Abstract
Th2 cell development is critically dependent on the presence of interleukin-4 (IL-4) at priming. The cellular origin and the mechanisms regulating this early production of IL-4 at the site of naive T-cell priming are extensively investigated. We previously reported that anti-CD3–activated and CD28-costimulated naive human CD4+ T cells themselves release very low but sufficient levels of IL-4 to support their development into high IL-4–producing cells. We show here that ligation of OX40 Ag, a member of the tumor necrosis factor receptor (TNF-R) family, on activated umbilical cord blood CD4+ T cells upregulates IL-4 production at priming and thereby promotes their development into effector cells producing high levels of the type 2 cytokines IL-4, IL-5, and IL-13. OX40 ligation increases four times the expression of IL-4 mRNA after 48 hours of anti-CD3/B7.1 activation and significantly augments the release of IL-4 and IL-13 in primary cultures. The effects of OX40 costimulation on Th cell differentiation are observed in the presence of optimal and suboptimal CD28 stimulation. Because OX40 ligand is expressed on dendritic cells, the OX40 costimulation pathway may be involved in the physiological regulation of Th cell development by augmenting the differentiation of IL-4–producing cells.
© 1998 by The American Society of Hematology.
IN VIVO AND IN VITRO observations have indicated that Th subset development is regulated very early in the course of the immune response, at the time when naive CD4+T cells first encounter Ag presented by dendritic cells (DC).1,2 Several factors have been implicated in the regulation of naive T-cell differentiation into Th1 or Th2 effectors, including (1) the intensity and the nature of T-cell receptor (TCR)-mediated activation signal3-8; (2) the strength and the nature of costimulatory signals9-12; (3) the cytokine and hormonal milieu in which T cells are primed1,2,13; and (4) the genetic background of the naive T cells.14 Among all these factors, cytokines appear to exert the most important role, with interleukin-4 (IL-4) and IL-12 promoting Th2 and Th1 responses, respectively.15,16 The other factors may act by influencing the production of these two cytokines at priming. Whereas the cellular origin and the mechanisms leading to the early production of IL-12 at the time of T-cell priming have been elucidated,17-19 the origin and the regulation of IL-4 production at priming are still under investigation.20Depending on the experimental system used to induce a Th2 response, different types of cells were shown to be involved in the early production of IL-4, including CD4+ NK1.1+ T cells, γδ T cells, and FcεR1-bearing cells.21-24 More recent studies showed that naive T cells themselves may be the original source of IL-4, which they release in very small but sufficient amounts to promote their development into high IL-4 producers via an autocrine pathway.5,6,8,25,26 For example, cell-sorted purified and phenotypically naive murine CD4+ T cells were shown to release IL-4 at priming and to develop into Th2 effectors upon in vitro priming with a low concentration of Ag or altered peptide ligands.8 Moreover, the acquisition of a Th2 phenotype was abolished by neutralization of IL-4 in priming cultures. In agreement with these observations, we previously reported that virtually every naive human CD4+ T cell of neonatal or adult origin grown in single-cell culture developed into high IL-4/IL-5 producers after multiple cycles of stimulation with anti-CD3 monoclonal antibody (MoAb) immobilized on CD32/B7.1-transfected L cells and IL-2 expansion.27 That IL-4 was released during the first 3 days of anti-CD3/B7.1 activation was evidenced by the following three observations: (1) the addition of anti–IL-4R neutralizing Ab to primary cultures reduced the acquisition of IL-4/IL-5–producing capacity while increasing that of interferon-γ (IFN-γ)28; (2) IL-4 mRNA was detected after 48 hours of naive CD4+ T-cell stimulation with a mixture of soluble anti-CD3 and anti-CD28 MoAb; and (3) IL-4 protein could be measured in the supernatant fluids of priming cultures performed in the presence of anti–IL-4R blocking MoAb, preventing IL-4 consumption by activated naive CD4+ T cells.29 Further studies in the murine as well as in the human system showed that IL-4 production at priming was critically dependent on CD28 costimulation.8,29,30 Indeed, IL-4 production during primary activation of naive TCR Tg T cells by altered peptide ligand was strictly dependent on CD28/B7 costimulation.8Similarly, IL-4 release by anti-CD3–activated human naive T cells was significantly higher when the anti-CD3 MoAb was immobilized on CD32/B7.1 L transfectants expressing high levels of B7.1 than when it was immobilized on CD32 single L transfectants, expressing low but functionally sufficient levels of mouse B7.1.29 We are here reporting a novel mechanism that may regulate IL-4 production at priming in the presence of both optimal and suboptimal CD28 costimulation. We provide evidence that ligation of OX40 Ag, a member of the tumor necrosis factor receptor (TNF-R) family,31 increases IL-4 production by naive T cells and promotes their development into effector cells producing high levels of the Th2 cytokines IL-4, IL-5, and IL-13.
MATERIALS AND METHODS
Reagents.
Anti-CD3 MoAb (64.1) was a gift of Bristol-Myers (Seattle, WA). Anti-OX40 MoAbs (315 and 131) and anti-OX40L/gp34 MoAb (5A8) were described previously.32,33 Anti-OX40 MoAb (Act35) was purchased from PharMingen (Mississauga, Ontario, Canada). Neutralizing anti–IL-4 receptor MoAb (MoAb 230) was from R&D Systems (Minneapolis, MN). Isotype-matched negative control MoAbs (mouse IgG1 and mouse IgG2a) were prepared in our laboratory. Recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF), rhIL-4, rhIL-2, and rhIL-12 were kindly provided by Dr D. Bron (Institute Bordet, Brussels, Belgium), Dr C. Maliszewski (Immunex Corp, Seattle, WA), Dr F.K. Kahn (Hoffmann-La Roche, Nutley, NJ), and Dr M. Gately (Hoffmann-La Roche), respectively. Recombinant human tumor necrosis factor-α (rhTNF-α) was purchased from Genzyme (Cambridge, MA). CD32 and B7.1 double-transfected mouse L fibroblasts were prepared and selected, as described,28 for the expression of high levels of B7.1. Human OX40L cDNA in pDC410 generated by Immunex Corp was subcloned into pBJ expression vector.34 OX40L transfected L fibroblasts were prepared as described.35
CD4+ T-cell purification and culture conditions.
CD4+ T cells were isolated from umbilical cord blood of healthy neonates as described.28 Briefly, mononuclear cells were obtained by centrifugation on Lymphoprep (Nycomed Pharma As, Oslo, Norway) and were treated with L-Leucine methyl ester to remove monocytes and natural killer (NK) cells. Cell preparation was enriched in T cells by E-rosetting and CD4+ cells were obtained by treating rosette-forming cells with Lympho-kwik T helper (One Lambda, Canoga Park, CA). The resulting populations were greater than 98% viable (trypan blue negative); were greater than 98% CD3+, CD4+/CD8−, and CD45RA+; and contained no detectable CD45ROhi, CD25+, CD19+, and CD56+ cells.
CD4+ T cells (1 × 106/mL) were cultured in 48-well culture plates in 0.4 mL of RPMI 1640 medium containing 10% fetal calf serum (FCS), 5 mmol/L L-glutamine, 50 IU penicillin G, and 50 μg streptomycin and were primed with anti-CD3 MoAb (100 ng/mL) and anti-OX40 MoAb (5 μg/mL) or control mouse IgG in the presence of mitomycin C-treated CD32/B7.1 L cells (0.25 × 106/mL). Alternatively, T cells were stimulated with anti-CD3 (100 ng/mL) and mitomycin C-treated OX40L L cells or L cells (0.25 × 106/mL) in 24-well plates (1 mL/ well). After 3 days, cells were washed and cultured at 0.25 × 106/mL in culture medium supplemented with 50 U/mL rhIL-2 in 24-well plates. After 4 days of IL-2 expansion, cells were washed and stimulated for cytokine production.
Dendritic cell preparation and mixed leukocyte cultures (MLR).
Monocyte-derived DC (Mo-DC) were prepared as previously described.36 Monocytes were isolated from peripheral blood mononuclear cells (PBMC) from healthy donors by cold aggregation followed by E-rosetting and plastic plate adhesion. The adherent cells were cultures in RPMI/10% FCS supplemented with 800 U/mL GM-CSF and 25 ng/mL IL-4. On day 4, the cultures were fed by replacing two thirds of supernatant fluids with fresh medium containing GM-CSF, IL-4, and TNF-α (final concentration, 10 ng/mL). After 7 days of culture, the cells were washed and treated with mitomycin C (25 μg/mL for 30 minutes).
Primary MLR were conducted in 96-well U-bottom tissue culture plates by adding different numbers of mitomycin C-treated Mo-DC to 2 × 105 allogenic CD4+ T cells in 200 μL culture medium in the presence of anti-OX40L MoAb (5A8) or control mouse IgG1 (10 μg/mL). After 5 days of culture, 100 μL of supernatants was replaced with fresh medium containing 100 U/mL of IL-2. On days 7 and 9, cultures were split and expanded in the presence of 50 U/mL of IL-2. After 5 days of IL-2 expansion, cells were washed and tested for cytokine production.
Flow cytometric analysis.
Cells were stained with fluorescein isothiocyanate (FITC)-conjugated Act35 or control mouse IgG1. Stained cells were analyzed with a FACSort (Becton Dickinson, Mountain View, CA).
Cytokine measurements.
To determine cytokine production capacity, T cells (1 × 106/mL) were restimulated with anti-CD3 (100 ng/mL) immobilized on mitomycin C-treated CD32/B7.1 L cells (0.25 × 106/mL) and the cell-free supernatants were collected after 24 or 48 hours, as indicated. IL-4, IL-5, and IFN-γ were measured using a two-site sandwich enzyme-linked immunosorbent assay (ELISA) or radioimmunoassay (RIA), exactly as described.28 IL-13 and IL-4 in primary cultures were measured with Quantikine IL-13 and Quantikine HS IL-4 (R&D Systems), respectively.
IL-4 mRNA analysis.
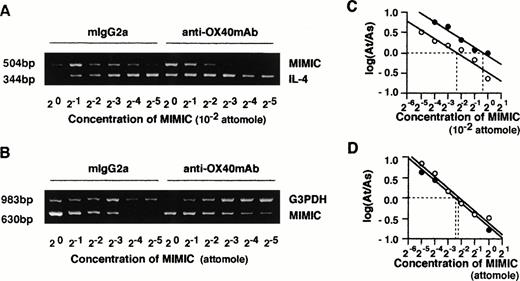
T cells were collected after 48 hours of anti-CD3/B7.1 stimulation and total RNA was prepared with RNAeasy Total RNA kit (Qiagen, Chasworth, CA). One microgram of RNA from each sample was reverse transcribed by GeneAmp RNA polymerase chain reaction (PCR) kit from Perkin Elmer (Cetus, Emeryville, CA) with oligo d(T)16 as the first-strand cDNA primer. One twentieth volume of reverse-transcription product was mixed with known quantities of serially diluted competitive internal standards (PCR MIMICs; Clontech, Palo Alto, CA) and was subjected to quantitative reverse transcriptase-PCR (RT-PCR) according to the manufacturer’s protocol. Target-specific primer pairs of IL-4 and G3PDH were also purchased from Clontech. After 40 cycles of amplification, the PCR products were resolved on a 1.8% agarose gel containing ethidium bromide. The intensities of competitor-generated bands and the cDNA sample-generated bands were compared to determine the quantity of target gene product. The amount of target cDNA was ascertained by determining the amount of competitor required to produce equal molar quantities of target and competitor products. The photographs of agarose gels were further analyzed by computer imaging (NIH image, version 1.61; National Institutes of Health, Bethesda, MD) and the ratio of mean histogram of target-generated to competitor-generated band was calculated. The specificity of the amplified bands was validated by their predicted size.
Statistical analysis.
The paired t-test was used to determine statistical significance of the data. Vaules of P < .05 were chosen for rejection of the null hypothesis. The cDNA concentrations were calculated by linear regression test after logarithmic transformation of the concentrations of competitors and the ratio of gel band intensities. The 95% confidence limits are shown in parenthesis.
RESULTS
Ligation of OX40 promotes the development of naive T cells into Th2-like effectors.
In initial studies we determined the time course expression of OX40 on neonatal CD4+ T cells activated with anti-CD3 MoAb immobilized on CD32/B7.1 L transfectants. OX40 was detected by flow cytometry using FITC-conjugated MoAb (clone Act35) after 1, 8, 24, 48, 72, and 168 hours of stimulation. As shown in Fig 1, OX40 was absent on freshly isolated T cells, became detectable after 8 hours of stimulation, reached maximal levels at 48 hours, and was still upregulated at 168 hours after activation. Further experiments showed that the addition of CTLA4-Ig to these cultures slightly delayed but did not reduce OX40 expression after 40 hours of activation, suggesting that TCR/CD3-mediated signals were sufficient for OX40 expression (data not detailed). The influence of OX40 ligation on Th cell development was next examined in three-step cultures in which neonatal CD4+T cells were first activated with anti-CD3 MoAb cross-linked on CD32/B7.1 L cells (refered to hereafter as anti-CD3/B7.1 activation) in the presence of anti-OX40 MoAb or isotype control mouse IgG. After 3 days of priming, cells were washed and expanded with IL-2 for 4 days. The cells were then washed and restimulated (anti-CD3/B7.1) for cytokine production. As seen in Fig 2A, cells primed in the presence of anti-OX40 MoAb (clone 315) produced much more IL-4 (3.7-fold increase), IL-13 (2.7-fold increase), and IL-5 (3.0-fold increase) but significantly less IFN-γ (75% inhibition) than did cells primed in the presence of control mouse IgG. Similar results were obtained by using another anti-OX40 MoAb (clone 131), whereas a third clone (Act35) used to stain the cells in Fig 1 was inactive. To ascertain that OX40 signaling may skew the cytokine production pattern of developing Th cells toward Th2, naive CD4+ T cells were primed with anti-CD3 MoAb (clone 64.1) in the presence of OX40 ligand (OX40L)-transfected L cells or untransfected L cells. As previously reported,29 64.1 anti-CD3 MoAb is capable of triggering naive T-cell proliferation and maturation when used in soluble form together with either anti-CD28 MoAb or untransfected L cells, expressing low but functional levels of mouse B7.1. The three experiments summarized in Fig 2B showed that cells primed in the presence of OX40L transfectants produced at least 10 times more IL-4 and much less IFN-γ than did those primed in the presence of L cells. As expected,29 the basal levels of IL-4 production by T cells primed with anti-CD3 together with L cells (Fig 2B) were lower than those of anti-CD3/B7.1–primed T cells (Fig 2A), confirming the enhancing effect of CD28 cosignal on IL-4 production. Collectively, these two series of experiments thus indicated that OX40 ligation favored the differentiation of IL-4–producing effector cells in the presence of both optimal (Fig 2A) or suboptimal (Fig 2B) CD28 costimulation. Because OX40L is expressed on DC,36 we next examined the role of OX40/OX40L costimulation pathway in the differentiation of naive CD4+ T cells stimulated with allogeneic DC. Neonatal CD4+ T cells were cocultured with allogeneic DC at low (15/1) or high (300/1) T cells/DC ratios in the presence of a blocking anti-OX40L MoAb (clone 5A8) or isotype control mouse IgG. These cultures were supplemented with IL-2 at day 5 and T cells were stimulated for cytokine production at day 10. As expected,19 cells primed at a high T/DC ratio produced significantly more IL-4 and IL-5 but slightly less IFN-γ than did those primed at a low T/DC ratio (Fig 2C). In each case, anti-OX40L MoAb significantly inhibited the development of IL-4/IL-5–producing cells, a finding consistent with the notion that signaling through OX40 enhances the acquisition of IL-4–producing capacity. Interestingly, anti-OX40L MoAb did not affect the development of IFN-γ–producing cells, indicating that blocking the OX40/OX40L pathway during naive T-cell/DC interaction suppressed the acquisition of IL-4– but not of IFN-γ–producing capacity.
Expression of OX40 Ag on naive T cells. Neonatal CD4+ T cells were stimulated with anti-CD3 MoAb and CD32/B7.1 L cells and stained after the indicated intervals with FITC-conjugated Act35 (dark lines) or control mouse IgG1 (faint lines).
Expression of OX40 Ag on naive T cells. Neonatal CD4+ T cells were stimulated with anti-CD3 MoAb and CD32/B7.1 L cells and stained after the indicated intervals with FITC-conjugated Act35 (dark lines) or control mouse IgG1 (faint lines).
OX40 costimulation at priming deviates the cytokine production profile of primed T cells. T cells were primed either with anti-CD3 and anti-OX40 (clone 315) or control mouse IgG2a together with CD32/B7.1 L cells (A) or with anti-CD3 and OX40L L cells (B). In (C), T cells were cocultured with allogenic DC in the presence of anti-OX40L or control mouse IgG1. In each case, cells were restimulated for cytokine production after IL-2 expansion. IL-4, IL-5, and IFN-γ were measured after 24 hours and IL-13 after 48 hours of stimulation. Shown are the mean ± SEM of five experiments. *P < .05; **P < .01.
OX40 costimulation at priming deviates the cytokine production profile of primed T cells. T cells were primed either with anti-CD3 and anti-OX40 (clone 315) or control mouse IgG2a together with CD32/B7.1 L cells (A) or with anti-CD3 and OX40L L cells (B). In (C), T cells were cocultured with allogenic DC in the presence of anti-OX40L or control mouse IgG1. In each case, cells were restimulated for cytokine production after IL-2 expansion. IL-4, IL-5, and IFN-γ were measured after 24 hours and IL-13 after 48 hours of stimulation. Shown are the mean ± SEM of five experiments. *P < .05; **P < .01.
OX40 ligation enhances IL-4 production at priming.
Given the essential role of IL-4 in the generation of Th2 cells, we examined whether the effects of OX40 ligation on naive T-cell maturation were dependent on the endogenous release of IL-4 in primary cultures. This appeared to be the case inasmuch as addition of neutralizing anti–IL-4R MoAb or anti–IL-4 MoAb to primary cultures markedly inhibited the effects of OX40 ligation on Th cell differentiation (Fig 3). These results not only indicated that the effects of OX40 costimulation were IL-4–dependent, but also supported the notion that IL-4 was produced in primary cultures. We next asked whether the OX40 cosignal enhanced the acquisition of IL-4–producing capacity by upregulating IL-4 expression during primary T-cell activation. IL-4 mRNA levels were measured 48 hours after anti-CD3/B7.1 activation of neonatal CD4+ T cells in the presence of anti-OX40 MoAb or control IgG by means of a quantitative RT-PCR assay using competitive PCR-MIMICS. These experiments (Fig 4) showed that OX40 ligation increased the expression of IL-4 transcription from 2.0 (1.3 to 3.3) to 8.1 (4.7 to 13.6) × 10−3 attomoles, corresponding to a fourfold increase. The decision to measure IL-4 transcripts after 48 hours of activation was based on previous studies, including ours,3,25 29showing that, whereas IL-4 mRNA is easily detected within a few hours after the activation of memory/effector CD4+ T cells, it becomes detectable only 36 to 48 hours after naive T-cell activation.
Effects of anti–IL-4R neutralizing Ab added at priming on the cytokine profile at restimulation. Cells were primed with anti-CD3, anti-OX40 (clone 315) or control mouse IgG2a, and CD32/B7.1 L cells in the presence of anti–IL-4R (5 μg/mL; ▧) or control mouse IgG2a (▧). Primed cells were restimulated for cytokine production as in the legend to Fig 2. Cells primed with anti-CD3 and CD32/B7.1 alone (□). Shown are the mean ± SEM of five experiments. *P < .05; **P < .01.
Effects of anti–IL-4R neutralizing Ab added at priming on the cytokine profile at restimulation. Cells were primed with anti-CD3, anti-OX40 (clone 315) or control mouse IgG2a, and CD32/B7.1 L cells in the presence of anti–IL-4R (5 μg/mL; ▧) or control mouse IgG2a (▧). Primed cells were restimulated for cytokine production as in the legend to Fig 2. Cells primed with anti-CD3 and CD32/B7.1 alone (□). Shown are the mean ± SEM of five experiments. *P < .05; **P < .01.
Quantitative analysis of IL-4 and G3PDH mRNA by competitive PCR. CD4+ T cells were stimulated with anti-CD3 MoAb and CD32/B7.1-transfected L cells in the presence of control mouse IgG2a or anti-OX40 MoAb (clone 315; 5 μg/mL) for 48 hours. Total RNAs of both groups of cells were prepared, and cDNA was synthesized. Quantitative PCR was performed in the presence of a twofold dilution of competitive internal standards (PCR MIMICs) of IL-4 (A) and G3PDH (B). The mean histogram of each band was analyzed by computer imaging. The ratio of target to competitors was plotted against the reciprocal of the concentrations of competitors added to the PCR reaction in log scale (C and D). Data were derived from RNA of (○) control IgG2a-treated cells and (•) anti-OX40 MoAb-treated cells.
Quantitative analysis of IL-4 and G3PDH mRNA by competitive PCR. CD4+ T cells were stimulated with anti-CD3 MoAb and CD32/B7.1-transfected L cells in the presence of control mouse IgG2a or anti-OX40 MoAb (clone 315; 5 μg/mL) for 48 hours. Total RNAs of both groups of cells were prepared, and cDNA was synthesized. Quantitative PCR was performed in the presence of a twofold dilution of competitive internal standards (PCR MIMICs) of IL-4 (A) and G3PDH (B). The mean histogram of each band was analyzed by computer imaging. The ratio of target to competitors was plotted against the reciprocal of the concentrations of competitors added to the PCR reaction in log scale (C and D). Data were derived from RNA of (○) control IgG2a-treated cells and (•) anti-OX40 MoAb-treated cells.
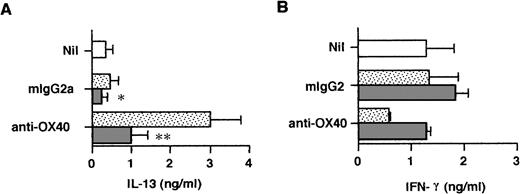
To examine the release of IL-4 in primary cultures, neonatal CD4+ T cells were stimulated for 3 days in the presence or absence of anti–IL-4R MoAb and the cell-free supernatant fluids were assayed by means of the ultrasensitive Quantikine HS IL-4 ELISA, allowing for the detection of 0.25 pg/mL of IL-4. As seen in Table 1, trace amounts of IL-4 were released by anti-CD3/B7–stimulated cells and these were marginally increased by anti-OX40 stimulation. However, the addition of IL-4R MoAb to priming cultures drastically increased IL-4 levels, indicating that endogenously produced IL-4 was consumed by activated naive T cells. Furthermore, OX40 ligation consistently increased the levels of IL-4 released in priming cultures. It is of note that the IL-4 levels measured in the five experiments summarized in Table 1 were quite variable and that the enhancing effect of OX40 costimulation on IL-4 protein production was less pronounced than that on IL-4 mRNA expression. A possible explanation is that IL-4 transcripts, unlike IL-4 protein, were measured after stimulation in the absence of IL-4R MoAb so that, as it has been suggested, endogenous IL-4 could upregulate IL-4 gene expression in an autocrine manner.37That naive T-cell–derived IL-4 may regulate cytokine production during the first 72 hours of primary activation was confirmed by measuring IL-13 and IFN-γ in the same culture supernatants as those used to measure IL-4 (Table 1). As seen in Fig 5, ligation of OX40 increased IL-13 and decreased IFN-γ production in primary culture and this effect was abolished by anti–IL-4R MoAb. Thus, the ability of OX40 costimulation to promote the development of anti-CD3/B7.1–activated neonatal CD4+ T cells into Th2-like cells appeared to result from increased IL-4 production at priming.
IL-4 Production in Primary Cultures
| . | Exp. 1 . | Exp. 2 . | Exp. 3 . | Exp. 4 . | Exp. 5 . |
|---|---|---|---|---|---|
| Nil | 0.68 | 1.16 | 0.73 | <0.25 | 5.14 |
| mIgG2a + mIgG2a | 0.47 | 0.69 | 1.03 | <0.25 | 4.95 |
| mIgG2a + anti-OX40 | 0.95 | 1.93 | 1.30 | 3.14 | 4.52 |
| Anti–IL-4R + mIgG2a | 23.53 | 15.18 | 14.14 | 1.20 | 100.20 |
| Anti–IL-4R + anti-OX40 | 40.32 | 28.86 | 23.16 | 21.08 | 158.03 |
| . | Exp. 1 . | Exp. 2 . | Exp. 3 . | Exp. 4 . | Exp. 5 . |
|---|---|---|---|---|---|
| Nil | 0.68 | 1.16 | 0.73 | <0.25 | 5.14 |
| mIgG2a + mIgG2a | 0.47 | 0.69 | 1.03 | <0.25 | 4.95 |
| mIgG2a + anti-OX40 | 0.95 | 1.93 | 1.30 | 3.14 | 4.52 |
| Anti–IL-4R + mIgG2a | 23.53 | 15.18 | 14.14 | 1.20 | 100.20 |
| Anti–IL-4R + anti-OX40 | 40.32 | 28.86 | 23.16 | 21.08 | 158.03 |
IL-4 was measured after 72 hours of anti-CD3/B7.1 stimulation by means of Quantikine HS IL-4 ELISA. All antibodies were used at 5 μg/mL. Values are given as picograms per milliliter.
OX40 ligation regulates IL-13 and IFN-γ production at priming. Cells were primed with anti-CD3, anti-OX40 (315) or control mouse IgG2a, and CD32/B7.1 L cells in the presence of anti–IL-4R (5 μg/mL; ▧) or control mouse IgG2a (▧). Cells primed with anti-CD3 and CD32/B7.1 alone (□). Supernatants were collected after 3 days for cytokine measurement. Shown are the mean ± SEM of five experiments. *P < .05; **P < .01.
OX40 ligation regulates IL-13 and IFN-γ production at priming. Cells were primed with anti-CD3, anti-OX40 (315) or control mouse IgG2a, and CD32/B7.1 L cells in the presence of anti–IL-4R (5 μg/mL; ▧) or control mouse IgG2a (▧). Cells primed with anti-CD3 and CD32/B7.1 alone (□). Supernatants were collected after 3 days for cytokine measurement. Shown are the mean ± SEM of five experiments. *P < .05; **P < .01.
Effects of exogenous IL-12 on OX40-mediated Th2 cell development.
Because Th subset development is known to be critically dependent on the relative concentrations of IL-4 and IL-12 at priming, we next examined the influence of exogenous IL-12 on the maturation of anti-CD3/B7.1–activated and OX40-costimulated naive CD4+ T cells. Consistent with the notion that the effects of IL-4 dominate over those of IL-12, the experiments summarized in Fig 6 showed that, even at a high concentration, exogenous IL-12 did not affect the enhancing effect of OX40 costimulation on IL-4 production. However, IL-12 slightly but significantly reduced the OX40-mediated enhancement of IL-13 and IL-5 production, implicating that IL-12 may also downregulate these two Th2-type cytokines in an IL-4–independent manner. Previous studies in the mouse16 38 and in the human system (Byun and Delespesse, unpublished observation) have demonstrated that exogenous IL-4 reduces the ability of IL-12 to prime naive T cells for increased IFN-γ production. The data in Fig 6 showed that, in contrast to these reported effects of IL-4, OX40 costimulation did not inhibit the IL-12–mediated upregulation of IFN-γ production. These findings therefore implicate that OX40 costimulation may regulate naive T-cell development not only by increasing early IL-4 production, but also by additional unknown mechanisms. The ability of OX40 signaling to upregulate IL-4 without inhibiting IL-12–mediated upregulation of IFN-γ production suggests that, in priming conditions associated with high IL-12 production, the OX40 costimulation pathway may favor the early development of mixed populations of Th cells producing both type 1 and type 2 cytokines.
Interactions between exogenous IL-12 and OX40 costimulation at priming. Cells were primed with anti-CD3 and CD32/B7.1 L cells alone (□) or in the presence of anti-OX40 (315; ▧) or control mouse IgG2a (▧) together with the indicated concentrations of IL-12. Primed cells were examined for cytokine production. Shown are the mean ± SEM of five experiments. IL-12 significantly inhibits (P < .05) the enhancing effect of OX40 costimulation on IL-5 and IL-13, but not on IL-4 production.
Interactions between exogenous IL-12 and OX40 costimulation at priming. Cells were primed with anti-CD3 and CD32/B7.1 L cells alone (□) or in the presence of anti-OX40 (315; ▧) or control mouse IgG2a (▧) together with the indicated concentrations of IL-12. Primed cells were examined for cytokine production. Shown are the mean ± SEM of five experiments. IL-12 significantly inhibits (P < .05) the enhancing effect of OX40 costimulation on IL-5 and IL-13, but not on IL-4 production.
DISCUSSION
There is increasing evidence that conventional naive CD4+ T cells themselves may be the original source of IL-4 that is required for the development of Th2 response.5,6,8,25-27Phenotypically naive murine and human CD4+ T cells were shown to express IL-4 mRNA within 36 to 48 hours after primary activation and to release low levels of IL-4 protein after 3 to 4 days; moreover, their differentiation into IL-4–producing effectors is inhibited by the addition of neutralizing Abs to IL-4 or IL-4R in primary cultures. Thus, endogenously produced IL-4 appears to upregulate in an autocrine manner the acquisition of IL-4–producing capacity by developing Th cells. This notion is in sharp contrast with the demonstration that IL-4 production by differentiated Th2 cells is IL-4– and STAT-6–independent.39 Relatively little is known regarding the regulation of this early IL-4 production, with the noticeable exceptions that it is critically dependent on the strength of the TCR-mediated signals and that it may be upregulated by CD28 costimulation and APC-derived IL-6.8,25,29,30 Naive T cells stimulated with low concentrations of Ag or with low-affinity TCR binding altered peptides produce IL-4, provided that the T cells receive a CD28-mediated cosignal; in contrast, higher concentrations of Ag do not trigger IL-4 production, even in conjunction with CD28 costimulation.8 IL-6 was recently shown to promote Th2 development in vitro by enhancing IL-4 production at priming, a finding consistent with the earlier observation that NF–IL-6 and NF–IL-6β may regulate IL-4 gene promoter.25,40,41 The present results suggest an additional mechanism regulating IL-4 production at priming, involving the interaction between OX40 Ag, which is expressed on activated naive T cells, and its ligand, which is expressed on DC. The OX40 Ag is a member of the TNF-R superfamily and binds to high-affinity ligand (OX40L) expressed on APCs such as DC and activated B cells as well as endothelial cells.31-34,36Expression of OX40 is restricted to activated T cells, and engagement of OX40 by its ligand is known to costimulate the proliferation and the production of IL-2 and IL-4 by polyclonally activated adult T lymphocytes.31,34,42 OX40L, like the other members of the TNF superfamily (with the exception of TNF-β), is an integral type II membrane molecule capable of signaling the cells on which it is expressed.33,36,43 OX40L is constitutively expressed on a subset of differentiated DC and is inducible, via CD40 ligation, on more immature DC.36 Ligation of OX40L on CD40L-activated DC costimulates both their cytokine production (mainly IL-12, IL-1β, IL-6, and TNF-α) and their expression of surface costimulatory molecules; however, these effects are much more pronounced on immature than on differentiated DC.36 We have shown here that OX40 was expressed within 8 hours after anti-CD3/B7.1 activation of naive CD4+ T cells and that its ligation by selected MoAbs or by OX40L promoted the development of effector cells, producing much higher levels of the type 2 cytokines IL-4, IL-5, and IL-13. These effects of OX40 costimulation were observed in the presence of both infraoptimal and optimal CD28 costimulation; finally, the disruption of OX40/OX40L interaction in primary MLR between neonatal CD4+ T cells and DC markedly inhibited the development of IL-4/IL-5–producing allo-antigen primed cells. Two observations indicated that the effects of OX40 cosignal on naive T-cell maturation were secondary to increased IL-4 production at priming. First, they were abolished by the addition of neutralizing anti–IL-4R or anti–IL-4 MoAb to primary cultures (Fig3), indicating that they were dependent on endogenously released IL-4. Second, OX40 ligation significantly increased IL-4 mRNA expression and IL-4 secretion in primary cultures. The OX40-mediated upregulation of IL-4 at priming accounted for the increased production of IL-13 and the decreased production of IFN-γ in primary cultures. Because IL-13, like IL-4, downregulates IL-12 production by APC and because IFN-γ has the reverse effect,17 these findings suggest that OX40 may promote Th2 development not only via a direct effect on T cells, but also indirectly, by reducing IL-12 production by DC. However, and most interestingly, unlike exogenous IL-4, OX40 cosignal did not inhibit IL-12 priming for increased IFN-γ production,16,38 suggesting that this costimulation pathway may support the emergence of IL-4–producing effectors in the early stage of a polarized Th1 response against pathogens capable of triggering IL-12 production by APCs. Alternatively, it is also possible that, in the presence of IL-12, OX40 ligation favors the development of Th0 effectors, producing both IL-4 and IFN-γ, as described most recently.44 In fact, OX40/OX40L interaction is bidirectional. On one hand, engagement of OX40 on activated T cells by its ligand expressed on DC enhances their production of IL-4, whereas, on the other hand, ligation of OX40L on DC upregulates their production of IL-12.36
The mechanisms whereby OX40 upregulates IL-4 gene expression in naive T cells remain to be determined, and the OX40 signal transduction pathway is currently being investigated. Recent results showed that OX40 associates with TNF receptor-associated factor 2 (TRAF2) and TRAF3 and activates NF-κB as well as c-Jun, a component of AP-1.45Because these two transcription factors are known to upregulate IL-6 gene promoter activity,46 we have examined whether OX40 might increase IL-4 indirectly by increasing IL-6 expression. Indeed, naive T cells are capable of producing IL-6,47 and, as mentioned earlier, IL-6 promotes Th2 development presumably by augmenting IL-4 at priming.25 We found that OX40 ligation increased IL-6 mRNA expression in naive T cells by threefold (as shown by quantitative competitive RT-PCR) from 0.55 × 10−3 to 1.8 × 10−3 attomoles; however, the levels of IL-6 transcripts were one order of magnitude lower than those of IL-4 and, most importantly, neutralizing anti–IL-6R MoAb did not alter the effects of OX40 ligation on cytokine production (data not shown). Thus, the enhancing effect of OX40 ligation on IL-4 expression in naive T cells was not secondary to increased IL-6 production. There is increasing evidence that, in addition to being the most efficient APC to prime naive T cells, DC also play a decisive role in determining the fate of the activated T cells. As recently reviewed, DC may either initiate virogous T-cell–dependent immune response or induce T-cell tolerance/anergy.48 In addition to providing B7-dependent costimulation, which is critical for the development of Th2 response and enhances Th1 cell development,8 DC also regulate Th subset development by producing IL-1217,49 or by expressing costimulatory molecules belonging to the TNF/TNF receptor families. Some of these, such as OX40L, may promote the expression of Th2 cytokine, whereas others, such as 4-1BBL and CD40, favor Th1 cell development either directly or indirectly by inducing IL-12 production.11 50
Address reprint requests to Guy Delespesse, MD, PhD, Universitéde Montréal, Centre de recherche Louis-Charles Simard, Laboratoire de recherche en Allergie (M4211-K), Hôpital Notre-Dame, 1560 Sherbrooke St E, Montreal, Quebec H2L 4M1, Canada; e-mail: delespeg@ere.umontreal.CA.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal