Abstract
Soluble (s) CD14, a marker for monocyte/macrophage activation and a mediator of bacterial lipopolysaccharide (LPS) action, was elevated in serum from human immunodeficiency virus type 1 (HIV- 1)-infected individuals (n = 92) compared with seronegative controls. The highest levels were found in patients with advanced clinical and immunological disease. Patients with ongoing clinical events had significantly higher sCD14 levels than symptomatic HIV-1-infected individuals without clinical events, with especially elevated levels in patients infected with Mycobacterium avium complex (MAC). On longitudinal testing of patients (n = 26) with less than 100 × 106CD4 lymphocytes/L at baseline, we found that increasing sCD14 serum concentrations per time unit were associated with death, whereas no differences in CD4 cell number decrease were found between survivors and nonsurvivors. In vitro studies showed that HIV-1 glycoprotein 120 and purified protein derivative (PPD) from M avium (MAC-PPD) stimulated normal monocytes to release sCD14. Furthermore, MAC-PPD induced tumor necrosis factor (TNF) release from monocytes through interactions with CD14 and, importantly, the addition of sCD14 enhanced this MAC-PPD stimulatory effect. Our findings suggest that the CD14 molecule may be involved in the immunopathogenesis of HIV-1 infection, and it is conceivable that serial determination of sCD14 may give useful predictive information concerning disease progression and survival in HIV-1-infected patients.
© 1998 by The American Society of Hematology.
AN IMPORTANT QUESTION regarding the pathogenesis of human immunodeficiency virus type 1 (HIV-1) infection is the altered activation status and reaction pattern for different cell populations of the immune system. Monocytes and macrophages are reservoirs for HIV-1, and these cells produce altered levels of cytokines1 and have impaired ability to kill microorganisms such as Mycobacterium avium.2 CD14 (reviewed by Ziegler Heitbrock and Ulevitch3) is a glycoprotein expressed mainly on the surface of monocytes/macrophages and, to a lesser extent, on neutrophils.3,4 Membrane (m) CD14 is a receptor for bacterial lipopolysaccharide (LPS), but soluble (s) CD14 may also bind LPS directly and enhance LPS responses in cells that lack mCD14.5,6 However, both enhancing7,8 and inhibitory9;10 effects are observed when sCD14 is added together with LPS to monocyte cell cultures depending on type and concentration of sCD14. Recently, a cell surface structure distributed on many cell types was postulated as a receptor for sCD14/LPS complexes, and this structure may also be involved in interactions with mCD14.11 CD14 has been implicated in the activation of cells by molecules other than LPS, eg, streptococcal rhamnose glucose polymers,12 cell wall components from Staphylococcus aureus,13-15 lipoarabinomannan (LAM) fromMycobacterium,16,17 and mannuronan isolated from seaweed or Pseudomonas aeruginosa.18,19 It has also been identified as a phospholipid transfer molecule20; additionally, a role of CD14 in monocyte interleukin-2 (IL-2) signaling has been reported.21
sCD14, which exists in two different forms with molecular weights of 48 and 54 kD, respectively, is either shedded from the cell surface or released from intracellular pools.22 Increased release of sCD14 from monocytes is observed after stimulation with various agents,22-25 and sCD14 may therefore be a marker for activation of monocytes/macrophages. In normal serum, sCD14 is present in relatively high concentrations (>1 μg/mL) and elevated serum levels have been reported in disorders such as trauma,26sepsis,27,28 and rheumatoid arthritis.29;30There is also one report demonstrating elevated levels in HIV-1 infection in a cross-sectional study.31 Earlier, we and others have described increased concentrations of activation parameters, such as tumor necrosis factor α (TNF), soluble TNF receptors (sTNFR), and neopterin in sera from HIV-1-infected patients.32-34 This may reflect monocyte/macrophage hyperactivity. To further investigate this phenomenon, we studied serum levels of sCD14 in a large number of HIV-1-infected individuals, both with longitudinal and cross-sectional testing. Furthermore, we examined the in vitro release of sCD14 from normal monocytes after stimulation with viral and bacterial components and the involvement of CD14 in monocyte activation by components of M avium.
PATIENTS AND METHODS
Patients
From September 1991 through December 1994, serum samples were obtained from consecutively recruited HIV-1-infected patients from The Section of Clinical Immunology and Infectious Diseases, Medical Department A, the National Hospital (Oslo, Norway). The patients were clinically classified according to Center for Disease Control and Prevention (CDC)-1993 revision35 in groups of asymptomatic HIV-1 infection (CDC group A, n = 29; median, 30 years; range, 19 to 34 years), symptomatic non-acquired immunodeficiency syndrome (AIDS) (CDC group B, n = 22; median, 35 years; range, 25 to 56 years), and AIDS (CDC group C, n = 41; median, 38 years; range, 16 to 54 years). Twenty-one healthy, HIV-1-seronegative blood donors served as controls (median, 36 years; range, 22 to 60 years). Of the HIV-1-infected patients, 42 received zidovudine in combination with Pneumocystis carinii prophylaxis, and 7 of those received didanosine in addition. None of the patients had initiated therapy or changed dosage regimen during the last 6 months. For the longitudinal study, patients (n = 26) followed twice a year were included in the study the first time their blood CD4 lymphocytes were less than 100 × 106/L. After inclusion, three to five blood samples were taken, with median intervals of 160 days. All serum samples were prepared as earlier described32 and were stored at −70°C until assaying for sCD14. Informed consent for blood sampling was obtained from each patient.
Enzyme-Linked Immunosorbent Assay (ELISA) for sCD14
Measurements of sCD14 were performed using a sandwich ELISA with CD14-specific monoclonal antibodies (MoAbs) 3C10 and 5C5. Immunoplates (Maxisorp, Nunc, Denmark) were coated for 12 hours with 10 μg/mL MoAb 3C1036 (hybridoma obtained from American Type Culture Collection, Manassas, VA; ATCC TIB-228) diluted in phosphate-buffered saline (PBS). Nonspecific binding was blocked with 0.5% bovine serum albumin (BSA; RIA grade, Sigma, St Louis, MO) in PBS for 1 hour at room temperature (RT). After washing the plates three times with PBS/0.05% Tween 20 (Merck, Darmstadt, Germany), the plates were incubated for 1 hour at RT with 50 μL of samples or standard (recombinant soluble CD14; a kind gift from Drs H. Lichenstein and M. Zukowski, Amgen Inc, Boulder, CO) diluted in 0.1% BSA, 0.05% Tween 20 in PBS. The MoAb 5C5 (non-LPS neutralizing CD14 MoAb from a hybridoma developed in our laboratory after immunization of Balb/c mice with sCD14) was labeled with digoxigenin (DIG antibody labeling kit; Boehringer Mannheim, Mannheim, Germany), diluted to 1 μg/mL in the BSA/Tween 20/PBS buffer, and added to the immunoplates after washing. Furthermore, after 1 hour of incubation at RT and subsequent washing, peroxidase-conjugated antidigoxigenin Fab fragments (Boehringer Mannheim) were used for detection with ortho-phenylene diamine (OPD; Dako, Glostrup, Denmark) as peroxidase substrate. The detection limit of the assay was 0.8 ng/mL, and LPS, LPS-binding protein, HIV-1 glycoprotein 120 (gp120), and purified protein derivative (PPD) from M avium had no effect on sCD14 quantification in the assay. Interassay and intra-assay variations were less than 10%. Serum samples from 1 patient were analyzed on the same plate to minimize run-to-run variations.
Analysis of Blood CD4 Lymphocytes
The number of CD4 lymphocytes in blood were determined by immunomagnetic quantification as described.37
Measurement of HIV-1 RNA Copies in Plasma
HIV-1 RNA copy numbers in plasma were measured by quantitative reverse transcriptase polymerase chain reaction (PCR; Amplicor HIV monitor; Roche Diagnostic Systems, Branchburg, NJ). The detection limit of the assay was 200 copies/mL.
Stimulation of Cells
Cells.
Monolayer cultures of adherent human monocytes from healthy blood donors were prepared using gradient centrifugation of buffy coat (The Blood Bank, University Hospital of Trondheim, Trondheim, Norway) on Lymphoprep (Nycomed, Oslo, Norway). The peripheral blood mononuclear cell fraction (2 × 106 cells/well) was incubated in 24-well cell culture plates (Costar, Cambridge, MA) for 1.5 hours in culture medium (see below) containing 40 μg/mL gentamicin and 1% glutamin. Nonadherent cells were removed by washing three times with Hank’s Balanced Salt Solution (GIBCO, Paisley, UK). This method gave typically more than 92% CD14+ cells as determined by flow cytometry (results not shown). Cell isolations yielded 4 to 8 × 105 monocytes/well.
Stimulations with HIV-1 gp120.
Recombinant HIV-1 IIIB gp120 derived from baculovirus-infected insect cells (obtained through MRC AIDS Reagent Project, Potters Bar, UK) was added to monocytes in RPMI 1640 medium (GIBCO) containing 10% fetal calf serum (FCS; GIBCO) for 72 hours. For immunodepletion of gp120, 100 μL goat antimouse sepharose gel (Zymed, San Francisco, CA) was incubated in the presence or absence (for control) of 50 μg gp120 MoAb (obtained through MRC AIDS Reagent Project, UK) with end-over-end rotation for 4 hours. The gel was washed, and nonspecific binding was blocked with 0.1% BSA in PBS and incubated with a 10 μg/mL gp120 solution for 14 hours with end-over-end rotation. The gp120 content in solution after depletion was analyzed in an ELISA with recombinant sCD4 (Intracel, London, UK) coating, sample incubation, and detection with polyclonal rabbit anti-gp120 antibodies (IgG fraction; Intracel) and peroxidase-conjugated goat anti-rabbit antibodies/OPD substrate (Dako). The ELISA demonstrated removal of more than 99.6% of gp120 in the immunodepletion. Equal amounts of liquid from the gp120-immunodepleted solution and control (equivalent to 0.4 and 0.2 μg/mL gp120 according to the ELISA) were then added to monocytes and incubated for 72 hours. By use of Limulus amoebocyte lysate assay (Chromogenix, Mölndal, Sweden), endotoxin contamination was measured to 8 pg/μg for gp120. Cell supernatants were analyzed in the sCD14 ELISA as described above.
Stimulations with purified protein derivative from M avium(MAC-PPD) and LPS.
Adherent monocytes were stimulated with PPD from M avium(MAC-PPD) or LPS from Escherichia coli O26:B6 (catalogue no. L8274; Sigma) in AIM V serum-free medium (GIBCO). MAC-PPD (generously provided by Reidar Bjørlo, The National Veterinary Institute, Oslo, Norway) was produced from M avium strain D4ER (provided by Central Veterinary Laboratory, Weybridge, UK) after main principles, as described.38 Briefly, the bacteria were grown on Sutong-medium, and PPD was prepared from filtrate after precipitation with 40% trichloroacetic acid and subsequent washings with 2% trichloroacetic acid, aceton, and ether. For study of sCD14-release, cells were stimulated for 72 hours. In separate experiments, monocytes were stimulated for 8 hours to investigate the release of TNF. To modulate the stimulatory response mediated by MAC-PPD, the following reagents were used: LPS-neutralizing MoAb 3C10 against CD14, control MoAb 6H8 against a widely distributed myeloid cell surface protein (B. Naume and T. Espevik, unpublished results), and recombinant sCD14 (Amgen). Supernatants were analyzed for bioactive TNF in the WEHI 164 clone 13 bioassay, as described.39 The endotoxin content of MAC-PPD was 0.7 pg/μg as measured by the Limulus assay (Chromogenix).
Statistics
For statistical analysis, the Wilcoxon rank-sum (Mann-Whitney U) test was used for comparing two groups. The Kruskal-Wallis test was used for comparing more than two groups, and correlation analysis was performed using Spearman’s rank correlation coefficients. Results are given as medians with the corresponding 25 to 75 percentiles, unless otherwise specified. For calculations of change in parameters over time, linear regression was used. The influence of different parameters on survival was studied using Kaplan-Meyer analysis and the log rank test.P values less than .05 were considered significant. Data were analyzed with the SPSS for Windows statistical software (SPSS Inc, Chicago, IL).
RESULTS
Increasing Levels of sCD14 Throughout HIV-1 Infection
We found a significant increase of serum sCD14 levels in all clinical stages of HIV-1 infection compared with controls (Fig 1). In fact, the increase in sCD14 was more pronounced in patients with AIDS (CDC group C) than in individuals from the asymptomatic group (CDC group A) or the symptomatic non-AIDS group (CDC group B). The differences between sCD14 levels in AIDS (CDC group C) and non-AIDS (CDC groups A and B, respectively) patients were highly significant.
Serum sCD14 levels are increasing during HIV-1 infection. The highest concentration of serum sCD14 is found in the AIDS patients (CDC group C, n = 41), but symptomatic non-AIDS patients (CDC group B, n = 22) and asymptomatic HIV-1-infected individuals (CDC group A, n = 29) also show increased levels of sCD14 in serum compared with controls (n = 21). Bars represent medians and 25 to 75 percentiles. Differences are highly significant (P < .001) in comparisons between all CDC groups and controls and for comparing CDC group C with groups A and B, respectively.
Serum sCD14 levels are increasing during HIV-1 infection. The highest concentration of serum sCD14 is found in the AIDS patients (CDC group C, n = 41), but symptomatic non-AIDS patients (CDC group B, n = 22) and asymptomatic HIV-1-infected individuals (CDC group A, n = 29) also show increased levels of sCD14 in serum compared with controls (n = 21). Bars represent medians and 25 to 75 percentiles. Differences are highly significant (P < .001) in comparisons between all CDC groups and controls and for comparing CDC group C with groups A and B, respectively.
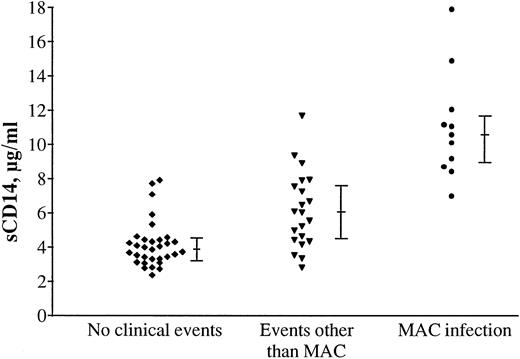
Our earlier studies have indicated elevated activation markers in patients with clinical events and especially in patients with MAC infection.32 34 To relate sCD14 levels to ongoing clinical events (3 weeks before to 1 week after blood sampling), the symptomatic non-AIDS and AIDS patients (CDC groups B and C) were divided into three groups: (1) patients without clinical events (n = 31); (2) patients with events other than MAC infection (n = 21; 6 patients with symptomatic cytomegalovirus [CMV] infection, 4 with disseminated Kaposi’s sarcoma, 3 with bacterial pneumonia, 2 with Pneumocystis carinii pneumonia, 1 patient with Cryptosporidiumenteritis, 1 with non-Hodgkin’s lymphoma, 1 with AIDS dementia complex, 1 with Toxoplasmosis gondii encephalitis, 1 with disseminated cutaneous Herpes simplex virus type 1 infection, and 1 with Salmonella enteritis); and (3) patients with MAC infection (n = 11). As shown in Fig 2, serum sCD14 levels were increased particularly in patients with MAC infection, but also in patients with other events compared with symptomatic individuals without clinical events. MAC-infected patients had significantly higher sCD14 levels than the patients with other clinical events.
High levels of serum sCD14 in relation to ongoing clinical events. Levels of sCD14 in serum from symptomatic HIV-1 patients (CDC groups B and C, n = 63) are especially associated with secondary clinical events. The highest sCD14 concentrations were found in serum from patients (n = 11) with MAC infection (P < .001 compared with patients with other clinical events), but patients with events other than MAC infection (n = 21) also had significant higher sCD14 serum levels than did symptomatic patients without ongoing clinical events (n = 31, P < .001). The bars indicate medians and 25 to 75 percentiles.
High levels of serum sCD14 in relation to ongoing clinical events. Levels of sCD14 in serum from symptomatic HIV-1 patients (CDC groups B and C, n = 63) are especially associated with secondary clinical events. The highest sCD14 concentrations were found in serum from patients (n = 11) with MAC infection (P < .001 compared with patients with other clinical events), but patients with events other than MAC infection (n = 21) also had significant higher sCD14 serum levels than did symptomatic patients without ongoing clinical events (n = 31, P < .001). The bars indicate medians and 25 to 75 percentiles.
Thus, HIV-1 infection seems to induce an increase in sCD14 serum levels. In addition, patients in CDC-groups B and C with ongoing clinical events had significantly increased serum sCD14 compared with patients without clinical events, with especially high levels in patients with MAC infection. This indicates that both the virus infection itself and secondary clinical events reflect activation of the monocyte/macrophage system, reflected in increased production of sCD14.
Correlation to Other Immunological Parameters
Some of the patients (n = 66) participated in a previous study.32 When correlation analysis was performed with sCD14 as a new parameter, we found inverse correlations to numbers of CD4 lymphocytes (r = -.66) and CD8 lymphocytes (r = -.49) and correlations to neopterin (r = .59), HIV-1 p24 antigen (r = .58), immunoactive TNF (r = .53), p55 sTNFR (r = .77), and p75 sTNFR (r = .78; P < .001 for all correlations). Thus, increased serum levels of sCD14 appeared not only to be associated with advanced clinical disease, but also with serious immunological disturbances. The strong correlation between sCD14 and sTNFR is particularly interesting, because of the prognostic value of these parameters.32,33 40 Soluble CD14 may have a role in characterization of total immunological status in HIV-1 infection.
Correlation of sCD14 Concentrations to Viral Load
To correlate amounts of sCD14 in serum to the viral load in patients, we measured HIV-1 RNA copy numbers by PCR in plasma samples from 49 patients in the study (median, 50,954 copies/mL; 25 to 75 percentile, 6,790 to 311,751 copies/mL). Interestingly, we found a highly significant correlation (r = .69, P < .001) between sCD14 and the amount of plasma HIV-1 RNA, as shown in Fig 3. In addition to the relationship between sCD14 and different immunological parameters, this further demonstrates the close correlation between disease activity and serum sCD14 concentrations.
Correlation between serum sCD14 and plasma HIV-1 RNA copy numbers. Plasma HIV-1 RNA copy numbers were measured in 49 patients, and the relationship between HIV-1 RNA copy numbers and sCD14 concentration is plotted. The correlation is highly significant (r = .69, P < .001).
Correlation between serum sCD14 and plasma HIV-1 RNA copy numbers. Plasma HIV-1 RNA copy numbers were measured in 49 patients, and the relationship between HIV-1 RNA copy numbers and sCD14 concentration is plotted. The correlation is highly significant (r = .69, P < .001).
Longitudinal Testing
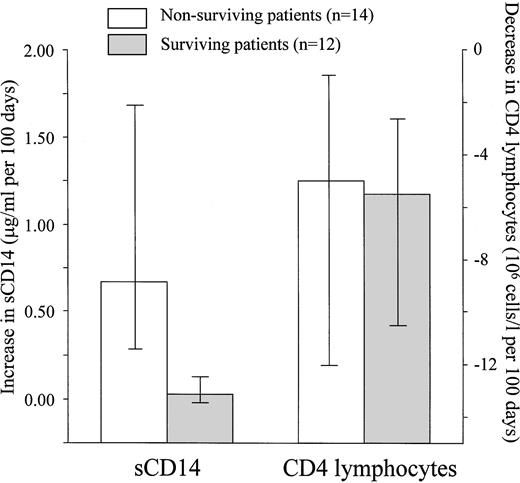
For HIV-1-infected patients with markedly reduced numbers of CD4 lymphocytes, the CD4 cell number is not a good prognostic marker.41 42 To investigate if levels of sCD14 might give predictive information concerning survival, some of the HIV-1-infected patients were tested longitudinally. Twenty-six patients were included in the study when their blood CD4 cell numbers decreased to less than 100 × 106/L. Sera from the HIV-1-infected individuals were collected at different time points, and the patients were divided into two groups: one group of the patients who died during the study period (up to 6 months after the last serum collection; n = 14) and a second group of the patients who survived the same period (n = 12). As shown in Fig 3, the patients who died had a significant increase in sCD14 per time unit compared with the patients who survived. Patients in both groups had similar values for decrease in CD4 lymphocyte number per time unit (Fig 4). At the time of inclusion, survivors and nonsurvivors did not differ significantly in serum sCD14 concentrations (nonsurvivors, 4.01 μg/mL [3.55 to 4.76 μg/mL]; survivors, 3.95 μg/mL [3.31 to 4.92 μg/mL]). They also did not differ significantly in CD4 lymphocyte numbers (nonsurvivors, 43 × 106 cells/L [19 to 63 × 106 cells/L]; survivors, 52 × 106cells/L [43 to 68 × 106 cells/L]). However, at the last blood sampling, nonsurvivors had significantly higher sCD14 values (6.07 μg/mL [4.58 to 9.67 μg/mL]) compared with the inclusion sample (P = .001), and the number of blood CD4 lymphocytes was also significantly lower (15 × 106 cells/L [9 to 31 × 106 cells/L]) than at the first sampling time point for these patients (P = .003). In contrast, the surviving patients did not have a significant difference in sCD14 concentrations at the last time point (4.25 μg/mL [3.74 to 5.24 μg/mL]) compared with the time of inclusion, whereas the CD4 cell numbers also in these patients were significantly depressed at the last sampling time point (19 × 106 cells/L [11 to 24 × 106cells/L]) compared with the first (P = .01). Additionally, at the last sampling time point, the nonsurviving patients had significantly higher serum sCD14 concentrations than the patients who survived the study period (P = .008), whereas there were no difference between the two groups in the number of blood CD4 cells.
Nonsurviving HIV-1-infected patients have markedly increasing serum sCD14 concentrations over time. In a longitudinal study, serum samples from patients (n = 26) with blood CD4 lymphocytes less than 100 × 106/L were collected at different time points. The patients could be divided into two groups: patients who survived the study period (up to 6 months after last serum collection; n = 12; ▧) and patients who died during the study period (n = 14, □). When using linear regression analysis, variations in sCD14 serum concentration and blood CD4 lymphocyte number could be analyzed and expressed as changes per time unit (100 days). The results showed increasing sCD14 levels in the patents who died and relatively stable levels in the surviving patients (P < .001 comparing the 2 groups). In contrast, both nonsurvivors and survivors had similar decreasing number of CD4 lymphocytes in blood during the study period. Shown are medians with corresponding 25 to 75 percentiles.
Nonsurviving HIV-1-infected patients have markedly increasing serum sCD14 concentrations over time. In a longitudinal study, serum samples from patients (n = 26) with blood CD4 lymphocytes less than 100 × 106/L were collected at different time points. The patients could be divided into two groups: patients who survived the study period (up to 6 months after last serum collection; n = 12; ▧) and patients who died during the study period (n = 14, □). When using linear regression analysis, variations in sCD14 serum concentration and blood CD4 lymphocyte number could be analyzed and expressed as changes per time unit (100 days). The results showed increasing sCD14 levels in the patents who died and relatively stable levels in the surviving patients (P < .001 comparing the 2 groups). In contrast, both nonsurvivors and survivors had similar decreasing number of CD4 lymphocytes in blood during the study period. Shown are medians with corresponding 25 to 75 percentiles.
To study the importance of different parameters on survival time, we divided the patients into groups according to the median values for all 26 patients participating in the longitudinal study, and the data were analyzed by Kaplan-Meyer survival analysis. Kaplan-Meyer curves for sCD14 versus survival are shown in Fig 5, in which patients are divided into two groups with either higher or lower sCD14 increase compared with median. The log rank test showed that sCD14 increase (median, 0.24 μg/mL per 100 days) was predictive for death (P < .001), as was the sCD14 concentration at the last sampling time (median, 5.11 μg/mL; P = .003). In contrast, the other parameters, such as CD4 lymphocyte decrease (median, −5 × 106 cells/L per 100 days), the number of CD4 cells at first/last sampling time (medians, 50 × 106 cells/L and 16 × 106 cells/L, respectively), and the sCD14 concentration at the first sampling time (median, 4.01 μg/mL) were not predictive.
Kaplan-Meyer survival analysis for patients in the longitudinal study. Patients (n = 26) are divided into groups with higher or lower increase of serum sCD14 per time unit compared with median (0.24 μg/mL per 100 days). The Kaplan-Meyer curves indicate that patients with higher sCD14 increase have much shorter survival times than patients with a lower increase in serum sCD14 concentrations. The figure shows cumulative survival plotted against time (until death or censoring). Censored cases are indicated (X).
Kaplan-Meyer survival analysis for patients in the longitudinal study. Patients (n = 26) are divided into groups with higher or lower increase of serum sCD14 per time unit compared with median (0.24 μg/mL per 100 days). The Kaplan-Meyer curves indicate that patients with higher sCD14 increase have much shorter survival times than patients with a lower increase in serum sCD14 concentrations. The figure shows cumulative survival plotted against time (until death or censoring). Censored cases are indicated (X).
Changes in serum sCD14 and number of blood CD4 lymphocytes for 6 patients are shown in Fig 6. Patients A and C with MAC infections had high levels of sCD14 and a rapid increase in sCD14 serum concentrations over time. MAC-infected patients had high sCD14 values in serum (Fig 2) and had a tendency to a higher increase of sCD14 per unit time than the other patient groups (not shown).
Variations in serum sCD14 concentrations and blood CD4 lymphocyte number for some patients in the longitudinal study. Changes over time in serum sCD14 concentration (—) and blood CD4 lymphocyte number (––) for 6 patients are shown. Patients A (with MAC infection), B (with disseminated Kaposi’s sarcoma [KS]), and C (MAC infection) died during the study period and all had increasing sCD14 values and decreasing number of blood CD4 lymphocytes. Patients D (no clinical events), E (with disseminated cutaneous Herpes simplex virus type 1 infection), and F (no clinical events) survived the study period and all had relatively stable sCD14 levels and decreasing CD4 cell numbers. Arrows indicate sampling time point with ongoing clinical event and CDC classification; death is indicated with a cross for patients A, B, and C.
Variations in serum sCD14 concentrations and blood CD4 lymphocyte number for some patients in the longitudinal study. Changes over time in serum sCD14 concentration (—) and blood CD4 lymphocyte number (––) for 6 patients are shown. Patients A (with MAC infection), B (with disseminated Kaposi’s sarcoma [KS]), and C (MAC infection) died during the study period and all had increasing sCD14 values and decreasing number of blood CD4 lymphocytes. Patients D (no clinical events), E (with disseminated cutaneous Herpes simplex virus type 1 infection), and F (no clinical events) survived the study period and all had relatively stable sCD14 levels and decreasing CD4 cell numbers. Arrows indicate sampling time point with ongoing clinical event and CDC classification; death is indicated with a cross for patients A, B, and C.
Viral and Mycobacterial Components Stimulate Monocytes to the Release of sCD14 In Vitro
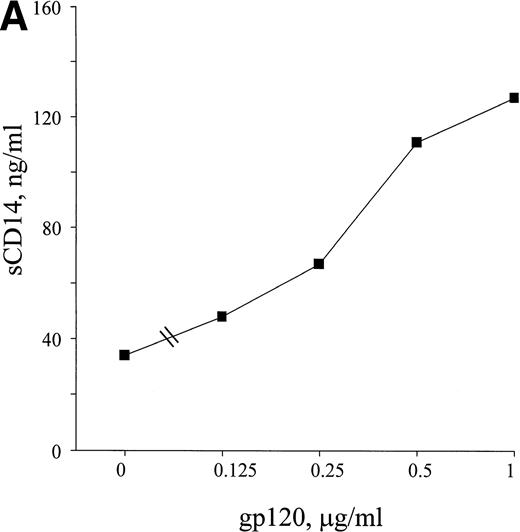
HIV-1 envelope glycoprotein 120 (gp120) is able to stimulate monocytes to release soluble mediators such as TNF, IL-1, IL-6, and endothelin-1.43-45 To investigate if viral components could be responsible for some of the increased release of sCD14 seen in HIV-1 infection, monocytes from healthy donors were stimulated with recombinant HIV-1 gp120. The results from the experiments showed an enhanced release of sCD14 from monocytes after 72 hours of treatment with gp120 (Fig 7A). The stimulatory effect was due to gp120, because immunodepletion of gp120 using a gp120 MoAb removed the sCD14 response (Table 1).
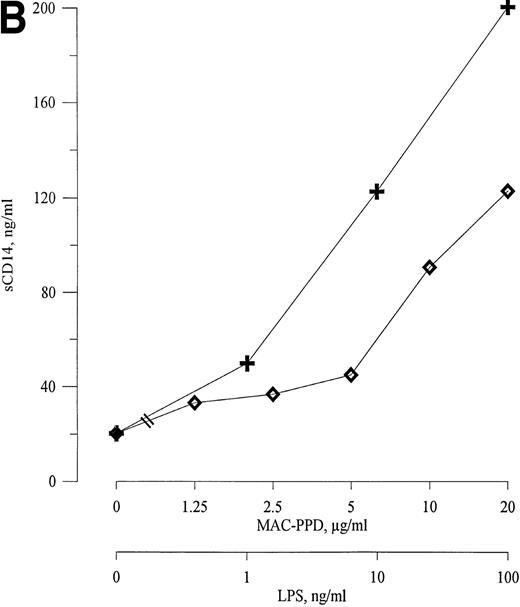
HIV-1 gp120, MAC-PPD, and LPS induce release of sCD14 from monocytes. Freshly isolated monocytes from healthy donors release sCD14 after 72 hours of stimulation with viral and bacterial components. (A) Recombinant HIV-1 gp120 induces release of sCD14 from monocytes in a dose-dependent manner. (B) MAC-PPD (◊) stimulated monocytes to release sCD14, although not as potently as LPS (+). 0 means unstimulated cells. The mean of duplicates is shown; data are from one experiment representative of three performed.
HIV-1 gp120, MAC-PPD, and LPS induce release of sCD14 from monocytes. Freshly isolated monocytes from healthy donors release sCD14 after 72 hours of stimulation with viral and bacterial components. (A) Recombinant HIV-1 gp120 induces release of sCD14 from monocytes in a dose-dependent manner. (B) MAC-PPD (◊) stimulated monocytes to release sCD14, although not as potently as LPS (+). 0 means unstimulated cells. The mean of duplicates is shown; data are from one experiment representative of three performed.
Immunodepletion of gp120 Blocks sCD14 Release From Monocytes
| gp120 (μg/mL) . | gp120 MoAb in Immunodepletion . | sCD14 (ng/mL) . |
|---|---|---|
| 0.4 | − | 76 |
| 0.4 | + | 46 |
| 0.2 | − | 65 |
| 0.2 | + | 49 |
| gp120 (μg/mL) . | gp120 MoAb in Immunodepletion . | sCD14 (ng/mL) . |
|---|---|---|
| 0.4 | − | 76 |
| 0.4 | + | 46 |
| 0.2 | − | 65 |
| 0.2 | + | 49 |
A separate experiment showed that immunodepletion of gp120 with a gp120-specific MoAb removed the stimulating effect on sCD14-release from monocytes. Adherent monocytes from healthy donors were treated for 72 hours with gp120-immunodepleted solution and compared with equal amounts of liquid from the control gp120 solution (without gp120 MoAb in immunodepletion procedure) in sCD14-releasing ability. Numbers are the mean of duplicates. The concentration of sCD14 in unstimulated culture was 49 ng/mL.
AIDS patients with MAC infection were found to have markedly elevated serum levels of sCD14 compared with patients with other clinical events (Fig 2). Therefore, we tested the ability of MAC-PPD to induce sCD14 release from monocytes. As can be seen from Fig 7B, MAC-PPD stimulated monocytes to increased release of sCD14 in a dose-dependent manner, but, however, with less potency than LPS. This demonstrates that mycobacterial components may contribute to the raised levels of serum sCD14 found in AIDS patients with MAC infection.
Components From M avium Stimulate Monocytes Via the CD14 Pathway
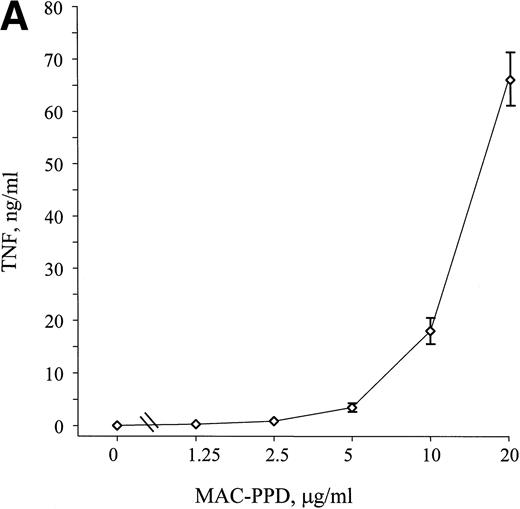
Mycobacterial components such as LAM have the ability to stimulate monocytes through interactions with mCD14.16 17 MAC-PPD induced release of TNF from adherent monocytes in a dose-dependent manner after 8 hours of stimulation (Fig8A); to study if this stimulation is mediated via the CD14 pathway, adherent monocytes were stimulated with MAC-PPD in the absence or presence of the LPS neutralizing CD14 MoAb 3C10. When added 15 minutes before MAC-PPD, 3C10 clearly inhibited MAC-PPD-induced release of TNF from the monocytes (Fig 8B), indicating that mCD14 may be directly involved in the signaling mediated by cell components from MAC. To assess the role of sCD14 in this system, we added recombinant sCD14 together with MAC-PPD and found a significant increase in TNF release in these monocyte cell cultures (Fig 8B). Thus, both mCD14 and sCD14 seem to play important roles in the stimulation of monocytes by components from MAC and may also thereby have clinical significance during MAC infection.
MAC-PPD induces TNF release from monocytes through the CD14 pathway. Monocytes from healthy donors were stimulated with MAC-PPD for 8 hours, and supernatants were analyzed for bioactive TNF. (A) MAC-PPD induced TNF production from monocytes in a dose-dependent manner. (B) The LPS-neutralizing CD14 MoAb 3C10 (10 μg/mL) inhibited the stimulatory effect of MAC-PPD, in contrast to the control MoAb 6H8. Incubation of MAC-PPD together with recombinant sCD14 (100 ng/mL) led to a significant increase in TNF production from the monocytes. 0 means unstimulated cells. The mean of triplicates ± SD is shown; data are from one experiment representative of four performed.
MAC-PPD induces TNF release from monocytes through the CD14 pathway. Monocytes from healthy donors were stimulated with MAC-PPD for 8 hours, and supernatants were analyzed for bioactive TNF. (A) MAC-PPD induced TNF production from monocytes in a dose-dependent manner. (B) The LPS-neutralizing CD14 MoAb 3C10 (10 μg/mL) inhibited the stimulatory effect of MAC-PPD, in contrast to the control MoAb 6H8. Incubation of MAC-PPD together with recombinant sCD14 (100 ng/mL) led to a significant increase in TNF production from the monocytes. 0 means unstimulated cells. The mean of triplicates ± SD is shown; data are from one experiment representative of four performed.
DISCUSSION
In the present study, we show that there is a marked increase in serum concentrations of sCD14 in all clinical stages of HIV-1 infection, with the highest levels in the AIDS group. Particularly high levels were found in patients with ongoing disseminated MAC infection. Furthermore, increased levels of sCD14 were significantly correlated with the degree of immunodeficiency and HIV-1 replication, as measured by number of blood CD4+ lymphocytes and plasma HIV-1 copy numbers, respectively. Also, we found a strong correlation between sCD14 and sTNFR, earlier shown to be important immunological prognostic markers both in HIV-1 infection32 as well as other diseases.46 47 The fact that the number of HIV-1 RNA copies in plasma also showed a highly significant correlation to sCD14 serum concentrations (Fig 3) further highlights that sCD14 serum concentrations reflect disease activity and viral load.
In addition to virus-bound HIV-1 gp120, free gp120 can be detected in circulation.48 Therefore, in vitro cell responses to this envelope glycoprotein may be relevant to the situation found in vivo during HIV-1 infection. Recombinant or natural glycoproteins are shown to stimulate the release of inflammatory cytokines from monocytes43,44; in our experiments, treatment of monocytes with recombinant gp120 resulted in increased release of sCD14. These experiments show that monocytes constantly exposed to virus-bound or circulating free viral antigens may release elevated levels of sCD14 and that these viral glycoproteins may be partly responsible for the increased serum sCD14 levels found in HIV-1-infected individuals.
Infection with the opportunistic pathogen MAC results in a poor prognosis for HIV-1-infected patients. The MAC infection in AIDS is characterized by high morbidity, weight loss, fever, and short survival times.49 HIV- 1-infected patients with MAC infection showed significant higher serum sCD14 concentrations than other patients, including symptomatic HIV-1-infected patients with clinical events other than MAC. These results may, at least in part, be explained by directly stimulated release of sCD14 from monocytes by mycobacterial antigens, as shown in the present study. Serum levels of inflammatory cytokines and cytokine receptors, as TNF/sTNFR, are also elevated in HIV-1-infected patients with MAC infection,34,50 and mononuclear cells from these patients release more TNF, IL-6, and IL-1 when challenged with LPS.50 Our findings demonstrating enhancement of MAC-PPD-induced TNF-release from monocytes by sCD14 suggest that sCD14 may directly contribute to immunological disturbances in MAC patients.
Monocytes from HIV-1-infected patients show reduced ability to take up and kill MAC.2 This may be a result of direct interaction between monocytes and HIV-1 or virus components, such as gp120.51,52 The monocyte response to LAM17 or MAC-PPD (Fig 8B) is increased in the presence of sCD14; in addition, mCD14 may play a role in cell activation by components from MAC, because CD14 MoAbs inhibited MAC-PPD stimulatory effects. A consequence of LPS or MAC stimulation of cells in HIV-1-infected patients in vivo may therefore be enhanced replication of virus, because CD14 is involved in the LPS-induced upregulation of HIV-1 replication.53 Activation of monocytes/macrophages can lead to the release of cytokines such as TNF, which in turn may further activate these cells with both increased HIV-1 replication and subsequent effector molecule release. The involvement of sCD14 and mCD14 in cell stimulation therefore may indicate that these molecules may be parts of a vicious circle leading to more advanced disease in HIV-1-infected individuals. Interestingly, a recent study stressed the important role of cells of the macrophage lineage in HIV-1 infection as high-producers of virus.54 Furthermore, infection with common opportunistic pathogens such as Pneumocystis carinii and MAC dramatically increased the virus production by macrophages.54 Monitoring of monocyte/macrophage activity may therefore be of high clinical relevance in later stages of HIV-1 infection, in which monocytes/macrophages may be important reservoirs of HIV-1.
A recent report shows that mice with a disrupted and dysfunctional CD14 gene have increased resistance to dissemination of bacteria and lethality caused by gram-negative infection.55 CD14 is involved in phagocytosis of bacteria into monocytes,56 and high concentrations of sCD14 can block uptake of Mycobacterium tuberculosis, a common pathogen in HIV-1-infected patients, by microglia.57 In addition, the modulation of cell response to bacteria may be dependent on the concentration of LPS-binding protein.58 These facts show the complex pattern of reactions involving CD14 and indicate a possible important role of CD14 both in dissemination and control of certain secondary infections during HIV-1 disease.
In the longitudinal study, the prognostic value of serially measured sCD14 was emphasized. Increased serum sCD14 concentrations over time were associated with death in the patient group with blood CD4 lymphocytes less than 100 × 106/L, whereas no differences in the decrease of CD4 cells were observed between surviving and nonsurviving patients. In chronic diseases such as systemic lupus erythematosus59 and rheumatoid arthritis,29;30 sCD14 levels have been associated with changes in immunological and clinical status. Our findings suggest that the CD14 molecule may be involved in the immunopathogenesis of HIV-1 infection. Therefore, it is interesting that, in the present study, we have demonstrated that an increase in serum levels of sCD14 over time was associated with death in the patients. Thus, it is conceivable that serial determination of sCD14 might give useful predictive information concerning disease progression and survival in HIV-1-infected patients.
Lines of evidence demonstrate increased levels of activation parameters during HIV-1 infection,60 and, as this study shows, the soluble monocyte/macrophage activation marker sCD14 is found in increasing concentrations in serum during disease progression. Monocytes/macrophages are the most important producers of sCD14, and these cells also harbor HIV-1. Therefore, activation of monocytes/macrophages may result in both enhanced virus replication and in release of sCD14, which may further aggravate the disease. The direct role of sCD14 in HIV-1 infection and secondary clinical events remains unclear, but with the ability of sCD14 to modulate interactions between cells and bacterial components, this molecule may have an important role in regulating immune functions.
ACKNOWLEDGMENT
The authors thank Mari Sørensen and Bodil Lunden for outstanding technical assistance.
Supported by The Research Council of Norway, Odd Kåre Rabben’s Memorial Fund for AIDS research, and The Norwegian Cancer Society.
Address reprint requests to Egil Lien, PhD, Boston University Medical Center, The Maxwell Finland Laboratory for Infectious Diseases, 774 Albany St, Boston, MA 02118; e-mail: egil.lien@bmc.org.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.




![Fig. 6. Variations in serum sCD14 concentrations and blood CD4 lymphocyte number for some patients in the longitudinal study. Changes over time in serum sCD14 concentration (—) and blood CD4 lymphocyte number (––) for 6 patients are shown. Patients A (with MAC infection), B (with disseminated Kaposi’s sarcoma [KS]), and C (MAC infection) died during the study period and all had increasing sCD14 values and decreasing number of blood CD4 lymphocytes. Patients D (no clinical events), E (with disseminated cutaneous Herpes simplex virus type 1 infection), and F (no clinical events) survived the study period and all had relatively stable sCD14 levels and decreasing CD4 cell numbers. Arrows indicate sampling time point with ongoing clinical event and CDC classification; death is indicated with a cross for patients A, B, and C.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/6/10.1182_blood.v92.6.2084/4/m_blod41826006x.jpeg?Expires=1765921138&Signature=4xoU8esHcAhwHub-ncQlxhyKNWVVWre9-uzFdJ1QWytyyOC7UpJqoCRcjD9nx3A4nA0RsKLtM5XIz6BAZxr7-n4Hkul4f0f3y97fF0nsQ8QA3YfOrZQjnfLJFlV-FYIJlKOiyow2G3b-N~k1bMj0KFSt4cQM26dy47lwrN-MAxbBTKEuoZALACFU7McCgz7AEX3wVfnLjYkrxvwvNCeanE2BaEkWmRmLp~GcoL3sX5jVfv4EweUxJ8aze8uFKu3ain6Sb6MLm~~RnSVf9EYSPTx9yZhbwwiMCpGAtSWGWiqroiowwsJcC7uT1f68zcj9Mv10uSdJ0seapcMUz-q1eg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal