Abstract
Recombinant human granulocyte colony-stimulating factor (G-CSF; filgrastim) shortens the time to neutrophil recovery after intensive chemotherapy, but its role in the treatment of adults with acute lymphoblastic leukemia (ALL) is uncertain. We randomly assigned 198 adults with untreated ALL (median age, 35 years; range, 16 to 83) to receive either placebo or G-CSF (5 μg/kg/d) subcutaneously, beginning 4 days after starting intensive remission induction chemotherapy and continuing until the neutrophil count was ≥1,000/μL for 2 days. The study assignment was unblinded as individual patients achieved a complete remission (CR). Patients initially assigned to G-CSF then continued to receive G-CSF through 2 monthly courses of consolidation therapy. Patients assigned to placebo received no further study drug. The median time to recover neutrophils ≥1,000/μL during the remission induction course was 16 days (interquartile range [IQR], 15 to 18 days) for the patients assigned to receive G-CSF and 22 days (IQR, 19 to 29 days) for the patients assigned to placebo (P< .001). Patients in the G-CSF group had significantly shorter durations of neutropenia (<1,000/μL) and thrombocytopenia (<50,000/μL) and fewer days in the hospital (median, 22 daysv 28 days; P = .02) compared with patients receiving placebo. The patients assigned to receive G-CSF had a higher CR rate and fewer deaths during remission induction than did those receiving placebo (P = .04 by the chi-square test for trend). During Courses IIA and IIB of consolidation treatment, patients in the G-CSF group had significantly more rapid recovery of neutrophils ≥1,000/μL than did the control group by approximately 6 to 9 days. However, the patients in the G-CSF group did not complete the planned first 3 months of chemotherapy any more rapidly than did the patients in the placebo group. Overall toxicity was not lessened by the use of G-CSF. After a median follow-up of 4.7 years, there were no significant differences in either the disease-free survival (P = .53) or the overall survival (P = .25) for the patients assigned to G-CSF (medians, 2.3 years and 2.4 years, respectively) compared with those assigned to placebo (medians, 1.7 and 1.8 years, respectively). Adults who received intensive chemotherapy for ALL benefited from G-CSF treatment, but its use did not markedly affect the ultimate outcome.
© 1998 by The American Society of Hematology.
INTENSIVE MULTI-AGENT chemotherapy programs produce complete remissions (CR) in the majority of adults with acute lymphoblastic leukemia (ALL). The major cause of treatment-related morbidity and mortality is infection due in part to bone marrow suppression by cytotoxic therapy. In a recent Cancer and Leukemia Group B (CALGB) trial using five chemotherapy drugs in combination, 84% of adult ALL patients achieved a CR, but 9% died during remission induction.1 This myelosuppressive regimen caused, on average, 21 days of severe granulocytopenia (<500 neutrophils/μL) and 19 days of thrombocytopenia (<50,000/μL) during the initial remission induction course. Therefore, the CALGB designed a study to test the effectiveness of filgrastim (granulocyte colony-stimulating factor [G-CSF]) in reducing the complications of treatment by potentially shortening the time to neutrophil recovery following courses of remission induction chemotherapy and postremission consolidation treatment.
The primary objectives of this randomized, double-blind, clinical trial were to compare the time to bone marrow recovery, the incidence of infections, the days of hospitalization, and the side effects of treatment after intensive chemotherapy for ALL in patients treated either with G-CSF or with placebo. In addition, we determined the effect of G-CSF on the rate and duration of CR and the incidence of death during treatment. We also compared the dose intensity of chemotherapy that was delivered to patients assigned to receive G-CSF or placebo during the first 3 months of treatment. Finally, we continued to investigate the prognostic significance of disease and patient entry characteristics for disease-free survival (DFS) using this treatment program.
MATERIALS AND METHODS
Eligibility.
Patients 15 years or older with previously untreated ALL were eligible for entry onto the trial. To support the diagnosis, cytochemistry and immunophenotyping studies performed at each institution had to be consistent with the diagnosis of ALL as defined by the French-American-British (FAB) classification system.2 3Emergency treatment of rapidly progressive hyperleukocytosis with hydroxyurea for up to 72 hours before entry on the study or the emergency use of leukapheresis was permissible. A single dose of cranial irradiation for central nervous system (CNS) leukostasis was also permissible. Unless directly attributable to leukemia, patients were required to have a total bilirubin concentration and serum creatinine level of less than 1.5 times the upper limits of normal at each institution.
Study entry.
Written informed consent was obtained from all patients before entry on the study. Patients were registered and randomly assigned to one of the two treatment groups via a telephone call to the CALGB Statistical Center before treatment. The diagnosis of ALL was confirmed by central review of pretreatment blood smears and bone marrow specimens for cytological and cytochemical features according to the FAB criteria. It was recommended that pretreatment blood and marrow specimens be submitted for cytogenetic analysis, including central review of the karyotypes (CALGB study 8461). A lumbar puncture for spinal fluid examination was performed only when there was clinical suspicion of CNS disease.
Immunophenotyping (CALGB study 8364) was performed by multiparameter flow cytometry in a central CALGB laboratory, using a panel of monoclonal antibodies and indirect immunofluorescence.1 The criterion for surface marker positivity was expression by at least 20% of the leukemia blast cell population. B-lineage was defined as CD19 or CD20 positivity. T-lineage was defined by CD2 or CD7 expression together with CD1, CD3, CD4, CD5, or CD8 reactivity. Myeloid (My) antigen expression included CD13 or CD33. Expression of the common ALL antigen was assessed by CD10 reactivity. Cases coexpressing lymphoid and myeloid antigens (BMy or TMy) were generally classified according to their lymphoid lineage (B- or T-cell, respectively). Cases expressing combinations of both B- and T-lineage antigens were classified as BT, BTMy, or miscellaneous. Cases expressing surface membrane immunoglobulin were considered FAB-L3 (Burkitt-type ALL) and were not included among the other B-lineage cases in subsequent analyses. Patients diagnosed with L3 ALL at the treating institution were not excluded from this trial but were recommended for entry onto a different CALGB trial running concurrently. Patients with myeloperoxidase negative blasts that expressed only myeloid antigens (and not B- or T-lymphoid antigens) were considered acute myeloid leukemia (AML), subtype M0, and were classified as ineligible.
Treatment protocol.
Drugs and dosages used in the induction (Course I), consolidation (Courses IIA and IIB), late intensification (Course IV), and maintenance (Courses III and V) phases of treatment are listed in Table 1. A complete report of the outcome of 214 patients treated with this same chemotherapy regimen in a prior study (CALGB study 8811) has been previously published.1
Chemotherapy Regimen for ALL in Adults
| Course I: Induction (4 wk) | |||
| Cyclophosphamide* | IV | 1,200 mg/m2 | Day 1 |
| Daunorubicin* | IV | 45 mg/m2 | Days 1, 2, 3 |
| Vincristine | IV | 2 mg | Days 1, 8, 15, 22 |
| Prednisone* | PO/IV | 60 mg/m2/d | Days 1-21 |
| L-Asparaginase (E coli) | SC/IM | 6,000 IU/m2 | Days 5, 8, 11, 15, 18, 22 |
| *For patients ≥60 years old: | |||
| Cyclophosphamide | 800 mg/m2 | Day 1 | |
| Daunorubicin | 30 mg/m2 | Days 1, 2, 3 | |
| Prednisone | 60 mg/m2/d | Days 1-7 | |
| Course IIA: Early Intensification (4 wk; repeat once for Course IIB) | |||
| Intrathecal methotrexate | 15 mg | Day 1 | |
| Cyclophosphamide | IV | 1,000 mg/m2 | Day 1 |
| 6-Mercaptopurine | PO | 60 mg/m2/d | Days 1-14 |
| Cytarabine | SC | 75 mg/m2/d | Days 1-4, 8-11 |
| Vincristine | IV | 2 mg | Days 15, 22 |
| L-Asparaginase (E coli) | SC/IM | 6,000 IU/m2 | Days 15, 18, 22, 25 |
| Course III: CNS Prophylaxis and Interim Maintenance (12 wk) | |||
| Cranial irradiation | 2,400 cGy | Days 1-12 | |
| Intrathecal methotrexate | 15 mg | Days 1, 8, 15, 22, 29 | |
| 6-Mercaptopurine | PO | 60 mg/m2/d | Days 1-70 |
| Methotrexate | PO | 20 mg/m2 | Days 36, 43, 50, 57, 64 |
| Course IV: Late Intensification (8 wk) | |||
| Doxorubicin | IV | 30 mg/m2 | Days 1, 8, 15 |
| Vincristine | IV | 2 mg | Days 1, 8, 15 |
| Dexamethasone | PO | 10 mg/m2/d | Days 1-14 |
| Cyclophosphamide | IV | 1,000 mg/m2 | Day 29 |
| 6-Thioguanine | PO | 60 mg/m2/d | Days 29-42 |
| Cytarabine | SC | 75 mg/m2/d | Days 29-32, 36-39 |
| Course V: Prolonged Maintenance (until 24 mo from diagnosis) | |||
| Vincristine | IV | 2 mg | Day 1 of every 4 wk |
| Prednisone | PO | 60 mg/m2/d | Days 1-5 of every 4 wk |
| 6-Mercaptopurine | PO | 60 mg/m2/d | Days 1-28 |
| Methotrexate | PO | 20 mg/m2 | Days 1, 8, 15, 22 |
| Course I: Induction (4 wk) | |||
| Cyclophosphamide* | IV | 1,200 mg/m2 | Day 1 |
| Daunorubicin* | IV | 45 mg/m2 | Days 1, 2, 3 |
| Vincristine | IV | 2 mg | Days 1, 8, 15, 22 |
| Prednisone* | PO/IV | 60 mg/m2/d | Days 1-21 |
| L-Asparaginase (E coli) | SC/IM | 6,000 IU/m2 | Days 5, 8, 11, 15, 18, 22 |
| *For patients ≥60 years old: | |||
| Cyclophosphamide | 800 mg/m2 | Day 1 | |
| Daunorubicin | 30 mg/m2 | Days 1, 2, 3 | |
| Prednisone | 60 mg/m2/d | Days 1-7 | |
| Course IIA: Early Intensification (4 wk; repeat once for Course IIB) | |||
| Intrathecal methotrexate | 15 mg | Day 1 | |
| Cyclophosphamide | IV | 1,000 mg/m2 | Day 1 |
| 6-Mercaptopurine | PO | 60 mg/m2/d | Days 1-14 |
| Cytarabine | SC | 75 mg/m2/d | Days 1-4, 8-11 |
| Vincristine | IV | 2 mg | Days 15, 22 |
| L-Asparaginase (E coli) | SC/IM | 6,000 IU/m2 | Days 15, 18, 22, 25 |
| Course III: CNS Prophylaxis and Interim Maintenance (12 wk) | |||
| Cranial irradiation | 2,400 cGy | Days 1-12 | |
| Intrathecal methotrexate | 15 mg | Days 1, 8, 15, 22, 29 | |
| 6-Mercaptopurine | PO | 60 mg/m2/d | Days 1-70 |
| Methotrexate | PO | 20 mg/m2 | Days 36, 43, 50, 57, 64 |
| Course IV: Late Intensification (8 wk) | |||
| Doxorubicin | IV | 30 mg/m2 | Days 1, 8, 15 |
| Vincristine | IV | 2 mg | Days 1, 8, 15 |
| Dexamethasone | PO | 10 mg/m2/d | Days 1-14 |
| Cyclophosphamide | IV | 1,000 mg/m2 | Day 29 |
| 6-Thioguanine | PO | 60 mg/m2/d | Days 29-42 |
| Cytarabine | SC | 75 mg/m2/d | Days 29-32, 36-39 |
| Course V: Prolonged Maintenance (until 24 mo from diagnosis) | |||
| Vincristine | IV | 2 mg | Day 1 of every 4 wk |
| Prednisone | PO | 60 mg/m2/d | Days 1-5 of every 4 wk |
| 6-Mercaptopurine | PO | 60 mg/m2/d | Days 1-28 |
| Methotrexate | PO | 20 mg/m2 | Days 1, 8, 15, 22 |
Course I: Patients received study drug (G-CSF 5 μg/kg or placebo) in a double-blinded fashion subcutaneously once per day starting on Day 4 and continuing until the ANC was ≥1,000/μL on two consecutive determinations >24 hours apart. Course IIA: Patients who had been assigned to G-CSF received G-CSF in an open-label fashion at 5 μg/kg subcutaneously daily, starting on day 2 and continuing for at least 14 days and until the ANC was ≥5,000/μL (see text). Patients who had been assigned to placebo received no further study drug.
The total duration of treatment was 24 months. Testicular biopsies were not required at the end of therapy, and testicular radiation was not administered prophylactically. Patients who had an isolated CNS relapse while continuing in marrow remission were counted as relapses. However, they continued to receive systemic chemotherapy on protocol after treatment with additional intrathecal chemotherapy. Patients with unfavorable cytogenetic features [ie, the Philadelphia (Ph) chromosome, t(9;22), t(4;11), t(8;14), or a variant] were recommended to be withdrawn from this trial in first remission and undergo allogeneic bone marrow transplantation, if it were feasible.
The use of oral nonabsorbable antibiotics, the management of febrile episodes and transfusions, and the criteria for hospitalization were not prescribed by the protocol, but rather were left to institutional guidelines. Prophylactic systemic antibacterial antibiotics were not permitted during Courses I and II of treatment, but oral nonabsorbable antifungal drugs could be used prophylactically. Co-trimoxazole or aerosolized pentamidine were recommended for pneumocystis prophylaxis, starting in Course III.
No hematopoietic growth factors were permitted for supportive care except as specified as part of the chemotherapy treatment on this protocol.
On day 4, approximately 12 to 24 hours after the third dose of daunorubicin, patients received the first subcutaneous injection of the study drug (either G-CSF at 5 μg/kg/d or placebo). The subcutaneous injections continued once daily for at least 7 days and until the absolute neutrophil count (ANC) was ≥1,000/μL for two consecutive determinations more than 24 hours apart. If the patient were otherwise eligible for earlier discharge from the hospital, he or she was allowed to self-administer the study drug subcutaneously at home. Once the study drug was discontinued, it was not restarted again during that particular treatment course, even if the neutrophil count fell below 1,000/μL.
After evaluation of the bone marrow exam on day 29 of the initial induction course, the study drug assignment was unblinded by the treating physician and pharmacist. Patients who had been randomly assigned to placebo during Course I no longer received study drug injections during Course II. Patients who had been randomly assigned to receive G-CSF in a blinded fashion during Course I continued to receive G-CSF at the same dose (5 μg/kg/d) in an unblinded, open-label fashion during Course II. Patients or family members were taught to administer daily subcutaneous injections at home.
During Courses IIA and IIB, the G-CSF injections began on day 2, ie, the day after the cyclophosphamide dose. The G-CSF treatment continued concurrently with the chemotherapy daily for at least 14 days after which time it was discontinued if the ANC were ≥5,000/μL for two consecutive determinations more than 24 hours apart. In every case the G-CSF was stopped at least 2 days before the next cyclophosphamide treatment. Priority was given to starting the cyclophosphamide treatment in Course IIB on schedule, ie, 29 days after starting Course IIA. Thus, for patients receiving G-CSF who had recovered ≥1,000 neutrophils/μL and ≥50,000 platelets/μL, the cyclophosphamide for Course IIB was administered on day 29 of Course IIA even if the ANC had not yet reached ≥5,000/μL by day 27.
Data quality control.
All data on this study were required to be submitted to the CALGB Data Management Center on specified forms. CALGB central data management personnel were responsible for quality assurance of the submitted data. Eligibility criteria were verified for all patients and an evaluation of treatment, response, and toxicity was made by the study chair (R.A.L.). In addition, as part of the group data-monitoring program, members of the CALGB Data Audit Committee made periodic site visits to all institutions to verify compliance with federal regulations and protocol requirements, including eligibility, treatment, response data, and follow-up.4 A random subset of 54 patients (27%) treated on this study had such an on-site review of their medical records.
Criteria for response.
Patients were considered to be in CR when the neutrophil count was ≥1,500/μL, the platelet count was ≥100,000/μL, the results of a bone marrow examination were normal (with <5% blasts), and all extramedullary disease had resolved. Patients with >25% lymphoblasts remaining in the marrow after Course I were removed from this protocol. All patients were required to have achieved CR by the end of Course IIA to remain on the study. Relapse was confirmed by the reappearance of >25% lymphoblasts in the bone marrow or blood or the presence of leukemia cells in the spinal fluid after remission.
Randomization.
Patients were stratified by institution and randomly assigned in a double-blinded fashion at the time of registration on the study to treatment with either G-CSF or placebo. At each institution only one investigational drug pharmacist who was not directly involved in the patient’s care was aware of the treatment assignment.
Statistical methods.
The primary analyses in this study followed the intention-to-treat principle, which requires all patients who are randomized to be included in the analysis regardless of eligibility status or the treatment actually received. Because all enrolled patients were randomized, no patients were excluded from the primary analyses.
The primary outcome measure was the number of days from the start of treatment until the neutrophil count exceeded 1,000/μL during the induction course and was maintained. Additional outcome measures were the number of days from the start of treatment in Courses IIA and IIB until the neutrophil count exceeded 1,000/μL, the duration of neutropenia (the number of days with an ANC <1,000/μL), the number of days from the start of treatment until the platelet count exceeded 50,000/μL, and the duration of thrombocytopenia (<50,000/μL) during each of the first three treatment courses (I, IIA, and IIB). In addition, we measured the number of days of hospitalization during each treatment course, the number of febrile days (>38.5°C), and the number of days required to complete the planned induction and consolidation therapy courses (ideally, 85 days). We also compared the nonhematologic toxicity observed in the two treatment groups.
For the primary endpoint, the recovery date was the first day after day 1 of chemotherapy with an ANC ≥1,000/μL when a sustained recovery was documented; that is, the ANC continued to increase steadily thereafter and did not drop again during that course to <1,000/μL. The study was designed to provide an 80% power to detect a 7-day difference in the time to recover an ANC ≥1,000/μL during the induction course (eg, a median of 14 days in the experimental groupv 21 days in the placebo group) with a significance level of 0.05. A total of 76 patients per treatment group (total, 152 patients) was the target. The duration of neutropenia was measured by counting each interval day from the first recorded date in each chemotherapy course when the ANC fell below 1,000/μL up to, but not including, the first recorded date with a sustained ANC ≥1,000/μL. Actual dates of recovery were used, and no data were derived from interpolation. Complete blood counts were required at least three times per week during Course I and weekly thereafter. In practice, blood counts were generally obtained daily while patients were hospitalized and at least once per week while they were outpatients. Estimations of the distributions of time to recovery of hematologic endpoints were performed via the Kaplan-Meier method,5 where patients who died before the endpoint was reached were censored. The variation in these trials was expressed in terms of the interquartile range (IQR, 25th to 75th percentiles).
The rate of CR and the duration of DFS and length of survival were additional outcome measures. Differences in proportions of CRs among patient subgroups were analyzed using Fisher’s exact test. In addition, for the analysis of CR rate and death rate during induction, the chi-square test for trend was used where the qualitative ordering was CR > alive with refractory disease > death.6 The duration of DFS was defined to be the time from achieving a CR to relapse (bone marrow, blood, CNS, or testicular), death, or date of last follow-up. Patients still at risk or lost to follow-up were censored for the analysis of DFS. Survival was defined as the time from study entry to the date of last follow-up. Probabilities of surviving and remaining in CR were estimated by the Kaplan-Meier method.5 Ninety-five percent confidence intervals (CI) for these probabilities were obtained using the method of Simon and Lee.7 Median follow-up time was estimated as the median survival time of all patients still at risk. Differences in survival or DFS between patient subgroups were tested using the log-rank statistic.8
In accordance with the study objectives, the prognostic significance of age, white blood cell (WBC) count, platelet count, mediastinal mass, organomegaly, lymphadenopathy, FAB classification, immunophenotype, and cytogenetics (presence or absence of the Ph chromosome) were assessed with respect to CR rate and duration and survival. For the joint analysis of these variables, regression analyses were used. The Cox proportional hazards model was used to estimate hazard ratios with respect to CR duration and survival for the randomized treatment groups, as well as to test for an age-treatment interaction in time to hematologic recovery.9 10 All reported P values are nominal, two-sided values unless otherwise stated. The analyses were based on all data available as of January 1998.
RESULTS
Conduct of the study.
Between June 24, 1991, and July 30, 1993, 198 patients were registered on CALGB study 9111 from 25 main member institutions and their affiliated hospitals. No single institution enrolled more than 10% of the total patients.
Before starting induction chemotherapy, 102 patients were randomly assigned to receive G-CSF and 96 to receive placebo. The groups were comparable with respect to pretreatment characteristics (Table 2). The median age was 35 years (range, 16 to 79), and the median WBC count was 16,800/μL (range, 200 to 373,000).
Clinical and Biological Features of 198 Patients Enrolled on CALGB Study 9111
| Characteristic at Diagnosis . | No. of Patients (%) . | ||
|---|---|---|---|
| G-CSG Group . | Placebo Group . | Total . | |
| 102 | 96 | 198 | |
| Age (yrs) | |||
| <30 | 45 (44) | 30 (31) | 75 (38) |
| 30-59 | 36 (35) | 46 (48) | 82 (41) |
| ≥60 | 21 (21) | 20 (21) | 41 (21) |
| Sex | |||
| Male | 61 (60) | 44 (46) | 105 (53) |
| Female | 41 (40) | 52 (54) | 93 (47) |
| Performance status (CALGB) | |||
| 0-1 | 80 (78) | 74 (78) | 154 (78) |
| 2-3 | 22 (22) | 21 (22) | 43 (22) |
| Mediastinal mass (chest radiograph) | |||
| Present | 7 (7) | 8 (9) | 15 (8) |
| Absent | 94 (93) | 85 (91) | 179 (92) |
| Lymphadenopathy (palpable) | |||
| Present | 32 (32) | 36 (40) | 68 (36) |
| Absent | 69 (68) | 54 (60) | 123 (64) |
| Splenomegaly (palpable) | |||
| Present | 27 (27) | 27 (29) | 54 (28) |
| Absent | 72 (73) | 66 (71) | 138 (72) |
| Leukocytes | |||
| <30,000/μL | 66 (65) | 57 (60) | 123 (62) |
| ≥30,000/μL | 36 (35) | 38 (40) | 74 (38) |
| Platelets | |||
| <50,000/μL | 53 (52) | 45 (48) | 98 (50) |
| ≥50,000/μL | 49 (48) | 49 (52) | 98 (50) |
| FAB subtype | |||
| L1 | 44 (44) | 39 (43) | 83 (44) |
| L2 | 37 (37) | 34 (37) | 71 (37) |
| L3 | 2 (2) | 2 (2) | 4 (2) |
| Other ALL | 11 (11) | 8 (9) | 19 (10) |
| AML/not ALL | 5 (5) | 8 (9) | 13 (7) |
| Immunophenotype | |||
| B or BMy | 61 (81) | 55 (77) | 116 (79) |
| T or TMy | 14 (19) | 16 (23) | 30 (21) |
| Cytogenetics | |||
| Ph+ | 12 (29) | 14 (28) | 26 (28) |
| Ph− | 30 (71) | 36 (72) | 66 (72) |
| Characteristic at Diagnosis . | No. of Patients (%) . | ||
|---|---|---|---|
| G-CSG Group . | Placebo Group . | Total . | |
| 102 | 96 | 198 | |
| Age (yrs) | |||
| <30 | 45 (44) | 30 (31) | 75 (38) |
| 30-59 | 36 (35) | 46 (48) | 82 (41) |
| ≥60 | 21 (21) | 20 (21) | 41 (21) |
| Sex | |||
| Male | 61 (60) | 44 (46) | 105 (53) |
| Female | 41 (40) | 52 (54) | 93 (47) |
| Performance status (CALGB) | |||
| 0-1 | 80 (78) | 74 (78) | 154 (78) |
| 2-3 | 22 (22) | 21 (22) | 43 (22) |
| Mediastinal mass (chest radiograph) | |||
| Present | 7 (7) | 8 (9) | 15 (8) |
| Absent | 94 (93) | 85 (91) | 179 (92) |
| Lymphadenopathy (palpable) | |||
| Present | 32 (32) | 36 (40) | 68 (36) |
| Absent | 69 (68) | 54 (60) | 123 (64) |
| Splenomegaly (palpable) | |||
| Present | 27 (27) | 27 (29) | 54 (28) |
| Absent | 72 (73) | 66 (71) | 138 (72) |
| Leukocytes | |||
| <30,000/μL | 66 (65) | 57 (60) | 123 (62) |
| ≥30,000/μL | 36 (35) | 38 (40) | 74 (38) |
| Platelets | |||
| <50,000/μL | 53 (52) | 45 (48) | 98 (50) |
| ≥50,000/μL | 49 (48) | 49 (52) | 98 (50) |
| FAB subtype | |||
| L1 | 44 (44) | 39 (43) | 83 (44) |
| L2 | 37 (37) | 34 (37) | 71 (37) |
| L3 | 2 (2) | 2 (2) | 4 (2) |
| Other ALL | 11 (11) | 8 (9) | 19 (10) |
| AML/not ALL | 5 (5) | 8 (9) | 13 (7) |
| Immunophenotype | |||
| B or BMy | 61 (81) | 55 (77) | 116 (79) |
| T or TMy | 14 (19) | 16 (23) | 30 (21) |
| Cytogenetics | |||
| Ph+ | 12 (29) | 14 (28) | 26 (28) |
| Ph− | 30 (71) | 36 (72) | 66 (72) |
There were major protocol violations involving 24 patients. These included 13 patients who were considered to be ineligible after central review of all pretreatment data; 10 were determined to have had AML, 2 had lymphocytic lymphoma, and 1 had T-cell lymphoma. Nevertheless, these patients were all treated according to the protocol and were evaluated for response. One patient withdrew consent before receiving the study drug. Because of errors in the treatment assignments at four institutions, one patient randomized to receive G-CSF actually received placebo during the induction course and then no study drug during the consolidation courses. Three patients who received G-CSF in an appropriately blinded fashion during the induction course did not receive any further study drug during their consolidation courses. Five patients who correctly received placebo injections during the induction course were incorrectly treated with G-CSF during the two consolidation courses. Regardless of these variations, the analysis of the responses reported here includes all enrolled patients according to intention-to-treat principles. A secondary analysis that excluded ineligible patients and those who received the incorrect study drug was also performed to determine whether these exclusions changed the conclusions.
Remission induction.
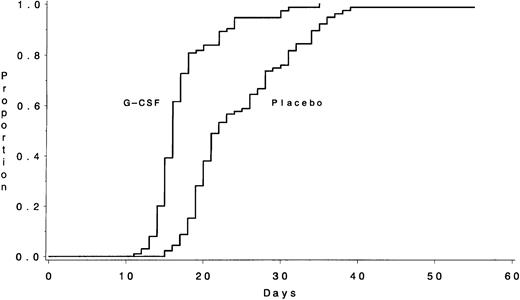
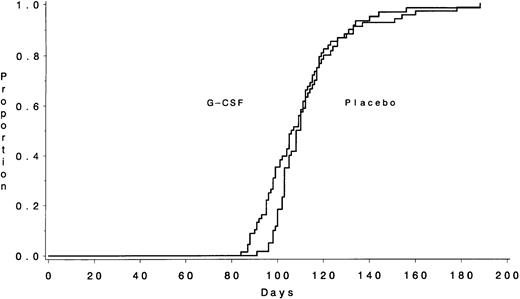
Data on the time required to recover an ANC ≥1,000/μL, the primary outcome measure, were available for 195 of the 198 patients (Table 3). The median times to recover an ANC ≥1,000/μL were 16 days (IQR, 15 to 18) for the 102 patients assigned to receive G-CSF and 22 days (IQR, 19 to 29) for the 93 patients assigned to the placebo (P < .001) (Fig 1). The duration of severe neutropenia (ANC < 1,000/μL) was also significantly shorter for those in the G-CSF group (median, 13 days [IQR, 10 to 16] v 20 days [IQR, 15 to 27] for the placebo group; P < .001). The median number of days with fever >38.5°C was 3 days (IQR, 1 to 5) for the G-CSF group and also 3 days (IQR, 2 to 7) for the placebo group (P = .44). Patients randomized to receive G-CSF had a median hospitalization of 22 days (IQR, 18 to 29) compared with 28 days (IQR, 22 to 33) for the placebo group (P = .02).
Hematologic Recovery After Chemotherapy and Clinical Outcome by Treatment Assignment
| Endpoint . | Median Days (IQR)* . | ||
|---|---|---|---|
| G-CSF Group . | Placebo Group . | P . | |
| Course I | |||
| Recovery to ANC >1,000/μL | 16 (15-18) | 22 (19-29) | <.001 |
| Duration of neutropenia | 13 (10-16) | 20 (15-27) | <.001 |
| Recovery to platelets >50,000/μL | 16 (14-20) | 19 (15-23) | .003 |
| Duration of thrombocytopenia | 14 (9-17) | 17 (11-22) | .008 |
| Fever >38.5°C | 3 (1-5) | 3 (2-7) | .44 |
| Hospitalization | 22 (18-29) | 28 (22-33) | .02 |
| Course IIA | |||
| Recovery to ANC >1,000/μL | 20 (6-25) | 29 (22-31) | <.001 |
| Duration of neutropenia | 5 (0-12) | 13 (6-18) | <.001 |
| Recovery to platelets >50,000/μL | 20 (17-22) | 20 (18-22) | .53 |
| Duration of thrombocytopenia | 7 (1-9) | 5 (2-7) | .21 |
| Fever >38.5°C | 0 (0-5) | 0 (0-3) | .78 |
| Hospitalization | 7 (0-17) | 3 (0-14) | .32 |
| Course IIB | |||
| Recovery to ANC >1,000/μL | 25 (15-32) | 31 (27-39) | <.001 |
| Duration of neutropenia | 11 (4-17) | 14 (10-25) | .001 |
| Recovery to platelets >50,000/μL | 24 (21-31) | 22 (0-28) | .03 |
| Duration of thrombocytopenia | 10 (7-20) | 7 (0-15) | .02 |
| Fever >38.5°C | 1 (0-3) | 1 (0-6) | .26 |
| Hospitalization | 4 (0-21) | 2 (0-15) | .17 |
| Course III | |||
| Interval on study to the beginning of Course III | 106 (96-117) | 108 (103-117) | .60 |
| Endpoint . | Median Days (IQR)* . | ||
|---|---|---|---|
| G-CSF Group . | Placebo Group . | P . | |
| Course I | |||
| Recovery to ANC >1,000/μL | 16 (15-18) | 22 (19-29) | <.001 |
| Duration of neutropenia | 13 (10-16) | 20 (15-27) | <.001 |
| Recovery to platelets >50,000/μL | 16 (14-20) | 19 (15-23) | .003 |
| Duration of thrombocytopenia | 14 (9-17) | 17 (11-22) | .008 |
| Fever >38.5°C | 3 (1-5) | 3 (2-7) | .44 |
| Hospitalization | 22 (18-29) | 28 (22-33) | .02 |
| Course IIA | |||
| Recovery to ANC >1,000/μL | 20 (6-25) | 29 (22-31) | <.001 |
| Duration of neutropenia | 5 (0-12) | 13 (6-18) | <.001 |
| Recovery to platelets >50,000/μL | 20 (17-22) | 20 (18-22) | .53 |
| Duration of thrombocytopenia | 7 (1-9) | 5 (2-7) | .21 |
| Fever >38.5°C | 0 (0-5) | 0 (0-3) | .78 |
| Hospitalization | 7 (0-17) | 3 (0-14) | .32 |
| Course IIB | |||
| Recovery to ANC >1,000/μL | 25 (15-32) | 31 (27-39) | <.001 |
| Duration of neutropenia | 11 (4-17) | 14 (10-25) | .001 |
| Recovery to platelets >50,000/μL | 24 (21-31) | 22 (0-28) | .03 |
| Duration of thrombocytopenia | 10 (7-20) | 7 (0-15) | .02 |
| Fever >38.5°C | 1 (0-3) | 1 (0-6) | .26 |
| Hospitalization | 4 (0-21) | 2 (0-15) | .17 |
| Course III | |||
| Interval on study to the beginning of Course III | 106 (96-117) | 108 (103-117) | .60 |
*25th to 75th percentiles.
The number of days from the start of chemotherapy until the recovery of an ANC >1,000/μL during Course I is shown according to treatment assignment. The medians were 16 days for the G-CSF group and 22 days for the placebo group (P < .001).
The number of days from the start of chemotherapy until the recovery of an ANC >1,000/μL during Course I is shown according to treatment assignment. The medians were 16 days for the G-CSF group and 22 days for the placebo group (P < .001).
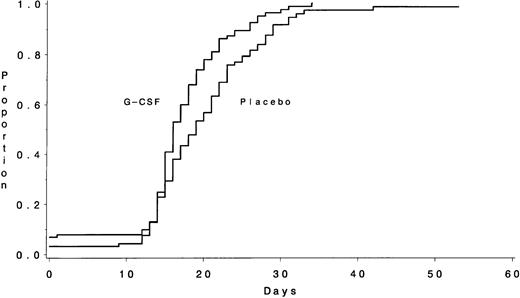
Platelet recovery was also more rapid in the patients receiving G-CSF (Fig 2). The median times to recover platelets ≥50,000/μL were 16 days (IQR, 14 to 20) for the G-CSF group and 19 days (IQR, 15 to 23) for the placebo group (P = .003). The median durations of thrombocytopenia <50,000/μL were 14 days (IQR, 9 to 17) and 17 days (IQR, 11 to 22), respectively (P = .008). A secondary analysis that excluded the 13 ineligible patients and the 2 who either received the wrong study drug treatment or withdrew from the study before receiving the study drug yielded similar results, both for neutrophil recovery and for platelet recovery (data not shown).
The number of days from the start of chemotherapy until the recovery of platelets >50,000/μL during Course I is shown according to treatment assignment. The medians were 16 days for the G-CSF group and 19 days for the placebo group (P = .003).
The number of days from the start of chemotherapy until the recovery of platelets >50,000/μL during Course I is shown according to treatment assignment. The medians were 16 days for the G-CSF group and 19 days for the placebo group (P = .003).
Of the 102 patients randomly assigned to receive G-CSF, 89 (87%) achieved a CR, 8 (8%) had refractory disease, and 5 (5%) died during the induction course (Table 4). Of the 96 patients assigned to placebo, 74 (77%) achieved a CR, 11 (11%) survived treatment but had refractory disease, and 11 (11%) died early. Using the chi-square test for trend for these three outcomes between the two treatment groups, there is a higher than expected proportion of CR patients in the G-CSF group and a higher than expected proportion of deaths in the placebo group (P = .04). Using the chi-square test for proportions, the P value is .07 for the comparison of CR with no CR in favor of the G-CSF treatment. Considering only the 185 eligible ALL patients by the treatment actually received, the CR rate was 87 of 97 patients (90%) with G-CSF versus 71 of 88 patients (81%) with placebo (P = .10). The CR rates for these 185 eligible patients according to pretreatment clinical and biological characteristics of ALL are shown in Table 5. The CR rate was 87% for patients <60 years old and 77% for patients ≥60 years old (P = .18).
Outcome According to Treatment Assignment
| . | No. of Patients (%) . | ||
|---|---|---|---|
| G-CSF Group . | Placebo Group . | Total . | |
| All patients enrolled | 102 | 96 | 198 |
| Died during induction | 5 (5) | 11 (11) | 16 (8) |
| Refractory disease | 8 (8) | 11 (11) | 19 (10) |
| CR | 89 (87) | 74 (77) | 163 (82) |
| All eligible patients | 97 | 88 | 185 |
| Died during induction | 4 (4) | 10 (11) | 14 (8) |
| Refractory disease | 6 (6) | 7 (8) | 13 (7) |
| CR | 87 (90) | 71 (81) | 158 (85) |
| Withdrawn for BMT | 8 | 6 | 14 |
| Died in CR | 8 | 5 | 13 |
| Relapsed | 35 | 39 | 74 |
| Alive in CCR | 35 (41) | 22 (31) | 57 (36) |
| . | No. of Patients (%) . | ||
|---|---|---|---|
| G-CSF Group . | Placebo Group . | Total . | |
| All patients enrolled | 102 | 96 | 198 |
| Died during induction | 5 (5) | 11 (11) | 16 (8) |
| Refractory disease | 8 (8) | 11 (11) | 19 (10) |
| CR | 89 (87) | 74 (77) | 163 (82) |
| All eligible patients | 97 | 88 | 185 |
| Died during induction | 4 (4) | 10 (11) | 14 (8) |
| Refractory disease | 6 (6) | 7 (8) | 13 (7) |
| CR | 87 (90) | 71 (81) | 158 (85) |
| Withdrawn for BMT | 8 | 6 | 14 |
| Died in CR | 8 | 5 | 13 |
| Relapsed | 35 | 39 | 74 |
| Alive in CCR | 35 (41) | 22 (31) | 57 (36) |
Abbreviations: BMT, bone marrow transplantation; CCR, continuous CR.
Clinical Characteristics in Relation to CR Rates, DFS, and Overall Survival for 185 Patients With ALL
| Variable . | N (%) . | CR (%) . | P* . | DFS From CR . | Overall Survival . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Median (mos) . | Probability of DFS at 3 yrs (95% CI) . | P4-151 . | Median (mos) . | Probability of Survival at 3 yrs (95% CI) . | P4-151 . | ||||
| Total | 185 | 158 (85) | 23 | 0.40 (0.33-0.48) | 23 | 0.43 (0.36-0.50) | |||
| Age (yrs) | |||||||||
| <30 | 73 (39) | 66 (90) | .17 | 29 | 0.46 (0.34-0.59) | .02 | 47 | 0.57 (0.46-0.68) | <.01 |
| 30-59 | 77 (42) | 65 (84) | 20 | 0.43 (0.32-0.55) | 21 | 0.40 (0.30-0.51) | |||
| ≥60 | 35 (19) | 27 (77) | 11 | 0.19 (0.09-0.35) | 12 | 0.17 (0.09-0.31) | |||
| Gender | |||||||||
| Male | 99 (54) | 90 (91) | .02 | 28 | 0.44 (0.34-0.55) | .14 | 32 | 0.49 (0.40-0.59) | .007 |
| Female | 86 (46) | 68 (79) | 20 | 0.35 (0.25-0.47) | 16 | 0.35 (0.26-0.45) | |||
| Mediastinal mass | |||||||||
| Present | 14 (8) | 13 (93) | .70 | >29 | 0.54 (0.29-0.77) | .38 | >39 | 0.64 (0.39-0.84) | .14 |
| Absent | 168 (92) | 143 (85) | 23 | 0.39 (0.31-0.47) | 23 | 0.41 (0.34-0.49) | |||
| Leukocytes | |||||||||
| <30,000 | 116 (63) | 102 (88) | .28 | 29 | 0.45 (0.35-0.55) | .10 | 31 | 0.48 (0.39-0.57) | .06 |
| ≥30,000 | 69 (37) | 56 (81) | 12 | 0.32 (0.21-0.46) | 15 | 0.33 (0.23-0.45) | |||
| Immunophenotype | |||||||||
| B or BMy | 115 (80) | 98 (85) | 1.00 | 20 | 0.39 (0.30-0.49) | .05 | 18 | 0.40 (0.32-0.49) | .03 |
| T or TMy | 29 (20) | 25 (86) | >34 | 0.60 (0.39-0.77) | >38 | 0.62 (0.43-0.77) | |||
| Cytogenetics | |||||||||
| Ph+ | 26 (30) | 22 (85) | .44 | 13 | 0.18 (0.08-0.36) | .27 | 15 | 0.19 (0.09-0.36) | .10 |
| Ph− | 60 (70) | 55 (92) | 13 | 0.34 (0.23-0.47) | 18 | 0.40 (0.28-0.52) | |||
| Variable . | N (%) . | CR (%) . | P* . | DFS From CR . | Overall Survival . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Median (mos) . | Probability of DFS at 3 yrs (95% CI) . | P4-151 . | Median (mos) . | Probability of Survival at 3 yrs (95% CI) . | P4-151 . | ||||
| Total | 185 | 158 (85) | 23 | 0.40 (0.33-0.48) | 23 | 0.43 (0.36-0.50) | |||
| Age (yrs) | |||||||||
| <30 | 73 (39) | 66 (90) | .17 | 29 | 0.46 (0.34-0.59) | .02 | 47 | 0.57 (0.46-0.68) | <.01 |
| 30-59 | 77 (42) | 65 (84) | 20 | 0.43 (0.32-0.55) | 21 | 0.40 (0.30-0.51) | |||
| ≥60 | 35 (19) | 27 (77) | 11 | 0.19 (0.09-0.35) | 12 | 0.17 (0.09-0.31) | |||
| Gender | |||||||||
| Male | 99 (54) | 90 (91) | .02 | 28 | 0.44 (0.34-0.55) | .14 | 32 | 0.49 (0.40-0.59) | .007 |
| Female | 86 (46) | 68 (79) | 20 | 0.35 (0.25-0.47) | 16 | 0.35 (0.26-0.45) | |||
| Mediastinal mass | |||||||||
| Present | 14 (8) | 13 (93) | .70 | >29 | 0.54 (0.29-0.77) | .38 | >39 | 0.64 (0.39-0.84) | .14 |
| Absent | 168 (92) | 143 (85) | 23 | 0.39 (0.31-0.47) | 23 | 0.41 (0.34-0.49) | |||
| Leukocytes | |||||||||
| <30,000 | 116 (63) | 102 (88) | .28 | 29 | 0.45 (0.35-0.55) | .10 | 31 | 0.48 (0.39-0.57) | .06 |
| ≥30,000 | 69 (37) | 56 (81) | 12 | 0.32 (0.21-0.46) | 15 | 0.33 (0.23-0.45) | |||
| Immunophenotype | |||||||||
| B or BMy | 115 (80) | 98 (85) | 1.00 | 20 | 0.39 (0.30-0.49) | .05 | 18 | 0.40 (0.32-0.49) | .03 |
| T or TMy | 29 (20) | 25 (86) | >34 | 0.60 (0.39-0.77) | >38 | 0.62 (0.43-0.77) | |||
| Cytogenetics | |||||||||
| Ph+ | 26 (30) | 22 (85) | .44 | 13 | 0.18 (0.08-0.36) | .27 | 15 | 0.19 (0.09-0.36) | .10 |
| Ph− | 60 (70) | 55 (92) | 13 | 0.34 (0.23-0.47) | 18 | 0.40 (0.28-0.52) | |||
*Fisher’s exact test.
Log-rank test.
The impact of G-CSF on hematologic recovery in older patients (≥60 years old) and younger patients (<60 years old) was analyzed by testing for an age-treatment interaction in a Cox regression model. There were no detectable treatment effects for the ANC endpoints or hospitalization in the two age groups. However, there was a significant interaction with respect to recovery of platelets (P = .04). Older patients who received placebo had a median time to platelet recovery of 26 days, whereas the older patients who received G-CSF had a median time to recovery of 17 days. The younger patients had median times to recovery of 16 days in each treatment group. A similar relationship was found for duration of thrombocytopenia.
For the 21 patients ≥60 years old assigned to G-CSF treatment, the CR rate was 81%, and 2 patients (10%) died during induction. For the 20 patients ≥60 years old assigned to placebo, the CR rate was 55%, and 5 patients (25%) died during induction. These differences were not statistically significant (P = .10 and .24, respectively). For patients <60 years old, the CR rate was 89% with G-CSF and 83% with placebo (P = .36), whereas the induction death rates were 4% and 8%, respectively (P = .32).
Remission consolidation courses.
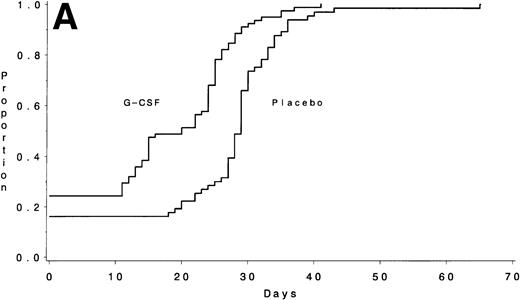
Data on neutrophil recovery after consolidation chemotherapy are available on 146 of the 154 patients who entered Course IIA and 129 of the 143 patients who entered Course IIB. During course IIA, the median times to recover an ANC ≥1,000/μL were 20 days (IQR, 6 to 25) with G-CSF and 29 days (IQR, 22 to 31) for the placebo group (P < .001) (Fig 3A). The duration of neutropenia was also significantly shorter with G-CSF: a median of 5 days (IQR, 0 to 12) for G-CSF and 13 days (IQR, 6 to 18) for placebo (P < .001). Of the patients receiving G-CSF, 35% never had an ANC <500/μL during this course compared with 19% of those on the control arm. The median times to recover platelets ≥50,000/μL were not different: 20 days (IQR, 17 to 22) with G-CSF and 20 days (IQR, 18 to 22) for placebo (P = .53). Forty-six percent of the patients receiving G-CSF had a fever >38.5°C compared with 45% of those on the control arm (P = 1.0). There were no significant differences in the number of days of hospitalization: a median of 7 days with G-CSF and 3 days for the placebo group (P= .32). Of the patients receiving G-CSF, 35% did not require hospitalization during this course compared with 40% of those on the control arm.
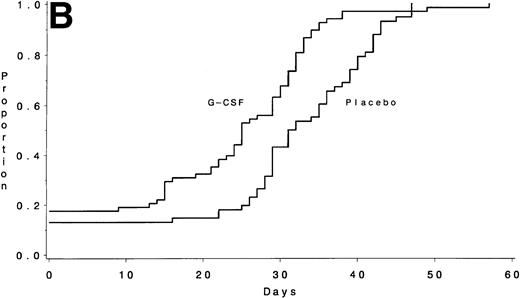
The number of days from the start of chemotherapy until the recovery of an ANC >1,000/μL is shown for Course IIA (A) and for Course IIB (B) according to treatment assignment. The medians were 20 days for the G-CSF group and 29 days for the placebo group for Course IIA (P < .001) and 25 days and 31 days, respectively, for Course IIB (P < .001).
The number of days from the start of chemotherapy until the recovery of an ANC >1,000/μL is shown for Course IIA (A) and for Course IIB (B) according to treatment assignment. The medians were 20 days for the G-CSF group and 29 days for the placebo group for Course IIA (P < .001) and 25 days and 31 days, respectively, for Course IIB (P < .001).
During course IIB, the median times to recover an ANC ≥1,000/μL were 25 days (IQR, 15 to 32) with G-CSF and 31 days (IQR, 27 to 39) for the placebo group (P < .001) (Fig 3B). The duration of neutropenia was also significantly shorter with G-CSF: a median of 11 days (IQR, 4 to 17) for G-CSF and 14 days (IQR, 10 to 25) for placebo (P = .001). Of the patients receiving G-CSF, 28% never had an ANC <500/μL compared with 24% on the control arm. The median times to recover platelets ≥50,000/μL were 24 days (IQR, 21 to 31) with G-CSF and 22 days (IQR, 0 to 28) for placebo (P = .03), and the duration of thrombocytopenia was also significantly different: a median of 10 days (IQR, 7 to 20) with G-CSF and 7 days (IQR, 0 to 15) for placebo (P = .02). There was no obvious explanation for the delay in platelet recovery observed on the G-CSF arm during this course. Forty-eight percent of the patients receiving G-CSF had a fever >38.5°C compared with 55% of those on the control arm (P= .73). There were no significant differences in the number of days of hospitalization: a median of 4 days with G-CSF and 2 days for the placebo group (P = .17). Of the patients receiving G-CSF, 38% did not require hospitalization during this course compared with 44% of those on the control arm.
Effects of G-CSF on treatment toxicity.
The G-CSF treatment itself was very well tolerated. Unfortunately, there was no evidence that the G-CSF significantly reduced the nonhematologic complications that occur during intensive chemotherapy treatment of ALL. The incidence of severe, life-threatening, and fatal toxicities (grades 3, 4, and 5) according to treatment assignment is shown in Table 6. Infectious complications were not different in the two groups. Probably as a consequence, the patients who were assigned to receive G-CSF were not able to complete the first 3 months of planned chemotherapy any more rapidly than those assigned to placebo. A median of 106 days (IQR, 96 to 117) was required by the G-CSF group to complete the first three courses of treatment compared with 108 days (IQR, 103 to 117) for the placebo group (P= .60) (Fig 4).
Toxicity of Remission Induction Therapy According to Treatment Assignment
| Grade 3, 4, or 5 Toxicity . | Percentage of Patients . | |||
|---|---|---|---|---|
| G-CSF Group (n = 100) . | Placebo Group (n = 93) . | Total (n = 193) . | P . | |
| WBC (<1,000/μL) | 98 | 97 | 97 | .67 |
| Platelets (<25,000/μL) | 97 | 95 | 96 | .71 |
| Hemoglobin (<6.5 g/dL) | 93 | 86 | 90 | .16 |
| Infection | 78 | 87 | 82 | .13 |
| Nausea | 23 | 28 | 26 | .41 |
| Bilirubin (>1.5 × normal) | 44 | 51 | 47 | .39 |
| Transaminases (>5 × normal) | 35 | 35 | 35 | 1.00 |
| Malaise/fatigue (PS > 2) | 16 | 25 | 20 | .15 |
| Motor neuropathy | 18 | 22 | 20 | .59 |
| Pain | 21 | 14 | 18 | .26 |
| Hyperglycemia (>250 mg/dL) | 33 | 35 | 34 | .88 |
| Hypofibrinogenemia (<0.5 × normal) | 26 | 18 | 22 | .16 |
| Grade 3, 4, or 5 Toxicity . | Percentage of Patients . | |||
|---|---|---|---|---|
| G-CSF Group (n = 100) . | Placebo Group (n = 93) . | Total (n = 193) . | P . | |
| WBC (<1,000/μL) | 98 | 97 | 97 | .67 |
| Platelets (<25,000/μL) | 97 | 95 | 96 | .71 |
| Hemoglobin (<6.5 g/dL) | 93 | 86 | 90 | .16 |
| Infection | 78 | 87 | 82 | .13 |
| Nausea | 23 | 28 | 26 | .41 |
| Bilirubin (>1.5 × normal) | 44 | 51 | 47 | .39 |
| Transaminases (>5 × normal) | 35 | 35 | 35 | 1.00 |
| Malaise/fatigue (PS > 2) | 16 | 25 | 20 | .15 |
| Motor neuropathy | 18 | 22 | 20 | .59 |
| Pain | 21 | 14 | 18 | .26 |
| Hyperglycemia (>250 mg/dL) | 33 | 35 | 34 | .88 |
| Hypofibrinogenemia (<0.5 × normal) | 26 | 18 | 22 | .16 |
Only toxic effects with an incidence of >20% in either group are included. Data were incomplete on two patients in the G-CSF group and three patients in the placebo group. The statistical test accounts for missing values.
The interval in days required from the start of chemotherapy until the beginning of Course III is shown according to treatment assignment. The medians were 106 days for the G-CSF group and 108 days for the placebo group (P = .60).
The interval in days required from the start of chemotherapy until the beginning of Course III is shown according to treatment assignment. The medians were 106 days for the G-CSF group and 108 days for the placebo group (P = .60).
DFS.
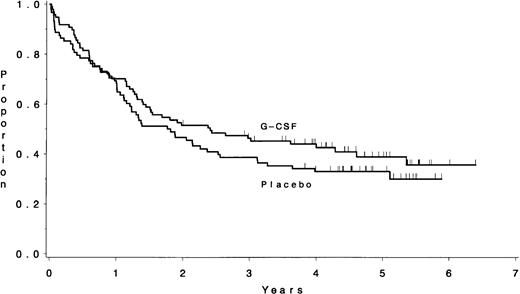
The median DFS for the 158 eligible ALL patients who achieved a CR on this study was 23 months; 40% (95% CI, 33% to 48%) were estimated to remain in continuous CR longer than 3 years. The DFS results for various clinical and biological subsets of patients are shown in Table5. There were no significant differences in the DFS according to treatment assignment: a median of 2.3 years for those assigned to G-CSF and 1.7 years for placebo (P = .53) (Fig 5). Treatment with G-CSF did not affect the DFS for patients who had coexpression of myeloid markers on their lymphoblasts. The median DFS is 4.0 years for the 20 patients with a BMy or TMy immunophenotype assigned to the G-CSF treatment and 1.3 years for the 12 similar patients assigned to placebo (P = .21). Similarly, the DFS was not different between the 11 patients who had Ph+ ALL and were assigned to G-CSF (median, 0.8 years) and the 11 similar patients who were assigned to placebo (median, 1.1 years; P = .98).
There was no difference in the DFS between the patients assigned to G-CSF (median, 2.3 years) and those assigned to placebo (median, 1.7 years) (P = .53).
There was no difference in the DFS between the patients assigned to G-CSF (median, 2.3 years) and those assigned to placebo (median, 1.7 years) (P = .53).
Survival.
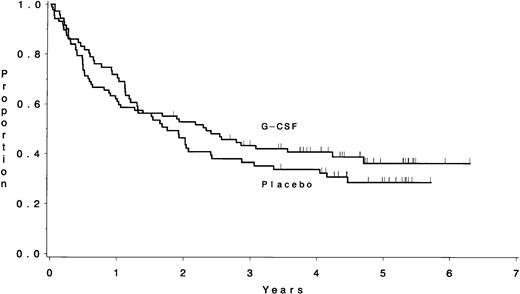
The median overall survival for the 185 eligible ALL patients enrolled on this study was 23 months; 43% (95% CI, 36% to 50%) of patients were estimated to be alive longer than 3 years (Table 5). Survival was not different according to treatment assignment: a median of 2.4 years for patients assigned to G-CSF and 1.8 years for placebo (P = .25) (Fig 6). Similarly, survival did not differ among the 39 patients with a BMy or TMy immunophenotype or the 26 patients known to have Ph+ ALL according to treatment assignment (data not shown). The median follow-up time for these analyses was 4.7 years (range, 2.0 to 6.4 years).
There was no difference in the survival between the patients assigned to receive G-CSF (median, 2.4 years) and those assigned to placebo (median, 1.8 years) (P = .25).
There was no difference in the survival between the patients assigned to receive G-CSF (median, 2.4 years) and those assigned to placebo (median, 1.8 years) (P = .25).
DISCUSSION
G-CSF is a potent stimulator of granulopoiesis as well as early hematopoietic progenitor cells.11 In this double-blinded, randomized, placebo-controlled clinical trial in adults with ALL, we have shown that G-CSF significantly shortens the time required to recover normal numbers of peripheral blood neutrophils after intensive combination chemotherapy. The duration of neutropenia was significantly shortened after each of three courses of treatment. The duration of thrombocytopenia was also shortened significantly after the first course but was no different after the second, and a modest delay in platelet recovery was observed during the third course. We observed no significant decreases either in the incidence or severity of nonhematologic toxicity such as severe infections, mucositis, or bleeding or in the number of febrile days during each course. This is probably because these chemotherapy-induced complications generally occur early in the treatment course at the nadir of the WBC count before the marrow has had time to respond to growth factor stimulation. Nevertheless, the more rapid recovery in the neutrophil count may have led to more rapid resolution of toxicity because our patients receiving G-CSF spent significantly fewer days in the hospital during the remission induction course than did those receiving placebo.
A recently published randomized trial of G-CSF (10 μg/kg/d) in children with ALL also reported on the accelerated rate of recovery of granulocytes after myelosuppressive remission induction chemotherapy, but this did not result in a decreased rate of hospitalization for neutropenic fever, lower costs of supportive care, or a higher probability of event-free survival.12 However, the use of G-CSF was significantly associated with a lower incidence of documented infections, shorter median hospital stays, and fewer delays in starting the consolidation chemotherapy on schedule.
The impact of G-CSF treatment is likely to be most apparent on the subsets of patients who otherwise have the slowest hematologic recovery. This includes elderly patients and may also include those with infection or malnutrition and those receiving prolonged courses of other myelosuppressive medications. In our study, fewer patients on the G-CSF arm than on the placebo arm had very prolonged neutrophil recovery times or very prolonged hospitalizations.
The ultimate clinical benefit of more rapid hematologic recovery is less apparent. Patients who received G-CSF had a higher response rate and fewer deaths during the remission induction course than did those receiving placebo. However, patients in the G-CSF group were not able to complete their first 3 months of prescribed chemotherapy any more rapidly than those in the placebo group. Thus, we were not able to increase the intensity of antileukemia therapy by shortening the time required to deliver the treatment safely. The remission durations and survival of the two treatment groups are not different after a median follow-up time of 4.7 years, although the study was not designed to detect significant differences in these two outcomes. If the apparent difference in the median survivals between the two treatment groups were true (Fig 6), we would need to have accrued 2.25-fold more patients to show statistical significance.
The German ALL study group has reported on a trial in which 76 adults with ALL were randomly allocated to receive either G-CSF in an open-label fashion or no growth factor during the last 4 weeks of an 8-week remission induction regimen.13 The median duration of neutropenia (ANC < 1,000/μL) was 8 days in the G-CSF arm and 12.5 days in the control group (P < .002). There was no significant difference in the incidence of infections. However, prolonged interruptions of chemotherapy administration were less frequent; delays of 2 weeks or more occurred in only 24% of patients receiving G-CSF but in 46% of patients in the control arm (P = .01). For this reason, the planned chemotherapy was completed more rapidly with the use of G-CSF (median, 39 v 44 days; P= .008), but this small interval is not likely to have clinical importance. No difference in the DFS was observed between the two patient groups.
We did not detect any disadvantage from the use of G-CSF in any subgroup of patients with ALL. It is known that some cases of Ph+ ALL and some ALL cells that coexpress myeloid antigens have surface receptors for G-CSF.14,15 However, neither the rate of hematologic recovery nor the clinical outcomes were different overall when Ph+ ALL patients were assigned to G-CSF or to placebo. Two patients who had Ph+ ALL were observed to have increasing numbers of lymphoblasts in the peripheral blood while receiving G-CSF during the induction course. After the G-CSF was discontinued, one patient entered a CR and one had residual ALL in the marrow. There were also no significant differences observed according to treatment assignment among patients who had myeloid antigens expressed on the surface of their lymphoblasts. Table 5 shows the treatment outcomes for the commonly used prognostic subsets of adults with ALL. These data confirm the results previously reported with this multiagent regimen.1
In this trial, the G-CSF treatment was begun only after the administration of the most myelosuppressive chemotherapy drugs, ie, on the fourth day of the induction course. Thus, we were able to avoid the possible toxicity of concurrent administration of a hematologic growth factor with weekly daunorubicin, a schedule widely used in other treatment programs.16 Importantly, the use of G-CSF concurrent with antimetabolite therapy (low doses of cytarabine and daily 6-mercaptopurine) during Courses IIA and IIB did not lead to more severe myelosuppression. Nor did the use of G-CSF in an earlier course lead to greater cytopenia, lack of cytokine-responsiveness, or “marrow exhaustion” in subsequent courses.
G-CSF is an expensive drug. The typical pharmacy cost for an adult is $120 to $180 per day. An economic analysis was not an objective of this trial, but we are now collecting these data retrospectively for study.17 Despite its lack of significant benefit for overall survival, the potential clearly exists for G-CSF to increase the CR rate and to reduce the overall cost of care by reducing hospitalization during remission-induction treatment for ALL. Although the clinical benefits were found largely among older patients in this trial, current CALGB trials for adults with ALL routinely use G-CSF for all patients during the induction course. Because prolonged hospitalization was rarely required during the consolidation chemotherapy courses and because there has been no prolongation of remission duration or survival, we are not routinely using G-CSF during postremission treatment.
ACKNOWLEDGMENT
We thank the many physicians, nurses, and data managers at each of the CALGB institutions and their affiliated hospitals for their assistance with the conduct of this clinical trial. We also thank Audrey McKinnon, CALGB data coordinator for this study, for her expertise in central data management and quality assurance. The study drug was supplied by Amgen to the National Cancer Institute, Cancer Therapy Evaluation Program.
APPENDIX
The following institutions (principal investigators) participated in the study: Wake Forest University School of Medicine, Winston-Salem, NC (M. Robert Cooper, MD; CA03927); Central Massachusetts Oncology Group, Worcester, MA (F. Marc Stewart, MD); Dana-Farber Cancer Institute, Boston, MA (George P. Canellos, MD; CA32291); Dartmouth Medical School — Norris Cotton Cancer Center, Lebanon, NH (L. Herbert Maurer, MD; CA04326); Duke University Medical Center, Durham, NC (Jeffrey Crawford, MD; CA47577); Long Island Jewish Medical Center, New York, NY (Marc Citron, MD; CA11028); Massachusetts General Hospital, Boston, MA (Michael Grossbard, MD; CA12449); McGill Department of Oncology, Montreal, Canada (Brian Leyland-Jones, MD; CA31809); Mount Sinai Hospital, New York, NY (James F. Holland, MD; CA04457); New York Hospital — Cornell Medical Center, New York, NY (Ted Szatrowski, MD; CA07968); North Shore University Hospital, Manhasset, NY (Daniel R. Budman, MD; CA 35279); Rhode Island Hospital, Providence, RI (Louis A. Leone, MD; CA 08025); Roswell Park Cancer Institute, Buffalo, NY (Ellis Levine, MD; CA 59518); SUNY Health Science Center at Syracuse, NY (Stephan Graziano, MD; CA 21060); University of Alabama, Birmingham, AL (Robert Diasio, MD; CA47545); University of California at San Diego, CA (Stephen Seagren, MD; CA 11789); University of Chicago, Chicago, IL (Nicholas Vogelzang, MD; CA41287); University of Iowa, Iowa City, IA (Gerald Clamon, MD; CA 47642); University of Maryland Cancer Center, Baltimore, MD (Ernest Borden, MD; CA 31983); University of Minnesota, Minneapolis, MN (Bruce Peterson, MD; CA 16450); University of Missouri/Ellis Fischel Cancer Center, Columbia, MO (Michael C Perry, MD; CA 12046); University of North Carolina at Chapel Hill, NC (Thomas Shea, MD; CA47559); Walter Reed Army Medical Center, Washington, DC (Nancy Dawson, MD; CA26806); Washington University — Barnes Hospital, St Louis, MO (Daniel Ihde, MD; CA47546).
Supported in part by grants from the National Cancer Institute to the CALGB (CA31946 and CA37027) and the CALGB Statistical Center (CA33601) and from the Coleman Leukemia Research Fund.
Address reprint requests to Richard A. Larson, MD, University of Chicago Medical Center, 5841 S Maryland Ave, MC2115, Chicago, IL 60637-1470; e-mail:ralarson@mcis.bsd.uchicago.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal