Abstract
The jumping translocation (JT) is a rare chromosomal abnormality in which a specific chromosomal segment translocates onto the ends of various chromosomes (jumps). In most cases, the region distal to 1q21 jumps onto numerous different telomeres. Here we report a molecular study of the JT involving 1q21 found in a patient with acute myelomonocytic leukemia that had transformed from myelodysplastic syndrome (MDS). This is the first report describing the analysis of the molecular structure of the JT. We demonstrated the presence of a stretch of telomeric repeats at the breakpoint by means of a fluorescence in situ hybridization experiment, molecular cloning, and nucleotide sequencing of the fused region. A significant amount of variant telomeric repeats (a telomeric sequence having one-base mismatch within the authentic telomeric repeat TTAGGG) was found in this region. The variant telomeric repeat has been shown to be present in the proximal region of telomeres and does not perform telomeric functions by itself. Therefore, these results indicated that the telomeres had already been critically shortened when the jumps occurred. We suggest that the extended proliferation of cancer cells during the premalignant stage, such as MDS, results in chromosomal instability due to the loss of telomeric functions.
TELOMERES PLAY a critical role in chromosomal stability.1 In most somatic cells, the telomere length is shortened each time the cell divides.2 When the telomere length reaches a critical point, the cells cease to divide which leads to senescence. However, cancer cells overcome this negative regulation of cell division induced by the shortened telomeres, and continue to proliferate.3 The resultant chromosome instability, caused by the loss of telomeric functions, has been proposed as a mechanism of cancer cell evolution.4 However, there has been no report actually implicating this hypothesis in human clinical cancers. An example of a chromosomal abnormality that apparently involves the telomere is the jumping translocation (JT).5 JT is an unbalanced translocation of a specific chromosomal segment onto the ends of various chromosomes (jumps). About 20 cases of JT have been reported in leukemia patients.5-15In most cases, the donor segments originated from the long arm of chromosome 1. In contrast, the acceptor telomeres varied greatly among patients and even within any one patient. These observations suggest that some general features of telomeres, such as insufficient telomeric function, may have caused JT. However, molecular cloning of the fusion points has not yet been reported, and thus it was unknown whether jumps occurred toward the telomeres, subtelomeric regions, or nontelomeric regions.
Here we report the molecular structure of the JT fusion point. We have demonstrated the presence of telomeric repeats at the fusion point. Interestingly, these telomeric repeats consist of so-called variant telomeric repeats and the authentic TTAGGG telomeric repeats were only found to a limited degree. Variant telomeric repeats have been reported to be located at the inner regions of native telomeres and do not perform telomeric functions in vivo. Therefore, this study suggests that the excessive proliferation of cancer cells results in the exposure of the variant telomeric repeats and telomere dysfunction leading to the chromosomal abnormality of JT.
MATERIALS AND METHODS
Probes.
The telomeric repeat (TTAGGG)n was generated by polymerase chain reaction (PCR).16 In brief, a PCR reaction was performed in a 0.1-mL volume containing 10 pmol of the primers (TTAGGG)4 and (CCCTAA)4, 2 U ofTaq polymerase, 0.2 mmol/L of each dNTP, and a standard buffer. Amplification consisted of 10 cycles of 1 minute at 94°C, 30 seconds at 55°C, and 1 minute at 72°C, followed by 30 cycles of 1 minute at 94°C, 30 seconds at 60°C, 90 seconds at 72°C, and one final step of 5 minutes at 72°C.
A JT-specific, nontelomeric sequence (probe A) was prepared from theEcoT14 I clone by EcoT14 I and Bgl I digestion. The resultant 330 bp was purified from an agarose gel and used as a probe for Southern blot analysis and for screening of the human genomic library.
Cytogenetic analysis.
Cytogenetic analyses were performed according to standard procedures. Giemsa-banding was used for routine analysis of metaphase preparations.
A fluorescence in situ hybridization (FISH) experiment was performed according to standard procedure. In brief, a probe was labeled with biotin-21-dUTP using a nick translation kit. The slides were denatured in 70% formamide in 2 × SSC (1 × SSC = 0.15 mol/L NaCl, 0.015 mol/L sodium citrate, pH 7.0) at 75°C for 10 minutes. After dehydration of the slides, the labeled probe was applied at a concentration of 0.5 μg/mL in 4 × SSC, 50% formamide, 20% dextran sulfate with 100 μg/mL herring sperm DNA. The slides were hybridized overnight at 42°C and then washed at 37°C in 2 × SSC, 50% formamide for 15 minutes, followed by 2 × SSC, 1 × SSC for 15 minutes.
The biotin-labeled probe was detected using fluorescein avidin and a biotinylated anti-avidin antibody. Chromosomes were counterstained with propidium iodide and visualized using a microscope (Zeiss, Thornwood, NY) with appropriate filters. Metaphase spreads were captured using a CCD camera system (Photometrics, Tucson, AZ).
Southern blot analysis.
Approximately 10 μg of human genomic DNA was digested with BAL31 in a standard buffer for 70 minutes (the final concentration was 20 U/mL) at 30°C. After phenol extraction and ethanol precipitation, the BAL31-digested human genomic DNAs were digested with Hinf I for 6 hours at 37°C. Restriction fragments were separated on a 0.6% agarose gel run at 20 V for 16 hours in 1× Tris/Borate/EDTA (TBE) buffer. The gel was then stained with dilute EtBr, denatured in 0.4 N NaOH for 30 minutes, and the DNAs were transferred by capillary action onto a Hybond-N+ nylon membrane (Amersham, Arlington Heights, IL). The blot was rinsed in 20× SSC, and hybridized at 42°C for 20 hours in 50% formamide, 1% sodium dodecyl sulfate (SDS), 5× SSC, 5× Denhart's solution, and 10 μg/mL herring sperm DNA against a DNA probe labeled with [32P] dCTP by nick translation. Filters were then washed for 10 minutes at room temprature in 0.1× SSC and 0.1% SDS, dried, wrapped, and exposed to Hyperfilm (Amersham) for 2 to 3 days at −80°C.
Genomic cloning.
Genomic DNAs were digested (Tsp509 I or EcoT14 I), ligated to an EcoRI linker, size-selected in a low melting point agarose gel (6 to 9 kb for the JT-specific fragment, or 2 to 3 kb for a normal allele), purified using β-agarase, and ligated to lambda ZAP II/EcoRI. These subgenomic libraries were screened using the telomeric repeat or the nontelomeric, JT-specific sequence (probe A). A λ phage genomic library was constructed according to standard procedure using the λ GEM12 half site vector.
RESULTS
Karyotypes of the jumping translocation.
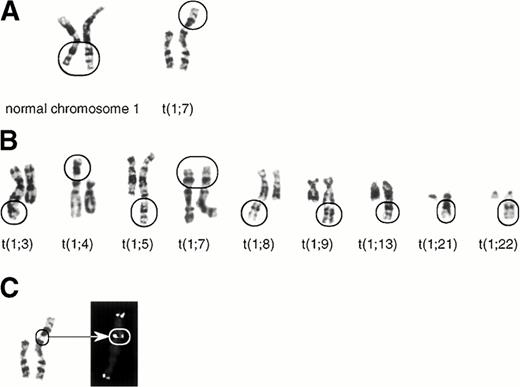
We studied one leukemia patient showing typical JT karyotype anomalies. The 55-year-old patient was diagnosed as acute myelomonocytic leukemia (AMMoL) in June 1994. She was treated by intermittent courses of the low-dose AraC regimen with minimal response, and died of massive bleeding in June 1995. Surface marker analysis showed that the leukemic cells were positive for CD13, CD14, CD33, CD34, and HLA-DR. The karyotypes and hematological findings of the patient during the clinical course are shown in Table 1. The leukemic cells showed a normal karyotype in June 1994, developed a variety of JTs in September 1994 and March 1995. The basic JT karyotype involved the donor chromosomal segment distal to 1q21 jumping onto the tip of the short arm of chromosome 7 [46, XX, der(7)t(1; 7)(q21; p22), see Table 1 and Fig 1A]. In most cells, an additional JT involving the same donor segment of 1q jumping onto the tip of 3q, 4p, 5q, 7p, 8q, 9q, 13q, 21q, or 22q was also present (Fig1B). These karyotypes resulted in 1q tetrasomy in most cells. Interestingly, the frequency of the different sets of JTs varied between September 1994 and March 1995: A particular set of JTs involving 7p and 5q showed 88% domination of the leukemic population in March 1995. However, leukemic cells with JTs were taken over by a clone with a completely different karyotype [46, XX, add(16)(q24)] in May 1995 (Table 1).
The Karyotype Alteration of the Jumping Translocation and Hematological Findings
| Date . | June 1994 . | September 1994 . | March 1995 . | May 1995 . |
|---|---|---|---|---|
| Karyotype | % | % | % | % |
| 46,XX | 100 | 2 | 0 | 0 |
| 46,XX,der(7)t(1;7)(q21;p22) | 0 | 10 | 4 | 0 |
| 46,XX,der(7)t(1;7)(q21;p22),der(3)t(1;3)(q21;q29) | 0 | 10 | 0 | 0 |
| 46,XX,der(7)t(1;7)(q21;p22),der(4)t(1;4)(q21;p16) | 0 | 8 | 0 | 0 |
| 46,XX,der(7)t(1;7)(q21;p22),der(5)t(1;5)(q21;q35) | 0 | 34 | 88 | 8 |
| 46,XX,der(7)t(1;7)(q21;p22),der(7)t(1;7)(q21;p22) | 0 | 4 | 4 | 0 |
| 46,XX,der(7)t(1;7)(q21;p22),der(8)t(1;8)(q21;q24.3) | 0 | 10 | 4 | 0 |
| 46,XX,der(7)t(1;7)(q21;p22),der(9)t(1;9)(q21;q35) | 0 | 14 | 0 | 0 |
| 46,XX,der(7)t(1;7)(q21;p22),der(13)t(1;13)(q21;q34) | 0 | 4 | 0 | 0 |
| 46,XX,der(7)t(1;7)(q21;p22),der(21)t(1;21)(q21;q22) | 0 | 2 | 0 | 0 |
| 46,XX,der(7)t(1;7)(q21;p22),der(22)t(1;22)(q21;q13) | 0 | 2 | 0 | 0 |
| 46,XX,add(16)(q24) | 0 | 0 | 0 | 92 |
| No. of cells analyzed | 19 | 50 | 27 | 13 |
| Peripheral blood WBC count (×109/L) | 12.9 | 27.5 | 23.2 | 32.9 |
| (% blast) | (34.7) | (83.0) | (82.5) | (79.5) |
| Date . | June 1994 . | September 1994 . | March 1995 . | May 1995 . |
|---|---|---|---|---|
| Karyotype | % | % | % | % |
| 46,XX | 100 | 2 | 0 | 0 |
| 46,XX,der(7)t(1;7)(q21;p22) | 0 | 10 | 4 | 0 |
| 46,XX,der(7)t(1;7)(q21;p22),der(3)t(1;3)(q21;q29) | 0 | 10 | 0 | 0 |
| 46,XX,der(7)t(1;7)(q21;p22),der(4)t(1;4)(q21;p16) | 0 | 8 | 0 | 0 |
| 46,XX,der(7)t(1;7)(q21;p22),der(5)t(1;5)(q21;q35) | 0 | 34 | 88 | 8 |
| 46,XX,der(7)t(1;7)(q21;p22),der(7)t(1;7)(q21;p22) | 0 | 4 | 4 | 0 |
| 46,XX,der(7)t(1;7)(q21;p22),der(8)t(1;8)(q21;q24.3) | 0 | 10 | 4 | 0 |
| 46,XX,der(7)t(1;7)(q21;p22),der(9)t(1;9)(q21;q35) | 0 | 14 | 0 | 0 |
| 46,XX,der(7)t(1;7)(q21;p22),der(13)t(1;13)(q21;q34) | 0 | 4 | 0 | 0 |
| 46,XX,der(7)t(1;7)(q21;p22),der(21)t(1;21)(q21;q22) | 0 | 2 | 0 | 0 |
| 46,XX,der(7)t(1;7)(q21;p22),der(22)t(1;22)(q21;q13) | 0 | 2 | 0 | 0 |
| 46,XX,add(16)(q24) | 0 | 0 | 0 | 92 |
| No. of cells analyzed | 19 | 50 | 27 | 13 |
| Peripheral blood WBC count (×109/L) | 12.9 | 27.5 | 23.2 | 32.9 |
| (% blast) | (34.7) | (83.0) | (82.5) | (79.5) |
Abbreviation: WBC, white blood cell.
The karyotypes of the jumping translocation. (A) The normal 1q21-ter (left) and the fused 1q21-ter onto the telomere of 7p (right). (B) Other fused 1q21-ter onto the telomeres of a variety of chromosomes (3q, 4p, 5q, 7p, 8q, 9q, 13q, 21q, and 22q). (C) FISH using the telomeric repeat, (TTAGGG)n. The twin spot signals at the fusion point of der(7)t(1;7)(q21;p22) are indicated by an arrow.
The karyotypes of the jumping translocation. (A) The normal 1q21-ter (left) and the fused 1q21-ter onto the telomere of 7p (right). (B) Other fused 1q21-ter onto the telomeres of a variety of chromosomes (3q, 4p, 5q, 7p, 8q, 9q, 13q, 21q, and 22q). (C) FISH using the telomeric repeat, (TTAGGG)n. The twin spot signals at the fusion point of der(7)t(1;7)(q21;p22) are indicated by an arrow.
Identification of the telomeric repeats at the fusion point.
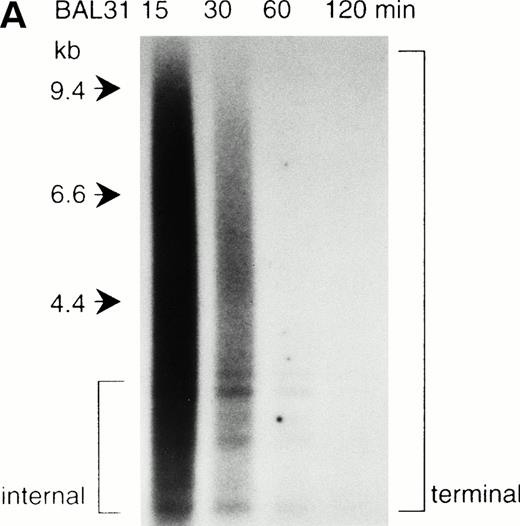
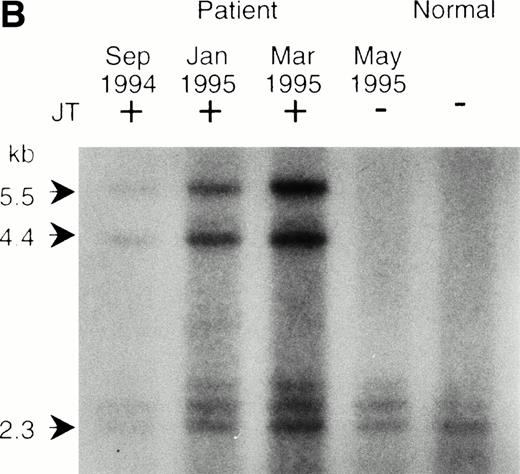
To determine whether the donor segment actually jumped and fused with the telomeric repeat of the various acceptor chromosomes, we performed a fluorescence in situ hybridization of the telomeric repeat (TTAGGG)n, with the metaphase chromosomes of JT. In normal human cells, the telomeric repeat signal is only observed at the chromosome ends.17 As indicated in Fig 1C, we detected the telomeric repeat at the fusion point in addition to the two terminal telomeres in the JT chromosome. This result was further confirmed by Southern blot analysis. Genomic DNAs from the peripheral blood cells of the patient and a normal individual were first digested by the exonuclease BAL31 to erase the terminal telomeric repeat. This was followed by restriction digestion with Hinf I. These digested DNAs were blotted onto a nylon membrane and hybridized with the telomeric repeat. Generally, the BAL31-insensitive hybridization signals represent internal, nonterminal sequences (Fig2A). We observed two hybridization signals (4.4 kb and 5.5 kb) specifically associated with the DNAs from the JT patient (Fig 2B). These hybridization signals did not appear in the DNA sample obtained after the frequency of JT had decreased (April 1995, see Table 1), or in DNA from the normal individual. Similar additional signals specific to JTs were also observed when the DNAs were digested with Tsp509 I (6 kb and 7 kb) or EcoT14 I (6 kb and 7 kb) (data not shown). We considered these BAL31-insensitive, JT-specific fragments to be derived from the fusion points of JT chromosomes. These results indicate that JT comprises the donor region fused with the telomeric repeats of each acceptor chromosome.
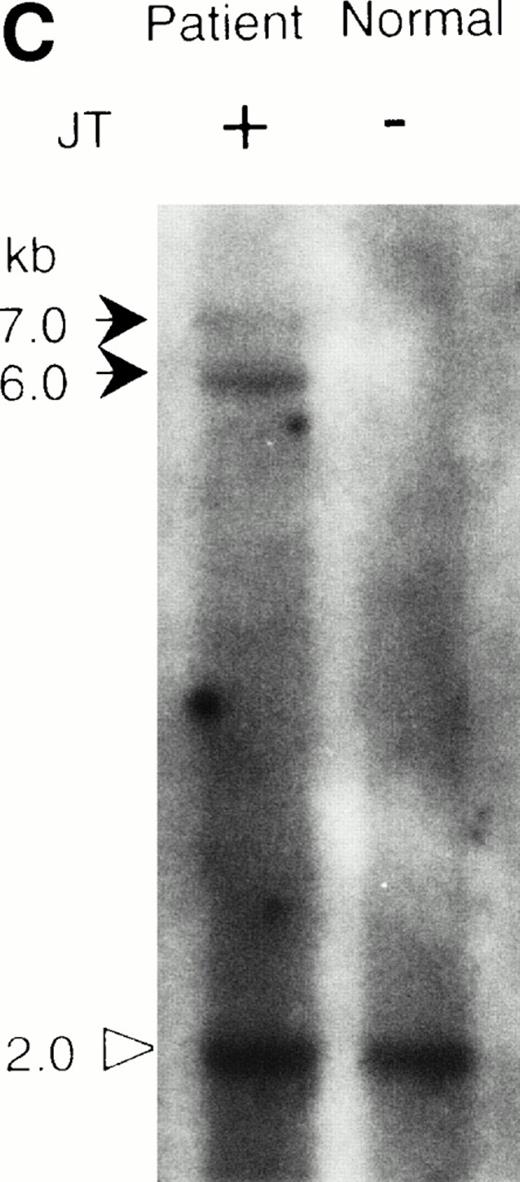
Southern blot analysis of the jumping translocation. (A) BAL31 exonuclease treatment of normal genomic DNA. A longer incubation time with BAL31 exonuclease (indicated in minutes at the top of the lanes) reduces the signal of the terminal telomeric repeat and results in the appearance of the internal TTAGGG-fragments. (B) Southern blot analysis of DNAs from the JT patient and a normal individual using the telomeric repeat as a probe. The DNAs were digested with BAL31 exonuclease, followed by the Hinf I restriction. Arrows indicate the JT-specific fragments (4.4 kb and 5.5 kb). (C) Southern blot analysis of DNAs from the patient and a normal individual using the nontelomeric probe A (see Fig 3a). The DNAs were digested withEcoT14 I. Arrows indicate the JT-specific fragments (6.0 kb and 7.0 kb). The arrowhead indicates the fragment derived from the normal allele (2 kb).
Southern blot analysis of the jumping translocation. (A) BAL31 exonuclease treatment of normal genomic DNA. A longer incubation time with BAL31 exonuclease (indicated in minutes at the top of the lanes) reduces the signal of the terminal telomeric repeat and results in the appearance of the internal TTAGGG-fragments. (B) Southern blot analysis of DNAs from the JT patient and a normal individual using the telomeric repeat as a probe. The DNAs were digested with BAL31 exonuclease, followed by the Hinf I restriction. Arrows indicate the JT-specific fragments (4.4 kb and 5.5 kb). (C) Southern blot analysis of DNAs from the patient and a normal individual using the nontelomeric probe A (see Fig 3a). The DNAs were digested withEcoT14 I. Arrows indicate the JT-specific fragments (6.0 kb and 7.0 kb). The arrowhead indicates the fragment derived from the normal allele (2 kb).
Molecular cloning of the fusion point.
To isolate these JT-specific, TTAGGG-positive DNA fragments, genomic libraries were constructed from size-fractionated Tsp509 I- orEcoT14 I-restricted DNAs derived from the patient, and these were screened using the telomeric probes. Two overlapping clones were obtained and a composite restriction map was made (indicated as jumping translocation in Fig 3A). A region with a unique nucleotide sequence was found adjacent to the telomeric repeats. Southern blot analysis using this nontelomeric sequence (indicated as “probe A” in Fig 3A) identified three DNA fragments in theEcoT14I-restricted patient's DNA. Two bands of 6 kb and 7 kb were specifically associated with the JT DNA, but not with the DNA derived from a normal individual (Fig 2C). These bands were identical with the EcoT14 I-restricted bands detected by the telomeric probe described above. A third band of 2 kb detected by the probe A was present in both the patient and the normal individual, and this was not detected by the telomeric probe (Fig 2C and data not shown). These results suggested that the 6- and 7-kb bands were derived from the fusion points of JTs involving different acceptor chromosomes, and that the 2-kb band originated from the normal allele of the jumped sequence, that is 1q21. A panel of mouse/human hybrid cells was examined with probe A by PCR and Southern blot analysis. The results were consistent with the hypothesis that the probe A was derived from chromosome 1. λ Clones were isolated from a human genomic library using probe A. Sequence analysis of these clones showed that the location of the fusion point is between D1S3384 and D1S2463. These two STS markers were found in a CEPH mega-YAC clone, 955_e_11 that had been previously mapped to 1q21.18 Taken together, these results indicated that the JT-specific fragments we cloned represent the fusion point of the JT chromosomes.
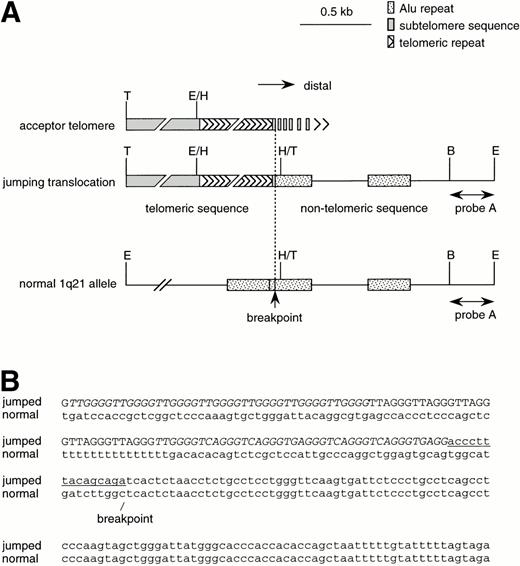
Structure of the fusion point. (A) Restriction maps of the JT-specific fragment and the normal 1q21 allele. The restriction enzymes used are: B, Bgl I; E, EcoT14 I; H,Hinf I; T, Tsp509 I. The position of probe A is shown. (B) The nucleotide sequences of the fusion point of the jumping translocation. The fusion point of 1q21 was determined by a sequence comparison between the JT-specific fragment and the normal 1q21 allele. The telomeric regions (indicated by capital letters) found at the fusion point were composed of variant repeats, such as TTGGGG, TGAGGG, and TCAGGG (indicated by italic letters), along with the authentic telomeric repeats (TTAGGG). The subtelomeric sequence distal to the variant repeat is underlined. The unique nucleotide sequence derived from 1q21 is indicated by small letters.
Structure of the fusion point. (A) Restriction maps of the JT-specific fragment and the normal 1q21 allele. The restriction enzymes used are: B, Bgl I; E, EcoT14 I; H,Hinf I; T, Tsp509 I. The position of probe A is shown. (B) The nucleotide sequences of the fusion point of the jumping translocation. The fusion point of 1q21 was determined by a sequence comparison between the JT-specific fragment and the normal 1q21 allele. The telomeric regions (indicated by capital letters) found at the fusion point were composed of variant repeats, such as TTGGGG, TGAGGG, and TCAGGG (indicated by italic letters), along with the authentic telomeric repeats (TTAGGG). The subtelomeric sequence distal to the variant repeat is underlined. The unique nucleotide sequence derived from 1q21 is indicated by small letters.
Critically shortened telomeres involved in JT.
We determined the nucleotide sequences of the regions around the fusion point both in the JT and the normal allele of chromosome 1 (Fig 3B). The JT sequence had regions which consisted of telomeric repeats (indicated by capital letters in Fig 3B) and of a unique sequence (small letters). A short subtelomeric sequence was found between these two regions (underlined). A comparison of this JT sequence with the normal 1q21 sequence enabled us to pinpoint the breakpoint (Fig 3B). Significantly, the telomeric repeats found in the JT sequence consisted of a large number of the variant telomeric repeats TTGGGG, TGAGGG, and TCAGGG (italics), along with the authentic TTAGGG repeats. The variant repeats have been shown to be present in the internal region of the telomere19-22 and proximal to a subtelomeric sequence.23,24 This result suggested that the functional telomere, composed of pure TTAGGG-repeats, had been completely lost when the JT occurred.23 It has been reported that DNA transfection of variant telomeric repeats does not result in telomeric functions.25 Therefore, it is possible that the variant repeats exposed at the telomeres in the leukemic cells were unable to protect the chromosomal ends from recombination; thus, JT fusion eventually occurred.
DISCUSSION
The patient studied here had myelodysplastic syndrome (MDS) for about 10 years before the onset of the leukemia. MDS is characterized by ineffective hematopoiesis: Although the hematopoietic stem cells in the bone marrow continue to proliferate extensively, systemic anemia ensues from a maturation defect in the hematopoietic precursor cells. During this long period of MDS, the telomeres of the patient's hematopoietic cells may have been reduced to a level at which telomere function was actually lost in some chromosomes.26,27 These telomere insufficiencies may have led to JT in these chromosomal tips. Excessive telomere shortening has been reported in MDS associated with complex karyotype and disease evolution.28
Significantly, the leukemia clones having the JT chromosomes were taken over by an unrelated clone having a distinct karyotype during the terminal stage (Table 1). This type of transient JT has been reported with a case of Down syndrome.29 In this case, the patient presented a congenital leukemoid reaction at birth. The leukemia cells showed a normal karyotype except for the presence of trisomy 21. The leukemoid reaction underwent a spontaneous remission, followed by the development of acute myeloid leukemia. The leukemia cells initially possessed a JT chromosome where the region distal to 1q21 had jumped to the telomere of 22q. This JT appeared only transiently: the JT soon disappeared and an unrelated leukemic clone with a complex abnormal karyotype without JT dominated the population. The two cases of the transient JT, the case reported in this study and the reported case of AML in Down syndrome, share an interesting feature. During the transition from the JT karyotype to another karyotype, the leukemia cells did not show any apparent phenotypic change. This observation leads to speculation that the transient JT may have not contributed to the progression of disease, but may have been a simple reflection of the chromosomal instability caused by telomere insufficiencies. If this is the case, the transient nature of JT may be expected, because chromosome abnormalities that actually contribute to the progression of disease would eventually appear and dominate the population. It is a possibility that the chromosomal instability represented by the JTs was the driving force promoting this clonal evolution of leukemic cells. As JT appears transiently, JT would be a more frequent event than previously observed: JTs may be unstable and lost from proliferating cancer cells before they can be detected.
In conclusion, the molecular structure of the chromosomal abnormality that resulted from a loss of telomeric functions (LTF) has been analyzed for the first time. LTF has been suggested to play a major role in the production of abnormal chromosomes.4,27 In rodent cells, it has been shown that gene amplifications occur via breakage-fusion-bridge cycles that are caused by LTF.30However, there has been no direct evidence indicating that LTF plays a role in the progression of human cancers. This study strongly suggests that LTF, possibly due to the sustained cell proliferation during the premalignant stage, may actually cause chromosomal instability and contribute to cancer cell evolution (Table 1).
ACKNOWLEDGMENT
We are grateful to Dr E.A. Kamei for critical reading of and comments on the manuscript. The excellent secretarial work of M. Fukuda is also acknowledged.
Supported by a Grant-in-Aid for Specially Promoted Research from the Ministry of Education, Science, Sports and Culture of the Japan, by a Grant-in-Aid of Special Coordination Funds for Promoting Science and Technology from the Science and Technology Agency of Japan, and by a Grant-in-Aid from Uehara Memorial Foundation. S.H. is supported by the Japan Society for the Promotion of Science.
Address reprint requests to Fuyuki Ishikawa, MD, Department of Life Science, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama 226, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be here-by marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal