Abstract
Single-chain urokinase plasminogen activator (scuPA), the unique form secreted by cells, expresses little intrinsic plasminogen activator activity. scuPA can be activated by proteolytic cleavage to form a two-chain enzyme (tcuPA), which is susceptible to inhibition by plasminogen activator inhibitor type I (PAI-1). scuPA is also activated when it binds to its cellular receptor (uPAR), in which case the protein remains as a single chain molecule with less susceptibility to PAIs. Fibrin clots are invested with PAI-1 derived from plasma and from activated platelets. Therefore, we compared the fibrinolytic activity of complexes between scuPA and recombinant soluble uPAR (suPAR) to that of scuPA, tcuPA, and tcuPA/suPAR complexes. scuPA/suPAR complexes mediated the lysis of plasma-derived fibrin clots 14-fold more extensively than did equimolar concentrations of scuPA and threefold more extensively than did tcuPA or tcuPA/suPAR, respectively. The enhanced catalytic activity of scuPA/suPAR required that all three domains of the receptor be present, correlated with its PAI-1 resistance, was not dependent on fibrin alone, and required a plasma cofactor that was identified as IgG. Human IgG bound specifically to suPAR and scuPA/suPAR as determined by using affinity chromatography and immunoprecipitation. Plasma depleted of IgG lost most of its capacity to promote the fibrinolytic activity of scuPA/suPAR, and the activity of the complex was restored by adding plasma concentrations of purified IgG. These studies indicate that scuPA/suPAR can function as a plasminogen activator in a physiological milieu.
© 1998 by The American Society of Hematology.
UROKINASE-TYPE PLASMINOGEN activator (uPA) has been implicated in several important biological processes including angiogenesis1-5; wound healing6; inflammation7; ovulation and placental development8-10; atherosclerosis, aneurysm, and neointima formation11-15; and the formation of tumor metastases.16-19 The results of recent studies also show that mice with targeted disruption of the uPA gene are fertile, develop normally, and show little tendency towards spontaneous thrombosis20,21 but are more prone to form thrombi when exposed to endotoxin20 or hypoxia22 or when the uPA gene is disrupted in otherwise healthy tissue-type plasminogen activator (tPA)−/− mice.23Downregulation of uPA expression in wild-type mice contributes to fibrin deposition in response to endotoxin as well.24 Taken together, these data suggest an important and unappreciated role for uPA in fibrinolysis. However, the mechanism by which uPA promotes fibrinolysis has not been resolved.
uPA is synthesized as a single chain molecule that expresses little25 or essentially no26 intrinsic plasminogen activator activity. There is evidence to suggest that the activity of single-chain uPA (scuPA) may be promoted by fibrin clots,27,28 but not nearly to the same extent as might be inferred from the difference in the propensity of transgenic mice lacking both tPA and uPA to develop thrombosis compared with mice lacking tPA alone.21,23 scuPA can be activated by proteolytic cleavage at leu158-ile159converting it to a two-chain molecule.29 However, two-chain enzyme uPA (tcuPA) is rapidly and essentially irreversibly inactivated by plasminogen activator inhibitor type I (PAI-1), which is present in plasma in molar excess.30 PAI-1 is present in even higher concentrations in clots as a result of its release locally by activated platelets31 and vascular cells,32 and its activity is stabilized by clot-bound vitronectin.33 34These observations raise a question as to how tcuPA could play such a seemingly important role in intravascular clot lysis.
One insight into the mechanism by which uPA may contribute to clot lysis comes from the in vitro observation that scuPA can also be activated when it binds to its receptor.35,36 Fibrin clots typically contain neutrophils and macrophages37,38 that express urokinase receptors39 and that accelerate uPA-mediated clot lysis.37,38,40 In accord with this, lysis of fibrin clots is impaired when macrophages from patients with paroxysmal nocturnal hemoglobinemia, which lack uPA receptor (uPAR), are compared with cells from normal individuals.41 These studies suggest that the binding of scuPA to uPAR may facilitate clot lysis at plasma concentrations of the reactants.
scuPA bound to its receptor expresses enzymatic activity comparable with that of tcuPA although it remains a single chain molecule.35 Further, the enzymatically active complex between scuPA and uPAR is relatively resistant to PAI-1.42However, to date, the activation of scuPA by its receptor has been shown only in the presence of the synthetic plasmin substrate H-D-norleucyl-hexahydrotyrosyl-lysine-p-nitroanilide D-lactate.36 43 This peptide inhibits plasmin-mediated conversion of scuPA to tcuPA, but the possibility that it also exerts a direct effect on the activity of the scuPA/uPAR complex has not been excluded. This leaves open the question as to whether plasma clots contain a physiological activator of the scuPA/uPAR complex and whether this activator may contribute to the seemingly critical role of uPA in fibrinolysis.
To address these issues, we asked whether complexes between scuPA and its receptor are capable of lysing plasma clots in the absence of potentially activating synthetic peptides. Our data indicate that plasma clots contain a soluble cofactor that stimulates the fibrinolytic activity of scuPA/suPAR complexes to levels that exceed the activity of tcuPA.
MATERIALS AND METHODS
Materials.
Recombinant human scuPA, the amino terminal fragment of scuPA (amino acids 1-143; ATF), and low molecular weight scuPA (amino acids 144 to 411; LMWscuPA) prepared from recombinant human scuPA, recombinant human soluble uPA receptor (suPAR; amino acids 1-281), and chymotryptic fragments of suPAR consisting of either domain 1 alone (amino acids 1-87; DI) or domains 2 and 3 (amino acids 88-281; DII-III) were all the gifts of Drs Jack Henkin and Andrew Mazar (Abbott Laboratories, Abbott Park, IL) and have been characterized in detail in previous publications.35,42-45 The plasmin-insensitive scuPA variant, scuPA-glu158, was kindly provided by Dr Bradford Schwartz (University of Wisconsin, Madison, WI).46Lyophilized human fibrinogen essentially free of plasminogen and thrombin (catalogue # F-4883) and human α-thrombin were obtained from Sigma (St Louis, MO). Bovine fibrinogen containing trace amounts of plasminogen was obtained from Calbiochem-Novabiochem (San Diego, CA). Recombinant active mutant PAI-1 (Catalogue # 1094), α2-antiplasmin, and high molecular weight human tcuPA (Catalogue # 124) were purchased from American Diagnostica (Greenwich, CT). Protein G-Agarose was purchased from GIBCO BRL, (Bethesda, MD). Purified human IgG was obtained from Organon Teknika Corp (West Church, PA). Plasminogen was prepared from normal human plasma as described.47 To prepare plasma, 450 mL of blood was drawn from healthy volunteers into 63 mL citrate-phosphate-dextrose solution (CPD; 1.66 g sodium citrate, 61 g dextrose, 206 mg citric acid, and 140 mg monobasic sodium phosphate).
Fibrinolysis assessed by clot size.
In some experiments clots were prepared by adding human α-thrombin (0.2 NIH u/mL final concentration) for 1 hour at 37°C to fibrinogen containing trace amounts of plasminogen that had been reconstituted to a concentration of 3 mg/mL in phosphate-buffered saline (PBS), pH 7.4. In other experiments, clots were prepared by adding thrombin (0.4 NIH U/mL final concentration) to citrated human plasma for 1 hour at 37°C. In a third set of experiments, human or bovine fibrinogen was resuspended in human serum to a final concentration of 3 mg/mL, and thrombin (0.4 NIH U/mL) was added as above. In each case, clots were incubated for 1 hour at room temperature in 24-well tissue culture plates (Costar, Cambridge, MA). Aliquots of PBS containing scuPA (10 pmol in 10 μL PBS) in the presence or absence of an equimolar concentration of suPAR was added to the surface of each clot for 2 hours at 37°C by which time digestion was evident. The clots were then washed several times with PBS, incubated overnight with 0.2% of trypan blue, rinsed four times with PBS, and photographed. The pictures were scanned by using a Hoefer GS 300 densitometer (Amersham Pharma Biotech, Piscataway, NJ) and the size of the lytic zones were calculated by using the NIH Image program. In other experiments, the effect of scuPA, scuPA/suPAR, scuPA/suPAR/ATF, LMWscuPA/suPAR, and tcuPA on the lysis of plasma clots was compared as described in detail below.
Fibrinolysis assessed by release of radioactivity.
Purified human fibrinogen was radiolabeled with125I48 and resuspended to a specific activity of 30,000 cpm/mL in either PBS containing 3 mg/mL fibrinogen and 0.5 μmol/L Glu-plasminogen or in plasma. Clots were formed from 0.4 mL soluble fibrinogen or from plasma placed in 16-mm diameter tissue culture wells (Costar) by adding thrombin (0.2 and 0.4 U/mL final concentration, respectively). Each plasminogen activator (10 pmol in 10 μL PBS) was then added directly to the center of the formed clot for 2 hours, the clots were washed with PBS, and the radioactivity released into the lavage solution at specific times was measured. In other experiments, fibrinolysis was measured as previously described.49 Briefly, radiolabeled fibrin or plasma clots were overlaid with serum or PBS containing 25 nmol/L plasminogen activator (scuPA, scuPA/suPAR complex, or tcuPA) for varying periods of time at 37°C, and the release of radiolabeled soluble fibrin degradation products was measured. The percent lysis was calculated to take into account any theoretical changes in the volume of distribution of the released cpms; however, no significant differences were observed when aliquots were taken from different samples at the various time points or from the same well sequentially.
Effect of PAI-1 on fibrinolysis.
Purified fibrin clots trace labeled with 125I were prepared as described above. PBS (400 μL) containing 25 nmol/L plasminogen activator (scuPA/suPAR, tcuPA, or tcuPA/suPAR) was added to each clot in the presence or absence of 50 nmol/L PAI-1 for 200 minutes at 37°C. An aliquot was then removed from the supernatant and counted for radioactivity.
Preparation of suPAR-Sepharose.
suPAR synthesized in SP2/0 cells was purified as described previously.42,44 46 suPAR (5 mg/mL in 0.5 mol/L NaHCO3, pH 8.5) was linked to CNBr-Sepharose (Sigma) according to the manufacturer’s instructions. The resin (1.5 × 50 cm) was washed with 10 column volumes of PBS, 10 column volumes of PBS containing 1 mol/L KCl, 10 column volumes of PBS, 10 column volumes of PBS containing 0.2 mol/L glycine, pH 3.0, and finally with sufficient PBS to re-equilibrate the column to pH 7.4 before use. The coupling efficiency was greater than 95%, yielding 2.85 mg suPAR/mL gel.
Binding of plasma proteins to immobilized suPAR.
suPAR-Sepharose (1 mL) was suspended in PBS in the presence or absence of 100 nmol/L scuPA for 1 hour at 4°C. Ten milliliters of citrated plasma or PBS was added for 1 hour at 4°C. The beads were centrifuged, washed four times with PBS and once with PBS/glycine buffer, pH 3.0, and resuspended in PBS containing 4% sodium dodecyl sulfate (SDS). One aliquot of the solubilized proteins was analyzed by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described below. From another aliquot, the amino acid sequences of its constituent proteins were determined (see below). In another set of experiments, an aliquot of the suspension, before incubation with glycine buffer, was added to a125I-fibrinogen–derived clot containing 0.5 μmol/L Glu-plasminogen and the fibrinolytic activity was determined as described above.
Amino acid sequencing.
Proteins bound to suPAR-Sepharose (50 μL) that had been preincubated with plasma as described above were analyzed by adding 0.5 mol/L Tris, pH 6.5 containing 10% SDS, 0.1% Coomasie Blue and 5% β-mercaptoethanol. The samples were boiled for 2 minutes and 25-μL aliquots were applied to an SDS-12% polyacrylamide gel under reducing conditions as described.50 Individual bands were then cut out for sequencing. Sequencing was performed at the Bletterman Macromolecular Research Laboratory of the Hebrew University in the Interdepartmental Equipment Unit of the Faculty of Medicine using a Perkin-Elmer Applied Biosystems Division model 429 Precise Microsequencer System (The Perkin-Elmer Corp, Norwalk, CT).
Binding of suPAR and IgG.
Five milliliters of normal human plasma was incubated with 1 mL of protein G-Agarose for 2 hours at 4°C. The beads were centrifuged at 5,000 rpm for 10 minutes and the plasma was decanted. No residual IgG was detected in the fall-through fraction on Western blotting. The capacity of this IgG-depleted plasma and normal plasma to promote clot lysis by scuPA/suPAR (as described above) and to precipitate suPAR (see below) were then compared. suPAR was radiolabeled with 125I (New England Nuclear, Cambridge, MA) as described.51125I-suPAR (50 nmol/L) was incubated with 0.1 mL normal citrated plasma (as a source of IgG) or with purified IgG (13 μmol/L) and 0.5 mL protein G-Agarose for 2 hours at 4°C. The mixture was centrifuged as above, the precipitate was washed four times with PBS, and the radioactivity precipitated by protein G-Agarose was measured. Nonspecific binding was determined by using plasma that had been depleted of IgG and by measuring the precipitation of an irrelevant125I-labeled protein (plasminogen). All experiments were repeated at least three times.
RESULTS
suPAR stimulates the fibrinolytic activity of scuPA on plasma clots.
The purpose of this study was to ask whether complexes formed between scuPA and its receptor are capable of mediating the lysis of plasma clots by activating plasminogen. Further, we asked whether this activity was comparable with that of tcuPA.
To address this question, we began by studying the capacity of scuPA and scuPA/suPAR to mediate the lysis of fibrin clots formed by adding thrombin to purified unlabeled bovine fibrinogen, unlabeled human fibrinogen, or radiolabeled purified human fibrinogen, each of which contained 0.5 μmol/L human plasminogen. suPAR inhibited scuPA-mediated plasminogen activator activity determined by using unlabeled bovine (Fig 1A) and human (not shown) fibrinogen and 125I-labeled human fibrinogen by approximately 40% and 50% (Fig 2A), respectively. This inhibition is similar in extent to that seen previously by using the small chromogenic peptide S-2251 as the substrate.43
Effect of suPAR on scuPA-mediated clot lysis: zymography. (A) Lysis of clots prepared from purified bovine fibrinogen. Clots were prepared by adding thrombin (0.2 NIH U/mL final concentration) to bovine fibrinogen (3 mg/mL) in PBS for 60 minutes at room temperature. scuPA (10 pmol in 10 μL PBS or equimolar concentrations of suPAR and scuPA were added for 2 hours at 37°C and the size of each lytic area was measured. The lytic areas generated by scuPA were 1.60 and 1.63 cm2, respectively; the corresponding areas for scuPA/suPAR were 1.19 and 1.05 cm2, respectively. In this and in each panel below, the experiment shown is representative of three so performed. (B) Lysis of clots prepared from human plasma. Clots were prepared by adding thrombin (0.4 NIH U/mL final concentration) to citrated human plasma for 60 minutes at room temperature. scuPA, scuPA/suPAR or suPAR (10 pmol in 10 μL) were added for 2 hours at 37°C. The size of the lytic areas generated by scuPA/suPAR were 0.94 and 0.90 cm2. Effect of ATF on suPAR/scuPA-induced lysis of plasma clots. (C) scuPA/suPAR (10 pmol in 10 μL PBS) was added to the plasma clots for 2 hours at 37°C in the absence or presence of 500 pmol ATF.
Effect of suPAR on scuPA-mediated clot lysis: zymography. (A) Lysis of clots prepared from purified bovine fibrinogen. Clots were prepared by adding thrombin (0.2 NIH U/mL final concentration) to bovine fibrinogen (3 mg/mL) in PBS for 60 minutes at room temperature. scuPA (10 pmol in 10 μL PBS or equimolar concentrations of suPAR and scuPA were added for 2 hours at 37°C and the size of each lytic area was measured. The lytic areas generated by scuPA were 1.60 and 1.63 cm2, respectively; the corresponding areas for scuPA/suPAR were 1.19 and 1.05 cm2, respectively. In this and in each panel below, the experiment shown is representative of three so performed. (B) Lysis of clots prepared from human plasma. Clots were prepared by adding thrombin (0.4 NIH U/mL final concentration) to citrated human plasma for 60 minutes at room temperature. scuPA, scuPA/suPAR or suPAR (10 pmol in 10 μL) were added for 2 hours at 37°C. The size of the lytic areas generated by scuPA/suPAR were 0.94 and 0.90 cm2. Effect of ATF on suPAR/scuPA-induced lysis of plasma clots. (C) scuPA/suPAR (10 pmol in 10 μL PBS) was added to the plasma clots for 2 hours at 37°C in the absence or presence of 500 pmol ATF.
Effect of suPAR on scuPA-mediated clot lysis: release of radioactivity. (A) Lysis of fibrin versus plasma clots. Left side: clots prepared from purified bovine fibrinogen. scuPA (10 pmol in 10 μL PBS; open bar) or equimolar concentrations of scuPA/suPAR complex (solid bar) was incubated with clots prepared from125I-labeled human fibrinogen for 2 hours at 37°C, and the radioactivity released into the supernatant was measured. The data are expressed relative to the condition that produced maximal fibrinolysis. In this and in each panel below, the mean ± standard error of the mean (SEM) of three experiments is shown. Right side: plasma clots. Plasma clots, trace labeled with125I-fibrinogen, were prepared as described in Fig 1B. scuPA (open bar) or scuPA/suPAR (solid bar) was added for 2 hours at 37°C, and the radioactivity released into the supernatant was measured. (B) Lysis of plasma clots. Left side: effect of ATF.125I-labeled plasma clots were incubated with scuPA/suPAR (10 pmol in 10 μL PBS) alone or in the presence of 500 pmol ATF for 2 hours at 37°C, and the radioactivity released into the supernatant was measured. Right side: Effect of scuPA-glu158. scuPA-glu158 (10 pmol in 10 μL PBS; designated *scuPA) was added to 125I-labeled plasma clots in the absence or presence of equimolar concentrations of suPAR for 2 hours at 37°C, and the radioactivity released into the supernatant was measured.
Effect of suPAR on scuPA-mediated clot lysis: release of radioactivity. (A) Lysis of fibrin versus plasma clots. Left side: clots prepared from purified bovine fibrinogen. scuPA (10 pmol in 10 μL PBS; open bar) or equimolar concentrations of scuPA/suPAR complex (solid bar) was incubated with clots prepared from125I-labeled human fibrinogen for 2 hours at 37°C, and the radioactivity released into the supernatant was measured. The data are expressed relative to the condition that produced maximal fibrinolysis. In this and in each panel below, the mean ± standard error of the mean (SEM) of three experiments is shown. Right side: plasma clots. Plasma clots, trace labeled with125I-fibrinogen, were prepared as described in Fig 1B. scuPA (open bar) or scuPA/suPAR (solid bar) was added for 2 hours at 37°C, and the radioactivity released into the supernatant was measured. (B) Lysis of plasma clots. Left side: effect of ATF.125I-labeled plasma clots were incubated with scuPA/suPAR (10 pmol in 10 μL PBS) alone or in the presence of 500 pmol ATF for 2 hours at 37°C, and the radioactivity released into the supernatant was measured. Right side: Effect of scuPA-glu158. scuPA-glu158 (10 pmol in 10 μL PBS; designated *scuPA) was added to 125I-labeled plasma clots in the absence or presence of equimolar concentrations of suPAR for 2 hours at 37°C, and the radioactivity released into the supernatant was measured.
We next asked whether suPAR has a similar effect on the capacity of scuPA to mediate the lysis of clots formed by adding thrombin to plasma (Fig 1B) or to plasma supplemented with trace amount of125I-human fibrinogen (Fig 2A). The fibrinolytic activity initiated by scuPA on plasma clots was less than 10% of that seen by using clots prepared from purified fibrinogen (compare Fig 1A v1B and Fig 2A, left v right side). However, in contrast to the modest inhibition seen when purified fibrin was used as a substrate, suPAR caused a 14-fold enhancement of scuPA-mediated fibrinolysis of plasma clots (Fig 2A). Fibrinolysis was stimulated to the same extent when plasma clots were incubated overnight with 0.5 nmol/L scuPA/suPAR compared with scuPA alone (not shown).
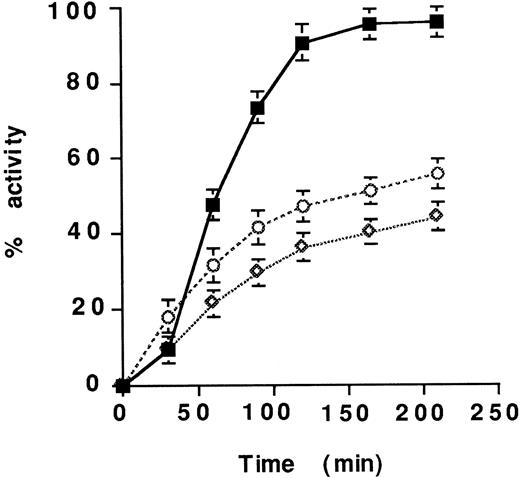
The time course of clot lysis by scuPA and scuPA/suPAR was then examined. Plasma-derived clots were overlaid with serum or with PBS containing scuPA or scuPA/suPAR. Plasma clots suspended in either medium were lysed more rapidly by scuPA/suPAR than by scuPA alone (Fig 3); half-maximal fibrinolysis was achieved at 54 minutes by scuPA/suPAR compared with 210 minutes by scuPA. The same result was obtained when clots were overlaid with plasma (not shown). scuPA/suPAR-mediated fibrinolysis was dose dependent. Doubling the concentration of the complex decreased the time needed for half maximal fibrinolysis from 54 to 33 minutes, whereas a 50% decrease in the concentration of the complex prolonged the time to achieve half maximal fibrinolysis to 102 minutes (not shown).
Time course of lysis of plasma clots by scuPA/suPAR.125I-labeled clots prepared from plasma were overlaid with serum and incubated with 25 nmol/L scuPA in the presence (solid symbols) or absence (open symbols) of equimolar concentrations of suPAR for 210 minutes at 37°C. The radioactivity released into the supernatant was measured. The clots were overlaid with serum (▧ and □) or PBS (△ and ▵), which contained either scuPA alone (□ and ▵) scuPA/suPAR (▧ and △), suPAR alone (◍) or scuPA/suPAR + 250 nM ATF in PBS (*). The mean ± SEM of three experiments is shown.
Time course of lysis of plasma clots by scuPA/suPAR.125I-labeled clots prepared from plasma were overlaid with serum and incubated with 25 nmol/L scuPA in the presence (solid symbols) or absence (open symbols) of equimolar concentrations of suPAR for 210 minutes at 37°C. The radioactivity released into the supernatant was measured. The clots were overlaid with serum (▧ and □) or PBS (△ and ▵), which contained either scuPA alone (□ and ▵) scuPA/suPAR (▧ and △), suPAR alone (◍) or scuPA/suPAR + 250 nM ATF in PBS (*). The mean ± SEM of three experiments is shown.
Four sets of experiments were then performed to ask whether the stimulatory effect of suPAR on scuPA-mediated lysis of plasma clots was induced by the cleavage of scuPA to tcuPA by contaminating proteases. First, the addition of ATF, which inhibits the interaction of scuPA with suPAR, almost completely blocked the stimulatory effect of suPAR on scuPA-mediated fibrinolysis (Figs 1C, 2B, and 3). Second, the plasminogen activator activity of LMWscuPA, which is unable to bind to suPAR because it lacks the ATF but which is as readily cleaved to a two-chain enzyme as is full length scuPA, was not affected by the presence of suPAR (not shown). Third, suPAR enhanced the lysis of plasma clots mediated by the plasmin-resistant variant, scuPA-glu158, more than 20-fold (Fig 2B). Fourth, the plasminogen activator activity of scuPA/suPAR on plasma clots exceeded that of tcuPA or tcuPA/suPAR (Fig 4).
Comparison of fibrinolysis mediated by tcuPA/suPAR and scuPA/suPAR. 125I-plasma–derived clots overlaid with serum were incubated with 25 nmol/L scuPA/suPAR (▪), tcuPA (◊), or suPAR/tcuPA (○) at 37°C for the indicated times, and the radioactivity released into the supernatant was measured. The mean ± SEM of three experiments is shown.
Comparison of fibrinolysis mediated by tcuPA/suPAR and scuPA/suPAR. 125I-plasma–derived clots overlaid with serum were incubated with 25 nmol/L scuPA/suPAR (▪), tcuPA (◊), or suPAR/tcuPA (○) at 37°C for the indicated times, and the radioactivity released into the supernatant was measured. The mean ± SEM of three experiments is shown.
All three domains of suPAR were required to enhance scuPA-mediated fibrinolysis. suPAR fragments that contain DI or DII-DIII can bind scuPA and stimulate its activity in the presence of a small plasmin substrate.44 However, neither fragment, alone or together, stimulated scuPA activity in the presence of plasma-derived clot nor inhibited scuPA-mediated cleavage of the fibrinogen-derived clot (not shown).
Mechanism of stimulation of suPAR/scuPA by plasma.
These experiments show that suPAR stimulates the enzymatic activity of scuPA in the presence of clots formed from human plasma, whereas it modestly inhibits scuPA-mediated lysis of clots formed from purified fibrinogen. Experiments were then performed to address two nonmutually exclusive mechanisms to explain these results. First, the possibility was considered that the activity of scuPA in the absence of suPAR may depend on its conversion to tcuPA, which is then rapidly inhibited by PAI-1 in plasma and within the clot. In this case, the enhanced fibrinolytic activity of scuPA/suPAR on plasma clots may result, in part, from its relative resistance to PAI-1.42 52Consistent with this hypothesis, the lysis of 125I-fibrin clots enriched with PAI-1 (50 nmol/L) by tcuPA and suPAR/tcuPA were each inhibited approximately 80%, whereas the activity of scuPA/suPAR was inhibited less than 20% (Fig 5).
Susceptibility of fibrinolysis to PAI-1.125I-labeled fibrin clots in PBS (−) or PBS supplemented with 50 nmol/L PAI-1 (+) were incubated with 25 nmol/L scuPA/suPAR, tcuPA, or tcuPA/suPAR for 200 minutes at 37°C, and the radioactivity released into the supernatant was measured. The mean ± SEM of three experiments is shown.
Susceptibility of fibrinolysis to PAI-1.125I-labeled fibrin clots in PBS (−) or PBS supplemented with 50 nmol/L PAI-1 (+) were incubated with 25 nmol/L scuPA/suPAR, tcuPA, or tcuPA/suPAR for 200 minutes at 37°C, and the radioactivity released into the supernatant was measured. The mean ± SEM of three experiments is shown.
We then considered a second possibility, ie, that plasma contains one or more factors that are not present in purified fibrinogen that promote the activity of the scuPA/suPAR complex directly. To test this possibility, clots were formed by adding thrombin to purified bovine or human fibrinogen suspended in human serum. scuPA/suPAR caused the lysis of this serum-enriched clot to a greater extent than did scuPA alone, the same pattern seen by using clots prepared directly from plasma (not shown). Further, lysis of serum-enriched clots scuPA/suPAR was abolished by ATF. The same results were obtained when scuPA/suPAR or scuPA were added to fibrin clots covered with serum or plasma compared with PBS. The stimulatory activity of plasma was not caused by anticoagulants, because the addition of CPD to clots formed by fibrinogen did not inhibit fibrinolysis mediated by scuPA nor did it stimulate fibrinolysis by scuPA/suPAR (not shown).
Isolation of a plasma stimulatory cofactor.
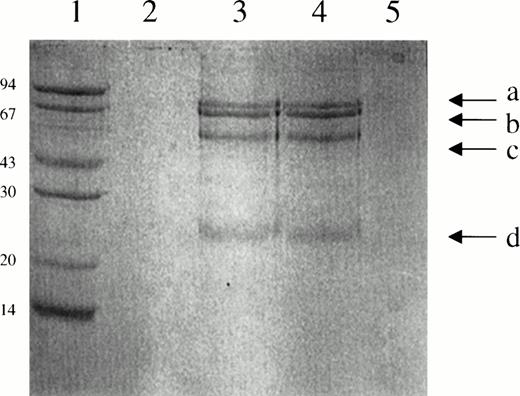
One explanation for the latter set of results is that plasma contains one or more proteins that bind to suPAR or to scuPA/suPAR and promote its activity. To identify such a potential cofactor, plasma was passed over suPAR-Sepharose, or suPAR-Sepharose preincubated with scuPA, and the tightly adherent proteins (resistant to elution at pH 3.0) were analyzed by SDS-PAGE (Fig 6). Four distinct bands were identified in the absence (lane 3) or presence (lane 4) of scuPA. Identical results were obtained when the experiment was repeated with plasma from four different donors. Bands a, c, and d were not seen when the incubation was performed in the presence of PBS (± scuPA) instead of plasma (lanes 2 and 5) indicating the proteins derived from the added plasma and not from the column itself. None of these bands were seen when plasma was incubated with a control Sepharose column (not shown). Only band b was seen when suPAR-Sepharose was analyzed by SDS-PAGE.
SDS-PAGE analysis of the proteins bound to suPAR-Sepharose. Lane 1, molecular weight markers; lane 2, suPAR-Sepharose incubated with PBS; lane 3, suPAR-Sepharose incubated with plasma; lane 4, suPAR-Sepharose incubated with scuPA and plasma; lane 5, suPAR-Sepharose incubated with scuPA and PBS.
SDS-PAGE analysis of the proteins bound to suPAR-Sepharose. Lane 1, molecular weight markers; lane 2, suPAR-Sepharose incubated with PBS; lane 3, suPAR-Sepharose incubated with plasma; lane 4, suPAR-Sepharose incubated with scuPA and plasma; lane 5, suPAR-Sepharose incubated with scuPA and PBS.
We then asked whether the ligand(s) that bound to scuPA/suPAR-Sepharose stimulated the fibrinolytic activity of the complex. To address this issue, plasma was incubated with scuPA/suPAR-Sepharose and the column was washed extensively. The washed gel was then added directly to a fibrinogen-derived clot supplemented with plasminogen. The results shown in Fig 7 indicate that the fibrinolytic activity mediated by scuPA/suPAR-Sepharose preincubated with plasma and then washed was considerably higher than that of scuPA/suPAR-Sepharose incubated with PBS and washed in the same manner. suPAR-Sepharose incubated with plasma and washed had no significant plasminogen activator activity.
Effect of suPAR-plasma suspension on the lysis of fibrin clots by scuPA/suPAR. An aliquot (10 μL) of the washed plasma/suPAR-Sepharose suspension (analyzed by SDS-PAGE in Fig 6) was added to 125I-labeled fibrin clots for 2 hours at 37°C and the release of radioactivity into the supernatant was measured. The first lane (solid bar) shows the activity of suPAR/scuPA on the fibrin clot. The second, middle lane, (open bar) shows the activity of scuPA/suPAR in the presence of the plasma/suPAR-Sepharose suspension. The third lane (hatched bar) shows the fibrinolytic activity of the plasma/suPAR-Sepharose suspension alone. The mean ± SEM of three experiments is shown.
Effect of suPAR-plasma suspension on the lysis of fibrin clots by scuPA/suPAR. An aliquot (10 μL) of the washed plasma/suPAR-Sepharose suspension (analyzed by SDS-PAGE in Fig 6) was added to 125I-labeled fibrin clots for 2 hours at 37°C and the release of radioactivity into the supernatant was measured. The first lane (solid bar) shows the activity of suPAR/scuPA on the fibrin clot. The second, middle lane, (open bar) shows the activity of scuPA/suPAR in the presence of the plasma/suPAR-Sepharose suspension. The third lane (hatched bar) shows the fibrinolytic activity of the plasma/suPAR-Sepharose suspension alone. The mean ± SEM of three experiments is shown.
We then performed amino-terminal sequence analysis of the two fastest migrating bands (c and d). The sequences of the proteins were EVQLVESGGXLVQPGXS and EIVMTQSPXTLS, respectively, where X refers to an ambiguous signal at that location (Table1). By using the Wisconsin Package Version 9.0-UNIX, the d sequence was identified as the amino terminus of the variable region of kappa V-III of human IgG KV3F. The N-terminal amino acid sequence of band c is identical to that of human IgG heavy chain V-III HV3T (Table 1). Bands a, b, and c comigrated with bands found on SDS-PAGE analysis of purified human IgG under reducing conditions (not shown). Band b migrated at a higher molecular weight than suPAR alone, but identically with suPAR-Sepharose, and was not found in the eluate from Sepharose-albumin; therefore, this band appears to represent trace amounts of the protein-Sepharose complex that dissociated from the column.
N-Terminal Amino Acid Sequence of the Proteins Bound to suPAR-Sepharose and the Isolated Chains of a Human IgG
| Band “c” | E V Q L V E S G G X L V Q P G X S |
| VH− IIIT | E V Q L V E S G G D L V Q P G R S |
| Band “d” | E I V M T Q S P X T L S |
| κ-V II IF | E I V M T Q S P V T L S |
| Band “c” | E V Q L V E S G G X L V Q P G X S |
| VH− IIIT | E V Q L V E S G G D L V Q P G R S |
| Band “d” | E I V M T Q S P X T L S |
| κ-V II IF | E I V M T Q S P V T L S |
Top: The amino acid sequence of band “c” from Fig 6 and an IgG heavy chain V-III are compared. Bottom: The amino acid sequence of band “d” and an IgG light kappa chain are compared. The term “X” refers to an ambiguous signal at that position.
Effect of IgG on scuPA/suPAR-mediated fibrinolytic activity.
This unexpected result led us to perform a series of experiments to determine whether the plasma cofactor activity could indeed be ascribed to IgG. The first question that was asked was whether IgG bound avidly to suPAR in a plasma environment. To study this, 125I-suPAR was incubated with plasma and then with protein G-Agarose. The results shown in Fig 8A indicate that suPAR was immunoprecipitated by protein G-Agarose in plasma, whereas under the same conditions radiolabeled plasminogen was not. When the same experiment was performed with IgG-depleted plasma, precipitation of suPAR was reduced by greater than 75% (not shown). Similar results were obtained when serum or IgG-depleted serum was used instead of plasma or IgG-depleted plasma (not shown). Similar results were also seen when purified human IgG (13 μmol/L) was substituted for plasma (Fig 8B). No specific immunoprecipitation of 125I-suPAR was seen when rabbit serum was used as a source of IgG instead of human serum (not shown).
(A) Immunoprecipitation of suPAR by plasma IgG.125I-suPAR or 125I-plasminogen (final concentration 50 nmol/L) was added to 0.5 mL native plasma [black bars: (+)] or to IgG-depleted plasma [open bars: (−)]. The mixture was incubated with protein G-Agarose, centrifuged, and washed repeatedly with PBS, and the precipitated radioactivity was measured. The mean ± SEM of three experiments is shown. (B) Immunoprecipitation of suPAR by purified IgG. Protein G-Agarose (0.5 mL) was added to a mixture containing either 50 nmol/L 125I-suPAR or 50 nmol/L125I-plasminogen plus 1 mg/mL IgG (black bars) or 1 mg/mL BSA (open bars), and the precipitated radioactivity was measured as above. The mean ± SEM of three experiments is shown.
(A) Immunoprecipitation of suPAR by plasma IgG.125I-suPAR or 125I-plasminogen (final concentration 50 nmol/L) was added to 0.5 mL native plasma [black bars: (+)] or to IgG-depleted plasma [open bars: (−)]. The mixture was incubated with protein G-Agarose, centrifuged, and washed repeatedly with PBS, and the precipitated radioactivity was measured. The mean ± SEM of three experiments is shown. (B) Immunoprecipitation of suPAR by purified IgG. Protein G-Agarose (0.5 mL) was added to a mixture containing either 50 nmol/L 125I-suPAR or 50 nmol/L125I-plasminogen plus 1 mg/mL IgG (black bars) or 1 mg/mL BSA (open bars), and the precipitated radioactivity was measured as above. The mean ± SEM of three experiments is shown.
We then asked whether IgG stimulates the fibrinolytic activity of scuPA/suPAR, simulating the effect of plasma. We found that IgG indeed stimulates the fibrinolytic activity of scuPA/suPAR in a dose-dependent manner (Fig 9), whereas no effect on the activity of scuPA alone was observed (not shown). Maximal stimulation was approached at physiological plasma IgG concentrations (Fig 9). The stimulatory effect of IgG required the presence of scuPA as well as plasminogen (not shown), excluding either plasminogen activator activity in the preparation or a direct proteolytic effect on fibrin. Further, α2PI (1.5 μmol/L) inhibited scuPA/suPAR-mediated fibrinolysis to the same extent in the presence or in the absence of IgG (not shown), consistent with fibrinolysis being mediated through the elaboration of plasmin.
Effect of IgG-addition on lysis of plasma-derived clots.125I-fibrin clots were incubated with 25 nmol/L scuPA/suPAR in the presence of the indicated concentrations of purified human IgG for 2 hours at 37°C, and the radioactivity released into the supernatant was measured. The mean ± SEM of three experiments is shown.
Effect of IgG-addition on lysis of plasma-derived clots.125I-fibrin clots were incubated with 25 nmol/L scuPA/suPAR in the presence of the indicated concentrations of purified human IgG for 2 hours at 37°C, and the radioactivity released into the supernatant was measured. The mean ± SEM of three experiments is shown.
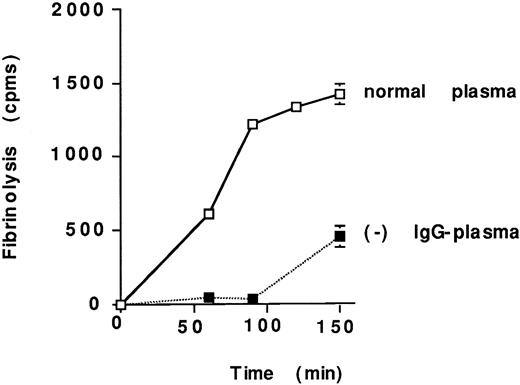
Lastly, we compared the lysis of plasma clots prepared from normal and IgG-depleted plasma. scuPA/suPAR-mediated fibrinolysis of clots prepared from IgG-depleted plasma was clearly delayed relative to those prepared from normal plasma (Fig 10) and the lytic activity was restored totally when IgG-depleted plasma was supplemented with Sepharose-suPAR–bound IgG or purified IgG (13 μmol/L; not shown).
Effect of IgG-depletion on lysis of plasma-derived clots. scuPA/suPAR complex (25 nmol/L) was added to125I-labeled clots formed by the addition of thrombin to normal plasma or to IgG-depleted plasma (−). Fibrinolysis was determined as described in the legend to Fig 2. The mean ± SEM of three experiments is shown. The error bars for certain data points were too small to be appreciated on the graph.
Effect of IgG-depletion on lysis of plasma-derived clots. scuPA/suPAR complex (25 nmol/L) was added to125I-labeled clots formed by the addition of thrombin to normal plasma or to IgG-depleted plasma (−). Fibrinolysis was determined as described in the legend to Fig 2. The mean ± SEM of three experiments is shown. The error bars for certain data points were too small to be appreciated on the graph.
DISCUSSION
The results of this study show that scuPA/suPAR mediates the lysis of plasma clots faster and to a greater extent than does equimolar concentrations of scuPA or tcuPA alone or tcuPA/suPAR in this experimental system. The fibrinolytic activity of scuPA/suPAR was not caused by proteolytic conversion of scuPA to tcuPA for several reasons. First, ATF, which blocks binding of scuPA to its receptor, totally inhibited the activity of scuPA/suPAR but did not inhibit the fibrinolytic activity of tcuPA. Second, suPAR did not stimulate the fibrinolytic activity of LMWscuPA, which cannot bind to the receptor but which is readily converted to LMWtcuPA. Third, the activity of scuPA/suPAR exceeded that of either tcuPA or tcuPA/suPAR. Fourth, suPAR stimulated the fibrinolytic activity of the plasmin-insensitive scuPA variant, scuPA-glu158.53
The greater potency of scuPA/suPAR compared with tcuPA to promote the lysis of plasma clots was explained, in part, by the presence of fibrinolytic inhibitors in the clot, such as PAI-1, to which the complex is relatively resistant.42 However, the enhanced activity is also compatible with the possibility that plasma clots contain factors that augment the activity of scuPA/suPAR. This latter possibility is supported by the observation that fibrinolysis mediated by scuPA/suPAR is actually enhanced when plasma or serum is added to clots prepared from purified fibrin. This finding also indicates that the regulator is present in plasma in an active form and is not activated further by clotting.
The finding that one plasma factor that promotes suPAR/scuPA-mediated fibrinolysis in vitro is IgG was unexpected. However, this conclusion was supported by several findings. First, IgG was retained specifically when plasma was passed over a suPAR-Sepharose column. Second, IgG specifically immunoprecipitated radiolabeled suPAR but not labeled plasminogen when studied in parallel. Third, scuPA/suPAR mediated considerably greater fibrinolytic activity on clots prepared from normal plasma than from IgG-depleted plasma, whereas no effect of IgG depletion on the activity of scuPA was observed. Fourth, IgG stimulated scuPA/suPAR mediated fibrinolysis directly in a dose-dependent manner but had no effect on the activity of scuPA or tcuPA. Fifth, IgG restored the fibrinolytic activity of scuPA/suPAR on clots prepared from IgG-depleted plasma.
There is precedent for finding a link between IgG and the fibrinolytic system. It has previously been reported that plasminogen binds to plasmin-cleaved Fab fragments of IgG and IgG-containing immune complexes and that these fragments provide a template for tPA-mediated plasminogen activation.54 In our experiments,125I-plasminogen was not precipitated from plasma by protein G-Agarose nor did plasminogen copurify with IgG on suPAR-Sepharose or on scuPA/suPAR-Sepharose, most likely because sufficient IgG had not undergone proteolytic cleavage or aggregation54 under these conditions. However, our findings and those of others54 raise the possibility that IgG may both promote the adhesion of leukocytes that express both Fcγ receptor and uPA receptors to fibrin clots as well as promoting cell migration by providing a surface on which urokinase and its substrate can assemble.40,41 55 The finding of a single amino acid sequence suggests that only a subset of immunoglobulin molecules associate tightly with suPAR and the mechanism of this attachment remains unsettled. Thus, it is evident that considerably more study is required to understand the mechanism by which IgG may modulate clot lysis and its importance relative to other plasma proteins that are incorporated into fibrin clots.
Irrespective of the exact mechanism of stimulation and/or the presence of additional plasma cofactors, our studies show that scuPA/suPAR can mediate the lysis of plasma clots and that the activity of this complex is regulated by processes that are distinct from those that control the activity of tcuPA.
Supported in part through grants from the National Institutes of Health (HL40387, HL50970, HL49839), Grant No. 960105000 from the American Heart Association, and a grant from the Joint Research Fund of the Hebrew University and Hadassah.
Address reprint requests to Abd Al-Roof Higazi, MD, Department of Clinical Biochemistry, Hadassah University Hospital, Jerusalem, Israel 91120; e-mail: abd@md2.huji.ac.il.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.






![Fig. 8. (A) Immunoprecipitation of suPAR by plasma IgG.125I-suPAR or 125I-plasminogen (final concentration 50 nmol/L) was added to 0.5 mL native plasma [black bars: (+)] or to IgG-depleted plasma [open bars: (−)]. The mixture was incubated with protein G-Agarose, centrifuged, and washed repeatedly with PBS, and the precipitated radioactivity was measured. The mean ± SEM of three experiments is shown. (B) Immunoprecipitation of suPAR by purified IgG. Protein G-Agarose (0.5 mL) was added to a mixture containing either 50 nmol/L 125I-suPAR or 50 nmol/L125I-plasminogen plus 1 mg/mL IgG (black bars) or 1 mg/mL BSA (open bars), and the precipitated radioactivity was measured as above. The mean ± SEM of three experiments is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/6/10.1182_blood.v92.6.2075/4/m_blod41808008x.jpeg?Expires=1767755136&Signature=kyDsx0PpAafb8kxiOKUDIN2vGKiNm2G2HxyCbQpTOLLGaBsG8DLt0PHEa-~VKFw9lZhdabv29-WaBT9UVUFnNFkuosGcJ6b~uWCp96j5NnxjF9YcRfT2SQTW2~fK4h4Xc9ISqzSU5dNJVSz4umzbyiYvUPYjTC~h3FHF99UVeXNW7u6l98Z6xTexU8agDzHnoXUYqVKS1Caeepn-F6xwqFBwfXfY2eL4C8y~eaL9UfZc6-SHyIcBY01kMqTaZ30zc57yWJ1zmy~9UhVSzJAmBE~GMRWXKVpg1BQZABDUdIshIA4LypdyMNElI7HqDjDrT0qPs6qrM3srpV8j60QjUA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal