Abstract
Although in utero transplantation (IUT) has been shown to be effective in treating human severe combined immune deficiency (SCID), the relative merit of IUT as compared with postnatal bone marrow transplantation (BMT) for SCID is unknown. Therefore, comparative studies were undertaken in mice to determine the engraftment outcome in these two settings. Because T-cell depletion (TCD) reduces graft-versus-host disease (GVHD) severity but compromises alloengraftment, studies were performed with TCD or non-TCD BM and GVHD risk was assessed using a tissue scoring system and by the adoptive transfer of splenocytes from engrafted mice into secondary recipients. Non-SCID recipients received pre-BMT irradiation to simulate those circumstances in which conditioning is required for alloengraftment. IUT recipients of non-TCD and especially TCD BM cells in general had higher levels of donor T-cell and myeloid peripheral blood (PB) engraftment than nonconditioned SCID recipients. Increased TCD or non-TCD BM cell numbers in adult SCID recipients resulted in similar levels of PB engraftment as IUT recipients. However, under these conditions, mean GVHD scores were higher than in IUT recipients. The majority of adoptive transfer recipients of splenocytes from IUT recipients were GVHD-free, consistent with the in vitro evidence of tolerance to host alloantigens. Total body irradiation (TBI)-treated mice that had the highest engraftment had evidence of thymic damage as denoted by a higher proportion of thymic and splenic T cells with a memory phenotype as compared with IUT recipients. IUT mice had vigorous thymic reconstitution by 3 weeks of age. Our data indicate that IUT has a number of advantages as compared with postnatal BMT. Future studies examining the fine specificity of immunoreconstitution in IUT versus postnatal BMT are indicated.
IMMUNOLOGICAL correction of human severe combined immune deficiency (SCID) has recently been accomplished by in utero transplantation (IUT) with HLA-disparate bone marrow (BM).1 At birth, T-cell engraftment and function were readily demonstrated, whereas B-cell engraftment was low. The alternative to IUT for SCID is postnatal bone marrow transplantation (BMT). HLA-disparate donor BM administered postnatally to SCID patients can engraft and restore immune function in some patients.2-16 However, uniform engraftment is not observed in this setting. When engraftment does occur, T cells typically engraft far better than B cells or myeloid cells. This split (donor/host) chimerism can leave the recipient with humoral immune deficiency if the recipient has few B cells or if the recipient’s B-cell dysfunction is not corrected by normal donor T cells. Pre-BMT conditioning regimens will improve the likelihood of B-cell and myeloid cell engraftment but are associated with damage to organs such as the lung, liver, developing central nervous system (CNS), and the endocrine system.16-19 In addition, conditioning regimens may damage the lymphoid microenvironment, which further hampers immune system development.
IUT offers the possibility of avoiding some of the problems associated with haploidentical BMT for SCID. Theoretically, IUT would have the benefit of allowing the immune system development to occur in utero. Alloengraftment might be easier than postnatal BMT, because the fetal immune system is naive and, at least in terms of immunological rejection mechanism(s), the fetus is preimmune. Immune restoration may be easier after IUT than after postnatal BMT with myeloablation, because conditioning regimens may damage the microenvironment necessary for optimal immune development. There is an opportunity for immune system recovery in utero so that, by birth, as shown in the X-SCID case, elements of the immune system may have been restored.1 Graft-versus-host (GVH) risk may be reduced after IUT if the fetal microenvironment is not permissive for the expansion of adult T-cell effectors.
Although many theoretical reasons have been offered suggesting that IUT is preferable to postnatal BMT for SCID, there are not sufficient data upon which to make a definitive conclusion. We have established a murine IUT model for SCID. Allogeneic adult BM transferred into fetal SCID recipients results in multilineage progenitor cell engraftment. We have focused on the engraftment and graft-versus-host disease (GVHD) effects of a large series of SCID recipients of IUT. Although T-cell depletion (TCD) is most often used to reduce the GVHD risk in SCID recipients of HLA-disparate donor BM, TCD BM may be more difficult to engraft in some recipients than non-TCD (NTCD) BM. Thus, IUT SCID recipients received either TCD or NTCD BM to determine the effect of donor T cells on alloengraftment and GVHD risk.
Because postnatal BMT is an alternative to IUT, in many instances comparative data to IUT recipients were obtained for adult SCID recipients of TCD or NTCD BM. An additional option in postnatal recipients is the use of conditioning therapy pre-BMT. Some SCID patients are conditioned to promote initial BM engraftment or in instances in which a second BM graft is needed. Therefore, mice that received total body irradiation (TBI) and postnatal BMT also were examined. The overall goal of this study was to determine the comparative engraftment, GVHD risk, and rapidity of reconstitution in IUT as compared with postnatal BMT recipients. We present data that show that IUT compares favorably with postnatal BMT for the treatment of SCID recipients with respect to lymphoid engraftment and GVHD risk.
MATERIALS AND METHODS
Mouse strains.
C57BL/6 (B6:H2b) and BALB/c (H-2d) were purchased from the National Institutes of Health (Bethesda, MD). BALB/c-SCID (H-2d, BALB/c background genes) were purchased from Taconic (Germantown, NY). B10.BR (H2k) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Donors and postnatal BMT recipients were 6 to 12 weeks of age. All mice were housed in microisolator cages under specific pathogen-free conditions and were fed ad libitum according to University of Minnesota Research Animal Resources guidelines.
In utero intraperitoneal microinjections.
Our procedure has been previously described in detail.20 21In brief, BM cells were harvested from 6- to 12-week-old B6 (allogeneic) or BALB/c (congenic) donors by flushing cells from the hind leg bones with phosphate-buffered saline (PBS). Cells were filtered through nylon mesh (Nitex HC3-41; Tetko, Chicago, IL) to remove clumps before injection. Where indicated, BM was TCD by treatment with anti-Thy1.2 (clone 30-H-12; provided by Dr David Sachs, Cambridge, MA) + complement, as previously described. TCD or NTCD BM cells (1 to 4 × 106) were suspended in 5 to 10 μL for injection into each BALB/c-scid/scid fetus. Pregnant females at days 15 and 16 of a 21-day gestation period were anesthetized with sodium pentobarbital injected intraperitoneally. The abdomen was entered with a midline ventral approach, and the uterine horns were exteriorized with jeweller’s forceps. Cells were injected intraperitoneally into each fetus through hand-drawn pipettes. The muscle layers were closed with nonabsorbable synthetic 4-0 sutures, and metal surgical clips were used to close the skin.
Postnatal BMT.
Nonconditioned BALB/c-SCID recipients were infused intravenously with the indicated number of TCD or NTCD (range, 5 to 100 × 106) BM. For TBI studies, BALB/c mice were conditioned with 600 cGy TBI from an x-ray source (dose rate, 41 cGy/min) on day −1 and then infused with 5 to 100 × 106 TCD BM cells intravenously on day 0.
Flow cytometry.
Whole PB cells or single-cell suspensions of BM, spleen, lymph node (LN), or thymus were prepared in buffer (PBS + 5% colostrum-free bovine serum + 0.015% sodium azide). Pelleted cells were incubated for 15 minutes at 4°C with 0.4 μg of an anti-Fc receptor monoclonal antibody (MoAb; clone 2.4G2, rat IgG2b) to prevent Fc binding. Two-color or three-color flow cytometry using directly conjugated (fluorescein isothiocyanate, phycoerythrin) MoAbs or biotinylated MoAb with pridinin chlorophyll protein (PerCP) was performed to assess chimerism and lineage content of lymphohematopoietic cells. Optimal concentrations of directly conjugated MoAbs were added to a total volume of 100 to 130 μL and incubated for 1 hour at 4°C. The following MoAbs were included: anti-H2b specific MoAb (clone EH-144, mouse IgG) and anti-H2d specific MoAb (clone 34-5-8S, mouse IgG2a). Additional MoAbs obtained from PharMingen (San Diego, CA) included lineage-specific markers: anti-CD4 (clone GK1.5, rat IgG2b), anti-CD8 (clone 53-6.7, rat IgG2a), pan-B cell (B220: clone Ra3-6B2, rat IgG2a or CD19:clone 1D3, rat IgG2a), and CD44 as an indicator of memory cell phenotype. All samples were analyzed on a FACScalibur (Becton Dickinson, Palo Alto, CA) using Cell Quest software. Forward and 90° side-scatter were used to identify and gate PB cells with lymphocyte, monocyte, and granulocyte characteristics. A minimum of 10,000 events was examined. Background subtraction using a directly conjugated irrelevant antibody control was performed for each sample.
GVHD assessment by tissue scoring and adoptive transfer into secondary recipients.
Recipients were monitored for the occurrence of GVHD symptomatology, including ruffled fur, diarrhea, hunched posture, and lethargy,22 and by twice weekly quantitation of body weights (for postnatal BMT mice). Liver, lung, colon, skin, and spleen were obtained for histological assessment using a semiquantitative scoring system (0.5 to 4.0 grades) as shown below (with each grade followed by its histological features).
Grade 0: normal.
Grade 0.5: minimal perivascular cuffing (liver, lung); occasional necrotic cells (spleen); occasional necrotic crypt cell, minimal infiltration in lamina propria and submucosa (colon).
Grade 1: perivascular cuffing, 1 to 2 cells in thickness, involving up to 15% of vessels (liver, lung); necrotic/apoptotic cells, up to 10 cells/mm2 of tissue (spleen); necrotic cells in up to 15% of crypts, minor infiltration of up to 20% of lamina propria (1 to 2 cell thickness in intermucosal areas and submucosa) (colon).
Grade 1.5: perivascular cuffing, 1 to 2 cells in thickness, involving up to 15% of vessels and infiltration into parenchyma proper (liver, lung); Necrotic/apoptotic cells, up to 10 cells/mm2 of tissue and occasional hemolysis (spleen); necrotic cells in up to 15% of crypts, minor infiltration of less than or equal to one third of the lamina propria (1 to 2 cell thickness in intermucosal areas and submucosa) (colon).
Grade 2: perivascular cuffing, 2 to 3 cells in thickness, involving up to 25% of vessels and infiltration into parenchyma proper (liver, lung); necrotic/apoptotic cells, ≤20 cells/mm2 of tissue, and occasional hemolysis with abnormal architecture (spleen); necrotic cells in ≤25% of crypts, infiltration of less than or equal to one third of the lamina propria (3 cell thickness in intermucosal areas and submucosa) (colon).
Grade 2.5: perivascular cuffing, 2 to 3 cells in thickness, involving 25% to 50% of vessels and infiltration into parenchyma proper (liver, lung); necrotic/apoptotic cells, ≤20 cells/mm2 of tissue, and hemolysis in ≤25% of the tissue with abnormal architecture (spleen); necrotic cells in 25% to 50% of crypts, infiltration of less than or equal to one third of lamina propria (3 to 4 cell thickness in intermucosal areas and submucosa) (colon).
Grade 3: perivascular cuffing, 4 to 5 cells in thickness, involving 25% to 50% of vessels, peribronchiolar cuffing (2 to 3 cells) and infiltration into parenchyma proper (liver, lung); necrotic/apoptotic cells, ≤40 cells/mm2 of tissue, hemolysis in 25% to 50% of tissue with abnormal architecture and areas of leukopenia involving ≤25% of tissue, formation of fibrous bands (spleen); necrotic cells in greater than 50% of crypts, infiltration of lamina propria (5 to 6 cell thickness in intermucosal areas and submucosa) with loss of ≤25% of goblet cells (colon).
Grade 3.5: perivascular cuffing, 6 to 7 cells in thickness, involving greater than 50% of vessels, peribronchiolar cuffing (4 to 5 cells, lung), necrotic foci (liver) and infiltration into parenchyma proper with severe disruption of structure (liver, lung); necrotic/apoptotic cells, up to 40 cells/mm2 of tissue, hemolysis evident in greater than 50% of tissue with abnormal architecture and areas of leukopenia involving 25% to 50% of tissue, formation of fibrous bands (spleen); necrotic cells in greater than 50% of crypts, infiltration of lamina propria resulting in displacement of ≤50% of mucosa with loss of 50% of goblet cells (colon).
Grade 4: perivascular cuffing, greater than 8 cells in thickness, involving greater than 50% of vessels, peribronchiolar cuffing (>6 cells, lung), large necrotic foci (liver), and infiltration into parenchyma proper with necrotic lesions (liver, lung); large areas of necrosis and hemolysis evident in greater than 50% of tissue with abnormal architecture and large areas of leukopenia involving greater than 50% of tissue (spleen); necrotic cells in greater than 50% of crypts, infiltration of lamina propria resulting in displacement of greater than 50% of mucosa with loss of 75% to 100% of goblet cells (colon).
Mice were monitored daily for survival. GVHD risk also was analyzed in vivo by secondary transfer experiments. Splenocytes (107per recipient) obtained from long-term chimeras that were GVHD-free by clinical assessment were infused into BALB/c-SCID secondary recipients pretreated with anti-asialo-GM1 antisera (Wako Chemicals, Richmond, VA) at a dose of 25 μL on days −4 and −2 to deplete host NK cells that might participate in graft resistance.
In vitro assessment of anti-host responsiveness.
T-cell function was measured using an in vitro mixed lymphocyte reaction (MLR) culture of splenocytes obtained from long-term chimeras. Single-cell suspensions of splenocytes were washed and mixed with irradiated (30 Gy) splenocyte stimulators from B6 (donor strain), BALB/c (host strain), or B10.BR (third-party strain) mice. The mixture was resuspended in Dulbecco’s Minimal Essential Medium (Bio Whittaker, Walkersville, MD), 10% fetal calf serum (Hyclone, Logan, UT), 2 mercaptoethanol (5 × 10−5 mol/L; Sigma, St Louis, MO), 10 mmol/L HEPES buffer, 1 mmol/L sodium pyruvate (GIBCO BRL, Grand Island, NY), and amino acid supplements (1.5 mmol/L L-glutamine, L-arginine, and L-asparagine; Sigma), antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin; Sigma). For MLR analysis, 2 × 105 cells responders and 5 × 105 stimulators were plated into 96-well round-bottom (Costar, Acton, MA) plates and placed at 37°C and 10% CO2 for 2 to 6 days. Tritiated thymidine (1 μCi) was added 16 hours before harvesting and counting in the absence of scintillant on a beta counter. For assessment of cytokine levels produced during an MLR reaction, supernatants were harvested from bulk cultures established in 24-well plates and analyzed for interleukin-2 (IL-2), IL-4, IL-10, and interferon γ (IFNγ) protein concentrations by enzyme-linked immunosorbent assay (ELISA; R & D Systems, Minneapolis, MN).
Statistical analysis.
The Kaplan-Meier product-limit method was used to assess the survival of mice. The log-rank statistic was used to test differences between groups.
RESULTS
PB engraftment in recipients treated via IUT or postnatal BMT: Role of donor BM-derived T cells and postnatal TBI on donor T-, B-, and myeloid cell engraftment.
A large series of experiments was performed in which the same donor-recipient allogeneic strain combination (B6 → BALB/c background) was used for IUT and for postnatal BMT. IUT recipients were treated at a fixed dose (4 × 106) of BM. To determine the role of donor BM-derived T cells in alloengraftment, in utero or postnatal recipients received either TCD or NTCD BM. Because some human SCID recipients undergoing first BMT with TCD HLA-disparate donor BM and many recipients undergoing second BMT receive pre-BMT conditioning therapy, a group of non-SCID murine recipients received TBI to simulate the effect of TBI conditioning therapy on engraftment and outcome in humans. An additional group of IUT treated mice received syngeneic donor NTCD BM as an indication of the extent of lymphoid reconstitution achievable by IUT in the absence of an allogeneic effect.
H2 phenotyping of whole PB performed 6 to 9 weeks after BM transfer in IUT recipients demonstrated high levels of PB donor cells (Fig 1A). Approximately one third of injected mice are live-born (typical range, 15% to 68%). Control noninjected pregnant mice in our colony give rise to a day-7 postnatal survival rate of 50% to 75%, which is higher than that of IUT-injected recipients. The proportion of donor PB cells in adult SCID recipients of TCD or NTCD BM was higher in recipients receiving a larger dose of BM cells. At the lowest BM cell dose (5 × 106), adult SCID recipients of NTCD BM had a higher mean percentage of PB donor cells than those that received TCD BM. At a BM cell dose of 25 × 106, the mean percentage of PB donor cells in adult SCID recipients of either TCD or NTCD BM was comparable to that of IUT recipients. Engraftment, as defined by the presence of ≥1% H2b+ PB cells, was 100% in IUT recipients and 98% to 100% of postnatal SCID recipients. The overall incidence of low level (1% to 10% PB cells of donor origin) chimeras was 5% (7/153) or 4% (4/107) for IUT recipients of NTCD or TCD BM, respectively. For nonconditioned adult SCID recipients of low numbers of NTCD or TCD BM cells (5 × 106), the incidence of low level chimeras was 13% or 21%, respectively. The infusion of higher numbers of BM cells was associated with a reduced incidence of low-level chimeras in nonconditioned adult SCID recipients of NTCD BM (0/34) or TCD (1/20). TBI conditioning of BALB/c recipients provides the highest percentage of donor PB cells and none of the TBI-treated recipients was a low-level chimera. Because engraftment of IUT and postnatal SCID recipients was found to be stable at time periods greater than 5 months posttransfer, only the initial age 6- to 9-week PB typing data, which are available on all mice, are shown.
Comparative PB engraftment analysis in IUT and postnatal BMT recipients. BALB/c-SCID recipients were treated via IUT or postnatal BMT, with the indicated B6 BM cell dose shown in parentheses. Recipients received TCD or NTCD BM as indicated. Additional control groups consisted of BALB/c-SCID recipients of BALB/c BM (congenic [cong.]) and BALB/c recipients were treated with TBI and received B6 TCD BM. Where indicated, controls are non-BMT B6 mice. The mean proportion of PB-expressing H2b (A), CD4 (C), CD8 (D), and B220 (B cells) (E) antigens are listed on the y-axis. The mean percentage of donor myeloid engraftment (B) was calculated by dividing the percentage of H2b+ Mac1+ cell population by the total Mac1+ cells. A composite graph (F) of the relative mean proportion of donor CD4+, CD8+, B220+, Mac1+, and host Mac1+ cells in individual mice in each group is shown. The number of recipients studied (no.) in each group is listed at the bottom of (F). Symbols for (F): (▪) CD4; (▨) CD8; (□) B220; (▧) H2b Mac1; (▥) H2d Mac1. One standard error of the mean values ranged 0% to 4% for all values shown, except for a value of 9% for H2b expression in SCID TCD25 recipients. *Significant differences between IUT TCD recipients and other groups. For the assessment of the proportion of PB expressing donor lymphoid markers, the (#) indicates significant differences between the indicated groups and the non-BMT B6 control mice.
Comparative PB engraftment analysis in IUT and postnatal BMT recipients. BALB/c-SCID recipients were treated via IUT or postnatal BMT, with the indicated B6 BM cell dose shown in parentheses. Recipients received TCD or NTCD BM as indicated. Additional control groups consisted of BALB/c-SCID recipients of BALB/c BM (congenic [cong.]) and BALB/c recipients were treated with TBI and received B6 TCD BM. Where indicated, controls are non-BMT B6 mice. The mean proportion of PB-expressing H2b (A), CD4 (C), CD8 (D), and B220 (B cells) (E) antigens are listed on the y-axis. The mean percentage of donor myeloid engraftment (B) was calculated by dividing the percentage of H2b+ Mac1+ cell population by the total Mac1+ cells. A composite graph (F) of the relative mean proportion of donor CD4+, CD8+, B220+, Mac1+, and host Mac1+ cells in individual mice in each group is shown. The number of recipients studied (no.) in each group is listed at the bottom of (F). Symbols for (F): (▪) CD4; (▨) CD8; (□) B220; (▧) H2b Mac1; (▥) H2d Mac1. One standard error of the mean values ranged 0% to 4% for all values shown, except for a value of 9% for H2b expression in SCID TCD25 recipients. *Significant differences between IUT TCD recipients and other groups. For the assessment of the proportion of PB expressing donor lymphoid markers, the (#) indicates significant differences between the indicated groups and the non-BMT B6 control mice.
Because myeloid lineage cells are short-lived and there is not a competitive advantage for donor over host myeloid cells in SCID recipients, assessment of donor myeloid engraftment provides a more reliable indicator of pluripotent hematopoietic progenitor cell engraftment than the lymphoid compartment. Because recipients have myeloid (but not lymphoid) cells, the degree of myeloid engraftment can be quantified by comparing the proportion of myeloid cells derived from donor (H2b) BM as opposed to the recipient (H2d). The beneficial effect of donor T cells on myeloid engraftment in IUT and postnatal BMT SCID recipients is readily demonstrable (Fig 1B). Although there is a titratable effect of BM cell dose on the percentage of of myeloid cells derived from donor rather than host BM in adult SCID recipients of either TCD or NTCD BM, adult SCID recipients of very high doses TCD BM (100 × 106) were unable to achieve the level of myeloid cell engraftment observed in IUT recipients of 4 × 106 NTCD BM. Adult SCID recipients of 25 × 106 but not 5 × 106 TCD BM cells had comparable levels of myeloid engraftment as IUT recipients of TCD BM, albeit significantly lower than IUT recipients of NTCD BM. Although TBI-treated mice achieved full donor myeloid engraftment, these recipients had significant morbidity (27% loss in pre-BMT body weight in the first week post-BMT) and mortality (30% by 6 weeks post-BMT) from the conditioning process. Collectively, these data indicate that a high level of myeloid engraftment requires either donor T cells, high numbers of BM cells, or TBI conditioning (for postnatal BMT recipients).
The goal of SCID therapy by BMT is to reconstitute the immune system. Donor T- and B-cell engraftment is necessary for this purpose in SCID patients with combined T- and B-cell defects. The proportion of PB composed of CD4+ or CD8+ T cells or B cells was examined as an indication of lymphoid engraftment, because recipients do not have lymphoid cells and therefore all lymphoid cells are of donor origin. As compared with non-BMT B6 controls, IUT recipients of either TCD allogeneic or NTCD congenic or allogeneic BM had similar proportions of PB CD4+ T cells (Fig 1C). Similar findings were observed in adult SCID recipients that received higher BM cell doses (25 to 100 × 106). IUT recipients of allogeneic donor grafts had a mean percentage of PB CD8+ T cells that most closely approximated values observed in non-BMT controls (Fig 1D). Adult SCID recipients of NTCD BM or small doses of TCD BM had lower values and TBI-treated mice had the lowest values for donor PB CD8+ T cells. Because the proportion of the PB that is of myeloid origin is low (average approximately 20% of PB), it is not surprising that the mean percentage of PB B cells in the various groups was inversely related to the degree of T-cell engraftment, because the PB is primarily composed of T or B cells (Fig 1E and F). In all groups, ≥10% of the PB was composed of B cells.
Multiorgan system engraftment in IUT or postnatal BMT recipients.
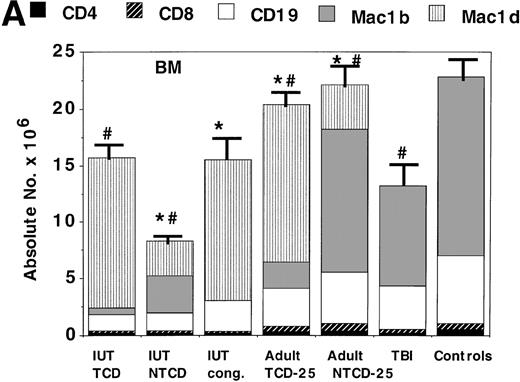
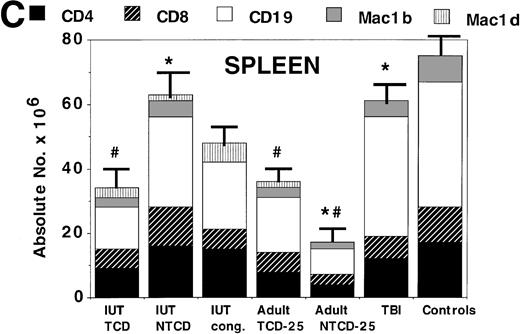
To obtain a quantitative assessment of donor cell engraftment, BM, spleen, and thymus were examined in representative IUT and postnatal BMT recipients at 3 to 5 months of age. Adult SCID recipients of low BM cell doses had significantly lower levels of donor engraftment than those receiving higher doses. Some of the adult SCID recipients of the highest doses of NTCD had clinical GVHD. Therefore, to obtain the comparative data of IUT versus postnatal BMT for SCID, we elected to focus on the comparison of adult SCID recipients of 25 × 106 TCD or NTCD BM to IUT recipients.
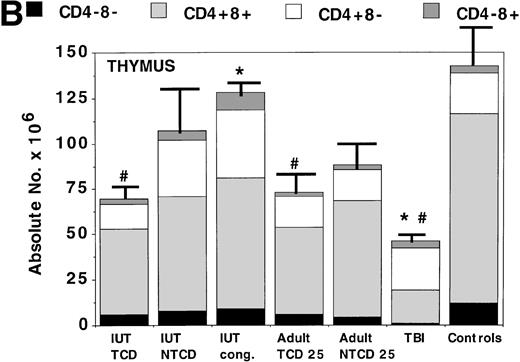
BM (Fig 2A) and thymus (Fig 2B) were examined as the primary sites for the generation of T cells, B cells, and myeloid cells. In the BM, donor myeloid lineage engraftment was highest in TBI-treated mice and adult SCID recipients of NTCD BM, with progressively lower levels seen in IUT recipients of NTCD BM, adult SCID recipients of TCD BM, and then IUT recipients of TCD BM. Thus, pre-BMT conditioning or the inclusion of donor BM T cells provided the highest levels of donor myeloid engraftment. In general, IUT recipients had lower levels of myeloid engraftment than adult SCID recipients. Donor CD19+ B cells were found in both IUT and postnatal BMT recpients, with higher levels observed in the latter groups. Thymic reconstitution was vigorous in all groups except for postnatal mice that received TBI. TBI-treated mice were noted to have few immature CD4−8− cells and a low number of CD4+8+ thymocytes, although more mature CD4+8− and CD4−8+ cells were comparable in number to the other groups.
Assessment of alloengraftment in central (BM, thymus) and peripheral (spleen) lymphohematopoietic compartments. BM (A), thymus (B), and spleen (C) was obtained from 4- to 6-month-old chimeras (n = 5 to 6 mice/group) and analyzed for the presence of donor cells. In BM and spleen, the number of donor CD4+, CD8+, CD19+ B cells, donor Mac1+(Mac1b), and host Mac1+ (Mac1d) cells were quantified. The thymus was analyzed for T-cell differentiation consisting of immature CD4−8−, intermediate CD4+8+, and mature CD4+8− or CD4−8+ cells. The absolute number of cells is shown on the y-axis. One standard error of the mean values for absolute cell number are designated by bars. *Significant differences between IUT TCD recipients and other groups. #Significant differences between the indicated groups and the non-BMT B6 control mice.
Assessment of alloengraftment in central (BM, thymus) and peripheral (spleen) lymphohematopoietic compartments. BM (A), thymus (B), and spleen (C) was obtained from 4- to 6-month-old chimeras (n = 5 to 6 mice/group) and analyzed for the presence of donor cells. In BM and spleen, the number of donor CD4+, CD8+, CD19+ B cells, donor Mac1+(Mac1b), and host Mac1+ (Mac1d) cells were quantified. The thymus was analyzed for T-cell differentiation consisting of immature CD4−8−, intermediate CD4+8+, and mature CD4+8− or CD4−8+ cells. The absolute number of cells is shown on the y-axis. One standard error of the mean values for absolute cell number are designated by bars. *Significant differences between IUT TCD recipients and other groups. #Significant differences between the indicated groups and the non-BMT B6 control mice.
The spleen (Fig 2C) was examined as a primary site for quantifying T-cell, B-cell, and myeloid peripheralization. In the spleen, donor myeloid and B-cell numbers were both highest in IUT recipients of NTCD BM or TBI-conditioned postnatal BMT recipients, with the lowest values observed in adult SCID recipients of NTCD BM. Comparable values for CD4+ and CD8+ T-cell reconstitution were observed in all groups except for the adult SCID recipients of NTCD BM. These data are consistent with an ongoing GVH process in the latter group. To determine whether IUT and postnatal BMT recipients were equally capable of generating naive T cells, T cells localized to the spleen and LN of clinically healthy mice were phenotyped for the expression of cell surface determinants associated with a naive or memory cell (CD44hi) phenotype. CD4+ and CD8+ T cells in the spleen and LN from TBI-treated postnatal BMT recipients had a higher density of CD44 antigen as compared with IUT recipients of NTCD BM or normal controls (Table 1). CD44 antigen density on CD4+ or CD8+ T cells in spleen or LN of IUT mice was comparable to controls.
Generation of Naive and Memory T Cells in IUT Versus TBI-Conditioned Recipients
| Group . | Spleen . | Lymph Node . | ||||||
|---|---|---|---|---|---|---|---|---|
| CD44 . | CD8 . | CD4 . | CD8 . | |||||
| No. . | CD44 . | No. . | CD44 . | No. . | CD44 . | No. . | CD44 . | |
| IUT NTCD | 21 (3) | 435 (43) | 17 (2) | 524 (46) | 3 (2) | 398 (48) | 2 (1) | 357 (33) |
| TBI TCD | 16 (3) | 587 (32)* | 9 (1)* | 780 (49)* | 7 (1) | 545 (15)* | 4 (1) | 612 (17)* |
| Controls | 21 (3) | 436 (19) | 14 (2) | 470 (37) | 7 (1) | 360 (90) | 5 (1) | 398 (93) |
| Group . | Spleen . | Lymph Node . | ||||||
|---|---|---|---|---|---|---|---|---|
| CD44 . | CD8 . | CD4 . | CD8 . | |||||
| No. . | CD44 . | No. . | CD44 . | No. . | CD44 . | No. . | CD44 . | |
| IUT NTCD | 21 (3) | 435 (43) | 17 (2) | 524 (46) | 3 (2) | 398 (48) | 2 (1) | 357 (33) |
| TBI TCD | 16 (3) | 587 (32)* | 9 (1)* | 780 (49)* | 7 (1) | 545 (15)* | 4 (1) | 612 (17)* |
| Controls | 21 (3) | 436 (19) | 14 (2) | 470 (37) | 7 (1) | 360 (90) | 5 (1) | 398 (93) |
Spleen and LN from 5 high-level chimeras per group were individually analyzed for CD4+ and CD8+ T-cell content. The absolute numbers (No.) × 10−6 are shown. CD4+ and CD8+ T cells were analyzed by FACS for the expression of CD44 as an indicator of memory cell generation. The mean channel fluorescence data are shown. The standard error of the mean is shown in parentheses.
A significant (P < .05) difference between IUT NTCD and other groups.
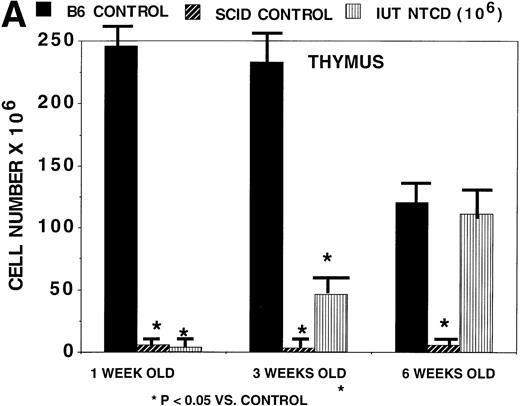
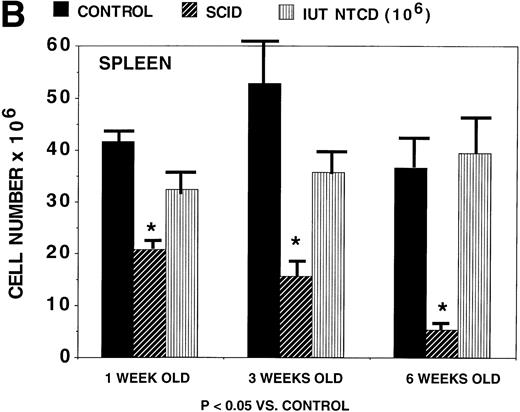
A potential advantage of IUT versus postnatal BMT is the possibility of achieving immunological reconstitution during the time period before birth. We therefore sought to determine how quickly thymic and peripheral (splenic) reconstitution occurs after IUT. Thymic reconstitution in normal and SCID controls was compared with IUT recipients at 1, 3, and 6 weeks of age. By 3 weeks of age (Fig 3A), IUT recipients had significantly higher numbers of thymocytes than SCID controls, and by 6 weeks of age, IUT recipients had comparable numbers of thymocytes as normal controls. Splenocyte numbers were significantly higher in IUT than in SCID recipients at all time periods (Fig 3B). These data are consistent with rapid lymphoid engraftment that is evident by 3 to 6 weeks of age.
Rapidity of thymic and splenic reconstitution in SCID recipients treated via IUT. BALB/c-SCID fetal recipients received 106 B6 TCD BM cells on day 15 to 16 of gestation. Controls consisted of noninjected BALB/c-SCID and noninjected BALB/c wild-type mice. At the indicated times postnatally, 4 to 8 mice were studied for thymic (A) and splenic (B) cellularity. *Significant differences in cellularity as compared with normal age-matched B6 controls. The absolute cell number is listed on the y-axis. The bars designate one standard error of the mean.
Rapidity of thymic and splenic reconstitution in SCID recipients treated via IUT. BALB/c-SCID fetal recipients received 106 B6 TCD BM cells on day 15 to 16 of gestation. Controls consisted of noninjected BALB/c-SCID and noninjected BALB/c wild-type mice. At the indicated times postnatally, 4 to 8 mice were studied for thymic (A) and splenic (B) cellularity. *Significant differences in cellularity as compared with normal age-matched B6 controls. The absolute cell number is listed on the y-axis. The bars designate one standard error of the mean.
Anti-host responses of IUT and post-BMT recipients.
IUT reconstituted mice were uniformly healthy appearing at all time periods of observation. To search for subclinical GVH responses, coded tissue (liver, lung, colon, and spleen) samples were examined for histological evidence of GVHD. Groups of mice selected for analysis were chosen according to the criteria used for multiorgan phenotyping (Fig 2). IUT recipients of TCD or NTCD or TBI recipients of TCD BM had minimal evidence of GVHD (Table 2). In contrast, SCID recipients of either high doses of TCD or NTCD BM had GVHD of moderate severity. Although IUT mice were not weighed during the time period of weaning, these mice appeared healthy without obvious weight loss. TBI-treated mice had an early loss in body weight that was regained in less than 1 month post-BMT. SCID recipients of TCD BM had a transient 6% decrease in mean weights at 1 month post-BMT. SCID recipients of NTCD BM had a 10% decrease in mean body weights beginning 1 month post-BMT that never recovered to their peak post-BMT body weights. These data are consistent with the mean GVHD scores of liver, lung, and spleen, which were significantly higher in the adult SCID recipients as compared with the IUT recipients of congenic BM. For each tissue analyzed, there was either a significant difference or a statistical trend (.1 = P > .05) toward more severe GVH in adult SCID recipients as compared with IUT recipients of TCD BM.
Assessment of GVHD Tissue Damage in IUT and Postnatal BMT Recipients
| Group . | BM Dose (×106) . | Liver . | Lung . | Colon . | Spleen . |
|---|---|---|---|---|---|
| IUT congenic | 4.0 | 0.0 (0.0) | 0.3 (0.3) | 0.3 (0.3) | 0.0 (0.0) |
| IUT TCD | 4.0 | 0.0 (0.0) | 0.6 (0.2) | 0.8 (0.2) | 0.5 (0.2)* |
| IUT NTCD | 4.0 | 0.0 (0.0) | 1.2 (0.4) | 0.1 (0.1) | 0.4 (0.2) |
| SCID TCD | 25.0 | 1.4 (0.7)† | 2.3 (0.2)* | 1.1 (0.4) | 1.0 (0.0)* |
| SCID NTCD | 25.0 | 2.4 (0.3)* | 1.9 (0.5)* | 1.0 (0.6) | 1.8 (0.3)* |
| TBI TCD | 4.0 | 0.1 (0.1) | 0.3 (0.3) | 0.5 (0.2) | 0.0 (0.0) |
| Group . | BM Dose (×106) . | Liver . | Lung . | Colon . | Spleen . |
|---|---|---|---|---|---|
| IUT congenic | 4.0 | 0.0 (0.0) | 0.3 (0.3) | 0.3 (0.3) | 0.0 (0.0) |
| IUT TCD | 4.0 | 0.0 (0.0) | 0.6 (0.2) | 0.8 (0.2) | 0.5 (0.2)* |
| IUT NTCD | 4.0 | 0.0 (0.0) | 1.2 (0.4) | 0.1 (0.1) | 0.4 (0.2) |
| SCID TCD | 25.0 | 1.4 (0.7)† | 2.3 (0.2)* | 1.1 (0.4) | 1.0 (0.0)* |
| SCID NTCD | 25.0 | 2.4 (0.3)* | 1.9 (0.5)* | 1.0 (0.6) | 1.8 (0.3)* |
| TBI TCD | 4.0 | 0.1 (0.1) | 0.3 (0.3) | 0.5 (0.2) | 0.0 (0.0) |
Coded tissue samples from 4 high-level chimeras per group were analyzed using a semiquantitative GVH scoring system. Data are the mean values of the individual scores and in parentheses is shown one standard error of the mean.
P ≤ .05 versus IUT congenic recipients.
.05 < P < .1 versus IUT congenic recipients.
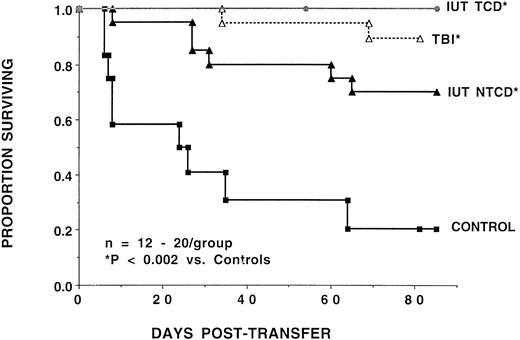
To more rigorously test whether IUT and TBI-treated recipients were tolerant to host alloantigens in vivo, splenocytes (107) from representative high-level chimeric mice from these groups were adoptively transferred into SCID recipients (Fig 4). Phenotyping of donor splenocytes used for adoptive transfer did not show any significant differences in the proportions of CD4+ or CD8+ T cells in the various groups, including the non-BMT controls. Splenocytes from IUT recipients of TCD BM were unable to cause lethal GVHD in secondary recipients, in contrast to the 78% lethality observed after the infusion of splenocytes from non-BMT controls. Splenocytes from IUT recipients of NTCD and TBI-treated recipients had a 30% and 10% lethality rate, respectively. Secondary recipients of splenocytes from IUT or postnatal BMT had mean weights that exceeded pre-BMT values in all groups except the recipients of control splenocytes. Adoptive transfer experiments were not performed with adult SCID, because these recipients already had evidence of GVHD clinically and by histological evaluation.
The adoptive transfer of splenocytes from high-level chimeras as an assessment of alloreactivity in vivo. Splenocytes (107) from B6 controls, IUT recipients of TCD or NTCD, or TBI-conditioned BALB/c recipients were transferred into NK-depleted BALB/c-SCID recipients. The number of mice per group and the Pvalue comparison with B6 controls are indicated. On the x-axis is days posttransfer and on the y-axis is the proportion of mice surviving.
The adoptive transfer of splenocytes from high-level chimeras as an assessment of alloreactivity in vivo. Splenocytes (107) from B6 controls, IUT recipients of TCD or NTCD, or TBI-conditioned BALB/c recipients were transferred into NK-depleted BALB/c-SCID recipients. The number of mice per group and the Pvalue comparison with B6 controls are indicated. On the x-axis is days posttransfer and on the y-axis is the proportion of mice surviving.
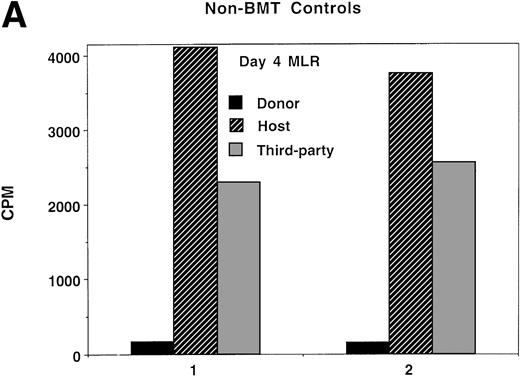
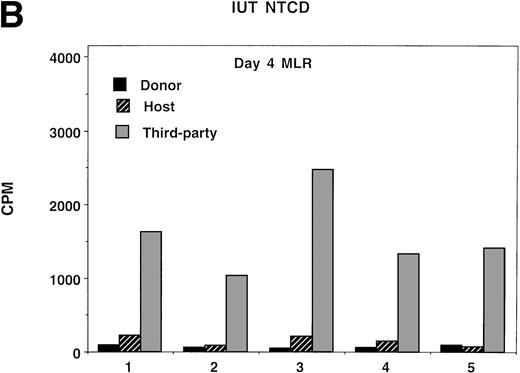
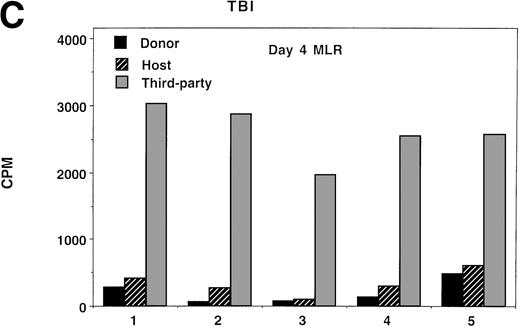
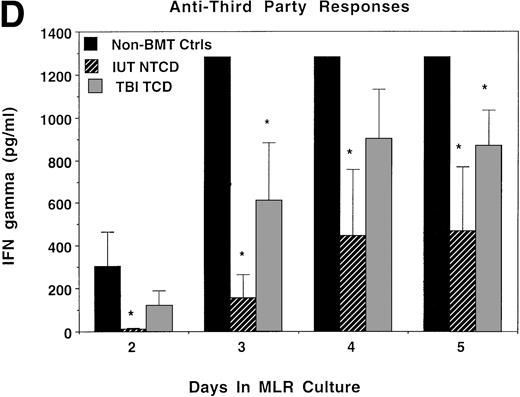
Because splenocytes obtained from IUT recipients of NTCD BM resulted in the highest incidence of GVHD in secondary recipients, we proceeded to determine whether splenocytes from this group of donors had in vitro evidence of anti-host alloresponsiveness. For comparison purposes, non-BMT controls and TBI-treated recipients of TCD BM were concurrently analyzed. Day 4, the time of peak responses, is represented (Fig 5), although similar trends were present at all time points analyzed (data not shown). As compared with normal controls, bulk MLR cultures established from IUT recipients and from TBI-treated recipients showed anti-host responses to be of a similar magnitude to anti-donor responses. Anti-third party responses were intact, although modestly lower in the IUT NTCD as compared with the TBI-treated group.
Ex vivo assessment of anti-host and anti–third-party alloreactivity. MLR cultures were established using splenocytes obtained from nonmanipulated B6 controls (A) or 4- to 6-month-old BALB/c-SCID recipients receiving B6 NTCD via IUT (B) or TBI-conditioned BALB/c recipients of B6 TCD BM (C). Day-4 peak MLR responses to B6 (donor strain), BALB/c (host strain), or B10.BR (third-party strain) alloantigen-bearing stimulator cells were quantified. Mean values are shown. In all instances, one standard deviation of the mean was less than 84 cpm. Similar findings were observed on days 3 and 5 (not shown). The individual recipient animal number is presented as indicated on the x-axis. On the y-axis are the cpm obtained in the absence of scintillant amplification.
Ex vivo assessment of anti-host and anti–third-party alloreactivity. MLR cultures were established using splenocytes obtained from nonmanipulated B6 controls (A) or 4- to 6-month-old BALB/c-SCID recipients receiving B6 NTCD via IUT (B) or TBI-conditioned BALB/c recipients of B6 TCD BM (C). Day-4 peak MLR responses to B6 (donor strain), BALB/c (host strain), or B10.BR (third-party strain) alloantigen-bearing stimulator cells were quantified. Mean values are shown. In all instances, one standard deviation of the mean was less than 84 cpm. Similar findings were observed on days 3 and 5 (not shown). The individual recipient animal number is presented as indicated on the x-axis. On the y-axis are the cpm obtained in the absence of scintillant amplification.
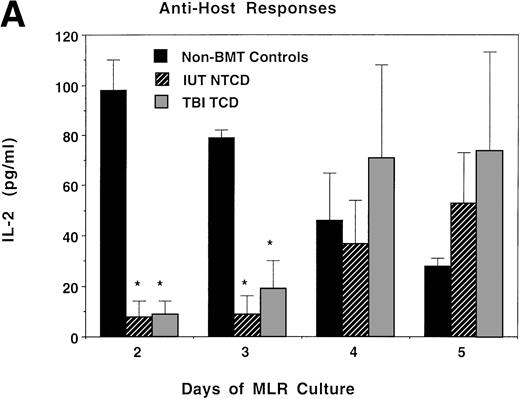
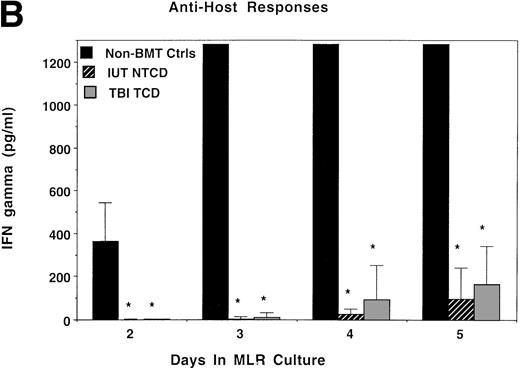
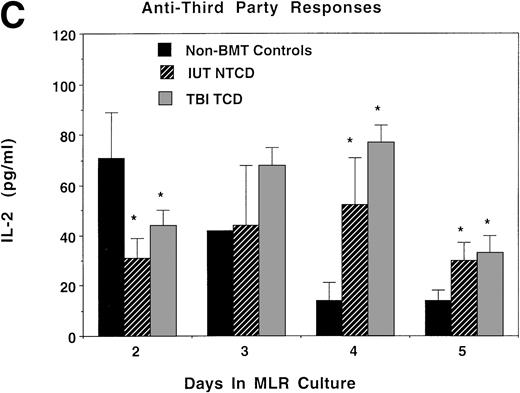
Consistent with the anti-host hyporesponsiveness seen in the IUT NTCD group, as compared with control cultures, supernatants from these bulk cultures had markedly lower levels of IL-2 and IFNγ in both the IUT NTCD BM and TBI TCD BM recipients on days 2 and 3 of culture (Fig 6A and B). Anti-host responses in IUT recipients peaked later than controls (data not shown), consistent with a lower precursor frequency of anti-host reactive cells. Therefore, it is not surprising that IL-2 levels in the supernatant reached control levels later in culture (day 4), past the time of peak proliferative response of controls. IL-2 and IFNγ responses to third-party alloantigens in both groups peaked later than non-BMT controls, with responses in TBI recipients being of a modestly higher magnitude at the time of peak proliferative response (Fig 6C and D). The consistently low IFNγ levels in response to host (Fig 6B) and third-party (Fig 6D) alloantigens in the IUT and TBI groups could be related to an intrinsic immune dysfunction in these two groups. Mean values for IL-4 and IL-10 protein concentration were less than 15 pg/mL in all three groups in response to donor, host, or third-party stimulators, except for an IL-10 value of 27 pg/mL obtained from day-5 MLR cultures containing splenocytes from TBI-treated recipients and third-party stimulators. The modest differences in IL-2 and IFNγ cytokine levels cannot be readily explained by the splenic T-cell content in these cultures, because control and IUT NTCD recipients had comparable percentages of CD4+ and CD8+ T cells that were both higher than in the splenocytes from the TBI-treated group. Because the mean CD44 antigen density on CD4+ and especially CD8+ T cells was higher in splenocytes obtained from the TBI-treated group as compared with non-BMT controls or IUT recipients, it is possible that the modest differences in proliferative and cytokine responses observed using splenocytes obtained from TBI or IUT recipients could be due to a higher proportion of T cells with a memory cell phenotype in the latter group.
IL-2 and IFNγ protein concentrations in supernatants obtained from bulk MLR cultures. Splenocytes obtained from recipients analyzed in Fig 5 also were studied for the production of IL-2 or IFNγ in bulk MLR cultures. Supernatants obtained at the indicated time periods were analyzed by ELISA for the concentration of IL-2 (A and C) or IFNγ protein (B and D) released in response to host (A and B) or third-party (C and D) alloantigen-bearing stimulator cells. On the x-axis is the days of MLR culture. On the y-axis is the protein concentration in picograms per milliliter. *Significant differences between the indicated groups and nonmanipulated B6 controls.
IL-2 and IFNγ protein concentrations in supernatants obtained from bulk MLR cultures. Splenocytes obtained from recipients analyzed in Fig 5 also were studied for the production of IL-2 or IFNγ in bulk MLR cultures. Supernatants obtained at the indicated time periods were analyzed by ELISA for the concentration of IL-2 (A and C) or IFNγ protein (B and D) released in response to host (A and B) or third-party (C and D) alloantigen-bearing stimulator cells. On the x-axis is the days of MLR culture. On the y-axis is the protein concentration in picograms per milliliter. *Significant differences between the indicated groups and nonmanipulated B6 controls.
The high level of engraftment observed after IUT with either TCD or NTCD is associated with low anti-host alloresponses in vivo and in vitro. IUT recipients of TCD BM appear to be at a lower risk for GVHD than comparably engrafted adult SCID recipients of NTCD BM, whereas IUT recipients of NTCD BM have a relatively low but not absent risk of GVHD.
DISCUSSION
We have provided the first extensive comparative analysis of the alloengraftment and GVHD side effects of adult B6 NTCD or TCD BM infused in utero or postnatally into BALB/c-SCID recipients. In this study, we have shown that IUT can lead to high levels of PB, thymic, and splenic lymphoid alloengraftment in SCID recipients, with minimal histological evidence of GVHD. The presence of low numbers of BM-derived donor T cells facilitated alloengraftment in IUT recipients without significantly increasing GVHD-induced tissue destruction. At comparable levels of PB lymphoid alloengraftment, nonconditioned postnatal SCID recipients had more GVH-induced tissue destruction than IUT recipients. TBI conditioning of nonimmune deficient postnatal recipients that provided the highest level of alloengraftment damaged the thymus and compromised the generation of naive T cells that repopulate the lymph node and spleen of recipients. IUT reconstituted the thymus and spleen between 3 and 6 weeks of age. Thus, IUT provides a rapid means of achieving lymphoid reconstitution at an early age of life and suggests that SCID recipients treated by IUT have certain advantages over adult SCID recipients treated by postnatal BMT.
It is possible that the high levels of PB alloengraftment seen in IUT recipients are due to the relatively large BM dose infused. The infusion of higher numbers of BM cells (≥25 × 106) was required in nonconditioned SCID recipients to prevent the development of low-level (1% to 10% PB of donor origin) chimeras that would be an insufficient immune reconstitution setting in human SCID recipients to provide immune surveillance against infectious agents. IUT recipients had a smaller incidence of low level chimeras than adult nonconditioned SCID recipients, regardless of whether these mice received NTCD or TCD BM. Additional studies indicated that none of 31 (0%) IUT recipients of NTCD BM at a dose of 106 cells failed to engraft or was a low-level chimera. At a dose of 106 and a weight of 1 to 2 g (day-15 fetus), the estimated NTCD BM dose is 0.5 to 1 × 109/kg. For adult nonconditioned SCID recipients of 5 × 106 NTCD BM cells, which did not preclude graft rejection, the estimated NTCD BM cell dose is 0.2 × 109/kg; for recipients of 25 × 106, which did preclude graft rejection, the estimated NTCD BM dose is 1 × 109/kg. Because very young SCID mice would have lower NK activity, which does not fully develop until 4 to 5 weeks of age, it is possible that engraftment rates would be improved if very young SCID mice were used. Although the high BM cell dose alone could be responsible for the high level of alloengraftment observed in IUT recipients, as discussed below, nonconditioned adult SCID recipients of comparable NTCD BM cell doses have more GVHD-induced tissue destruction than IUT recipients.
Alloengraftment of SCID recipients via IUT was not entirely due to a selective expansion of mature lymphoid cells or their progenitors but rather involved the engraftment of multilineage progenitor and stem cell populations. In a different type of severe combined immune deficiency (Janus-3 kinase [Jak3K]), small numbers of Jak3K-expressing lymphoid cells will repopulate the recipient’s lymphoid system, whereas there is no competitive advantage of donor myeloid cells in Jak3K deficient mice.23 Because myeloid cells have a short half-life and SCID mice do not have a known defect in myeloid progenitor cells, evaluation of the myeloid compartment provides a better indicator of the requirement of donor T cells in facilitating donor stem cell alloengraftment. Our data show that myeloid engraftment, a reflection of multilineage progenitor and stem cell engraftment, is achievable in IUT or postnatal SCID recipients of sufficient numbers of BM cells. A clear effect of the inclusion of donor BM-derived T cells was observed in PB myeloid engraftment in both the IUT and nonconditioned adult SCID recipients. Adult SCID recipients of high numbers of BM cells (25 × 106) had a greater degree of BM myeloid engraftment than IUT recipients, although IUT recipients of NTCD BM had higher myeloid engraftment and less GVHD than adult SCID recipients of TCD BM.
The inclusion of donor BM-derived T cells for transfer into murine IUT recipients facilitated alloengraftment without increasing GVHD risk. The role of donor T cells in facilitating alloengraftment also has been examined in fetal sheep (reviewed in Zanjani et al24). The transfer of T-cell–containing cord blood cells, newborn BM, or adult BM permitted a higher degree of alloengraftment. However, GVHD was increased in each instance. In baboons, the infusion of T-replete BM was associated with GVHD. The T-cell content of the progenitor cells infused into sheep or baboons would have exceeded the proportion of T cells present in murine BM (2% to 4%). In the sheep model, TCD BM avoided GVHD but led to diminished alloengraftment. In a recent report of a human SCID recipient successfully treated by IUT, the repetitive infusion of rigorously TCD, stem cell-enriched haploidentical BM permitted high levels of T cells with low levels of myeloid engraftment and no GVHD.1 Murine IUT with BM may represent a situation in which there are sufficient BM-derived T cells to promote alloengraftment but insufficient numbers to cause significant GVHD. In that regard, Archer et al25 showed that 44% to 66% of NOD/SCID mice, deficient in NK and macrophage activity, had a mean of 30% donor PB at 4 weeks of age after intraperitoneal injection on day 13.5 with purified lineage-depleted BM (0.8 × 106cells). Although direct comparisons cannot be made between our study and that of Archer et al25 due to potential differences in day of injection and technical aspects of the injection process, lineage-depleted BM would contain fewer T cells than TCD BM. Because we have found that NTCD BM engrafts better than TCD BM in IUT recipients, the higher PB alloengraftment reported in our series may be due to the presence of higher proportions of donor BM-derived T cells infused in our experiments.
The high engraftment and low GVHD of the murine IUT studies reported here may be due to the fact that the microenvironment of the murine fetus may be more conducive to the alloengraftment of mouse BM than the generation of GVHD. Higher doses of NTCD or TCD BM administered to nonconditioned SCID recipients that resulted in comparable alloengraftment were associated with more GVHD-induced tissue destruction. Consistent with the greater histological evidence of GVHD, both thymic and splenic cellularity were reduced in adult SCID recipients of these high BM cell doses, presumably due to GVHD-induced involution. The use of TBI to condition postnatal recipients provided the highest level of PB and BM myeloid alloengraftment. At the TBI dose administered, TCD BM readily engrafted. TBI damaged the central (thymus) and peripheral (spleen, lymph node) lymphoid compartments. The generation of naive T cells was compromised. Significant morbidity and mortality was observed in TBI-treated recipients. Thus, TBI conditioning, which offers the highest likelihood of successful alloengraftment, is associated with substantial side effects and leads to the impaired naive T-cell repopulation of the lymph node and splenic compartments.
An important aspect of the present studies was the demonstration that IUT SCID recipients had rapid recovery of the thymus and spleen by 3 to 6 weeks of age; IUT provides an advantage of beginning the alloengraftment process before birth. The extent to which the pregnancy and fetal risks associated with IUT outweigh waiting to perform BMT in newborn SCID recipients is unknown and will require further investigation. However, it is noteworthy that the human SCID recipient treated with TCD haploidentical BM had normal numbers of CD8+ T cells and B cells at birth.1CD4+ T cells, which were low at birth, progressively increased in number, reaching normal numbers at 5 months of age. T cells were of donor origin and B cells of host origin, an important distinction to the results obtained in murine SCID recipients treated by IUT. It is not known whether the high levels of donor B-cell engraftment seen in IUT-treated murine SCID recipients is due to an earlier defect in B-cell development than in the human IL-2Rγc–deficient SCID recipient and/or an overall higher alloengraftment seen in murine as compared with human recipients. Because myeloid cells in the human SCID recipient were of host origin and, in the mouse, a mean of 20% to 40% of myeloid cells were of donor origin in the PB of murine SCID recipients, at least part of the explanation would appear to include a more ready engraftment of the mouse than the human fetus. Despite the high engraftment results reported in murine SCID recipients, it is worth emphasizing that the engraftment of non-SCID, non-stem cell defective IUT recipients with allogeneic BM has been problematic in murine series reported to date. In our previous studies with transplacental injections, alloengraftment was transient in this setting.26 Carrier et al27 reported alloengraftment in 50% of day 12 to 13 gestational recipients injected ip with day 11 to 13 fetal liver cells, although the levels ranged from 0.0001% to 0.6%. IUT treatment of nonimmunodeficient sheep typically provides alloengraftment levels of approximately 15% donor cells, although repetitive infusions can increase mean levels to 30%.24 A challenge in the field will be to identify strategies that are sufficient to routinely repopulate a fetal microenvironment in which there is not a competitive advantage to a particular lineage of progenitor cell, as is the case for murine SCID recipients.
In our murine studies as well as the human SCID patient,1donor T cells have a markedly blunted response to host alloantigens in MLR culture while retaining third-party responses. The adoptive transfer of splenocytes from murine IUT recipients into host-strain SCID recipients was not able to uncover an alloresponse in recipients of TCD BM, although some recipients of NTCD did experience GVHD. Although we have previously observed that IUT NTCD reconstituted SCID mice have circulating levels of IgM at least as high as control B6 mice,20 additional studies will be required to determine the fine specificity of T-cell and B-cell responses to antigenic challenge and to determine whether there are unique mechanism(s) responsible for tolerance induction after IUT versus postnatal BMT.
In summary, we have shown that, at comparable levels of T-cell, B-cell, and myeloid PB alloengraftment, IUT recipients had less GVHD tissue destruction than nonconditioned adult SCID recipients. TCD of donor BM did not preclude alloengraftment of IUT recipients. TBI, which resulted in the highest alloengraftment when used to condition non-SCID recipients, impaired thymic T-cell production and the generation of naive T cells. IUT recipients of TCD or NTCD BM were tolerant to host alloantigen-bearing cells as assessed in vitro and in vivo. Because SCID recipients treated by IUT had rapid reconstitution of the thymic and splenic compartments, our data would indicate that IUT treatment of fetal SCID recipients has a number of potential advantages as compared with BMT of nonconditioned adult SCID recipients. Because the major limitation for the more widespread use of IUT is alloengraftment, approaches to improve alloengraftment can be readily tested in SCID recipients that are permissive for lymphoid but less permissive for myeloid and stem cell engraftment.
Supported in part by National Institutes of Health Grants No. R01-HL49997 and R01-HL52952.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Bruce Blazar, Box 109 Mayo Bldg, University of Minnesota Hospital, Minneapolis, MN 55455.

![Fig. 1. Comparative PB engraftment analysis in IUT and postnatal BMT recipients. BALB/c-SCID recipients were treated via IUT or postnatal BMT, with the indicated B6 BM cell dose shown in parentheses. Recipients received TCD or NTCD BM as indicated. Additional control groups consisted of BALB/c-SCID recipients of BALB/c BM (congenic [cong.]) and BALB/c recipients were treated with TBI and received B6 TCD BM. Where indicated, controls are non-BMT B6 mice. The mean proportion of PB-expressing H2b (A), CD4 (C), CD8 (D), and B220 (B cells) (E) antigens are listed on the y-axis. The mean percentage of donor myeloid engraftment (B) was calculated by dividing the percentage of H2b+ Mac1+ cell population by the total Mac1+ cells. A composite graph (F) of the relative mean proportion of donor CD4+, CD8+, B220+, Mac1+, and host Mac1+ cells in individual mice in each group is shown. The number of recipients studied (no.) in each group is listed at the bottom of (F). Symbols for (F): (▪) CD4; (▨) CD8; (□) B220; (▧) H2b Mac1; (▥) H2d Mac1. One standard error of the mean values ranged 0% to 4% for all values shown, except for a value of 9% for H2b expression in SCID TCD25 recipients. *Significant differences between IUT TCD recipients and other groups. For the assessment of the proportion of PB expressing donor lymphoid markers, the (#) indicates significant differences between the indicated groups and the non-BMT B6 control mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3949/4/m_blod42226001ax.jpeg?Expires=1769219475&Signature=mQMMcS4dwOf-62lRjriVIq7l6y1iFTZrwPeXM7l32F1tyVfFAmo8FR09P7mKNf9ncGVufFNaxD6iVnEck-9VgwBxeckUUsTrTktGQem8zfdO9ABqHUFy-eNLJRGkUOnw0oP8QRofb25Kxo0Hi8OgVGMUTKS2UB4FhHOHS48gu67-DcKHkPz~IltyC~W0R4bPGZ3rF3xdFSrB3XOffCK5A7V~g~o9sR2gFYA~q8edhAHGH7W-T1jh9jzS2LPED0GcRQMBRS585fjii0-gtsy8AnYHbK5mazNK7~woqCAcxrbP4NcyuzSPS~rTIFfAv2xt5Vyi7XtzxR35ijYzN-zL3g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Comparative PB engraftment analysis in IUT and postnatal BMT recipients. BALB/c-SCID recipients were treated via IUT or postnatal BMT, with the indicated B6 BM cell dose shown in parentheses. Recipients received TCD or NTCD BM as indicated. Additional control groups consisted of BALB/c-SCID recipients of BALB/c BM (congenic [cong.]) and BALB/c recipients were treated with TBI and received B6 TCD BM. Where indicated, controls are non-BMT B6 mice. The mean proportion of PB-expressing H2b (A), CD4 (C), CD8 (D), and B220 (B cells) (E) antigens are listed on the y-axis. The mean percentage of donor myeloid engraftment (B) was calculated by dividing the percentage of H2b+ Mac1+ cell population by the total Mac1+ cells. A composite graph (F) of the relative mean proportion of donor CD4+, CD8+, B220+, Mac1+, and host Mac1+ cells in individual mice in each group is shown. The number of recipients studied (no.) in each group is listed at the bottom of (F). Symbols for (F): (▪) CD4; (▨) CD8; (□) B220; (▧) H2b Mac1; (▥) H2d Mac1. One standard error of the mean values ranged 0% to 4% for all values shown, except for a value of 9% for H2b expression in SCID TCD25 recipients. *Significant differences between IUT TCD recipients and other groups. For the assessment of the proportion of PB expressing donor lymphoid markers, the (#) indicates significant differences between the indicated groups and the non-BMT B6 control mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3949/4/m_blod42226001bx.jpeg?Expires=1769219475&Signature=sK5ZBTNlKOAtKJwWlYYAkGNk0ByeIYir6KQKvK01cpJTQ4KI~JxuAS5LYN9VFeqR0GEfgUR3z9ZHhLTpDS~QQPJPniQ~TBZdAozkrW4qydBjAwS957Vq1huBQ3thubAdAjioAou3~TxIY-B02Z~ghFlYaDncXWNXGTF4M3S~kAncaLBMNojRbe1p1Pkb5CvsxJrbYsgBNQtHKQlVZedTWBanrRs~fDKmDFNIia96ShxbDkRRzIMYc5YkvOw~~YKrFBvzhr2tX7jfm0gs9uYM9DEFpzLI9xdO91gy011q9O-hmBA58SgZqomnvAXtWHB2ifKaf5nzTPGk2t7dUh5kyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Comparative PB engraftment analysis in IUT and postnatal BMT recipients. BALB/c-SCID recipients were treated via IUT or postnatal BMT, with the indicated B6 BM cell dose shown in parentheses. Recipients received TCD or NTCD BM as indicated. Additional control groups consisted of BALB/c-SCID recipients of BALB/c BM (congenic [cong.]) and BALB/c recipients were treated with TBI and received B6 TCD BM. Where indicated, controls are non-BMT B6 mice. The mean proportion of PB-expressing H2b (A), CD4 (C), CD8 (D), and B220 (B cells) (E) antigens are listed on the y-axis. The mean percentage of donor myeloid engraftment (B) was calculated by dividing the percentage of H2b+ Mac1+ cell population by the total Mac1+ cells. A composite graph (F) of the relative mean proportion of donor CD4+, CD8+, B220+, Mac1+, and host Mac1+ cells in individual mice in each group is shown. The number of recipients studied (no.) in each group is listed at the bottom of (F). Symbols for (F): (▪) CD4; (▨) CD8; (□) B220; (▧) H2b Mac1; (▥) H2d Mac1. One standard error of the mean values ranged 0% to 4% for all values shown, except for a value of 9% for H2b expression in SCID TCD25 recipients. *Significant differences between IUT TCD recipients and other groups. For the assessment of the proportion of PB expressing donor lymphoid markers, the (#) indicates significant differences between the indicated groups and the non-BMT B6 control mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3949/4/m_blod42226001cx.jpeg?Expires=1769219475&Signature=3ZWZHFRRu7A0i-Fl4j1qEBv8K3zp-5q8nogdI5YrIQFtkfkSQtwyNN6gkk2iMgf6Hy07x9sNKaMjMYk-MtNYNAyOAjxu~jjO6JHQ0jfyNecKjZreGagvXiBzKpiHjA5ApUTVPN4upnaWzs-Z-gxviaBmHiC-jleJYPo0-G9x8wjNOzQnMpq42L96moQAfCszNwHO7Qa1ajpvGBD6~E~pXxvGXzilAbKD4M6OuHD~ZE-RPISiGIUNh3baHxFj6ZFJRWibAzAP3CEoiRK5nN~SsFMnU9UviOPHyqVM8Nl-tGRukjT0PQqoI1OB8wYEWUxBQW~zRJguGKICWZs7c6HMlg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Comparative PB engraftment analysis in IUT and postnatal BMT recipients. BALB/c-SCID recipients were treated via IUT or postnatal BMT, with the indicated B6 BM cell dose shown in parentheses. Recipients received TCD or NTCD BM as indicated. Additional control groups consisted of BALB/c-SCID recipients of BALB/c BM (congenic [cong.]) and BALB/c recipients were treated with TBI and received B6 TCD BM. Where indicated, controls are non-BMT B6 mice. The mean proportion of PB-expressing H2b (A), CD4 (C), CD8 (D), and B220 (B cells) (E) antigens are listed on the y-axis. The mean percentage of donor myeloid engraftment (B) was calculated by dividing the percentage of H2b+ Mac1+ cell population by the total Mac1+ cells. A composite graph (F) of the relative mean proportion of donor CD4+, CD8+, B220+, Mac1+, and host Mac1+ cells in individual mice in each group is shown. The number of recipients studied (no.) in each group is listed at the bottom of (F). Symbols for (F): (▪) CD4; (▨) CD8; (□) B220; (▧) H2b Mac1; (▥) H2d Mac1. One standard error of the mean values ranged 0% to 4% for all values shown, except for a value of 9% for H2b expression in SCID TCD25 recipients. *Significant differences between IUT TCD recipients and other groups. For the assessment of the proportion of PB expressing donor lymphoid markers, the (#) indicates significant differences between the indicated groups and the non-BMT B6 control mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3949/4/m_blod42226001dx.jpeg?Expires=1769219475&Signature=NkCTxwCBHSLB~dLdbTyvGax~frWXuXwkMc8crHKbcCWR0QAXkfwfy5romIZq6YRDyt4qht3meyR-KS2MHQhLEGhHsQRwTlgNihJZ92v5SPxlf0y~k-gashzy7CpYmSzftcbjV0K1Jgn4HN~yX5Z3AYOZ8sUe3hvzf~kU1atUPfBFjvApbdPm81i3oHkhAedb85yc8txKbOqL8TFiq6utlyykSQKRwpenhcXY1bS5saAugq4~QAawJxYxM5y2yaMxC-9PX2TmcuxgVmhdt2U~vIT3vfmbDRY5U9fB57~WeU4Lk747eJt4O17wQGiqhBcvLtvqBxP~MmyNTHk7OCA6Lg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Comparative PB engraftment analysis in IUT and postnatal BMT recipients. BALB/c-SCID recipients were treated via IUT or postnatal BMT, with the indicated B6 BM cell dose shown in parentheses. Recipients received TCD or NTCD BM as indicated. Additional control groups consisted of BALB/c-SCID recipients of BALB/c BM (congenic [cong.]) and BALB/c recipients were treated with TBI and received B6 TCD BM. Where indicated, controls are non-BMT B6 mice. The mean proportion of PB-expressing H2b (A), CD4 (C), CD8 (D), and B220 (B cells) (E) antigens are listed on the y-axis. The mean percentage of donor myeloid engraftment (B) was calculated by dividing the percentage of H2b+ Mac1+ cell population by the total Mac1+ cells. A composite graph (F) of the relative mean proportion of donor CD4+, CD8+, B220+, Mac1+, and host Mac1+ cells in individual mice in each group is shown. The number of recipients studied (no.) in each group is listed at the bottom of (F). Symbols for (F): (▪) CD4; (▨) CD8; (□) B220; (▧) H2b Mac1; (▥) H2d Mac1. One standard error of the mean values ranged 0% to 4% for all values shown, except for a value of 9% for H2b expression in SCID TCD25 recipients. *Significant differences between IUT TCD recipients and other groups. For the assessment of the proportion of PB expressing donor lymphoid markers, the (#) indicates significant differences between the indicated groups and the non-BMT B6 control mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3949/4/m_blod42226001ex.jpeg?Expires=1769219475&Signature=vDX1YmgaSlvq5whf5EmT9EmfsymBfuafFOaWQNWQtUUO7xyeleK0y0fAZcSp2A~p6cYnKG8oVLeon8EOHOtX4e~NRUD~cZmUQyVRGbYx~f2ftXWvil986eY41Oxo4Xiiv7XEzeP1xpxVzKJVndHFr7db94hA-z9CXmp2x3~8g313YtVRD8FCjTt5qCMIWMDXRWQxzEniYmJncMNLgPFtk6aTQc91J9ouHpyGRH8~D9dZPZCkC5DgA8-P2TtY6K3QU0H23gmfvdd2ZAYoAP8W7467plIYwejQXY~sKFxVcdb5uh3CtutFTou4YyZxJCg5Bv-BPiSDvH4d6K~PRnIyaw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Comparative PB engraftment analysis in IUT and postnatal BMT recipients. BALB/c-SCID recipients were treated via IUT or postnatal BMT, with the indicated B6 BM cell dose shown in parentheses. Recipients received TCD or NTCD BM as indicated. Additional control groups consisted of BALB/c-SCID recipients of BALB/c BM (congenic [cong.]) and BALB/c recipients were treated with TBI and received B6 TCD BM. Where indicated, controls are non-BMT B6 mice. The mean proportion of PB-expressing H2b (A), CD4 (C), CD8 (D), and B220 (B cells) (E) antigens are listed on the y-axis. The mean percentage of donor myeloid engraftment (B) was calculated by dividing the percentage of H2b+ Mac1+ cell population by the total Mac1+ cells. A composite graph (F) of the relative mean proportion of donor CD4+, CD8+, B220+, Mac1+, and host Mac1+ cells in individual mice in each group is shown. The number of recipients studied (no.) in each group is listed at the bottom of (F). Symbols for (F): (▪) CD4; (▨) CD8; (□) B220; (▧) H2b Mac1; (▥) H2d Mac1. One standard error of the mean values ranged 0% to 4% for all values shown, except for a value of 9% for H2b expression in SCID TCD25 recipients. *Significant differences between IUT TCD recipients and other groups. For the assessment of the proportion of PB expressing donor lymphoid markers, the (#) indicates significant differences between the indicated groups and the non-BMT B6 control mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/10/10.1182_blood.v92.10.3949/4/m_blod42226001fx.jpeg?Expires=1769219475&Signature=WkcO97xvQvELwoK-7gc0l06oUlx1ejJh7cBe7jXi-4w3Yqwh2UCj2EeBozhcU7zxZI9lPYX4dxSfuSpkscB-8LtJf25fmBpS26DpsLjwbHTX0GpH~A0cuw9LT0hpikOnzgphpVbDYCAc6PncHTniSWR8uygCfBEjRiWDpPmP7RCAaF9Dick7PLFjDLLUjJKQx4MMJ~5d14qj7y1rhva-48NQWtNa0Hc6122H~L7PaksdWFd08BDSy5r2xMAwRRA5xwXmORADEsUk6hb2FkVuEtMrW5Y5Zbd3gOj87XqyU~AlqDzHaqqobgUqxJVTIZDQy6dY7fdHNwH5a2-wSgi0Mg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal