Abstract
We have investigated the interleukin-12 (IL-12) and tumor necrosis factor- (TNF)-induced regulation of human natural killer (NK) cell function and their relationship with nitric oxide (NO) generation. We demonstrate that both cytokines were efficient to trigger the transcription of the inducible nitric oxide synthase (iNOS) mRNA, as detected by reverse transcriptase-polymerase chain reaction (RT-PCR). Western blot analysis and intracytoplasmic fluorescence showed that iNOS protein was also induced by both cytokines. However, our data indicate that NO does not play a significant role in the effector phase of the cytotoxic activity mediated by NK-stimulated cells, inasmuch as the lytic activity was not affected in the presence of specific NO synthase inhibitors. When aminoguanidine (AMG), an inhibitor of iNOS, was added during the afferent phase of NK stimulation with IL-12 and TNF, a subsequent increase in the lytic potential of the effector cells towards the NK-sensitive target cells (K562) and lymphokine-activated killer (LAK) target cells (Daudi) was observed. Conversely, the addition of chemical NO donors during the afferent step resulted in a dose-dependent inhibition of the NK and LAK cytotoxicity. Our data suggest that the enhancement of NK-cell cytotoxic activity resulting from iNOS inhibition may be correlated, at least in part, to an increase in interferon-γ production and granzyme B expression.
© 1998 by The American Society of Hematology.
NATURAL KILLER (NK) cells play an important role in the early defense against viral infection and malignant transformation.1,2 Their activity is characterized as nonadaptive and major histocompatibility complex (MHC) unrestricted and is thought to play an important role in immune surveillance. It is now well established that the lytic function of these cells is regulated by a complex network of cytokines acting either independently3-5 or synergistically.6-8 Several mechanisms have been proposed to account for NK cytotoxicity. Exocytosis of granules containing perforin (cytolysin or pore-forming protein) and serine esterases (or granzymes) is believed to be the primary mediator of the cellular cytotoxicity exhibited by NK cells.9-11 Additional studies demonstrated a granule-independent killing pathway involving Fas ligand (FasL).12-14
The existence of a new pathway for induction of the lethal injury to target cells by NK cells has been suggested. In murine model, a role of nitric oxide (NO) in the generation of LAK cells has been reported.15 Moreover, several lines of evidence suggested that nitric oxide synthase (NOS) pathway may mediate NK-cell cytotoxicity in rats and mice.16 17
NO is synthesized from the oxidation of the terminal guanido nitrogen atom of L-arginine by a family of nicotinamide adenine dinucleotide phosphate (NADPH)-dependent enzyme, the NOS.18-21 In addition to the existence of two constitutive isoforms (neuronal NOS and endothelial NOS), a third isoform, the inducible NOS (iNOS), can be elicited in different cell types by various stimuli such as bacteria, microbial products, and/or cytokines.22,23 NO, a short-lived molecule, has been identified as a potent biological mediator.24 It plays an active role in many physiological processes such as vasodilatation, neural function, and inhibition of platelet aggregation, as well as in pathological processes such as inflammation.25,26 Evidence has been provided indicating that activated murine mononuclear phagocytes synthesize NO, which contributes in part to their cytotoxic activity against tumor cells and bacteria.27 Similarly, it has been reported that, in vitro, the tumoricidal function of interleukin-2 (IL-2)-activated rodent NK cells depends, at least in part, on their NO- synthesizing ability. Deprival of L-arginine in the medium or blocking of NO synthesis with NG-methyl-L- arginine (NMMA) reduced the killer function of these cells.16 In contrast, it is unclear whether NO plays a role in the tumoricidal and antimicrobial activities of human NK cells.
In the present study, we have investigated the expression of NOS in human NK cells as well as the involvement of NO in the regulation of the cytotoxic activity of these cells by IL-12 and tumor necrosis factor-α (TNFα). We demonstrate that, whereas treatment of NK cells with IL-12 and TNFα triggers the expression of the iNOS, NO does not seem to be involved in the basal or induced cytotoxic activity of human NK cells. Our data suggest that NO plays an inhibitory role on the IL-12- and TNFα-induced stimulation of NK cells.
MATERIALS AND METHODS
Reagents.
L-arginine and aminoguanidine (AMG) hemisulfate salt were purchased from Sigma (St Louis, MO). AMG is a structural analogue of L-arginine that was reported to be more active against the iNOS.28 The14C-labeled L-arginine was purchased from Amersham (Les Ulis, France) or from NEN (Dreieich, Germany). The strong acid cation exchange resin, binding to L-arginine but not to L-citrulline (AG 50W-X8, 100 to 200 mesh, H form) was purchased from Biorad Laboratories (Richmond, CA). A second NOS inhibitor S,S′-(1,3-Phenylenebis (1,2-ethanediyl)) bisisothiourea, or 1,3 PB-ITU, that was reported to be highly selective for iNOS versus ecNOS29 was purchased from Alexis Biochemicals (San Diego, CA). The chemical NO donor 3-morpholino-sydnonimine (Sin-1) and its inactive control N-morpholino-imino-acetonitril (Sin-1C) were kind gifts from Dr J. Winicki (Laboratoires Hoechst, Paris, France); sodium nitroprusside (SNP) was purchased from Sigma.
Cytokines and monoclonal antibodies (MoAbs).
Recombinant IL-12 (specific activity, 5 × 106 U/mg) was kindly provided by S. Wolf (Genetic Institute, Boston, MA). Highly purified (99%) recombinant TNFα (specific activity, 6.63 × 106 U/mg protein) was kindly provided by Dr I. Apfler (Bender Wien, Austria). A polyclonal rabbit antirat iNOS that recognizes the human enzyme30 was kindly provided by V. Riveros-Moreno and S. Moncada (The Cruciform Project, London, UK); a preimmune rabbit antiserum was used as control. Fluorescein isothiocyanate (FITC)-conjugated goat antirabbit IgG (H+L) F(ab′)2 fragments were purchased from Jackson Immunoresearch (West Grove, PA). The FITC-conjugated mouse monoclonal anti-macNOS (IgG2a) that recognizes the human iNOS was purchased from Transduction Laboratories (Lexington, KY). The FITC-labeled isotypic control (FITC-IgG2a) was from Becton Dickinson (Mountain View, CA). The mouse monoclonal antihuman granzyme B and the neutralizing antihuman interferon γ (IFNγ) polyclonal rabbit antibody were kindly provided by M. Sasportes (Hopital Saint Louis, Paris, France) and J. Wietzerbin (Institut Curie, Paris, France), respectively.
Cell preparation.
Peripheral blood lymphocytes (PBL) were isolated from healthy donors (Banque du sang, Hopital Saint-Louis, Paris, France) by Ficoll Hypaque gradient centrifugation followed by plastic adherence. NK cells were enriched by Percoll density gradients, as previously described.7 Cells were then incubated for 30 minutes at 4°C with a cocktail of antibodies: OKT3, OKT4, OKT8 (Ortho Diagnostics Systems, Inc, Westwood, MA), which recognize CD3, CD4, and CD8 molecules, respectively; A13 (MoAb, IgG1) and TiVδ2 (MoAb, IgG1), which react with Vδ1 and Vδ2 gene products, respectively31; and MY4 (anti-CD14) and B4 (anti-CD20) (Coulter/Immunotech, Marseille, France). The cells were resuspended in RPMI complete medium supplemented with 10% human serum, incubated with magnetic beads (Dynal, Oslo, Norway) for 20 minutes, and passed over a magnet. This separation was performed twice and the resulting cell preparation contained 99% CD3− cells. Alternatively, NK cells were purified as positively sorted CD3−CD56+ NK cells using fluorescence-activated cell sorting after magnetic bead depletion.
Determination of L-citrulline, nitrate/nitrite concentration, and cytokine assay.
NK cells were cultured for 3 days without or with IL-12 or/and TNFα in the absence or in the presence of AMG. L-citrulline levels were determined in the cell-free supernatants from NK cultures by colorimetric detection, as described previously.32 Briefly, L-citrulline was measured by the colorimetric reaction of carbamido groups with diacetyl monoxime in acid solution. A total of 30 μL of urease (25 U/mL) was added to 300 μL of supernatants for 1 hour of incubation at 37°C. After the addition of 37.5 μL of trichloroacetic acid (TCA) (59% vol/vol), the precipitated proteins were removed by 5 minutes of centrifugation at 11,000 rpm in an Eppendorf centrifuge. A total of 250 μL of supernatants was harvested and 300 μL of a 1:1 (vol/vol) mixture of 240 mmol/L diacetyl monoxime and a solution of phenazone (3 g in 104 mL H2O reacted with 12 mg FeSO4 and 21 mL H2SO4 36 N) was added for 15 minutes of incubation at 90°C in the dark. A total of 200 μL was collected and transferred to a microtitration plate for measurement of the optical density (OD) at 492 nm using an autoreader (Dynatech Laboratories Inc, Boulogne, Billancourt, France). A calibration curve was performed in parallel with a standard solution of citrulline. Supernatants were also tested for the presence of nitrite/nitrate after reduction of nitrate into nitrite using a Nitric Oxide Assay kit (R&D Systems Europe, Oxon, UK).
The same supernatants were also tested for IFNγ production by enzyme-linked immunosorbent assay (ELISA; Genzyme, Cambridge, MA or Immunotech/Coulter, Marseille, France).
Analysis of iNOS gene expression.
Total RNA was isolated from freshly harvested cells using RNAzol (Bioprobe System, Montreuil-sous-bois, France) procedure based on the method of Chomczynski and Sacchi.33 iNOS mRNA expression was investigated with reverse transcriptase-polymerase chain reaction (RT-PCR), as described.34 The low expression level of iNOS mRNA does not allow its detection by classical Northern blot analysis and usually required a more sensitive technique, such RT-PCR.35 The sequences of the intron-spanning oligonucleotide primer sets in these experiments were as follows: iNOS mRNA sense (5′ TCCGAGGCAAACAGCACATTCA 3′) and iNOS mRNA antisense (5′ GGGTTGGGGGTGTGGTGATGT 3′) and β-actin mRNA sense (5′ GGGTCAGAAGGATTCCTATG 3′) and mRNA antisense (5′ GGGTCAGAAGGATTCCTAATG 3′). The iNOS message is represented by a 371-bp band, and a 237- bp band indicates the β-actin message. The PCR conditions were the following: 3 minutes of denaturation at 94°C, 1 minute of annealing at 60°C, and 1 minute of elongation at 72°C for 42 cycles using a GeneAmp 96000 PCR system (Perkin-Elmer Cetus, Norwalk, CT). The amplified products were analyzed on 0.8% agarose gel containing 0.5 mg/mL ethidium bromide.
Immunofluorescence analysis.
Detection of intracellular protein iNOS was performed with FIX & PERM cell permeabilization kits (Caltag Laboratories, Burlingame, CA) for fixing in suspension and then permealizing the cells. The cells were incubated with appropriate dilution of the anti-iNOS antibody or control rabbit serum for 30 minutes at 4°C. After washes in phosphate-buffered saline supplemented with 1% bovine serum albumin (PBS-BSA), the cells were incubated with FITC-labeled goat antirabbit F(ab′)2 fragment for an additional 30 minutes at 4°C. Alternatively, direct fluorescence was performed using the FITC-conjugated anti-macNOS MoAb. Cells were then extensively washed and analyzed using an EPICS C (Coulter Counter). Fluorescence data were collected on 5 × 103 viable cells, as determined by forward light scatter intensity. Background fluorescence was determined in each case by using an isotype-matched antibody.
Western blot analysis for iNOS and granzyme B.
Determination of iNOS protein in NK cells, cultured for various times (from 4 to 24 hours) without or with IL-12 or/and TNFα, was performed by Western blotting of cytosolic protein extracts. The cells were lysed in 10 mmol/L Tris-HCl, pH 7.4, buffer heated to 90°C. The buffer contained 1% sodium dodecyl sulfate (SDS), 10 μg/mL leupeptin, 2 mmol/L phenylmethylsulfonyl fluoride (PMSF), 2 μg/mL aprotinin, 10 μg/mL pepstatin A, and 2 mmol/L phenanthroline. Samples of 10 or 50 μg of protein were electrophoresed under reducing conditions on 7.5% SDS-polyacrylamide minigels. The proteins were then electroblotted to 0.2-mm vinylidene difluoride and the membrane was blocked with 10 mmol/L Tris-HCl, 100 mmol/L NaCl, pH 7.5 (TBS), containing 1% BSA and 0.1% Tween 20. Blots were then incubated with the rabbit antimouse iNOS antibody at a final concentration of 1/500 vol/vol.36The blots were then washed with 1% TBS containing 0.1% Tween 20 and incubated with a goat antirabbit Ig-conjugated to horseradish peroxidase, and bands were shown by luminol-dependent chemiluminescence (ECL; Amersham). The maximum light emission is at a wavelength of 428 nm and was detected by short exposure to blue light-sensitive autoradiography film (Hyperfilm ECL, Paris, France). For granzyme B protein, NK cells were cultured for 12 hours with IL-12 and TNFα, in the presence or absence of AMG. The cells were lysed in the buffer containing 20 mmol/L Tris/HCl, pH 8, 1 mmol/L EDTA, pH 8, 150 mmol/L NaCl, 1% NP40, 10% glycerol, 0.2 mmol/L PMSF, 1 mmol/L dithiothreitol (DTT), and 20 μL/mL of Protease Inhibitor Cocktail tablets (Complete; Boehringer Mannheim, Mannheim, Germany). Samples of 10 μg were electrophoresed on 12.5% SDS-polyacrylamide minigels and the blot was incubated with the mouse antihuman granzyme B at a final concentration of 1/600 and shown as previously described with a peroxidase-conjugated antimouse Ig (Santa Cruz Biotechnology, Inc, Santa Cruz, CA).
Enzymatic iNOS activity.
NK cells (2 × 106) were stimulated with IL-12 or/and TNFα in presence or absence of AMG. Cultures were performed in Iscove medium (1 mL/well) supplemented with glutamine (2 mmol/L), penicillin (100 U/mL), streptomycin (100 μg/mL), 20 mmol/L HEPES, 5% normal human serum, and L-arginine to obtain a concentration of 1 mmol/L in the medium. Cell extracts were prepared by 4 cycles of freezing on liquid nitrogen and thawing in a 50 mmol/L Tris, HCl buffer, pH 7.5, containing a cocktail of protease inhibitors and centrifuged at 14,000g for 15 minutes at 4°C to remove debries. Supernatants were collected, their protein concentration was determined, and iNOS activity was measured by quantifying the conversion of [14C] L-arginine into [14C] L-citrulline according to a technique described elsewhere.34 [14C] L-citrulline in the eluate was quantitated by scintillation counting.
Cytotoxicity assay.
NK cells were stimulated for 3 days in complete medium (CM; RPMI 1640 supplemented with 10% normal human serum, 100 UI/mL penicillin, 100 μg/mL streptomycin, and 2 mmol/L L-glutamine) in the presence of the indicated cytokines. L-arginine and AMG were added in CM at final concentrations of 2 and 4 mmol/L, respectively. AMG was added either during the time of incubation with the cytokines or during the51Cr- release assay. After the culture period, serial dilutions of effector cells were then distributed in duplicates (0.1 mL per well) into round-bottomed microwell plates. K562 and Daudi target cells were labeled with 200 mCi of Na251CrO4 (5 μCi/mL; Amersham) for 1 hour at 37°C and washed three times, and 5,000 cells per well (in 0.1 mL volume) were distributed in round-bottom microwells with 0.1 mL of effector cells at various effector to target cell (E/T) ratios. After 4 hours of incubation at 37°C, the plates were centrifuged at 2,000g for 2 minutes and cell-free supernatants were collected using a cell harvester (Skatron Inc, Sterling, VA). Supernatant radioactivity was assayed using an automated gamma counter (Packard Instrument Co, Meriden, CT). Spontaneous release was determined by incubating target cells in medium alone. Maximum release was determined by adding 0.1 mL of 1 mol/L HCl to the target cell suspension. The percentage of specific lysis was calculated as follows: 100 × (Experimental 51Cr Release − Spontaneous 51Cr Release)/(Maximum 51Cr Release − Spontaneous51Cr Release).
Statistical analysis.
Significant differences between cytokine-induced NK-cell activation with or without the inhibitor of iNOS were determined by the Student’st-test adapted for a small number of samples. The data were analyzed with use of the InStat 1.4 software (StatSoft Inc, Tulsa, OK). Comparisons were considered significant for a corresponding P≤ .05.
RESULTS
Effect of IL-12 and TNFα on citrulline and nitrate/nitrite release by human NK cells.
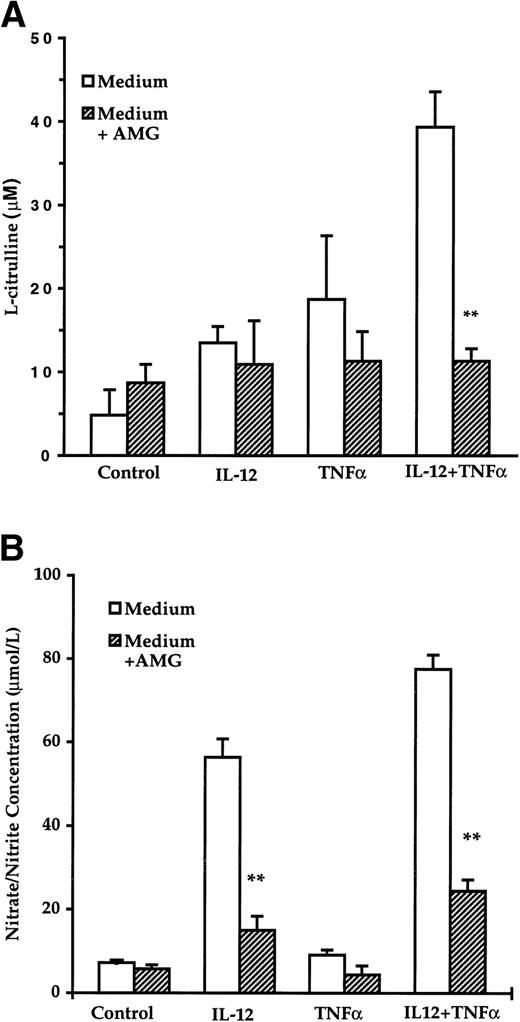
The effect of IL-12 and TNFα on NOS-derived arginine metabolite generation in highly purified human NK cells was investigated. Because NO by itself is a very labile molecule, it is difficult to perform direct determination of its concentration. The amount of the other product of the NOS enzymatic reaction, namely the citrulline released in the supernatants, was quantitated by a colorimetric technique. As seen in Fig 1A, a slight increase in L-citrulline production was observed in the presence of IL-12 or TNFα. However, in the presence of both cytokines, a marked accumulation of citrulline was detected that was greatly reduced in the presence of AMG (4 mmol/L), a specific inhibitor of the iNOS. The amount of nitrite/nitrate released by cultured cells was also measured by NO assay. IL-12 or IL-12 and TNFα induced an increase in NO2−/NO3− that was significantly abrogated by the addition of AMG (4 mmol/L; Fig 1B). The levels of citrulline and nitrite/nitrate released were roughly of the same order of magnitude, taking into account the individual variations between different blood donors.
Production of citrulline and nitrate/nitrite in cultures of NK cells. Purified NK cells (106 cells/mL) were incubated in Dulbecco’s modified Eagle’s medium (DMEM; arginine content, 0.4 mmol/L) with IL-12 (5 U/mL) or/and TNF (20 ng/mL) without or with AMG (4 mmol/L) for 3 days. Supernatants were then collected and production of citrulline (A) and nitrate/nitrite (B) levels was determined as described in Materials and Methods. The results are expressed as the mean ± SE of three different experiments. The asterisks denote a statistically significant difference (P < .01) between IL-12 + AMG and IL-2 or/and IL-12 + TNF + AMG and IL-12 + TNF, as determined by the Student’s t-test.
Production of citrulline and nitrate/nitrite in cultures of NK cells. Purified NK cells (106 cells/mL) were incubated in Dulbecco’s modified Eagle’s medium (DMEM; arginine content, 0.4 mmol/L) with IL-12 (5 U/mL) or/and TNF (20 ng/mL) without or with AMG (4 mmol/L) for 3 days. Supernatants were then collected and production of citrulline (A) and nitrate/nitrite (B) levels was determined as described in Materials and Methods. The results are expressed as the mean ± SE of three different experiments. The asterisks denote a statistically significant difference (P < .01) between IL-12 + AMG and IL-2 or/and IL-12 + TNF + AMG and IL-12 + TNF, as determined by the Student’s t-test.
iNOS gene expression in human NK cells.
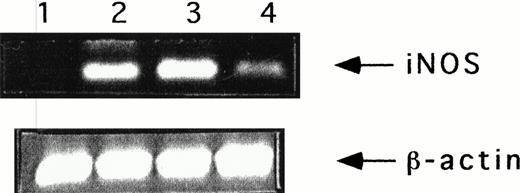
Expression of iNOS mRNA was analyzed in human NK cells upon the addition of human recombinant IL-12 or/and TNFα by reverse transcription of RNA followed by DNA polymerase chain reaction (RT-PCR) using specific human iNOS primers. β-Actin primers were used as a control of amplification. PCR products were analyzed on ethidium-stained agarose gel. As shown in Fig 2, unstimulated NK cells do not express any iNOS mRNA, although a faint band could be observed for some donors. After stimulation with TNFα, IL-12, or their combination, an amplification fragment corresponding to the expected size of 371 bp was visualized. These results demonstrate that iNOS expression is mostly not constitutive in purified NK cells but can be induced after the addition of human recombinant IL-12 or/and TNFα.
iNOS gene expression in unstimulated and IL-12- or/and TNF-treated NK cells. NK cells were stimulated for 7 hours in the absence (lane 1) or presence of IL-12 (5 U/mL) (lane 2), IL-12 and TNF (lane 3), or TNF (20 ng/mL) (lane 4), and then mRNA was extracted. The presence of iNOS mRNA was analyzed by RT-PCR, as described in Materials and Methods. Data are from one representative experiment of seven that gave comparable results.
iNOS gene expression in unstimulated and IL-12- or/and TNF-treated NK cells. NK cells were stimulated for 7 hours in the absence (lane 1) or presence of IL-12 (5 U/mL) (lane 2), IL-12 and TNF (lane 3), or TNF (20 ng/mL) (lane 4), and then mRNA was extracted. The presence of iNOS mRNA was analyzed by RT-PCR, as described in Materials and Methods. Data are from one representative experiment of seven that gave comparable results.
IL-12 and TNFα synergize in inducing iNOS protein and activity by human NK cells.
The expression of iNOS was investigated at the protein level by immunofluorescence on permeabilized cells and fluorescence-activated cell sorting (FACS) analysis using a specific polyclonal anti-iNOS antiserum. As shown in Fig 3, cells stimulated with the combination of IL-12 and TNFα displayed higher iNOS immune reactivity in their cytoplasm than cells stimulated with IL-12 or TNFα alone. Unstimulated NK cells failed to express the iNOS protein, and the staining was determined at the same level as the control antibody. These experiments were confirmed on positively selected FACS-sorted CD56+ NK cells using the commercial FITC-conjugated anti-macNOS MoAb (not shown). The presence of iNOS protein in stimulated NK cells was also analyzed by specific Western blotting. Whereas the expression of iNOS was undetectable or weakly detectable in unstimulated NK cells, depending on the donors, a major band of 135 kD was detected after incubation of these cells for 24 hours with either IL-12 or TNFα alone. This band was clearly more intense after cell treatment by the combination of IL-12 and TNFα (Fig 4). This observation was confirmed by immunocytochemistry and similar data were also obtained using a mouse MoAb against human iNOS.
iNOS protein expression in stimulated NK cells. NK cells were stimulated for 24 hours in the presence or absence of IL-12 (5 U/mL) or/and TNF (20 ng/mL) and then harvested. The cells were washed, permeabilized, and stained with a polyclonal rabbit anti-iNOS and then with a (FITC)-conjugated F(ab’)2 fragment goat antirabbit IgG, as described in Materials and Methods. Fluorescence data were collected on 5,000 viable cells. (Open area) untreated NK cells (profile identical to that obtained with the preimmune serum, indicative that these cells were negative for the expression of this marker). (Shaded area) NK cells stimulated with TNF (upper panel), IL-12 (middle panel), or both cytokines (lower panel). The percentage represents the change in the mean log fluorescence for this experiment. Student’s t-test after comparison of the values obtained with IL-12 + TNF with the corresponding values obtained with medium, IL-12, or TNF results in P < .05. Similar results were obtained in three other experiments.
iNOS protein expression in stimulated NK cells. NK cells were stimulated for 24 hours in the presence or absence of IL-12 (5 U/mL) or/and TNF (20 ng/mL) and then harvested. The cells were washed, permeabilized, and stained with a polyclonal rabbit anti-iNOS and then with a (FITC)-conjugated F(ab’)2 fragment goat antirabbit IgG, as described in Materials and Methods. Fluorescence data were collected on 5,000 viable cells. (Open area) untreated NK cells (profile identical to that obtained with the preimmune serum, indicative that these cells were negative for the expression of this marker). (Shaded area) NK cells stimulated with TNF (upper panel), IL-12 (middle panel), or both cytokines (lower panel). The percentage represents the change in the mean log fluorescence for this experiment. Student’s t-test after comparison of the values obtained with IL-12 + TNF with the corresponding values obtained with medium, IL-12, or TNF results in P < .05. Similar results were obtained in three other experiments.
Western blot analysis of iNOS expression in NK cells. NK cells were treated with the indicated cytokines for 24 hours, lysed, and analyzed by Western blot with a polyclonal rabbit antirat iNOS that cross-reacts with human iNOS, as described in Materials and Methods. The fold induction over the basal activity was quantitated with video densitometry.
Western blot analysis of iNOS expression in NK cells. NK cells were treated with the indicated cytokines for 24 hours, lysed, and analyzed by Western blot with a polyclonal rabbit antirat iNOS that cross-reacts with human iNOS, as described in Materials and Methods. The fold induction over the basal activity was quantitated with video densitometry.
After the demonstration of iNOS mRNA and protein induction in NK cells, we next asked whether the detected iNOS was functional. For this purpose, the ability of cellular extracts to convert L-[14C]arginine to L-[14C]citrulline was measured. Results in Fig 5 show that no catalytic NOS activity was detected in extracts from unstimulated NK cells. However, 48 hours of incubation with IL-12 was efficient to induce iNOS activity that was potentiated through simultaneous addition of TNFα. The enzymatic activity was markedly suppressed (60% to 75%) in the presence of AMG during the assay.
iNOS activity in human NK cells. NOS activity was determined by monitoring of [14C] L-citrulline produced per milligram of cellular protein per minute. NK cells were stimulated with IL-12 (5 U/mL) or/and TNF (20 ng/mL) for 48 hours, and then cell lysates were prepared and tested for their NOS catalytic activity by measuring their capacity to convert radiolabeled arginine into citrulline, as described in Materials and Methods. Results are expressed as the mean ± SD of three representative experiments. Significance of inhibition was assessed by the Student’st-test after comparison of the values obtained with IL-12 or/and TNF + AMG, with the corresponding values obtained in each experiment with IL-12 or/and TNF (**P < .01; *P< .05).
iNOS activity in human NK cells. NOS activity was determined by monitoring of [14C] L-citrulline produced per milligram of cellular protein per minute. NK cells were stimulated with IL-12 (5 U/mL) or/and TNF (20 ng/mL) for 48 hours, and then cell lysates were prepared and tested for their NOS catalytic activity by measuring their capacity to convert radiolabeled arginine into citrulline, as described in Materials and Methods. Results are expressed as the mean ± SD of three representative experiments. Significance of inhibition was assessed by the Student’st-test after comparison of the values obtained with IL-12 or/and TNF + AMG, with the corresponding values obtained in each experiment with IL-12 or/and TNF (**P < .01; *P< .05).
Differential effect of NO on LAK generation and LAK lytic function.
Inasmuch as NK cells stimulated with IL-12 or with IL-12 and TNFα display an increased cytotoxic activity towards NK-sensitive and -resistant targets, the role of NO in the differentiation of LAK cells as well as its involvement in the lytic function of both NK and LAK cells was examined. For this purpose, the iNOS inhibitor AMG was added to purified human NK cell cultures, either during the incubation with or without IL-12 and/or TNFα, or during the 51Cr release assay against the classical NK target (K562) or against the LAK target (Daudi).
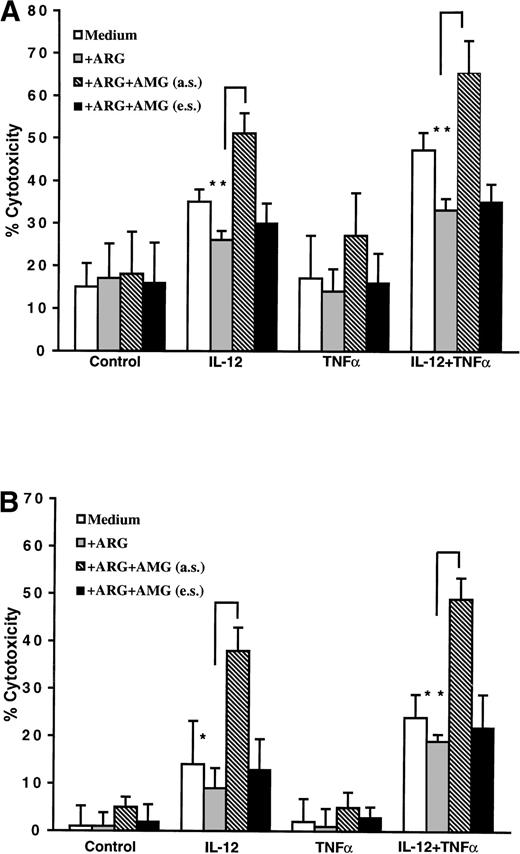
The NK cells were stimulated with IL-12 or/and TNFα, without or with 4 mmol/L AMG. Experiments were performed either in regular medium RPMI-1640 (L-arginine concentration, 0.94 mmol/L) supplemented with 10% human AB serum or in the same medium adjusted to a L-arginine concentration of 2 mmol/L to alleviate a possible depletion of the substrate during the incubation time and/or to favor the catalytic activity of the iNOS. After 3 days of culture, the recovered cells were tested for NK and LAK activities. As seen in Fig 6A, the adjustment at 2 mmol/L of the L-arginine concentration during the time of incubation with the cytokines led to a faint inhibition of the lytic activity as compared with the nonsupplemented cultures. The augmented activity of IL-12- and TNFα-incubated NK cells was not altered by the presence of the NOS inhibitor AMG during the 51Cr- release assay. Strikingly, this cytotoxicity was markedly augmented when AMG was present during the 3 days of culture.
Effects of AMG on the NK cells cytotoxic activity. NK cells were purified by negative selection as described in Materials and Methods and stimulated for 3 days with 5 U/mL IL-12 or/and 20 ng/mL TNF in the regular medium RPMI 1640 or in the same medium adjusted to a L-arginine concentration of 2 mmol/L. AMG (4 mmol/L) was added during the afferent step of sensitization (a.s.) or during the effector step (e.s). Cytotoxicity against K562 target cells (A) and Daudi target cells (B) was tested in a standard 4-hour 51Cr- release assay at an E:T ratio of 12.5:1 for K562 cells and 25:1 for Daudi cells. Bars represent the means ± SD for three independent experiments. *P < .05, **P < .01 by the Student’st-test comparing IL-12/IL-12 + TNF in the presence or absence of AMG (a.s.)
Effects of AMG on the NK cells cytotoxic activity. NK cells were purified by negative selection as described in Materials and Methods and stimulated for 3 days with 5 U/mL IL-12 or/and 20 ng/mL TNF in the regular medium RPMI 1640 or in the same medium adjusted to a L-arginine concentration of 2 mmol/L. AMG (4 mmol/L) was added during the afferent step of sensitization (a.s.) or during the effector step (e.s). Cytotoxicity against K562 target cells (A) and Daudi target cells (B) was tested in a standard 4-hour 51Cr- release assay at an E:T ratio of 12.5:1 for K562 cells and 25:1 for Daudi cells. Bars represent the means ± SD for three independent experiments. *P < .05, **P < .01 by the Student’st-test comparing IL-12/IL-12 + TNF in the presence or absence of AMG (a.s.)
Data depicted in Fig 6B demonstrate that AMG was devoid of effect when added during LAK cytotoxicity assay, yet its presence during the sensitization phase resulted in a marked increase of the subsequent LAK activity. The observed increase in cytotoxicity after incubation of NK cells with AMG could not be attributed to a direct lytic effect of AMG on the target cells, inasmuch as the effector cells were washed before the chromium release assay and AMG was devoid of any toxic effect when added directly to the target cells. Similar conclusions were obtained using another specific iNOS inhibitor of a different class, 1,3 PB-ITU, which, when added together with IL-12 and TNFα during the sensitization phase, was also found to induce an augmentation of NK and LAK activity.
Effect of NO donors on NK and LAK cytotoxicity.
Conversely, chemical NO donors were tested for their potential effect on NK cytotoxicity and LAK generation. When added at the onset of the 3-day sensitization phase of incubation of NK cells with the cytokines, SNP was found to elicit a dose-dependent inhibition of the resulting cytotoxicity, as measured by a classical 4-hour chromium release assay (Table 1). The SNP at the concentrations used was not toxic for the effector or target cells, with some toxicity being observed only for concentrations of SNP greater than 100 μg/mL. Similar results were obtained with another NO donor of a different structure, Sin-1, as shown in Table 1, whereas control Sin 1C was inactive (not shown).
Effect of NO Donors on the Stimulation of NK and LAK Activity by IL-12 and TNF
| Culture Conditions . | % of Cell Lysis . | |
|---|---|---|
| NK Activity . | LAK Activity . | |
| Medium | 15 ± 4.5 | 3 ± 2.3 |
| IL-12 + TNFα | 61 ± 9.3 | 22 ± 7.5 |
| IL-12 + TNFα + SNP (10 μmol/L) | 59 ± 2.3 | 15 ± 5.4 |
| IL-12 + TNFα + SNP (20 μmol/L) | 46 ± 2.1 | 7 ± 3.2 |
| IL-12 + TNFα + SNP (50 μmol/L) | 32 ± 3.0 | 6 ± 1.2 |
| Medium | 31 ± 1.05 | ND |
| IL-12 + TNFα | 65 ± 3.01 | 47 ± 1.5 |
| IL-12 + TNFα + SIN-1 (10 μM) | 52 ± 1.1 | 27 ± 0.6 |
| IL-12 + TNFα + SIN-1 (20 μM) | 32 ± 2.0 | 15 ± 2.6 |
| IL-12 + TNFα + SIN-1 (50 μM) | 28 ± 0.7 | 10 ± 0.2 |
| Culture Conditions . | % of Cell Lysis . | |
|---|---|---|
| NK Activity . | LAK Activity . | |
| Medium | 15 ± 4.5 | 3 ± 2.3 |
| IL-12 + TNFα | 61 ± 9.3 | 22 ± 7.5 |
| IL-12 + TNFα + SNP (10 μmol/L) | 59 ± 2.3 | 15 ± 5.4 |
| IL-12 + TNFα + SNP (20 μmol/L) | 46 ± 2.1 | 7 ± 3.2 |
| IL-12 + TNFα + SNP (50 μmol/L) | 32 ± 3.0 | 6 ± 1.2 |
| Medium | 31 ± 1.05 | ND |
| IL-12 + TNFα | 65 ± 3.01 | 47 ± 1.5 |
| IL-12 + TNFα + SIN-1 (10 μM) | 52 ± 1.1 | 27 ± 0.6 |
| IL-12 + TNFα + SIN-1 (20 μM) | 32 ± 2.0 | 15 ± 2.6 |
| IL-12 + TNFα + SIN-1 (50 μM) | 28 ± 0.7 | 10 ± 0.2 |
Purified NK cells were stimulated for 3 days with IL-12 + TNFα and in the presence or not of various concentrations of the NO donors SNP or SIN-1. At the end of the incubation, the effector cells were washed and tested for their cytotoxic activity on NK (K562 at an E/T ratio 12.5:1) (left column) and LAK (Daudi at an E/T ratio 25:1) (right column) target cells. Results are expressed as the mean ± SD of triplicate samples of one representative experiment of five (Sin-1) or three (SNP) performed.
Abbreviation: ND, not determined.
AMG potentiates the IFNγ production by NK cells stimulated with IL-12 and TNFα.
The regulation of NK activity has been reported to be often associated with IFNγ production.37 38 We asked whether the regulation of NK cells by AMG involves IFNγ production by these cells. As shown in Fig 7, stimulation of purified NK cells with IL-12 resulted in a marked induction of IFNγ. The latter was significantly increased (∼50%) in the presence of the iNOS inhibitor AMG. Moreover, in the presence of TNFα, which by itself does not stimulate IFNγ release nor potentiate that elicited by IL-12, AMG was found to yield a higher amount of IFNγ. Therefore, it would appear that NO generated during the sensitization of NK cells with IL-12 and/or TNFα inhibits, in part, the secretion of IFNγ by these cells. The addition of anti-IFNγ during the sensitization phase with IL-12 and TNFα resulted in a partial inhibition of NK and LAK function. The increased cytotoxicity observed when AMG is present during the afferent phase was also suppressed to a comparable level by the neutralizing anti-IFNγ antibody, suggesting that the augmentation of lytic activity elicited by inhibition of NOS activity was effectively due, at least in part, to the elevated production of IFNγ (data not shown).
Effect of AMG on IFNγ production by NK cells. Purified NK cells (106 cells/mL) were incubated with the indicated cytokines in presence or absence the AMG for 3 days. Supernatants were collected and IFNγ production was measured by ELISA. Results are expressed as the mean ± SD of three different donors. Asterisks denote statistically significant differences between IL-12 + TNF + AMG and IL-12 + TNF (*P < .05), as determined by the Student’s t-test.
Effect of AMG on IFNγ production by NK cells. Purified NK cells (106 cells/mL) were incubated with the indicated cytokines in presence or absence the AMG for 3 days. Supernatants were collected and IFNγ production was measured by ELISA. Results are expressed as the mean ± SD of three different donors. Asterisks denote statistically significant differences between IL-12 + TNF + AMG and IL-12 + TNF (*P < .05), as determined by the Student’s t-test.
AMG stimulates the expression of granzyme B in cytokine-stimulated NK cells.
The cytotoxic granule component, granzyme B, is associated with NK-mediated cytolytic activity.39 40 We therefore analyzed the effect of AMG on the expression of this cytotoxic molecule upon stimulation with IL-12 and TNFα. Extracts from stimulated NK cells were probed by Western blot with mouse antihuman granzyme B. As shown in Fig 8, granzyme B protein was constitutively expressed by unstimulated NK cells. After incubation with AMG, a slight increase of the basal level of granzyme B was observed. The data in Fig 8 also demonstrate that, after stimulation of NK cells with the combination of IL-12 and TNFα, a significant increase in granzyme B expression was observed. The addition of AMG resulted in a potentiation of IL-12/TNFα-induced granzyme B. This suggests that NO production may inhibit the cytokines-driven increase in cytolytic activity through downregulation of granzyme B, which can be overcome in the presence of an iNOS inhibitor.
Effect of AMG on granzyme B protein in NK cells. NK cells were cultured in RPMI complete medium during 12 hours in the absence (lane 1) or presence of AMG (lane 2) or with IL-12 + TNF (lane 3) and IL-12 + TNF + AMG (lane 4); the cells were then lysed. The cell lysate was analyzed by Western blot with an anti-granzyme B antibody, as described in Materials and Methods. Blot was quantitated by video densitometry and fold induction over the basal activity was indicated. Similar results were obtained in two other experiments.
Effect of AMG on granzyme B protein in NK cells. NK cells were cultured in RPMI complete medium during 12 hours in the absence (lane 1) or presence of AMG (lane 2) or with IL-12 + TNF (lane 3) and IL-12 + TNF + AMG (lane 4); the cells were then lysed. The cell lysate was analyzed by Western blot with an anti-granzyme B antibody, as described in Materials and Methods. Blot was quantitated by video densitometry and fold induction over the basal activity was indicated. Similar results were obtained in two other experiments.
DISCUSSION
The killing mediated by NK cells represents an important mechanism in the immune defense against tumors, virus, infected cells, and parasites.1,2 These cells have the ability to mediate cytotoxicity and also to produce cytokines.4 Recently, evidence has been provided supporting a role for NO in murine NK-cell-mediated lysis.15- 17 The present studies indicate that, in most instances, iNOS is not expressed in unstimulated purified human NK cells, either at the mRNA or protein level. However, upon incubation of these cells with IL-12, which is known to stimulate NK cytotoxicity and generate LAK cells,41 iNOS expression could be detected, both at the mRNA and protein level. This effect was potentiated in the presence of TNFα. The iNOS protein was catalytically active, as evidenced by the accumulation of citrulline and nitrite/nitrate in the supernatants and the direct measurement of NOS enzymatic activity in cell extracts. The iNOS induction is unlikely to be due to the small percentage of contaminating monocytes remaining in our preparations of NK cells, because NK sorted cell culture gave similar results (data not shown). Indeed, even if activated NK cells release IFNγ, the latter cytokine, at variance with rodents monocytes, does not induce NO production by human monocytes/macrophages, even though the presence of iNOS mRNA and eventually protein may be detected in some instances.42-44The combination of IL-12 and TNFα appears to be more potent for inducing iNOS protein, as measured by flow cytometry and Western blotting, than either cytokine alone, despite the fact that IL-12 appears to be much more potent than TNFα in eliciting iNOS mRNA. The induction of iNOS by IL-12 and TNFα in NK cells is consistent with earlier findings of Hibbs et al45and Ochoa et al46 indicating that IL-2 was also a potent inducer of high-output NO synthase in human patients treated for advanced malignancy. However, the cellular origin and contribution of NO to IL-2-mediated tumor regression remains to be established.
Huang et al47 have reported that a high seric concentration of NO in MRL/lpr mice was correlated with a high level of IL-12, as compared with MRL/+ mice. Upon stimulation of splenic or peritoneal cells from MRL/lpr mice with IFNγ and lipopolysaccharide (LPS), an increase of NO was observed, subsequent to an upregulation of IL-12 production. The effect of IL-12 on NO production was found to be mostly dependent on the presence of NK cells. Yet, the putative contribution of NK-associated NO production in vivo against tumor cells remains controversial. Recently, in a model of gene therapy with granulocyte-macrophage colony-stimulating factor (GM-CSF) in a mouse injected with B16 melanoma cells, the protective effect of this cytokine appeared mainly to be mediated by activated macrophages actively synthesizing NO, whereas the effect of NK, LAK, and CTL was little.48 In a model of intestinal graft-versus-host reaction in mouse, NG-mono-methyl-L-arginine (L- NMMA), an inhibitor of the iNOS pathway, was found to reduce the enhanced activity of NK cells that occurs in this graft complication.49 Cifone et al16 have also observed that stimulation of rat NK cells with IL-2 and/or through NKR-P1 triggering resulted in LAK activity that was sensitive to NOS inhibitors and to depletion of L- arginine in the assay buffer.
Our data indicate that the IL-12/TNFα combination was efficient to trigger the induction of iNOS in NK cells. Despite the time-correlated effects, the release of NO does not seem to be involved in the enhanced lytic potential of NK cells. Indeed, as stated above, the presence of a specific iNOS inhibitor, such as AMG, during the efferent stage of51Cr-release did not significantly affect the cytotoxic potential of the effector cells. Interestingly, we found that the presence of AMG and of 1,3 PB-ITU, two specific iNOS inhibitors, during the sensitization phase of NK cells with IL-12 or IL-12 and TNFα markedly increased the cytotoxic ability of these cells. Conversely, the addition of chemical NO donors resulted in an impairment of the subsequent lytic activity mediated by NK and LAK cells. Taken together, these data suggest that NO endogenously produced by the iNOS elicited in NK cells after stimulation by IL-12 and TNFα may contribute to downregulate stimulation of lytic potential.
Our observations are somewhat different from those of studies that were performed using mouse NK cells, in which the depletion of arginine in the lytic assay medium resulted in an abrogation of NK cytotoxicity.17 This discrepancy may be due to differences in the experimental conditions related to NK purification, stimulation, and/or to species differences. In this regard, it should be noted that human and murine NK cells are differentially regulated. For instance, whereas IL-4 activates NK cells into LAK effectors in a murine model,50 we have previously reported that this cytokine was efficient in inhibiting IL-2-induced human LAK generation.51
Our data delineate an important role of endogenously produced NO in the regulation of NK and LAK activity. Indeed, after the induction of iNOS in NK cells upon stimulation with IL-12, alone or in combination with TNFα, the presence of iNOS inhibitors yields effector cells with increased cytotoxic potential towards both NK and LAK targets. This indicates that the NO generated inhibits, to some extent, the cytokine-driven increase of the lytic potential of NK cells and their differentiation into LAK effectors. A possible mechanism for the increased NK lytic activity detected after cytokine stimulation in the presence of an iNOS inhibitor may be due to the observed enhancement of the secretion of IFNγ. It is well established that one of the most potent functions of IL-12 is its ability to induce NK cells to produce lymphokines, particularly IFNγ, which is known to upregulate the NK lytic activity.37 38 Indeed, as expected, the addition of a neutralizing anti-IFNγ antibody was found to reduce the increase in cytotoxic activity elicited by the treatment with IL-12 and TNFα, inasmuch as this combination of cytokines is known to induce IFNγ production by NK cells, which contributes to the enhanced lytic activity. Moreover, the anti-IFNγ was found to reduce to a similar level the increase in cytotoxicity evoked in the presence of iNOS inhibitor, indicating an involvement of IFNγ in this process.
The addition of an iNOS inhibitor during the phase of stimulation with cytokines also resulted in an enhanced expression of granzyme B protein, which plays a relevant role in cell-mediated cytotoxicity. Granzyme B is constitutively expressed in NK cells and its level of expression in cytotoxic lymphocytes is regulated by several cytokines. Whether NO could affect some transcription factors involved in the expression of the IFNγ and granzyme B genes by IL-12 and TNFα is currently under investigation. In this regard, it has been reported that the promoter of IFNγ gene contains an NF-κB-related site that binds NF-κB protein family members.52 Because NO has been shown to inhibit NF-κB through stabilization of IκBα53, this could contribute to the observed increased production of IFNγ by NK cells stimulated by IL-12 and TNFα in the presence of AMG.
Overall, these findings suggest that the NO generated in human NK cell in response to cytokine stimulation inhibits the lytic potential of these cells. Therefore, the development of NO inhibitors to circumvent its production and action could potentially contribute to the design of appropriate strategies for cytokine-based immune-intervention.
ACKNOWLEDGMENT
The authors are indebted to Dr M. Sasportes and Dr Y. Zhang for kindly providing granzyme B antibody, to Dr J. Wietzerbin for anti-IFNγ antibody, and to Dr R. Webber (R&D) for iNOS antibody. We acknowledge Dr A. Caignard, Dr F.M. Chouaib, and Dr V. Shatrov for critical reading of the manuscript and F. Gay for excellent technical assistance.
Supported by grant from INSERM, IGR, ARC (6227), ARC (6957), la Ligue Nationale Française de Recherche contre le Cancer. O.S. is supported by la Société de Secours des Amis des Sciences.
Address reprint requests to Salem Chouaib, PhD, Laboratoire Cytokines et Immunologie des Tumeurs Humaines, U 487 INSERM, Institut Gustave-Roussy, 39 rue Camille Desmoulins, 94805 Villejuif Cedex, France; e-mail: chouaib@igr.fr.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.



![Fig. 5. iNOS activity in human NK cells. NOS activity was determined by monitoring of [14C] L-citrulline produced per milligram of cellular protein per minute. NK cells were stimulated with IL-12 (5 U/mL) or/and TNF (20 ng/mL) for 48 hours, and then cell lysates were prepared and tested for their NOS catalytic activity by measuring their capacity to convert radiolabeled arginine into citrulline, as described in Materials and Methods. Results are expressed as the mean ± SD of three representative experiments. Significance of inhibition was assessed by the Student’st-test after comparison of the values obtained with IL-12 or/and TNF + AMG, with the corresponding values obtained in each experiment with IL-12 or/and TNF (**P < .01; *P< .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/6/10.1182_blood.v92.6.2093/4/m_blod41831005x.jpeg?Expires=1767918788&Signature=gNgoKJimC0AXPuiy7TMhI7YawwkUz9uxc3xIzzoRSfiiDv2fhZrltnGU9oeh0mtztzu62e2XcvoqHLhadwfRpZEg6Bhgk6~kjel4wSdv4hUlEs7NBCYgm1tqSyMkryda~i4kPsCaYbAzsr22SkEXPJjs7d83yUZ-Dm5Oaf9vADrK0uLUel3KnPJxZOsR52~eM7pNYxg-0pXwuYIAAfOzW3QtMYBhZPWODYDvgf79AxgOEmQLw9gcScnFPP8mnCcNi7aMmGQCzUebSDs-gYg2VRgARG0UhP3qRPSY8NAPT8JLCs7E4BRudQ-xAB0ZxmNxCrJzkNR32jFscMnp8Vfu5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal