Abstract
Recently, we have demonstrated that antibodies that block the function of the β2-integrin leukocyte function-associated antigen-1 (LFA-1) completely abrogate the rapid mobilization of hematopoietic progenitor cells (HPC) with colony-forming and radioprotective capacity induced by interleukin-8 (IL-8) in mice. These findings suggested a direct inhibitory effect of these antibodies on LFA-1–mediated transmigration of stem cells through the bone marrow endothelium. Therefore, we studied the expression and functional role of LFA-1 on murine HPC in vitro and in vivo. In steady state bone marrow ± 50% of the mononuclear cells (MNC) were LFA-1neg. Cultures of sorted cells, supplemented with granulocyte colony-stimulating factor (G-CSF)/granulocyte-macrophage colony-stimulating factor (GM-CSF)/IL-1/IL-3/IL-6/stem cell factor (SCF) and erythropoietin (EPO) indicated that the LFA-1neg fraction contained the majority of the colony-forming cells (CFCs) (LFA-1neg 183 ± 62/7,500 cells v LFA-1pos 29 ± 17/7,500 cells,P < .001). We found that the radioprotective capacity resided almost exclusively in the LFA-1neg cell fraction, the radioprotection rate after transplantation of 103, 3 × 103, 104, and 3 × 104 cells being 63%, 90%, 100%, and 100% respectively. Hardly any radioprotection was obtained from LFA-1pos cells. Similarly, in cytokine (IL-8 and G-CSF)–mobilized blood, the LFA-1neg fraction, which comprised 5% to 10% of the MNC, contained the majority of the colony-forming cells, as well as almost all cells with radioprotective capacity. Subsequently, primitive bone marrow-derived HPC, represented by Wheat-germ-agglutinin (WGA)+/Lineage (Lin)−/Rhodamine (Rho)− sorted cells, were examined. More than 95% of the Rho− cells were LFA-1neg. Cultures of sorted cells showed that the LFA-1neg fraction contained all CFU. Transplantation of 150 Rho− LFA-1neg or up to 600 Rho−LFA-1pos cells protected 100% and 0% of lethally irradiated recipient mice, respectively. These results show that primitive murine HPC in steady-state bone marrow and of cytokine-mobilized blood do not express LFA-1.
THE PROLIFERATION and differentiation of hematopoietic progenitor cells (HPC) is highly dependent on interactions with components of the bone marrow microenvironment. Adhesion molecules have been implicated to play a key role in this complex network.1 Among these, β1- and β2-integrins are involved in the cellular interactions between HPC, stromal cells, and the extracellular matrix (ECM).2 A number of studies has indicated the expression of the β1-integrins very late antigen (VLA)-4 (CD49d) and VLA-5 (CD49e) on colony-forming HPC.3-8
Leukocyte function-associated antigen −1 (LFA-1) (CD11a) has been reported to be expressed on human committed progenitor cells and also on a subset of more immature cells in vitro.2,9-13 In long-term bone marrow cultures (LTBMC), the CD34+LFA-1neg fraction generated more colony-forming cells (CFCs) than LFA-1pos cells, indicating that CD34+ LFA-1neg cells are more primitive than CD34+ LFA-1pos cells.11 LFA-1 also plays a role in the attachment of CD34+ cells to stromal cells via one of its ligands, intercellular adhesion molecule (ICAM)-12. In addition, it has been reported that CD34+ LFA-1neg cells express LFA-1 within 24 hours of culture, without losing their growth capacity.13 These results could explain the inhibition of anti–LFA-1 antibodies on the generation of CFCs by CD34+cells in stromal layers.11
Although these human studies have demonstrated that the in vitro expression of LFA-1 is confined to the more mature HPC, no such studies are reported on murine HPC. Recently, we have demonstrated that anti–LFA-1 blocking antibodies completely prevent the interleukin-8 (IL-8)–induced mobilization of HPC with colony-forming or radioprotective capacity in mice, without interfering with stem cell homing.14 These experiments showed the direct involvement of the β2-integrin LFA-1 in cytokine-induced mobilization. From these studies, however, it remained unclear whether LFA-1 expression on progenitor cells or on accessory cells was responsible for this activity.
In the present report, we studied the expression and functional role of LFA-1 on murine HPC in vitro and in vivo. We found that the LFA-1neg fraction of BM-derived, as well as cytokine-mobilized, blood mononuclear cells (MNC) contain the majority of the CFCs and of cells with radioprotective capacity. Thus, murine HPC with colony-forming or radioprotective capacity do not express LFA-1.
MATERIALS AND METHODS
Mice.
BALB/c mice, with an age ranging between 8 to 12 weeks, were purchased from Broekman BV, Someren, The Netherlands. Male donor animals were fed commercial rodent chow and acidified water ad libitum. Recipient female animals were maintained in a pathogen-free environment and fed water containing ciprofloxacin 1 mg/mL (Bayer Nederland BV, Mijdrecht, The Netherlands), polymyxin-B 70 μg/mL and saccharose 2 g/100 mL.
Preparation of cell suspensions.
Mice were killed by CO2 asphyxiation. Blood was obtained by intracardiac puncture. Bone marrow cells were obtained by flushing the femur under sterile conditions with RPMI 1640 containing 500 μg/mL penicillin, 250 μg/mL streptomycin, and 2% fetal bovine serum (FBS; GIBCO, Grand Island, NY). Cell counts were performed on a Sysmex F800 (TOA Medical Electronics Co, LTD, Kobe, Japan). Manual neutrophil counts were performed after May Grünwald-Giemsa staining. Blood and bone marrow-derived MNC suspensions were obtained by Ficoll separation as described earlier.15
Stem cell purification.
Bone marrow and peripheral blood cells were separated by density gradient centrifugation (Ficoll Isopaque; specific density [SD] 1.077g/cm3) at 4°C. Low-density cells were labeled with the monoclonal rat-antimouse antibody H154.163 (anti-CD11a, IgG2a, directed against the adhesion molecule LFA-1, kindly provided by Dr M. Pierres, Centre D’ Immunologie De Marseille-Luminy, Marseille, France16). Cells were washed once and labeled with phycoerythrin (PE)-conjugated goat antirat-IgG (GaRa-Pe) (Caltag, San Francisco, CA). In some experiments, further purification of bone marrow–derived low-density cells was performed as described earlier.17 In short, low-density cells were labeled with a biotin-conjugated myelomonocytic monoclonal antibody (LY-6C), and PE-conjugated CD3e and CD45R/B220 (Pharmingen, San Diego, CA). After washing once, the cells were further labeled with Streptavidin-Pe (Becton Dickinson, San José, CA) and fluorescein-conjugated wheat-germ-agglutinin (WGA, 0.2 μg/mL, Vector, Burlingame, CA). WGA+/Lin− cells (±5%) were fluorescence-activated cell sorted (FACS) using a FACSTAR cytometer (Becton Dickinson) tuned at 488 nm. Sorted cells were stained with rhodamine 123 (Rho; Molecular Probes Inc, Eugene, OR) at a concentration of 0.1 μg/mL for 20 minutes at 37°C. Cells were washed twice and incubated in medium without Rho for 20 minutes at 37°C. The Rho− fraction was sorted using the FACSTAR tuned at 514 nm. Sorted cells were used for colony cultures or transplantation within 8 hours after killing the donor mice.
Cytokines.
Recombinant human IL-8 was purified from Escherichia coliexpressing a synthetic gene18 and kindly provided by Dr I.J.D. Lindley of the Novartis Forschungsinstitut, Vienna, Austria. IL-8 had no colony-stimulating activity as reported previously.19 The concentration of endotoxin was less than 0.05 EU/mL as determined by the Limulus amoebocyte lysate assay. Recombinant granulocyte colony-stimulating factor (G-CSF) was purchased from Amgen, Thousand Oaks, CA. For in vivo experiments, all agents were diluted to the desired concentration in endotoxin-free phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) and administered as an intraperitoneal (IP) injection.
Progenitor cell assays.
To determine the clonogenic capacity of cells from purified cell fractions, 7,500 sorted cells were cultured in 35 mm tissue culture dishes in Iscove’s modified Dulbecco’s medium (IMDM), containing 30% FBS, 1.1% methylcellulose 2 × 10−5β-mercaptoethanol, human transferrin (0.47 g/L) saturated with FeCl3.H2O in the presence of various growth factors. Growth factors used included recombinant human (rhu) IL-1β, 10 ng/mL (kindly provided by Hoffman-La Roche, Nutley, NJ); recombinant murine (rmu) IL-3, 25 ng/mL (kindly provided by Novartis, Basel, Switzerland); rhu-IL-6, 10 ng/mL (kindly provided by Dr L. Aarden, CLB, Amsterdam, The Netherlands); rhu-G–CSF, 10 ng/mL (Amgen); rmu-granulocyte-macrophage colony-stimulating factor (GM-CSF) 10 ng/mL (kindly provided by Dr E. Liehl, Novartis Forschungsinstitut, Vienna, Austria); rhu-stem cell factor (SCF), 25 ng/mL (kindly provided by Amgen); and rhu-erythropoietin (EPO), 2 U/mL (Organon Technica N.V., Turnhout, Belgium). Colony-forming unit-granulocyte macrophage (CFU-GM) were cultured as described previously.15 Briefly, peripheral blood MNC were cultured in 3.5 cm dishes containing 5 × 105 cells per mL in semisolid medium in the presence of murine GM-CSF (1.25 ng/mL). After 6 or 7 days of culture in a fully humidified atmosphere of 37°C containing 5% CO2, the number of colonies was scored using an inverted microscope. Colonies were defined as aggregates of at least 20 cells.
Experimental design.
Mobilization of HPC by IL-8 was induced by a single IP injection of 30 μg of IL-8.20 After 20 minutes, the mice were killed by CO2 asphyxiation and peripheral blood was obtained by cardiac puncture. Mobilization by G-CSF was induced by IP administration of 5 μg G-CSF once daily for 3 days. At day 4, the animals were killed and blood was obtained as described. In transplantation experiments, recipient mice were placed in a polymethylmetaacetate (PMMA) box and given total body irradiation (8.75 Gy, Philips SL 75-5/6 mV linear accelerator; Philips Medical Systems, Best, The Netherlands), divided in two parts in posterior-anterior and anterior-posterior position, at a dose rate of 4 Gy/min. Decreasing cell numbers of sorted bone marrow or mobilized blood cells were injected in the tail vein of lethally irradiated recipients. In each experiment, groups of 10 mice for each cell dose and cell fraction were transplanted. The experimental protocol was approved by the institutional ethical committee on animal experiments.
Late phase of engraftment.
Long-term repopulating ability (LTRA) of bone marrow–derived, as well as cytokine-mobilized, blood LFA-1neg cells was assessed by long-term bone marrow function, ie, the level of circulating mature blood cells. Furthermore, the percentage of male cells was determined in blood using fluorescence in situ hybridization with the Y-chromosome-specific probe M34 as previously described.17To assess multilineage engraftment, blood-derived granulocytes, T lymphocytes, and B lymphocytes were sorted from individual mice.21 Of each cell fraction, 200 nuclei were scored using a Leitz Diaplan microscope (Leica, Wetzlar, Germany).
Statistical analysis.
Differences were evaluated using the Student’s t-test.P values of < .05 were considered statistically significant.
RESULTS
Expression of LFA-1 on steady-state bone marrow–derived cells and cytokine-mobilized blood cells.
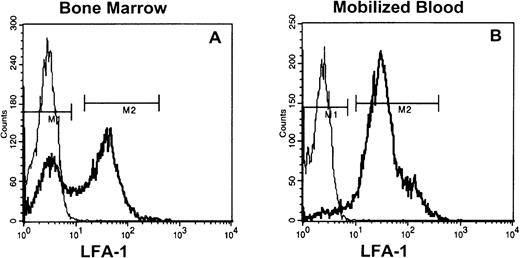
Staining of low-density steady-state bone marrow cells (8% to 15%) with anti–LFA-1 antibodies showed that 40% to 60% was LFA-1neg, whereas in G-CSF–, as well as IL-8–mobilized blood, only 5% to 10% (Fig 1) and in steady-state peripheral blood < 5% of the MNC were LFA-1neg (data not shown).
LFA-1 fluorescence histograms of steady state bone marrow (A) or cytokine (G-CSF and IL-8)–mobilized blood MNC (B). The LFA-1neg cell fraction comprised 40% to 60% and 5% to 10% of appropriately gated MNC from bone marrow and blood, respectively.
LFA-1 fluorescence histograms of steady state bone marrow (A) or cytokine (G-CSF and IL-8)–mobilized blood MNC (B). The LFA-1neg cell fraction comprised 40% to 60% and 5% to 10% of appropriately gated MNC from bone marrow and blood, respectively.
Colony-forming capacity of LFA-1 sorted bone marrow and cytokine-mobilized blood cells.
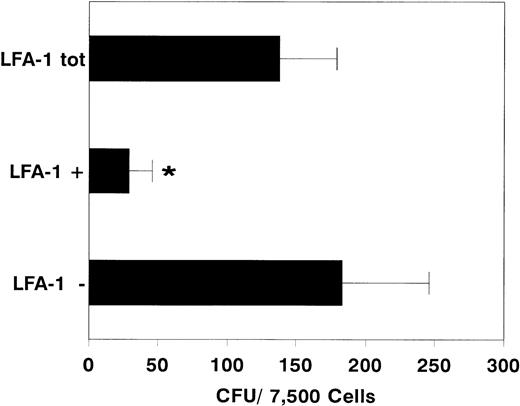
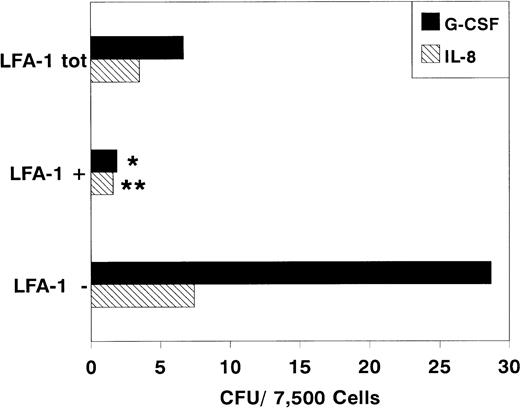
After sorting, we determined the clonogenic capacity of bone marrow–derived LFA-1neg and LFA-1pos cells in medium containing a cocktail of growth factors (G-CSF, GM-CSF, IL-1, IL-3, IL-6, SCF, and EPO). Figure 2 shows that the majority of the CFCs resided in the LFA-1negfraction (LFA-1neg 183 ± 62, n = 10 vLFA-1pos 29 ± 17 CFU per 7,500 sorted cells; mean ± standard deviation [SD], n = 6, P < .001). Similar results were obtained with cytokine-mobilized blood cells (Fig 3). Although the frequency of CFCs was lower in all fractions, colonies were also predominantly found in the LFA-1neg fraction (LFA-1neg 28.5 ± 18v LFA-1pos 2 ± 1.5 CFU/7,500 sorted cells for G-CSF; mean ± SD, n = 5, P < .05 and LFA-1neg 7.5 ± 3 v LFA-1pos 1.5 ± 1 CFU/7,500 sorted cells for IL-8, respectively; mean ± SD, n = 7, P < .01, Fig 3).
Number of colonies formed by 7,500 sorted LFA-1 negative (LFA-1−), positive (LFA-1+), or total (LFA-1tot) bone marrow MNC. Results are expressed as mean ± SD. *P < .001.
Number of colonies formed by 7,500 sorted LFA-1 negative (LFA-1−), positive (LFA-1+), or total (LFA-1tot) bone marrow MNC. Results are expressed as mean ± SD. *P < .001.
Number of colonies formed by 7,500 sorted LFA-1−, LFA-1+, or LFA-1totcytokine-mobilized blood MNC. Mice were treated with daily IP injections of 5 μg G-CSF for 3 days or with a single IP injection of 30 μg IL-8. Results are expressed as mean ± SD. *P < .05 and **P < .01.
Number of colonies formed by 7,500 sorted LFA-1−, LFA-1+, or LFA-1totcytokine-mobilized blood MNC. Mice were treated with daily IP injections of 5 μg G-CSF for 3 days or with a single IP injection of 30 μg IL-8. Results are expressed as mean ± SD. *P < .05 and **P < .01.
Radioprotective capacity of LFA-1 sorted bone marrow cells.
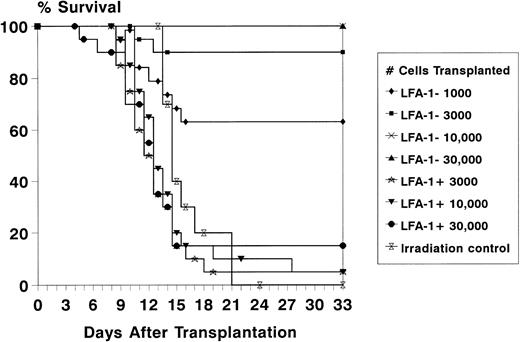
To assess the radioprotective capacity of the LFA-1neg and LFA-1pos cell fractions, lethally irradiated recipient mice were transplanted with increasing numbers of LFA-1neg and LFA-1pos sorted bone marrow–derived MNC. The radioprotective capacity resided almost entirely in the LFA-1neg cell fraction, as radioprotection after transplantation of 103, 3 × 103, 104, and 3 × 104LFA-1neg cells resulted in 63%, 90%, 100%, and 100% survival, respectively (Fig 4). In contrast, after transplantation of 3 × 103, 104, and 3 × 104 LFA-1poscells, a radioprotection rate of 5%, 5%, and 15% was obtained (Fig4).
Survival of lethally irradiated (8.75 Gy) recipient mice for at least 4 weeks after transplantation of increasing numbers of LFA-1neg and LFA-1pos sorted bone marrow MNC. Survival data are expressed as absolute percentages of two experiments with 10 mice transplanted per group in each experiment.
Survival of lethally irradiated (8.75 Gy) recipient mice for at least 4 weeks after transplantation of increasing numbers of LFA-1neg and LFA-1pos sorted bone marrow MNC. Survival data are expressed as absolute percentages of two experiments with 10 mice transplanted per group in each experiment.
Radioprotective capacity of cytokine-mobilized LFA-1 sorted blood cells.
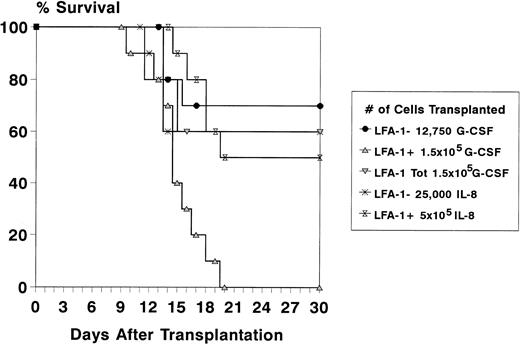
To study the radioprotective capacity of the mobilized LFA-1 sorted MNC, recipient mice were lethally irradiated and transplanted with a fixed number of LFA-1 sorted cells. Because the majority of CFCs was confined to the LFA-1neg cell fraction, cell doses of LFA-1neg and LFA-1pos cell fractions were chosen in relation to their relative frequency and to the total number of MNC required for radioprotection (1.5 × 105 for G-CSF– and 5 × 105 for IL-8–mobilized cells).20 22 Because after mobilization with G-CSF the LFA-1neg fraction comprised 8.5% of the MNC, 8.5% of 1.5 × 105 (12,750) LFA-1neg cells were transplanted and 1.5 × 105 LFA-1poscells. The survival rate of animals transplanted with LFA-1neg cells was similar to animals transplanted with the LFA-1 unsorted fraction (60% for total MNC and 70% for the LFA-1neg fraction). No animals survived in the group transplanted with LFA-1pos cells (n = 10 mice per group, Fig 5). After mobilization with IL-8, the LFA-1neg fraction comprised 5% of the MNC, resulting in transplantation of 5 × 105 LFA-1pos cells and 25,000 LFA-1neg cells (5% of 5 × 105). The survival rate of recipient mice transplanted with IL-8–mobilized LFA-1neg MNC was 60%. A 50% survival rate was observed after transplantation of 5 × 105LFA-1pos MNC (n = 10 mice per group, Fig 5).
Survival data of lethally irradiated (8.75 Gy) recipient mice for at least 4 weeks after transplantation of LFA-1 sorted cytokine-mobilized blood cells. Donor mice were pretreated with daily IP injections of 5 μg G-CSF for 3 days or a single IP injection of 30 μg IL-8. With G-CSF, a total number of 1.5 × 105 MNC (LFA-1tot), 1.5 × 105 LFA-1pos, and 12,750 LFA-1neg cells were transplanted and with IL-8, a total number of 5 × 105 LFA-1pos and 25,000 LFA-1neg cells were transplanted. Survival data are expressed as percentages of 10 mice transplanted per group in one experiment.
Survival data of lethally irradiated (8.75 Gy) recipient mice for at least 4 weeks after transplantation of LFA-1 sorted cytokine-mobilized blood cells. Donor mice were pretreated with daily IP injections of 5 μg G-CSF for 3 days or a single IP injection of 30 μg IL-8. With G-CSF, a total number of 1.5 × 105 MNC (LFA-1tot), 1.5 × 105 LFA-1pos, and 12,750 LFA-1neg cells were transplanted and with IL-8, a total number of 5 × 105 LFA-1pos and 25,000 LFA-1neg cells were transplanted. Survival data are expressed as percentages of 10 mice transplanted per group in one experiment.
LTRA of bone marrow–derived and cytokine-mobilized blood-derived LFA-1neg cells.
In addition to radioprotection or short-term repopulating ability (STRA), also LTRA was assessed by long-term bone marrow function of bone marrow–derived, as well as G-CSF– and IL-8–mobilized LFA-1neg cells (Table 1). The time interval after transplantation between the three groups varied, ie, 8 months for BM recipients, 7 months for IL-8, and 5 months for recipients transplanted with G-CSF–mobilized cells. To exclude lineage-specific chimerism, three mice from each group were killed and blood was obtained by cardiac puncture. Granulocytes, B lymphocytes, and T lymphocytes were sorted from individual mice. The mean percentage of male cells for the three lineages was 99%, 98%, and 84% for BM, 76%, 91%, and 76% for IL-8, and 75%, 73%, and 59% for G-CSF–transplanted recipient mice, showing that chimerism was multilineage.
Peripheral Blood Cell Counts in Recipient Mice After Transplantation of LFA-1neg MNC
| . | Transplanted Cell Populations . | ||
|---|---|---|---|
| Bone Marrow . | G-CSF– Mobilized . | IL-8– Mobilized . | |
| Months since transplantation | 8 | 5 | 7 |
| No. LFA-1neg MNC transplanted | 10,000 | 12,750 | 25,000 |
| White blood cells × 109/L | 16.7 ± 2.5 | 17.4 ± 0.9 | 16.0 ± 6.7 |
| Red blood cells × 1012/L | 8.3 ± 0.8 | 7.5 ± 1.5 | 7.4 ± 1.8 |
| Platelets × 109/L | 598 ± 237 | 429 ± 54 | 531 ± 155 |
| . | Transplanted Cell Populations . | ||
|---|---|---|---|
| Bone Marrow . | G-CSF– Mobilized . | IL-8– Mobilized . | |
| Months since transplantation | 8 | 5 | 7 |
| No. LFA-1neg MNC transplanted | 10,000 | 12,750 | 25,000 |
| White blood cells × 109/L | 16.7 ± 2.5 | 17.4 ± 0.9 | 16.0 ± 6.7 |
| Red blood cells × 1012/L | 8.3 ± 0.8 | 7.5 ± 1.5 | 7.4 ± 1.8 |
| Platelets × 109/L | 598 ± 237 | 429 ± 54 | 531 ± 155 |
Results are expressed as means ± SD of three mice per group.
Expression and function of LFA-1 on primitive HPC.
Because LFA-1 is expressed on all leukocytes,23LFA-1pos MNC will contain mainly lymphocytes, monocytes, and mature committed progenitor cells. We therefore studied the expression and functional role of LFA-1 on primitive HPC with repopulating ability, represented by bone marrow-derived WGA+/Lin−/Rho−cells.17 More than 95% of the sorted cells were LFA-1neg. Cultures of 750 sorted cells showed that the LFA-1neg fraction contained all CFU with a high plating efficiency of ± 33% (247 v 1, mean, n = 4 experiments). Transplantation of 150 Rho− LFA-1neg or up to 600 Rho− LFA-1pos cells protected 100% and 0% of lethally irradiated recipient mice, respectively (n = 10 mice per group in one experiment).
DISCUSSION
A number of studies has indicated the variable expression of LFA-1 on human CD34+ cells in steady state bone marrow, as well as cytokine-mobilized, blood.13,24-28 In addition, several reports have demonstrated that the in vitro expression of LFA-1 is confined to the more mature HPC.9,11,13 However, no such studies are reported on murine HPC. Therefore, we first examined the expression of LFA-1 on bone marrow–derived and cytokine-mobilized MNC. In accordance with Miller et al,29 approximately 40% to 50% of murine bone marrow MNC were LFA-1neg. In cytokine-mobilized blood, 5% to 10% of IL-8– as well as G-CSF–mobilized, blood MNC were LFA-1neg. Virtually no expression of LFA-1 was found on primitive HPC. The differences in LFA-1 expression between cytokine-mobilized peripheral blood (90% to 95%), steady-state bone marrow (50% to 60%), and primitive HPC (<5%) coincides with the expected number of mature leukocytes in these fractions, which all express LFA-123 and is reflected in the different plating efficiencies of sorted LFA-1negcells from these fractions (cytokine-mobilized blood 0.1% to 0.4%, bone marrow 2.4%, and primitive HPC 33%).
Several studies report the induction of LFA-1 on primitive LFA-1neg bone marrow cells during in vitro culture in humans13 and mice.30 The acquisition of LFA-1 did not preclude the multilineage colony-forming potential of these cells.13 In contrast, the growth potential of CD34+LFA-1pos sorted BM cells was much less and consisted of small macrophage-like colonies.13 Therefore, it was postulated for human HPC that LFA-1 is expressed by default, providing maturing bone marrow cells with adhesion molecules enabling migration into the peripheral blood. Our data do not support the active downregulation of LFA-1 on HPC in the bone marrow microenvironment, as in cytokine-mobilized blood, both colony formation and radioprotective capacity were confined to the LFA-1neg cell fraction, indicating that in vivo circulating stem cells do not acquire LFA-1 after mobilization.
The possibility was considered that the results of transplantation experiments with mobilized blood were determined by the absolute numbers of repopulating cells present in the LFA-1neg and positive cell fractions, ie, it was conceivable that the absolute number of repopulating cells in the LFA-1pos fraction was equal or even higher than in the LFA-1neg fraction. Cell doses of the LFA-1neg and LFA-1pos fractions were therefore chosen in relation to the total number of MNC required for radioprotection.20 22 A number of 150,000 G-CSF–mobilized MNC resulted in 60% radioprotection. An equal number of LFA-1pos cells did not mediate radioprotection, whereas transplantation of 12,750 LFA-1neg cells resulted in 70% survival. From these data, we concluded that the cells responsible for radioprotection after transplantation of unpurified blood resided completely in the LFA-1neg cell fraction.
In conclusion, we showed that the majority of murine bone marrow– and cytokine–mobilized blood-derived CFCs, cells with radioprotective capacity, and primitive HPC do not express LFA-1. In accordance with these results, a recent report defines the phenotype of a Sca+/Lin− engrafting murine progenitor cell as LFA-1neg.31 Our data are compatible with human in vivo studies, as patients with the leukocyte adhesion deficiency syndrome (LAD), characterized by mutations in the integrin β2 chain, lack defects in early hematopoiesis.32 Furthermore, treatment with anti–LFA-1 antibodies as part of the conditioning regimen for allogeneic bone marrow transplantation in immunodeficient children improved engraftment.33,34 As stem cells appear not to express LFA-1, whereas almost all leukemias do,35 negative selection for LFA-1 could be a useful addition in purging of autologous bone marrrow transplants.36
Recently, we reported that anti–LFA-1 blocking antibodies completely prevent the rapid IL-8–induced mobilization of HPC,14suggesting a direct effect of the antibody on LFA-1 expressed on HPC. However, our results clearly show that HPC with both colony-forming and radioprotective capacity does not express LFA-1 and therefore these cells are unable to function as direct targets for the blocking antibody. These data seem to indicate that an accessory cell, expressing LFA-1, as well as IL-8 receptors, plays an important role in IL-8–induced mobilization. IL-8 is a potent inducer of the release of matrix metalloproteinases as gelatinase B (MMP-9) by neutrophils.37 Furthermore, preliminary experiments in monkeys have indicated the rapid induction of MMP-9 by IL-8 and neutralizing antibodies directed against MMP-9 block the IL-8–induced mobilization of HPC.38 Taken together, our data support the hypothesis that neutrophils, expressing LFA-1, may serve as intermediate cells in the induction of mobilization. In accordance with this hypothesis, Liu et al39 have found severely impaired IL-8–induced mobilization in G-CSF receptor-deficient mice. Further studies are underway to study the possible role of neutrophils and metalloproteinases as key regulators in IL-8–induced stem cell mobilization.
ACKNOWLEDGMENT
The authors thank Maarten van der Keur and Arie van der Marel for their technical assistance in the cell sorting experiments, Peter de Jong for his excellent animal care, Karin Kleiverda for performing the in situ hybridizations, and Sandra van Vliet for purifying the LFA-1 antibodies.
Supported by the Dutch Cancer Society (NKB) Grant No. RUL 95-1091.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Johannes F.M. Pruijt, MD, Department of Hematology, Leiden University Medical Center, Bldg 1, C2-R, PO Box 9600, 2300 RC Leiden, The Netherlands.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal