Abstract
Macrophage-derived chemokine (MDC) is a CC chemokine that recognizes the CCR4 receptor and is selective for T helper 2 (Th2) versus T helper 1 (Th1) cells. The present study was designed to investigate the effect of the prototypic Th2/Th1 cytokines, interleukin-4 (IL-4) and interferon-γ (IFN-γ), on the production of MDC by human monocytes. IL-4 and IL-13 caused a time-dependent (plateau at 24 hours) and concentration-dependent (EC50 2 and 10 ng/mL, respectively) increase of MDC mRNA levels in monocytes. Increased expression of MDC mRNA was associated with protein release in the supernatant. MDC expression and production induced by IL-4 and IL-13 were inhibited by IFN-γ. IFN-γ also suppressed the constitutive expression of MDC in mature macrophages and dendritic cells. These results delineate an amplification loop of polarized Th2 responses based on differential regulation of MDC production by IL-4 and IL-13 versus IFN-γ and on the selectivity of this chemokine for polarized Th2 cells.
© 1998 by The American Society of Hematology.
THE CHEMOKINE SUPERFAMILY consists of chemotactic cytokines that may be subdivided in four families, CXC, CC, C, and CX3C, depending on the position of the first two cysteine residues.1-3 The CXC and CC families are composed by many members, whereas the C and CX3C families are each composed by one member.4,5 Chemokines exert their action by binding G-protein–coupled receptors and five CXC chemokine receptors, nine CC chemokine receptors, and one CX3C receptor have been identified.3 6
Most chemokines, such as monocyte chemotactic protein-1 (MCP-1),3 are produced by tissue cells and infiltrating leukocytes in response to microbial, immune, or inflammatory signals and are key mediators for leukocyte extravasation and migration to sites of inflammation.7 Other chemokines are constitutively produced and may play a role in the maturation of leukocytes in the bone marrow,8 in lymphocyte homing to lymphoid areas,9 and in the regulation of lymphocyte traffic.10-14
Macrophage-derived chemokine (MDC) is a CC chemokine recently identified in macrophages.10,15 It has also been purified from supernatants of CD8+ T lymphocytes acting as a suppressive factor for human immunodeficiency virus (HIV) infection.16 MDC is expressed in lymphoid organs and, among hematopoietic and nonhematopoietic cells, in macrophages and dendritic cells.10 MDC binds CCR4.17 CCR4 is preferentially expressed on type 2 helper T (Th2) lymphocytes, which migrate selectively in response to its ligands MDC and thymus and activation-regulated chemokine (TARC).18,19 Therefore, CCR4, together with CCR320,21 and CCR8,22characterizes polarized Th2 lymphocytes, whereas CCR5 and CXCR3 are preferentially expressed by polarized Th1 lymphocytes.18,23,24 Th1 and Th2 lymphocytes are differentiated on the basis of the cytokines they produce, and cytokines cross-regulate each other’s development and activity. Typically, Th1 lymphocytes produce interferon-γ (IFN-γ), whereas Th2 lymphocytes produce interleukin-4 (IL-4).25 26
The present study was designed to investigate the regulation of production of MDC, a chemokine selectively active on polarized Th2 cells.18 We found that IL-4 (and IL-13) induces MDC production in monocytes, whereas IFN-γ inhibits it. These findings outline an MDC-based amplification circuit of polarized Th2 responses.
MATERIALS AND METHODS
Cytokines and antibodies.
Human recombinant IL-4 and IL-13 were from Schering-Plough (Kenilworth, NJ) and Sanofi Elf Bio Recherches (Labège, France), respectively. IFN-γ (specific activity, 20 × 106 U/mL) was obtained from Roussel Uclaf (Romainville, France). Cytokines were endotoxin free as assessed by Limulus Amebocyte assay. Monoclonal antibodies (MoAbs) 252Y, 252Z, and 272D were generated against recombinant human MDC. These antibodies were shown to be specific for human MDC by a variety of criteria including immunoprecipitation, Western blotting, and neutralization of biological activity (Chantry et al26a). These reagents were used in a sandwich enzyme-linked immunosorbent assay (ELISA) using as chromogen substrate ABTS (Kirkegaard and Perry, Gaithersburg, MD).
Cell preparation.
Monocytes were obtained from buffy coats of healthy blood donors through the courtesy of Centro Trasfusionale, Ospedale Sacco (Milan, Italy). Blood was washed once with saline at 300g to remove plasma and platelets and then centrifuged on Ficoll (Biochrom, Berlin, Germany) at 400g for 30 minutes at room temperature. Monocytes were purified by centrifugation on 46% iso-osmotic Percoll (Pharmacia, Uppsala, Sweden) gradient, as previously described.27 These cells were more than 95% monocytes, as evaluated by morphological analysis. Cells (5 × 106/mL) were resuspended in RPMI 1640 medium (Biochrom) with 10% fetal calf serum (FCS; Hyclone, Logan, UT) and incubated in Petriperm dishes (Haereus, Austria) with different concentrations of IL-4, IL-13, and IFN-γ as specified in the text.
Dendritic cells were obtained incubating monocytes for 7 days at 1 × 106/mL in 6-well multiwell tissue culture plates (Falcon, Becton Dickinson, Franklin Lakes, NJ) in RPMI with 10% FCS supplemented with 50 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) and 10 ng/mL IL-13. These cells were greater than 80% CD1a+, greater than 90% major histocompatibility complex (MHC) class II+, less than 10% CD14+, less than 2% CD3+, and less than 4% CD20+.28 Macrophages were obtained incubating monocytes in Petriperm dishes for 7 days in RPMI with 50% of autologous serum.29
Northern blot analysis.
Monocytes were prepared has described above and total RNA was extracted by the guanidinium thiocyanate method, blotted, and hybridized as described.30 Probes were labeled by Megaprime DNA labeling system (Amersham, Buckinghamshire, UK) with α32P-dCTP (3,000 Ci/mmol; Amersham). Membranes were preybridized at 42°C in Hybrisol (Oncor, Inc, Gaithersburg, MD) and hybridized overnight with 1 × 106 cpm/mL of 32P-labeled probe. Membranes were then washed three times with 2× SSC (1× SSC = 0.15 mol/L NaCl, 0.015 mol/L sodium citrate, pH 7.0) at room temperature for 10 minutes, twice with 2× SSC, 1% sodium dodecyl sulfate (SDS) at 60°C for 20 minutes, and then with 0.1× SSC for 5 minutes, before being autoradiographed using Kodak XAR-5 films (Eastman Kodak, Rochester, NY) and intesifyer screens at −80°C. MDC probe was obtained as described.10
Detection of MDC protein.
Ten micrograms of anti-MDC MoAb (252Y) was added to 3 mL of culture supernatant from human monocyte preparations (stimulated as described above) and incubated overnight at 4°C. After overnight incubation, 20 μL of protein G sepharose was added and incubated for an additional 45 minutes at 4°C. Immune complexes were washed in Tris-buffered saline containing 0.1% tween 20 (TBS-Tw), boiled in 2× Laemmli buffer, and separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were then transferred to polyvinylidene difluoride (PVDF) membrane (300 mA for 30 minutes). The membrane was then blocked with TBS-Tw for 15 minutes at room temperature, probed in the same buffer with 1 μg/mL 252Y for 1 hour, washed three times in TBS-Tw, and probed with 200 ng/mL goat antimouse horseradish peroxidase (Transduction Labs, Lexington, KY) for 30 minutes, washed three times with TBS-Tw, and detected using electro-chemiluminescence (Renaissance ECL; NEN Life Science, Boston, MA) and autoradiography (Hyperfilm; Eastman Kodak).
RESULTS AND DISCUSSION
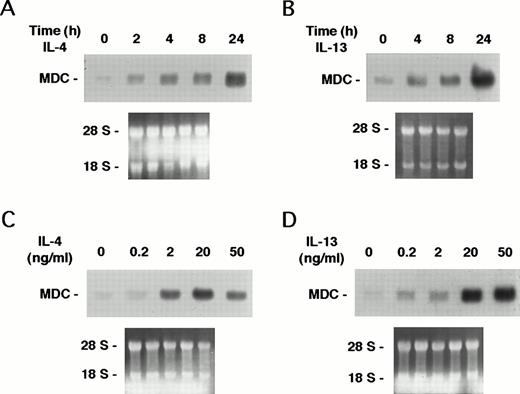
Under resting conditions, human monocytes express low levels of MDC mRNA, as shown in Fig 1. Incubation of monocytes with IL-4 or IL-13 (20 ng/mL) strongly increased MDC mRNA expression, evaluated by Northern analysis, with maximal stimulation after 24 hours of incubation (Fig 1A and B). Monocytes were stimulated with the two cytokines at concentrations ranging from 0.2 to 50 ng/mL for 24 hours (Fig 1C and D). The effect was dose-dependent, with an EC50 of 2 and 10 ng/mL for IL-4 and IL-13, respectively. At a concentration of 20 ng/mL and incubation time of 24 hours, IL-4 and IL-13 caused a 26.5-fold (n = 6; range, 16- to 35-fold) and a 30.3-fold (n = 5; range, 21.8- to 43.8-fold) stimulation of MDC expression as evaluated by densitometric analysis, respectively.
Induction of MDC expression by IL-4 and IL-13. Monocytes were incubated for 24 hours with increasing concentrations of IL-4 and IL-13 (dose-response) or with IL-4 or IL-13 (20 ng/mL) for different times (time-course). Ten micrograms of total RNA was used in Northern blot analysis. Results of one experiment representative of two performed are shown. Autoradiography were obtained after 12 hours of exposure. Etidium bromide staining of the membrane is shown in the lower part of the figure. (A) Time-course of IL-4. (B) Time-course of IL-13. (C) Dose-response of IL-4. (D) Dose-response of IL-13.
Induction of MDC expression by IL-4 and IL-13. Monocytes were incubated for 24 hours with increasing concentrations of IL-4 and IL-13 (dose-response) or with IL-4 or IL-13 (20 ng/mL) for different times (time-course). Ten micrograms of total RNA was used in Northern blot analysis. Results of one experiment representative of two performed are shown. Autoradiography were obtained after 12 hours of exposure. Etidium bromide staining of the membrane is shown in the lower part of the figure. (A) Time-course of IL-4. (B) Time-course of IL-13. (C) Dose-response of IL-4. (D) Dose-response of IL-13.
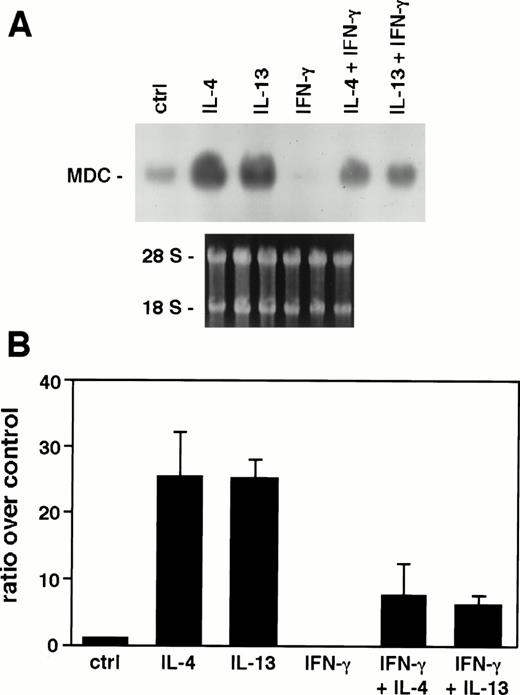
To address whether Th1 cytokines regulate MDC mRNA expression, monocytes were stimulated with IFN-γ (500 U/mL). IFN-γ did not induce MDC expression and completely inhibited the basal level. Moreover, IFN-γ suppressed MDC expression in IL-4– and IL-13–stimulated monocytes (Fig 2A). Figure 2B shows the mean of densitometric analysis of four Northern blot experiments in which cells were treated for 24 hours, as indicated. IL-4 and IL-13 induced an increase of 25.4- ± 6.7-fold (P < .01 using the paired Student’s t-test) and 25.2- ± 2.7-fold (P < .001) over control, respectively. When monocytes were stimulated with IL-4 and IL-13 together with IFN-γ, MDC expression was reduced being only 7.5- ± 4.8-fold (P < .004) and 6- ± 1.4-fold (P < .007) over the control. Thus, these results indicate that IFN-γ reverts the stimulatory effect of IL-4 and IL-13.
Effect of IFN-γ on MDC mRNA expression. Ten micrograms of total RNA from monocytes stimulated for 24 hours with or without IL-4, IL-13 (20 ng/mL), and IFN-γ (500 U/mL) were analyzed with Northern blot. (A) Autoradiography of a representive experiment obtained after 6 hours of exposure. (B) Densitometric analysis of four independent experiments. Error bars indicate the SD.
Effect of IFN-γ on MDC mRNA expression. Ten micrograms of total RNA from monocytes stimulated for 24 hours with or without IL-4, IL-13 (20 ng/mL), and IFN-γ (500 U/mL) were analyzed with Northern blot. (A) Autoradiography of a representive experiment obtained after 6 hours of exposure. (B) Densitometric analysis of four independent experiments. Error bars indicate the SD.
MDC was shown to be constitutively expressed by in vitro-derived macrophages and dendritic cells in the absence of deliberate stimulation.10 It was therefore important to assess whether constitutive expression by macrophages and dendritic cells was also affected by IFN-γ. In one experiment performed, IFN-γ abolished MDC expression in mature macrophages and dendritic cells (data not shown).
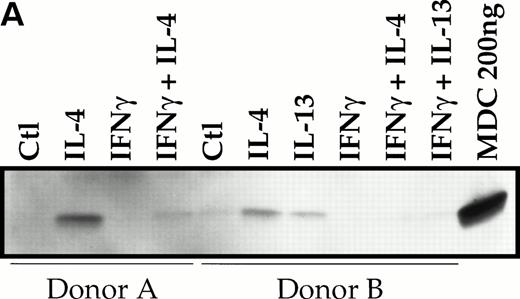
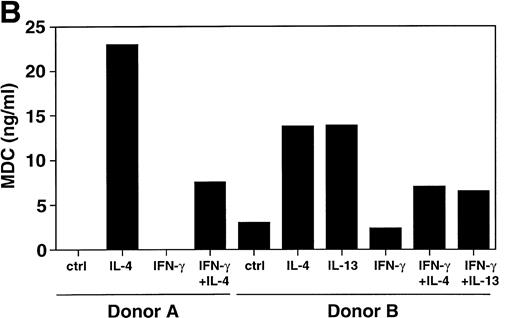
To evaluate whether MDC mRNA increase was associated with protein release, the supernatants of monocytes stimulated with IL-4, IL-13, IFN-γ and their combination were examinated using an anti-MDC MoAb. Production of MDC was clearly induced by IL-4 and IL-13, as shown by immunodetection of a 6-kD band (Fig 3A). IFN-γ completely abolished the IL-4/IL-13–induced MDC production. Similar results were obtained when MDC production was assessed by ELISA (Fig 3B).
Divergent effect of IL-4 and IFN-γ on MDC protein production. Supernatants of monocytes stimulated with or without IL-4 (20 ng/mL), IL-13 (20 ng/mL), and IFN-γ (500 U/mL) were examined for immunoreacting MDC using MoAb 252Y (A) or by MDC specific ELISA (B). Each panel shows the results of two independent experiments.
Divergent effect of IL-4 and IFN-γ on MDC protein production. Supernatants of monocytes stimulated with or without IL-4 (20 ng/mL), IL-13 (20 ng/mL), and IFN-γ (500 U/mL) were examined for immunoreacting MDC using MoAb 252Y (A) or by MDC specific ELISA (B). Each panel shows the results of two independent experiments.
The results presented here show that IL-4 and IFN-γ have opposite effects on the expression of the CC chemokine MDC in human mononuclear phagocytes, with induction by IL-4 (and IL-13) and inhibition by IFN-γ. MDC is not inducible by LPS in fresh monocytes,31suggesting that expression of the MDC gene is regulated by a limited set of signals.
IFN-γ and IL-4 are central mediators involved in the induction and expression of polarized Th1 versus Th2 responses.25,26Recent results indicate that chemokines are part of the distinguishing features of polarized Th1 versus Th2 responses. It was shown that chemokine receptors are differentially expressed in Th1 versus Th2 cells and that, accordingly, polarized T-cell populations show differential responsiveness to chemokines.18-24 In particular, it was found that CCR4 is expressed at high levels in Th2, but not in Th1 cells, and that the CCR4 agonists MDC and TARC are selective Th2 attractants.18 19
Given the constitutive expression of MDC in mature macrophages and DC in vitro,10 the preferential attraction of Th2 cells by this chemokine could not be placed in a pathophysiological context. The results presented here show that IL-4 and IL-13 induce MDC, whereas IFN-γ inhibits it. Thus, the present observations outline an amplification circuit of polarized Th2 responses, based on induction of MDC by IL-4/IL-13, counterbalanced by IFN-γ, and selective responsiveness to this chemokine of polarized CCR4-expressing Th2 cells.
ACKNOWLEDGMENT
The authors thank Susan Pederson for developing the MDC ELISA and running the samples.
Supported by Istituto Superiore di Sanità, AIDS Project, and Italy-US Program on Therapy of tumors and by 40% fund from MURST Italy. The generous contribution of the Italian Association for Cancer Research (AIRC) is gratefully acknowledged. R.B. is a recipient of a Fondazione A. Valenti fellowship and has been carried out in part under a research contract with Consorzio Autoimmunita Tandiva C.A.U.T. (Pomezia, Italy) within the “Programma Nazionale Farmaci-seconda fase” of the Italian Ministry of the University Scientific and Technological Research.
Address reprint requests to Alberto Mantovani, MD, Istituto di Ricerche Farmacologiche “Mario Negri,” via Eritrea 62, 20157 Milan, Italy; e-mail: Mantovani@irfmn.mnegri.it.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal