Abstract
Keratinocyte growth factor (KGF) is important in tissue repair and wound healing and its administration can abrogate chemical- and radiation-induced tissue damage in rodents. We investigated KGF as a therapeutic agent for the prevention of graft-versus-host disease (GVHD)-induced tissue damage, morbidity, and mortality in an established murine allogeneic bone marrow transplantation (BMT) model. B10.BR (H2k) recipient mice were lethally irradiated and transplanted with C57BL/6 (H2b) bone marrow (BM) with spleen cells (BMS) as a source of GVHD-causing T cells. KGF-treated mice (5 mg/kg/d subcutaneously days −6, −5, and −4 pre-BMT) receiving BMS exhibited better survival than those not receiving KGF (P = .0027). Cyclophosphamide (Cy), a common component of total body irradiation (TBI)-containing regimens, was administered to other cohorts of mice at a dose of 120 mg/kg/d intraperitoneally on days −3 and −2 before BMT. KGF-treated mice again exhibited a better survival rate than those not receiving KGF (P = .00086). However, KGF-treated recipients receiving TBI or Cy/TBI BMS were not GVHD-free, as shown by lower body weights compared with BM groups. GVHD target tissues were assessed histologically during a 38-day post-BMT observation period. KGF ameliorated GVHD-induced tissue damage in the liver, skin, and lung (completely in some recipients) and moderately so in the spleen, colon, and ileum, even with Cy conditioning. These studies demonstrate that KGF administration, completed before conditioning, has potential as an anti-GVHD therapeutic agent.
GRAFT-VERSUS-HOST disease (GVHD) is caused by a donor antihost alloreactivity that involves both donor and host immune responses and is a major cause of morbidity and mortality post-bone marrow transplantation (BMT). To prevent GVHD, most therapies rely on the elimination of donor T cells from the graft or immunosuppressing the host. Unfortunately, these approaches can result in poor engraftment, a loss of graft-versus-leukemia (GVL) activity, and/or higher risk of leukemic relapse. Measures to hinder tissue damage and the subsequent adhesion, extravasation, and recruitment of the cells responsible for the initial inflammatory insult in the early post-BMT period may provide alternative strategies.
Humans and rodents that receive intense conditioning regimens develop manifestations of an acute-phase reaction, including cachexia, weight loss, and fever early post-BMT. The acute injury to endothelial and epithelial cells causes the expulsion of intracellular contents, acute-phase reactants, and cytokines (eg, interleukin-1 [IL-1], IL-6, and tumor necrosis factor α [TNFα]) that initiate the inflammatory response necessary for tissue repair. Not only do these cytokines induce the expression of endothelial surface proteins (eg, P-selectin, E-selectin, and intercellular adhesion molecule-1 [ICAM-1]) conducive to the tethering, adhesion, and transmigration of inflammatory cells, but the damage caused by the acute injury is also conducive to the prolonged exposure of alloreactive and/or host cells to normally sequestered antigens. Contributing to this injury cascade is endotoxin released from the intestinal bacterial flora now accessible to the circulation through the damaged gut epithelium. With endothelial injury to the liver, clearance of toxins is compromised, further amplifying manifestations of GVH disease. In the response of the lung to toxic injury, type I epithelial pneumocytes are most sensitive to death from injury and type II cells proliferate to replace them in the repair process. If the extent of damage to type II cells exceeds the ability for re-epithelialization, the deposition of extracellular matrix (collagen) and fibrosis ensues leading to respiratory insufficiency and idiopathic pneumonia. These failing target organs thus become contributory factors culminating in GVHD-induced morbidity and mortality.
Keratinocyte growth factor (KGF), also called fibroblast growth factor 7 (FGF-7), is a mediator of epithelial cell proliferation1and a growth factor for hepatocytes2 and type II pneumocytes.3,4 KGF has been shown to be protective in lethal models of radiation- and bleomycin-induced lung injury in rats,5 possibly by facilitating repair of DNA damage in alveolar epithelial cells.6 Other studies have shown that KGF is protective against cyclophosphamide (Cy)-induced ulcerative hemorrhagic cystitis of the bladder in rats7 and also against chemotherapy- and radiation-induced gastrointestinal injury (mucositis) and mortality in mice8 (possibly by increasing intestinal stem cell survival9). Particularly noteworthy is a recent study that demonstrated that KGF did not cause significant proliferation of various human squamous cell tumor lines, including those that were KGF receptor positive.10 Therefore, by preventing chemotherapy- and radiation-induced side effects such as mucositis and lung injury, KGF has the potential for reducing GVHD complications and increasing the therapeutic index of chemoradiotherapy of cancer without stimulating the cancer cells. Few studies have focused on the prevention of the damage to host tissues caused by the conditioning chemoradiation regimen administered pre-BMT. The current study was performed to determine whether exogenous in vivo administration of KGF, completed before conditioning, could prevent, ameliorate, or delay GVHD after allogeneic BMT in mice.
MATERIALS AND METHODS
Mice.
B10.BR (H2k) and C57BL/6 (H2b) mice were purchased from The Jackson Laboratories (Bar Harbor, ME). Mice were housed in microisolator cages in the SPF facility of the University of Minnesota and cared for according to the Research Animal Resources guidelines of our institution. For BMT, donors were 8 to 12 weeks of age and recipients were used at 8 to 10 weeks of age.
KGF production.
Pre-BMT conditioning.
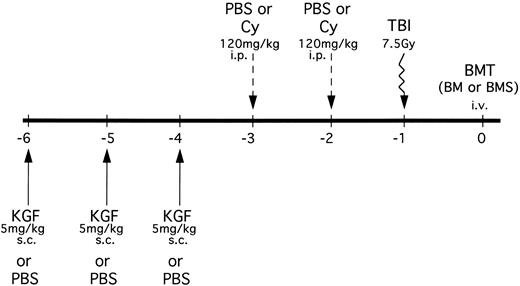
B10.BR mice received PBS or KGF (5 mg/kg/d subcutaneously [sc]) on days −6, −5 and −4 pre-BMT (Fig 1). Mice were then segregated into those receiving either phosphate-buffered saline (PBS) or Cy (Cytoxan; Bristol Myers Squibb, Seattle, WA) at 120 mg/kg/d as a conditioning regimen pre-BMT on days −3 and −2. All mice were lethally irradiated on the day before BMT (7.5 Gy total body irradiation [TBI]) by x-ray at a dose rate of 0.41 cGy/min, as described.12 In an alternative treatment schedule, some mice also received KGF on days 1, 2, and 3 post-BMT.
KGF treatment and conditioning schedule for BMT experiments. KGF (5 mg/kg/d) or PBS was administered sc to B10.BR recipients on days −6, −5, and −4. Mice were then further segregated into those receiving Cy (120 mg/kg/d) or PBS intraperitoneally on days −3 and −2. All recipients were irradiated (7.5 Gy) on the day before transplant (day −1). Groups were segregated into those receiving C57BL/6 BM alone or BM with allogeneic spleen cells (BMS) intravenously. In some mice, KGF treatment was also administered on days 1, 2, and 3 posttransplant in the context of Cy/TBI conditioning.
KGF treatment and conditioning schedule for BMT experiments. KGF (5 mg/kg/d) or PBS was administered sc to B10.BR recipients on days −6, −5, and −4. Mice were then further segregated into those receiving Cy (120 mg/kg/d) or PBS intraperitoneally on days −3 and −2. All recipients were irradiated (7.5 Gy) on the day before transplant (day −1). Groups were segregated into those receiving C57BL/6 BM alone or BM with allogeneic spleen cells (BMS) intravenously. In some mice, KGF treatment was also administered on days 1, 2, and 3 posttransplant in the context of Cy/TBI conditioning.
BMT.
Our BMT protocol has been described previously.13 Briefly, donor C57BL/6 BM was T-cell depleted (TCD) with anti-Thy 1.2 monoclonal antibody (clone 30-H-12, rat IgG2b; kindly provided by Dr David Sachs, Cambridge, MA) + complement (Nieffenegger Co, Woodland, CA). Recipient mice were transplanted via caudal vein with 20 × 106 TCD C57BL/6 (H-2b) marrow with or without 15 × 106 NK cell depleted (PK136, anti-NK1.1 + complement) spleen cells (BMS) as a source of GVHD-causing T cells. All mice were monitored for survival and clinical evidence of GVHD (ie, weight loss, ruffled fur, cachexia, alopecia, and diarrhea). In other experiments, cohorts of mice were killed on a periodic basis for histologic evaluation of GVHD target tissues (3 mice/group/time point; see below).
Frozen tissue preparation.
After anesthesia with sodium pentobarbitol, mice were killed by cervical dislocation and the GVHD target organs were removed by dissection. Spleen, liver, colon, small intestine, cecum, skin, and lungs were arranged in aluminum foil cups, snap-frozen in liquid nitrogen, and stored at −80°C. The lungs were first infused via the trachea with a mixture of 0.5 mL Optimal Cutting Temperature medium (OCT; Miles Inc, Elkhart, IN):PBS (3:1) before freezing.
Histological assessment.
Frozen sections were cut 4-μm thick, mounted onto glass slides, fixed for 5 minutes in acetone, and stained with hematoxylin and eosin (H&E). Tissues were scored on a scale of 0 to 4+ for GVHD based on standard GVHD criteria.13-19
Statistical analysis.
Kaplan-Meier plots of survival data were analyzed by Mantel-Peto-Cox summary of χ2.20 Other data were analyzed using the Student’s t-test. Probability (P) values less than or equal to .05 were considered statistically significant.P values greater than .05 and less than .1 were considered a statistical trend.
RESULTS
KGF ameliorates GVHD-induced lethality and weight loss after allogeneic BMT.
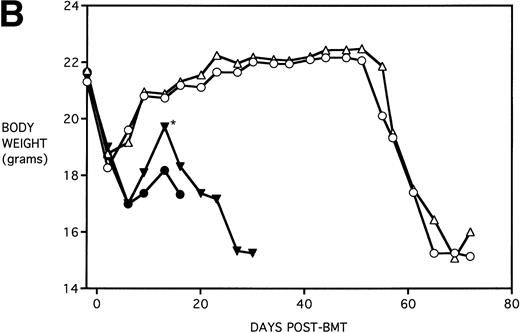
To determine whether KGF administration would benefit BMT recipients under sublethal GVHD conditions, experiments were begun using a nonuniformally lethal dose of allogeneic spleen cells (ie, not all recipients die). Figure 2A depicts the survival curves of PBS- or KGF-pretreated (days −6, −5 and −4 pre-BMT), lethally irradiated adult B10.BR (H2k) mice infused with C57BL/6 bone marrow cells alone (BM groups) or with 5 × 106 spleen cells as a source of GVHD-causing T cells (BMS groups), which corresponded to a 50% lethal dose in this experiment. BMS recipients receiving KGF exhibited a statistical trend toward improved survival (P = .070). Nonetheless, Fig 2B depicts the very significant difference in body weights between BMS groups receiving KGF or PBS. Beginning on day 19 post-BMT, the weights of BMS mice receiving KGF diverged from those not receiving KGF to a significant degree (P = .009 on day 19). Furthermore, these recipients gained weight post-BMT at a rate equal to those mice receiving BM without spleen cells.
Amelioration of mortality (A) and weight loss (B) by KGF following allogeneic BMT with a nonuniformly lethal dose of allogeneic spleen cells. B10.BR recipient mice (n = 8/group) were lethally irradiated (7.5 Gy) on day −1 and infused on day 0 with C57BL/6 BM either without (BM) or with 5 × 106 spleen cells (BMS) as indicated. For (A), (□) BM ± KGF, (▪) BMS + PBS, and (▴) BMS + KGF. For (B), (□) BM + PBS, (▵) BM + KGF, (▪) BMS + PBS, and (▴) BMS + KGF. *.0003 < P < .01 from this point.
Amelioration of mortality (A) and weight loss (B) by KGF following allogeneic BMT with a nonuniformly lethal dose of allogeneic spleen cells. B10.BR recipient mice (n = 8/group) were lethally irradiated (7.5 Gy) on day −1 and infused on day 0 with C57BL/6 BM either without (BM) or with 5 × 106 spleen cells (BMS) as indicated. For (A), (□) BM ± KGF, (▪) BMS + PBS, and (▴) BMS + KGF. For (B), (□) BM + PBS, (▵) BM + KGF, (▪) BMS + PBS, and (▴) BMS + KGF. *.0003 < P < .01 from this point.
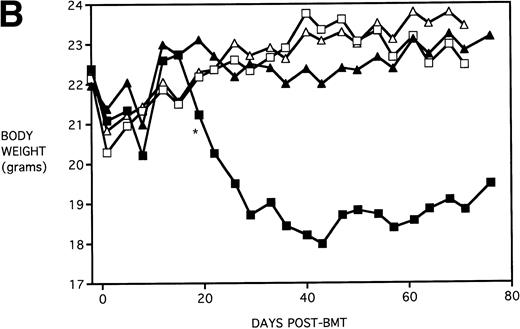
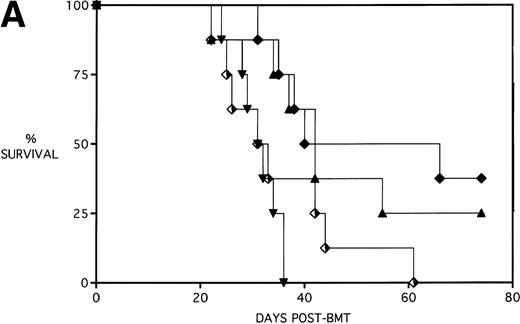
Next, KGF was studied under a more aggressive, 100% lethal condition induced by administration of 15 × 106 spleen cells. Figure 3A depicts the survival curves of lethally irradiated adult B10.BR mice infused with C57BL/6 BM or BMS. As indicated, these groups were further segregated into those receiving pretreatments of PBS or KGF. Mice receiving KGF (BMS+KGF) exhibited a higher actuarial survival rate that was statistically significant (P = .0027) compared with the GVHD control group (BMS+PBS). Figure 3B shows the post-BMT body weights. Although KGF had no significant effect on the weights of the recipients for the first 4 weeks post-BMT, it did prevent the subsequent weight loss in surviving mice. However, these mice did not regain their pre-BMT body weights, implying that they were not GVHD-free. In the absence of allogeneic T cells, Figs 2 and 3 (open symbols) demonstrate that KGF is not deleterious to survival or weight gain post-BMT.
Amelioration of mortality (A) and weight loss (B) by KGF after allogeneic BMT with a uniformly 100% lethal dose of allogeneic spleen cells. B10.BR recipient mice (n = 26/group) were lethally irradiated (7.5 Gy) on day −1 and infused on day 0 with C57BL/6 BM either without (BM) or with 15 × 106 spleen cells (BMS) as indicated. Groups were further segregated into those receiving PBS or KGF (5 mg/kg/d) on days −6, −5, and −4 pre-BMT. Data compiled from two representative experiments are shown. For (A), (□) BM ± KGF, (▪) BMS + PBS, and (▴) BMS + KGF. For (B), (□) BM + PBS, (▵) BM + KGF, (▪) BMS + PBS, and (▴) BMS + KGF.
Amelioration of mortality (A) and weight loss (B) by KGF after allogeneic BMT with a uniformly 100% lethal dose of allogeneic spleen cells. B10.BR recipient mice (n = 26/group) were lethally irradiated (7.5 Gy) on day −1 and infused on day 0 with C57BL/6 BM either without (BM) or with 15 × 106 spleen cells (BMS) as indicated. Groups were further segregated into those receiving PBS or KGF (5 mg/kg/d) on days −6, −5, and −4 pre-BMT. Data compiled from two representative experiments are shown. For (A), (□) BM ± KGF, (▪) BMS + PBS, and (▴) BMS + KGF. For (B), (□) BM + PBS, (▵) BM + KGF, (▪) BMS + PBS, and (▴) BMS + KGF.
KGF delays the Cytoxan-induced acceleration of lethality post-BMT.
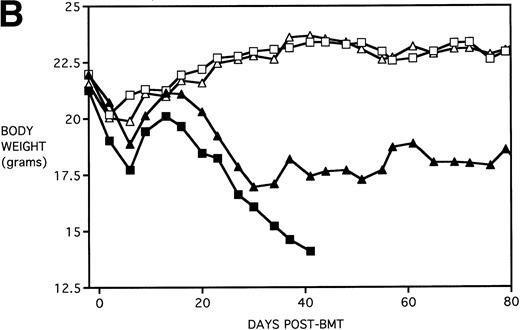
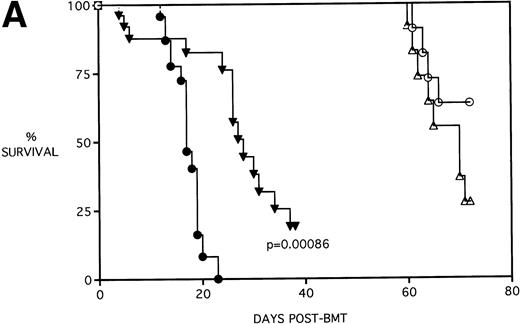
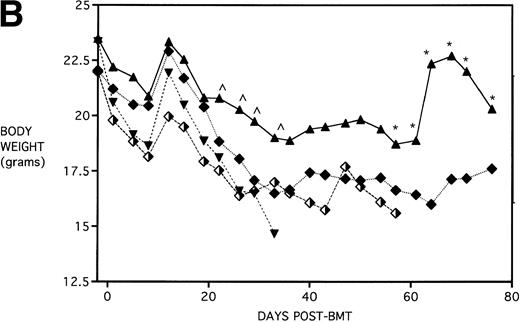
Because the majority of BMT protocols in humans entail the use of Cy and TBI as a conditioning regimen, experiments were set up to determine the effects of KGF on this combined regimen. The dose of Cy used (120 mg/kg/d for 2 days) was previously shown by this laboratory21 to be well tolerated in the early post-BMT period and biologically effective in facilitating the engraftment of TCD allogeneic BM when combined with 7.5 Gy TBI. We have also previously shown that, in the presence of allogeneic T cells, Cy accelerates GVHD-induced mortality and weight loss and leads to the most significant pulmonary dysfunction.22Figure 4A shows that KGF delays the Cy-induced acceleration of GVHD mortality to a significant degree (P = .00086). In fact, this actuarial survival rate is no different from the BMS + PBS group of Fig 3A (from the same pool of experiments) that received TBI alone (P = .5). Therefore, it appears that, as far as GVHD mortality is concerned, KGF has negated the deleterious effects of the combined Cy/TBI regimen.
KGF delays the Cytoxan-induced acceleration of mortality (A) and weight loss (B). B10.BR recipient mice (n = 26/group) were lethally irradiated (7.5 Gy) on day −1 and infused on day 0 with C57BL/6 BM either without (BM) or with 15 × 106 spleen cells (BMS) as indicated. Groups treated with Cy received 120 mg/kg/d on days −3 and −2. Groups were further segregated into those receiving PBS or KGF (5 mg/kg/d) on days −6, −5, and −4 pre-BMT. Data compiled from two representative experiments are shown. For (A), (○) BM + CY + PBS, (▵) BM + CY + KGF, (•) BMS + CY + PBS, and (▾) BMS + CY + KGF. For (B), (○) BM + CY + PBS, (▵) BM + CY + KGF, (•) BMS + CY + PBS, and (▾) BMS + CY + KGF. *P < .05 versus no KGF.
KGF delays the Cytoxan-induced acceleration of mortality (A) and weight loss (B). B10.BR recipient mice (n = 26/group) were lethally irradiated (7.5 Gy) on day −1 and infused on day 0 with C57BL/6 BM either without (BM) or with 15 × 106 spleen cells (BMS) as indicated. Groups treated with Cy received 120 mg/kg/d on days −3 and −2. Groups were further segregated into those receiving PBS or KGF (5 mg/kg/d) on days −6, −5, and −4 pre-BMT. Data compiled from two representative experiments are shown. For (A), (○) BM + CY + PBS, (▵) BM + CY + KGF, (•) BMS + CY + PBS, and (▾) BMS + CY + KGF. For (B), (○) BM + CY + PBS, (▵) BM + CY + KGF, (•) BMS + CY + PBS, and (▾) BMS + CY + KGF. *P < .05 versus no KGF.
Figure 4B shows that KGF had a beneficial effect on weight loss during the second week post-BMT (P < .05) using this Cy/TBI regimen in combination with a uniformally lethal spleen cell dose. This finding was highly reproducible in two separate experiments. However, after the second week, body weights did not differ between groups. KGF had no effect on the late Cy-induced death and weight loss in the BM groups not receiving allogeneic spleen cells (Fig 4, open symbols). Although the cause of this late mortality22 is still under investigation, we have concluded that these deaths are not GVHD-mediated as assessed histologically and neither are they due to bone marrow aplasia22 or inflammatory mediators such as those induced by sepsis as assessed by systemic levels of IL-1β, IL-6, TNFα, and interferon γ (IFNγ). It is suspected that the mortality may be caused by malnutrition due to tooth deformities arising from the toxic side effects of Cy on tooth eruption. The time course of the mortality is consistent with the observations of others23 24 concerning this problem, and our inspections of the recipients’ teeth at the time of death support this hypothesis.
The dose of KGF used (5 mg/kg/d) before cytoablative therapy was consistent with that used for other studies in the literature.5,7,8 There is evidence that KGF can be even more effective if administered both before and immediately after irradiation or chemotherapy.25 26 To determine whether the beneficial effect of KGF on survival could be improved upon, an additional KGF dosing schedule was tested. Mice received KGF on days −6, −5 and −4 pre-BMT only or also on days +1,+2, and +3 post-BMT. To compare the effect of KGF schedule on the different pre-BMT conditioning regimens, mice were further segregated into those receiving TBI alone or Cy/TBI. Figure 5A shows that KGF administered pre-BMT and post-BMT did not further improve the actuarial survival rate over that of mice receiving KGF pre-BMT only (P = .32 and .19 comparing KGF schedules for TBI groups and Cy/TBI groups, respectively). In addition, TBI BMS recipients receiving KGF pre-BMT tended to have body weights higher than their counterparts receiving KGF pre-BMT and post-BMT, with some recipients reaching their pre-BMT body weights (Fig 5B). Therefore, it appears that an additional post-BMT dose schedule of KGF does not add to survival and may be detrimental to body weight post-BMT.
Effect of additional post-BMT KGF administration on mortality (A) and weight loss (B) after allogeneic BMT with a uniformly 100% lethal dose of allogeneic spleen cells. B10.BR recipient mice (n = 8/group) were lethally irradiated (7.5 Gy) on day −1 and infused on day 0 with C57BL/6 BM with 15 × 106 spleen cells (BMS). Groups treated with Cy received 120 mg/kg/d on days −3 and −2. Groups were further segregated into those receiving KGF (5 mg/kg/d) on days −6, −5, and −4 pre-BMT (KGFpre) or those receiving KGF on days −6, −5, and −4 pre-BMT and days 1, 2, and 3 post-BMT (KGFpre&post). In (A), P = .32 and .19 comparing KGF schedules for TBI groups and Cy/TBI groups, respectively. In (B), for BMS + KGFpre versus BMS + KGFpre&post, .05 < P < .1 () and .01 < P < .05 (*). (▴) BMS + KGFpre, (⧫) BMS + KGFpre and post, (▾) BMS + CY + KGFpre, and (⬗) BMS + CY + KGFpre and post.
Effect of additional post-BMT KGF administration on mortality (A) and weight loss (B) after allogeneic BMT with a uniformly 100% lethal dose of allogeneic spleen cells. B10.BR recipient mice (n = 8/group) were lethally irradiated (7.5 Gy) on day −1 and infused on day 0 with C57BL/6 BM with 15 × 106 spleen cells (BMS). Groups treated with Cy received 120 mg/kg/d on days −3 and −2. Groups were further segregated into those receiving KGF (5 mg/kg/d) on days −6, −5, and −4 pre-BMT (KGFpre) or those receiving KGF on days −6, −5, and −4 pre-BMT and days 1, 2, and 3 post-BMT (KGFpre&post). In (A), P = .32 and .19 comparing KGF schedules for TBI groups and Cy/TBI groups, respectively. In (B), for BMS + KGFpre versus BMS + KGFpre&post, .05 < P < .1 () and .01 < P < .05 (*). (▴) BMS + KGFpre, (⧫) BMS + KGFpre and post, (▾) BMS + CY + KGFpre, and (⬗) BMS + CY + KGFpre and post.
KGF ameliorates GVHD-induced pathology in the liver, lung, and skin but only delays GVHD in the spleen, colon, and ileum.
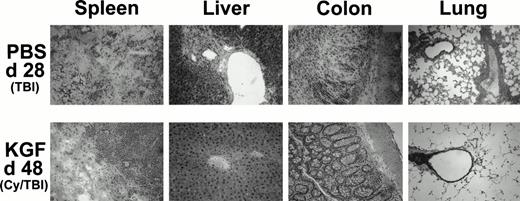
Because it was evident from the weight curves of Figs 3B and 4B that surviving KGF-treated animals were not GVHD-free, representative recipients from each group were examined to assess possible effects on GVHD-induced pathology in target organs. Recipients receiving pretreatment with KGF, Cy/TBI, and high-dose (15 × 106) spleen cells had reduced histological signs of GVHD in the target organs (liver, colon, and lung) on day 48 post-BMT compared with recipients not receiving KGF. Representative photomicrographs are shown in Fig 6 that illustrate the dramatic difference that pretreatment with KGF can effect on GVHD-induced tissue destruction. The infiltrates are predominantly composed of monocytes, CD4+ T cells, CD8+ T cells, and neutrophils (immunohistochemistry not shown). KGF pretreatment did not affect the composition of the infiltrates, when present. There is minimal perivascular mononuclear cell infiltration in the liver and lung and only moderate infiltration in the colonic mucosa. The spleen did show evidence of destruction, but this was surrounded by normal appearing post-BMT splenic tissue not seen in the GVHD-positive control (moribund at day 28 post-BMT) that did not even receive Cy in the conditioning regimen.
Histologic assessment of GVHD in BMS (15 × 106 spleen cells) recipients on day 28 (PBS, TBI group) and day 48 (KGF, Cy/TBI group) after allogeneic BMT. H&E-stained cryosections of spleen, liver, colon, and lung of a representative mouse taken from the indicated treatment groups at these time points show that KGF can abrogate GVHD-induced manifestation in the liver and lung and moderately so in the spleen and colon (original magnification × 100; resolution power, 40× objective lens).
Histologic assessment of GVHD in BMS (15 × 106 spleen cells) recipients on day 28 (PBS, TBI group) and day 48 (KGF, Cy/TBI group) after allogeneic BMT. H&E-stained cryosections of spleen, liver, colon, and lung of a representative mouse taken from the indicated treatment groups at these time points show that KGF can abrogate GVHD-induced manifestation in the liver and lung and moderately so in the spleen and colon (original magnification × 100; resolution power, 40× objective lens).
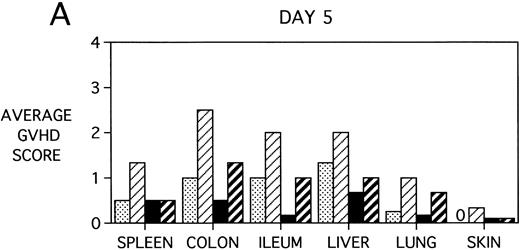
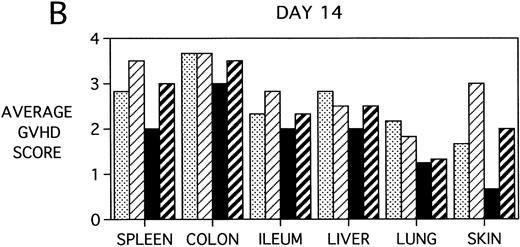
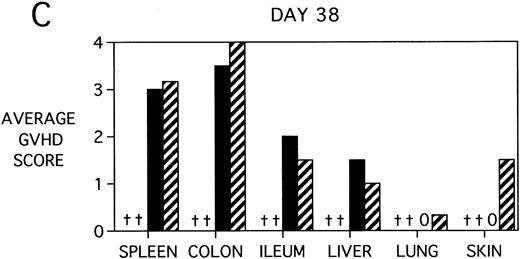
To determine whether KGF was preventing, ameliorating, or delaying GVHD-induced pathology, a kinetic histologic analysis was performed on cohorts of BMT recipients of BM or BMS comparing KGF pretreatment on both TBI and Cy/TBI conditioning regimens on days 5, 14, 18, and 38 post-BMT. The average GVHD scores (3 mice/group) assessed from H&E stains of cryosections of BMS recipient organs removed on days 5, 14, and 38 post-BMT are depicted in Fig 7. At day 5 post-BMT (Fig 7A), KGF-treated mice had average GVHD scores lower than their PBS-treated counterparts. KGF-pretreatment of Cy/TBI BMS recipients (thick hatched bars) resulted in lowering GVHD scores to the level of PBS-treated BMS recipients not receiving Cy (dotted bars) for most target organs. This is consistent with the findings of Fig 4A in which KGF negated the Cy-induced accelerated mortality. In the absence of Cy, KGF treatment (solid bars) again resulted in lower scores. However, on days 14 and 18 post-BMT, KGF-treated recipients had moderate GVHD scores, albeit slightly lower than non-KGF recipients, in the lung and skin, while exhibiting moderate to severe lesions in the spleen, colon, ileum, and liver equivalent to the PBS control recipients (Fig 7B, day 14 shown). It is obvious that this KGF treatment regimen, although it significantly delayed mortality, did not prevent GVHD-induced pathology. However, when graphed on a linear time scale, GVHD scoring results showed that KGF delayed the time it took to reach a moderate GVHD score of 2+ by 3 to 7 days for all organs (not shown). On day 38 post-BMT, a time when recipients not treated with KGF had all died of severe GVHD, Fig 7C shows that KGF-treated survivors had severe GVHD lesions in the spleen and gut but little to moderate pathology in the liver, lung, and skin. In fact, KGF-treated BMS TBI recipients exhibited no GVHD-induced pathology in the lung and skin (ie, appeared normal) at this time point. Thus, it appears that these KGF-treated recipients were able to survive despite severe splenic destruction and colitis, which would account for the inability of KGF to prevent weight loss at this high dose of allogeneic T cells. All recipients of allogeneic BM without spleen cells had GVHD scores of either 0 or 0.5+ in all target organs for all time points, with few exceptions (highest score was 1+).
GVHD scores in target organs of B10.BR recipients pretreated with PBS or KGF on days −6, −5, and −4 pre-BMT, conditioned with Cy/TBI (days −3, −2, and −1) or TBI alone (day −1), and transplanted with C57BL/6 BM with 15 × 106spleen cells. Scores are averages of 3 mice/group on days 5, 14, and 38 post-BMT. (†) indicates that all mice in the group had died by this time point. (0) indicates that all mice in the group exhibited normal histological appearance of the target organ. (▧) BMS + PBS, (▨) BMS + CY + PBS, (▪) BMS + KGF, (▨) BMS + CY + KGF.
GVHD scores in target organs of B10.BR recipients pretreated with PBS or KGF on days −6, −5, and −4 pre-BMT, conditioned with Cy/TBI (days −3, −2, and −1) or TBI alone (day −1), and transplanted with C57BL/6 BM with 15 × 106spleen cells. Scores are averages of 3 mice/group on days 5, 14, and 38 post-BMT. (†) indicates that all mice in the group had died by this time point. (0) indicates that all mice in the group exhibited normal histological appearance of the target organ. (▧) BMS + PBS, (▨) BMS + CY + PBS, (▪) BMS + KGF, (▨) BMS + CY + KGF.
DISCUSSION
A major contribution of this study is that it shows for the first time that KGF administration, when administered and completed before conditioning for murine allogeneic BMT recipients, results in a significant anti-GVHD effect, entirely inhibiting GVHD lesions in the lungs and skin of long-term survivors. When administered as a preconditioning treatment, KGF ameliorated tissue damage and, consequently, mice exhibited a higher survival rate, reduced weight loss, and reduced cellular infiltration post-BMT. This approach is unique as KGF is administered only as a preconditioning therapy, in contrast to neutralization with monoclonal antibodies or cytokine protein administration administered during or after conditioning, which may also affect antileukemia or engraftment properties.27-29 KGF pretreatment of irradiated recipients of BM with a 50% lethal dose of allogeneic spleen cells resulted in higher survival (88% v 50% on day 62 post-BMT) and prevented GVHD-induced weight loss. Under more aggressive GVHD conditions using a uniformly 100% lethal dose of allogeneic spleen cells, KGF significantly prolonged survival (P = .0027), but recipients never reached their pre-BMT body weights. However, KGF did prevent lethal weight loss in some mice, ie, KGF-treated long-term survivors maintained sufficient body weights to prevent morbidity.
Another major observation was that KGF inhibited the morbidity/mortality induced by Cy conditioning. KGF pretreatment of BMT recipients conditioned with the combined Cy/TBI regimen again resulted in a significant increase in the actuarial survival rate (P = .00086). In fact, KGF negated the Cy-induced acceleration of lethality so that the survival of these recipients became equivalent to BMS recipients conditioned with TBI alone (and no KGF). KGF-treatment significantly ameliorated GVHD-induced weight loss using this aggressive conditioning regimen, but its effects were not maintained after 2 weeks post-BMT.
When KGF was administered post-BMT in addition to the pretreatment regimen, no additional beneficial effect on survival or body weights were attained. In fact, body weights tended to be lower with the additional post-BMT KGF treatment. The reason for this paradox is unclear. It has been reported30 that cytokines mitogenic for epithelial cells may actually promote inflammation during tissue repair when the epithelium is injured and producing inflammatory mediators such as IL-1β and IL-8 either directly or indirectly via fibroblast activation. Hence, the timing of the KGF administration may be critical to realizing its benefits, and we are currently investigating the effects of KGF on cytokine release in our murine BMT model.
Kinetic histological analysis demonstrated that KGF did not prevent the generation of GVHD-induced inflammatory cell infiltrations and lesions in the target organs studied (spleen, colon, ileum, liver, lung, and skin), although it appeared that it delayed the generation of moderate lesions in affected recipients by 3 to 7 days. Surprisingly, KGF-treated survivors (day 38 post-BMT) exhibited no pathology in the lung and skin (and liver in some recipients, see also Fig 6). Because of the manner in which this study was executed, it could not be determined whether KGF pretreatment effected a resolution of the GVHD-induced lesions in the lungs and skin (and liver in many cases) or whether these survivors never had GVHD manifestations in these organs. However, on days 14 and 18 post-BMT, all KGF recipients had moderate GVHD scores in the liver, lungs, and skin. Severe splenic lesions and colitis were manifest in all KGF recipients, even those surviving post 38 days, equivalent to those lesions present in moribund BMT recipients not pretreated with KGF. This would account for the inability of KGF-treated mice to regain their pre-BMT body weights. Nonetheless, surviving KGF-treated recipients were able to tolerate these severe lesions. This implies that KGF ameliorated the GVHD-induced manifestations in the liver, lung, and skin, so that, when combined with the manifestations in the other organs, they were insufficient to tip the survival threshold.
It is possible that the route of injection (sc) was not suitable for sufficient KGF to reach the intestinal and colonic epithelium in the face of severe GVHD-induced destruction. However, this sc route has been used in other studies and shown to be effective for KGF-mediated protection of irradiation and chemotherapy-induced intestinal injury,8 9 but in the absence of allogeneic, GVHD-causing cells. The administration of KGF via another route, eg, intraperitoneally or intravenously, should be investigated.
We have not, to date, investigated the mechanism by which KGF ameliorates GVHD in our mouse model. KGF, a member of the fibroblast growth factor family, is produced by mesenchymal cells11and intraepithelial γδ T cells31 and acts predominantly on epithelial cells via binding to KGF receptors.32 It is presumed to play an important role as a mediator of mesenchymal-epithelial cell interactions during embryogenesis.33,34 The binding of KGF to its receptor activates a phosphorylation cascade35 entailing phospholipase C-γ and mitogen-activated protein kinases.36 KGF can increase repair of irradiation-induced DNA damage via DNA polymerases-α, -δ, and -ε6 in addition to protecting cells from reactive oxygen-induced apoptosis,4 possibly by upregulating expression of glutathione peroxidase,37 which detoxifies reactive oxygen species. It can facilitate wound repair in various injury-inducing models through its proliferative effects on epithelial cells in the skin (keratinocytes),38 hepatocytes in the liver,2 alveolar type II cells in the lung,3-5gastrointestinal,2,8,9 urothelial,7,39pancreatic,40 and mammary epithelium.41 These effects also have the benefit of helping to maintain functional epithelial barriers, as has been shown in lung42permeability studies.
In the current study, KGF had the greatest effect on those target organs that have been shown to express relatively high levels of KGF receptor, specifically the lung and skin.33 We have yet to determine whether KGF is affecting the GVHD properties of allogeneic T cells via direct signaling, but the expression of KGF receptors on lymphocytes has not been directly addressed. KGF is most likely ameliorating GVHD by reducing conditioning-induced injury, hence lowering the release of acute-phase reactants and dampening the ensuing inflammatory cascade.
The potential use of recombinant human KGF for minimizing the toxicities of chemotherapy and/or radiation in the clinic holds great promise, especially in view of the results of recent studies demonstrating that exogenous KGF does not stimulate the proliferation of tumor cells10 (even those bearing KGF receptors) or provide a selective growth advantage or radioprotection.10 43
ACKNOWLEDGMENT
The expert technical assistance of Sumiko Yonegi, Claudia DeLlano, John Hermanson, Chris Lees, Kelly Coffey, Stacey Hermanson, and Tamra Knutson is greatly appreciated. We also thank Dr Patricia A. Taylor for critical review of this manuscript.
Supported by National Institutes of Health Grant No. HL 55209 and by the Viking Children’s Fund.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Angela Panoskaltsis-Mortari, PhD, University of Minnesota, Department of Pediatrics, Division of Heme/Onc/Bone Marrow Transplantation, Box 484 UMHC, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: panos001@maroon.tc.umn.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal