Abstract
A number of studies have suggested that an immune response to human leukocyte antigen (HLA) alloantigens may contribute to protection against HIV infection. In the present study, we examined the effect of alloantigen-stimulated cell lines obtained from peripheral blood mononuclear cells (PBMC) of HIV-uninfected (HIV−) individuals and the soluble factors produced by these cell lines on HIV-1 replication. Multiple in vitro restimulation with irradiated allogeneic PBMC from HIV− donors resulted in the expansion of CD8+ T-cell lines that inhibited HIV-1 replication when cocultured with either autologous or heterologous in vitro–infected phytohemagglutinin (PHA) blasts. Supernatants from the alloantigen-stimulated cell lines also inhibited HIV replication in both PHA blasts and a chronically infected cell line. The alloantigen-stimulated cell lines and the factors they produced inhibited both T-cell–tropic (T) and macrophage-tropic (M) isolates of HIV-1. Blocking experiments using anti-chemokine antibodies suggested that this inhibition of HIV replication was not due to the β-chemokines present in cocultures of cell lines with HIV-infected blasts. These results indicate that alloantigen-stimulation of PBMC from HIV−individuals activates CD8+ T cells that produce soluble factor(s) that inhibit HIV replication of a wide spectrum of HIV-1 isolates through a chemokine-independent mechanism.
This is a US government work. There are no restrictions on its use.
EFFORTS TO DEVELOP an effective anti-HIV vaccine have predominantly concentrated on the development of subunit vaccines as well as inactivated and live attenuated virus vaccines.1,2 However, considerable evidence suggests that immune responses to major histocompatibility complex antigens (MHC) can contribute to protection against HIV-1 infection.3-7 The demonstration that immunization of macaques with allogeneic cynomolgous lymphocytes can protect against challenge infection with SIV grown in simian cells raises the possibility that responses to foreign MHC might have implications for AIDS vaccine design.1 The role of MHC in protection against HIV infection is further supported by the observation that immunization with purified human leukocyte antigens (HLA) can protect macaques against challenge with human cell-grown virus.8,9 In addition, cellular proteins, including HLA antigens, have been identified on free virions, and these virus-associated antigens can be detected on the surface of infected cells.10 Three dimensional homologies between HIV-1 envelope proteins and HLA antigens have been reported and raise the possibility that anti-HLA responses will induce cross-reactive immune responses to HIV envelope determinants.11-13 The recent report that HIV-1 replication can be inhibited by alloantigen-stimulated cells adds an additional potential protective component to alloimmunization.14
The production of soluble anti-HIV factors by non-cytolytic CD8+ T cells, originally described by Walker et al,15-19 has become a topic of intense investigation.20-22 The C-C chemokines RANTES, MIP-1α, and MIP-1β were recently identified as major components of an HIV-suppressive factor produced by immortalized and primary CD8+ T cell lines.22 These chemokines suppress infection by primary and monocyte/macrophage (M)-tropic HIV-1 isolates, whereas T-cell–adapted viral strains appear to be resistant to the chemokine effects.23 24
In the present study we analyzed the effect of T-cell lines generated by stimulation with allogeneic peripheral blood mononuclear cells (PBMC) and the alloantigen-stimulated factors they produce on the replication of HIV-1 in human T-cell blasts and a chronically infected cell line. Our results show that allogeneic stimulation can induce production of anti–HIV-1 soluble factor(s) that suppress T-cell (T)–tropic and M-tropic viral isolates and that the chemokines recently reported to inhibit HIV-1 replication are not responsible for this antiviral effect.
MATERIALS AND METHODS
Cells.
Mononuclear cells were isolated by density gradient centrifugation from heparinized peripheral blood from healthy HIV-seronegative (HIV−) blood donors who were volunteers for platelets at the National Institutes of Health Department of Transfusion Medicine. The PBMC were washed twice in phosphate-buffered saline (PBS; Life Technologies, Gaithersburg, MD) and were resuspended at 3 × 106/mL in RPMI 1640 (Life Technologies) supplemented with 10% heat-inactivated fetal calf serum (FCS; Hyclone, Logan, UT), 2 mmol/L L-glutamine, penicillin (100 U/mL), and streptomycin (100 μg/mL).
Viral stocks.
The primary isolates HIV-1BZ167, HIV-1CD, and HIV-1EV were grown in human phytohemagglutinin (PHA) blasts, as previously described.25 HIV-1Ba-Lwas grown in monocyte-derived macrophages. Cell-free supernatants were titered for p24 core Ag by enzyme-linked immunosorbent assay (ELISA; AIDS Vaccine Program, NCI, Frederick MD).
To determine viral coreceptor usage, primary isolates (HIV-1BZ167, HIV-1CD, and HIV-1EV) were used to infect U87 cells stably transfected with CD4 and CCR1, CCR2B , CCR3, CCR4, CCR5, or CXCR4. U87 cells expressing CD4 linked to the neomycin resistance gene and individual chemokine receptors for CCR1, CCR2B, CCR4, CCR5, or CXCR4 cloned into the pBABE-puro plasmid were selected in 300 mg/mL G418 (GIBCO, Grand Island, NY) and 1mg/mL puromycin (Sigma, St Louis, MO). Lines were plated in 6-well dishes and grown for 1 to 2 days until 50% confluent before infection. Infections were performed with 10 ng viral p24, corresponding to a multiplicity of infection (MOI) of 2 to 5, in DMEM containing 20% fetal calf serum (FCS), 2 mmol/L L-glutamine, and 50 mg/mL gentamicin. Cultures were observed daily for at least 1 week and refed every other day when samples were taken for p24 analysis. The maximum p24 level attained during the duration of the infection was recorded. Dual-tropic viruses were cloned by limiting dilution on U87/CXCR4 and U87/CCR5 cells cultured in 96-well microtiter plates and determined by this system to have approximately equivalent TCID50s, whether grown on CXCR4 or CCR5 cells. The viruses were scored according to the p24 shed after infection.
Generation of alloantigen-stimulated cell lines.
PBMC (3 × 106/mL) from randomly selected HIV-uninfected individuals were incubated with a pool of irradiated (50 Gy) allogeneic PBMC (1 × 106/mL) from four unrelated HIV-uninfected donors in tissue culture flasks (Costar, Cambridge, MA) for 7 days at 37°C in 7% CO2 humidified atmosphere. Seven days after the first round of stimulation, viable cells were obtained by density gradient centrifugation and restimulated with the same allogeneic cell pool. Three days after restimulation, media was changed and interleukin-2 (IL-2; 10 U/mL; Boehringer Mannheim, Indianapolis, IN) was added to the media. Five days after the fourth weekly round of allostimulation, the cells were harvested, washed in RPMI 1640, and used in cocultures with HIV-1–infected PHA-stimulated blasts.
In some experiments, we depleted the alloantigen-stimulated cell lines of CD4+ T cells using CD4 magnetic beads (Dynal, Lake Success, NY), according to manufacturer’s recommendations. After enrichment, the population contained about 92% CD8+ T cells and 5% CD4+ cells.
Generation of HIV-suppressive factors in vitro.
The cell lines (3 × 106 cells/mL) described above were stimulated in vitro with the pools of irradiated allogeneic PBMC (1 × 106 cells/mL). Supernatants were collected 2, 4, or 7 days after stimulation, filtered, and frozen at −70°C. Their anti-HIV activity was tested on in vitro HIV-infected PHA blasts, as described below. Unless otherwise stated, the final concentration of supernatant used in all experiments was 50% (vol/vol).
In some experiments, PBMC (3 × 106/mL) were stimulated with tetanus toxoid (1:400; Connaught Laboratories, Swiftwater, PA) or with immobilized anti-CD3 monoclonal antibody (Ortho-Biotech, Raritan, NJ; 10 μg/mL) for 5 days. Supernatants were obtained and tested for anti-HIV activity.
HIV-1 infection of PHA blasts.
PBMC were adjusted to 2 × 106/mL cells in RPMI 1640 supplemented with 10% heat-inactivated FCS and stimulated with PHA (2 μg/mL; Sigma) for 3 days at 37°C. The PHA blasts were then washed twice and the pellet was incubated for 1 hour with HIV-1 (at the indicated concentrations of virus), resuspended in RPMI 1640/FCS, and washed three times.
HIV-infected blasts (1 × 105 cells) were then cocultured with either allostimulated cell lines (1 × 105 cells) or supernatants derived from the alloantigen-stimulated cell lines in RPMI 1640 containing 10% FCS and 10 U/mL of IL-2 (in a total volume of 200 μL) in three or four replicates in flat-bottom 96-well plates (Costar). Supernatants (100 μL) of these cultures were collected and inactivated with 100 μL of 2% Triton X-100 (Sigma) 3 and 6 days post-infection and frozen at −20°C, and fresh complete media or cell line supernatants were added to each well. p24 antigen levels in these supernatants were determined by ELISA (Coulter, Westbrook, ME).
Experiments were performed using cell lines that were derived from the same donor cells as the PHA blasts (autologous) used for in vitro infection, or from cell lines derived from cells obtained from an unrelated donor (heterologous).
In some experiments, supernatants derived from allostimulated cell lines (100 μL) were incubated for 3 days with an HIV-1 chronically infected H9 cell line (2 × 104 cells/100 μL) (H9/HTLVIIIMN NIH 1984; AIDS Research and Reference Reagent Program, Rockville, MD) in flat bottom 96-well plates (Costar). Supernatants of culture were obtained and assayed for HIV-1 p24 antigen content as described above.
Quantitation of cytokine production after in vitro antigenic stimulation.
Cytokine levels from the culture supernatants were determined by ELISA. The following ELISA kits were used in this study: interferon-γ (IFN-γ; Genzyme, Cambridge, MA), tumor necrosis factor-α (TNF-α; PharMingen, San Diego, CA), IL-2 (Genzyme DuoSet, Cambridge, MA), IL-4, IL-16, IFN-α (Biosource International, Camarillo, CA), IL-10 (Genzyme), MIP-1α, MIP-1β, and RANTES (R&D, Cambridge, MA). All assays were performed in duplicate according to manufacturer’s instructions, and the cytokine levels were calculated by comparison to standard curves using recombinant cytokines.
Cytokine neutralization experiments of alloantigen-stimulated supernatants were performed using anti–MIP-1α, –MIP-1β, and -RANTES neutralizing antibodies (R&D; 50 μg/mL) or anti–IFN-γ antibody (Genzyme; 25 μg/mL) or mouse and goat isotype control antibodies (R&D; 50 μg/mL).
Flow cytometry analysis.
Immunofluorescence staining was performed by incubating 1 × 106 cells in wells of 96-well round-bottom plates in PBS containing 1% bovine serum albumin (BSA) and 0.1% NaN3. The cells were incubated with the relevant antibody (anti-CD3, anti-CD4, or anti-CD8; Becton Dickinson, Mountain View, CA) and control monoclonal antibodies directly conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE) at 4°C, for 30 minutes in the dark. After incubation, cells were washed three times with buffer, fixed with 2% paraformaldehyde in PBS, and analyzed by fluorescence-activated cell sorter (FACS; Becton Dickinson).
Chromium release assays.
Autologous uninfected and 3-day HIV-1BZ167–infected PHA blasts were used as targets in a standard chromium release assay. In some experiments, Epstein-Barr virus (EBV)–transformed B-cell lines pulsed with the HIV-1 envelope peptides T1, T2, TH4.1, P18 MN, P18 IIIB (5 μmol/L), or a specificity control myoglobin peptide were used as targets, as previously described.26 Briefly,51Cr-labeled target cells were incubated for 4 hours with the alloantigen-stimulated cell line at several E:T ratios, and51Cr release was determined in a γ-counter. The results are presented as percent specific lysis determined by the following equation:
where maximum release and spontaneous release were the 51Cr released during incubation with 2% Triton X-100 and medium alone, respectively.
RESULTS
Inhibition of HIV replication by allostimulated cell lines.
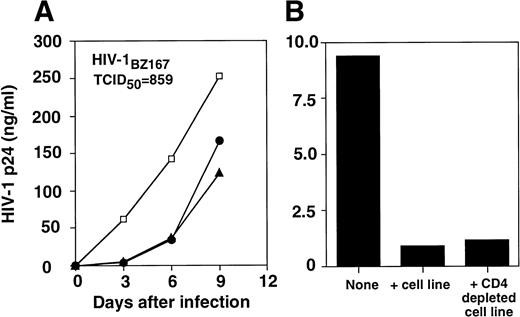
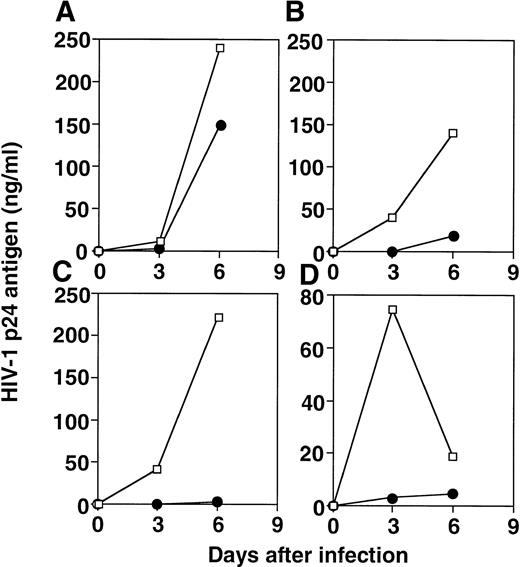
We investigated the effect of allostimulated cell lines obtained from different healthy blood bank donors on HIV replication in vitro using autologous or heterologous PHA-stimulated T-cell blasts. After six rounds of stimulation, the cell lines were comprised of 97% of CD3+ T cells (9% CD4+CD8−; 32% CD4+CD8+; 56% CD4−CD8+ for one representative cell line). The extracellular release of HIV-1 p24 core antigen assessed on culture supernatants at day 3, 6, and 9 postinfection is shown in Fig 1. The replication of the HIV-1BZ167 isolate, which uses predominantly CXCR4 and CCR3 coreceptors (G. Blatner and D.I. Cohen, manuscript submitted), was suppressed fivefold on day 6 in the wells of HIV-infected blasts in the presence of allostimulated cell lines. This antiviral activity was generated against HIV-infected PHA-activated targets that were autologous or heterologous to the alloantigen-stimulated cells (Fig 1A). A similar suppressive effect on viral replication was observed when infection was performed with 172 TCID50/105 cells (data not shown). The inhibitory effect observed on HIV-1BZ167 replication appears to be primarily mediated by CD8+ T cells (Fig 1B), because depletion of CD4+ T cells from alloantigen-stimulated cell lines, using anti-CD4 coated magnetic beads, did not appreciably alter the inhibitory effect on viral replication observed by the same cells before depletion of CD4+ T cells. This pattern of inhibition on viral replication was verified in five independent alloantigen-stimulated cell lines. Figure 2 illustrates the spectrum of suppressive effects of four different alloantigen-stimulated lines tested for their ability to inhibit viral replication, 3 and 6 days after infection.
Inhibition of HIV-1BZ167 replication by alloantigen-stimulated cell lines. (A) PHA blasts were infected with 859 TCID50/105 cells, as described in Materials and Methods section. Infected PHA blasts were cultured in the absence (□) or presence of an alloantigen-stimulated cell line derived from the same cells donor as the PHA blasts (autologous, •) or an alloantigen-stimulated cell line derived from an unrelated donor (heterologous, ▴). HIV-1 p24 core antigen was determined by ELISA. The results represent means of triplicate cultures. (B) HIV-1BZ167–infected PHA blasts (172 TCID50/105 cells) were incubated in the absence or presence of an alloantigen-stimulated cell line before and after depletion of CD4+ T cells, using anti-CD4–coated magnetic beads. Results are expressed as p24 antigen production from one experiment determined by ELISA.
Inhibition of HIV-1BZ167 replication by alloantigen-stimulated cell lines. (A) PHA blasts were infected with 859 TCID50/105 cells, as described in Materials and Methods section. Infected PHA blasts were cultured in the absence (□) or presence of an alloantigen-stimulated cell line derived from the same cells donor as the PHA blasts (autologous, •) or an alloantigen-stimulated cell line derived from an unrelated donor (heterologous, ▴). HIV-1 p24 core antigen was determined by ELISA. The results represent means of triplicate cultures. (B) HIV-1BZ167–infected PHA blasts (172 TCID50/105 cells) were incubated in the absence or presence of an alloantigen-stimulated cell line before and after depletion of CD4+ T cells, using anti-CD4–coated magnetic beads. Results are expressed as p24 antigen production from one experiment determined by ELISA.
Effect of four different alloantigen-stimulated cell lines on HIV-1 infection. PHA blasts were infected with HIV-1BZ167 (172 TCID50/105 cells) and cultured in the absence (□) or the presence (•) of alloantigen-stimulated cell lines, derived from cells obtained from the same donor as the one used to generate the blasts, at a ratio of 1:1. The respective percentages of inhibition on days 3 and 6 of culture were: (A) 82% and 39%; (B) 100% and 87%; (C) 100% and 99%; and (D) 96% and 74%. The results represent means of triplicate cultures.
Effect of four different alloantigen-stimulated cell lines on HIV-1 infection. PHA blasts were infected with HIV-1BZ167 (172 TCID50/105 cells) and cultured in the absence (□) or the presence (•) of alloantigen-stimulated cell lines, derived from cells obtained from the same donor as the one used to generate the blasts, at a ratio of 1:1. The respective percentages of inhibition on days 3 and 6 of culture were: (A) 82% and 39%; (B) 100% and 87%; (C) 100% and 99%; and (D) 96% and 74%. The results represent means of triplicate cultures.
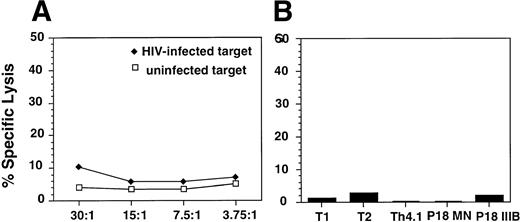
The antiviral effect observed was not due to cytolytic activity of the effector cells against the HIV-infected targets as determined by chromium-release assays using either HIV-infected targets (Fig 3A) or autologous EBV-transformed B-cell lines that were pulsed with HIV envelope peptides (Fig 3B), previously shown to be epitopes for HIV-specific cytolytic T cells in HIV-infected patients or in uninfected but exposed individuals.26 27 Furthermore, the supernatants obtained from alloantigen-stimulated cell lines did not inhibit the viability of the infected PHA blasts (data not shown).
Analysis of CTL responses of alloantigen-stimulated cells using a standard 51Cr release assay. (A) Uninfected (□) or HIV-1BZ167–infected (⧫) blasts were used as targets. The targets were incubated with the effector cells at the indicated E:T ratios for 4 hours. The results are expressed as percent specific lysis as described in Materials and Methods. (B) EBV-transformed B-cell lines from the same donor as the alloantigen-stimulated cell line were used as targets. Targets were pulsed overnight with the HIV-1 envelope peptides T1, T2, Th4.1, P18MN, P18 IIIB (5 μmol/L) and then incubated with the alloantigen-stimulated cell line for 4 hours. The results are represented as percent specific lysis observed at an E:T ratio of 60:1.
Analysis of CTL responses of alloantigen-stimulated cells using a standard 51Cr release assay. (A) Uninfected (□) or HIV-1BZ167–infected (⧫) blasts were used as targets. The targets were incubated with the effector cells at the indicated E:T ratios for 4 hours. The results are expressed as percent specific lysis as described in Materials and Methods. (B) EBV-transformed B-cell lines from the same donor as the alloantigen-stimulated cell line were used as targets. Targets were pulsed overnight with the HIV-1 envelope peptides T1, T2, Th4.1, P18MN, P18 IIIB (5 μmol/L) and then incubated with the alloantigen-stimulated cell line for 4 hours. The results are represented as percent specific lysis observed at an E:T ratio of 60:1.
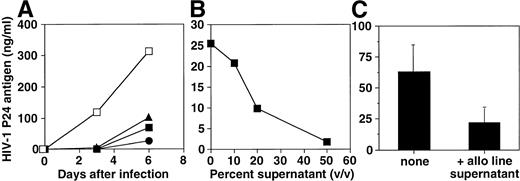
To investigate whether the antiviral effect was due to a soluble factor(s) induced by the alloantigenic stimulation, HIV-infected blasts were incubated in the presence of supernatants obtained from alloantigen-stimulated cell lines 2 to 7 days after stimulation (Fig 4A). These supernatants inhibited infection in a pattern similar to that observed for the cell lines, and the inhibitory effect was dose dependent (Fig 4B). To further address the mechanism of inhibition of HIV-1 replication by the soluble factor(s) generated by alloantigen stimulation, an alloantigen-stimulated supernatant was cocultured with an HIV-1MN chronically infected H9 cell line. Interestingly, we observed that this supernatant inhibited the HIV-1 replication of the chronically infected cell line, assessed by p24 antigen determination (Fig 4C).
Effect of supernatants from an alloantigen-stimulated cell line on HIV-1 infection. (A) PHA blasts were infected with HIV-1BZ167 (172 TCID50/105 cells) and cultured in the absence (□) or the presence of supernatants that were obtained 2 (•), 4 (▴), and 7 days (▪) after cell line stimulation with allogeneic cells. The results are expressed as mean of triplicate cultures and are representative of one of two independent experiments. (B) PHA blasts were infected with HIV-1BZ167(172 TCID50/105 cells) and cultured with different concentrations (vol/vol) of allostimulated cell line–derived supernatant collected 4 days after stimulation. p24 antigen production was assayed at 3 days postinfection by ELISA. The results are expressed as mean of triplicate cultures using a representative supernatant. (C) Effect of allostimulated cell line supernatant (1:2 dilution) on viral replication in the HIV-1MN chronically infected H9 cell line after 3 days of coculture. p24 antigen production was determined by ELISA. Results represent means ± SEM of three different experiments.
Effect of supernatants from an alloantigen-stimulated cell line on HIV-1 infection. (A) PHA blasts were infected with HIV-1BZ167 (172 TCID50/105 cells) and cultured in the absence (□) or the presence of supernatants that were obtained 2 (•), 4 (▴), and 7 days (▪) after cell line stimulation with allogeneic cells. The results are expressed as mean of triplicate cultures and are representative of one of two independent experiments. (B) PHA blasts were infected with HIV-1BZ167(172 TCID50/105 cells) and cultured with different concentrations (vol/vol) of allostimulated cell line–derived supernatant collected 4 days after stimulation. p24 antigen production was assayed at 3 days postinfection by ELISA. The results are expressed as mean of triplicate cultures using a representative supernatant. (C) Effect of allostimulated cell line supernatant (1:2 dilution) on viral replication in the HIV-1MN chronically infected H9 cell line after 3 days of coculture. p24 antigen production was determined by ELISA. Results represent means ± SEM of three different experiments.
To determine whether antigenic stimuli other than allogeneic PBMC would generate supernatants with anti-HIV activity, we stimulated PBMC with tetanus toxoid or with immobilized anti-CD3 and tested the antiviral activity on HIV-1BZ167–infected PHA blasts. We found that tetanus toxoid– or anti-CD3–stimulated supernatants did not appreciably inhibit HIV-1BZ167 replication when compared with the effect of alloantigen-stimulated supernatants (Table 1). Similarly, supernatants generated by an HTLV-1 tax peptide-specific CD8+ T cell clone28 did not inhibit HIV-1 replication, showing that not all antigen-stimulated CD8+ T cells produce this soluble factor(s) (data not shown). Furthermore, addition of autologous PBMC to the HIV-1 cultures, instead of the allostimulated cell lines, did not significantly decrease viral replication in infected cells as compared with the cultures in the presence of the alloantigen-stimulated cell lines or cell line supernatant (5 ± 14% and 88 ± 6% inhibition of viral replication in three experiments, respectively).
Effect of Supernatants Obtained From Several Types of Stimulation on HIV-1 p24 Antigen Production by Infected Lymphoblasts
| . | HIV-1 p24 Antigen (ng/mL) . |
|---|---|
| Control unstimulated supernatant | 66.9 ± 14.1 |
| Tetanus-stimulated supernatant | 124.3 ± 10.6 |
| Anti-CD3–stimulated supernatant | 46.7 ± 4.7 |
| Control alloantigen supernatant | 56.2 ± 38.3 |
| Alloantigen-stimulated supernatant | <2 |
| . | HIV-1 p24 Antigen (ng/mL) . |
|---|---|
| Control unstimulated supernatant | 66.9 ± 14.1 |
| Tetanus-stimulated supernatant | 124.3 ± 10.6 |
| Anti-CD3–stimulated supernatant | 46.7 ± 4.7 |
| Control alloantigen supernatant | 56.2 ± 38.3 |
| Alloantigen-stimulated supernatant | <2 |
The results represent average ± SD of two experiments performed in triplicate. PHA blasts were infected with HIV-1BZ167(172 TCID50/105 cells) and cultured in the presence of supernatants derived from alloantigen-stimulated cell lines, tetanus toxoid-, and anti-CD3–stimulated PBMC, as described in Materials and Methods. p24 antigen production was determined by ELISA 3 days post-infection. Control cultures for tetanus toxoid- or anti-CD3–stimulated supernatants were supernatants derived from unstimulated cells. Control cultures for alloantigen-stimulated cell line supernatant were culture media.
Inhibition of M-tropic and T-tropic isolates of HIV by alloantigen-stimulated supernatants.
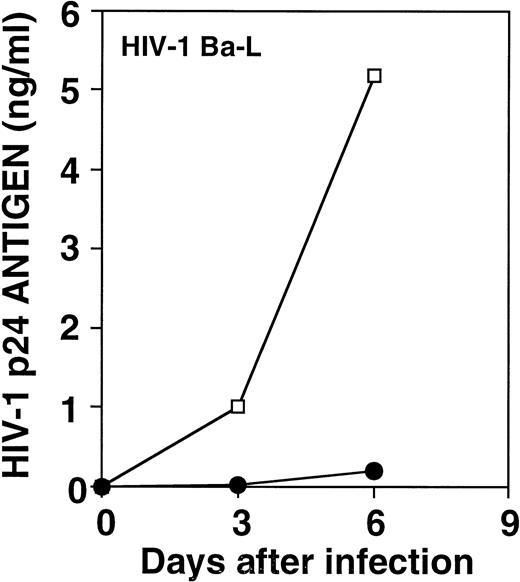
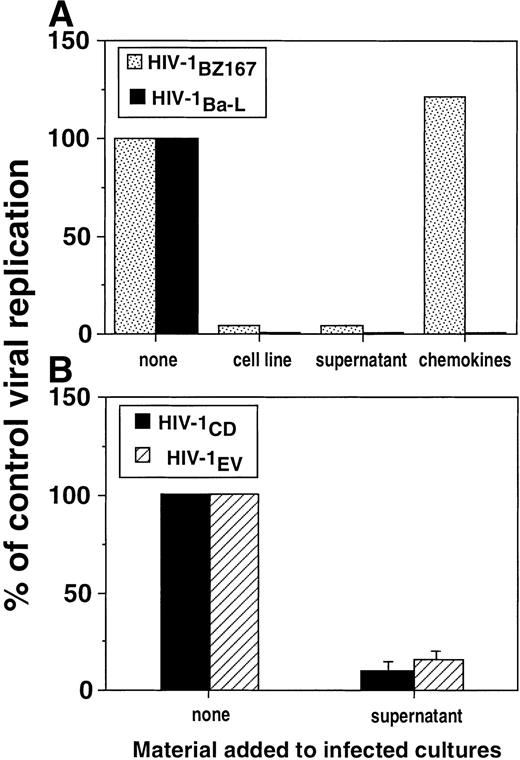
To test whether this suppression of HIV-1 replication would also inhibit an M-tropic isolate, we performed experiments in which HIV-1Ba-L was used to infect target cells. As shown in Fig 5, the alloantigen-stimulated cell line inhibited viral replication in PHA blasts infected by HIV-1Ba-L by a factor of 26-fold on day 6. In fact, allostimulated cell lines as well as their supernatants strongly inhibited HIV-1Ba-L replication 3 days post-infection in a similar fashion as observed for HIV-1BZ167(Fig 6A). Addition of β-chemokines at 200 ng/mL, a dose that was shown to efficiently inhibit HIV-1 replication of several primary isolates,24 suppressed the replication of HIV-1 Ba-L (Fig 6A), which has been previously described as a β-chemokine–sensitive isolate.22 However, the chemokines did not inhibit replication of HIV-1BZ167 under conditions in which allostimulated cell lines and their supernatants potently suppressed p24 antigen production (Fig 6A). The antiviral effect of an allostimulated cell line supernatant was also investigated on the viral isolates HIV-1CD (T-cell–tropic) and HIV-1EV (M-tropic), to address the extent of its activity. As shown in Fig 6B, this supernatant inhibited the replication of both viral isolates to identical levels.
Effect of an alloantigen-stimulated cell line on HIV-1Ba-L replication. PHA blasts were infected with HIV-1Ba-L (570 TCID50/105 cells) in the absence (□) or the presence (•) of an allostimulated cell line. Results represent means of triplicate cultures from one of four experiments with similar results.
Effect of an alloantigen-stimulated cell line on HIV-1Ba-L replication. PHA blasts were infected with HIV-1Ba-L (570 TCID50/105 cells) in the absence (□) or the presence (•) of an allostimulated cell line. Results represent means of triplicate cultures from one of four experiments with similar results.
Inhibition of replication of T-tropic and M-tropic HIV-1 isolates by alloantigen-stimulated cell lines and supernatants. (A) PHA blasts were infected with HIV-1BZ167 (▧, 172 TCID50/105 cells) or HIV-1Ba-L(▪, 570 TCID50/105 cells) and cultured with an alloantigen-stimulated cell line, its supernatant (1:2 dilution), and the β-chemokines MIP-1, MIP-1β, and RANTES (200 ng/mL of each). p24 antigen production was assayed at 3 days post-infection by ELISA. Results are expressed as the percentage of p24 production by infected cultures in the absence of any treatment and represent means of triplicate cultures. (B) PHA blasts were infected with HIV-1CD (▪) and HIV-1EV (▧) (20 ng p24/106 cells) and cultured with a supernatant derived from an alloantigen-stimulated cell line collected 4 days after alloantigenic stimulation. p24 production was assayed 3 days after infection by ELISA. Results are expressed as the percentage of p24 production by infected cultures in the absence of allostimulated supernatant. Results represent means ± SEM of two experiments performed in triplicate.
Inhibition of replication of T-tropic and M-tropic HIV-1 isolates by alloantigen-stimulated cell lines and supernatants. (A) PHA blasts were infected with HIV-1BZ167 (▧, 172 TCID50/105 cells) or HIV-1Ba-L(▪, 570 TCID50/105 cells) and cultured with an alloantigen-stimulated cell line, its supernatant (1:2 dilution), and the β-chemokines MIP-1, MIP-1β, and RANTES (200 ng/mL of each). p24 antigen production was assayed at 3 days post-infection by ELISA. Results are expressed as the percentage of p24 production by infected cultures in the absence of any treatment and represent means of triplicate cultures. (B) PHA blasts were infected with HIV-1CD (▪) and HIV-1EV (▧) (20 ng p24/106 cells) and cultured with a supernatant derived from an alloantigen-stimulated cell line collected 4 days after alloantigenic stimulation. p24 production was assayed 3 days after infection by ELISA. Results are expressed as the percentage of p24 production by infected cultures in the absence of allostimulated supernatant. Results represent means ± SEM of two experiments performed in triplicate.
Cytokine production by alloantigen-stimulated cell lines.
To characterize the cytokine profile of the antiviral supernatants derived from allostimulated cell lines, levels of IFN-γ, TNF-α, IL-2, IL-4, IL-10, and the HIV-1 suppressive chemokines MIP-1α, MIP-1β, and RANTES were determined by ELISA. The results obtained with an allostimulated cell line are shown in Table 2. High levels of IFN-γ and IL-2 were produced by this cell line. IFN-γ is not likely to be solely responsible for the antiviral effect, because day 7 data (Table 2) show the lowest level of IFN-γ production but a high percent of inhibition of HIV-1 replication by that same supernatant. The β-chemokines, IL-10, IL-16, and IFN-α generated by allostimulation (Table 2) were lower than the levels reported to have significant inhibitory effects on HIV-1 replication.22,24 29-31
Cytokine Levels Present in Supernatants Derived From an Alloantigen-Stimulated Cell Line With Antiviral Activity
| . | Cytokine Levels (ng/mL) . | ||
|---|---|---|---|
| Day 2 . | Day 4 . | Day 7 . | |
| IFN-γ | 6.27 | 27.43 | 2.63 |
| IFN-α | 0.05 | 0.05 | 0.02 |
| TNF-α | 0.25 | 0.17 | 0.34 |
| IL-2 | 5.14 | 1.61 | 0.67 |
| IL-4 | 0.02 | 0.007 | 0.005 |
| IL-10 | 0.77 | 0.68 | 0.68 |
| IL-16 | 0.47 | 1.3 | 1.0 |
| MIP-1α | 9.85 | 7.45 | 5.41 |
| MIP-1β | 11.48 | 11.44 | 9.30 |
| RANTES | 9.52 | 1.25 | 1.63 |
| % inhibition of HIV replication | 99.7 | 95.6 | 98.5 |
| . | Cytokine Levels (ng/mL) . | ||
|---|---|---|---|
| Day 2 . | Day 4 . | Day 7 . | |
| IFN-γ | 6.27 | 27.43 | 2.63 |
| IFN-α | 0.05 | 0.05 | 0.02 |
| TNF-α | 0.25 | 0.17 | 0.34 |
| IL-2 | 5.14 | 1.61 | 0.67 |
| IL-4 | 0.02 | 0.007 | 0.005 |
| IL-10 | 0.77 | 0.68 | 0.68 |
| IL-16 | 0.47 | 1.3 | 1.0 |
| MIP-1α | 9.85 | 7.45 | 5.41 |
| MIP-1β | 11.48 | 11.44 | 9.30 |
| RANTES | 9.52 | 1.25 | 1.63 |
| % inhibition of HIV replication | 99.7 | 95.6 | 98.5 |
Supernatants from allostimulated cell lines were obtained at the indicated days after stimulation with allogeneic cells, as indicated in Materials and Methods. Cytokine levels in the supernatants were determined by ELISA. Results represent means of duplicates.
Effect of neutralizing anti-cytokine antibodies on the HIV-suppressive activity of alloantigen-stimulated cell lines and supernatants.
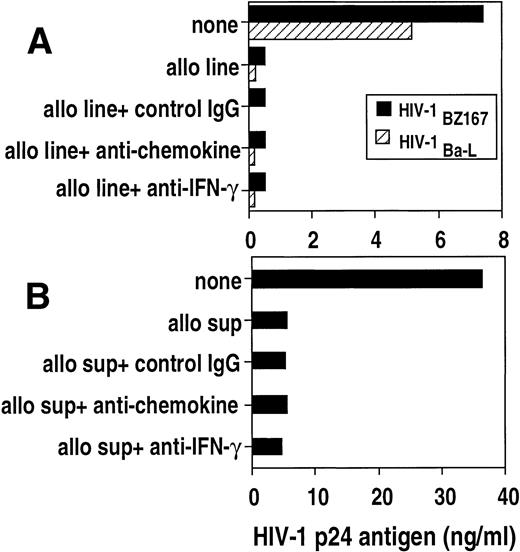
To further address whether β-chemokines are responsible for the antiviral effect, a pool of monoclonal antibodies against MIP-1α, ΜIP-1β, and RANTES were added to cocultures of HIV-infected blasts with either allostimulated cell lines (Fig7A) or with cell line–derived supernatants (Fig 7B). The anti-chemokine antibodies did not reverse the suppressive effect of cell lines on HIV-1BZ167 or HIV-1Ba-Lreplication. Furthermore, the antiviral effect of the cell lines was not altered by the presence of neutralizing anti–IFN-γ antibody (Fig7A).
Effect of neutralizing anti-cytokine antibodies on the anti–HIV-1 activity of an allostimulated cell line and supernatant. (A) PHA blasts were infected with HIV-1BZ167 (▪, 172 TCID50/105 cells) and HIV-1Ba-L(▧,570 TCID50/105 cells) and cultured in the absence or presence of an allostimulated cell line. Cultures containing the alloantigen-stimulated cell line were also incubated with a pool of antichemokine (anti–MIP-1, –MIP-1β, and -RANTES) antibodies (50 μg/mL), anti–interferon-γ (25 μg/mL), or IgG control antibodies (50 μg/mL). HIV p24 antigen was measured on day 3 (HIV-1BZ167) or day 6 (HIV-1Ba-L) post-infection by ELISA. Results represent means of duplicate cultures from one experiment. (B) PHA blasts infected with HIV-1BZ167 were cultured with an allostimulated supernatant in the presence or the absence of neutralizing anticytokine antibodies as above. HIV-1 p24 was measured on day 3 post-infection by ELISA. Results represent means of duplicate cultures from one of two separate experiments with similar results.
Effect of neutralizing anti-cytokine antibodies on the anti–HIV-1 activity of an allostimulated cell line and supernatant. (A) PHA blasts were infected with HIV-1BZ167 (▪, 172 TCID50/105 cells) and HIV-1Ba-L(▧,570 TCID50/105 cells) and cultured in the absence or presence of an allostimulated cell line. Cultures containing the alloantigen-stimulated cell line were also incubated with a pool of antichemokine (anti–MIP-1, –MIP-1β, and -RANTES) antibodies (50 μg/mL), anti–interferon-γ (25 μg/mL), or IgG control antibodies (50 μg/mL). HIV p24 antigen was measured on day 3 (HIV-1BZ167) or day 6 (HIV-1Ba-L) post-infection by ELISA. Results represent means of duplicate cultures from one experiment. (B) PHA blasts infected with HIV-1BZ167 were cultured with an allostimulated supernatant in the presence or the absence of neutralizing anticytokine antibodies as above. HIV-1 p24 was measured on day 3 post-infection by ELISA. Results represent means of duplicate cultures from one of two separate experiments with similar results.
Similarly, anti-chemokine and anti–IFN-γ monoclonal antibodies did not inhibit the suppressive activity of the supernatants derived from the allogeneic cell line (Fig 7B). These data suggest that the soluble antiviral factor that we are studying is not one of the three β-chemokines studied or IFN-γ. The blocking activity of these antibodies was verified by chemokine or IFN-γ–specific ELISA. Neutralizing antibodies to β-chemokines reduced the supernatant levels of RANTES by 67.6% (from 11.9 to 3.0 ng/mL), MIP-1α by 65.4% (from 9.4 to 3.1 ng/mL), and MIP-1β by 74.7% (from 29.5 to 10 ng/mL). The addition of antibodies to IFN-γ reduced the levels of IFN-γ from 21.3 to 5.3 ng/mL. Neutralizing anti–β-chemokines antibodies were also able to reverse the inhibitory effect of exogenous β-chemokines on the replication of HIV-1 Ba-L (from 77.8% inhibition in the absence of neutralizing antibodies to 23% inhibition in the presence of the neutralizing antibodies).
DISCUSSION
The present study has investigated the effect of alloantigen-stimulated T-cell lines and their soluble factor(s) on HIV replication in PHA-activated T-cell blasts. We observed anti-HIV activity in cell lines derived from five different, randomly selected healthy (HIV-uninfected) blood bank donors. These cell lines, comprised mainly of CD8+ T cells, as well as the supernatants derived from these lines inhibited HIV replication of both T-tropic and M-tropic isolates of HIV-1. This HIV-suppressive effect was not due to allostimulated β-chemokines or IFN-γ, which suggests that the alloantigen-stimulated factor(s) might be similar to the CD8-suppressive factor described by Walker et al.15Furthermore, we showed that the allostimulated supernatants inhibited p24 production by the HIV-1MN chronically infected H9 cell line.
We observed a spectrum of suppressive activity among different cell lines from different blood donors. Based on the small number of individuals tested thus far, we were unable to determine whether different HLA-haplotypes might be associated with different antiviral potency. It will be important to determine whether recognition of allogeneic HLA class I, class II, or both are necessary or optimal for generating the anti-HIV activity. It is also noteworthy that in vitro primary alloantigen stimulation of PBMC from one of four blood bank donors that we tested induced factor(s) that inhibited HIV-1 replication (81.7% inhibition; L.A.P. and G.M.S., unpublished data, December 1996). This observation raises the possibility that cells from certain individuals might produce higher amounts of HIV suppressive molecules following primary alloantigen exposure in vitro and that in vivo alloimmunization may generate stronger allostimulated anti-HIV inhibition.
No detectable CTL activity was observed against uninfected or HIV-infected autologous PHA blasts and envelope peptide-pulsed EBV-transformed targets. These results indicate that the inhibition of viral replication observed was not due to a cytolytic effect of the CD8+ T cells against HIV-infected targets. The potent antiviral effect of cell lines that were autologous to the HIV-infected blasts excludes the possibility that this activity was mediated by cytolytic effectors against HLA alloantigens.
The observation that anti-HIV activity is achieved not only by allostimulated cell lines but also by supernatants further indicates that it is unlikely that the mechanism of inhibition is mediated by CTL. Titration of supernatants indicated that the antiviral effect observed was concentration dependent. Although other studies have reported anti-HIV factor production by CD8+ T cells from HIV-infected15-17 and uninfected individuals,20 21 these allostimulated T cells represent the first antigen-stimulated production of β-chemokine–independent anti–HIV-1 factor(s). This HIV-suppressive effect appears to be dependent on alloantigen stimulation because supernatants derived from tetanus or anti-CD3–stimulated cultures as well as from an HTLV-1tax peptide-specific CD8+ T-cell clone did not inhibit HIV replication.
Different strains of HIV-1 were analyzed to investigate whether the viral suppressive effect of the supernatants derived from alloantigen-stimulated cell lines extends to isolates exhibiting different cellular tropism. The fact that the supernatants contain factor(s) that inhibit T-cell–tropic and M-tropic virus is relevant in view of the observations that M-tropic isolates of virus appear to be more important in transmission of HIV infection,32 and predominate in the early stages of infection,33,34 whereas T-tropic isolates were reported to appear later in the spectrum of HIV-associated disease.35,36 A change in coreceptor usage with disease progression was recently reported.37 Although there is not an exact correlation between cell tropism and chemokine receptor usage,38 the use of isolates with different coreceptor usage for infection in our study indicates that the allostimulated cell line supernatants can inhibit isolates that use predominantly CCR5 (HIV-1Ba-L and HIVEV), as well as isolates that use CXCR4 (HIV-1CD) or preferentially CXCR4 and CCR3 (HIV-1BZ167; G. Blatner and D.I. Cohen, manuscript submitted). The broad spectrum of activity of these supernatants was further supported by the observation that the supernatants of alloantigen-stimulated cell lines inhibit p24 production by the chronically infected H9 cell line, which suggests that these soluble factors inhibit HIV-1 at a post-entry level. Studies are in progress to determine the mechanism and site of inhibition of HIV-1 infection. Interestingly, studies using CD8+antiviral factor (CAF) have shown that CD8+ cells can inhibit both syncytium and nonsyncytium-inducing strains of HIV.39
Analysis of cytokine levels in supernatants with HIV-suppressive activity showed that the cytokines IL-2, IL-4, IFN-γ decreased in concentration during 7 days of culture, whereas the inhibitory effects of the supernatants remained constant. This difference in kinetics suggests that these cytokines are not involved in the in vitro biologic activity of the supernatants. The β-chemokines RANTES, MIP-1α, and MIP-1β were also present in the alloantigen-stimulated supernatants. It was reported that the C-C chemokines RANTES, MIP-1α, and MIP-1β produced by immortalized and primary patient CD8+ T cells inhibit in vitro HIV replication of M-tropic isolates but not T-cell–line adapted strains.22 However, the concentrations of chemokines produced by the alloantigen-stimulated cell lines was 5- to 10-fold lower than the levels reported to be necessary to efficiently block HIV-1 replication.22,24,40 Furthermore, the RANTES concentration was highest in the day-2 supernatant, although the inhibitory effect of the supernatant remained high at all three time points tested after allostimulation. Blocking experiments with anti–MIP-1α, –MIP-1β, -RANTES, and –IFN-γ had no effect on the antiviral effect of the supernatants or the allostimulated cell lines, suggesting that this inhibitory effect on HIV replication is not chemokine- or IFN-γ–mediated. Also, addition of exogenous chemokines at concentrations reported to inhibit HIV replication did not suppress HIV-1BZ167 replication under conditions in which both cell lines and their supernatants strongly inhibited viral replication. Because this HIV strain uses as coreceptor for infection preferentially CXCR4 and CCR3 and weakly CCR5 (G. Blatner and D.I. Cohen, manuscript submitted) the lack of efficacy of β-chemokines on inhibition of viral replication is not surprising. Nevertheless, at the same concentrations, chemokines inhibit HIV-1Ba-L replication, an M-tropic strain of HIV that uses CCR5 as coreceptor for infection,41 and these results agree with previous published data.22 IL-10, IL-16, and IFN-α have been shown to inhibit HIV-1 infection of T cells.29-31However, it is unlikely that these cytokines play a major role in the antiviral activity detected in our study because the levels of these cytokines in the supernatants of alloantigen-stimulated cultures are several logs lower than the amount reported to suppress HIV replication.
Preliminary analysis of specificity of the alloantigen-stimulated supernatants indicates that these supernatants did not inhibit HTLV-1 replication, although they reduced cytomegalovirus (CMV) infection of susceptible cell lines (L.A.P. and G.M.S., unpublished data, October 1996). This latter observation raises the possibility that allostimulated factor(s) could have a dual effect by inhibiting not only HIV, but also CMV, an AIDS-associated opportunistic infectious virus that may synergize with HIV.42
In conclusion, the present study shows that PBMC from randomly selected, HIV-uninfected individuals can be stimulated with irradiated PBMC from randomly selected uninfected individuals to generate CD8+ T cell lines that produce a factor(s) that interferes with HIV-1 replication in vitro. This factor, different from HIV-suppressive β-chemokines, inhibits T-cell–tropic and M-tropic isolates of HIV-1, illustrating its versatility against a spectrum of viral isolates. Studies are in progress to attempt to isolate and identify the soluble anti-HIV factor(s) as well as to determine whether its suppressive effect extends to other viruses.
ACKNOWLEDGMENT
The authors thank Dr Susan Zolla-Pazner, New York University Medical Center, New York, NY, for providing HIV-1BZ167; Dr William Biddisson, National Institutes of Health, MD, for the supernatants from HTLV-1 tax-specific clones; and Dr Jay A. Berzofsky, National Institutes of Health, MD, for the HIV-1 envelope peptides.
Address reprint requests to Gene M. Shearer, PhD, National Cancer Institute, National Institutes of Health, Experimental Immunology Branch, Bldg 10, Room 4B36, Bethesda, MD 20892; e-mail:ShearerG@exchange.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal