulating microparticles did actually not originate from lysed RBC to a significant extent. On the other hand, very high levels of particles of platelet origin were detected in several PNH samples. Hence, platelet activation, already reported in PNH,8,9 17 could be one of the main causes of the high incidence of thrombosis associated with PNH. It has to be emphasized that platelet-derived microparticles were easily detectable in control samples and probably account for an important part of the basal particle level.
The absence of RBC-derived particles in PNH samples led us to investigate the ability of RBC to externalize phosphatidylserine and to vesiculate. An impaired ability of RBC to vesiculate has already been reported by Whitlow et al24 for 2 PNH patients lacking CD59 and CD55. We also observed a very weak ability of some PNH RBC to vesiculate using a functional prothrombinase assay, and we assume that it could explain the absence of circulating RBC-derived microparticles. The heterogeneity of the responses of the RBC samples to ionophore is probably linked to the random selection of the patients. The different stages of evolution of the disease and current treatments might, at least in part, explain such an heterogeneity. The impaired ability to vesiculate might also be related with the resistance to apoptosis observed in PNH granulocytes.25
The use of the prothrombinase assay concomitantly with flow cytometry showed apparent contradictory results with the RBC sample P1. Vesiculation was lower than in control samples when measured by flow cytometry, but normal in a functional prothrombinase assay. This observation points to the fact that the two analyses do not measure the same parameters. Flow cytometry enables us to estimate the proportion of particles, whereas the prothrombinase assay detects their procoagulant potential. Therefore, the P1 RBC actually show an impaired ability to vesiculate, but the microparticles shed in the supernatant might bear an increased procoagulant potential. Interestingly, patient P1 recently experienced several thrombotic events. On the other hand, P4 RBC showed impaired ability to externalize phosphatidylserine and to vesiculate by prothrombinase assay, but patient P4 also developed thrombosis.
Whitlow et al24 also evidenced an impaired ex vivo vesiculation of platelets completely lacking GPI-linked proteins, whereas Wiedmer et al9 were able to induce complement-mediated platelet membrane vesiculation in platelet samples lacking CD59 antigen. Here, we have detected very high levels of in vivo circulating platelet-derived microparticles in several PNH blood samples. In our case, platelet vesiculation was not directly investigated using isolated platelets. It can be reasonably assumed that our assay measures a balance between the shedding of particles in the blood flow and elimination by the various clearance systems. Therefore, it is conceivable that significant levels of particles of platelet origin are consistently released in the blood flow, although higher or equivalent amounts of particles of RBC origin are shed, but, for unknown reasons, the latter might be more efficiently eliminated from the circulation. The description by Simons and Ikonen26 of functional sphingolipid-cholesterol rafts in cell membranes might explain such differences, especially in a context of absence of GPI-anchored proteins. CD55 and CD59 have been precisely shown to be sorted and shed in exosomes during reticulocyte maturation.27
Circulating particles, giving rise to disseminated potential prothrombotic seats, are certainly not neutral with respect to the response of the coagulation system of an individual. In several PNH patients investigated, these particles seem to originate massively from platelets and their amount could be responsible in part for thrombotic events. Moreover, platelet microparticles released after complement activation were shown to be enriched in the membrane receptor for coagulation factor Va,15 the latter involving phosphatidylserine.28
On a therapeutic point of view, the measurement of the proportion of circulating microparticles can be of interest with regard to the choice of the anticoagulant treatment. The balance between hemorrhage and thrombosis is believed to be subtle in the PNH pathology. It is probably related to the frequent association of pancytopenia with severe cell activation. Therefore, if high levels of circulating microparticles increase the thrombotic tendency, anti-vitamin K treatment should be the more appropriate preventive approach. Moreover, PNH patients with pancytopenia and high levels of circulating microparticles should not be at risk of bleeding episodes, whereas PNH patients with pancytopenia but low levels of circulating microparticles might probably be. Elevated levels of circulating platelet microparticles were precisely found protective against bleeding in patients with autoimmune thrombocytopenia but were associated with the occurrence of small cerebral vessel infarcts when very high.29 Helpful indications can be deduced from the new biological parameter consisting of the level of circulating procoagulant microparticles. This system may enable us to assess an instant in vivo thrombotic risk associated with PNH owing to the possibility of massive release of procoagulant microparticles shed from activated platelets in the blood flow. However, it would be interesting to investigate both circulating microparticles and other coagulation parameters in more homogeneous groups of patients who previously developed thrombosis to evaluate the multifactorial character of the mechanisms of thrombosis in the PNH pathology. But, the relative rare occurrence of this disorder is certainly a limit for such studies.
Supported by Grants from the Institut National de la Santé et de la Recherche Médicale, the Université Louis Pasteur de Strasbourg, and the Fondation pour la Recherche Médicale.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Marie-Lorraine Scrobohaci, MD, Laboratoire Central d’Hématologie, Hôpital Saint-Louis, 1, avenue Claude Vellefaux, 75475 Paris Cedex 10, France.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal