Homozygous type I plasminogen deficiency has been identified as a cause of ligneous conjunctivitis. In this study, 5 additional patients with ligneous conjunctivitis are examined. Three unrelated patients (1 boy, 1 elderly woman, and 1 man) had plasminogen antigen levels of less than 0.4, less than 0.4, and 2.4 mg/dL, respectively, but had plasminogen functional residual activity of 17%, 18%, and 17%, respectively. These subjects were compound-heterozygotes for different missense mutations in the plasminogen gene: Lys19 → Glu/Arg513 → His, Lys19 → Glu/Arg216 → His, and Lys19 → Glu/Leu128 → Pro, respectively. The other 2 patients, a 14-year-old boy and his 19-year-old sister, who both presented with a severe course of the disease, exhibited plasminogen antigen and functional activity levels below the detection limit (<0.4 mg/dL and <5%, respectively). These subjects were compound-heterozygotes for a deletion mutation (del Lys212) and a splice site mutation in intron Q (Ex17 + 1del-g) in the plasminogen gene. These findings show that certain compound-heterozygous mutations in the plasminogen gene may be associated with ligneous conjunctivitis. Our findings also suggest that the severity of clinical symptoms of ligneous conjunctivitis and its associated complications may depend on the amount of plasminogen functional residual activity.
LIGNEOUS CONJUNCTIVITIS is an uncommon form of chronic conjunctivitis that may initially present with unspecific symptoms such as tearing and conjunctival hyperemia. At the beginning, pseudomembranes develop on the palpebral surfaces that may later form thick nodular masses with subsequent loss of the normal mucosa. In 1847, the first report of ligneous conjunctivitis describes a 46-year-old man with bilateral pseudomembraneous conjunctivitis.1 Because the pseudomembranes may have a wood-like consistency, the disease was later termed “ligneous” conjunctivitis.2 In addition to the ocular lesions, many patients show similar pseudomembranes in the mucosa of the mouth, the tongue, the nasopharynx, the tracheobronchial tree, and the female genital tract.3-8 Histological examination of pseudomembranes from affected eyes or mucosal tissue from the genital tract exhibits a disrupted epithelium replaced by a massive deposition of fibrin and amorphous hyalin-like eosinophilic material, accompanied by an inflammatory cellular infiltration.6,9-14 Ligneous conjunctivitis has recently been linked to severe type I plasminogen deficiency by Mingers et al.8,15 In 3 patients with ligneous conjunctivitis distinct homozygous mutations in the plasminogen gene were identified.16,17 Similar fibrin-rich conjunctival lesions that are indistinguishable from human ligneous conjunctivitis were also described in mice with homozygously disrupted plasminogen genes (Plg−/− mice) or in mice homozygously deficient for both the urokinase-type plasminogen activator (u-PA) and the tissue-type plasminogen activator (t-PA) genes (u-PA−/−/t-PA−/−mice).18 Ligneous conjunctivitis has been also reported after use of the antifibrinolytic agent tranexamic acid during treatment of menorrhagia.19 Tranexamic acid blocks the lysine binding sites on the kringle domains of plasminogen and leads to dissociation of the fibrin(ogen)-plasminogen complex. These findings point to the central role of functional plasmin(ogen) deficiency in the pathogenesis of ligneous conjunctivitis.
We report here distinct compound-heterozygous mutations in the plasminogen gene in 5 patients with ligneous conjunctivitis.
MATERIALS AND METHODS
Patients
Patient no. 1.
This 5-year-old boy is the first child of healthy nonconsanguinous German parents. Episodes of venous thrombosis were not observed in any member of the family.
At 13 months of age, he developed chronic bilateral tarsal ligneous conjunctivitis (Table 1) after an infection with streptococci group A. Repeated surgical excision of conjunctival pseudomembranes and treatment with topical antibiotics and chymotrypsin resulted in complete recovery of the left eye, but only transient and short-term improvement of the right eye. Histological examination of the pseudomembrane exhibited massive exsudation of fibrin with an inflammatory cellular infiltration (mainly CD4+ T cells and CD19+ B cells), a disrupted and infolded epithelium, granulation tissue, and an amorphous, eosinophilic, hyaline material.
Clinical Findings in Five Patients With Ligneous Conjunctivitis
| . | Patient No. 1 . | Patient No. 2 . | Patient No. 3 . | Patient No. 4 (brother of patient no. 3) . | Patient No. 5 . |
|---|---|---|---|---|---|
| Current age | 5 yrs | 71 yrs | 17 yrs | 14 yrs | 23 yrs |
| Sex | Male | Female | Female | Male | Male |
| Affected tissues/organs | Conjunctivae | Conjunctivae | Conjunctivae Gingiva Vocal cords | Conjunctivae Gingiva Pharynx | Conjunctivae |
| Larynx | |||||
| Tracheobronchial tree | Upper gastrointestinal tract | ||||
| Kidney | Kidney | ||||
| Age of first manifestation | 13 mos | 69 yrs | 3 wks | 9 mos | 9 mos |
| Thrombosis | None | None | None | None | None |
| . | Patient No. 1 . | Patient No. 2 . | Patient No. 3 . | Patient No. 4 (brother of patient no. 3) . | Patient No. 5 . |
|---|---|---|---|---|---|
| Current age | 5 yrs | 71 yrs | 17 yrs | 14 yrs | 23 yrs |
| Sex | Male | Female | Female | Male | Male |
| Affected tissues/organs | Conjunctivae | Conjunctivae | Conjunctivae Gingiva Vocal cords | Conjunctivae Gingiva Pharynx | Conjunctivae |
| Larynx | |||||
| Tracheobronchial tree | Upper gastrointestinal tract | ||||
| Kidney | Kidney | ||||
| Age of first manifestation | 13 mos | 69 yrs | 3 wks | 9 mos | 9 mos |
| Thrombosis | None | None | None | None | None |
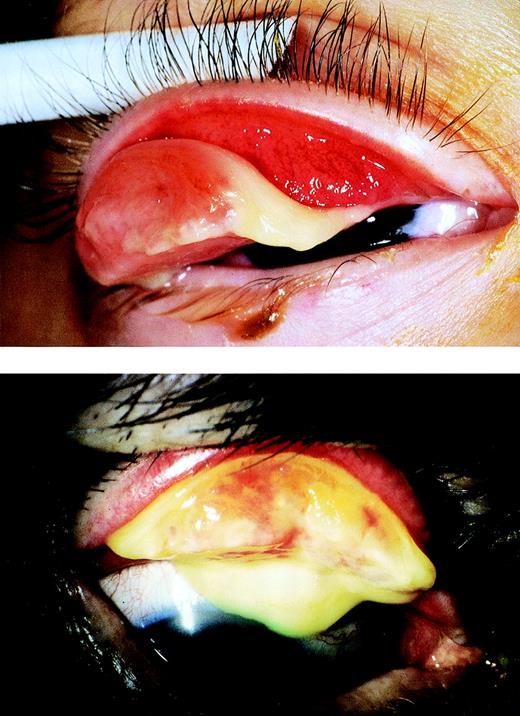
At 3.5 years of age, a large pedunculated fibrin-rich pseudomembranous lesion of the right upper tarsal conjunctiva (Fig 1A) developed that was completely excised, followed by intensive topical administration of steroids and long-term topical heparin (5,000 U/mL) according to the recommendations of De Cock et al.11 Within 6 months, the conjunctival pseudomembrane had almost completely disappeared and has not recurred.
(A) Ligneous conjunctivitis in patient no. 1. (B) Ligneous conjunctivitis in patient no. 2.
(A) Ligneous conjunctivitis in patient no. 1. (B) Ligneous conjunctivitis in patient no. 2.
Patient no. 2.
This previously healthy 71-year-old woman first developed unilateral ligneous conjunctivitis at the age of 69 years (Fig 1B and Table 1). At that time, a pseudomembrane developed over the palpebral conjunctiva of the right upper eye lid. It recurred rapidly after surgical removal. Treatment with topical heparin eye drops (1,000 to 5,000 U/mL), topical cyclosporine A (2%), and corticosteroids did not prevent recurrences of the pseudomembranes and surgical excision was performed three times. During this time period, visual acuity decreased from 20/30 to 20/70. Other mucous membranes were unaffected. Histopathology of excised pseudomembrane showed a disrupted conjunctival epithelium and massive fibrinous tissue with infiltration of inflammatory cells, mainly lymphocytes, granulocytes, and some macrophages. The family history is unremarkable, without eye disease or venous thrombosis.
Patients no. 3 and 4.
This 19-year-old white female (patient no. 3) first developed conjunctivitis at 3 weeks of age (Table 1) and was treated with multiple eye drops, including corticosteroids. At 3 years of age, she developed bilateral conjunctival pseudomembranes that were surgically removed; a diagnosis of ligneous conjunctivitis was made. These membranes have recurred repeatedly, necessitating surgical removal on 18 different occasions, the last removal occurring 2 years ago. However, the rate of conjunctival membrane formation has decreased in recent years. At 5 years of age, she developed hoarseness and was noted to have a ligneous membrane on the vocal cords. She also showed asthma-like symptoms. She continued to have intermittent lung problems and at 8 years of age she developed a pneumomediastinum. At 8 years of age, she had her first of 20 bronchoscopies to remove thickened membranes from her laryngo-tracheobronchial tree. At 16 years of age, she developed an abscess of the left lung necessitating bronchoscopic drainage of this area. She also had had gingival membranes and nodular, calcified masses in the renal collecting system, demonstrable by ultrasound and pyelography. Treatment with multiple eye drops, corticosteroids, local heparin, and multiple courses of antibiotics have been ineffective.
Her 14-year-old brother (patient no. 4) developed conjunctivitis at 9 months of age that became severe at 4 years of age. At 5 years of age, he had surgical removal of the ligneous conjunctival membranes. These have recurred frequently necessitating surgery on 15 occasions, the last time occurring 1 year ago. There is some scarring of the upper lids with symmetrical ptosis. Since 5 years of age, he has had membranes on the gums associated with intermittent bleeding, geographic tongue, and sinusitis. He also had membrane formation in the pharynx and in the renal collecting system. Furthermore, duodenal ulceration and an eosinophilic gastric infiltration were observed. He has been treated in the same way as his sister and, in addition, has received intravenous Igs and thalidomide without dramatic effects. Papers concerning the clinical course of patients no. 3 and 4 and the bronchial lesions of patient no. 3 have already been published.3 7
Patient no. 5.
This currently 23-year-old man developed unilateral ligneous conjunctivitus on the right upper eyelid at 9 months of age (Table 1). After surgical excision, pseudomembranes recurred within 8 months. During the first 10 years of life the patient required local excision of recurrent pseudomembranes combined with cryotherapy on 12 occasions. At 12 years of age, he gradually developed bilateral limbal dots and itching suggestive of bilateral vernal conjunctivitis. At this time, the disease could be adequately controlled by a combination of topical steroids, antihistamines, and levocabastine. After a few years, limbal disease activity gradually decreased and treatment was discontinued. There has been no formation of extraocular pseudomembranes during the entire course of disease and there have been no signs of active conjunctival disease for the past 5 years.
Coagulation Studies
Quantitative determination of plasminogen antigen in citrated plasma of patients was performed by 1% agarose immunoelectrophoresis20 using a polyclonal rabbit antiplasminogen antiserum (OSCB; Behringwerke AG, Marburg, Germany). The lower limit of detection of plasminogen antigen in human plasma is 0.4 mg/dL.
Plasminogen functional activity in citrated plasma after activation with streptokinase was assessed using a chromogenic assay with p-nitroaniline as substrate (Berichrom Plasminogen; Behringwerke AG). The lower limit of detection is 5% plasminogen functional activity. Quantitative determination of t-PA in citrated plasma was performed using enzyme-linked immunosorbent assay (ELISA; Asserachrom t-PA; Boehringer Mannheim, Mannheim, Germany). Quantitative determination of u-PA in citrated plasma was performed by ELISA (IMUBIND uPA Strip-well ELISA kit; American Diagnostica GmbH, Pfungstadt, Germany). Plasminogen activator inhibitor type 1 (PAI-1) activity in citrated plasma was determined by a chromogenic assay (Baxter Diagnostics AG, Düdingen, Switzerland).
Mutation Analysis
Genomic DNA was prepared from peripheral blood samples of all patients with ligneous conjunctivitis as well as from healthy family members. Thereafter, DNA samples were amplified by polymerase chain reaction (PCR) using a set of primer pairs flanking all 19 exons, including intron boundaries of the human plasminogen gene.21,22 To screen for plasminogen gene mutations, we used the method of single-strand conformation polymorphism (SSCP) analysis, which permits the detection of point mutations and other sequence alterations according to a variant migration pattern of the mutant versus the wild-type DNA fragment in gel electrophoresis, as described previously.16 Amplified and subsequently denatured PCR segments were electrophoresed in a nondenaturing 5% polyacrylamide gel (Roth, Karlsruhe, Germany) containing 5% glycerol in 0.5× TBE buffer at room temperature for 7 hours at 10 W. The gel was dried and exposed to Kodak XAR-5 films (Eastman Kodak, Rochester, NY). Variant bands of single-stranded DNA fragments that differed in their migration pattern were excised from dried gels, dissolved in 1× TE buffer, purified with MicroSpin columns (Pharmacia, Freiburg, Germany), and reamplified. Amplified PCR products were again purified with MicroSpin columns and directly cycle-sequenced by the dideoxy-termination method using 35S-dATP and the Exo(−)Pfu Cyclist DNA Sequencing Kit (Stratagene, Heidelberg, Germany) according to the manufacturer’s directions. Samples were electrophoresed on 6% polyacrylamide/8.3 mol/L urea sequencing gels (Roth), dried, and exposed to Kodak XAR-5 films.
Direct Analysis of Plasminogen Mutations by Amplification Mutagenesis and by Restriction Fragment Length Polymorphism (RFLP) Analysis
For the direct detection of the plasminogen gene mutation Arg216 → His in exon 7 (patient no. 2), genomic DNA was amplified by PCR with the following primer pair flanking the mutation in exon 7: 5′-AACCTGAAGAAGAATTACTGTG-3′ (forward) and 5′-CGGGATCCTGGAATCTGGGTGTGCATCATAC-3′ (reverse). The first primer has a single sequence mismatch at position 779 (C → G) that results in the introduction of an artificialBstE II restriction site into only the wild-type exon 7 allele. Amplified PCR products were digested with BstE II (GIBCO, Eggenstein, Germany), electrophoresed in a 2.5% agarose gel, and visualized by ethidium bromide staining as described recently.16 The wild-type BstE II-digested PCR fragment has a size of 119 bp; the mutant (uncut) PCR fragment has a size of 141 bp.
The missense mutation Arg513 → His (G → A at position 1671) in exon 13 of plasminogen gene (patient no. 1) disrupts the restriction site for the restriction enzyme MaeIII. To detect this mutation directly, amplified PCR products of plasminogen exon 13 were digested with Mae III (Boehringer Mannheim), electrophoresed in a 2% agarose gel, and visualized by ethidium bromide staining. The (uncut) mutant PCR fragment has a size of 184 bp, the wild-type Mae III-digested PCR fragment has a size of 139 bp.
The deletion mutation of a guanosine at position +1 in intron Q of the plasminogen gene (mutation name, Ex17 + 1del-g) in patients no. 3 and 4 disrupts the restriction site for the restriction enzyme Sty I. To detect this mutation directly, amplified PCR products of plasminogen exon 17/intron Q were digested with Sty I (Boehringer Mannheim), electrophoresed in a 2% agarose gel, and visualized by ethidium bromide staining. The (uncut) mutant PCR fragment has a size of 203 bp; the wild-type Sty I-digested PCR fragment has a size of 143 bp.
The missense mutation Leu128 → Pro (T → C at position 516) in exon 5 of the plasminogen gene (patient no. 5) produces a restriction site for the restriction enzyme Msp I. To detect this mutation directly, amplified PCR products of plasminogen exon 5 were digested with Msp I (Boehringer Mannheim), electrophoresed in a 2% agarose gel, and visualized by ethidium bromide staining. The (uncut) wild-type PCR fragment has a size of 221 bp, whereas the mutant Msp I-digested PCR fragment has a size of 144 bp.
RESULTS
Patients No. 1 Through 5 With Ligneous Conjunctivitis
Coagulation studies in patient no. 1 and his family.
The coagulation system of patient no. 1 at the age of 4 years and 4 months showed a plasminogen functional activity of 17% (normal range, 80% to 120%) and a plasminogen immunoreactive antigen level of less than 0.4 mg/dL (normal range, 6 to 25 mg/dL; Table 2). Fibrinogen, t-PA (Table 2), u-PA (Table 2), and PAI-1 of the patient and all healthy family members tested were within the normal range (data not shown). Plasminogen functional activities in the mother, father, and the brother (1 year and 8 months old) were 54%, 88%, and 121%, respectively. Plasminogen antigen concentrations were 7.0, 9.0, and 15.0 mg/dL, respectively (Table 2).
Plasminogen Values in Plasma and Molecular Genetic Findings in Five Patients With Ligneous Conjunctivitis and One Healthy Female With Heterozygous Type I Plasminogen Deficiency
| Subjects . | t-PA (ng/mL)* . | u-PA (ng/mL)† . | Plasminogen Activity (%)‡ . | Plasminogen Antigen (mg/dL)2-153 . | Mutations in the Plasminogen Gene . | ||
|---|---|---|---|---|---|---|---|
| First Allele . | / . | Second Allele . | |||||
| Patient no. 1 (male) | 0.8 | 1.1 | 17 | <0.4 | Lys19 → Glu | / | Arg513 → His |
| Mother | 0.9 | 0.7 | 54 | 7 | Wild-type | / | Arg513 → His |
| Father | 4.5 | 0.8 | 88 | 9 | Lys19 → Glu | / | Wild-type |
| Brother | 0.3 | 1.7 | 121 | 15 | Wild-type | / | Wild-type |
| Patient no. 2 (female) | 12 | 0.9 | 18 | <0.4 | Lys19 → Glu | / | Arg216 → His |
| Patient no. 3 (female) | 4.3 | 1.4 | <5 | <0.4 | del Lys2122-155 | / | Ex17 + 1del-g2-154 |
| Patient no. 4 (male) | 4.4 | 6.3 | <5 | <0.4 | del Lys212 | / | Ex17 + 1del-g |
| Mother | 6.2 | 1.0 | 52 | 6 | del Lys212 | / | Wild-type |
| Father | 3.2 | 1.9 | 52 | 12 | Ex17 + 1del-g | / | Wild-type |
| Patient no. 5 (male) | 3.8 | 1.7 | 17 | 2.4 | Lys19 → Glu | / | Leu128 → Pro |
| Mother | 0.3 | 0.8 | 57 | 7 | Wild-type | / | Leu128 → Pro |
| Father | 7 | 1.0 | 70 | 14 | Lys19 → Glu | / | Wild-type |
| Subject H (female) | 3 | 1.0 | 57 | 7.5 | Thr9 → Asn | / | Wild-type |
| Subjects . | t-PA (ng/mL)* . | u-PA (ng/mL)† . | Plasminogen Activity (%)‡ . | Plasminogen Antigen (mg/dL)2-153 . | Mutations in the Plasminogen Gene . | ||
|---|---|---|---|---|---|---|---|
| First Allele . | / . | Second Allele . | |||||
| Patient no. 1 (male) | 0.8 | 1.1 | 17 | <0.4 | Lys19 → Glu | / | Arg513 → His |
| Mother | 0.9 | 0.7 | 54 | 7 | Wild-type | / | Arg513 → His |
| Father | 4.5 | 0.8 | 88 | 9 | Lys19 → Glu | / | Wild-type |
| Brother | 0.3 | 1.7 | 121 | 15 | Wild-type | / | Wild-type |
| Patient no. 2 (female) | 12 | 0.9 | 18 | <0.4 | Lys19 → Glu | / | Arg216 → His |
| Patient no. 3 (female) | 4.3 | 1.4 | <5 | <0.4 | del Lys2122-155 | / | Ex17 + 1del-g2-154 |
| Patient no. 4 (male) | 4.4 | 6.3 | <5 | <0.4 | del Lys212 | / | Ex17 + 1del-g |
| Mother | 6.2 | 1.0 | 52 | 6 | del Lys212 | / | Wild-type |
| Father | 3.2 | 1.9 | 52 | 12 | Ex17 + 1del-g | / | Wild-type |
| Patient no. 5 (male) | 3.8 | 1.7 | 17 | 2.4 | Lys19 → Glu | / | Leu128 → Pro |
| Mother | 0.3 | 0.8 | 57 | 7 | Wild-type | / | Leu128 → Pro |
| Father | 7 | 1.0 | 70 | 14 | Lys19 → Glu | / | Wild-type |
| Subject H (female) | 3 | 1.0 | 57 | 7.5 | Thr9 → Asn | / | Wild-type |
Tissue-type plasminogen activator (normal range, 0.3 to 12 ng/mL).
Urokinase-type plasminogen activator (normal range, 0.6 to 7 ng/mL).
Plasminogen activity (normal range, 80% to 120%).
Plasminogen antigen (normal range, 6 to 25 mg/dL).
Deletion of amino acid lysine at position 212.
Deletion of a guanosine at position +1 in intron Q (name of mutation: Ex17 + 1del-g).
Molecular genetic findings in patient no. 1 and his family.
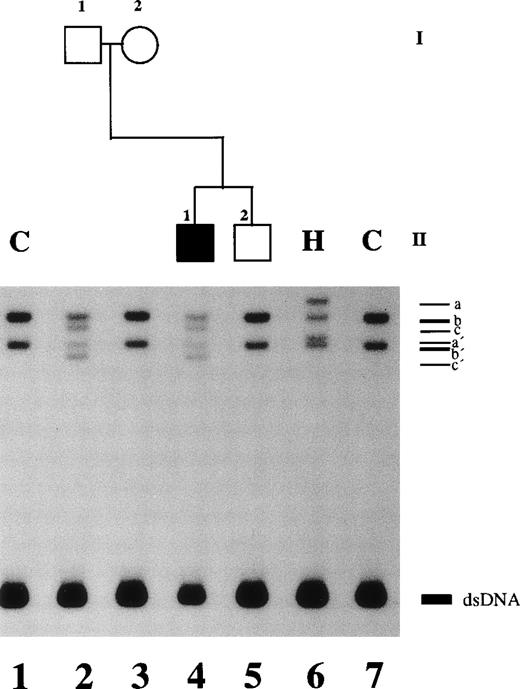
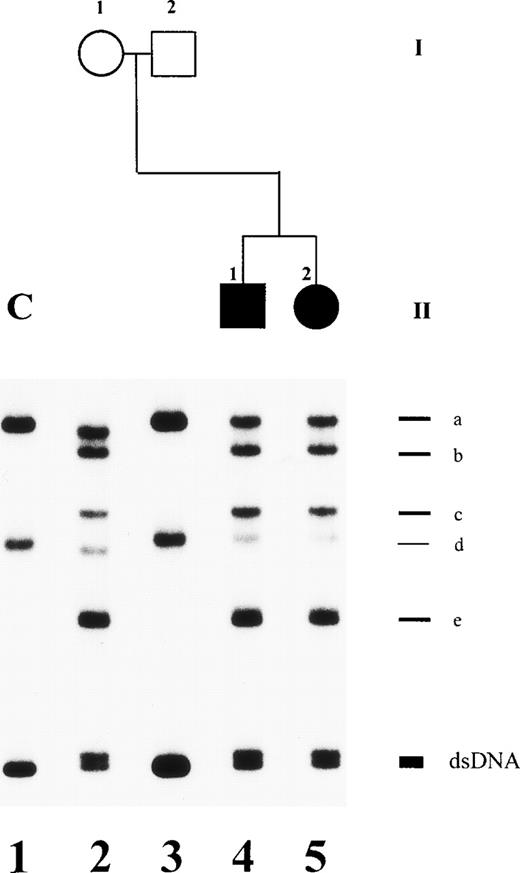
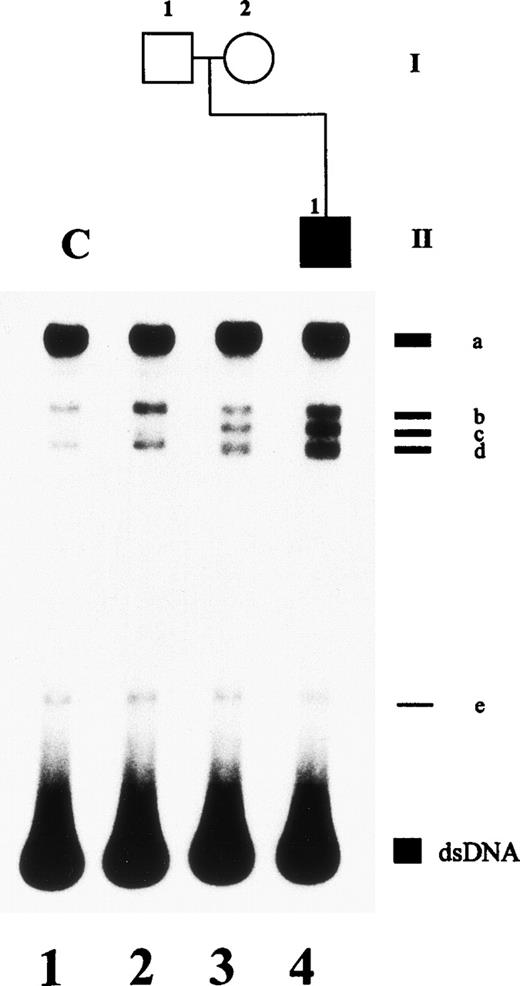
An altered SSCP band pattern was found in this family when primers specific for plasminogen exon 2 (Fig 2), exon 7 (data not shown), and exon 13 (data not shown) were used. Direct sequencing of abnormal single strands of plasminogen exon 2 from patient no. 1 (Fig 2, lane 4; band c and c′) demonstrated an A → G point mutation at position 188 leading to an amino acid exchange at position 19 (Lys19 → Glu; not shown). The father was shown to be heterozygous (Table 2). This mutation is located in the proactivation peptide (NH2-terminal acidic domain of plasminogen) 59 amino acids upstream of the Lys77-Lys78 cleavage site.21Examination of the younger brother and the mother and 50 healthy controls showed the wild-type pattern of plasminogen exon 2 (data not shown).
SSCP analysis of plasminogen gene exon 2 in patient no. 1, his healthy parents, and his healthy brother and in healthy subject H. Radiolabeled single-stranded PCR products of plasminogen exon 2 were electrophoresed in a nondenaturing 5% polyacrylamide gel as described in Materials and Methods. Lane 1, healthy control (C); lane 2, father of patient no. 1; lane 3, mother of patient no. 1; lane 4, patient no. 1 with ligneous conjunctivitis; lane 5, brother of patient no. 2; lane 6, healthy subject H; lane 7, healthy control. dsDNA, double-stranded DNA.
SSCP analysis of plasminogen gene exon 2 in patient no. 1, his healthy parents, and his healthy brother and in healthy subject H. Radiolabeled single-stranded PCR products of plasminogen exon 2 were electrophoresed in a nondenaturing 5% polyacrylamide gel as described in Materials and Methods. Lane 1, healthy control (C); lane 2, father of patient no. 1; lane 3, mother of patient no. 1; lane 4, patient no. 1 with ligneous conjunctivitis; lane 5, brother of patient no. 2; lane 6, healthy subject H; lane 7, healthy control. dsDNA, double-stranded DNA.
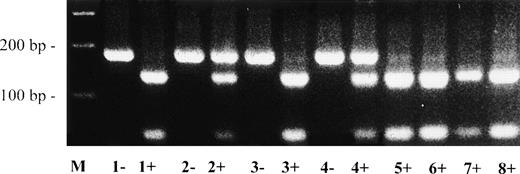
Direct sequencing of abnormal single strands of plasminogen exon 13 from patient no. 1 demonstrated a G → A point mutation at position 1671 leading to an amino acid exchange at position 513 (Arg513 → His; data not shown). The mother was also heterozygous for this Arg513 → His mutation. The Arg513 → His mutation could be easily differentiated from the wild-type allele by RFLP analysis (Fig 3).
Direct detection of missense mutation Arg513→ His in plasminogen exon 13 by RFLP analysis. Amplified PCR products of plasminogen exon 13 were digested with Mae III, electrophoresed in a 2% agarose gel, and visualized by ethidium bromide staining. The (uncut) mutant type PCR fragment has a size of 184 bp; the wild-type Mae III-digested PCR fragment has a size of 139 bp. −, no Mae III added to PCR product; +,Mae III added to PCR product. Lane M, 100-bp ladder; lane 1, father of patient no. 1; lane 2, mother of patient no. 1; lane 3, brother of patient no. 1; lane 4, patient no. 1; lanes 5 through 8, healthy controls. The larger (mutant type) allele is found afterMae III-digestion in patient no. 1 and his mother (lanes 2+ and 4+, heterozygous), but not in the father and the brother of patient no. 1 (lanes 1+ and 3+) and not in 4 healthy controls (lanes 5+, 6+, 7+, and 8+).
Direct detection of missense mutation Arg513→ His in plasminogen exon 13 by RFLP analysis. Amplified PCR products of plasminogen exon 13 were digested with Mae III, electrophoresed in a 2% agarose gel, and visualized by ethidium bromide staining. The (uncut) mutant type PCR fragment has a size of 184 bp; the wild-type Mae III-digested PCR fragment has a size of 139 bp. −, no Mae III added to PCR product; +,Mae III added to PCR product. Lane M, 100-bp ladder; lane 1, father of patient no. 1; lane 2, mother of patient no. 1; lane 3, brother of patient no. 1; lane 4, patient no. 1; lanes 5 through 8, healthy controls. The larger (mutant type) allele is found afterMae III-digestion in patient no. 1 and his mother (lanes 2+ and 4+, heterozygous), but not in the father and the brother of patient no. 1 (lanes 1+ and 3+) and not in 4 healthy controls (lanes 5+, 6+, 7+, and 8+).
This mutation is located in the kringle 5 domain of plasminogen, downstream from the intrakringle disulfide bridge Cys512-Cys536.21 Examination of the younger brother and the father and 50 healthy controls showed the wild-type pattern of plasminogen exon 13 (data not shown).
In this family, two different plasminogen gene mutations were independently transmitted from the father (Lys19 → Glu) and the mother (Arg513 → His) to patient no. 1, who therefore is a compound-heterozygote for these mutations. The combination of these mutations leads to undetectable plasminogen antigen (<0.4 mg/dL) and markedly decreased functional activity (17%). However, due to the limited sensitivity of the assays, we cannot exclude traces of plasminogen antigen in this subject (Table 2).
In the mother, who is heterozygous for the Arg513→ His mutation, plasminogen values (antigen concentration, 7.0 mg/dL; functional activity, 54%) are reduced by approximately 50% (heterozygous range).
In the father, who is heterozygous for the Lys19 → Glu mutation, plasminogen values (antigen concentration, 9.0 mg/dL; functional activity, 88%) are in the low normal range (antigen concentration, 6 to 25 mg/dL; activity, 80% to 120%).
Coagulation studies in patient no. 2.
Molecular genetic findings in patient no. 2.
Altered SSCP band patterns in genomic DNA of patient no. 2 were found by SSCP analysis when primers specific for plasminogen exon 2 and exon 7 were used (data not shown). Direct sequencing of the altered exon 7 single strands from patient no. 2 exhibited a G → A point mutation at position 780 leading to an amino acid substitution at position 216 (Arg216 → His; data not shown). The Arg216 → His mutation could be easily differentiated from the wild-type allele by amplification mutagenesis (Fig 4). Examination of 50 healthy controls by this method showed only the wild-type allele (data not shown).
Direct detection of plasminogen exon 7 mutation in patient no. 2 by amplification mutagenesis. A part of plasminogen exon 7 containing the mutation (G → A at position 780) was amplified by PCR as described in Materials and Methods. The first primer has a single sequence mismatch that results in the introduction of an artificial BstE II restriction site into the wild-type allele only. Amplified PCR products were digested with BstE II, electrophoresed in a 2.5% agarose gel, and visualized by ethidium bromide staining. The wild-type BstE II-digested PCR fragment has a size of 119 bp; the mutant (uncut) PCR fragment has a size of 141 bp. −, no BstE II added to PCR product; +, BstE II added to PCR product. Lane M, 100-bp ladder; lane 1, healthy control; lane 2, homozygous Turkish girl with ligneous conjunctivitis16; lane 3, patient no. 2. The larger (mutant) allele is found after BstE II-digestion in an unrelated girl with ligneous conjunctivitis (lane 2+, homozygous)16 and in patient no. 2 (lane 3+, heterozygous), but not in the healthy control (lane 1+).
Direct detection of plasminogen exon 7 mutation in patient no. 2 by amplification mutagenesis. A part of plasminogen exon 7 containing the mutation (G → A at position 780) was amplified by PCR as described in Materials and Methods. The first primer has a single sequence mismatch that results in the introduction of an artificial BstE II restriction site into the wild-type allele only. Amplified PCR products were digested with BstE II, electrophoresed in a 2.5% agarose gel, and visualized by ethidium bromide staining. The wild-type BstE II-digested PCR fragment has a size of 119 bp; the mutant (uncut) PCR fragment has a size of 141 bp. −, no BstE II added to PCR product; +, BstE II added to PCR product. Lane M, 100-bp ladder; lane 1, healthy control; lane 2, homozygous Turkish girl with ligneous conjunctivitis16; lane 3, patient no. 2. The larger (mutant) allele is found after BstE II-digestion in an unrelated girl with ligneous conjunctivitis (lane 2+, homozygous)16 and in patient no. 2 (lane 3+, heterozygous), but not in the healthy control (lane 1+).
This Arg216 → His mutation is located in the kringle 2 domain of plasminogen, downstream from the intrakringle disulfide bridge Cys215-Cys238.21It has been shown in another family with type I plasminogen deficiency that heterozygous Arg216 → His mutation in the plasminogen gene leads to reduction of plasminogen functional activity and plasminogen antigen by approximately 50%.16
Direct sequencing of the altered exon 2 single strands from patient no. 2 exhibited a A → G point mutation at position 188 leading to an amino acid exchange at position 19 (Lys19 → Glu). Examination of 50 healthy controls by SSCP-PCR analysis showed only the wild-type pattern of plasminogen exon 2 (data not shown).
Coagulation studies in patients no. 3 and 4 and their family.
When the coagulation system of patients no. 3 and 4 was examined at 14 and 19 years of age, respectively, no plasminogen functional activity was found (<5%; normal range, 80% to 120%). Plasminogen antigen levels were below the detection limit of the assay used (<0.4 mg/dL; normal range, 6 to 25 mg/dL; Table 2). Plasminogen functional activities in both the healthy mother and the healthy father were 52%, whereas plasminogen antigen concentrations were 6.0 and 12.0 mg/dL, respectively. Fibrinogen, t-PA (Table 2), u-PA (Table 2), and PAI-1 were within the normal range in all family members (data not shown).
Molecular genetic findings in patients no. 3 and 4 and other members of family 3.
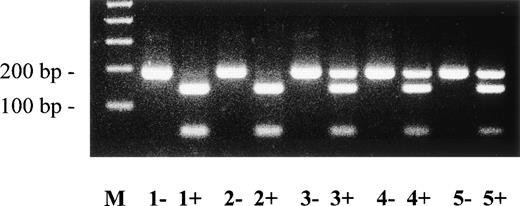
An altered SSCP band pattern was found in patients no. 3 and 4 when primers specific for plasminogen exon 7 (Fig 5) and exon 17, including intron boundaries (data not shown), were used. Direct sequencing of abnormal single strands of plasminogen exon 7 from patients no. 3 and 4 (Fig 5, lanes 4 and 5; band b) demonstrated a (heterozygous) 3-bp deletion (AAG) at position 767-769 leading to deletion of a lysine residue at position 212 (del Lys212; data not shown). The mother is heterozygous for this Lys212-deletion (Table 2). Examination of 50 healthy controls showed only the wild-type pattern of plasminogen exon 7 (data not shown).
SSCP analysis of plasminogen gene exon 7 in patients no. 3 and 4 and her parents. Radiolabeled single-stranded PCR products of plasminogen exon 7 were electrophoresed in a nondenaturing 5% polyacrylamide gel as described in Materials and Methods. Lane 1, healthy control (C); lane 2, mother; lane 3, father; lane 4, patient no. 4 with ligneous conjunctivitis; lane 5, patient no. 3. dsDNA, double-stranded DNA. The double-stranded DNA of patients no. 3 and 4 (lanes 4 and 5) and of the mother (lane 2) shows two bands. The shorter band is the mutant allele with a 3-bp deletion (AAG) at position 767-769. Note that alleles a and d of the mother (lane 2) are slightly faster migrating when compared with bands a and d of a healthy control with the wild-type sequence (lane 1). This is due to two polymorphisms, a silent C → T base exchange at position 847 in plasminogen exon 7 (Cys238) and a T → G base exchange at position −15 in plasminogen intron F.
SSCP analysis of plasminogen gene exon 7 in patients no. 3 and 4 and her parents. Radiolabeled single-stranded PCR products of plasminogen exon 7 were electrophoresed in a nondenaturing 5% polyacrylamide gel as described in Materials and Methods. Lane 1, healthy control (C); lane 2, mother; lane 3, father; lane 4, patient no. 4 with ligneous conjunctivitis; lane 5, patient no. 3. dsDNA, double-stranded DNA. The double-stranded DNA of patients no. 3 and 4 (lanes 4 and 5) and of the mother (lane 2) shows two bands. The shorter band is the mutant allele with a 3-bp deletion (AAG) at position 767-769. Note that alleles a and d of the mother (lane 2) are slightly faster migrating when compared with bands a and d of a healthy control with the wild-type sequence (lane 1). This is due to two polymorphisms, a silent C → T base exchange at position 847 in plasminogen exon 7 (Cys238) and a T → G base exchange at position −15 in plasminogen intron F.
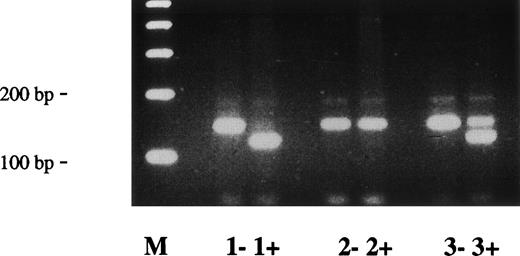
Direct sequencing of plasminogen exon 17/intron Q from patients no. 3 and 4 and their father demonstrated a (heterozygous) 1-bp deletion of a guanosine at position +1 in intron Q (name of mutation: Ex17+1del-g), probably resulting in an aberrant splicing (data not shown). This mutation could be easily differentiated from the wild-type allele by RFLP analysis after digestion of PCR fragments with the restriction enzyme Sty I (Fig 6).
Direct detection of deletion mutation (Ex17 + 1del-g) in plasminogen intron Q by RFLP analysis. Amplified PCR products of plasminogen exon 17/intron Q were digested with Sty I, electrophoresed in a 2% agarose gel, and visualized by ethidium bromide staining. The (uncut) mutant type PCR fragment has a size of 203 bp; the wild-type Sty I-digested PCR fragment has a size of 143 bp. −, no Sty I added to PCR product; +, Sty I added to PCR product. Lane M, 100-bp ladder; lane 1, healthy control; lane 2, mother of patients no. 3 and 4; lane 3, father of patients no. 3 and 4; lane 4, patient no. 4; lane 5, patient no. 3. The larger (mutant) allele is found after Sty I-digestion in patients no. 3 and 4 (lanes 4+ and 5+, heterozygous) and their father (lane 3+, heterozygous), but not in the healthy control and not in the mother of patients no. 3 and 4 (lanes 1+ and 2+).
Direct detection of deletion mutation (Ex17 + 1del-g) in plasminogen intron Q by RFLP analysis. Amplified PCR products of plasminogen exon 17/intron Q were digested with Sty I, electrophoresed in a 2% agarose gel, and visualized by ethidium bromide staining. The (uncut) mutant type PCR fragment has a size of 203 bp; the wild-type Sty I-digested PCR fragment has a size of 143 bp. −, no Sty I added to PCR product; +, Sty I added to PCR product. Lane M, 100-bp ladder; lane 1, healthy control; lane 2, mother of patients no. 3 and 4; lane 3, father of patients no. 3 and 4; lane 4, patient no. 4; lane 5, patient no. 3. The larger (mutant) allele is found after Sty I-digestion in patients no. 3 and 4 (lanes 4+ and 5+, heterozygous) and their father (lane 3+, heterozygous), but not in the healthy control and not in the mother of patients no. 3 and 4 (lanes 1+ and 2+).
Coagulation studies in patient no. 5 and his family.
The coagulation system of patient no. 5 at the age of 23 years showed a plasminogen functional activity of 17% (normal range, 80% to 120%) and a plasminogen immunoreactive antigen level of 2.4 mg/dL (normal range, 6 to 25 mg/dL; Table 2). Fibrinogen, t-PA (Table 2), u-PA (Table2), and PAI-1 were within the normal range in all family members (data not shown). Plasminogen functional activities in the mother and the father were 57% and 70%, respectively. Plasminogen antigen concentrations were 7.0 and 14.0 mg/dL, respectively (Table 2).
Molecular genetic findings in patient no. 5 and his family.
An altered SSCP band pattern was found in this family when primers specific for plasminogen exon 2 (data not shown) and exon 5 (Fig 7) were used. Direct sequencing of abnormal single strands of plasminogen exon 2 from patient no. 5 demonstrated an A → G point mutation at position 188 leading to an amino acid exchange at position 19 (Lys19 → Glu; data not shown). The father is heterozygous for this mutation (Table 2). Examination of the mother and 50 healthy controls showed the wild-type pattern of plasminogen exon 2 (data not shown).
SSCP analysis of plasminogen gene exon 5 in patient no. 5 and his healthy parents. Radiolabeled single-stranded PCR products of plasminogen exon 5 were electrophoresed in a nondenaturing 5% polyacrylamide gel as described in Materials and Methods. Lane 1, healthy control (C); lane 2, father of patient no. 5; lane 3, mother of patient no. 5; lane 4, patient no. 5. dsDNA, double-stranded DNA.
SSCP analysis of plasminogen gene exon 5 in patient no. 5 and his healthy parents. Radiolabeled single-stranded PCR products of plasminogen exon 5 were electrophoresed in a nondenaturing 5% polyacrylamide gel as described in Materials and Methods. Lane 1, healthy control (C); lane 2, father of patient no. 5; lane 3, mother of patient no. 5; lane 4, patient no. 5. dsDNA, double-stranded DNA.
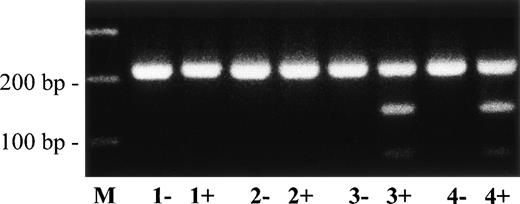
Direct sequencing of the abnormal single strands of plasminogen exon 5 from patient no. 5 (Fig 7, lane 4; band c) demonstrated a T → C point mutation at position 516 leading to an amino acid exchange at position 128 (Leu128 → Pro; data not shown). The mother (Fig 7, lane 3) was also heterozygous for this mutation. The Leu128 → Pro mutation could be easily differentiated from the wild-type allele by RFLP analysis (Fig 8). Examination of the the father and 50 healthy controls showed the wild-type pattern of plasminogen exon 5.
Direct detection of missense mutation Leu128→ Pro in plasminogen exon 5 of patient no. 5 by RFLP analysis. Amplified PCR products of plasminogen exon 5 were digested withMsp I, electrophoresed in a 2% agarose gel, and visualized by ethidium bromide staining. The (uncut) wild-type PCR fragment has a size of 221 bp; the mutant Msp I-digested PCR fragment has a size of 144 bp. −, no Msp I added to PCR product; +,Msp I added to PCR product. Lane M, 100-bp ladder; lane 1, healthy control; lane 2, father of patient no. 5; lane 3, mother of patient no. 5; lane 4, patient no. 5. The shorter (mutant type) allele is found after Msp I-digestion in patient no. 5 and his mother (lanes 3+ and 4+, heterozygous), but not in his father (lane 2+) and not in a healthy control (lane 1+).
Direct detection of missense mutation Leu128→ Pro in plasminogen exon 5 of patient no. 5 by RFLP analysis. Amplified PCR products of plasminogen exon 5 were digested withMsp I, electrophoresed in a 2% agarose gel, and visualized by ethidium bromide staining. The (uncut) wild-type PCR fragment has a size of 221 bp; the mutant Msp I-digested PCR fragment has a size of 144 bp. −, no Msp I added to PCR product; +,Msp I added to PCR product. Lane M, 100-bp ladder; lane 1, healthy control; lane 2, father of patient no. 5; lane 3, mother of patient no. 5; lane 4, patient no. 5. The shorter (mutant type) allele is found after Msp I-digestion in patient no. 5 and his mother (lanes 3+ and 4+, heterozygous), but not in his father (lane 2+) and not in a healthy control (lane 1+).
Leu128 is located in the kringle 1 domain of plasminogen 5 amino acids upstream of the intrakringle disulfide bridge Cys133-Cys157.21
In this family, two different plasminogen gene mutations were independendly transmitted from the father (Lys19 → Glu) and the mother (Leu128 → Pro) to patient no. 5, who therefore is a compound-heterozygote for these mutations. The combination of these mutations leads to markedly decreased plasminogen antigen (2.4 mg/dL) and functional activity (17%; Table 2).
Polymorphisms in the plasminogen gene.
A silent C → T base exchange at position 847 in plasminogen exon 7 (Cys238)21,23 in combination with a T → G base exchange at position −15 in plasminogen intron F was found in a homozygous pattern in patient no. 1 and the father of patient no. 2; it was also found in a heterozygous pattern in patient no. 2, his healthy brother, patients no. 3 and 4, and their mother (not shown). The second allele of the mother of patients no. 3 and 4 exhibits the Cys238 polymorphism without the intron F polymorphism. A silent C → T base exchange at position 1018 (Phe295) and a silent A → G base exchange at position 1159 (Gln342) was found in a heterozygous pattern in patients no. 3 and 4.21 23 The mother was homozygous for the former and heterozygous for the second polymorphism (not shown).
Molecular genetic findings in a healthy subject with heterozygous type I plasminogen deficiency (subject H).
In this healthy woman, the plasminogen functional activity was 57% (normal range, 80% to 120%) and the plasminogen antigen level was 7.5 mg/dL (normal range, 6 to 25 mg/dL; Table 1). Molecular genetic studies showed a heterozygous C → A point mutation in plasminogen exon 2 at position 159 leading to an amino acid exchange (Thr9→ Asn; Fig 2, lane 6, SSCP pattern; sequencing data not shown; Table 2). This novel missense mutation lies in the NH2-terminal fragment of plasminogen, the proactivation peptide, which is released during the conversion of Glu-plasminogen to Lys-plasminogen by cleavage between Lys77-Lys78.21
DISCUSSION
We have shown that certain compound-heterozygous mutations in the plasminogen gene (Lys19 → Glu/Arg216→ His, Lys19 → Glu/Arg513→ His, Lys19 → Glu/Leu128→ Pro, and del Lys212/Ex17 + 1del-g) are associated with ligneous conjunctivitis. It is possible that, as a consequence of these mutations, mRNA transcription and/or the half-life of mRNA of the mutant plasminogen allele may be markedly decreased. It is also conceivable that the liver may synthesize a truncated plasminogen molecule that is rapidly cleared from the circulation. Some of the missense mutations in the plasminogen gene, described here, affect a lysine (Lys19 → Glu, del Lys212) or an arginine residue (Arg216→ His, Arg513 → His). It has been suggested that lysine and arginine side chains of plasminogen interact with the lysine binding sites of the kringles of the plasminogen molecule and stabilize specific protein conformations.24These mutations may therefore change the folding and conformation of the mutant plasminogen molecule, leading to impaired secretion from the liver into the blood stream. This mechanism has been recently demonstrated in vitro by genetic engineering techniques for type I plasminogen deficiency caused by a Ser572 → Pro mutation.25 Further in vitro studies are needed to show whether the plasminogen variants, described here, exhibit an altered binding capacity to cells and fibrinogen and whether the activation by t-PA, u-PA, or other plasminogen activators is altered.
The plasminogen gene is well conserved in humans and different animal species. The plasminogen gene mutations in patients with type I plasminogen deficiency described in this and other studies16,17 ( Table 3) have not been identified in different animals studied so far, except for His216, which has been found in mice.26 Amino acid residues Thr9, Leu128, Arg216, Arg513, Ser572, and Trp597 are conserved in all animal species studied so far: Rhesus monkey,27Macropus eugenii (GENBANK Accession No.AF012297), pig,28-30 ox,31-33horse,34,35 mouse (except for His216),26 and hedgehog.36,37Partial coding sequence of the N-terminal end of the plasminogen gene shows the conservation of Thr9 also in sheep, goat, rabbit, and dog.26,34 In the cat, Thr9 is deleted.26 Lys19 is conserved in Rhesus monkey,27Macropus eugenii (GENBANK accessionAF012297), rabbit, canine, hedgehog, and mouse, but is replaced by an arginine in pig, horse, ox, sheep, and goat.26,29,31 34
Current Classification of Plasminogen (Plg) Deficiency
| . | Immunoreactive Plg Antigen in Plasma (mg/dL) . | Total Functional Plg Activity in Plasma (%) . | Known Mutations in the Plasminogen Gene . | References . |
|---|---|---|---|---|
| Type I | Decreased | Decreased | Arg216 → His, Trp597 → Stop, Trp597 → Cys | 16 |
| Glu460 → Stop | 17 | |||
| Ser572 → Pro, Ala675 → Thr | 22, 38 | |||
| del Lys212, Arg513 → His, Thr9 → Asn, Ex17 + 1del-g | This report | |||
| Leu128 → Pro, Lys19 → Glu | ||||
| Type II | Normal or slightly decreased | Decreased | Ala601 → Thr, Val355 → Phe, Asp676 → Asn Gly732 → Arg | 23, 42-46 54 |
| . | Immunoreactive Plg Antigen in Plasma (mg/dL) . | Total Functional Plg Activity in Plasma (%) . | Known Mutations in the Plasminogen Gene . | References . |
|---|---|---|---|---|
| Type I | Decreased | Decreased | Arg216 → His, Trp597 → Stop, Trp597 → Cys | 16 |
| Glu460 → Stop | 17 | |||
| Ser572 → Pro, Ala675 → Thr | 22, 38 | |||
| del Lys212, Arg513 → His, Thr9 → Asn, Ex17 + 1del-g | This report | |||
| Leu128 → Pro, Lys19 → Glu | ||||
| Type II | Normal or slightly decreased | Decreased | Ala601 → Thr, Val355 → Phe, Asp676 → Asn Gly732 → Arg | 23, 42-46 54 |
Inherited plasminogen deficiency has been classified into two types, true deficiency (type I) and dysplasminogenemia (type II). In the former, the level of immunoreactive plasminogen is reduced in parallel with its functional activity. Six distinct plasminogen gene mutations leading to type I plasminogen deficiency have been previously described: Ser572 → Pro, Ala675→ Thr, Glu460 → Stop, Arg216 → His, Trp597 → Stop, and Trp597 → Cys (Table3).16,17,22,38 We have described here six new mutations in the plasminogen gene (Thr9 → Asn, Lys19 → Glu, Leu128 → Pro, del Lys212, Arg513 → His, and Ex17 + 1del-g) that lead to type I plasminogen deficiency (Table 3). The prevalence of type I plasminogen deficiency has been recently calculated as 0.26% (25 of 9,611 subjects) in a large epidemiological study in United Kingdom.39 The theoretically predicted prevalence of compound-heterozygotes (including homozygotes) would therefore be approximately 7 per 1,000,000. Most subjects with heterozygous type I plasminogen deficiency are clinically healthy. Two recent epidemiological studies suggest that heterozygous type I plasminogen deficiency by itself is not a risk factor for thrombosis.39,40 Furthermore, none of the 8 reported patients with ligneous conjunctivitis and homozygous or compound-heterozygous type I plasminogen deficiency developed thrombosis (this report and Schuster et al16 and Schott et al17).
In type II plasminogen deficiency, or dysplasminogenemia, the level of immunoreactive plasminogen is normal (or only slightly reduced), whereas the functional activity is markedly reduced (Table3).41 Four distinct plasminogen gene mutations (Ala601 → Thr, Val355 → Phe, Asp676 → Asn, and Gly732 → Arg) have been identified in patients with dysplasminogenemia (Table3).23,42-46,54 The prevalence of dysplasminogenemia has been calculated as 0.03% (3 of 9,611 subjects) in an epidemiologic study in United Kingdom.39 In Japan and China, the prevalence rate is greater than 2%.46 47 Only a small fraction of subjects with type II plasminogen deficiency will develop thrombosis, suggesting that this plasminogen variant by itself is not a risk factor for vascular occlusions.
Our previous15-17 and current results show that homozygous and compound-heterozygous type I plasminogen deficiency may be associated with ligneous conjunctivitis. In contrast, ligneous conjunctivitis has never been described in subjects with type II plasminogen deficiency.23 The severity of clinical symptoms in patients with homozygous or compound-heterozygous type I plasminogen deficiency seems to depend mainly on the amount of plasminogen functional residual activity. Five patients with ligneous conjunctivitis and severe type I plasminogen deficiency studied so far who showed undetectable plasminogen functional activity15-17 (patients no. 3 and 4 of the present report) exhibited severe multisystem disease. In contrast, 3 patients of this study who had a plasminogen functional residual activity of 17% and 18%, presented with a much milder course of disease. In these cases, formation of pseudomembranes was confined to the conjunctivae.
To our knowledge, 2 of the patients described in this report are the first cases in which the upper gastrointestinal tract (duodenal ulcer and eosinophilic gastric infiltration in patient no. 4) and the kidneys (patients no. 3 and 4) are also affected. Involvement of the kidneys has been documented so far only rarely in dogs with ligneous conjunctivitis48 and in plasminogen-deficient mice (Plg−/− mice)49 and occasionally in plasminogen activator-deficient mice (t-PA−/−mice and u-PA−/−/t-PA−/−mice).50 In contrast, gastric and duodenal ulcers are common in Plg−/− and u-PA−/−/t-PA−/−mice.49-53
Therapeutic options in the treatment of multisystem complications in patients with severe type I plasminogen deficiency are limited. In one boy with homozygous type I plasminogen deficiency and bilateral ligneous conjunctivitis treated so far, repeated intravenous infusions of Lys-plasminogen resulted in complete resolution of conjunctival pseudomembranes.17 For different reasons (short half-life of Lys-plasminogen and high costs), this procedure will not be possible in most cases. In patients with recurrent life-threatening complications (ie, severe bronchopneumonias, upper airway obstruction, imminent blindness), liver transplantation may be another therapeutic approach. In patients in whom formation of pseudomembranes is restricted to the conjunctivae, topical treatment with heparin, steroids, and α-chymotrypsin immediately after excision of pseudomembranse may lead to acceptable results.11 Further in vitro and in vivo studies may eventually help to develop plasminogen variants with higher specific activity and a longer half-life for therapeutic replacement therapy in patients with severe type I plasminogen deficiency.
Supported by the Deutsche Forschungsgemeinschaft (Grant Schu 560/4-1 to V.S.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Volker Schuster, MD, Children’s Hospital, University of Würzburg, Josef-Schneider-Straβe 2, D-97080 Würzburg, Germany; e-mail: Schuster@mail.uni-wuerzburg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal