Abstract
Although transcriptional activation of the c-fosproto-oncogene plays an intrinsic role in the mechanism of blood cell growth, it is still obscure how protein-tyrosine kinases (PTKs) regulate the cytokine-driven c-fos activation pathway. We present here that Tec PTK is tyrosine-phosphorylated and activated by granulocyte-macrophage colony-stimulating factor (GM-CSF) stimulation in a human GM-CSF–dependent cell line. Moreover, we could show that introduction of Tec into mouse BA/F3-hGMRαβ cells can profoundly activate the c-fos promoter in response to GM-CSF or to interleukin-3 (IL-3). In contrast, introduction of a kinase-deleted Tec could suppress cytokine-driven c-fos activation, indicating that Tec is directly involved in the regulation of c-fos transcription. Interestingly, strong activation by Tec of the c-fos promoter was blocked by the co-expression of dominant negative Jak2. The molecular interaction between Tec and Jak2 was then investigated both in mammalian and insect cell systems, revealing that they can not only bind to each other, but either of the two can phosphorylate the other. Thus, Tec and Jak2 can “cross-talk” in a complexed way to mediate cytokine-driven c-fos activation.
TEC PROTEIN-TYROSINE kinase (PTK) is the prototype of a recently emerging subfamily among nonreceptor PTKs including Tec, Btk, Emt/Itk/Tsk, Txk, and Bmx.1,2 In contrast to the Src-family kinases, none of the Tec-family members carry the myristylation signals or the C-terminal tyrosine residues corresponding to Tyr-527 in c-Src. One of the characteristic feature of the Tec-family members is that they (with the exception of Txk) contain a relatively long N-terminal unique region comprising a pleckstrin homology (PH) domain3 and a Tec homology (TH) domain.4 Tec-family members are, to date, the only PTKs containing the PH domain in their structures. Subsets of phospholipids have been shown to bind to the PH domain of Btk5,6 and Tec (T. Shirai and Y. Fukui, personal communication), and this PH-phospholipid interaction is supposed to play an important role in the recruitment of the PTKs to cell membrane and/or in the regulation of the kinase activities, as already proven in the case of a serine/threonine kinase, c-Akt/PKB/Racα.7
Although physiological roles of the Tec-family members are still to be revealed, accumulating evidence has suggested that Tec-family PTKs may be involved in the growth and/or differentiation mechanism of hematopoietic cells. First, many Tec-family members are abundantly expressed in blood cells; for instance, Btk in myeloid cells and B lymphocytes,8 Emt/Itk/Tsk in T lymphocytes,9and Tec in all the lineages.2 Second, mutations in Btk cause agammaglobulinemia in humans.10,11Third, many Tec-family kinases have been shown to be implicated in the intracellular signaling pathways of cytokines. We and our colleagues have shown that Tec can be tyrosine-phosphorylated and activated in response to interleukin-3 (IL-3), IL-6, stem cell factor (SCF), granulocyte colony-stimulating factor (G-CSF), erythropoietin (EPO), or thrombopoietin (TPO).12-17 Tec was shown to be physically associated (either directly or indirectly) with the receptors for SCF and IL-6. In addition to Tec, Btk and Emt/Itk/Tsk have also been shown to be activated by growth/differentiation signals of lymphocytes.18-20 Thus, currently one of the major concerns in the research of Tec-family kinases is in which part of intracellular machinery of cytokines they directly participate.
The c-fos proto-oncogene is one of the immediate early genes induced by a wide range of cytokine stimulations, and can encode a transcription factor containing a “leucine zipper” structure.21 Although transcriptional activation of c-fos is not directly involved in DNA synthesis, it is believed to be one of the important mechanisms to maintain the growth of blood cells.22 So far, two PTK-subfamilies have been suggested to play a regulatory role in the c-fos transcription. Minami et al23 have shown that Lck, a member of the Src-family, becomes activated by the stimulation with IL-2, and this PTK activation correlates well with the accumulation of c-fos transcripts. On the other hand, expression of a dominant negative form of Jak2 or Jak3 was demonstrated to suppress the c-fos transcription induced by granulocyte-macrophage CSF (GM-CSF)24 or IL-2,25 respectively. Although these data place Src-family and Jak-family members in the c-fos regulation pathways, little is still understood for the molecular mechanism by which they activate the c-fos promoter. We and others have already shown that Lyn, a member of the Src-family, can phosphorylate and activate Tec and Btk in cells.26 27 Thus, the Tec-family members are likely to work downstream of the Src-family kinases in vivo. Therefore, it would be an intriguing question whether Tec is directly involved in the regulation of the c-fospromoter activity in the hematopoietic system.
To address this issue, here we transiently introduced a reporter plasmid (pfos/luc), containing the promoter region of the c-fos gene and the luciferase cDNA, into mouse BA/F3-HGMRαβ cells28 which express the high-affinity receptors for human GM-CSF. pSRα plasmids carrying the cDNAs of various nonreceptor PTKs were co-introduced to compare the ability of each PTK to modulate c-fos promoter activity. Interestingly, we could observe that Tec was one of the most potent PTKs in the ability of the reporter gene activation. On the contrary, introduction of a kinase-deleted Tec could suppress the cytokine-driven c-fos activation in a dose-dependent manner. Because Jak2 expression also activated the c-fos promoter in our assay, we next investigated the functional and physical interaction between Tec and Jak2 in the context of c-fos activation mechanism. Co-expression of dnJak2 could block the Tec-driven c-fosactivation, suggesting that Jak2 may work at a point downstream of Tec. Surprisingly, in both 293 cells and insect cells we could show that Tec and Jak2 can not only associate with, but also phosphorylate, each other. Our data indicate that the Src-, Tec-, and Jak-family members functionally interact to transduce the cytokine-driven c-fosactivation mechanism.
MATERIALS AND METHODS
Cell lines.
BA/F3 cells29 were maintained in RPMI 1640 medium (GIBCO-BRL, Gaithersburg, MD) supplemented with 10% fetal calf serum (FCS) and 25 U/mL of mouse IL-3. The BA/F3-hGMRαβ cells and a human GM-CSF–dependent cell line, UT-7,30 were maintained in the same medium with 10% FCS and 1 ng/mL of human GM-CSF. 293 cells (American Type Culture Collection [ATCC], Rockville, MD) were maintained in Dulbecco's modified Eagle medium/F12 (DMEM/F12; GIBCO-BRL) containing 10% FCS and 2 mmol/L L-glutamine. Sf21 cells (Invitrogen, San Diego, CA) were grown in suspension at 28°C in the SF-900 II serum-free medium (GIBCO-BRL) without CO2 supply. For the stimulation experiments, UT-7 cells were cultured in the starvation medium (RPMI 1640 medium with 0.5% FCS, 100 μg/mL transferrin [Boehringer Mannheim, Mannheim, Germany] and 100 μg/mL bovine serum albumin [Boehringer Mannheim]) at the concentration of 5 × 105 cells/mL for 12 hours, then at the concentration of 1 × 107 cells/mL in the same medium for 0.5 hour. The cells were stimulated with 10 ng/mL of human GM-CSF for the period of 5 minutes unless otherwise indicated.
Immunoprecipitation and in vitro kinase assay.
The cDNA of mouse Tec type IV,12 mouse Jak2,31dominant negative Jak2,24 mouse Lyn A,32 Syk with an N-terminal gp120 epitope tag,33 or dominant negative Ras was ligated with the pSRα expression vector to generate pSRα-Tec, pSRα-Jak2, pSRα-dnJak2, pSRα-Lyn, pSRα-Syk, or pSRα-dnRas, respectively. To construct the cDNA encoding a kinase-deleted Tec (TecΔKD), Tec cDNA was digested byBpu1102I, blunt-ended by T4 DNA polymerase, and the 3′-fragment encoding the kinase domain was removed. Introduction of the expression plasmids into 293 cells was performed by the calcium phosphate method. UT-7 or 293 cells were rinsed once with ice-cold phosphate-buffered saline (PBS) supplemented with 0.1 mmol/L Na3VO4, and resuspended into the 1%-lysis buffer (1% Nonidet P-40, 50 mmol/L Tris-HCl, 7.4, 150 mmol/L NaCl, 1 mmol/L NaF, 1 mmol/L Na3VO4, 200 U/mL aprotinin, and 1 mmol/L phenylmethylsulfonyl fluoride). After incubation on ice for 30 minutes, cell extracts were centrifuged to remove insoluble materials. Tec or Jak2 was immunoprecipitated from 1.5 to 2 mg of the cell lysates by anti-Tec serum12 or anti-Jak2 sera (Santa Cruz Biotechnology, Santa Cruz, CA and Upstate Biotechnology, Lake Placid, NY), respectively, and was eluted into the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Where indicated, cells were solubilized by the 0.1%-lysis buffer containing 0.1% of NP-40 instead of 1%.
For the in vitro kinase assay, the immune complexes were washed three times with the 1%-lysis buffer, three times with the kinase buffer (20 mmol/L Tris-HCl, 7.4, 50 mmol/L NaCl, 10 mmol/L MgCl2, 2 mmol/L MnCl2), and finally incubated with 0.37 MBq of [γ-32P]ATP (Amersham, Arlington Heights, IL) for 15 minutes at 30°C. For the assay of Jak2 activity, a synthetic substrate of Jak2 (Upstate Biotechnology) was added to the reaction (20 μg/experiment). Samples of the Jak2 kinase assay were subjected to Tricine-SDS-PAGE.
Immunoblotting.
Total cell lysates (10 μg/lane) and the immune complexes were separated through 7.5% SDS-PAGE and electroblotted onto polyvinylidene difluoride (PVDF) membranes (Immobilon; Millipore, Bedford, MA). The membranes were incubated for 1 hour at room temperature in TBST (20 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 0.05% Tween 20) with 4% bovine serum albumin (Fraction V; Sigma, St Louis, MO). The membranes were then incubated with anti-Tec serum (1:10,000 dilution), anti-Jak2 serum, anti-Lyn serum,26 anti-gp120 epitope tag antibody (H902), or anti-phosphotyrosine antibody (4G10; Upstate Biotechnology) for 1 hour at room temperature in TBST. Specific bindings of the antibodies were visualized by the ECL detection system (Amersham) according to the manufacturer's instructions.
Metabolic labeling and phosphoamino acid analysis.
UT-7 cells were cultured at the concentration of 1 × 107cells/mL in phosphate-free RPMI 1640 medium (GIBCO-BRL) supplemented with 5% dialyzed FCS (GIBCO-BRL) and 37 MBq/mL of [32P]orthophosphate for 1 hour, and then stimulated with human GM-CSF for 5 minutes. Tec was immunoprecipitated from the cells, blotted onto a PVDF membrane, and incubated in 1 N KOH at 55°C according to the method of Kamps and Sefton.34 Phosphoamino acid contents of pp70Tec were determined as described earlier.12
Luciferase reporter assay.
With the c-fos promoter-luciferase plasmid (pfos/luc) as a reporter, the expression plasmid of each kinase was introduced into BA/F3-hGMRαβ cells by electroporation according to the method of Watanabe et al35 with minor modifications. Briefly, 1 × 107 of BA/F3-hGMRαβ cells were resuspended into 200 μL of OPTI-MEM I medium (GIBCO-BRL) and mixed with the expression vector DNAs (5 μg per construct unless otherwise indicated) plus the pfos/luc reporter plasmid (2 μg). Total amounts of plasmid DNAs in each set of electroporation were adjusted to be equal by adding the appropriate amounts of the blank vector DNA. After electroporation with the GenePulser apparatus (BioRad, Hercules, CA) at the condition of 200 V and 960 μF, cells were resuspended into 30 mL of RPMI 1640 medium with 10% FCS and cultured for 5 hours. The samples were further cultured for 5 hours either unstimulated or stimulated with 25 U/mL of mouse IL-3 or 5 ng/mL of human GM-CSF. The luciferase activities were measured by using the Luciferase Assay System (Promega, Madison, WI), and are shown as relative light units/min/μg of protein. The Elk activity was assayed in BA/F3 cells by using the PathDetect in vivo reporting system (Stratagene, La Jolla, CA). The MEK1 inhibitor (PD98059; New England Biolabs, Beverly, MA) was dissolved in dimethyl sulfoxide (DMSO) and add to the culture at the concentration of 50 μmol/L. The pfos/luc mutants were constructed by inserting the mutant promoter fragments36 into pGL3-Basic plasmid (Promega).
Recombinant baculoviruses.
The cDNAs of Tec and Jak2 were inserted into the pFastBacHT and pFastBac1 plasmids (both from GIBCO-BRL), respectively. The recombinant baculoviruses based on these plasmids were generated by the Bac-to-Bac baculovirus expression systems (GIBCO-BRL), and were used to infect Sf21 cells at the multiplicity of infection (MOI) of 1.0. After 48 to 72 hours of culture, cells were harvested and lysed as described above.
RESULTS
Tec is involved in the signaling pathway of GM-CSF receptor.
To investigate whether Tec is involved in the signaling mechanism mediated by GM-CSF receptor (GMR), Tec was immunoprecipitated from a human GM-CSF–dependent cell line, UT-7, with or without the GM-CSF stimulation, and was immunoblotted with anti-phosphotyrosine antibody (αP-Tyr Ab). As shown in the upper panel of Fig1A, GM-CSF stimulation of UT-7 cells for 5 minutes could clearly induce tyrosine-phosphorylation of Tec (indicated by an arrow) and a Tec-associated p56. The identity of this p56 is yet to be determined although we confirmed that p52shc and p56lyn, both of which are known to be associated with Tec, have the same electrophoretic mobility with that of the “p56.” The same membrane was reblotted with anti-Tec serum to prove that equivalent amounts of Tec were precipitated (lower panel). We could not detect a significant level of Btk expression in UT-7 cells by using an anti-Btk antibody (M-138; Santa Cruz Biotechnology). We next examined the time course of Tec phosphorylation. Tec was immunoprecipitated from UT7 cells with various periods of GM-CSF stimulation, and probed with αP-Tyr Ab. As shown in Fig 1B, tyrosine-phosphorylation of Tec was induced as rapidly as 1 minute after the stimulation, reached to the maximum level in 5 to 10 minutes, and decreased thereafter. Thus, the phosphorylation of Tec in response to GM-CSF is rapid and transient. To examine whether the kinase activity of Tec is also affected in response to GM-CSF, Tec was immunoprecipitated from UT-7 cells with or without GM-CSF stimulation and subjected to an in vitro kinase assay without exogenous substrates. As shown in Fig 1C and D, stimulation with GM-CSF for 5 minutes could enhance the auto-phosphorylation activity of Tec.
Tec is involved in the signaling pathway of GM-CSF receptor. (A) UT-7 cells (1 × 107) were cultured in the starvation medium for 12 hours and then stimulated with 10 ng/mL of human GM-CSF (+) for 5 minutes or left unstimulated (−). Tec was immunoprecipitated from each fraction (αTec), subjected to 7.5% SDS-PAGE, and immunoblotted with anti-phosphotyrosine antibody (αP-Tyr). Total cell lysates (TCL; 10 μg/lane) and the immunoprecipitates by normal rabbit serum (NRS) prepared from the same set of cells were also analyzed. The position of Tec is indicated by an arrow. The molecular weight standards (×10−3) are shown at the left. The same membrane was reblotted with anti-Tec serum to show the amounts of Tec precipitated (lower panel). (B) UT-7 cells were stimulated with GM-CSF (10 ng/mL) for 0, 1, 5, 10, or 20 minutes as indicated at the top. Tec was immunoprecipitated from each fraction (1 × 107 cells), and was immunoblotted with anti-phosphotyrosine antibody (αP-Tyr) or anti-Tec serum (αTec). (C) Tec was immunoprecipitated from 1 × 107 of UT-7 cells with (+) or without (−) 5 minutes of GM-CSF stimulation, and was subjected to an in vitro kinase assay. Autophosphorylation of pp70Tec is shown. (D) Specific kinase activity of the Tec protein (32P-incorporation/protein amount) with (+) or without (−) the GM-CSF stimulation was calculated by densitometric analysis and shown as arbitrary units. (E) Tec was immunoprecipitated from UT-7 cells (1 × 107), with (GM) or without (−) the GM-CSF stimulation (10 ng/mL), metabolically labeled with [32P]orthophosphate (37 MBq/mL), and was analyzed by 7.5% SDS-PAGE. The proteins were blotted onto a PVDF membrane, and heated in 1 N KOH to decrease the backgrounds of serine- and threonine-phosphorylation. The position of Tec is indicated. (F) pp70Tec in (E) was subjected to the phosphoamino acid analysis. The positions of free phosphate (Pi), phosphoserine (p-Ser), phosphothreonine (p-Thr), and phosphotyrosine (p-Tyr) are indicated at the right.
Tec is involved in the signaling pathway of GM-CSF receptor. (A) UT-7 cells (1 × 107) were cultured in the starvation medium for 12 hours and then stimulated with 10 ng/mL of human GM-CSF (+) for 5 minutes or left unstimulated (−). Tec was immunoprecipitated from each fraction (αTec), subjected to 7.5% SDS-PAGE, and immunoblotted with anti-phosphotyrosine antibody (αP-Tyr). Total cell lysates (TCL; 10 μg/lane) and the immunoprecipitates by normal rabbit serum (NRS) prepared from the same set of cells were also analyzed. The position of Tec is indicated by an arrow. The molecular weight standards (×10−3) are shown at the left. The same membrane was reblotted with anti-Tec serum to show the amounts of Tec precipitated (lower panel). (B) UT-7 cells were stimulated with GM-CSF (10 ng/mL) for 0, 1, 5, 10, or 20 minutes as indicated at the top. Tec was immunoprecipitated from each fraction (1 × 107 cells), and was immunoblotted with anti-phosphotyrosine antibody (αP-Tyr) or anti-Tec serum (αTec). (C) Tec was immunoprecipitated from 1 × 107 of UT-7 cells with (+) or without (−) 5 minutes of GM-CSF stimulation, and was subjected to an in vitro kinase assay. Autophosphorylation of pp70Tec is shown. (D) Specific kinase activity of the Tec protein (32P-incorporation/protein amount) with (+) or without (−) the GM-CSF stimulation was calculated by densitometric analysis and shown as arbitrary units. (E) Tec was immunoprecipitated from UT-7 cells (1 × 107), with (GM) or without (−) the GM-CSF stimulation (10 ng/mL), metabolically labeled with [32P]orthophosphate (37 MBq/mL), and was analyzed by 7.5% SDS-PAGE. The proteins were blotted onto a PVDF membrane, and heated in 1 N KOH to decrease the backgrounds of serine- and threonine-phosphorylation. The position of Tec is indicated. (F) pp70Tec in (E) was subjected to the phosphoamino acid analysis. The positions of free phosphate (Pi), phosphoserine (p-Ser), phosphothreonine (p-Thr), and phosphotyrosine (p-Tyr) are indicated at the right.
To directly estimate the phosphotyrosine contents, Tec was immunoprecipitated from UT-7 cells metabolically labeled with [32P]orthophosphate, separated through 7.5% SDS-PAGE, blotted onto a PVDF membrane, and incubated in 1 N KOH to enrich the signals of phosphotyrosine. Autoradiography of the membrane could show that GM-CSF can induce phosphorylation of pp70Tec (Fig 1E). The phosphoamino acid contents of this pp70Tec were then examined by thin-layer chromatography, showing that phosphorylation of tyrosine residues was actually induced by the stimulation with GM-CSF (Fig 1F). These data imply that Tec is involved in the intracellular signaling mechanism mediated by GMR.
Tec is involved in cytokine-driven activation of c-fostranscription.
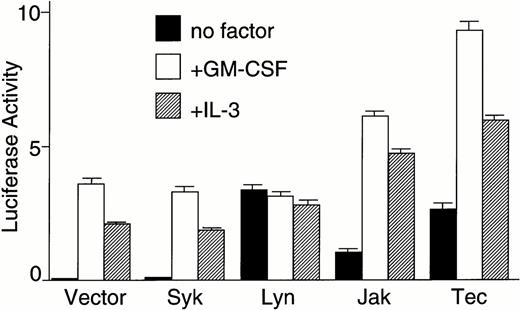
To examine whether Tec mediates cytokine-driven activation of the c-fos gene, the pfos/luc plasmid in which the luciferase expression is controlled by the c-fos promoter was transfected into BA/F3-hGMRαβ cells by electroporation together with the pSRα-based expression plasmid of Syk, Lyn, Jak2, or Tec. As shown in Fig 2A,stimulation of the vector-transfected BA/F3-hGMRαβ cells with either GM-CSF or IL-3 could enhance the luciferase reporter activity. Co-introduction of the Syk kinase with an N-terminal tag33 did not affect the luciferase activity, suggesting Syk is not involved in the c-fos activation mechanism in BA/F3 cells. In contrast, introduction of Lyn kinase significantly elevated the luciferase activity of the unstimulated basal level. However, cytokine stimulation of the cells could not further enhance the reporter activity. This lack of cytokine-responsiveness in Lyn-transfected cells was confirmed in repeated experiments. As previously reported, introduction of Jak2 could elevate the reporter activity of the unstimulated state as well as of cytokine-stimulated states. Interestingly, Tec introduction elevated the reporter activity of the unstimulated state similar to the level obtained by the Lyn-transfection. In contrast to the case of Lyn, Tec expression could also strongly enhance the reporter activity in response to GM-CSF or IL-3. Appropriate expression of each kinase was confirmed by the immunoblot analysis of the total cell lysates (Fig2B).
Tec is involved in the cytokine-driven activation of c-fos proto-oncogene. (A) BA/F3-hGMRαβ cells (1 × 107) were transfected with the pfos/luc reporter plasmid (2 μg) together with 5 μg each of the pSRα (Vector), pSRα-Syk (Syk), pSRα-Lyn (Lyn), pSRα-Jak2 (Jak), or pSRα-Tec (Tec). After 5 hours of incubation in cytokine-free medium, the cells were further cultured for 5 hours without (no factor) or with 5 ng/mL of human GM-CSF (+GM-CSF) or 25 U/mL of mouse IL-3 (+IL-3). Luciferase activity was assayed in each fraction and calculated as relative light units (RLU)/min/μg of protein. The mean value plus SD of the luciferase activities in triplicate samples from each fraction is shown as arbitrary units. (B) BA/F3-hGMRαβ cells were transfected with pSRα-Syk, pSRα-Lyn, pSRα-Jak2, or pSRα-Tec, and cultured for 24 hours in the presence of IL-3. Total cell lysates (10 μg/lane) were prepared from each set (+) and untransfected BA/F3-hGMRαβ cells (−), and were immunoblotted with the antibodies against the corresponding kinases.
Tec is involved in the cytokine-driven activation of c-fos proto-oncogene. (A) BA/F3-hGMRαβ cells (1 × 107) were transfected with the pfos/luc reporter plasmid (2 μg) together with 5 μg each of the pSRα (Vector), pSRα-Syk (Syk), pSRα-Lyn (Lyn), pSRα-Jak2 (Jak), or pSRα-Tec (Tec). After 5 hours of incubation in cytokine-free medium, the cells were further cultured for 5 hours without (no factor) or with 5 ng/mL of human GM-CSF (+GM-CSF) or 25 U/mL of mouse IL-3 (+IL-3). Luciferase activity was assayed in each fraction and calculated as relative light units (RLU)/min/μg of protein. The mean value plus SD of the luciferase activities in triplicate samples from each fraction is shown as arbitrary units. (B) BA/F3-hGMRαβ cells were transfected with pSRα-Syk, pSRα-Lyn, pSRα-Jak2, or pSRα-Tec, and cultured for 24 hours in the presence of IL-3. Total cell lysates (10 μg/lane) were prepared from each set (+) and untransfected BA/F3-hGMRαβ cells (−), and were immunoblotted with the antibodies against the corresponding kinases.
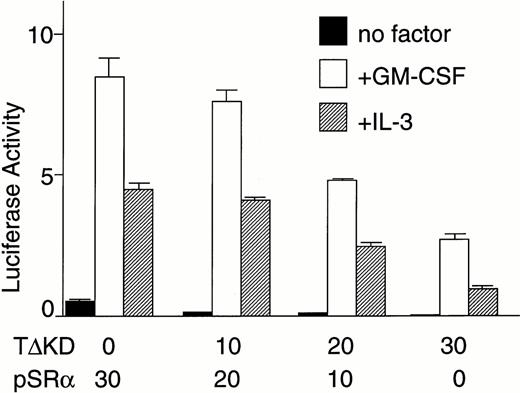
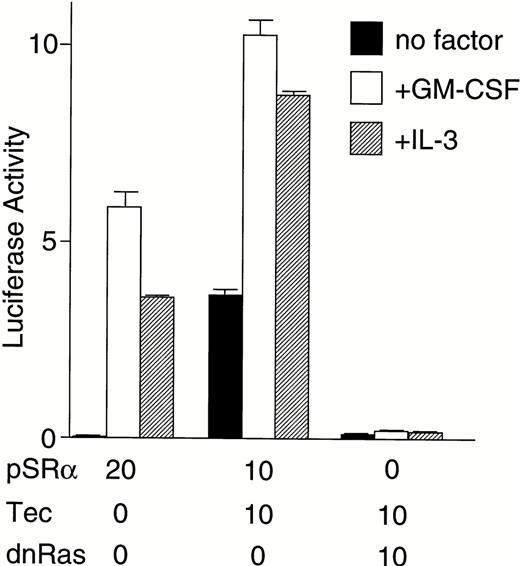
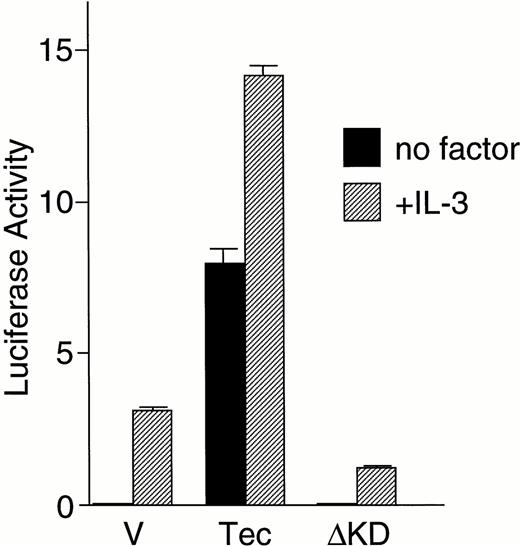
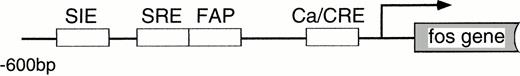
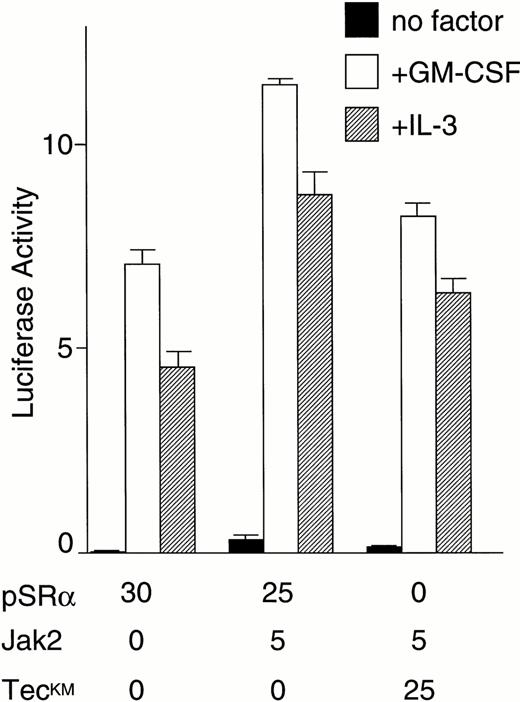
We then directly tested whether Tec is an intermediate in the cytokine-driven c-fos activation pathway by using a kinase-deleted Tec (TecΔKD). As shown in Fig 2C, introduction of pSRα-TecΔKD into BA/F3-hGMRαβ cells suppressed the c-fos promoter activity stimulated by GM-CSF or IL-3 in a dose-dependent manner. These data strongly support the idea that Tec directly mediates the cytokine-driven c-fos activation. It is widely known that c-fos transcription is regulated via the Ras-MAPK pathway. Therefore, we checked whether the Tec-driven c-fos activation is transduced through Ras by coexpressing a dominant negative form of Ras (dnRas). As shown in Fig 2D, coexpression of dnRas could totally block the Tec-driven activation of the c-fos gene. Thus, Tec is likely to drive the c-fosactivation through a Ras-regulated mechanism. By using the PathDetect in vivo reporting system (Stratagene), we then asked whether Elk, a transcriptional factor acting downstream of Ras, is involved in the Tec-mediated c-fos activation. The pFA-Elk plasmid, encoding the fusion protein consisting of the DNA binding domain of yeast GAL4 and the activation domain of Elk, was transfected into BA/F3 cells together with Tec-expression plasmids and the reporter pFR-luc plasmid in which expression of luciferase is controlled by a promoter containing the GAL4-binding sites (Fig 2E). In the pSRα-transfected cells (“V” part), IL-3 stimulation resulted in the elevation of luciferase activity, which suggests that Elk is activated in response to IL-3. Introduction of Tec markedly increased the reporter activity both in the unstimulated and stimulated states (“Tec” part). In contrast, transfection of TecΔKD suppressed the luciferase activity, indicating that Elk-pathway is involved in the Tec-driven c-fosactivation process. We also tested whether MEK1, an intermediate between Ras and Elk, plays a role in this c-fos activation mechanism. After electroporation with pFA-Elk and pFR-luc, BA/F3 cells were cultured for 4 hours without IL-3, and then for 1 hour with an MEK1 inhibitor, PD98059, before the IL-3 stimulation. As shown in Fig2F, treatment with PD98059 significantly suppressed the Tec-driven Elk-activation. In a separate line of experiment, we investigated what kind of transcriptional factor(s) is responsible for the Tec-mediated c-fos transcription. The c-fos promoter fragment is known to contain four cis-regulatory elements, namely, thesis-inducible element (SIE), the serum response element (SRE), the c-fos AP-1 binding element (FAP), and the calcium and cyclic AMP response element (Ca/CRE) (Fig 2G). These regulatory sequences are presumed to work in concert to control the c-fostranscription in a tissue- and stimulus-specific fashion.36By using the pfos/luc mutants in which point mutations were introduced into individual regulatory elements (kind gifts of T. Curran), we here analyzed how each element contributes to the Tec-driven c-fos activation (Fig 2H). In accordance with the results in Fig 2E, mutations at SRE, the binding site of the Elk/TCF complex, significantly decreased the Tec-driven activation of c-fos transcription. These lines of evidence support the idea that Tec activates c-fos promoter through, at least in part, the Ras-MEK1-Elk pathway.
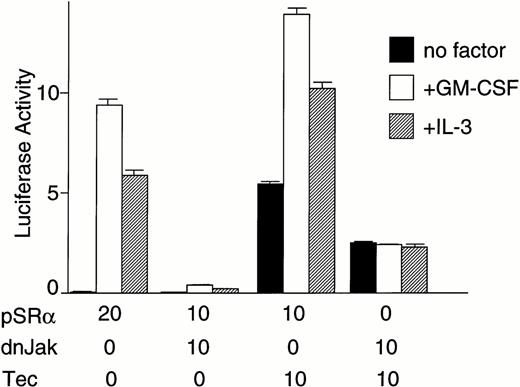
Because both of the Tec and Jak2 kinases could enhance cytokine-driven c-fos activation, we then tried to clarify whether Tec and Jak2 work in the same pathway or in a parallel manner to drive the c-fos promoter. First, Tec was introduced into BA/F3-hGMRαβ cells with or without dominant negative Jak2 (dnJak2) to examine whether Jak2 is involved in the Tec-driven pathway (Fig 2I). Interestingly, expression of dnJak2 could suppress the cytokine-driven as well as Tec-driven luciferase activity, indicating that Jak2 acts downstream of Tec in the c-fos activation mechanism. We have also tested the possibility of the Tec-Jak2 interaction in the reverse direction. As shown in Fig 2J, introduction of a kinase-dead TecKM (Lys-397 at the ATP-binding site is replaced with Met) could slightly suppress the Jak2-driven activation of the c-fos gene. Although we could reproducibly observe this weak suppression (about 20% reduction), we do not yet have a strong proof that Tec is involved in a part of the Jak2-driven mechanism in the c-fos regulation.
Tec can phosphorylate Jak2 in cells.
To understand how Tec and Jak2 can functionally interact with each other, we first examined the possibility that the former directly phosphorylates the latter. A kinase-dead Jak2 (Jak2KE: Lys-882 in the ATP-binding site is replaced with Glu) was expressed in 293 cells with or without Tec, immunoprecipitated by anti-Jak2 serum and blotted with αP-Tyr Ab. To our surprise, as shown in the upper panel of Fig 3A, Jak2KE could be phosphorylated by Tec in intact cells. Interestingly, the tyrosine-phosphorylated p70Tec was also identified in the anti-Jak2 immunoprecipitate, suggesting the physical interaction between the two PTKs. The same membrane was reprobed with anti-Jak2 serum to prove that equivalent amounts of Jak2 were precipitated (lower panel). This Tec phosphorylation of Jak2KE is not likely to arise from a nonspecific reaction by over-expressed Tec proteins, because Syk could not phosphorylate Jak2KE in a similar experiment in 293 cells (data not shown). We also examined the ability of Tec to phosphorylate Jak2 in the insect cell system. Sf21 cells derived from Spodoptera frugiperda were infected with the recombinant baculovirus expressing Jak2KE alone or in combination with the Tec-expressing or TecKM-expressing virus. After 2 days of incubation, Jak2KE was immunoprecipitated from the cells and probed with αP-Tyr Ab (upper panel of Fig 3B). As expected, phosphorylation of Jak2KEcould be identified only when Jak2KE was coexpressed with kinase-active Tec. In contrast, coexpression of TecKM did not confer detectable tyrosine-phosphorylation on Jak2KE. The same membrane was reblotted with anti-Jak2 serum to estimate the amounts of Jak2 immunoprecipitated. These data favor the idea that Jak2 is a direct substrate of Tec in vivo. In these experiments, Jak2-phosphorylation by Tec was clearly and reproducibly observed when we used, for immunoprecipitation, anti-Jak2 serum against the C-terminal tail of Jak2 (C-20; Santa Cruz Biotechnology), not the one against amino acid positions 758-776 (Upstate Biotechnology), which may imply that the target site(s) of Tec is localized within or very close to the 758-776 region.
Tec can phosphorylate Jak2 in both mammalian and insect cells. (A) Jak2KE was immunoprecipitated from 2 × 106 of 293 cells expressing Jak2KE with (T) or without (−) Tec. Total cell lysates (TCL, 10 μg/lane) and anti-Jak2 immunoprecipitates (Jak IP) were electrophoresed and probed with anti-phosphotyrosine antibody (αP-Tyr) or anti-Jak2 serum (αJak2). The positions of Jak2 (Jak2), Tec (Tec), and the Ig heavy chain (IgH) are indicated at the right. (B) Jak2KE was immunoprecipitated from Sf21 cells infected with Jak2KEexpressing baculovirus (JE) alone or in combination with Tec-expressing (T) or TecKM-expressing (TM) virus. The immunoprecipitates were separated through 7.5% SDS-PAGE and probed with anti-phosphotyrosine antibody (αP-Tyr) or anti-Jak2 serum (αJak2). The positions of Jak2 (Jak2) and Tec (Tec) are indicated at the right. (C) Jak2 was immunoprecipitated from Sf21 cells expressing Jak2 (J) or Jak2KE (JE) either alone or in combination with Tec (T). The immunoprecipitates were incubated with [γ-32P]ATP and the synthetic Jak2-substrate, and subjected to Tricine-SDS-PAGE. Phosphorylation of the Jak2-substrate is shown. (D) Total cell lysates (TCL: 10 μg/lane) and the anti-Jak2 immunoprecipitates (Jak IP) were prepared from parental BA/F3 cells (P) and two BA/F3 clones (1 and 2) stably expressing Tec▵SH3, and immunoblotted with αP-Tyr Ab (upper panel) or anti-Jak2 serum (lower panel). The position of Jak2 is indicated at the right. The positions of molecular weight standards (×10−3) are also shown at the left.
Tec can phosphorylate Jak2 in both mammalian and insect cells. (A) Jak2KE was immunoprecipitated from 2 × 106 of 293 cells expressing Jak2KE with (T) or without (−) Tec. Total cell lysates (TCL, 10 μg/lane) and anti-Jak2 immunoprecipitates (Jak IP) were electrophoresed and probed with anti-phosphotyrosine antibody (αP-Tyr) or anti-Jak2 serum (αJak2). The positions of Jak2 (Jak2), Tec (Tec), and the Ig heavy chain (IgH) are indicated at the right. (B) Jak2KE was immunoprecipitated from Sf21 cells infected with Jak2KEexpressing baculovirus (JE) alone or in combination with Tec-expressing (T) or TecKM-expressing (TM) virus. The immunoprecipitates were separated through 7.5% SDS-PAGE and probed with anti-phosphotyrosine antibody (αP-Tyr) or anti-Jak2 serum (αJak2). The positions of Jak2 (Jak2) and Tec (Tec) are indicated at the right. (C) Jak2 was immunoprecipitated from Sf21 cells expressing Jak2 (J) or Jak2KE (JE) either alone or in combination with Tec (T). The immunoprecipitates were incubated with [γ-32P]ATP and the synthetic Jak2-substrate, and subjected to Tricine-SDS-PAGE. Phosphorylation of the Jak2-substrate is shown. (D) Total cell lysates (TCL: 10 μg/lane) and the anti-Jak2 immunoprecipitates (Jak IP) were prepared from parental BA/F3 cells (P) and two BA/F3 clones (1 and 2) stably expressing Tec▵SH3, and immunoblotted with αP-Tyr Ab (upper panel) or anti-Jak2 serum (lower panel). The position of Jak2 is indicated at the right. The positions of molecular weight standards (×10−3) are also shown at the left.
We then tested whether this trans-phosphorylation of Jak2 by Tec affects the kinase activity of the Jak2 protein. Jak2 was expressed in Sf21 cells with or without Tec, immunoprecipitated by anti-Jak2 serum, and was subjected to an in vitro kinase assay with a synthetic substrate peptide. As shown in Fig 3C, coexpression of Tec did not affect the phosphorylation of the Jak2-substrate. Because the immunoprecipitated Jak2KE could not phosphorylate the peptide at all (lane “JE + T”), phosphorylation of the peptide in the other lanes was supposed to be carried out by Jak2, not by the coprecipitated kinases from Sf21 cells. We observed that Jak2 was expressed in equal amounts in each Sf21 fraction, as judged from the immunoblotting of the total cell lysates with anti-Jak2 serum (data not shown). Similar results of the in vitro kinase assay were obtained with Jak2 expressed in 293 cells (data not shown). Phosphorylation of Jak2 without the modulation of its activity may be used in vivo for collecting signaling molecules to Jak2 protein. We checked this possibility by using the BA/F3 cells expressing an SH3-deleted active Tec (TecΔSH3).37 Jak2 was immunoprecipitated from parental BA/F3 cells and two BA/F3 transfectants stably expressing TecΔSH3, and blotted with αP-Tyr Ab. As shown in Fig 3D, many tyrosine-phosphorylated proteins become associated with Jak2 only when TecΔSH3 is coexpressed, which may indirectly support the possibility above.
Jak2 can phosphorylate Tec at Tyr-518.
To investigate the phosphorylation reaction in the reverse direction between the two kinases, TecKM was introduced into 293 cells with or without Jak2, and was analyzed for tyrosine-phosphorylation (upper panel, Fig4A). Because it was already known that Tec can be directly phosphorylated and activated by Lyn PTK, a coexpression experiment of Lyn kinase was used as a positive control. To our surprise again, Tec could be in vivo phosphorylated by Jak2 as well as by Lyn. The same membrane was then probed with anti-Tec serum to estimate the amounts of Tec precipitated (lower panel). Therefore, Tec and Jak2 can trans-phosphorylate each other.
Jak2 can phosphorylate Tec at Tyr-518. (A) The kinase-dead TecKM (TM) was expressed in 293 cells either alone or in combination with Jak2 (Jak2) or Lyn (Lyn) kinase. Tec was immunoprecipitated from each fraction, and probed with anti-phosphotyrosine antibody (αP-Tyr) or anti-Tec serum (αTec). (B) TecKM (TM), TecKM▵SH3 (TM▵3), or TecKM,YF (TM,518F) was expressed in 293 cells either alone (−) or in combination with Jak2 (J) or Lyn (L). Tec was immunoprecipitated from each fraction, and blotted with anti-phosphotyrosine antibody (αP-Tyr) or anti-Tec serum (αTec). The positions of full-length Tec (T) and SH3-deleted (▵SH3) forms are indicated at the right. (C) The amino acid sequences of the Tec-family kinases, surrounding the tyrosine residues corresponding to Tyr-518 in mouse Tec, are compared. The asterisk indicates the position of the phosphorylated tyrosine. At the left shown are the numbers of amino acid positions of mouse Tec,2 human Btk,10,11 mouse Emt/Itk/Tsk, 9,41,42 human Bmx,43 and human Txk.44 (D) pSRα (V), pSRα-Tec (T), or pSRα-TecKM (TM) was transfected into 293 cells with or without pSRα-Jak2 (J). Tec was immunoprecipitated from each fraction, and incubated with [γ−32P]ATP without exogenous substrates. Autophosphorylation of pp70Tec in each sample is shown.
Jak2 can phosphorylate Tec at Tyr-518. (A) The kinase-dead TecKM (TM) was expressed in 293 cells either alone or in combination with Jak2 (Jak2) or Lyn (Lyn) kinase. Tec was immunoprecipitated from each fraction, and probed with anti-phosphotyrosine antibody (αP-Tyr) or anti-Tec serum (αTec). (B) TecKM (TM), TecKM▵SH3 (TM▵3), or TecKM,YF (TM,518F) was expressed in 293 cells either alone (−) or in combination with Jak2 (J) or Lyn (L). Tec was immunoprecipitated from each fraction, and blotted with anti-phosphotyrosine antibody (αP-Tyr) or anti-Tec serum (αTec). The positions of full-length Tec (T) and SH3-deleted (▵SH3) forms are indicated at the right. (C) The amino acid sequences of the Tec-family kinases, surrounding the tyrosine residues corresponding to Tyr-518 in mouse Tec, are compared. The asterisk indicates the position of the phosphorylated tyrosine. At the left shown are the numbers of amino acid positions of mouse Tec,2 human Btk,10,11 mouse Emt/Itk/Tsk, 9,41,42 human Bmx,43 and human Txk.44 (D) pSRα (V), pSRα-Tec (T), or pSRα-TecKM (TM) was transfected into 293 cells with or without pSRα-Jak2 (J). Tec was immunoprecipitated from each fraction, and incubated with [γ−32P]ATP without exogenous substrates. Autophosphorylation of pp70Tec in each sample is shown.
We then tried to map the phosphorylation site of Tec by Jak2. Yamashita et al37 previously demonstrated that the deletion of the internal SH3 domain results in hyperphosphorylation and activation of Tec in vivo. In addition, Tec kinase has a tentative autophosphorylation site (Tyr-518) in the activation loop of its catalytic domain, corresponding to Tyr-416 in c-Src. Therefore, we investigated the possibility that either of the SH3 domain or Tyr-518 is the target site of Jak2 and Lyn kinases. TecKM, TecKMΔSH3 (the SH3 domain of TecKM is deleted), or TecKM,YF (Tyr-518 of TecKM is replaced with Phe) was expressed in 293 cells either alone or in combination with Jak2 or Lyn. Tec was then immunoprecipitated and probed with αP-Tyr Ab. As shown in the upper panel of Fig 4B, internal deletion of the SH3 domain did not decrease the phosphorylation of TecKM by Jak2. On the other hand, the Phe-substitution for Tyr-518 nearly completely abolished the phosphorylation of TecKM protein (“TM,518F” part). Hence, Tyr-518 of Tec is the target site of both Jak2 and Lyn. The same membrane was reblotted with anti-Tec serum to prove that equivalent amounts of Tec proteins were immunoprecipitated (lower panel). It is not likely that Jak2 phosphorylated Tec indirectly through Lyn (or other Src-family kinases), because the kinase activity of Lyn was not affected by the coexpression of Jak2 when transiently expressed in 293 cells (data not shown). The amino acid sequences surrounding this Tyr-518 position in Tec-family kinases are well conserved (Fig 4C). Therefore, it would not be surprising if other members of the Tec-family are also controlled by Src- and Jak-family kinases through a similar phosphorylation mechanism.
Because Jak2 and Lyn can phosphorylate the same residue (Tyr-518) of Tec, we speculated that Jak2 may activate Tec as in the case of Lyn. Tec or TecKM was expressed in 293 cells either alone or in combination with Jak2. Tec was immunoprecipitated from each set and subjected to an in vitro kinase assay to test its auto-phosphorylation activity. Unexpectedly, as shown in Fig 4D, autophosphorylation level of p70Tec was not altered irrespective of the presence of Jak2 PTK. In contrast, coexpression of Lyn kinase could activate Tec as reported previously (data not shown). Thus, although Jak2 and Lyn can phosphorylate the same site of Tec, we observed only Lyn can activate the Tec kinase under the sensitivity of our assay.
Tec can bind to Jak2 in insect cells.
We then tested whether Tec and Jak2 can physically associate with each other in cells. Recombinant baculovirus expressing Tec or TecKM was used to infect Sf21 cells either alone or in combination with the virus expressing Jak2 or Jak2KE (Fig5). Jak2 was immunoprecipitated from the cells lysed by the 0.1% lysis buffer, and immunoblotted with anti-Tec serum. Tec could be identified very weakly in the Jak2-immune complex, and more clearly found was TecKM in the Jak2-immune complex (top panel). Also, Jak2KE was shown to associate with Tec irrespective of the Tec-activity. Thus, Tec can weakly bind to Jak2 in insect cells, and this interaction does not require the kinase activity of Tec and Jak2. It should be noted that co-introduction of kinase-active Tec always reduced the expression level of Jak2 in Sf21 cells (middle panel, and also confirmed in other repeated experiments). Therefore, difference of the amounts of coprecipitated Tec may have arisen from the different expression level of Jak2 (compare the intensities of the bands between the top and middle panels).
Tec can constitutively associate with Jak2 in Sf21 cells. Sf21 cells were infected with baculovirus expressing Tec (T), Jak2 (J), TecKM (TM), and Jak2KE(JE) in the combinations indicated at the top. Jak2 was immunoprecipitated from each cells lysed by the 0.1%-lysis buffer (αJak IP), and probed with either anti-Tec serum (αTec) or anti-Jak2 serum (αJak2). Total cell lysates (TCL: 10 μg/lane) of each fraction were also probed with anti-Tec serum to estimate the expression level of Tec.
Tec can constitutively associate with Jak2 in Sf21 cells. Sf21 cells were infected with baculovirus expressing Tec (T), Jak2 (J), TecKM (TM), and Jak2KE(JE) in the combinations indicated at the top. Jak2 was immunoprecipitated from each cells lysed by the 0.1%-lysis buffer (αJak IP), and probed with either anti-Tec serum (αTec) or anti-Jak2 serum (αJak2). Total cell lysates (TCL: 10 μg/lane) of each fraction were also probed with anti-Tec serum to estimate the expression level of Tec.
We could not test the Tec/Jak2 interaction in the reverse direction, since Jak2 nonspecifically bound to protein A-Sepharose beads and glutathione Sepharose 4B (and even to nickel agarose beads) when Jak2 was expressed abundantly in either 293 or Sf21 cells (data not shown).
DISCUSSION
In this report we have shown that Tec is involved in the signaling pathway of GMR, especially in the c-fos activation machinery. Because Jak2 was previously shown to be an intermediate in the cytokine-driven c-fos activation pathway, both of Jak2 and Tec should play a role in the regulation of c-fos transcription. Furthermore, our data with dnJak2 support an intriguing idea to place Jak2 downstream of Tec in the mechanism of c-fos activation.
How does Jak2 participate in the Tec-driven pathway to the c-fos gene? A simple hypothesis is that Jak2 becomes activated via the phosphorylation by Tec, and drives the c-fostranscription as an effector of the Tec kinase. However, this is unlikely because coexpression of Tec could not affect the activity of Jak2 in either mammalian or insect cells. The second explanation is that Jak2 may be required to fully activate Tec through the phosphorylation of Tec protein by Jak2. This assumption is again unlikely because (1) coexpression of Jak2 could not activate Tec in either 293 cells or Sf21 cells, and (2) coexpression of dnJak2 with Tec in 293 cells did not suppress the kinase activity or tyrosine-phosphorylation of Tec (data not shown). Therefore, Jak2 may not be a direct second messenger of Tec, but should be required for the appropriate function of Tec-substrates (“Substrate X” in Fig6). There are several possible scenarios for such interaction. Jak2 may be, for instance, prerequisite to recruit the substrates of Tec into the cytokine receptor complex. It is well known that cytokine receptors are the good substrates of Jak-family kinases both in vitro and in vivo, and that a variety of signaling molecules become associated with the receptors through the phosphotyrosine-SH2 domain (or phosphotyrosine-binding [PTB] domain) interaction.38 Thus, it is possible that the second messengers for c-fos activation can become accessible to Tec through the phosphorylation of receptors by Jak2. Another explanation may be that Tec collects its substrates by phosphorylating Jak2 and thereby making it bound to the Tec-substrates. In this scenario, Jak2 plays as a “bridge” to connect Tec and its effector molecules. There would be, again, the other possibility that Jak2 is required to phosphorylate the Tec-substrates and to let them associated with Tec. To determine which interaction really takes place in vivo, we have to identify the “Substrate X” responsible for the c-fosactivation, and we should also clarify the phosphorylation site(s) of Jak2 by the Tec kinase.
Cytokine-driven pathways to the c-fosproto-oncogene. When activated by Lyn, Tec phosphorylates “Substrate X” and triggers the signaling pathway linked to the c-fosactivation. Tec and Jak2 can trans-phosphorylate each other. The biological significance of this phosphorylation in the context of c-fos activation mechanism is not settled yet. Jak2 is required for the appropriate function of “Substrate X” as well as for the phosphorylation of STATs. The STATs activation may also have some roles in the regulation of the c-fos transcription.
Cytokine-driven pathways to the c-fosproto-oncogene. When activated by Lyn, Tec phosphorylates “Substrate X” and triggers the signaling pathway linked to the c-fosactivation. Tec and Jak2 can trans-phosphorylate each other. The biological significance of this phosphorylation in the context of c-fos activation mechanism is not settled yet. Jak2 is required for the appropriate function of “Substrate X” as well as for the phosphorylation of STATs. The STATs activation may also have some roles in the regulation of the c-fos transcription.
Analysis of various deletion mutants of human GMR common β chain (βc) showed that a central area in the cytoplasmic region of βc is necessary for cytokine-dependent Shc phosphorylation, activation of Ras, and induction of the c-fosgene.35,39 In fibroblasts, Shc is already known to bind to Grb2 in a phosphorylation-dependent manner, and thereby to trigger the recruitment of SOS guanine nucleotide exchanging factor and the Ras activation.40 Therefore, Shc/Ras may be a key component to drive the c-fos transcription also in blood cells. If this is the case, Shc would be an intriguing candidate for the “Substrate X” in Fig 6. Currently we have only a few data to support this hypothesis. First, because expression of dnRas could suppress the cytokine-driven as well as Tec-driven c-fos activation, Ras itself or the Ras-regulated machinery should be a relay point of the pathway to the c-fos gene. Second, we already proved that Shc can be associated (either directly or indirectly) with Tec in cells. However, it is yet to be shown whether Shc is a direct substrate of Tec in vivo, and whether Shc plays a central role in the activation of c-fos gene in the hematopoietic system.
Our mapping experiments evidenced that Tyr-518 is the major phosphorylation site of Tec by both Jak2 and Lyn. The fact that Jak2 is capable of phosphorylating Tec was also confirmed in another laboratory (T. Matsuda and J.N. Ihle, personal communication). However, only Lyn could enhance the kinase activity of Tec in our experiments. Although we do not have any evidence to explain this discrepancy, several possibilities can be raised. First, as shown in Fig 4A and B, stoichiometry of Tec-phosphorylation was always higher when coexpressed with Lyn than with Jak2. Thus, low level of Tyr-518 phosphorylation by Jak2 may not be sufficient to demonstrate the enhancement of autophosphorylation activity in the anti-Tec immunoprecipitates. On the other hand, a weak but still significant tyrosine-phosphorylation of TecKM,518F could be reproducibly observed in a longer exposure of the film, when coexpressed with either Lyn or Jak2 (data not shown). Thus, there may be additional phosphorylation sites by Jak2 and Lyn, and these minor sites may have a pivotal role in the regulation of Tec activity. It is also possible that Lyn and Jak2 bind to Tec at different sites, and that these bindings may render distinct allosteric effects on Tec molecules.
Our report has shown the presence of a “cross-talk” between Tec and Jak2 PTKs. Although Tec is the first PTK among non-Jak kinases shown capable of phosphorylating Jak2, it would not be surprising if the members of other PTK-families are also able to phosphorylate Jak kinases. Growth of blood cells would be controlled through these complexed networks among various PTKs, and our observation would be an important information to decipher the control mechanisms.
(C) BA/F3-hGMRαβ cells (1 × 107) were transfected with the pfos/luc reporter plasmid (0.5 μg) with various amounts of pSRα-Tec▵KD (T▵KD) as indicated at the bottom (in micrograms). After starvation in cytokine-free medium, cells were further cultured for 3 hours without (no factor) or with GM-CSF (+GM-CSF; 5 ng/mL) or mouse IL-3 (+IL-3; 25 U/mL). Total amount of plasmid DNA for each transfection was adjusted to be equal by adding the pSRα vector DNA (pSRα). The mean value plus SD of the luciferase activities in triplicate samples from each fraction is shown as arbitrary units. (D) BA/F3-hGMRαβ cells (1 × 107) were transfected with the pfos/luc reporter plasmid (2 μg) together with of pSRα (pSRα), pSRα-Tec (Tec), and pSRα-dnRas (dnRas) plasmids at the amounts indicated at the bottom (in micrograms). Luciferase activity was assayed in the samples from the BA/F3-hGMRαβ cells without (no factor) or with the stimulation of GM-CSF (+GM-CSF) or IL-3 (+IL-3). The mean value plus SD of the luciferase activities in triplicate samples from each fraction is shown as arbitrary units. (E) BA/F3 cells (1 × 107) were transfected with the pFR-luc reporter plasmid (1 μg) and the pFA-Elk fusion transactivator plasmid (0.1 μg) by electroporation together with 30 μg each of pSRα (V), pSRα-Tec (Tec), or pSRα-Tec▵KD (▵KD). After starvation in cytokine-free medium for 5 hours, cells were further cultured for 3 hours without (no factor) or with IL-3 (+IL-3; 25 U/mL), and subjected to the luciferase assay. (F) BA/F3 cells (1 × 107) were transfected with the pFR-luc plasmid (1 μg) plus the pFA-Elk plasmid (0.1 μg) together with 30 μg each of pSRα (V) or pSRα-Tec (Tec). After starvation in cytokine-free medium for 4 hours, cells were cultured for 1 hour with DMSO [PD(−)] or 50 μmol/L of PD98059/DMSO [PD(+)]. Cells were further cultured for 3 hours in the presence (+IL-3) or absence (no factor) of IL-3.(G) The structure of the c-fos promoter fragment. The c-fos promoter contains four known regulatory elements, namely, the sis-inducible element (SIE), serum response element (SRE), fos AP-1 binding element (FAP), and calcium and cyclic AMP response element (Ca/CRE). (H) BA/F3 cells (1 × 107) were transfected with 5 μg of pSRα-Tec (Tec) and 2 μg of pfos/luc (WT) or the pfos/luc mutants carrying point mutations at SIE (mSIE), SRE (mSRE), FAP (mFAP), or Ca/CRE site (mCRE). After starvation in cytokine-free medium for 5 hours, cells were further cultured for 3 hours without (no factor) or with IL-3 (+IL-3; 25 U/mL), and subjected to the luciferase assay. (I) BA/F3-hGMRαβ cells (1 × 107) were transfected with the pfos/luc reporter plasmid (2 μg) together with of pSRα (pSRα), pSRα-dnJak2 (dnJak) and pSRα-Tec (Tec) plasmids at the amounts indicated at the bottom (in micrograms). Luciferase activity was assayed in the samples from the BA/F3-hGMRαβ cells without (no factor) or with the stimulation of GM-CSF (+GM-CSF) or IL-3 (+IL-3). (J) BA/F3-hGMRαβ cells (1 × 107cells) were transfected with the pfos/luc reporter plasmid (2 μg) together with of pSRα (pSRα), pSRα-Jak2 (Jak2) and pSRα-TecKM (TecKM) plasmids at the amounts indicated at the bottom (in micrograms). Luciferase activity was assayed in the samples from the BA/F3-hGMRαβ cells without (no factor) or with the stimulation of GM-CSF (+GM-CSF) or IL-3 (+IL-3).
ACKNOWLEDGMENT
We are grateful to J.N. Ihle for the kind gifts of Jak2 cDNAs and for critical reading of this manuscript. We thank T. Curran for thefos-promoter constructs, A. Kikuchi for dominant negative Ras cDNA, T. Mustelin for tagged Syk cDNA, T. Yi for Lyn A cDNA, Kirin Brewery (Tokyo, Japan) for mouse IL-3, and Sumitomo Pharmaceutical Company (Osaka, Japan) for human GM-CSF. The H902-production hybridoma cell line is the reagent no. 521 from the NIH AIDS Research and Reference Program (Bethesda, MD).
Supported in part by the Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science and Culture, Japan, and by the grant from Haraguchi Memorial Cancer Research Fund.
Address reprint requests to Hiroyuki Mano, MD, PhD, Department of Molecular Biology, Jichi Medical School, Yakushiji 3311-1, Minami-kawachi-machi, Kawachi-gun, Tochigi 329-04, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be here-by marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Tec is involved in the signaling pathway of GM-CSF receptor. (A) UT-7 cells (1 × 107) were cultured in the starvation medium for 12 hours and then stimulated with 10 ng/mL of human GM-CSF (+) for 5 minutes or left unstimulated (−). Tec was immunoprecipitated from each fraction (αTec), subjected to 7.5% SDS-PAGE, and immunoblotted with anti-phosphotyrosine antibody (αP-Tyr). Total cell lysates (TCL; 10 μg/lane) and the immunoprecipitates by normal rabbit serum (NRS) prepared from the same set of cells were also analyzed. The position of Tec is indicated by an arrow. The molecular weight standards (×10−3) are shown at the left. The same membrane was reblotted with anti-Tec serum to show the amounts of Tec precipitated (lower panel). (B) UT-7 cells were stimulated with GM-CSF (10 ng/mL) for 0, 1, 5, 10, or 20 minutes as indicated at the top. Tec was immunoprecipitated from each fraction (1 × 107 cells), and was immunoblotted with anti-phosphotyrosine antibody (αP-Tyr) or anti-Tec serum (αTec). (C) Tec was immunoprecipitated from 1 × 107 of UT-7 cells with (+) or without (−) 5 minutes of GM-CSF stimulation, and was subjected to an in vitro kinase assay. Autophosphorylation of pp70Tec is shown. (D) Specific kinase activity of the Tec protein (32P-incorporation/protein amount) with (+) or without (−) the GM-CSF stimulation was calculated by densitometric analysis and shown as arbitrary units. (E) Tec was immunoprecipitated from UT-7 cells (1 × 107), with (GM) or without (−) the GM-CSF stimulation (10 ng/mL), metabolically labeled with [32P]orthophosphate (37 MBq/mL), and was analyzed by 7.5% SDS-PAGE. The proteins were blotted onto a PVDF membrane, and heated in 1 N KOH to decrease the backgrounds of serine- and threonine-phosphorylation. The position of Tec is indicated. (F) pp70Tec in (E) was subjected to the phosphoamino acid analysis. The positions of free phosphate (Pi), phosphoserine (p-Ser), phosphothreonine (p-Thr), and phosphotyrosine (p-Tyr) are indicated at the right.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1496/3/m_blod4053701a.jpeg?Expires=1763492363&Signature=WvEOKgaXdfdDQX-KpMl7Ldp2-REp7dUigoAD7pYqhsJHTMLuBVS1KNPRXDTDJ33VOkHPCL8WgTe9cXy0T64woWZ9YiqWWA7nYWNQY9C~MlbiZA~~sDkrrc5u1KX8Zhs58DuPoFHksoySU~T826PTGSZYCCRJBrMsuos6Q1WRJJZqig2yuT9KuilR80IB834URYrKMeyPQ7jD-bmQzbG6FcXcBL5xoC8LeXexY2NMN8EH-EWzMWjL3lgJ6x~B~OrTJ9cd1325dpkIRFQoaC7xzgniK17yPWifkGrV4O~8SF2oE3Ej7ct0pbQuyiJId5k8k5WUapDZSM96o8tNK06KuA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Tec is involved in the signaling pathway of GM-CSF receptor. (A) UT-7 cells (1 × 107) were cultured in the starvation medium for 12 hours and then stimulated with 10 ng/mL of human GM-CSF (+) for 5 minutes or left unstimulated (−). Tec was immunoprecipitated from each fraction (αTec), subjected to 7.5% SDS-PAGE, and immunoblotted with anti-phosphotyrosine antibody (αP-Tyr). Total cell lysates (TCL; 10 μg/lane) and the immunoprecipitates by normal rabbit serum (NRS) prepared from the same set of cells were also analyzed. The position of Tec is indicated by an arrow. The molecular weight standards (×10−3) are shown at the left. The same membrane was reblotted with anti-Tec serum to show the amounts of Tec precipitated (lower panel). (B) UT-7 cells were stimulated with GM-CSF (10 ng/mL) for 0, 1, 5, 10, or 20 minutes as indicated at the top. Tec was immunoprecipitated from each fraction (1 × 107 cells), and was immunoblotted with anti-phosphotyrosine antibody (αP-Tyr) or anti-Tec serum (αTec). (C) Tec was immunoprecipitated from 1 × 107 of UT-7 cells with (+) or without (−) 5 minutes of GM-CSF stimulation, and was subjected to an in vitro kinase assay. Autophosphorylation of pp70Tec is shown. (D) Specific kinase activity of the Tec protein (32P-incorporation/protein amount) with (+) or without (−) the GM-CSF stimulation was calculated by densitometric analysis and shown as arbitrary units. (E) Tec was immunoprecipitated from UT-7 cells (1 × 107), with (GM) or without (−) the GM-CSF stimulation (10 ng/mL), metabolically labeled with [32P]orthophosphate (37 MBq/mL), and was analyzed by 7.5% SDS-PAGE. The proteins were blotted onto a PVDF membrane, and heated in 1 N KOH to decrease the backgrounds of serine- and threonine-phosphorylation. The position of Tec is indicated. (F) pp70Tec in (E) was subjected to the phosphoamino acid analysis. The positions of free phosphate (Pi), phosphoserine (p-Ser), phosphothreonine (p-Thr), and phosphotyrosine (p-Tyr) are indicated at the right.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1496/3/m_blod4053701b.jpeg?Expires=1763492363&Signature=kOgLU8AXYV9CCdaKD6X1U6wsG57H1~-u5MGFE3cPBNYRhDvaiKoiQUlvqj6krLZVV6Fjz~MMmTiESvW5bevIx5CiOV10frySKHJaL2eRf6CzVmNqDAu7aamzqCiFA4o1lJrZh7ZBJ0JPCn-gENH9023TVHU6VvbRIhhYhZH6vXZ9UnwQQovy6I8yurtHc4diMiiDL2NwPn5smg8WMuBrX26Fm4EbIHkAJqccVBLJQVZKX9yyvdvx5WWPfaOz0U8Q~4hXhBtHDJBRDDqydhho4huXMejFYOJVRoU7IP~9VNcH5mS6VluOOaf~rhnveFKzkmXH5neU9ESRb-T~heUJPg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Tec is involved in the signaling pathway of GM-CSF receptor. (A) UT-7 cells (1 × 107) were cultured in the starvation medium for 12 hours and then stimulated with 10 ng/mL of human GM-CSF (+) for 5 minutes or left unstimulated (−). Tec was immunoprecipitated from each fraction (αTec), subjected to 7.5% SDS-PAGE, and immunoblotted with anti-phosphotyrosine antibody (αP-Tyr). Total cell lysates (TCL; 10 μg/lane) and the immunoprecipitates by normal rabbit serum (NRS) prepared from the same set of cells were also analyzed. The position of Tec is indicated by an arrow. The molecular weight standards (×10−3) are shown at the left. The same membrane was reblotted with anti-Tec serum to show the amounts of Tec precipitated (lower panel). (B) UT-7 cells were stimulated with GM-CSF (10 ng/mL) for 0, 1, 5, 10, or 20 minutes as indicated at the top. Tec was immunoprecipitated from each fraction (1 × 107 cells), and was immunoblotted with anti-phosphotyrosine antibody (αP-Tyr) or anti-Tec serum (αTec). (C) Tec was immunoprecipitated from 1 × 107 of UT-7 cells with (+) or without (−) 5 minutes of GM-CSF stimulation, and was subjected to an in vitro kinase assay. Autophosphorylation of pp70Tec is shown. (D) Specific kinase activity of the Tec protein (32P-incorporation/protein amount) with (+) or without (−) the GM-CSF stimulation was calculated by densitometric analysis and shown as arbitrary units. (E) Tec was immunoprecipitated from UT-7 cells (1 × 107), with (GM) or without (−) the GM-CSF stimulation (10 ng/mL), metabolically labeled with [32P]orthophosphate (37 MBq/mL), and was analyzed by 7.5% SDS-PAGE. The proteins were blotted onto a PVDF membrane, and heated in 1 N KOH to decrease the backgrounds of serine- and threonine-phosphorylation. The position of Tec is indicated. (F) pp70Tec in (E) was subjected to the phosphoamino acid analysis. The positions of free phosphate (Pi), phosphoserine (p-Ser), phosphothreonine (p-Thr), and phosphotyrosine (p-Tyr) are indicated at the right.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1496/3/m_blod4053701c.jpeg?Expires=1763492363&Signature=ZPwy9VXQk5nLZwurBkeLDv1FLd5xd2omS-tJpykcOrTIfHmOUBWqjO-YfPp646N6qqmh881Xan~eKhg-ynwV0UcHtqPPfwn8EXjnigGT~2DtRvHwlFnCqbULEbgzIPIs7~GtKaXvFiO-fBySpv0fSvhBYVEOXARaYIGHnwUYqwFImjtvXg~W5D3iokWCsqsVp3MmXc1jGiDNDAMFdYEwXEnwy51E~PFyUqW9Yu6Z8IrS0zNNfYIs4mqoipMLLGHBXbDGtcVv9rsScn~kMoxkead8XXMyJZomBBGUlufPSNjeNeE9Z6Z1~75DJ846qqFtbeWpWJbCFwPbGXuPHpsPxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Tec is involved in the signaling pathway of GM-CSF receptor. (A) UT-7 cells (1 × 107) were cultured in the starvation medium for 12 hours and then stimulated with 10 ng/mL of human GM-CSF (+) for 5 minutes or left unstimulated (−). Tec was immunoprecipitated from each fraction (αTec), subjected to 7.5% SDS-PAGE, and immunoblotted with anti-phosphotyrosine antibody (αP-Tyr). Total cell lysates (TCL; 10 μg/lane) and the immunoprecipitates by normal rabbit serum (NRS) prepared from the same set of cells were also analyzed. The position of Tec is indicated by an arrow. The molecular weight standards (×10−3) are shown at the left. The same membrane was reblotted with anti-Tec serum to show the amounts of Tec precipitated (lower panel). (B) UT-7 cells were stimulated with GM-CSF (10 ng/mL) for 0, 1, 5, 10, or 20 minutes as indicated at the top. Tec was immunoprecipitated from each fraction (1 × 107 cells), and was immunoblotted with anti-phosphotyrosine antibody (αP-Tyr) or anti-Tec serum (αTec). (C) Tec was immunoprecipitated from 1 × 107 of UT-7 cells with (+) or without (−) 5 minutes of GM-CSF stimulation, and was subjected to an in vitro kinase assay. Autophosphorylation of pp70Tec is shown. (D) Specific kinase activity of the Tec protein (32P-incorporation/protein amount) with (+) or without (−) the GM-CSF stimulation was calculated by densitometric analysis and shown as arbitrary units. (E) Tec was immunoprecipitated from UT-7 cells (1 × 107), with (GM) or without (−) the GM-CSF stimulation (10 ng/mL), metabolically labeled with [32P]orthophosphate (37 MBq/mL), and was analyzed by 7.5% SDS-PAGE. The proteins were blotted onto a PVDF membrane, and heated in 1 N KOH to decrease the backgrounds of serine- and threonine-phosphorylation. The position of Tec is indicated. (F) pp70Tec in (E) was subjected to the phosphoamino acid analysis. The positions of free phosphate (Pi), phosphoserine (p-Ser), phosphothreonine (p-Thr), and phosphotyrosine (p-Tyr) are indicated at the right.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1496/3/m_blod4053701d.jpeg?Expires=1763492363&Signature=rwsSUlzplFTTcZMnyDpn9ABSKdhTODWXUsynb1hq~tVC0wI23sQwLF0MCX~Pqf4G6vUwk9~fB1zIdW1dA5w2UdloStxOP~Mneu4yVeMzl7Rt4ctQAWxTiktfyOdEr~N8oxvb0KxqIM~Yi~bykHQJVI-6VKJY2XDeSPbKRjv~fPj7bT3BxHkKt2B98u9pi4Rf1Cl7A1Ma9bEufEswlMUwMk5CZZySoed3dxvXD25AgraYFytkllamh1yerExd3~AWVB5a~nkJ3m-MCGOtKRX9TeWI1Aw36uW2C1xK3g6YKyywmfS5NRmecnfWe3CZaGsqT56brm1r4uBsuQc3KKNsCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Tec is involved in the signaling pathway of GM-CSF receptor. (A) UT-7 cells (1 × 107) were cultured in the starvation medium for 12 hours and then stimulated with 10 ng/mL of human GM-CSF (+) for 5 minutes or left unstimulated (−). Tec was immunoprecipitated from each fraction (αTec), subjected to 7.5% SDS-PAGE, and immunoblotted with anti-phosphotyrosine antibody (αP-Tyr). Total cell lysates (TCL; 10 μg/lane) and the immunoprecipitates by normal rabbit serum (NRS) prepared from the same set of cells were also analyzed. The position of Tec is indicated by an arrow. The molecular weight standards (×10−3) are shown at the left. The same membrane was reblotted with anti-Tec serum to show the amounts of Tec precipitated (lower panel). (B) UT-7 cells were stimulated with GM-CSF (10 ng/mL) for 0, 1, 5, 10, or 20 minutes as indicated at the top. Tec was immunoprecipitated from each fraction (1 × 107 cells), and was immunoblotted with anti-phosphotyrosine antibody (αP-Tyr) or anti-Tec serum (αTec). (C) Tec was immunoprecipitated from 1 × 107 of UT-7 cells with (+) or without (−) 5 minutes of GM-CSF stimulation, and was subjected to an in vitro kinase assay. Autophosphorylation of pp70Tec is shown. (D) Specific kinase activity of the Tec protein (32P-incorporation/protein amount) with (+) or without (−) the GM-CSF stimulation was calculated by densitometric analysis and shown as arbitrary units. (E) Tec was immunoprecipitated from UT-7 cells (1 × 107), with (GM) or without (−) the GM-CSF stimulation (10 ng/mL), metabolically labeled with [32P]orthophosphate (37 MBq/mL), and was analyzed by 7.5% SDS-PAGE. The proteins were blotted onto a PVDF membrane, and heated in 1 N KOH to decrease the backgrounds of serine- and threonine-phosphorylation. The position of Tec is indicated. (F) pp70Tec in (E) was subjected to the phosphoamino acid analysis. The positions of free phosphate (Pi), phosphoserine (p-Ser), phosphothreonine (p-Thr), and phosphotyrosine (p-Tyr) are indicated at the right.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1496/3/m_blod4053701e.jpeg?Expires=1763492363&Signature=SXCvQ2LKtZ5f4NNHOiDtPurJxZFGHM5B4wieqtYuYMf6sTwOLcPQPDFnjEM0K1WU10CnfQyT1dCrRUsoFrKPycJf2On0jdQ92FR7Hvt00C8ilykQIWehlQTWbjQz3~CGj7gFuzo3G~q40~18PDIbBHoxmM67Oxv~Dbk5Xmprebupib0kq64A6RJ8Xu~o5ZJ3hNau4S5ONCBdA9eyb248-b2YY3TEN38IaabHqoV4JaLuu5j8crMQ0zgmlP5UnkKHcm5jw-CXpG8Eq24mOpZIffcgtu3DY~89CoCjw6xxD5MQ3eZtM2qrsUg1TMhNxG9SKm9u7qwqsye9ltLEQp6Pfg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Tec is involved in the signaling pathway of GM-CSF receptor. (A) UT-7 cells (1 × 107) were cultured in the starvation medium for 12 hours and then stimulated with 10 ng/mL of human GM-CSF (+) for 5 minutes or left unstimulated (−). Tec was immunoprecipitated from each fraction (αTec), subjected to 7.5% SDS-PAGE, and immunoblotted with anti-phosphotyrosine antibody (αP-Tyr). Total cell lysates (TCL; 10 μg/lane) and the immunoprecipitates by normal rabbit serum (NRS) prepared from the same set of cells were also analyzed. The position of Tec is indicated by an arrow. The molecular weight standards (×10−3) are shown at the left. The same membrane was reblotted with anti-Tec serum to show the amounts of Tec precipitated (lower panel). (B) UT-7 cells were stimulated with GM-CSF (10 ng/mL) for 0, 1, 5, 10, or 20 minutes as indicated at the top. Tec was immunoprecipitated from each fraction (1 × 107 cells), and was immunoblotted with anti-phosphotyrosine antibody (αP-Tyr) or anti-Tec serum (αTec). (C) Tec was immunoprecipitated from 1 × 107 of UT-7 cells with (+) or without (−) 5 minutes of GM-CSF stimulation, and was subjected to an in vitro kinase assay. Autophosphorylation of pp70Tec is shown. (D) Specific kinase activity of the Tec protein (32P-incorporation/protein amount) with (+) or without (−) the GM-CSF stimulation was calculated by densitometric analysis and shown as arbitrary units. (E) Tec was immunoprecipitated from UT-7 cells (1 × 107), with (GM) or without (−) the GM-CSF stimulation (10 ng/mL), metabolically labeled with [32P]orthophosphate (37 MBq/mL), and was analyzed by 7.5% SDS-PAGE. The proteins were blotted onto a PVDF membrane, and heated in 1 N KOH to decrease the backgrounds of serine- and threonine-phosphorylation. The position of Tec is indicated. (F) pp70Tec in (E) was subjected to the phosphoamino acid analysis. The positions of free phosphate (Pi), phosphoserine (p-Ser), phosphothreonine (p-Thr), and phosphotyrosine (p-Tyr) are indicated at the right.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1496/3/m_blod4053701f.jpeg?Expires=1763492363&Signature=M6FDfT7dD6DZHC1rppUu~dFexYMiiUPkuYtzVduAqRY5rTqvBto8F4bUcxSfDAJb9mmFJMtM1-hk9qdt2LwlXU-5PGRSMLEdlBW66OjwD9rHt1jeKCNVYOdIuUBH0yWTgLyI5TGuk7FcgwLzG~FMv88ENy1HYs-T4BZhgqZmlGR3JsmKDKKO-wSSpJvTB4sKBfYtH4fT5GX16NVs4qyiFgo9HsIug~lTxUl5LQBtPJmHLrkqDnDrvb-u-tmu-IJQqnmxugABFrToZKTqQThimKilxRAcM1nYPiDP69XoJObKCRZPcy~1sjQ8uo9XUAbYAnqBKohpT8IkqsxTOSl61w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Fig. 3. Tec can phosphorylate Jak2 in both mammalian and insect cells. (A) Jak2KE was immunoprecipitated from 2 × 106 of 293 cells expressing Jak2KE with (T) or without (−) Tec. Total cell lysates (TCL, 10 μg/lane) and anti-Jak2 immunoprecipitates (Jak IP) were electrophoresed and probed with anti-phosphotyrosine antibody (αP-Tyr) or anti-Jak2 serum (αJak2). The positions of Jak2 (Jak2), Tec (Tec), and the Ig heavy chain (IgH) are indicated at the right. (B) Jak2KE was immunoprecipitated from Sf21 cells infected with Jak2KEexpressing baculovirus (JE) alone or in combination with Tec-expressing (T) or TecKM-expressing (TM) virus. The immunoprecipitates were separated through 7.5% SDS-PAGE and probed with anti-phosphotyrosine antibody (αP-Tyr) or anti-Jak2 serum (αJak2). The positions of Jak2 (Jak2) and Tec (Tec) are indicated at the right. (C) Jak2 was immunoprecipitated from Sf21 cells expressing Jak2 (J) or Jak2KE (JE) either alone or in combination with Tec (T). The immunoprecipitates were incubated with [γ-32P]ATP and the synthetic Jak2-substrate, and subjected to Tricine-SDS-PAGE. Phosphorylation of the Jak2-substrate is shown. (D) Total cell lysates (TCL: 10 μg/lane) and the anti-Jak2 immunoprecipitates (Jak IP) were prepared from parental BA/F3 cells (P) and two BA/F3 clones (1 and 2) stably expressing Tec▵SH3, and immunoblotted with αP-Tyr Ab (upper panel) or anti-Jak2 serum (lower panel). The position of Jak2 is indicated at the right. The positions of molecular weight standards (×10−3) are also shown at the left.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1496/3/m_blod4053703a.jpeg?Expires=1763492363&Signature=nGp0Ntb~xQSJh7v8C9IWAYJYPDg-xnNlHVtJBdeTGYy6mwUCPStACUCozuI0Ao1B--C9PdxV~OnpwSFTdzIkAPr0XRobk-W2XsOGU5E7DL64aHVckOSdATq1jfrOwU-QflqblnBp3EGjbcTeIgZ5z4tbrfSRd4K6QnJP9ok-lhTzvG3L7IjYLPy~~h~OeVrXqNgeZOtd-E6gUGZT3fBkz~gmFgnMYpsSPKNzHnoUccKK8mjLNEEKQAajN6~4ytJ327lCxyElLZ5l-lKMOl-QGfJ~TGUSErT8u0RuqlTKrhYEjYsxiVf20TmLOKK1aKVzKHCD29CeFiBpOfSCs46J7w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Tec can phosphorylate Jak2 in both mammalian and insect cells. (A) Jak2KE was immunoprecipitated from 2 × 106 of 293 cells expressing Jak2KE with (T) or without (−) Tec. Total cell lysates (TCL, 10 μg/lane) and anti-Jak2 immunoprecipitates (Jak IP) were electrophoresed and probed with anti-phosphotyrosine antibody (αP-Tyr) or anti-Jak2 serum (αJak2). The positions of Jak2 (Jak2), Tec (Tec), and the Ig heavy chain (IgH) are indicated at the right. (B) Jak2KE was immunoprecipitated from Sf21 cells infected with Jak2KEexpressing baculovirus (JE) alone or in combination with Tec-expressing (T) or TecKM-expressing (TM) virus. The immunoprecipitates were separated through 7.5% SDS-PAGE and probed with anti-phosphotyrosine antibody (αP-Tyr) or anti-Jak2 serum (αJak2). The positions of Jak2 (Jak2) and Tec (Tec) are indicated at the right. (C) Jak2 was immunoprecipitated from Sf21 cells expressing Jak2 (J) or Jak2KE (JE) either alone or in combination with Tec (T). The immunoprecipitates were incubated with [γ-32P]ATP and the synthetic Jak2-substrate, and subjected to Tricine-SDS-PAGE. Phosphorylation of the Jak2-substrate is shown. (D) Total cell lysates (TCL: 10 μg/lane) and the anti-Jak2 immunoprecipitates (Jak IP) were prepared from parental BA/F3 cells (P) and two BA/F3 clones (1 and 2) stably expressing Tec▵SH3, and immunoblotted with αP-Tyr Ab (upper panel) or anti-Jak2 serum (lower panel). The position of Jak2 is indicated at the right. The positions of molecular weight standards (×10−3) are also shown at the left.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1496/3/m_blod4053703b.jpeg?Expires=1763492363&Signature=wsaNMHHOkYCE5xYE04x26-OrEbrr5swzmXSTcHPGII0XxXpqvaFc0P2nZcB5kTxqhGGPj0hYwa0knEYth~n-7gwmymvEn6HEHzsDmsnNPfelNY1WSRnxPmENRPu88la0AnLCg5B-FMsSn0-UOqQLxmfOpSCA0TvFlXgYQqVigxbj6kLowkw-mShGSiI8tQLjg74vKOVaHLy4KNIZ6td7qznxUjZ2goHaBXNgd8FvoGoDfiId905sSkZHyMoXHrBCt9JtS2hqI~wVvBG2oPgCSM3bUoS0qlolZXpsnG8xeSPW~UHBYitEce7VWdIDdNET6Ngj4InseRM5~jInHNa9eA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Tec can phosphorylate Jak2 in both mammalian and insect cells. (A) Jak2KE was immunoprecipitated from 2 × 106 of 293 cells expressing Jak2KE with (T) or without (−) Tec. Total cell lysates (TCL, 10 μg/lane) and anti-Jak2 immunoprecipitates (Jak IP) were electrophoresed and probed with anti-phosphotyrosine antibody (αP-Tyr) or anti-Jak2 serum (αJak2). The positions of Jak2 (Jak2), Tec (Tec), and the Ig heavy chain (IgH) are indicated at the right. (B) Jak2KE was immunoprecipitated from Sf21 cells infected with Jak2KEexpressing baculovirus (JE) alone or in combination with Tec-expressing (T) or TecKM-expressing (TM) virus. The immunoprecipitates were separated through 7.5% SDS-PAGE and probed with anti-phosphotyrosine antibody (αP-Tyr) or anti-Jak2 serum (αJak2). The positions of Jak2 (Jak2) and Tec (Tec) are indicated at the right. (C) Jak2 was immunoprecipitated from Sf21 cells expressing Jak2 (J) or Jak2KE (JE) either alone or in combination with Tec (T). The immunoprecipitates were incubated with [γ-32P]ATP and the synthetic Jak2-substrate, and subjected to Tricine-SDS-PAGE. Phosphorylation of the Jak2-substrate is shown. (D) Total cell lysates (TCL: 10 μg/lane) and the anti-Jak2 immunoprecipitates (Jak IP) were prepared from parental BA/F3 cells (P) and two BA/F3 clones (1 and 2) stably expressing Tec▵SH3, and immunoblotted with αP-Tyr Ab (upper panel) or anti-Jak2 serum (lower panel). The position of Jak2 is indicated at the right. The positions of molecular weight standards (×10−3) are also shown at the left.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1496/3/m_blod4053703c.jpeg?Expires=1763492363&Signature=MIXM9GKyvYA-T4qxK65PMCO-suiNd-5bfySoAvHQPhlAvzjg6k~j~EZyDO0xPJQ1fJqbWEWd~BX3EmP2~nZ7FHBzKl1L8ld7X7zlaMN59nSgfZtwAQB-wMY-k~PirSGMYGylrxdk3ZgRdwjTM-jnnI0GZHUCUbPEfhfmJU-bI1~7nqzN28ltKqKxApFDvds~Rqqgrjy7wpFZZ-e~LNmDT9ZyJqgFhqJ3Hdu4Sm6ck59grEbs9rw6xMQDNHZhYMN3xyS6EYtj8L5N8CwWGaNFq747nB4RI0hoPWRMHyiXbIjkG9i-U9OzA4rhwfZeUZj2g2pRO5HuDyH8WangUsv80A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Tec can phosphorylate Jak2 in both mammalian and insect cells. (A) Jak2KE was immunoprecipitated from 2 × 106 of 293 cells expressing Jak2KE with (T) or without (−) Tec. Total cell lysates (TCL, 10 μg/lane) and anti-Jak2 immunoprecipitates (Jak IP) were electrophoresed and probed with anti-phosphotyrosine antibody (αP-Tyr) or anti-Jak2 serum (αJak2). The positions of Jak2 (Jak2), Tec (Tec), and the Ig heavy chain (IgH) are indicated at the right. (B) Jak2KE was immunoprecipitated from Sf21 cells infected with Jak2KEexpressing baculovirus (JE) alone or in combination with Tec-expressing (T) or TecKM-expressing (TM) virus. The immunoprecipitates were separated through 7.5% SDS-PAGE and probed with anti-phosphotyrosine antibody (αP-Tyr) or anti-Jak2 serum (αJak2). The positions of Jak2 (Jak2) and Tec (Tec) are indicated at the right. (C) Jak2 was immunoprecipitated from Sf21 cells expressing Jak2 (J) or Jak2KE (JE) either alone or in combination with Tec (T). The immunoprecipitates were incubated with [γ-32P]ATP and the synthetic Jak2-substrate, and subjected to Tricine-SDS-PAGE. Phosphorylation of the Jak2-substrate is shown. (D) Total cell lysates (TCL: 10 μg/lane) and the anti-Jak2 immunoprecipitates (Jak IP) were prepared from parental BA/F3 cells (P) and two BA/F3 clones (1 and 2) stably expressing Tec▵SH3, and immunoblotted with αP-Tyr Ab (upper panel) or anti-Jak2 serum (lower panel). The position of Jak2 is indicated at the right. The positions of molecular weight standards (×10−3) are also shown at the left.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1496/3/m_blod4053703d.jpeg?Expires=1763492363&Signature=aaLhdssXjy-vi2WHYzKe0G7vBPNgh3LyIuikzv1lb9TB-1StHnfjRaFuo~gL2uSmsWrITc1GkbmT9zphCZLXcOofveZUc2Q-nAoTIodKd9iFoFCsJNuSC5boeHeaBKwNNKHNGtHpL1W2XKqntWGX3vO1VRM-LQnvLwzZ-Dr7eWwBiM0MTF8mb7KsbJjKaGJ~FQ1uFRYxRje1t3QQxFoCjWCPLxHFgALebFdGQxx3J7lpShazY2m5NwkY6oaGCRkF~P7eoQBr5jfXC0GNfBDjT4vzpe9Yh5hHdail9QBqsiyyzaYxSOS8AywUkCcVcP7mpa9UypXynCIoptnQih-AIA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Jak2 can phosphorylate Tec at Tyr-518. (A) The kinase-dead TecKM (TM) was expressed in 293 cells either alone or in combination with Jak2 (Jak2) or Lyn (Lyn) kinase. Tec was immunoprecipitated from each fraction, and probed with anti-phosphotyrosine antibody (αP-Tyr) or anti-Tec serum (αTec). (B) TecKM (TM), TecKM▵SH3 (TM▵3), or TecKM,YF (TM,518F) was expressed in 293 cells either alone (−) or in combination with Jak2 (J) or Lyn (L). Tec was immunoprecipitated from each fraction, and blotted with anti-phosphotyrosine antibody (αP-Tyr) or anti-Tec serum (αTec). The positions of full-length Tec (T) and SH3-deleted (▵SH3) forms are indicated at the right. (C) The amino acid sequences of the Tec-family kinases, surrounding the tyrosine residues corresponding to Tyr-518 in mouse Tec, are compared. The asterisk indicates the position of the phosphorylated tyrosine. At the left shown are the numbers of amino acid positions of mouse Tec,2 human Btk,1011 mouse Emt/Itk/Tsk, 9,41,42 human Bmx,43 and human Txk.44 (D) pSRα (V), pSRα-Tec (T), or pSRα-TecKM (TM) was transfected into 293 cells with or without pSRα-Jak2 (J). Tec was immunoprecipitated from each fraction, and incubated with [γ−32P]ATP without exogenous substrates. Autophosphorylation of pp70Tec in each sample is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1496/3/m_blod4053704a.jpeg?Expires=1763492363&Signature=iM8s7Gz1ZZx4sHcCdvaPFo5rRxikEs06OYh~6reOFuCxwellUgOTI0MYOQnyIGRVw7ZQGafoO~NUwn9T-DBSkQf993T~VDdjtjfSV3b6y9QGCMZFi5kp-f7ICzTY3dtAyKmrNSnIcHQ5q6qLHBP2VVa0faRWWkCGCZcPdvMgW~doNKtwPaxAhfseKIALkB~FS6TpOD2UVItfL2R4CR-sk8ppZ0GL7uzlEsfFvE4~Jkr~F6qRutG6Fr3TvMR69mOrdSh6lu5OeWfAzKaLk9a~XF4fKIxB9t7GXedUks2r7RBgptttx0o21Mc2~dX2-HDrRDCH7BNAwWD7JXWfG4VFvA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Jak2 can phosphorylate Tec at Tyr-518. (A) The kinase-dead TecKM (TM) was expressed in 293 cells either alone or in combination with Jak2 (Jak2) or Lyn (Lyn) kinase. Tec was immunoprecipitated from each fraction, and probed with anti-phosphotyrosine antibody (αP-Tyr) or anti-Tec serum (αTec). (B) TecKM (TM), TecKM▵SH3 (TM▵3), or TecKM,YF (TM,518F) was expressed in 293 cells either alone (−) or in combination with Jak2 (J) or Lyn (L). Tec was immunoprecipitated from each fraction, and blotted with anti-phosphotyrosine antibody (αP-Tyr) or anti-Tec serum (αTec). The positions of full-length Tec (T) and SH3-deleted (▵SH3) forms are indicated at the right. (C) The amino acid sequences of the Tec-family kinases, surrounding the tyrosine residues corresponding to Tyr-518 in mouse Tec, are compared. The asterisk indicates the position of the phosphorylated tyrosine. At the left shown are the numbers of amino acid positions of mouse Tec,2 human Btk,1011 mouse Emt/Itk/Tsk, 9,41,42 human Bmx,43 and human Txk.44 (D) pSRα (V), pSRα-Tec (T), or pSRα-TecKM (TM) was transfected into 293 cells with or without pSRα-Jak2 (J). Tec was immunoprecipitated from each fraction, and incubated with [γ−32P]ATP without exogenous substrates. Autophosphorylation of pp70Tec in each sample is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1496/3/m_blod4053704b.jpeg?Expires=1763492363&Signature=0nwC~5TmIJLZr2u~fNR-tg03fW0yiOeSl5fJI1N5H7TF6UoIJ85pCxQMaSiLM7wXMxaDs0fYbXYbiye7X6lcc9zLOiQj3s6BGwf0pPBzgZ7flv5kuFCLHW0dHkzTJopzuDLvtbq8y0vcUnWR7XBspAcN56mfQ8NCx488nOhfW98BFW5GR1Uy3IEWpj1H3-L5LgJZlJeuw90FBqWbqwmNCx5QiRlwOpNslkSn26yiG~DP3Ob783m3fhxRfdRDAWUTf0zh5h6gxBL1p7JKW86R5qF1gu84iH8CYmDQ~0N-kyVMjlwU5id5-JDxNWjFtoXDwALfJu87o9c6szajk8CYcA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Jak2 can phosphorylate Tec at Tyr-518. (A) The kinase-dead TecKM (TM) was expressed in 293 cells either alone or in combination with Jak2 (Jak2) or Lyn (Lyn) kinase. Tec was immunoprecipitated from each fraction, and probed with anti-phosphotyrosine antibody (αP-Tyr) or anti-Tec serum (αTec). (B) TecKM (TM), TecKM▵SH3 (TM▵3), or TecKM,YF (TM,518F) was expressed in 293 cells either alone (−) or in combination with Jak2 (J) or Lyn (L). Tec was immunoprecipitated from each fraction, and blotted with anti-phosphotyrosine antibody (αP-Tyr) or anti-Tec serum (αTec). The positions of full-length Tec (T) and SH3-deleted (▵SH3) forms are indicated at the right. (C) The amino acid sequences of the Tec-family kinases, surrounding the tyrosine residues corresponding to Tyr-518 in mouse Tec, are compared. The asterisk indicates the position of the phosphorylated tyrosine. At the left shown are the numbers of amino acid positions of mouse Tec,2 human Btk,1011 mouse Emt/Itk/Tsk, 9,41,42 human Bmx,43 and human Txk.44 (D) pSRα (V), pSRα-Tec (T), or pSRα-TecKM (TM) was transfected into 293 cells with or without pSRα-Jak2 (J). Tec was immunoprecipitated from each fraction, and incubated with [γ−32P]ATP without exogenous substrates. Autophosphorylation of pp70Tec in each sample is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1496/3/m_blod4053704c.jpeg?Expires=1763492363&Signature=kzPcqcNkdKd8xFYuqbQybhLT22qxWn6756AJOhQPvrVoAUP72DVtIut4tmi1BS-YQeOTDRau7j52Fyo5M2ve7z7P-RKHngX76bYgk~1AJR7JHjePaohT73K-Wib-~Gk5D~~~KADylET7zlIYh2If8d-5gBz1DH2yhTPVpf1NKrZl4XtyPYcaCclNKvC6-WA3AlRMqcpNG-I9~Zp~izk2gQB2mUcrJzKmocoxcXmfAQ37-cD0vz1cNyDFFS7UMAkafpOUqTvuOtAmuotSnQzWbDVkGxcVMq5dbhctsw8LxtKrVEDGIvmYplPp0d9AEHouXydLbMjaphPkk8czuCrInw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Jak2 can phosphorylate Tec at Tyr-518. (A) The kinase-dead TecKM (TM) was expressed in 293 cells either alone or in combination with Jak2 (Jak2) or Lyn (Lyn) kinase. Tec was immunoprecipitated from each fraction, and probed with anti-phosphotyrosine antibody (αP-Tyr) or anti-Tec serum (αTec). (B) TecKM (TM), TecKM▵SH3 (TM▵3), or TecKM,YF (TM,518F) was expressed in 293 cells either alone (−) or in combination with Jak2 (J) or Lyn (L). Tec was immunoprecipitated from each fraction, and blotted with anti-phosphotyrosine antibody (αP-Tyr) or anti-Tec serum (αTec). The positions of full-length Tec (T) and SH3-deleted (▵SH3) forms are indicated at the right. (C) The amino acid sequences of the Tec-family kinases, surrounding the tyrosine residues corresponding to Tyr-518 in mouse Tec, are compared. The asterisk indicates the position of the phosphorylated tyrosine. At the left shown are the numbers of amino acid positions of mouse Tec,2 human Btk,1011 mouse Emt/Itk/Tsk, 9,41,42 human Bmx,43 and human Txk.44 (D) pSRα (V), pSRα-Tec (T), or pSRα-TecKM (TM) was transfected into 293 cells with or without pSRα-Jak2 (J). Tec was immunoprecipitated from each fraction, and incubated with [γ−32P]ATP without exogenous substrates. Autophosphorylation of pp70Tec in each sample is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1496/3/m_blod4053704d.jpeg?Expires=1763492363&Signature=oYep3Tg6VTL~zG0WFJltVTZxzQQAJIaB0OeSHSwUSvCQlFEoh1GaSH4LKHtI-P8dNxawN3U372Rx30gHmvN6ap-lgq4clyUngDdzECrKS~jOz4IkC0hvBqGHVeaDK1CnNG-zwN9IHRFu~6cfEV3wiYmo3RaOx42gc1WhgciuIe7BCoHVq4-l~2WxI1nBn5XGYo4ptUuSN0YySlUltbiVyRxhrzl3jyIW7p5IgNGf~~WVI-02WqhCm1XKBdu48MlmeinZZncFJdGPktk66JxN-KGPcx7CsUV5Ic5QipnBjdrLN6k95ajCjialFMj~XR8t5PGH5iu3FHdUT~2MwMBDvA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
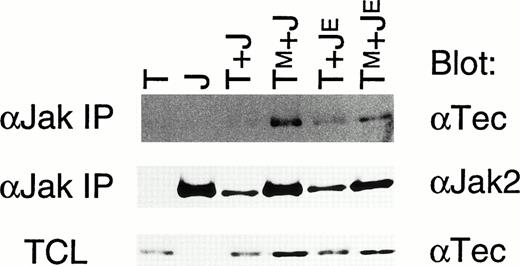
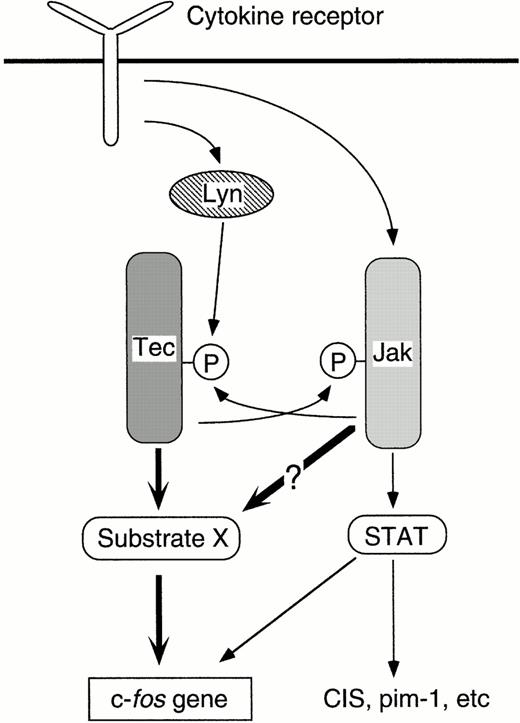
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal