Abstract
Optimization of retroviral gene transfer into hematopoietic cells of the dog will facilitate gene therapy of canine X-linked severe combined immunodeficiency (XSCID) and in turn advance similar efforts to treat human XSCID. Both canine and human XSCID are caused by defects in the common γ chain, γc, of receptors for interleukin-2 and other cytokines. In this study, normal dogs were given retrovirally transduced bone marrow cells with and without preharvest mobilization by the canine growth factors granulocyte colony-stimulating factor (G-CSF) and stem cell factor (SCF). Harvey sarcoma virus and Moloney murine leukemia virus constructs were used, both containing cDNA encoding human γc. The Harvey-based vector transduced into cytokine-primed marrow yielded persistent detectable provirus in bone marrow and blood and expression of human γc on peripheral lymphocytes. In three dogs, human γc expression disappeared after 19 to 34 weeks but reappeared and was sustained, in one dog beyond 16 months posttransplantation, upon immunosuppression with cyclosporin A and prednisone, with up to 25% of lymphocytes expressing human γc. The long-term expression of human γc in a high proportion of normal canine lymphocytes predicts that retrovirus-mediated gene correction of hematopoietic cells may prove to be of clinical benefit in humans affected with this XSCID.
This is a US government work. There are no restrictions on its use.
X-LINKED SEVERE combined immunodeficiency (XSCID) is an inherited primary immunodeficiency caused by mutations of the gene IL2RG.1-3IL2RGencodes the cytokine receptor common γ chain (γc), which is a member of several leukocyte cytokine receptor complexes, including the receptors for interleukin-2 (IL-2), IL-4, IL-7, IL-9, and IL-15.4 Male infants affected with XSCID have low numbers of T lymphocytes that are not responsive to antigenic stimulation. B-lymphocyte counts in XSCID patients are normal to high; however function of B cells is impaired, and specific antibody responses are absent. Chronic diarrhea, failure to thrive, and opportunistic infections lead to early death in the absence of immune reconstitution by allogeneic bone marrow transplantation (BMT).5 BMT, including T-cell–depleted haploidentical BMT, is often lifesaving, but graft-versus-host disease and poor posttransplant B-cell function are frequent.
XSCID is a favorable candidate disease for treatment by gene transfer into hematopoietic cells. There is an in vivo selective advantage for lymphocytes expressing functional γc. Female carriers ofIL2RG mutations show nonrandom X chromosome inactivation in their T, B, and natural killer cells, reflecting superior survival and proliferation of lymphoid progenitors with an active X chromosome bearing an intact copy of IL2RG.4,6 Moreover, an untreated boy with XSCID has been described who developed functional autologous T cells with spontaneous reversion of his inheritedIL2RG mutation to the correct nucleotide sequence.7This case was similar to that of a previously reported patient with SCID due to adenosine deaminase (ADA) deficiency who regained immune function after spontaneous in vivo reversion to normal of one mutant ADA allele.8
Another favorable feature of XSCID for gene therapy is that the primary defect causes profound immunologic incompetence, which renders affected patients unlikely to reject transduced hematopoietic cells newly expressing γc protein. Finally, in the mouse, γc is expressed on immature and mature cells of all hematopoietic lineages.9 10 Thus constituitive expression of γc from a retroviral long terminal repeat (LTR) in myeloid and erythroid cells should not be deleterious.
The ideal target cell for retrovirus-mediated gene therapy of XSCID would be the hematopoietic stem cell (HSC). Integration of a provirus containing γc into XSCID HSC would ensure continuous production of γc+ lymphocytes. Retroviral transduction of normalIL2RG cDNA into B-cell lines from XSCID patients has been shown to restore membrane expression of γc, intracellular signaling function in response to IL-2 and IL-4 stimulation, and proliferation in response to IL-2.11-13
Amphotropic retroviruses can transduce murine, primate, and canine HSC and have been approved for human gene therapy. However, transduction efficiency in this setting is poor, possibly related to the low levels of amphotropic receptor mRNA and the quiescent cell cycle status of HSC.14 The highest rates of amphotropic retrovirus transduction to date have been achieved by in vivo mobilization of donor HSC before harvest, followed by transduction in the presence of stem cell factor (SCF) and IL-6. In both mouse and nonhuman primate models, cytokine pretreatment of the donor increased both the number of HSC obtained and the efficiency with which they could be transduced.15
Animal models of XSCID include mice with targeted disruptions ofIL2RG16-18 and Basset hound and Welsh Corgi dogs with spontaneous frameshift mutations ofIL2RG.19,20 Both species can serve as preclinical models for the study of gene transfer. However, the phenotype of the dog model is more similar to human XSCID. Like humans, affected male dogs have nonfunctional B cells, whereas B cells are absent in the mouse. XSCID dogs also have failure to thrive, chronic diarrhea, fatal infections, and well-characterized T- and B-cell defects.21The challenge of transducing canine HSC with amphotropic retroviruses closely resembles the realities of human gene therapy. In this report we describe the transduction of cytokine-primed normal canine bone marrow using amphotropic retroviral vectors containing humanIL2RG. Long-term persistence of peripheral blood leukocytes and bone marrow containing the human γc provirus was achieved by using allogeneic BMT from cytokine pretreated donors. Moreover, human γc was detected on the surface of canine lymphocytes for up to 16 months.
MATERIALS AND METHODS
Animals.
Normal dogs were maintained under approved protocols at the University of Pennsylvania School of Veterinary Medicine. Puppies were 8 to 10 weeks old at the beginning of cytokine treatment and weighed about 4 kg. Donors in allogeneic experiments were matched at the canine major histocompatibility locus, DLA, by mixed lymphocyte culture or polymerase chain reaction (PCR) amplification of polymorphic DLA gene segments.22
Cytokine treatment of donor dogs.
Recombinant canine SCF (cSCF; kindly provided by Fred Fletcher, Amgen, Thousand Oaks, CA) was stored at −80°C until needed and then diluted in saline for injection of 25 mg/kg/d. Recombinant canine granulocyte colony-stimulating factor (cG-CSF; also from Fred Fletcher, Amgen) was stored at 4°C and diluted in saline for injection of 10 mg/kg/d. Cytokines were administered subcutaneously for 4 consecutive days.
Marrow harvest.
For allogeneic transplant experiments, the donor was killed for harvest of femoral marrow 10.5 days after the last cytokine injection. Under aseptic conditions, the ends of the bone were clipped and the marrow eluted with phosphate buffered saline (PBS). The marrow was washed repeatedly to remove acellular debris, and red cells were lysed by two washes in ammonium chloride lysis buffer (150 mmol/L NH4Cl, 10 mmol/L KHCO3, 100 mmol/L EDTA). On average 3.8 × 108 cells (n = 3) were obtained by this procedure. For autologous transplant experiments, marrow was aspirated from six sites on the pelvis and long bones, washed in PBS, and depleted of red cells by lysis as above. On average 3.2 × 108 cells (n = 4) were collected by this procedure.
Irradiation and infusion.
One day before graft infusion, recipients received a single sublethal dose of 200 cGy total body irradiation from parallel opposed portals in a 6–million electron volts (MEV) linear accelerator. Standard oral antibiotic prophylaxis was administered while white blood counts (WBC) were below 103 per μL. For transplantation, bone marrow cells were suspended in normal saline and infused intravenously.
Immunosuppressive treatment in the late posttransplant period.
Three dogs were given cyclosporin A, 14 to 24 mg/kg, and prednisone acetate, 1 to 2 mg/kg, daily in an attempt to minimize immune destruction of transduced cells. Doses of these agents were adjusted to maintain peripheral absolute lymphocyte counts near 1 × 103 per μL.
Retroviral vectors and producer cell lines.
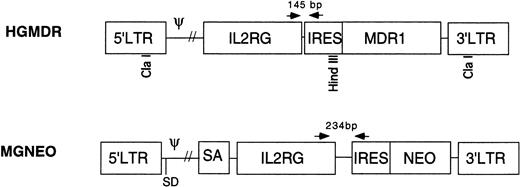
Two retroviral vectors were used (Fig 1). HGMDR contained human IL2RG cDNA cloned into theBamHI site of the retroviral expression construct pHa-MCS-IRES-MDR.23 This construct contains the Harvey murine sarcoma virus LTR and the human MDR1 gene driven from an internal ribosome entry site (IRES) from the encephalomyocarditis virus. The construct pHa-IL2RG-IRES-MDR was first transfected into the ecotropic producer cell line GP+E86.24Vincristine-resistant GP+E86 cells were then used to transduce the HGMDR provirus into the amphotropic producer cell line GP+AM12.25 26 GP+AM12 cells expressing high levels of γc were isolated by flow cytometry (FACS Vantage, Becton Dickinson, San Jose, CA) and plated at a density of 50 cells/mL for isolation of individual clones with high viral titers.
Retroviral vectors in this study. HGMDR: Harvey murine sarcoma virus backbone, human IL2RG cDNA, IRES, humanMDR1 cDNA. MGNEO: Moloney murine leukemia virus backbone, humanIL2RG cDNA, IRES, NEO. Selected restriction sites used for construction and analysis of HGMDR are indicated. Arrows represent PCR primers used to detect proviral DNA segments of indicated size.
Retroviral vectors in this study. HGMDR: Harvey murine sarcoma virus backbone, human IL2RG cDNA, IRES, humanMDR1 cDNA. MGNEO: Moloney murine leukemia virus backbone, humanIL2RG cDNA, IRES, NEO. Selected restriction sites used for construction and analysis of HGMDR are indicated. Arrows represent PCR primers used to detect proviral DNA segments of indicated size.
The second vector, MGNEO, contained human IL2RG cDNA cloned into the BamHI site of the retroviral expression construct pG1sam-EN.27 This construct contains the Moloney murine leukemia virus LTR and neomycin resistance gene (NEO) following the same IRES as in HGMDR. The new construct, pG1sam-ENIL2RG, was transfected into GP+E86 cells, and the resulting supernatant was used to transduce the amphotropic retrovirus producer cell line Ψ-Crip.28 Transduced Ψ-Crip cells were plated at limiting dilution, and individual clones were isolated by selection for resistance to the neomycin analog G418 (GIBCO-BRL, Gaithersburg, MD).
Producer cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 15% filtered, heat-inactivated newborn calf serum, penicillin/streptomycin, and L-glutamine, 400 mmol/L (all from GIBCO-BRL).
Retroviral transduction.
Retroviral supernatant was made by culturing nearly confluent retroviral producer cells overnight in DMEM, 15% fetal calf serum (Hyclone, Logan, UT), penicillin/streptomycin, and L-glutamine. Supernatant was supplemented with 1× Fungizone (GIBCO-BRL), 0.1 μg/mL cSCF, 0.1 μg/mL human IL-6 (Amgen), and 6 μg/mL polybrene (Sigma, St Louis, MO) and passed through a 0.45-mm filter (Nalgene, Rochester, NY) before addition to bone marrow cells, which were then plated at a density of 3.5 × 105 cells/mL in 150-mm polystyrene dishes (Corning, Corning, NY). The medium was replaced daily with freshly prepared retroviral supernatant. After the fourth day of transduction, cells were removed from the plates with a rubber cell scraper, pooled, washed in fresh medium, and suspended in normal saline for infusion.
Analysis of posttransplant expression.
Peripheral blood and bone marrow samples were obtained at regular intervals posttransplantation. Results were subjected to statistical analysis using Student’s t-test. For immunofluorescence analysis, 0.5 to 1 × 106 cells were divided into two 50 μL aliquots in PBS with 1 mg/mL human IgG. One aliquot was incubated 30 minutes with 2 μL of anti-human γc antibody TUGh4 conjugated to phycoerythrin (PE; Pharmingen, San Diego, CA) plus 4 μL of isotype control, a rat anti-mouse PE-conjugated IgG2bΚ (Pharmingen); the other aliquot received 4 μL of isotype control alone. The samples were washed and analyzed by flow cytometry (FACScan, Becton Dickinson). Lymphocytes were gated by size and granularity on forward and side scatter. Histograms were collected to record the increase in PE intensity on the cell surface due to specific expression of human γc. The percentage of anti-γc–stained lymphocytes brighter than the isotype control peak was used to estimate the proportion of cells bearing human γc.
To screen for canine anti-human γc antibody, canine serum was incubated with test human B-cell lines from a normal control (γc+) and an XSCID patient (γc−) followed by fluorochrome-labeled anti-canine IgG (Pharmingen).
PCR analysis of transduced cells.
DNA was extracted from peripheral blood and bone marrow of recipient dogs by standard methods. PCR was performed using primers complementary to sequences shared by both proviruses. The 5′ primer, 5′-CCCCATGTTACACCCTAAAGCCTGAA, was located in theIL2RG cDNA, whereas the 3′ primer, 5′-AGGTTTCCGGGCCCTCACATTG, was in the IRES (Fig 1). Due to differences in the lengths of the polylinkers in the vector constructs, the HGMDR-derived amplicon was 145 bp, the MGNEO-derived amplicon 234 bp. PCR conditions were 1′ at 94°C; 1′ at 63°C; 2′ at 72°C, for 35 cycles. As a control for DNA concentration and lane loading, a 134-bp fragment of the autosomal gene for canine corticotrophin releasing hormone (CRH) was amplified using the primers 5′-GGACGAGGCGCCGCAACTTTTT and 5′-CTCTCCTCTCCGGGGTCTCTT. PCR conditions were 1′ at 94°C; 1′ at 55°C; 2′ at 72°C, for 35 cycles. Each 50-μL reaction contained 400 ng of DNA in standard PCR buffer (Perkin Elmer, Norwalk CT), 400 ng of each primer, 300 μmol/L dNTPs (Perkin Elmer), 50 U Taq polymerase (Perkin Elmer), and a trace amount of 32P-labeled dCTP (Amersham, Arlington Heights, IL). PCR products were separated on 5% polyacrylamide gels and exposed to autoradiographic film. A phosphorimager was used to quantitate signal strength (Storm 9600; Molecular Diagnostics, Sunnyvale, CA). The percent of canine cells containing integrated vector DNA was estimated using the formula
whereV is the vector amplicon PCR signal in test sample, CRHis the CRH amplicon PCR signal in test sample, Vprod. line is the vector amplicon PCR signal in HGMDR producer line, CRHcontrol is the CRH amplicon PCR signal in untreated dog DNA, and 2 is the CRH copy number in canine diploid genome. Values used for Vprod. line andCRHcontrol were averages of five independent samples.
RESULTS
Retroviral producer cell lines.
The MGNEO producer line had a titer of 1.7 × 107colony-forming units (cfu)/mL on NIH 3T3 cells. Slot blot analysis was used to estimate the titer of HGMDR producer clones. By comparison of the hybridization signal of an IL2RG probe to viral RNA extracted from 1 mL of producer cell supernatant, it was estimated that the titer of the best HGMDR clone was 2.0 × 107cfu/mL. Southern blot analysis of Cla I–digested HGMDR producer cell line DNA showed the presence of an unrearranged proviral fragment. Digestion with HindIII, which cut once in the vector backbone (Fig 1), indicated that the HGMDR producer line had five proviral inserts (data not shown).
Transplantation and posttransplant course.
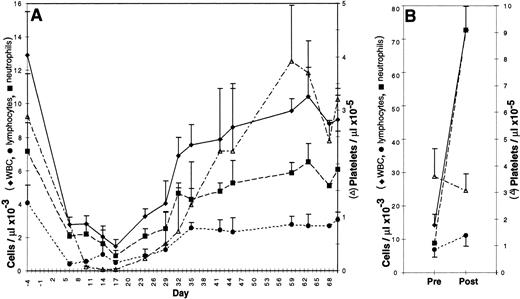
Bone marrow was either aspirated, transduced, and reinfused into the original autologous donor or obtained from the femur of a donor dog, transduced, and transplanted into a DLA-matched allogeneic recipient. Recipients were given a partially, but not totally, ablative dose of total body irradiation, 200 cGy, on day −1 and received 1.3 to 6 × 108 (mean 3.2 × 108) transduced cells on day 0. No infectious, hemorrhagic, or other complications were observed. As shown in Fig 2A, the nadir of the WBC, absolute neutrophil count, and platelet count for all dogs occurred between 10 and 17 days posttransplantation; these counts returned to normal values between 37 and 44 days. The nadir of the lymphocyte count was between 6 and 10 days. Hematocrits remained within normal ranges for age.
Peripheral blood counts in 3 to 6 canine subjects ± standard deviation. (A) Canine bone marrow recipients from 4 days before to 70 days after infusion of transduced cells. Each dog received 200 cGy total body irradiation on day −1. (B) Canine bone marrow donors before and after four daily subcutaneous injections of cSCF and cG-CSF.
Peripheral blood counts in 3 to 6 canine subjects ± standard deviation. (A) Canine bone marrow recipients from 4 days before to 70 days after infusion of transduced cells. Each dog received 200 cGy total body irradiation on day −1. (B) Canine bone marrow donors before and after four daily subcutaneous injections of cSCF and cG-CSF.
Retroviral marking in dogs receiving noncytokine-primed grafts.
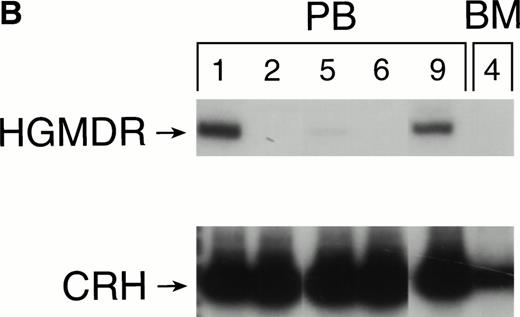
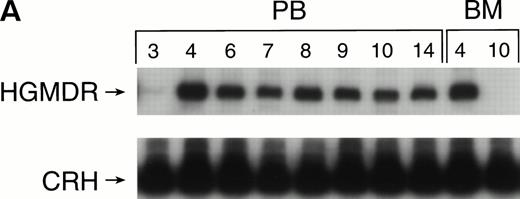
One autologous transplant and one allogeneic transplant were performed using bone marrow from donor dogs that did not receive G-CSF and SCF before harvest. The presence of proviral DNA in the transduced bone marrow cells was detected by PCR (Fig 3). Low levels of proviral DNA were detected in peripheral blood leukocytes of both dogs initially, but no marking was detected after 7 weeks in the autologous and 9 weeks in the allogeneic recipient. The HGMDR provirus was detected in the autologous recipient’s bone marrow at 4 weeks posttransplant but not in the allogeneic recipient’s bone marrow.
Proviral, HGMDR, and control genomic, CRH, signals amplified from peripheral blood (PB) or bone marrow (BM) DNA sampled sequentially from a dog receiving autologous (A) or allogeneic (B) noncytokine-primed bone marrow transduced with HGMDR. Numbers refer to weeks posttransplantation.
Proviral, HGMDR, and control genomic, CRH, signals amplified from peripheral blood (PB) or bone marrow (BM) DNA sampled sequentially from a dog receiving autologous (A) or allogeneic (B) noncytokine-primed bone marrow transduced with HGMDR. Numbers refer to weeks posttransplantation.
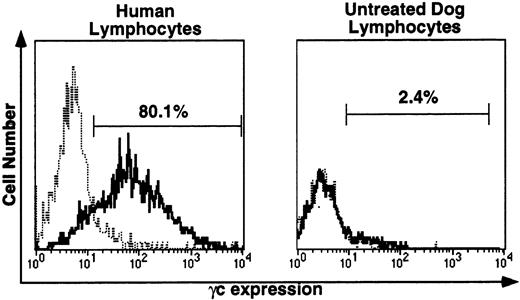
For immunofluorescence analysis of γc protein expression on peripheral blood lymphocytes, the species specificity of the anti-human γc antibody, TUGh4, was tested (Fig 4). This antibody detected human γc on the surface of all human lymphocytes (left), but did not detect canine lymphocyte γc (right). Blood samples from untreated dogs were processed in parallel with all posttransplantation samples. In untreated dog samples, histograms with and without TUGh4 were uniformly completely superimposable, as illustrated in Fig 4, right.
Immunofluorescence (IF) analysis of human (left) and canine (right) lymphocyte-gated peripheral blood cells stained with rat anti-human γc monoclonal antibody TUGh4 (solid tracings) or rat isotype control alone (dotted tracings). Bars indicating percentage of lymphocytes brighter than the control peak were used for comparative estimates of lymphocyte expression of human γc.
Immunofluorescence (IF) analysis of human (left) and canine (right) lymphocyte-gated peripheral blood cells stained with rat anti-human γc monoclonal antibody TUGh4 (solid tracings) or rat isotype control alone (dotted tracings). Bars indicating percentage of lymphocytes brighter than the control peak were used for comparative estimates of lymphocyte expression of human γc.
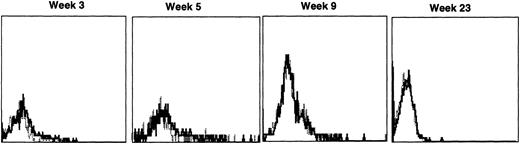
Both dogs receiving noncytokine-primed grafts transiently had detectable peripheral lymphocytes expressing human γc. The allogeneic recipient’s lymphocyte-gated histograms are shown in Fig 5. Human γc expression diminished to undetectable levels by 10 weeks in both autologous and allogeneic recipients.
Sequential IF analysis of peripheral blood lymphocytes in a dog transplanted with histocompatible, allogeneic noncytokine-primed bone marrow transduced with HGMDR. TUGh4 antibody (solid tracings); rat isotype control (dotted tracings).
Sequential IF analysis of peripheral blood lymphocytes in a dog transplanted with histocompatible, allogeneic noncytokine-primed bone marrow transduced with HGMDR. TUGh4 antibody (solid tracings); rat isotype control (dotted tracings).
Cytokine pretreatment of bone marrow donors.
Cytokine pretreatment of donors has been shown to improve the efficiency of retroviral gene transfer to bone marrow HSC in mouse and nonhuman primate models.14 Canine donors were subjected to a similar, but species-specific, cytokine pretreatment regimen consisting of 4 daily injections of 25 μg/kg cSCF and 10 μg/kg cG-CSF ending 10.5 days before marrow harvest. To monitor the effects of the cytokine pretreatment in the donor’s peripheral blood, complete and differential blood counts were obtained before treatment and on the final day of cytokine injection (Fig 2B). WBC increased fivefold from a pretreatment mean of 14 × 103 to 73 × 103 cells per μL (n = 6 dogs). This was largely due to an increase in neutrophils from a mean of 6.8 × 103 to 67 × 103 cells per μL. Lymphocytes increased modestly from 7.0 × 103 to 11.2 × 103 cells per μL, whereas platelet count and hematocrit were not significantly altered by cSCF and cG-CSF administration.
Marking in dogs receiving cytokine mobilized autologous bone marrow.
Three dogs were treated with cG-CSF and cSCF, followed by bone marrow collection, 200 cGy irradiation, and autologous transplantation of bone marrow cells that had been transduced in vitro with HGMDR. Compared with dogs receiving bone marrow from noncytokine-primed dogs, the only significant difference in cell counts was that these dogs had prolonged lymphocyte count depression (<600 cells per μL for a mean of 24, rather than 14, days posttransplantation).
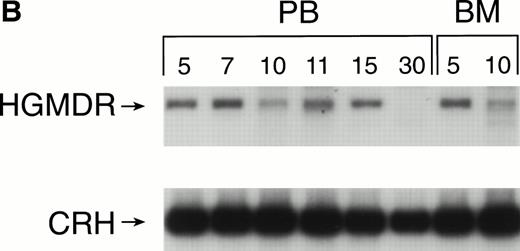
Recipients of autologous cytokine-primed marrow had detectable γc provirus in peripheral blood DNA for longer than recipients of noncytokine-primed marrow, with two of three dogs showing substantial marking at 14 and 15 weeks (Fig 6A and B) and one showing variable marking through 11 weeks (Fig 6C). Human γc proviral sequences were not detected in DNA from either peripheral blood or bone marrow of these dogs after 15 weeks (Fig 6).
Proviral, HGMDR, and control, CRH, signals amplified from blood (PB) or bone marrow (BM) DNA of three dogs (A, B, and C), sampled at weekly intervals after receiving autologous, cytokine-primed bone marrow transduced with HGMDR.
Proviral, HGMDR, and control, CRH, signals amplified from blood (PB) or bone marrow (BM) DNA of three dogs (A, B, and C), sampled at weekly intervals after receiving autologous, cytokine-primed bone marrow transduced with HGMDR.
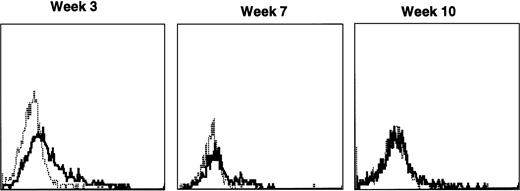
Immunofluorescence analysis of peripheral blood lymphocytes from dogs treated with autologous cytokine-primed marrow revealed that human γc was expressed at weeks 3 and 5 but had declined to minimal levels by week 9 and was undetectable at week 23, as shown for one dog in Fig 7.
Sequential IF analysis of peripheral lymphocytes from a dog transplanted with autologous, cytokine-primed bone marrow transduced with HGMDR. TUGh4 antibody (solid tracings); rat isotype control (dotted tracings).
Sequential IF analysis of peripheral lymphocytes from a dog transplanted with autologous, cytokine-primed bone marrow transduced with HGMDR. TUGh4 antibody (solid tracings); rat isotype control (dotted tracings).
Marking in dogs receiving cytokine-primed allogeneic bone marrow.
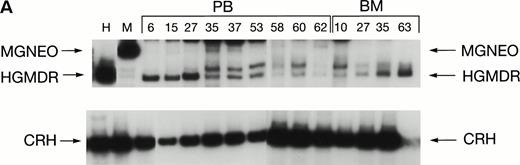
Two donor dogs were pretreated with cG-CSF and cSCF followed by femoral bone marrow collection. The bone marrow cells were transduced and transplanted into allogeneic histocompatible recipients 1 day after 200 cGy irradiation. Bone marrow cells from one animal were transduced with HGMDR alone. The bone marrow cells from the second animal were divided so that half of the marrow was transduced with HGMDR and half with MGNEO. The presence and expression of human γc in the latter dog has been followed for more than 16 months. The HGMDR and MGNEO provirus could be assessed by PCR because of the distinct sizes of the amplification products with the vector primer set (Fig 1). Although the two viruses were of comparable titer, MGNEO was detectable only sporadically and at low levels in peripheral blood DNA at weeks 15, 27, 35, 37, 58, and 62 (Fig 8A). In contrast, the HGMDR vector DNA was found continuously and at higher levels in blood and bone marrow DNA from both dogs (Fig 8A and B).
Proviral and control signals amplified from blood (PB) or marrow (BM) of two dogs transplanted with histocompatible, allogeneic, cytokine-primed marrow. Bands not at the specific sizes marked were considered artifacts and excluded from quantitation analysis. (A) Dogs receiving combined bone marrow aliquots, one transduced with HGMDR and one transduced with MGNEO. The two left lanes show signals from the HGMDR (H) and MGNEO (M) retroviral producer cell lines. (B) Dogs receiving marrow transduced with HGMDR only. The two left lanes show PCR amplification from donor bone marrow cells pretransduction and posttransduction.
Proviral and control signals amplified from blood (PB) or marrow (BM) of two dogs transplanted with histocompatible, allogeneic, cytokine-primed marrow. Bands not at the specific sizes marked were considered artifacts and excluded from quantitation analysis. (A) Dogs receiving combined bone marrow aliquots, one transduced with HGMDR and one transduced with MGNEO. The two left lanes show signals from the HGMDR (H) and MGNEO (M) retroviral producer cell lines. (B) Dogs receiving marrow transduced with HGMDR only. The two left lanes show PCR amplification from donor bone marrow cells pretransduction and posttransduction.
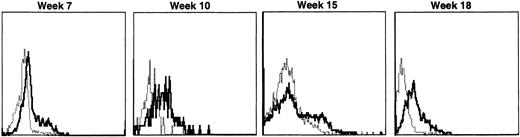
Lymphocyte expression of human γc protein was achieved in both of the dogs receiving cytokine-mobilized allogeneic grafts and persisted longer than in recipients of noncytokine-primed or autologous cytokine-primed transduced grafts. Representative histograms of lymphocytes from the dog that received two viruses and was followed longest are shown in Fig 9. Throughout 23 weeks posttransplantation, when dogs receiving other graft types had ceased to express human γc, these dogs exhibited substantial proportions of blood lymphocytes expressing the retrovirally transduced protein. Long-lasting bone marrow expression of human γc was also found (not shown). To assess directly which of the two retroviruses was responsible for γc expression, the brightest 10% of bone marrow cells at 15 weeks were sorted by flow cytometry. PCR of this population detected the 145-bp amplicon from HGMDR but not the 234-bp amplicon from MGNEO (not shown).
Sequential IF analysis of peripheral lymphocytes from a dog transplanted with matched, allogeneic, cytokine-primed bone marrow transduced with human IL2RG cDNA. TUGh4 antibody (solid tracings); rat isotype control (dotted tracings).
Sequential IF analysis of peripheral lymphocytes from a dog transplanted with matched, allogeneic, cytokine-primed bone marrow transduced with human IL2RG cDNA. TUGh4 antibody (solid tracings); rat isotype control (dotted tracings).
Immunosuppression was followed by re-expression of human γc.
Despite improved duration of expression of human γc in recipients of cytokine-primed grafts, this expression did decline. One recipient of a primed autologous-transduced transplant had no immunofluorescent (IF) staining above the isotype control by week 19, whereas two recipients of primed allogeneic grafts had baseline staining at weeks 24 and 27 posttransplantation. This loss of cell surface expression after the establishment of a strong, stable expression pattern, together with the persistence of the HGMDR vector sequence in the blood and bone marrow by PCR, suggested the development of an immune reaction to cells expressing either human γc and/or the P-glycoprotein encoded by the MDR gene. Serum from one dog was tested for anti-human γc, but none was detected.
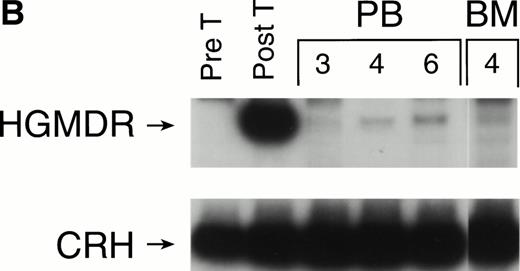
The three dogs were given a course of immunosuppressive therapy with prednisone and cyclosporin A in an attempt to minimize immune destruction of developing lymphoid progenitors expressing transduced foreign protein. After 8 to 10 weeks of treatment with doses adjusted to maintain lymphocyte counts near 1 × 103 per μL, lymphocytes expressing human γc reappeared in peripheral blood of all three dogs. Figure 10 shows negative IF staining histograms from before immunosuppressive treatment and examples of the strongly positive posttreatment patterns, sustained in all three dogs for at least 3 weeks. One recipient of allogeneic cytokine-primed transduced marrow (Fig 10B) had elevated liver enzymes prompting cessation of prednisone and cyclosporin A treatment after 10 weeks, following which lymphocyte counts rose and human γc expression again became undetectable.
Examples of IF analysis of peripheral blood lymphocytes from three recipients of transduced bone marrow transplant (BMT) before (left) and following (right) treatment with cyclosporin A and prednisone. Weeks after BMT are shown. (A) Recipient of cytokine mobilized autologous BMT. (B and C) Recipients of cytokine-mobilized, matched, allogeneic BMT.
Examples of IF analysis of peripheral blood lymphocytes from three recipients of transduced bone marrow transplant (BMT) before (left) and following (right) treatment with cyclosporin A and prednisone. Weeks after BMT are shown. (A) Recipient of cytokine mobilized autologous BMT. (B and C) Recipients of cytokine-mobilized, matched, allogeneic BMT.
Comparison of retroviral marking strategies.
The semiquantitative PCR estimates of the percent of blood leukocytes containing HGMDR provirus in each treatment group and estimates of the percentage of lymphocytes expressing human γc protein in different time intervals are summarized in Table 1. Both PCR and IF analyses detected transient marking in dogs receiving unprimed marrow and low levels of marking for longer periods of time in those receiving cytokine-primed autologous marrow. By far the best frequency of marking in every time interval was in two recipients of cytokine-primed allogeneic-transduced bone marrow. For these dogs, continuous long-term viral DNA was detected in an average of 19% of leukocytes through week 37. Throughout the first three posttransplant intervals shown in Table 1, an average of 25% to 34% of their peripheral lymphocytes expressed γc protein with IF brighter than the corresponding isotype control histogram. Although lymphocyte surface expression of transduced human γc eventually waned in all dogs, expression by IF following immunosuppression rebounded to 11% and 19% in recipients of cytokine-primed autologous and allogeneic-transduced marrow, respectively.
Quantitation of Marker DNA and Expression
| Type of Bone Marrow Transduced and Transplanted . | Cytopenic Early Posttransplant Period-150 . | Postengraftment Period-151 . | Late Follow-Up Period-152 . | Postimmunosuppressive Treatment . | ||||
|---|---|---|---|---|---|---|---|---|
| PCR % TBL-153 . | IF % LYM-155 . | PCR % TBL . | IF % LYM . | PCR % TBL . | IF % LYM . | PCR % TBL . | IF % LYM . | |
| No cytokine pretreatment | 2 ± 1¶ | 30 ± 7 | 1.4 ± 1 | 0 | NA | 0 | NA | NA |
| Cytokine pretreated autologous | 7 ± 2 | 18 ± 2 | 10 ± 2 | 1 ± 1 | 2 ± 1 | 0 | NA | 11 ± 4 |
| Cytokine pretreated allogeneic | 19# | 30 ± 7 | 28# | 25 ± 5 | 19 ± 2 | 34 ± 24 | 20 ± 4 | 19 ± 4 |
| Type of Bone Marrow Transduced and Transplanted . | Cytopenic Early Posttransplant Period-150 . | Postengraftment Period-151 . | Late Follow-Up Period-152 . | Postimmunosuppressive Treatment . | ||||
|---|---|---|---|---|---|---|---|---|
| PCR % TBL-153 . | IF % LYM-155 . | PCR % TBL . | IF % LYM . | PCR % TBL . | IF % LYM . | PCR % TBL . | IF % LYM . | |
| No cytokine pretreatment | 2 ± 1¶ | 30 ± 7 | 1.4 ± 1 | 0 | NA | 0 | NA | NA |
| Cytokine pretreated autologous | 7 ± 2 | 18 ± 2 | 10 ± 2 | 1 ± 1 | 2 ± 1 | 0 | NA | 11 ± 4 |
| Cytokine pretreated allogeneic | 19# | 30 ± 7 | 28# | 25 ± 5 | 19 ± 2 | 34 ± 24 | 20 ± 4 | 19 ± 4 |
Abbreviation: NA, no measurement available.
1 to 5 weeks.
6 to 15 weeks.
16 to 37 weeks.
Percent of total blood leukocytes carring retroviral signal.
Percent of lymphocyte-gated peripheral blood cells expressing human γc at the cell surface.
¶Mean ± standard error of 2 to 12 available measurements.
#Only one measurement available.
DISCUSSION
Previous in vitro marking studies indicated that canine hematopoietic colony-forming cells could express retrovirally transduced exogenous marker genes in maturing lineages.29,30 However, as with other large animal models, high levels of marking and expression in vivo have been more difficult to achieve. In vivo studies of bone marrow transplantation of transduced dog, like primate and human marrow, reported low levels of engraftment and poor expression, compromising therapeutic potential.31 These results are similar to our data using noncytokine-primed bone marrow. More recent canine gene transfer studies used bone marrow ablation of the recipient,32,33 long-term marrow culture to induce the division of otherwise noncycling progenitors,33-35and/or cytokine pretreatment of the bone marrow donor.36,37 Persistence in 0.1% of cells bearing transduced proviral DNA for up to 5 years and expression of retrovirally encoded protein for over 2 years posttransplant was achieved with a combination of cytokine mobilization of donor marrow and lethal irradiation of the recipient.38 Another approach involving in vitro activation and transduction of bone marrow cells for 21 days, followed by transplantation into nonablated recipient dogs, produced 5% hematopoietic colony-forming cells 2 years posttransplant.34
Our findings support previous studies in other species suggesting that pretreatment of donors with SCF and G-CSF improves transduction efficiency of canine long-term repopulating cells from bone marrow.14 In mouse models we have shown both an expansion of a normally quiescent population of pluripotent HSC and an increase in the level of the amphotropic retrovirus receptor mRNA in HSC.39 In mice, treatment with SCF and G-CSF first causes an increase in the peripheral blood leukocyte counts, followed 10 days later by an increase in bone marrow repopulating cells.40In our canine trial, we saw increases in WBC, neutrophil, and lymphocyte counts in response to cytokine pretreatment, but the effects of cytokine priming on the number of primitive progenitors in the bone marrow were not directly observed, because bone marrow sampling before marrow harvest was judged too invasive.
Semiquantitative PCR and flow cytometry analysis showed that cytokine priming of donors increased the duration of detectable transduced DNA and protein expression. The fact that lymphocyte repopulation was 10 days more rapid in dogs receiving noncytokine-primed grafts may reflect the presence of a greater proportion of committed and maturing lymphoid progenitors in the marrow when cytokines are not administered.
Two of the three dogs that received autologous cytokine primed marrow maintained a substantial number of retrovirally marked leukocytes through 15 weeks posttransplantation. However, lymphocytes bearing human γc protein were not detectable after 15 weeks. This would be expected if the cells bearing proviral transduced DNA in the PCR assay were predominantly granulocytes rather than lymphocytes. The PCR assay did not distinguish among cell types, whereas the IF assay of cell surface expression was gated on lymphocytes. Circulating granulocytes have a shorter life than lymphocytes; their maturation and removal would be consistent with the observed disappearance of peripheral blood marking. As in previous studies by our group and others in mouse models, there can be considerable variation in the proportion of marked cells of different lineages.41-45
The transient marking of autologous cytokine-primed bone marrow may reflect host responses to the cytokine mobilization itself. The mouse model would predict that dogs receiving cSCF and cG-CSF priming would have higher numbers of endogenous HSC 10 to 15 days later. After a relatively small 200 cGy dose of irradiation, these HSC could still outnumber the transduced, transplanted cells. It may be that higher dose irradiation or different timing could enhance engraftment of cytokine-primed autologous-transduced bone marrow.
Our results in 200-cGy irradiated allogeneic recipients of cytokine-primed marrow represent an improvement over previous reports in that we were able to detect proviral sequences in 27% of blood leukocytes more than 16 months posttransplant. Furthermore, up to 25% of lymphocytes expressed the transduced γc protein. Protocols based on our successful long-term marking with retrovirally transduced human γc have the potential to successfully treat canine XSCID. In direct comparison, our retrovirus constructed with the Harvey murine sarcoma virus backbone was superior to the one based on the Moloney murine leukemia virus in both persistence of proviral DNA and expression of human γc. Because the XSCID dog is not immune competent before treatment, an immune response to cells newly expressing γc protein would be unlikely. On the contrary, it would be expected that cell surface expression of γc on the lymphoid lineage progeny of transduced long-term repopulating cells would have a selective advantage over endogenous cells not expressing γc. If therapeutic benefit of IL2RG retroviral transduction of bone marrow HSC can be achieved in the canine model of XSCID, a clinical gene therapy trial for human XSCID could follow.
After 28 weeks, all of the dogs had lost detectable lymphocyte expression of human γc by IF, and expression did not spontaneously reappear during the following 10 to 20 weeks. A loss of cells expressing human γc after strong expression is consistent with an immune reaction against either transduced human γc or P-glycoprotein. The canine MDR protein sequence is not available, but the extracellular domains of human and canine γc differ at 16% of their amino acids.19 Moreover, the species specificity of the rat anti-human γc monoclonal antibody we used shows the existence of human γc epitopes that would be potentially immunogenic in the dog. The two allogeneic recipients of cytokine-primed transduced marrow as well as one recipient of autologous cytokine-primed marrow were therefore given a trial of immune suppressive treatment after the disappearance of lymphocytes expressing human γc. In all three cases, marked lymphocytes returned to levels close to the peak levels seen earlier. After termination of immune suppression in one dog, expression has again fallen to undetectable over a 10-week period, consistent with the hypothesis that an immune response contributes to elimination of cells expressing transduced protein. Immune responses to transduced proteins have been noted before in the dog46 and are a major issue in human gene transfer trials.47 Fortunately, in the gene therapy of XSCID, the underlying primary immunodeficiency is predicted to render the host incapable of immune rejection of transduced cells.
ACKNOWLEDGMENT
The authors thank Margie Weil and the University of Pennsylvania Veterinary Students for compassionate animal care; Fred Fletcher of Amgen for canine cytokines; Pat Miller-Wilson for canine irradiation; Stacie Anderson for flow cytometry and sorting; Ann Ferrero, Laurie Girard, and Brian Hartnett for technical assistance; and Ian Dubé, Peter Felsburg, and Michael Gottesman and for helpful discussions.
Supported by NIH Grant Nos. DK54481 and NS33526 to M.E.H. and AI33177 to P.S.H.
Address reprint requests to Jennifer M. Puck, MD, NHGRI/NIH, Bldg 49, Rm 3A14, 49 Convent Dr, Bethesda, MD 20892-4442; e-mail:jpuck@nhgri.nih.gov.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal