Abstract
Tumor cells are eradicated by several systems, including Fas ligand-Fas and tumor necrosis factor (TNF)-tumor necrosis factor receptor (TNFR). In the previous study, we purified an apoptosis-inducing factor (AIF) to homogeneity from a medium conditioned by PDBu-treated HL-60 cells. N-terminal sequence analysis showed that AIF is identical to endothelial interleukin-8 (IL-8). A novel apoptosis system, in which endothelial cells participate via endothelial IL-8 release, is identified here. Human umbilical vein cells (VE cells) produce and secrete IL-8 by stimulation of IL-1 and TNF-. Endothelial IL-8, which is secreted from VE cells by stimulation of IL-1 and TNF- , induces apoptosis in myelogenous leukemia cell line K562 cells. Monocyte-derived IL-8 could not induce apoptosis in K562 cells. Moreover, interaction between VE cells and K562 cells induces the release of endothelial IL-8 from VE cells, and the attached K562 cells undergo apoptosis. Moreover, interactions between VE cell and other cell lines, such as HL-60, U937, Jurkat, and Daudi, induce the secretion of endothelial IL-8 and the induction of apoptosis in cell lines. Endothelial IL-8 significantly inhibits tumor growth of intraperitoneal and subcutaneous tumor mass of K562 cells and induces apoptosis in their cells in vivo. Endothelial IL-8 plays an important role in apoptosis involving endothelial cells, which may provide us with a new therapy for hematological malignancies.
© 1998 by The American Society of Hematology.
APOPTOSIS IS AN ACTIVE process in which various types of cells are selectively deleted during embryonic development and in the adult multicellular organism under certain physiologic conditions.1 Cell death occurs during the development and regulation of the immune system, leading to deletion of self-reactive T and B lymphocytes, regulation of immunologic memory, and lysis of target cells by cytotoxic T lymphocytes and natural killer (NK) cells.2-4 Alteration of the genes that control apoptosis may lead to a variety of diseases such as autoimmune, malignant clonal growth, neurodegenerative diseases, as well as prolonged survival of cells during latent viral infection.5Cells with induced apoptosis appear shrunken, with condensed or fragmented nuclei. Apoptotic cells have fragmented DNA, with DNA ladders in electrophoresis.6 7
Tumor cells have systems to protect themselves from induction of apoptosis in various physiologic conditions.8-10 Alteration of bcl-2 or bcl-x, well-known as antiapoptosis genes, is observed in many tumor cells in leukemia, lymphoma, and other types of cancers.11,12 Overexpression of these genes in cancer cells leads to partial or complete resistance to several conditions such as the presence of chemotherapeutic agents and irradiation.13,14 Leukemic cells are known to undergo apoptosis under several conditions, and the basic strategy of leukemia therapy is the induction of apoptosis.15 Chemotherapeutic agents such as etoposide and all-trans retinoic acid can induce apoptosis, killing leukemic cells.15 Because cytosine arabinoside can reduce expression of bcl-2 or bcl-x in leukemic cells, their lifespan is shortened by induction of apoptosis.16 17
Some mechanisms induce apoptosis against tumor cells and leukemic cells, removing them from the body. Fas ligand and TNF-α, which physiologically exist in the body, are also inducers of apoptosis in leukemic cells and other cells.18,19 It has been reported that signals for some cytokines might select induction of apoptosis or proliferation in target cells,20 but no cytokine was able to induce apoptosis in leukemic cells and other tumor cells. Moreover, tumor cells are directly attacked by cytotoxic T cells, NK cells, and macrophages, inducing apoptosis.21,22 For example, cytotoxic T cells and NK cells attach to tumor cells and release perforin. A serine protease, granzyme B, is transferred into the cytosol of the target cells, and then apoptosis is induced.23,24 It has been reported that endothelial cells are also associated with inhibition of tumor cell invasion,25 but the mechanism is not clear.
The human myelogenous leukemia cell line HL-6026 can be induced to differentiate into monocyte/macrophage lineage by phorbol esters, undergoing apoptosis.27 In the previous study, we have purified an apoptosis-inducing factor (AIF) to homogene ity from a medium conditioned by PDBu-treated HL-60 cells.28N-terminal sequence analysis showed that AIF is identical to endothelial interleukin-8 (IL-8). Human recombinant endothelial IL-8 induces apoptosis in most leukemic cell lines, such as K562, HL-60, Jurkat, KG-1, U937, and THP-1, but monocyte-derived IL-8 does not. Endothelial IL-8, which has added five amino acids to the N terminus of monocyte-derived IL-8, is active in the induction of apoptosis, but its action against tumor cells in vivo has never been clarified.
To investigate the biological significance of endothelial IL-8, we observed the antitumor effect of endothelial cells in vitro and the antitumor effect of endothelial IL-8 in vivo.
MATERIALS AND METHODS
Reagents.
Lipopolysaccharide (LPS) was obtained from Sigma (St Louis, MO). Recombinant human IL-1α, recombinant human tumor necrosis factor-α (TNF-α), recombinant human interferon-γ (IFN-γ), and antihuman monoclonal IL-8 antibody were purchased from R & D Systems Inc (Minneapolis, MN). Recombinant human endothelial and monocyte-derived IL-8 were purchased from Genzyme (Cambridge, MA).
Cell lines and cell culture.
A human chronic myelogenous leukemia cell line, K562,29 was obtained from ATCC (Rockville, MD) and was maintained in GIT medium (Wako, Tokyo, Japan).30 Human myelogenous leukemic cell line HL-60, human monocytic leukemia cell line U937, human T-cell leukemia cell line Jurkat, and human myeloma cell line Daudi were also obtained from ATCC. Human umbilical venous cells (VE cells) were purchased from Cell Systems Co (Kirkland, WA) and were maintained in CS-C serum-free medium. Normal human monocytes were isolated in a nascent state from the peripheral blood of healthy volunteers, as described previously.31 In brief, collected mononuclear cells were resuspended in phosphate-buffered saline (PBS) and then incubated onto an MSP-P plate (JIMRO, Gunma, Japan) for 1 hour at 37°C. Adherent cells (monocyte; >90% purity) were collected and were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies Inc, Gaithersburg, MD). Monocytes (1 × 106cells/mL) and human umbilical venous cells (VE cells, confluent) were cultured with or without LPS (500 ng/mL), IL-1α (10 U/mL), TNF-α (10 ng/mL), IFN-γ (200 U/mL), and macrophage colony-stimulating factor (M-CSF; 100 ng/mL) in 24-well plates. Monocytes or VE cells was also cocultured with K562 cells with or without the factors listed above in 24-well plates with culture chambers (Intercell (Kurabo, Osaka).
Detection of IL-8.
For detection of IL-8 in culture media, an enzyme-linked immunosorbent assay (ELISA) system for human IL-8 (Amersham, Arlington Heights, IL) was used.32 In brief, endothelial cells or monocytes were cultured with various factors and the supernatants were collected by centrifugation at 15,000 rpm after 2 days. Samples were then applied to the wells of a microtiter plate coated with a specific monoclonal antibody for IL-8. After washing away any unbound sample proteins, an enzyme-linked polyclonal antibody specific for IL-8 was added to the wells and allowed to bind to the IL-8 that was bound during the incubation. After a wash to remove any unbound antibody-enzyme reagent, a substrate solution was added to the wells and color-developed in proportion to the amount of IL-8. Absorbance at 450 nm was determined by spectrophotometer (Bio-Rad, Hercules, CA). For detection of intracytoplasmic IL-8 in endothelial cells cultured with or without K562 cells, immunofluorescent staining for intracellular IL-8 (CytoStain kit; PharMingen, San Diego, CA) was performed. In brief, endothelial cells cultured with or without K562 cells for 3 days were fixed and permeabilized by Cytofix/Cytoperm solution and Perm/Wash solution. After washing, fixed/permeabilized cells were stained by fluorochrome-conjugated antihuman IL-8 antibody. After washing, flow cytometric analysis was performed by FACScan (Becton Dickinson, Mountain View, CA).
MTT assay.
K562 cells were seeded at 1 × 105 cells/mL onto confluent human umbilical venous cells in CS-C medium. After 3 days, K562 cells were collected and the ca pacity to reduce 3-[4, 5-Dimethylthiazol-2-yl]-2, 5-diphenylte trazolium bromide (MTT; Sigma) was determined.33 After adding 10 μL of MTT solution (5 mg/mL MTT in PBS), the preparation was incubated at 37°C for 4 hours. Cells with MTT for mazan were dissolved in 0.04 N HCl in 2-propanol, and color absorbance was measured at 595 nm by microplate reader (Bio-Rad).
TUNEL assay.
Additionally, residual cells were incubated with digoxigenin-dUTP terminaldeoxynucleotidyl transferase mixture and subsequently were stained with peroxidase-conjugated antibody to digoxigenin (Apop Tag PLUS; Oncor, Gaithersburg, MD),34counterstained with 1% methyl green in sodium acetate (pH 4.0), and mounted. Specimens were examined and photographed with a microscope. The percentage of apoptotic cells was determined by microscopically counting more than 200 cells. Statistical analysis was performed using the Student’s t-test.
In vivo experiments.
Male Balb/c nu/nu mice were purchased from Japan Charles River and were age-matched (5 weeks of age) at the onset of each experiment. Nude mice were inoculated with 106 K562 cells into the peritoneal space. Intraperitoneal tumor masses of K562 cells were injected daily with recombinant human endothelial IL-8 or monocyte-derived IL-8 for 2 days. As a control, saline was injected. Intraperitoneal cells were collected daily by washing with 5 mL PBS. Cell counting, Wright-Giemsa staining, and TUNEL assay were also performed. Mice were injected with 5 × 105 viable K562 cells by subcutaneous injection in a midline ventral position in a total volume of 0.1 mL PBS. Test mice bearing subcutaneously established K562 tumors (confirmed after 4 days of inoculation) were injected daily (for 11 days) into tumor with endothelial IL-8 or monocyte-derived IL-8 in a total volume of 0.1 mL saline. As a control, saline and TNF-α were injected. Tumor size was calculated using the formula described by Kyriazis et al,35 as follows: tumor volume = width2 × length × 0.4.
Tumors were resected in toto, fixed in 10% neutral formalin solution (Sigma), embedded in paraffin, sectioned at 4 mm, and stained with hematoxylin and eosin or with TUNEL assay. The injected dose of IL-8 was 100 ng per mouse per day. The dose of TNF-α was 200 U per mouse. Statistical analysis was performed using the Student’s t-test.
RESULTS
Secretion of IL-8 from monocytes and endothelial cells by various factors.
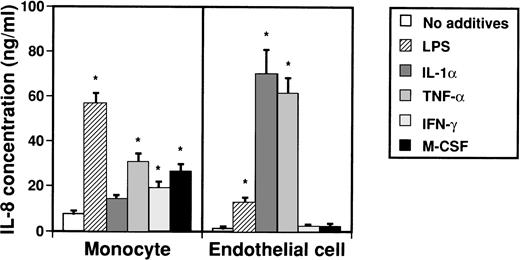
IL-8 takes several forms of various lengths that are produced by monocytes and endothelial cells. To examine by which factors monocytes and endothelial cells are induced to produce IL-8, possible inducers such as LPS, IL-1α, TNF-α, IFN-γ, and M-CSF were added to culture media of monocytes and endothelial cells (Fig 1). Concentrations of IL-8 in medium were then measured by an ELISA system after 2 days. When monocytes were stimulated with LPS, TNF-α, IFN-γ, and M-CSF, concentrations of IL-8 in culture medium significantly increased from 7.7 ± 1.2 ng/mL to 56.7 ± 4.5 ng/mL, 30.8 ± 3.6 ng/mL, 26.9 ± 3.0 ng/mL, and 19.4 ± 2.5 ng/mL, respectively. On the other hand, when LPS, IL-1α, and TNF-α were added to culture media of endothelial cells, concentrations of IL-8 in culture medium increased from 1.1 ± 1.0 ng/mL to 12.8 ± 2.3 ng/mL, 70.1 ± 10.6 ng/mL, and 61.5 ± 7.0 ng/mL, respectively.
Effect of various cytokines on production of IL-8 from monocytes and endothelial cells. Monocytes (1 × 106cells/mL) and human umbilical venous cells (VE cells, confluent) were cultured with or without LPS (500 ng/mL), IL-1 (10 U/mL), TNF- (10 ng/mL), IFN-γ (200 U/mL), and M-CSF (100 ng/mL) in 24-well plates. After 2 days, supernatants were collected and an ELISA system for IL-8 was performed. Columns represent the mean ± SD (bar) of three independent experiments. Statistical analysis was performed using the Student’s t-test. *P < .01.
Effect of various cytokines on production of IL-8 from monocytes and endothelial cells. Monocytes (1 × 106cells/mL) and human umbilical venous cells (VE cells, confluent) were cultured with or without LPS (500 ng/mL), IL-1 (10 U/mL), TNF- (10 ng/mL), IFN-γ (200 U/mL), and M-CSF (100 ng/mL) in 24-well plates. After 2 days, supernatants were collected and an ELISA system for IL-8 was performed. Columns represent the mean ± SD (bar) of three independent experiments. Statistical analysis was performed using the Student’s t-test. *P < .01.
Secretion of IL-8 from endothelial cells by attachment to K562 cells.
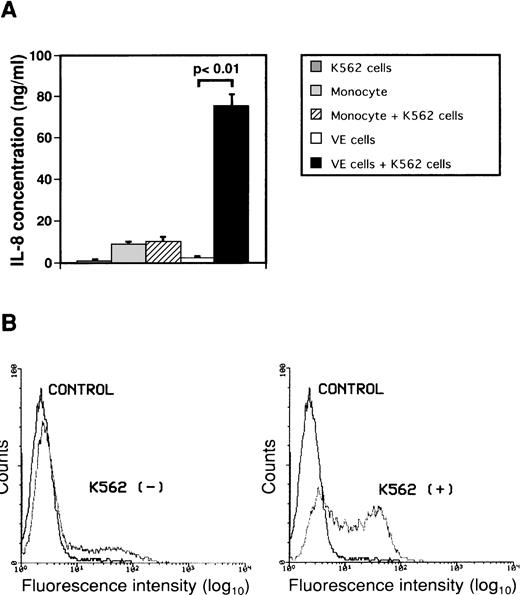
To investigate the effect of interaction between monocytes or endothelial cells and K562 cells on secretion of IL-8, monocytes or human umbilical endothelial cells (VE cells) were cultured with or without human leukemic cell line K562 cells for 2 days (Fig 2A). Concentrations of IL-8 in conditioned media did not increase significantly when monocytes were cultured with K562 cells. When VE cells were cultured with K562 cells, the concentration of IL-8 in culture medium increased from 2.1 ± 1.1 ng/mL to 75.1 ± 5.8 ng/mL as compared with that in culture medium of only VE cells. IL-8 in medium of K562 cells alone was 0.6 ± 0.3 ng/mL. Moreover, we observed a change in intracytoplasmic IL-8 of VE cells during interaction between VE cells and K562 cells using immunofluorescent staining by flow cytometric analysis to examine whether IL-8 is produced in VE cells (Fig 2B). The percentage of intracellular IL-8–positive VE cells was 22.7% ± 5.3% during culture of VE cells alone. When VE cells were cocultured with K562 cells for 2 days, the percentage of intracellular IL-8–positive VE cells increased to 55.5% ± 7.0%. These results suggest that VE cells can produce and secrete IL-8 during interaction with K562 cells but that monocytes cannot. Moreover, we examined concentrations of IL-8 when other cell lines such as HL-60, U937, Jurkat, and Daudi were cocultured with VE cells. When VE cells were cultured with HL-60, U937, Jurkat, or Daudi cells, concentration of IL-8 in culture medium increased from 2.1 ± 1.1 ng/mL to 74.2 ± 2.0 ng/mL, 71.2 ± 2.4 ng/mL, 48.1 ± 3.3 ng/mL, or 44.6 ± 3.9 ng/mL as compared with that in culture medium of only VE cells, respectively.
Effect of interaction between either monocytes or endothelial cells and K562 cells on secretion of IL-8 from endothelial cells. Monocytes (1 × 106 cells/mL) and human umbilical venous cells (VE cells, confluent) were cultured with or without K562 cells (5 × 104 cells/mL) for 2 days, and supernatants were then collected. (A) ELISA system for IL-8 was performed. Columns show the means of three independent experiments. Statistical analysis was performed using the Student’s t-test. (B) When VE cells were cocultured with (right) or without (left) K562 cells, the remaining VE cells, after removal of K562 cells, were collected and expression of intracytoplasmic IL-8 was examined as described in Materials and Methods. Purified mouse Ig G1 was used as a control antibody (CONTROL). The vertical axis indicates the frequency of fluorescence-positive cells. Intracytoplasmic IL-8 density is depicted on the horizontal axis. The representative data from three independent experiments are shown.
Effect of interaction between either monocytes or endothelial cells and K562 cells on secretion of IL-8 from endothelial cells. Monocytes (1 × 106 cells/mL) and human umbilical venous cells (VE cells, confluent) were cultured with or without K562 cells (5 × 104 cells/mL) for 2 days, and supernatants were then collected. (A) ELISA system for IL-8 was performed. Columns show the means of three independent experiments. Statistical analysis was performed using the Student’s t-test. (B) When VE cells were cocultured with (right) or without (left) K562 cells, the remaining VE cells, after removal of K562 cells, were collected and expression of intracytoplasmic IL-8 was examined as described in Materials and Methods. Purified mouse Ig G1 was used as a control antibody (CONTROL). The vertical axis indicates the frequency of fluorescence-positive cells. Intracytoplasmic IL-8 density is depicted on the horizontal axis. The representative data from three independent experiments are shown.
Endothelial IL-8 secreted by various factors induces apoptosis in K562 cells.
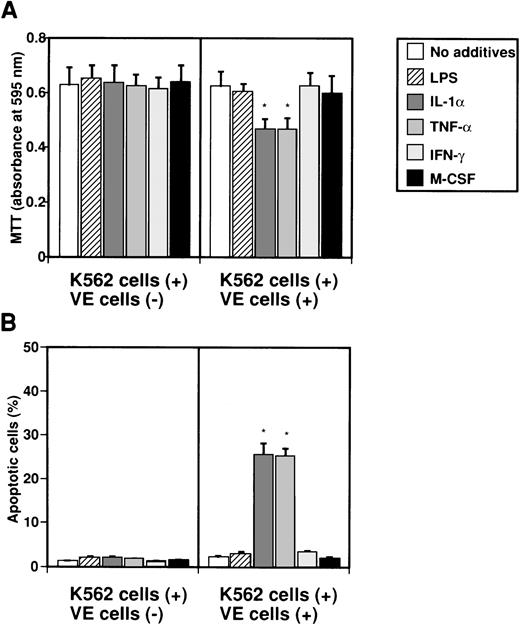
To examine whether IL-8 secreted from endothelial cells by several factors induces apoptosis in K562 cells, K562 cells in Intercell-insert were separately cultured with VE cells stimulated with various factors. K562 cells in Intercell were collected after 2 days, and MTT and TUNEL assays were performed (Fig 3). When K562 cells were cultured with various factors such as LPS, IL-1α, TNF-α, IFN-γ, and M-CSF, growth of K562 cells was not suppressed as measured by MTT assay (Fig 3A). However, when endothelial cells were cultured with IL-1α or TNF-α, growth of K562 cells was significantly suppressed to 74.2% or 74.6% as compared with control (no additives), respectively (Fig 3A). Moreover, when VE cells were cultured with IL-1α or TNF-α, the percentage of apoptotic cells in K562 cells increased from 1.2% ± 0.1% to 25.6% ± 2.5% or 25.3% ± 1.7%, respectively (Fig 3B). This effect was blocked by monoclonal anti–IL-8 antibody (data not shown). Our interpretation was that interaction between endothelial cells and K562 cells during separate culture by Intercell could neither inhibit cell growth nor induce apoptosis against K562 cells (Fig 3A and B). This suggests that endothelial IL-8, secreted from endothelial cells by various factors, induces apoptosis in K562 cells.
Effect of IL-8 from endothelial cells on induction of apoptosis in K562 cells. K562 cells alone (5 × 104cells/mL) or human umbilical venous cells (VE cells, confluent) and K562 cells (5 × 104 cells/mL) were cultured with or without LPS (500 ng/mL), IL-1 (10 U/mL), TNF- (10 U/mL), IFN-γ (200 U/mL), and M-CSF (100 ng/mL) in 24-well plates. Coculture of VE cells and K562 cells was mediated by Intercell. After 2 days, MTT assay (A) and TUNEL assay (B) were performed. Columns show the means of three independent experiments. Statistical analysis was performed using the Student’s t-test. *P < .01.
Effect of IL-8 from endothelial cells on induction of apoptosis in K562 cells. K562 cells alone (5 × 104cells/mL) or human umbilical venous cells (VE cells, confluent) and K562 cells (5 × 104 cells/mL) were cultured with or without LPS (500 ng/mL), IL-1 (10 U/mL), TNF- (10 U/mL), IFN-γ (200 U/mL), and M-CSF (100 ng/mL) in 24-well plates. Coculture of VE cells and K562 cells was mediated by Intercell. After 2 days, MTT assay (A) and TUNEL assay (B) were performed. Columns show the means of three independent experiments. Statistical analysis was performed using the Student’s t-test. *P < .01.
Interaction between endothelial cells and K562 cells induces apoptosis in K562 cells.
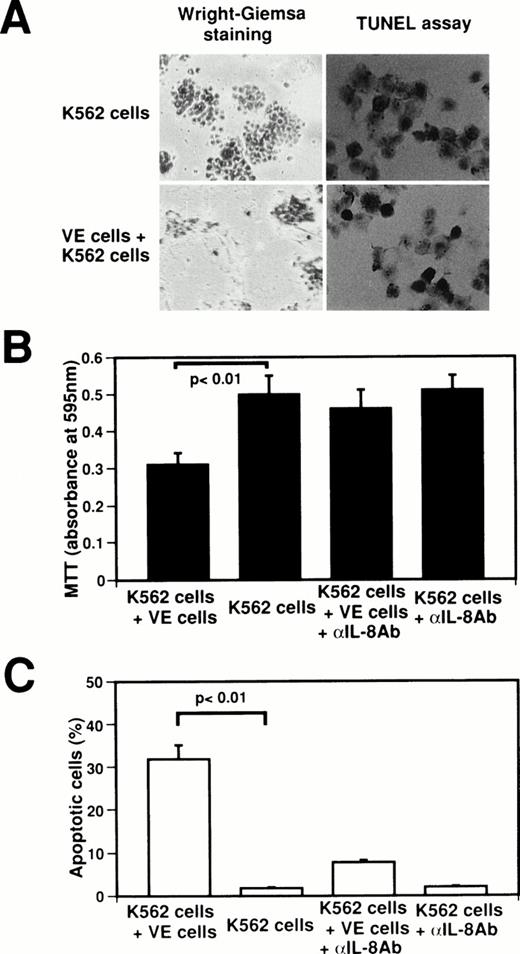
To identify the biological significance of endothelial IL-8, we examined the effect of interaction between normal human venous endothelial cells (VE cells) and K562 cells on the inhibition of cell growth and the induction of apoptosis (Fig4). When K562 cells were attached onto VE cells, growth of K562 cells was suppressed at 62.0% as compared with K562 cell culture alone (Fig4A and B), and 32.0% ± 3.1% of K562 cells underwent apoptosis (Fig 4A and C). Because the concentration of IL-8 in coculture medium of VE cells and K562 cells increased to 75.1 ng/mL from 2.1 ng/mL in medium of VE cells alone (Fig 2), we examined whether the effects of the interaction between VE cells and K562 cells on cell growth inhibition and apoptosis are blocked by anti–IL-8 antibody. When anti–IL-8 antibody was added to coculture media of VE cells and K562 cells, MTT reducing activity recovered to that of K562 cell culture alone (Fig 4B). Moreover, the percentage of apoptotic cells decreased to 7.8% ± 0.6% when anti–IL-8 antibody was added to coculture media of VE cells and K562 cells (Fig 4C). This result suggests that the inhibition of K562 cell growth and the induction of apoptosis were blocked by anti–IL-8 antibody. Western blot analysis also showed that endothelial IL-8 was detected in the culture medium of VE cells and K562 cells (data not shown). Moreover, when HL-60 cells, U937 cells, Jurkat cells, or Daudi cells were attached onto VE cells, 34.7% ± 3.1%, 31.0% ± 3.6%, 24.3% ± 4.0%, or 20.7% ± 3.1% of cells underwent apoptosis, respectively. These findings indicate that endothelial cells can induce apoptosis in the attached K562 cells or other cell lines by releasing endothelial IL-8.
Effect of interaction between human venous endothelial cells (VE cells) and K562 cells on secretion of endothelial IL-8 and apoptosis. K562 cells (5 × 104cells) were seeded with or without confluent VE cells in 24-well plates. Cell culture was boosted with or without anti–IL-8 antibody (5 μg/mL) every 24 hours. After 2 days, Wright-Giemsa staining was performed in 24-well plates (A). K562 cells and supernatants were collected, and MTT assay (B), TUNEL assay (C), and ELISA for IL-8 were performed. For (A), original magnification × 60. Data shown come from three independent experiments. Statistical analysis was performed using the Student’s t-test.
Effect of interaction between human venous endothelial cells (VE cells) and K562 cells on secretion of endothelial IL-8 and apoptosis. K562 cells (5 × 104cells) were seeded with or without confluent VE cells in 24-well plates. Cell culture was boosted with or without anti–IL-8 antibody (5 μg/mL) every 24 hours. After 2 days, Wright-Giemsa staining was performed in 24-well plates (A). K562 cells and supernatants were collected, and MTT assay (B), TUNEL assay (C), and ELISA for IL-8 were performed. For (A), original magnification × 60. Data shown come from three independent experiments. Statistical analysis was performed using the Student’s t-test.
Endothelial IL-8 suppresses cell growth of K562 cells and induces apoptosis in vivo.
Therefore, we investigated whether endothelial IL-8 can induce apoptosis or suppress cell growth in leukemic cells in vivo. Endothelial IL-8 was in jected daily for 2 days into intraperitoneal tumor masses of K562 cells in nude mice (Fig 5). Apoptosis and suppression of cell growth were observed after 2 days in intraperitoneal K562 cells (Fig 5B and C), and apoptotic cells were phagocytosed by macrophages (Fig 5A). After 2 days, apoptotic cells increased to 12.8% ± 2.2%. Moreover, numbers of K562 cells decreased to 33.3% of control. Monocyte-derived IL-8 did not significantly suppress cell growth or induce apoptosis in intraperitoneal K562 cells. In addition, we investigated whether endothelial IL-8 could suppress cell growth or induce apoptosis against subcutaneous K562 cells (Fig 6). Endothelial IL-8 was then injected daily from day 4 to day 11 into subcutaneous K562 cell tumors established in nude mice, and the antitumor and the apoptosis-inducible effects of endothelial IL-8 were examined. Mice showed a visible response to endothelial IL-8 intratumor inoculations characterized by apoptosis (Fig 6A). Therefore, tumor size of endothelial IL-8 treatment decreased in 52.2% of control (saline; Fig 6B). Tumor size of TNF-α treatment decreased in 41.3% of control (Fig 6B). On the other hand, monocyte-derived IL-8 treatment did not either induce apoptosis or suppress cell growth against subcutaneous K562 cells (Fig 6).
Inhibition of cell growth and induction of apoptosis on treatment of endothelial IL-8 in nude mice. The peritoneal space of nude mice (5 examined) were inoculated with K562 cells, and agents such as saline, endothelial IL-8 (IL-8 [E]), and monocyte-derived IL-8 (IL-8 [M]) were injected daily as described in Materials and Methods. (A) Morphology (Wright-Giemsa staining) and apoptotic cells (TUNEL assay) of intraperitoneal cells were collected. Arrows indicate the apoptotic cells. Original magnification × 160 and × 80. (B) Inhibition of cell growth of K562 cells by endothelial IL-8. Horizontal bars show the means. (C) The percentage of apoptotic cells was determined microscopically by counting more than 200 cells in situ on staining slides. Columns represent the means ± SD (bar) of three independent experiments. Statistical analysis was performed using the Student’s t-test.
Inhibition of cell growth and induction of apoptosis on treatment of endothelial IL-8 in nude mice. The peritoneal space of nude mice (5 examined) were inoculated with K562 cells, and agents such as saline, endothelial IL-8 (IL-8 [E]), and monocyte-derived IL-8 (IL-8 [M]) were injected daily as described in Materials and Methods. (A) Morphology (Wright-Giemsa staining) and apoptotic cells (TUNEL assay) of intraperitoneal cells were collected. Arrows indicate the apoptotic cells. Original magnification × 160 and × 80. (B) Inhibition of cell growth of K562 cells by endothelial IL-8. Horizontal bars show the means. (C) The percentage of apoptotic cells was determined microscopically by counting more than 200 cells in situ on staining slides. Columns represent the means ± SD (bar) of three independent experiments. Statistical analysis was performed using the Student’s t-test.
Endothelial IL-8 suppressed growth of subcutaneous K562 cell tumors. The subepiderms of nude mice (10 examined per group) were inoculated K562 cells, and agents were injected with endothelial IL-8 (IL-8 [E]), monocyte-derived IL-8 (IL-8 [M]), saline, and TNF- daily as described in Materials and Methods. As controls, monocyte-derived IL-8, saline, and TNF- were used. Data shown come from 10 nude mice. Statistical analysis was performed using the Student’s t-test. In (B), *P < .05 and **P< .005. (A) Photograph of suppression of subcutaneous K562 tumor by endothelial IL-8 (IL-8 [E]). As controls, saline, monocyte-derived IL-8 (IL-8 [M]), and TNF- were used.
Endothelial IL-8 suppressed growth of subcutaneous K562 cell tumors. The subepiderms of nude mice (10 examined per group) were inoculated K562 cells, and agents were injected with endothelial IL-8 (IL-8 [E]), monocyte-derived IL-8 (IL-8 [M]), saline, and TNF- daily as described in Materials and Methods. As controls, monocyte-derived IL-8, saline, and TNF- were used. Data shown come from 10 nude mice. Statistical analysis was performed using the Student’s t-test. In (B), *P < .05 and **P< .005. (A) Photograph of suppression of subcutaneous K562 tumor by endothelial IL-8 (IL-8 [E]). As controls, saline, monocyte-derived IL-8 (IL-8 [M]), and TNF- were used.
Antitumor effect of endothelial IL-8 is due to induction of apoptosis in K562 cells.
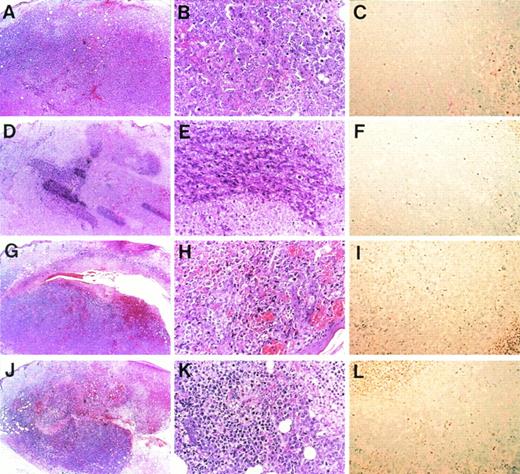
To investigate whether the antitumor effect of endothelial IL-8 is due to induction of apoptosis, pathological examination was performed by hematoxylin-eosin staining and TUNEL staining (Fig 7). Histologically, subcutaneous K562 tumors that responded to either endothelial IL-8 or TNF-α (10 mice of each were examined) generally displayed homogenous central necrosis with intratumor bleeding (Fig 7H and K). Within the viable tumor tissue, many tumor cells became smaller than control cells and showed either condensation or fragmentation of nuclei (Fig 7B and H). Neutrophil and lymphocyte infiltrations were unremarkable in both groups. Control K562 tumors (saline- and monocyte-derived IL-8 groups) displayed little or no tumor necrosis (Fig 7A and D), and their cells had no change in cell size or nuclei (Fig 7B and E). Tumor sections were stained with TUNEL assay specific for apoptotic cells. TUNEL assay showed that in 45.2% or 47.8% of K562 tumor cells inoculated with either endothelial IL-8 or TNF-α, apoptosis was induced (Fig 7I and L). However, control tumor cells showed little apoptosis (Fig 7C and F).
Microscopic morphology of progressive and regressing subcutaneous K562 tumors. Balb/c nu/nu mice were injected subcutaneously with K562 cells and were subsequently injected with saline (A through C), monocyte-derived IL-8 (D through F), endothelial IL-8 (G through I), and TNF- (J through L). Tumors were removed in toto, and hematoxylin and eosin staining (A, B, D, E, G, H, J, and K) or TUNEL assay (C, F, I, and L) was performed after 8 days. Original magnification × 5 for (A), (D), (G), and (J); × 20 for (B), (C), (E), (F), (H), (I), and (K).
Microscopic morphology of progressive and regressing subcutaneous K562 tumors. Balb/c nu/nu mice were injected subcutaneously with K562 cells and were subsequently injected with saline (A through C), monocyte-derived IL-8 (D through F), endothelial IL-8 (G through I), and TNF- (J through L). Tumors were removed in toto, and hematoxylin and eosin staining (A, B, D, E, G, H, J, and K) or TUNEL assay (C, F, I, and L) was performed after 8 days. Original magnification × 5 for (A), (D), (G), and (J); × 20 for (B), (C), (E), (F), (H), (I), and (K).
DISCUSSION
In this study, we observed that endothelial cells were able to secrete endothelial IL-8 after stimulation with IL-1α and TNF-α and that endothelial IL-8 was able to induce apoptosis in leukemic cells. Moreover, endothelial cells that attached to leukemic cells secreted IL-8, and endothelial IL-8 induced apoptosis in leukemic cells. In the previous study, we purified an apoptosis-inducing factor derived from differentiated HL-60 cells.28 This apoptosis-inducing factor is identical to endothelial IL-8. Human recombinant endothelial IL-8 is able to induce apoptosis in most leukemic cell lines, but monocyte-derived IL-8 is not.
IL-8 was originally isolated from culture supernatants of stimulated human monocytes and identified as a protein of 72 amino acids.36,37 The open reading frame of the IL-8 cDNA encodes for 99 amino acids,38 and the mature form is processed further at the N terminus, yielding several biologically active truncation analogs.39-41 The occurrence of N-terminal variants depends on cell type and culture conditions. Of the two major forms, the 72-amino acid form (monocyte-derived IL-8; SAKELRC…) predominates in cultures of monocytes and macrophages,40and the 77-amino acid form (endothelial IL-8;AVLPRSAKELRC…) prodominates in cultures of tissue cells such as endothelial cells42 and fibroblasts.43Endothelial IL-8 has five extra N-terminal amino acids lacking in monocyte-derived IL-8. Because endothelial IL-8 is converted to monocyte-derived IL-8 by serine proteases such as thrombin,44 monocyte-derived IL-8 exists mainly in plasma.45 Many previous reports have demonstrated that monocyte-derived IL-8 is an inflammatory chemoattractant for neutrophils and that several types of cells, such as monocytes, lymphocytes, and fibroblasts, produce IL-8 after stimulation with various agents.46 On the other hand, the chemotactic activity of endothelial IL-8 is one twentieth as strong as that of monocyte-derived IL-8. The difference of whether IL-8 has 5 extra N-terminal amino acids may decide the induction of chemotaxis or apoptosis. Moreover, it has been reported that leukemic cell dissemination to extravascular space is mediated by interaction between leukemic cells and endothelial cells.47 Our study demonstrated that endothelial IL-8 plays an important role in the antitumor action of endothelial cells.
Recently, it has been demonstrated that vascular cells are important participants in antitumor host defense. Vascular cells, which express nitric oxide synthase in response to IFN-γ and TNF-α, can kill leukemic cells.48 However, the mechanism of the antitumor host defense system associated with vascular cells has not been clarified. Many researchers have discussed angiogenesis for tumor vascularization and tumor metastasis.49 Tumor cells secrete metalloproteinase to destroy matrix proteins and to damage endothelial cells, and then they invade extravascular space.50 We demonstrated that endothelial IL-8 can protect against tumor invasion in this system. Because endothelial IL-8 is known to be secreted mainly by endothelial cells and fibroblasts,51 endothelial cells secreting IL-8 have the antitumor property of inducing apoptosis in contacting leukemic cells like other antitumor cells, such as macrophages and NK cells. This is a novel function of endothelial cells involving tumor cell eradication in the body, mainly via endothelial IL-8. Endothelial IL-8 may have an important role in induction of apoptosis in tumor cells in the blood stream when they anchor to endothelial cells.
Surprisingly, in our in vivo experiment, endothelial IL-8 inhibited growth of K562 cell tumors in the same manner as TNF-α. We demonstrated that TNF-α did not directly suppress cell growth or induce apoptosis in K562 cells, but that TNF-α allows endothelial cells to secrete IL-8 and can indirectly kill leukemic cells. It has been reported that TNF-α modulates expression of various biological molecules in endothelial cells.52 The in vivo effect of TNF-α on killing tumor cells may be explained by both a direct death signal mediated through its receptor and the indirect release of biological modulators such as endothelial IL-8 from endothelial cells. Therefore, it is possible that the antitumor effect of TNF-α in vivo may be mediated by secretion of endothelial IL-8 from intratumor endothelial cells. However, angiogenesis for tumor vascularization will aid tumor progression, and tumor cells produce and secrete some endogenous regulators such as endostatin and angiostatin.53 54 In this study, injected TNF-α acted on endothelial cells growing in tumors, and endothelial IL-8 might then be released from endothelial cells. There are two possible roles of IL-8 from endothelial cells in host defense against tumor cells. First, endothelial cells will secrete endothelial IL-8 when tumor cells in the vessels contact endothelial cells, and then the anchored tumor cells may be directly killed by endothelial IL-8. Second, when the growing endothelial cells in tumors are stimulated with some cytokines such as TNF-α, they will secrete endothelial IL-8 and induce apoptosis.
If we know how to stimulate release or production of endothelial IL-8, we may be able to develop new methods of treatment. In 10 of 14 clinical cases, we observed that apoptosis was significantly induced in fresh leukemic cells by endothelial IL-8 in vitro. Endothelial IL-8, with five extra N-terminal amino acids, can induce apoptosis, but monocyte-derived IL-8, which lacks them, cannot do so. Because most IL-8 in blood plasma exists in the form of monocyte-derived IL-845 and does not induce apoptosis in leukemic cells,28 this phenomenon will be important in leukemia therapy.
VP-16 is well-known to be an anticancer agent and an inducer of apoptosis in leukemic cells. When leukemic cells were treated with both 0.1 μmol/L VP-16 and 20 ng/mL endothelial IL-8 for 48 hours, apoptotic cells increased as compared with the treatment of only 0.1 μmol/L VP-16 (data not shown). This result suggests that endothelial IL-8 can enhance the effect of VP-16 on the induction of apoptosis and may therefore be clinically promising in combination with VP-16.
Understanding of the receptor for induction of apoptosis will be important to further research on chemokines, and we are currently investigating receptor(s) and apoptosis-signaling for them in our system. Anti–IL-8 receptor antibody or IL-8 receptor antagonist, or specific binding protein(s) for endothelial IL-8, should be characterized. About 20% of target cells (K562 cells) undergo apoptosis by endothelial IL-8; therefore, we need to have more susceptible cells as targets, using subclones of the cells. In a preliminary experiment, we separated whole K562 cells into three fractions of cell cycle-phases, ie, G0/G1-phase, S-phase, and G2/M-phase fractions by counterflow centrifugal elutriation system,30 and then examined the susceptibility of K562 cells in each cell-cycle phase to apoptosis induced by endothelial IL-8. This experiment demonstrated that susceptibility to apoptosis is higher in the G0/G1 phase of cell cycle than in the S and G2/M phases in K562 cells (data not shown). In our experiment, proliferation can be less changed but apoptosis markedly increased (Fig 3A). We showed bcl-2–overexpressing cells in any cell cycle phases lost susceptibility to apoptosis.30 Moreover, thymocyte apoptosis by methylpredonisolone and etoposide is independent of proliferation.55 This indicates that the antiapoptotic genes-expressing cells escaped from endothelial IL-8–induced apoptosis can proliferate.
In conclusion, endothelial cells involving a novel apoptotic system have been identified. In the mechanism of this system, endothelial IL-8 release plays an important role. It is not only an inflammatory mediator but also an apoptosis-inducing factor in leukemic cells.
ACKNOWLEDGMENT
The authors thank S. Kurokawa, H. Ishikawa, and T. Ishida for technical assistance.
Supported by a grant-in-aid from the Ministry of Education, Science and Culture of Japan; the Research on Advanced Medical Technology; the Japanese Foundation for Multidisciplinary Treatment of Cancer; and Jichi Medical School Young Investigator Award.
Address reprint requests to Kiyohiko Hatake, MD, PhD, Division of Hematology, Department of Internal Medicine, Jichi Medical School, 3311-1 Yakushiji, Minamikawachi-machi, Kawachi-gun, Tochigi 329-04, Japan; e-mail: kiyohiko@jichi.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 5. Inhibition of cell growth and induction of apoptosis on treatment of endothelial IL-8 in nude mice. The peritoneal space of nude mice (5 examined) were inoculated with K562 cells, and agents such as saline, endothelial IL-8 (IL-8 [E]), and monocyte-derived IL-8 (IL-8 [M]) were injected daily as described in Materials and Methods. (A) Morphology (Wright-Giemsa staining) and apoptotic cells (TUNEL assay) of intraperitoneal cells were collected. Arrows indicate the apoptotic cells. Original magnification × 160 and × 80. (B) Inhibition of cell growth of K562 cells by endothelial IL-8. Horizontal bars show the means. (C) The percentage of apoptotic cells was determined microscopically by counting more than 200 cells in situ on staining slides. Columns represent the means ± SD (bar) of three independent experiments. Statistical analysis was performed using the Student’s t-test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/8/10.1182_blood.v92.8.2672/5/m_blod42040005w.jpeg?Expires=1769107468&Signature=o8UxFqy6O09OFGniRmCQHHmNLn4eQgtVoqe77trUMvRQOEtyUj4oE3SInkKV4nQU91JXZG6id5iuLWOijRCew1dIujVpeLjR3GkEABhyNWXdnVH4EkBSLKtdbYbwSxfon5OdqSd-ThaVeDnPsQPPj0PjnBYstuXZxc2zE0LzRUVzpweV1NDgkbpiEcggcpyEET3XZepxppvR4f30PvOw91AFg2PRWuV5DYk1QpDrw8NOyZmuEOrtdRShcfpODRHIwpUNQf~DyDMwyQaImRCc2p5DwB960jPWIXuzZOGtj1l7~tH~b2TvMj-EgRsqZuGJwm129eRQJ5R5WkzO6Y3nAA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Endothelial IL-8 suppressed growth of subcutaneous K562 cell tumors. The subepiderms of nude mice (10 examined per group) were inoculated K562 cells, and agents were injected with endothelial IL-8 (IL-8 [E]), monocyte-derived IL-8 (IL-8 [M]), saline, and TNF- daily as described in Materials and Methods. As controls, monocyte-derived IL-8, saline, and TNF- were used. Data shown come from 10 nude mice. Statistical analysis was performed using the Student’s t-test. In (B), *P < .05 and **P< .005. (A) Photograph of suppression of subcutaneous K562 tumor by endothelial IL-8 (IL-8 [E]). As controls, saline, monocyte-derived IL-8 (IL-8 [M]), and TNF- were used.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/8/10.1182_blood.v92.8.2672/5/m_blod42040006w.jpeg?Expires=1769107468&Signature=MmJ6B0fHu6IWL7-tlwmx~KEAaS8wYy8MzsVF5eGdSj6HQ4iY-4PKL6EC07~pWTGtLo-DdOGNCr8C9d8QPG3EkB9LMKMnkudQPfMKbuxnMBLd3I6tXgUVnzBJV7oEsYZoqgUE9MenjZkviyJXGiMXP-Zh19puIKRadEiBhyOHgCLZonTr4-qHgWLSaYyWgV0B0CNtysQK-yxSxbm9lvsPLADGWlC-JppM~Mc-46iSd~awugCG91BhYH94QBCMAfBsKcmIkuiwvHRDloDnXUphWnAU0kqAiJl-mzUTg5TTP84KuE4i4tG8L~lEWGI5-BOwC9HyUNjxeL500FNBqTWglg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal