Abstract
The 110-kD lung resistance protein (LRP) is overexpressed in P-glycoprotein–negative multidrug-resistant cell lines and most likely involved in the multidrug resistance (MDR) of these cell lines. To determine the clinical significance of LRP, we have studied LRP expression of leukemic blasts and its association with clinical outcome in patients with de novo acute myeloid leukemia (AML). LRP expression of leukemic blasts obtained from peripheral blood or bone marrow of previously untreated patients (n = 86) was determined by immunocytochemistry by means of monoclonal antibody LRP-56. LRP expression at diagnosis was detected in 31 (36%) patients. LRP expression was independent of age and sex of the patients, French-American-British subtype, cytogenetic abnormalities, and lactate dehydrogenase levels, but correlated with white blood cell count (P = .01). Eighty-two patients received standard induction chemotherapy that included cytarabine and MDR drugs (daunorubicin in most patients, additional etoposide in the majority of patients). The complete remission rate of induction chemotherapy was 72% (95% confidence interval [CI] = 61% to 82%) for the total study population. The complete remission rate was 81% (95% CI = 67% to 91%) for patients without LRP expression but only 55% (95% CI = 36% to 74%) for patients with LRP expression (P = .01). Overall survival and disease-free survival were estimated according to Kaplan-Meier in 82 and 59 patients, respectively. Overall survival was significantly longer in patients without LRP expression than in patients with LRP expression. At a median follow-up of 16 months, median overall survival was 17 months (95% CI = 12 to 38 months) for LRP-negative patients but only 8 months (95% CI = 4 to 12 months) for -positive patients (P = .006). Disease-free survival was 9 months (95% CI = 7 to 11 months) for LRP-negative patients and 6 months (95% CI = 5 to 8 months) for -positive patients (P = .078). Outcome was best in patients lacking both LRP and P-glycoprotein expression. In conclusion, LRP predicts for poor outcome and thus theLRP gene appears to be another clinically relevant drug resistance gene in AML.
ACUTE MYELOID leukemia (AML) lends itself as a model disease for the evaluation of the clinically relevant mechanisms of resistance toward anticancer drugs. Potential mechanisms are those involved in the multidrug resistance (MDR) phenotype.1,2 They include the MDR1 gene and the recently characterized MRP (multidrug resistance protein) gene.1,3 The MDR1 gene codes for P-glycoprotein (P-gp), which functions as an energy-dependent drug efflux pump for natural hydrophobic compounds including anticancer drugs (eg, anthracyclines, epipodophyllotoxins).1 Like P-gp, MRP is a member of the ABC transporter family and mediates resistance to a similar spectrum of anticancer drugs as P-gp.4 Both genes are involved in MDR of cell lines.1,3,4 Expression ofMDR1 RNA as well as P-gp have been shown to be associated with worse outcome in AML.5-8 MRP is highly expressed in 26% of AML samples but, in contrast to P-gp, this expression does not predict for outcome of induction chemotherapy or survival of the patients.9 Only in the subgroup of patients with inversion in chromosome 16, patients with a deletion of the MRP gene had a longer overall and disease-free survival.10 WhetherMRP gene deletion is the reason for the good prognosis of patients with inversion in chromosome 16 remains unclear, because lack of MDR1 RNA/P-gp expression might also explain the good prognosis of patients with this AML subtype.11
Recently the lung resistance protein (LRP) was detected in MDR cell lines and its gene has been cloned.12,13 The LRPgene, located on chromosome 16 proximal to the MRPgene,13,14 is homologous to the major vault protein of the rat. Vaults are ribonucleoprotein particles that are located in the cytoplasm and probably involved in transport processes.15,16 LRP is thus believed to contribute to the drug resistance of these cell lines, probably via affecting drug transport. Consistent with this hypothesis, LRP overexpression was associated with resistance to doxorubicin, vincristine, carboplatin, cisplatin, and melphalan.17 LRP is overexpressed in normal colon tissue, normal lung tissue, renal proximal tubules, adrenal cortex, and macrophages,18 but its physiological function remains to be evaluated.
To determine whether LRP is a clinically relevant drug resistance gene in de novo AML, we have studied LRP expression of AML cells and its relationship to clinical outcome in previously untreated patients with de novo AML. Here we report the results of this study.
PATIENTS AND METHODS
Patients.
Eighty-six patients (37 females, 49 males) with de novo AML who were treated between January 1990 and February 1997 were studied after obtaining informed consent. Sixty-five of these patients had been included in a previous study on the clinical significance of MRP.9 Eighty-two patients received standard induction chemotherapy protocols, whereas 4 patients did not receive chemotherapy. Treatment consisted of daunorubicin 45 mg/m2daily on days 1-3 and cytarabine 200 mg/m2 daily on days 1-7 (DA protocol) in 21 patients and additional etoposide 100 mg/m2 daily on days 1-5 (DAE protocol) in 50 patients. Three patients were treated with idarubicin plus cytarabine (IA protocol). Six patients with French-American-British (FAB) subtype M3 received all-trans retinoic acid (ATRA) before chemotherapy. Two patients received intermediate-dose cytarabine followed by the DAE protocol as second induction chemotherapy cycle. Response to induction chemotherapy was assessed according to standard criteria.19Four patients did receive only one treatment cycle which did not result in complete remission (CR) and, therefore, these patients were classified as not evaluable. Fifty-six out of 59 patients in complete remission received consolidation therapy that included anthracylines and/or cytarabine. Seventeen patients underwent bone marrow transplantation.
Immunocytochemistry.
Mononuclear cells were isolated from either peripheral blood (n = 37), bone marrow (n = 36) aspirates, or both sources (n = 13) by Ficoll-Paque (Pharmacia, Uppsala, Sweden) gradient centrifugation. Smears were prepared from fresh samples and stored at −20°C until use. Cells were fixed in cold acetone (−20°C, 10 minutes), washed twice, and incubated in 3% H2O2 to block endogenous peroxidase activity. After two wash steps followed by a 20-minute incubation with normal goat serum (Dako, Glostrup, Denmark; diluted 1:20), cells were incubated for 2 hours with the monoclonal antibody (MoAb) LRP-56 (dilution 1:50) or MoAb C219 (Alexis, Läufelingen, Switzerland). LRP-56 detects LRP. C219 recognizes P-gp but also cross-reacts with theMDR2 gene product. Antibody binding was detected by the avidin-biotin-peroxidase method. Bound peroxidase was developed with 3-amino-9-ethylcarbazole (Sigma Chemical Co, St Louis, MO) and 0.1% H2O2 in acetate buffer pH 5.2. The slides were counterstained with Mayer's Hämalaun and mounted with Aquatex (Merck, Darmstadt, Germany). All washes were performed in phosphate-buffered saline.
To ensure specificity of staining, several controls were performed. Firstly, the small cell lung cancer cell line SW1573 and its drug-resistant variant SW1573/2R120 were used as negative and positive controls for LRP expression.12,13 Drug-sensitive KB-3-1 and multidrug-resistant KB-8-5 cells (provided by Drs I. Pastan and M.M. Gottesman, National Cancer Institute, Bethesda, MD) were used as negative and positive controls for P-gp expression.5Secondly, experiments without MoAbs were used as negative controls.
Karyotype analysis.
Cytogenetic analysis was performed as previously described.20 Inversion in chromosome 16 (inv16), t(8;21), or t(15;17) indicated good prognosis, and normal karyotype indicated intermediate prognosis. Cytogenetic abnormalities others than the ones described above were regarded as indicators of poor prognosis.
Survival analysis.
Overall survival and disease-free survival were estimated according to Kaplan-Meier.21 Overall survival was measured from the time of diagnosis until the time of either death or last control. Disease-free survival was measured from the time of complete remission until the time of relapse or death. Patients who underwent bone marrow transplantation were censored at the time of transplantation.
Statistical analysis.
Frequencies were tested by chi-squared analysis or exact chi-squared test. In addition, Kruskal-Wallis tests were performed. Comparisons of survival curves were done with the log-rank test.
RESULTS
LRP expression in de novo AML at diagnosis.
Eighty-six AML patients were studied for LRP expression in their leukemic cells at diagnosis. LRP expression was immunocytochemically determined by means of the MoAb LRP-56. Samples were scored LRP positive if ≥5% positive staining blasts were detected. LRP was positive in 31 of 86 (36%) patients (Table1). LRP was positive in 20 of 50 (40%) peripheral blood samples and 13 of 49 (27%) bone marrow samples. In those 13 cases where samples from both sources were studied, no differences between peripheral and bone marrow blasts were observed (data not shown).
Characteristics of Patients
| . | All Patients . | LRP− Patients n (%) . | LRP+ Patients n (%) . | P . |
|---|---|---|---|---|
| No. of patients | 86 | 55 (100) | 31 (100) | |
| Age (yr) | ||||
| Median | 56 | 54 | 61 | 0.1* |
| Range | 15-88 | 15-88 | 18-82 | |
| Patients >50 yr | 52 | 31 (56) | 21 (68) | 0.3-151 |
| Sex | ||||
| Males | 49 | 33 (60) | 16 (52) | 0.5-151 |
| Females | 37 | 22 (40) | 15 (48) | |
| White blood cell count | ||||
| Median | 21,750 | 14,380 | 52,300 | 0.01* |
| Range | 400-280,700 | 400-250,000 | 740-280,700 | |
| FAB subtype | ||||
| M0 | 5 | 2 (4) | 3 (10) | |
| M1 | 16 | 9 (17) | 7 (23) | |
| M2 | 22 | 17 (31) | 5 (16) | |
| M3 | 9 | 8 (15) | 1 (3) | 0.2-152 |
| M4 | 16 | 9 (16) | 7 (23) | |
| M4Eo | 5 | 3 (5) | 2 (6) | |
| M5 | 10 | 4 (7) | 6 (19) | |
| M6 | 3 | 3 (5) | 0 (0) | |
| Lactate dehydrogenase (U/L) | ||||
| Median | 463 | 433 | 583 | 0.1* |
| Range | 104-3,800 | 104-2,460 | 194-3,800 | |
| Karyotype (n = 79)-153 | ||||
| Good prognosis-155 | 20 | 16 (29) | 4 (13) | |
| Intermediate prognosis | 29 | 19 (35) | 10 (32) | 0.3-152 |
| Poor prognosis | 30 | 17 (31) | 13 (42) | |
| Induction therapy-153 | ||||
| DA | 21 | 14 (25) | 7 (23) | |
| DAE | 50 | 31 (56) | 19 (62) | |
| IA | 3 | 1 (2) | 2 (6) | 0.4-152 |
| ATRA + DA or DAE | 6 | 6 (11) | 0 (0) | |
| Intermed.-dose cytarabine + DAE | 2 | 1 (2) | 1 (3) | |
| None | 4 | 2 (4) | 2 (6) | |
| Consolidation therapy | ||||
| DA | 17 | 14 (33) | 3 (19) | |
| DAE | 25 | 16 (37) | 9 (56) | |
| Intermed.-dose cytarabine | 4 | 3 (7) | 1 (6) | |
| High-dose cytarabine | 6 | 4 (9) | 2 (13) | 0.9-152 |
| C-HAM | 1 | 1 (2) | 0 (0) | |
| DAE + intermed.-dose cytarabine | 2 | 2 (5) | 0 (0) | |
| DAE + high-dose cytarabine | 1 | 1 (2) | 0 (0) | |
| None | 3 | 2 (5) | 1 (6) | |
| Bone marrow transplantation | 17 | 13 (24) | 4 (13) | 0.2-151 |
| . | All Patients . | LRP− Patients n (%) . | LRP+ Patients n (%) . | P . |
|---|---|---|---|---|
| No. of patients | 86 | 55 (100) | 31 (100) | |
| Age (yr) | ||||
| Median | 56 | 54 | 61 | 0.1* |
| Range | 15-88 | 15-88 | 18-82 | |
| Patients >50 yr | 52 | 31 (56) | 21 (68) | 0.3-151 |
| Sex | ||||
| Males | 49 | 33 (60) | 16 (52) | 0.5-151 |
| Females | 37 | 22 (40) | 15 (48) | |
| White blood cell count | ||||
| Median | 21,750 | 14,380 | 52,300 | 0.01* |
| Range | 400-280,700 | 400-250,000 | 740-280,700 | |
| FAB subtype | ||||
| M0 | 5 | 2 (4) | 3 (10) | |
| M1 | 16 | 9 (17) | 7 (23) | |
| M2 | 22 | 17 (31) | 5 (16) | |
| M3 | 9 | 8 (15) | 1 (3) | 0.2-152 |
| M4 | 16 | 9 (16) | 7 (23) | |
| M4Eo | 5 | 3 (5) | 2 (6) | |
| M5 | 10 | 4 (7) | 6 (19) | |
| M6 | 3 | 3 (5) | 0 (0) | |
| Lactate dehydrogenase (U/L) | ||||
| Median | 463 | 433 | 583 | 0.1* |
| Range | 104-3,800 | 104-2,460 | 194-3,800 | |
| Karyotype (n = 79)-153 | ||||
| Good prognosis-155 | 20 | 16 (29) | 4 (13) | |
| Intermediate prognosis | 29 | 19 (35) | 10 (32) | 0.3-152 |
| Poor prognosis | 30 | 17 (31) | 13 (42) | |
| Induction therapy-153 | ||||
| DA | 21 | 14 (25) | 7 (23) | |
| DAE | 50 | 31 (56) | 19 (62) | |
| IA | 3 | 1 (2) | 2 (6) | 0.4-152 |
| ATRA + DA or DAE | 6 | 6 (11) | 0 (0) | |
| Intermed.-dose cytarabine + DAE | 2 | 1 (2) | 1 (3) | |
| None | 4 | 2 (4) | 2 (6) | |
| Consolidation therapy | ||||
| DA | 17 | 14 (33) | 3 (19) | |
| DAE | 25 | 16 (37) | 9 (56) | |
| Intermed.-dose cytarabine | 4 | 3 (7) | 1 (6) | |
| High-dose cytarabine | 6 | 4 (9) | 2 (13) | 0.9-152 |
| C-HAM | 1 | 1 (2) | 0 (0) | |
| DAE + intermed.-dose cytarabine | 2 | 2 (5) | 0 (0) | |
| DAE + high-dose cytarabine | 1 | 1 (2) | 0 (0) | |
| None | 3 | 2 (5) | 1 (6) | |
| Bone marrow transplantation | 17 | 13 (24) | 4 (13) | 0.2-151 |
*Kruskal-Wallis test.
Chi-square test.
Exact chi-squared test.
Protocols and karyotype classification are described in Patients and Methods.
Good prognosis versus all other karyotypes: P = .1.
LRP and clinical parameters.
Next we studied the association of LRP with clinical parameters. The major clinical and laboratory findings of the patients are summarized in Table 1. The median age of the patients was 56 years (range, 15 to 88 years). LRP expression correlated with white blood cell count (P = .01) but was independent of age, percentage of patients older than 50 years, sex, serum lactate dehydrogenase levels, and cytogenetic abnormalities (Table 1). Interestingly, 8 of 9 (89%) patients with promyelocytic leukemia (FAB M3) did not express LRP, but this was not significantly different from other FAB subtypes (P = .1). Karyotype was categorized into three prognostic groups (see Patients and Methods). LRP expression was not significantly different between these groups (Table 1), although patients with good prognosis karyotype showed a trend (P = .1) toward negative LRP (Table 1).
LRP expression and outcome of induction chemotherapy.
Eighty-two patients received standard induction chemotherapy that included cytarabine and MDR drugs. Four (5%) patients did not receive chemotherapy. The treatment protocols were not different between LRP-negative and LRP-positive patients (Table 1). The CR rate of induction chemotherapy was 72% (95% confidence interval [CI] = 61% to 82%) for the total study population. Resistant disease (after at least two treatment cycles) and early death (within 4 weeks after begin of treatment) occurred in 11% and 12% of the patients, respectively. Four (5%) patients were not evaluable for response. The complete remission rate was 81% (95% CI = 67% to 91%) for patients without LRP expression but only 55% (95% CI = 36% to 74%) for patients with LRP expression (P = .01) (Table 2). Resistant disease was seen in 4 (8%) (95% CI = 2% to 18%) LRP-negative and in 5 (17%) (95% CI = 6% to 36%) LRP-positive patients. Early death occurred in 5 (9%) (95% CI = 3% to 21%) negative and 5 (17%) (95% CI = 6% to 36%) positive patients (Table 2). When only patients who received induction chemotherapy with daunorubicin/cytarabine (DA) or daunorubicin/cytarabine/etoposide (DAE) (n = 77) were analyzed, similar results were obtained as CR rate was 80% for LRP-negative patients and 58% for LRP-positive patients (P = .03) (data not shown).
LRP and Outcome of Induction Chemotherapy
| . | No. of Patients . | Complete Remission . | Resistant Disease . | Early Death . | Not Evaluable . |
|---|---|---|---|---|---|
| Total | 82 | 59 (72%) | 9 (11%) | 10 (12%) | 4 (5%) |
| LRP− patients | 53 | 43 (81%) | 4 (8%) | 5 (9%) | 1 (2%) |
| LRP+ patients | 29 | 16 (55%) | 5 (17%) | 5 (17%) | 3 (11%) |
| . | No. of Patients . | Complete Remission . | Resistant Disease . | Early Death . | Not Evaluable . |
|---|---|---|---|---|---|
| Total | 82 | 59 (72%) | 9 (11%) | 10 (12%) | 4 (5%) |
| LRP− patients | 53 | 43 (81%) | 4 (8%) | 5 (9%) | 1 (2%) |
| LRP+ patients | 29 | 16 (55%) | 5 (17%) | 5 (17%) | 3 (11%) |
LRP expression of leukemic cells was compared with outcome of induction chemotherapy. Induction chemotherapy protocols are described in Patients and Methods. Statistical analysis (chi-squared test) for complete remission: P = .01.
LRP and survival.
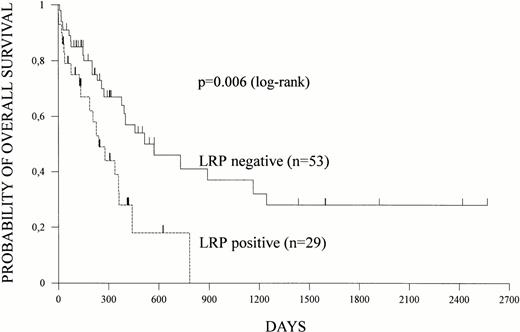
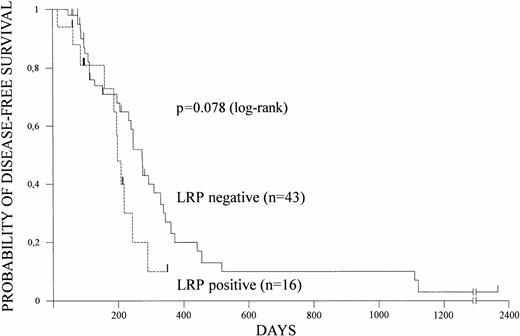
Overall survival and disease-free survival were estimated according to Kaplan-Meier in 82 and 59 patients, respectively. Relapses and deaths occurred in 41 and 54 patients, respectively. Both overall survival and disease-free survival were shorter in patients with LRP expression (Figs 1 and 2).At a median follow-up of 16 months, median overall survival was 17 months (95% CI = 12 to 38 months) for LRP-negative patients but only 8 months (95% CI = 4 to 12 months) for LRP-positive patients (P = .006). Nine of 10 patients surviving more than 2 years did not express LRP in their leukemic blasts. Median disease-free survival was 9 months (95% CI = 7 to 11 months) for LRP-negative and 6 months (95% CI = 5 to 8 months) for LRP-positive patients (P = .078).
LRP and overall survival. LRP expression of leukemic cells was determined by immunocytochemistry and overall survival was estimated according to Kaplan-Meier in 82 patients. Survival data based on LRP expression are shown.
LRP and overall survival. LRP expression of leukemic cells was determined by immunocytochemistry and overall survival was estimated according to Kaplan-Meier in 82 patients. Survival data based on LRP expression are shown.
LRP and disease-free survival. LRP expression of leukemic cells was determined by immunocytochemistry and disease-free survival was estimated according to Kaplan-Meier in 59 patients. Disease-free survival based on LRP expression is shown.
LRP and disease-free survival. LRP expression of leukemic cells was determined by immunocytochemistry and disease-free survival was estimated according to Kaplan-Meier in 59 patients. Disease-free survival based on LRP expression is shown.
LRP/P-gp status and clinical outcome.
Finally, we analyzed the combination of LRP and P-gp status in relation to clinical outcome (Table 3). Consistent with our previous studies on P-gp in AML,5,8 9 P-gp expression was high, intermediate, and low in 12%, 30%, and 58% of the patients, respectively (data not shown). High P-gp expression was associated with lower CR rates (75% v 44%, P = .05) and shorter survival (median overall survival: 13 months v 7 months, P = .03) (data not shown). No correlation was observed between high P-gp expression and LRP expression (data not shown). Response to induction chemotherapy was best (CR rate = 84%) in patients lacking expression of both genes, intermediate (CR rate = 57%) in those with expression of either of these two genes, and worst (CR rate = 40%) in patients expressing both genes (Table3). The CR rate was significantly higher in LRP−/P-gp− patients as compared to the remaining patients (P = .004). Overall survival and disease-free survival were also significantly longer for patients with LRP−/P-gp− leukemias as compared to the remaining patients with a median overall survival of 24 versus 7 months (P = .0002) and a median disease-free survival of 9 versus 6 months (P = .024) (data not shown).
LRP and P-Glycoprotein (P-gp) Status in Relation to Outcome of Induction Chemotherapy
| . | No. of Patients . | Complete Remission . | Resistant Disease . | Early Death . | Not Evaluable . |
|---|---|---|---|---|---|
| Total | 82 | 59 (72%) | 9 (11%) | 10 (12%) | 4 (5%) |
| LRP−/P-gp− | 49 | 41 (84%) | 4 (8%) | 3 (6%) | 1 (2%) |
| LRP+/P-gp−or LRP−/P-gp+ | 28 | 16 (57%) | 3 (11%) | 6 (21%) | 3 (11%) |
| LRP+/P-gp+ | 5 | 2 (40%) | 2 (40%) | 1 (20%) | 0 (0%) |
| . | No. of Patients . | Complete Remission . | Resistant Disease . | Early Death . | Not Evaluable . |
|---|---|---|---|---|---|
| Total | 82 | 59 (72%) | 9 (11%) | 10 (12%) | 4 (5%) |
| LRP−/P-gp− | 49 | 41 (84%) | 4 (8%) | 3 (6%) | 1 (2%) |
| LRP+/P-gp−or LRP−/P-gp+ | 28 | 16 (57%) | 3 (11%) | 6 (21%) | 3 (11%) |
| LRP+/P-gp+ | 5 | 2 (40%) | 2 (40%) | 1 (20%) | 0 (0%) |
LRP and P-gp expression of leukemic blasts was determined as described in Patients and Methods. Induction chemotherapy included MDR drugs (daunorubicin, etoposide) in most patients. Response to induction chemotherapy was assessed according to standard criteria. Response data are shown for patients without expression of these two genes, for patients with expression of either of these genes, and for patients with expression of both genes. Statistical analysis (chi-squared test) for complete remission: P = .004.
DISCUSSION
In the present study we have shown the clinical significance of LRP in AML on an unselected patient population. LRP expression of leukemic cells was observed in 36% of AML patients at diagnosis and did predict for both poor response to induction chemotherapy and shorter survival. The percentage of LRP-positive AMLs is similar to the percentages reported in two recent studies with a small sample size.18,22 Izquierdo et al18 reported LRP expression in 5 of 15 (25%) AML samples and List et al22in 7 of 21 (33%) de novo AML samples.
The association of LRP expression with poor outcome stresses the clinical relevance of LRP and also suggests that drug resistance in AML is multifactorial, involving at least P-gp and LRP. This multifactorial nature is further supported by the fact that prognosis is best in the absence and worst in the presence of both proteins (Table 3). Results similar to ours have recently been reported by List et al,22 who found a trend of LRP expression toward worse outcome on a heterogenous study population that included patients with de novo, secondary, or relapsed AML.
Although an association of LRP expression with high white blood cell counts was seen (Table 1), the poor outcome of LRP-positive patients cannot be explained by their higher white blood cell count because in our study white blood cell count (cut-off levels of 20,000 or 100,000) had no impact on outcome of induction chemotherapy or survival (data not shown). Lack of LRP expression was seen in most FAB M3 patients (Table 1) and this might contribute to the good response to anthracyclines as well as good prognosis of this AML subtype.23 Because LRP expression showed a trend toward CD7 expression,22 future studies will have to further address the association of LRP with surface markers. Future studies on large patient populations are also required to determine whether the impact of LRP expression on clinical outcome depends on the type of induction or consolidation chemotherapy.
LRP expression was also observed in several solid tumors.18,24 LRP predicted for both response to chemotherapy and prognosis in advanced ovarian carcinoma,24but the clinical relevance of LRP in other solid tumors remains to be determined. The mechanisms by which LRP exerts drug resistance will have to be elucidated in future studies.
In conclusion, the LRP gene appears to be another clinically relevant drug-resistance gene in AML and probably other malignancies. This will have to be taken into consideration in the planning of strategies to overcome drug resistance in cancer patients and warrants the pharmaceutical development of resistance modifiers not only of P-gp function25 26 but also of LRP function.
Supported by the ‘Fonds zur Förderung der wissenschaftlichen Forschung’ (Project No. P12264-MED).
Address reprint requests to Robert Pirker, MD, Associate Professor, Division of Oncology, Department of Internal Medicine I, University of Vienna Medical School, Währinger Gürtel 18-20, A-1090 Vienna, Austria.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be here-by marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal