THROMBOCYTOPENIA, anemia, lymphocytopenia, monocytopenia, and neutropenia and permutations of these abnormalities are found in most patients with acquired immunodeficiency syndrome (AIDS). In virtually all patients with advanced AIDS (group IV), pancytopenia is the rule. More than 90% of such patients are anemic and an equal fraction have either neutropenia or monocytopenia.1 Although immune thrombocytopenia is a common cause of low platelet counts in human immunodeficiency virus (HIV)-infected patients, the majority of other types of cytopenias usually reflect bone marrow (BM) dysfunction. Even so, the causes of these cytopenias are clearly heterogeneous and can, in a large number of cases, be attributed to the widely recognized hematopoietic suppressive effects of certain types of intercurrent infections (eg, cytomegalovirus, parvovirus, hepatitis virus, and mycobacterial infection), or of drugs commonly used in AIDS patients (zidovudine, gancyclovir, trimethoprim/sulfa). The multiple causes of marrow failure in patients with AIDS1-3 account for the mixed histological findings on BM examinations of these patients.1 BM biopsy samples and aspirates are most often nonspecifically abnormal. To be sure, certain findings are more than nonspecific. The discovery of abundant megakaryocytes in the BM of a patient with isolated thrombocytopenia, for example, is highly suggestive of idiopathic thrombocytopenic purpura (ITP). Erythroid hypoplasia suggests either parvovirus infection (a rare cause of anemia in patients with HIV infection4) or infection of the marrow with mycobacterium avium complex.1 Interestingly, frank hypocellularity is the exception and aplasia is rare. Taking these diagnostic complexities into account, it has been impossible, from examination of clinical material alone, to determine whether HIV-1 itself has any direct impact on hematopoietic activity.
We seek in this review not to catalogue the spectrum of hematopoietic defects that occur in patients with AIDS, but to focus specifically on the influence HIV-1 itself imposes on hematopoietic cells. For more than a decade, research teams worldwide have sought to identify a causal role for HIV-1 and its gene products in the hematopoietic defects that develop in patients with AIDS and more clear paradigms have emerged. The most important of these is that the greatest impact of viral infection on growth and differentiation of hematopoietic progenitor cells results from the capacity of the virus to infect and perturb the hematopoietic regulatory function of auxiliary cells, not from its capacity to infect progenitors and stem cells themselves. Here we review the evidence in support of this model. We will focus on two commonly encountered AIDS-related hematopoietic abnormalities in clinical practice, regenerative marrow failure and lymphoid neoplasms. Because BM failure and lymphomas are common consequences of AIDS progression, and limit both survival and quality of life, a clear pathophysiological picture of these disorders and the molecular mechanism(s) by which HIV-1 causes or sets the stage for them, is an essential prerequisite for the development of rational strategies for therapy and prevention.
THE ROLE OF HIV-1 IN REGENERATIVE MARROW FAILURE
Recovery from myelosuppression induced by treatment with antivirals and with antilymphoma regimens is consistently more toxic to hematopoietic tissues of AIDS patients than in those of non-AIDS patients. Although many AIDS patients have BM failure before therapy,5-12 a greater number experience inordinate myelosuppressive toxicity after conventional antilymphoma13 or antiviral14chemotherapy unless hematopoietic growth factors are used.14-16 Even when growth factors are used, conventional therapy is extremely toxic. Levine et al17 recently described the results of their study using ABVD (Adriamycin [Pharmacia & Upjohn, Kalamazoo, MI], bleomycin, Velban [Lilly, Indianapolis, IN], and Dacarbazine [Bayer, West Haven, CT]) with granulocyte colony-stimulating factor (G-CSF) support of 21 HIV+ patients with Hodgkin's disease, in which nearly every patient had either severe and prolonged thrombocytopenia and/or neutropenia.17 Theoretically, causes of regenerative failure might reflect dysfunction of the hematopoietic progenitor cell population, dysfunction of cells that control the regenerative response by releasing hematopoietic growth factors, or activation of gene programs that serve to inhibit one or both of these two cell types.18 The weight of evidence today quite clearly suggests that the progenitor populations are intact and largely uninfected, but that auxiliary cells are very consistently infected and broadly dysfunctional.
Infection of Hematopoietic Progenitor Cells Is Infrequent and not Substantially Involved in the Pathogenesis of Marrow Failure
Infection of hematopoietic progenitor cells can be demonstrated in one of a number of ways. One can infect a population of BM cells ex vivo or acquire marrow cells from seropositive patients and then either plate single highly enriched progenitor cells in multi-well plates or plate such cells in semisolid medium. Once wells with colonies are identified, provirus, viral mRNA transcripts, or viral proteins must be detected in all of the cells of the colony and, ideally, infectious virus should be detected in coculture assays. Alternatively, clones arising from plated single progenitor cells plucked from semisolid medium can be used in the same assays. If these steps are not taken, the appearance of infected macrophages in the cultures cannot be attributed to direct progenitor cell infection because they may have simply survived in vitro having been infected in vivo. Enrichment of CD34+ cells before plating in clonal assays does not sidestep this pitfall. Such BM cell populations may also contain microvascular endothelial cells (MVEC) which universally express CD34 and are consistently infected in marrow from seropositive patients.19 Thus, in vitro infection of more differentiated myeloid cells and even T cells20 could result from in vivo infection of contaminating MVEC when CD34+ cells are plated in clonal assays. Using these strict criteria, only 1 of the 36 studies listed in Tables 1 and2convincingly demonstrate that committed progenitor cells can be infected by HIV-1.21
Reports That Describe the Effects of Ex Vivo HIV-1 Infection on Hematopoietic Cells
| Reference . | Method . | Progenitor Growth . | HIV-1 Infection of Progenitors? . | HIV-1 Infection of Clonal Progeny? . | Comments . |
|---|---|---|---|---|---|
| Folks et al173 | HIV-1 challenge of CD34+ BM cells. Infectivity evaluated by RT assay and EM. | Not evaluated. | Evidence of HIV-1 infection at 1 mo pi (RT activity). Virus detected by EM at 40-60 d pi. | Not evaluated. | No convincing evidence of progenitor cell infection. HIV-1–infected CD34+ stromal cells or residual T cells/MΘ could infect differentiating BM cells. |
| Molina et al27 (see Table 2) | Unfractionated BM cells challenged by HIV-1 and seeded into colony assays. | Colony growth normal. | Not evaluated. | No HIV-1 DNA detected (gag PCR). | No evidence of progenitor cell infection. |
| Steinberg et al174 | NATD BM cells and CD34+ BM cells challenged with HIV-1. | ↓ CFU-GM ↓ BFU-E | HIV-1 DNA detected (gag PCR). Signal much weaker in NATD cells. | No HIV-1 DNA detected (gag PCR). | No convincing evidence of progenitor cell infection. Contaminating T cells/MΘ may yield PCR signal. Anti-gp 160 abrogates inhibition of colony growth. |
| Kojouharoff et al175 (see Table 2) | Coculture of CD34+ BM cells with HIV-1-infected M and seeding into colony assays. | Colony growth normal. | Not evaluated. | HIV-1 mRNA transcripts in “40% of CFU-GEMM and CFU-GM (in situ hybridization). | No convincing evidence of progenitor cell infection. MΘ-to-MΘ infection could not be excluded. |
| Zauli et al176 | CD34+ cells from PB infected and seeded into colony assays. | ↓ CFU-GM ↓ BFU-E ↓ CFU-Mk | Not evaluated. | No HIV-1 DNA in pooled colonies (gag PCR). | No evidence of progenitor cell infection. |
| Zauli et al177 | CD34+ BM cells exposed to recombinant gp160, gp120, gp41, & p24. | ↓ CFU-GM & ↓ CD34+ cells via gp160 & gp120. | Not evaluated. | Not evaluated. | Suggests a mechanism for HIV-1-induced cytopenia without direct infection. |
| Calenda et al178 | Challenge of LTBMC with HIV-1 strains LAV and NDK. | ↓↑ CFU-E ↓↑ BFU-E | Virus recovered from LTBMC by cocultured with PBMC. | Not evaluated. | No convincing evidence of progenitor cell infection. Variable results for colony growth (↓↑) depending on virus strain, moi, and time. |
| Steinberg et al8 | Human stromal cell line (BS-1) infected with HIV-1 and cocultured with murine BM progenitors. | ↓ BFU-E ↓ CFU-GM | Not evaluated. Murine lineage cells. | Not evaluated. Murine lineage cells. | Suppression of murine colony growth attributed to HIV-1 infection of stromal cells. |
| Cen et al179 | Challenge of LTBMC and CD34+ BM cells with HIV-1 strains LAV, NL4-3 and ICR 3. | ↓ CFU-GM | No HIV-1 DNA detected (gag/env PCR). | Not evaluated. | No evidence of progenitor cell infection. Treatment of inoculum with anti-gp120 abrogates inhibition of colony growth. Infection of cellular elements in LTBMC with T cell-tropic HIV-1. |
| Zauli et al33 | CD34+ cell line (TF-1) exposed to HIV-1 or gp120 plus anti-gp120 and anti-CD4 antibodies. | Not evaluated. | No viral DNA (gag/env PCR) or protein (p24 ELISA). | Not evaluated. | No evidence of progenitor cell infection. Treatment with gp120 & antibodies induced apoptosis; suggests mechanism for cytopenia. |
| Maciejewski et al167 | Total BM cells, MΘ-depleted BM cells and CD34+ BM cells challenged with HIV-1 or heat-inactivated HIV-1. | ↓ CFU-GM ↓ BFU-E in total BM cultures only. | Not evaluated. | Not evaluated. | Depletion experiments implicate MΘ. Heat-inactivated HIV-1 inhibits colony growth. Inhibition partially blocked by anti-gp120 or anti-TNFα. |
| Schwartz et al180 | BM stroma challenged with HIV-1 strain ADA and overlaid with CD34+ cells. | ↓ CFU-GM ↓ BFU-E | Not evaluated. | Not evaluated. | Factors from HIV-1-infected stroma supress colony growth. Neutralization of IFNα or IL-4 restores growth. |
| Marandin et al61 | Challenge of LTBMC with monocyte-tropic HIV-1. | Colony growth normal. | Not evaluated. | Not evaluated. | MΘ were only infected stromal cells detected. Cytokine profiles unaffected. |
| Chelucci et al21 | CD34+/Lin− PB cells challenged with HIV-1 and seeded into colony assays. | Colony growth normal. | Not evaluated. | HIV-1 DNA (PCR), RNA (RT-PCR) or protein (p24 ELISA) detected. | CFU-GM and BFU-E from PB infected in vitro. CD34+/CD4+ cells (5-20% of total) were presumed targets. |
| Zauli et al172 | CD34+ BM cells challenged with HIV-1 or heat-inactivated HIV-1. | ↓ CFU-GM | No HIV-1 DNA detected (gag PCR). | Not evaluated. | No evidence of progenitor cell infection. gp120 induction of TGFb inhibits colony growth. |
| Kaushal et al181 | CD34+ BM cells (K6.1 MAB selected) challenged with HIV-1. After 24 h reselected for CD34 with ICH3 MAB. | Not evaluated. | No HIV-1 RNA detected (RT-PCR for unspliced and spliced tat-rev-nef transcripts). | Not evaluated. | No evidence of progenitor cell infection. Two-step purification enhances purity of CD34 population. |
| Reference . | Method . | Progenitor Growth . | HIV-1 Infection of Progenitors? . | HIV-1 Infection of Clonal Progeny? . | Comments . |
|---|---|---|---|---|---|
| Folks et al173 | HIV-1 challenge of CD34+ BM cells. Infectivity evaluated by RT assay and EM. | Not evaluated. | Evidence of HIV-1 infection at 1 mo pi (RT activity). Virus detected by EM at 40-60 d pi. | Not evaluated. | No convincing evidence of progenitor cell infection. HIV-1–infected CD34+ stromal cells or residual T cells/MΘ could infect differentiating BM cells. |
| Molina et al27 (see Table 2) | Unfractionated BM cells challenged by HIV-1 and seeded into colony assays. | Colony growth normal. | Not evaluated. | No HIV-1 DNA detected (gag PCR). | No evidence of progenitor cell infection. |
| Steinberg et al174 | NATD BM cells and CD34+ BM cells challenged with HIV-1. | ↓ CFU-GM ↓ BFU-E | HIV-1 DNA detected (gag PCR). Signal much weaker in NATD cells. | No HIV-1 DNA detected (gag PCR). | No convincing evidence of progenitor cell infection. Contaminating T cells/MΘ may yield PCR signal. Anti-gp 160 abrogates inhibition of colony growth. |
| Kojouharoff et al175 (see Table 2) | Coculture of CD34+ BM cells with HIV-1-infected M and seeding into colony assays. | Colony growth normal. | Not evaluated. | HIV-1 mRNA transcripts in “40% of CFU-GEMM and CFU-GM (in situ hybridization). | No convincing evidence of progenitor cell infection. MΘ-to-MΘ infection could not be excluded. |
| Zauli et al176 | CD34+ cells from PB infected and seeded into colony assays. | ↓ CFU-GM ↓ BFU-E ↓ CFU-Mk | Not evaluated. | No HIV-1 DNA in pooled colonies (gag PCR). | No evidence of progenitor cell infection. |
| Zauli et al177 | CD34+ BM cells exposed to recombinant gp160, gp120, gp41, & p24. | ↓ CFU-GM & ↓ CD34+ cells via gp160 & gp120. | Not evaluated. | Not evaluated. | Suggests a mechanism for HIV-1-induced cytopenia without direct infection. |
| Calenda et al178 | Challenge of LTBMC with HIV-1 strains LAV and NDK. | ↓↑ CFU-E ↓↑ BFU-E | Virus recovered from LTBMC by cocultured with PBMC. | Not evaluated. | No convincing evidence of progenitor cell infection. Variable results for colony growth (↓↑) depending on virus strain, moi, and time. |
| Steinberg et al8 | Human stromal cell line (BS-1) infected with HIV-1 and cocultured with murine BM progenitors. | ↓ BFU-E ↓ CFU-GM | Not evaluated. Murine lineage cells. | Not evaluated. Murine lineage cells. | Suppression of murine colony growth attributed to HIV-1 infection of stromal cells. |
| Cen et al179 | Challenge of LTBMC and CD34+ BM cells with HIV-1 strains LAV, NL4-3 and ICR 3. | ↓ CFU-GM | No HIV-1 DNA detected (gag/env PCR). | Not evaluated. | No evidence of progenitor cell infection. Treatment of inoculum with anti-gp120 abrogates inhibition of colony growth. Infection of cellular elements in LTBMC with T cell-tropic HIV-1. |
| Zauli et al33 | CD34+ cell line (TF-1) exposed to HIV-1 or gp120 plus anti-gp120 and anti-CD4 antibodies. | Not evaluated. | No viral DNA (gag/env PCR) or protein (p24 ELISA). | Not evaluated. | No evidence of progenitor cell infection. Treatment with gp120 & antibodies induced apoptosis; suggests mechanism for cytopenia. |
| Maciejewski et al167 | Total BM cells, MΘ-depleted BM cells and CD34+ BM cells challenged with HIV-1 or heat-inactivated HIV-1. | ↓ CFU-GM ↓ BFU-E in total BM cultures only. | Not evaluated. | Not evaluated. | Depletion experiments implicate MΘ. Heat-inactivated HIV-1 inhibits colony growth. Inhibition partially blocked by anti-gp120 or anti-TNFα. |
| Schwartz et al180 | BM stroma challenged with HIV-1 strain ADA and overlaid with CD34+ cells. | ↓ CFU-GM ↓ BFU-E | Not evaluated. | Not evaluated. | Factors from HIV-1-infected stroma supress colony growth. Neutralization of IFNα or IL-4 restores growth. |
| Marandin et al61 | Challenge of LTBMC with monocyte-tropic HIV-1. | Colony growth normal. | Not evaluated. | Not evaluated. | MΘ were only infected stromal cells detected. Cytokine profiles unaffected. |
| Chelucci et al21 | CD34+/Lin− PB cells challenged with HIV-1 and seeded into colony assays. | Colony growth normal. | Not evaluated. | HIV-1 DNA (PCR), RNA (RT-PCR) or protein (p24 ELISA) detected. | CFU-GM and BFU-E from PB infected in vitro. CD34+/CD4+ cells (5-20% of total) were presumed targets. |
| Zauli et al172 | CD34+ BM cells challenged with HIV-1 or heat-inactivated HIV-1. | ↓ CFU-GM | No HIV-1 DNA detected (gag PCR). | Not evaluated. | No evidence of progenitor cell infection. gp120 induction of TGFb inhibits colony growth. |
| Kaushal et al181 | CD34+ BM cells (K6.1 MAB selected) challenged with HIV-1. After 24 h reselected for CD34 with ICH3 MAB. | Not evaluated. | No HIV-1 RNA detected (RT-PCR for unspliced and spliced tat-rev-nef transcripts). | Not evaluated. | No evidence of progenitor cell infection. Two-step purification enhances purity of CD34 population. |
Abbreviations: NATD, nonadherent T-cell depleted; MΘ, macrophage; EM, electron microscopy; PB, peripheral blood; pi, postinfection; LTBMC, long-term bone marrow cultures.
Reports That Describe Hematopoietic Function of Cells Obtained From HIV-1–Seropositive Patients
| Reference . | Method . | Progenitor Growth . | HIV-1 Infection of Progenitors? . | HIV-1 Infection of Clonal Progeny? . | Comments . |
|---|---|---|---|---|---|
| Stella et al40 | BM cells from HIV-1+ patients seeded into colony assays. | ↓ CFU-GM ↓ CFU-GEM ↓ BFU-E ↓ CFU-Mk | Not evaluated. | Not evaluated. | Depletion of T cells enhanced colony growth. Suggest a T-cell imbalance may contribute to impaired hematopoiesis. |
| Donahue et al182 | BM cells from HIV-1+patients seeded into colony assays in the presence/absence of anti–HIV-1 antisera. | ↓ CFU-GM & ↓ BFU-E only in presence of antisera. | Not evaluated. | HIV-1 in pooled colonies from 1/4 patients (RT activity in culture supernatants). | No convincing evidence of progenitor cell infection. More differentiated cells in culture may be virus source. Anti-gp120 antibodies may be suppressive elements in patient sera. |
| Lunardi-Iskandar et al11 | BM cells from HIV-1+ patients seeded into colony assays. | ↓ CFU-GM ↓ BFU-E | Not evaluated. | Not evaluated. | Depletion of adherent cells or T cells or addition of cytokines (GM-CSF, IL-3, Epo) did not restore colony growth). |
| Molina et al27 (see Table 1) | BM cells from HIV-1+ patients seeded into colony assays. | Colony growth normal. | Not evaluated. | No HIV-1 DNA detected (gag PCR). | No evidence of progenitor cell infection. Propose cytopenias due to alteration in BM microenvironment. |
| Von Laer et al183 | Culture of purified CD34+ BM cells from HIV-1+ patients. | Not evaluated. | HIV-1 DNA detected in 1/14 samples (gag PCR). | Not evaluated. | No convincing evidence of progenitor cell infection. Single positive attributed to CD4+ T-cell contamination. |
| Ganser et al184 | BM cells from HIV-1+ patients seeded into colony assays. | ↓ CFU-GM ↓ CFU-GEM ↓ BFU-E ↓ CFU-Mk | Not evaluated. | No HIV-1 detected (immunohistochemistry and in situ hybridization). | No evidence of progenitor cell infection. |
| Davis et al28 | Purified CD34+ BM cells from HIV-1+ patients seeded into colony assays. | ↓ CFU-GM ↓ CFU-E | HIV-1 DNA detected in 1/11 samples (gag/env PCR). | No HIV-1 DNA detected (gag/env PCR). | No convincing evidence of progenitor cell infection. Single positive attributed to CD4+ T-cell or MΘ contamination. |
| Kojouharoff et al175 (see Table 2) | BM cells from HIV-1+ patients seeded into colony assays. | Colony growth normal. | Not evaluated. | HIV-1 mRNA detected in CFU-GEM & CFU-GM in 2/6 patients (in situ hybridization) | No convincing evidence of progenitor cell infection. HIV-1+ cells may reflect infection of a more differentiated phenotype. |
| Zauli et al25 | Purified CD34+ BM cells from HIV-1+ patients seeded into colony assays. | ↓ CFU-GM ↓ CFU-E ↓ CFU-Mk | No HIV-1 DNA (gag PCR) or protein (p24 ELISA) detected. | Not evaluated. | No evidence of progenitor cell infection. HIV-1 detected in unfractionated bone marrow samples. A role for HIV-1 in the BM microenvironment proposed. |
| Zauli et al185 | Purified CD34+ BM cells from HIV-1+patients seeded into colony assays. | ↓ CFU-GM ↓ CFU-Mk | HIV-1 DNA detected in 2/17 samples (gag/env PCR). No p24 in culture supernatants. | Not evaluated. | No convincing evidence of progenitor cell infection. Two positives attributed to CD4+ T-cell or MΘ contamination. 9 CFU-Mk in all samples implies an indirect mechanism. |
| Stanley et al150 | Purified CD34+ BM cells from HIV-1+ patients seeded into colony assays. | ↓ CFU-GM ↓ BFU-E | HIV-1 DNA (gag/env PCR) & virus (co-culture) detected in 17/52 & 8/29 Zairian and 3/22 & 1/2 American samples. | Not evaluated. | HIV-1 detected only in subset of patients with advanced disease. 9 CFU-GM & 9 BFU-E in all samples implies an indirect mechanism. CD34+ accessory cells are potential source of HIV-1. |
| Louache et al186 | BM cells from 27 HIV-1+ patients seeded into colony assays. | ↓ CFU-GM ↓ BFU-E ↓ CFU-Mk | Not evaluated. | No HIV-1 DNA detected (gag PCR). | No evidence of progenitor cell infection. Anti-sense nef or tat increased clonal growth. |
| Kaczmarski et al187 | BM cells from HIV-1+ patients studied in LTBMC and colony assays. | Normal CFU-GEMM and CFU-GM. | Not evaluated. | HIV-1 DNA detected in pooled CFU-GEMM, CFU-GM & CFU-LTC (gag/env/tat PCR). | No convincing evidence of progenitor cell infection. No T-cell depletion so infection of differentiating cells could not be ruled out. |
| De Luca et al29 | Purified CD34+ BM cells from HIV-1+ patients seeded into colony assays. | ↓ CFU-GM ↓ BFU-E | No HIV-1 DNA detected (gag PCR). | Not evaluated. | No evidence of progenitor cell infection. |
| Hillyer et al7 | Purified CD34+ cells from SIV-infected macaques seeded into colony assays. | ↓ CFU-GM ↓ BFU-E | No SIV DNA detected (gag PCR). | SIV DNA detected in occasional pooled CFU-GM colonies. | No convincing evidence of progenitor cell infection. Rare positive signal attributed to CD4+ T-cell or MΘ contamination. |
| Neal et al34 | Purified CD34+ BM cells from HIV-1+ patients studied in LTBMC and colony assays. | Normal CFU-E, BFU-E, CFU-GM & cobblestone formation in LTBMC. | HIV-1 DNA detected in 2/10 patients (gag/pol PCR). | All pooled CFU-GM were HIV-1 DNA negative (gag/pol PCR). 1/5 pooled CFU-E/BFU-E were weakly HIV-1+ (<2 copies/75,000 cells). | No convincing evidence of progenitor cell infection. Low proviral DNA copy number in pooled colonies (2-5 copies/250,000 cells) suggests (CD4+ T-cell or MΘ contamination. |
| Dallalio et al188 | Purified CD34+ BM cells from 5 HIV-1+ patients seeded into CFU-E assays | Normal CFU-E. | Not evaluated. | Not evaluated. | CFU-E were not more sensitive to cytokine-mediated inhibition of colony formation. |
| Slobod et al31 | Mobilization of CD34+ BM cells from HIV-1+ patients. Nested PCR for evidence of HIV-1 infection. | Not evaluated. | HIV-1 DNA in 2/7 patients but in only 1/3 replicate samples (nested env PCR detecting 1 DNA copy/25,000 cells). | Not evaluated | No convincing evidence of progenitor cell infection. CD34+ cells at 92-99% purity so variable positives attributed to CD4+T-cell contamination. |
| Marandin et al189 | Primitive BM cells from HIV-1+ patients evaluated by flow cytometry. Fractionated CD34+ cells studied in LTBMC and colony assays. | ↓ CFU-GM ↓ BFU-E ↓ LTC-IC | No HIV-1 DNA detected (gag PCR). Included separate analysis of CD34+/CD4+, CD34+/CD38+ & CD34+/CD38− populations. | No HIV-1 DNA detected (gag PCR). | No significant change in percentage of CD34+ cells in HIV-1+ patients but proportions of CD34+/CD38− & CD34+/Thy 1+ cells decreased. Suggests loss of more primitive phenotype but not via direct infection. |
| Kearns et al151 | Purified CD34+ BM cells from HIV-1+ patients seeded into colony assays. | ↓ CFU-GM | HIV-1 DNA detected in rare samples. Semi-quantitative PCR estimates 1/100,000 cells infected. | Not evaluated. | No evidence of significant progenitor cell infection. Propose dysregulated hematopoiesis due to alterations in the BM microenvironment. |
| Bahner et al52 | Ex vivo infection of human marrow stromal cells with HIV-1 and stomal cells genetically modified to resist HIV-1 infection. Stroma overlayered with bone marrow cells. | ↓ CFU-GM (obtained after 3 wk on stroma) | Not evaluated. | Not evaluated. | Stroma is infectable. Reduced capacity of HIV-1-infected stroma to support hematopoietic progenitor cell growth. |
| Reference . | Method . | Progenitor Growth . | HIV-1 Infection of Progenitors? . | HIV-1 Infection of Clonal Progeny? . | Comments . |
|---|---|---|---|---|---|
| Stella et al40 | BM cells from HIV-1+ patients seeded into colony assays. | ↓ CFU-GM ↓ CFU-GEM ↓ BFU-E ↓ CFU-Mk | Not evaluated. | Not evaluated. | Depletion of T cells enhanced colony growth. Suggest a T-cell imbalance may contribute to impaired hematopoiesis. |
| Donahue et al182 | BM cells from HIV-1+patients seeded into colony assays in the presence/absence of anti–HIV-1 antisera. | ↓ CFU-GM & ↓ BFU-E only in presence of antisera. | Not evaluated. | HIV-1 in pooled colonies from 1/4 patients (RT activity in culture supernatants). | No convincing evidence of progenitor cell infection. More differentiated cells in culture may be virus source. Anti-gp120 antibodies may be suppressive elements in patient sera. |
| Lunardi-Iskandar et al11 | BM cells from HIV-1+ patients seeded into colony assays. | ↓ CFU-GM ↓ BFU-E | Not evaluated. | Not evaluated. | Depletion of adherent cells or T cells or addition of cytokines (GM-CSF, IL-3, Epo) did not restore colony growth). |
| Molina et al27 (see Table 1) | BM cells from HIV-1+ patients seeded into colony assays. | Colony growth normal. | Not evaluated. | No HIV-1 DNA detected (gag PCR). | No evidence of progenitor cell infection. Propose cytopenias due to alteration in BM microenvironment. |
| Von Laer et al183 | Culture of purified CD34+ BM cells from HIV-1+ patients. | Not evaluated. | HIV-1 DNA detected in 1/14 samples (gag PCR). | Not evaluated. | No convincing evidence of progenitor cell infection. Single positive attributed to CD4+ T-cell contamination. |
| Ganser et al184 | BM cells from HIV-1+ patients seeded into colony assays. | ↓ CFU-GM ↓ CFU-GEM ↓ BFU-E ↓ CFU-Mk | Not evaluated. | No HIV-1 detected (immunohistochemistry and in situ hybridization). | No evidence of progenitor cell infection. |
| Davis et al28 | Purified CD34+ BM cells from HIV-1+ patients seeded into colony assays. | ↓ CFU-GM ↓ CFU-E | HIV-1 DNA detected in 1/11 samples (gag/env PCR). | No HIV-1 DNA detected (gag/env PCR). | No convincing evidence of progenitor cell infection. Single positive attributed to CD4+ T-cell or MΘ contamination. |
| Kojouharoff et al175 (see Table 2) | BM cells from HIV-1+ patients seeded into colony assays. | Colony growth normal. | Not evaluated. | HIV-1 mRNA detected in CFU-GEM & CFU-GM in 2/6 patients (in situ hybridization) | No convincing evidence of progenitor cell infection. HIV-1+ cells may reflect infection of a more differentiated phenotype. |
| Zauli et al25 | Purified CD34+ BM cells from HIV-1+ patients seeded into colony assays. | ↓ CFU-GM ↓ CFU-E ↓ CFU-Mk | No HIV-1 DNA (gag PCR) or protein (p24 ELISA) detected. | Not evaluated. | No evidence of progenitor cell infection. HIV-1 detected in unfractionated bone marrow samples. A role for HIV-1 in the BM microenvironment proposed. |
| Zauli et al185 | Purified CD34+ BM cells from HIV-1+patients seeded into colony assays. | ↓ CFU-GM ↓ CFU-Mk | HIV-1 DNA detected in 2/17 samples (gag/env PCR). No p24 in culture supernatants. | Not evaluated. | No convincing evidence of progenitor cell infection. Two positives attributed to CD4+ T-cell or MΘ contamination. 9 CFU-Mk in all samples implies an indirect mechanism. |
| Stanley et al150 | Purified CD34+ BM cells from HIV-1+ patients seeded into colony assays. | ↓ CFU-GM ↓ BFU-E | HIV-1 DNA (gag/env PCR) & virus (co-culture) detected in 17/52 & 8/29 Zairian and 3/22 & 1/2 American samples. | Not evaluated. | HIV-1 detected only in subset of patients with advanced disease. 9 CFU-GM & 9 BFU-E in all samples implies an indirect mechanism. CD34+ accessory cells are potential source of HIV-1. |
| Louache et al186 | BM cells from 27 HIV-1+ patients seeded into colony assays. | ↓ CFU-GM ↓ BFU-E ↓ CFU-Mk | Not evaluated. | No HIV-1 DNA detected (gag PCR). | No evidence of progenitor cell infection. Anti-sense nef or tat increased clonal growth. |
| Kaczmarski et al187 | BM cells from HIV-1+ patients studied in LTBMC and colony assays. | Normal CFU-GEMM and CFU-GM. | Not evaluated. | HIV-1 DNA detected in pooled CFU-GEMM, CFU-GM & CFU-LTC (gag/env/tat PCR). | No convincing evidence of progenitor cell infection. No T-cell depletion so infection of differentiating cells could not be ruled out. |
| De Luca et al29 | Purified CD34+ BM cells from HIV-1+ patients seeded into colony assays. | ↓ CFU-GM ↓ BFU-E | No HIV-1 DNA detected (gag PCR). | Not evaluated. | No evidence of progenitor cell infection. |
| Hillyer et al7 | Purified CD34+ cells from SIV-infected macaques seeded into colony assays. | ↓ CFU-GM ↓ BFU-E | No SIV DNA detected (gag PCR). | SIV DNA detected in occasional pooled CFU-GM colonies. | No convincing evidence of progenitor cell infection. Rare positive signal attributed to CD4+ T-cell or MΘ contamination. |
| Neal et al34 | Purified CD34+ BM cells from HIV-1+ patients studied in LTBMC and colony assays. | Normal CFU-E, BFU-E, CFU-GM & cobblestone formation in LTBMC. | HIV-1 DNA detected in 2/10 patients (gag/pol PCR). | All pooled CFU-GM were HIV-1 DNA negative (gag/pol PCR). 1/5 pooled CFU-E/BFU-E were weakly HIV-1+ (<2 copies/75,000 cells). | No convincing evidence of progenitor cell infection. Low proviral DNA copy number in pooled colonies (2-5 copies/250,000 cells) suggests (CD4+ T-cell or MΘ contamination. |
| Dallalio et al188 | Purified CD34+ BM cells from 5 HIV-1+ patients seeded into CFU-E assays | Normal CFU-E. | Not evaluated. | Not evaluated. | CFU-E were not more sensitive to cytokine-mediated inhibition of colony formation. |
| Slobod et al31 | Mobilization of CD34+ BM cells from HIV-1+ patients. Nested PCR for evidence of HIV-1 infection. | Not evaluated. | HIV-1 DNA in 2/7 patients but in only 1/3 replicate samples (nested env PCR detecting 1 DNA copy/25,000 cells). | Not evaluated | No convincing evidence of progenitor cell infection. CD34+ cells at 92-99% purity so variable positives attributed to CD4+T-cell contamination. |
| Marandin et al189 | Primitive BM cells from HIV-1+ patients evaluated by flow cytometry. Fractionated CD34+ cells studied in LTBMC and colony assays. | ↓ CFU-GM ↓ BFU-E ↓ LTC-IC | No HIV-1 DNA detected (gag PCR). Included separate analysis of CD34+/CD4+, CD34+/CD38+ & CD34+/CD38− populations. | No HIV-1 DNA detected (gag PCR). | No significant change in percentage of CD34+ cells in HIV-1+ patients but proportions of CD34+/CD38− & CD34+/Thy 1+ cells decreased. Suggests loss of more primitive phenotype but not via direct infection. |
| Kearns et al151 | Purified CD34+ BM cells from HIV-1+ patients seeded into colony assays. | ↓ CFU-GM | HIV-1 DNA detected in rare samples. Semi-quantitative PCR estimates 1/100,000 cells infected. | Not evaluated. | No evidence of significant progenitor cell infection. Propose dysregulated hematopoiesis due to alterations in the BM microenvironment. |
| Bahner et al52 | Ex vivo infection of human marrow stromal cells with HIV-1 and stomal cells genetically modified to resist HIV-1 infection. Stroma overlayered with bone marrow cells. | ↓ CFU-GM (obtained after 3 wk on stroma) | Not evaluated. | Not evaluated. | Stroma is infectable. Reduced capacity of HIV-1-infected stroma to support hematopoietic progenitor cell growth. |
Abbreviations: NATD, nonadherent T-cell depleted; MΘ, macrophage.
It is important to emphasize that cells expressing CD34 on their surface are not all progenitor cells. Most committed progenitors express CD34 but only 10% to 20% of CD34+ cells from marrow will form colonies. Consequently, when studies are performed on CD34+ cells rather than their clonal progeny, only negative results are informative, vis-à-vis progenitor cell infection. In a frequently cited report, Folks et al173 reported that more than 1 month after ex vivo infection of CD34+ BM cells, monocyte progeny were productively infected by HIV-1. Although the report claimed to have documented infection of purified “progenitor” cells, and while the monocytes that remained in the cultures were likely derived from more primitive precursors, no direct test of progenitor cell infectivity was performed. In fact, in the past few years, most groups have reported that CD34+ cells and/or colony-forming units from seropositive patients are uninfected23-29 (see Table 2), results that nicely match studies performed on rhesus macaques infected with SIV smm9.7
Theoretically, the failure to discover infected progeny in clonal analyses might result from the induction of apoptosis in infected cells, but the weight of evidence is against this possibility. For example, although in the steady-state circulating committed progenitor cells (colony-forming unit megakaryocyte [CFU-Mk], CFU-granulocyte macrophage [CFU-GM], and burst-forming unit-erythroid [BFU-E]) are reduced in the blood of HIV-1 seropositive patients,30 progenitor reserves are sufficient to be released normally from hematopoietic niches after stimulation. Specifically, HIV-1–infected patients, even patients with advanced disease,31 respond appropriately to exogenous G-CSF by mobilizing progenitor cells of multiple lineages.31,32 More direct in vitro evidence exists against the notion of apoptosis in infected cells. For example, growth of a CD34+ progenitor cell line suppressed by exposure to HIV-1 can be rescued by interleukin-3 (IL-3) and GM-CSF.33 Finally, very direct evidence that progenitor cell apoptosis does not result from HIV-1 infection of progenitors can be found in the only published work that reports stringent progeny analysis from cultures of single cells, in which the potential impact of CD34+ auxiliary cells was minimized. Specifically, Chelucci et al21 purified CD34+/Lin− cells from peripheral blood of healthy volunteers, cultured the cells at densities of 104/mL in the presence of saturating quantities of Steel factor, erythropoietin (Epo), GM-CSF, and IL-3, pelleted the cells, and resuspended them in 50 mL in the presence of HIV-1, HIVIIIB, or NL4-3 strains for 2 hours. The cells were then diluted to 200 mL, cultured overnight, and were then washed and plated in methylcellulose medium at a density of one cell per plate. Colonies were plucked for p24 analysis (enzyme-linked immunosorbent assay [ELISA]) and for HIV-1 tat mRNA reverse transcriptase-polymerase chain reaction (RT-PCR). Twelve percent of CFU-GM colonies were p24 antigen+ and 23% were positive for tat mRNA. Seventeen percent of BFU-E colonies were tat mRNA+ but only 1 of 37 clones was positive for p24. No CFU-GEMM progeny were positive for virus. Appropriate control vectors, including heat-inactivated virus, were used. Consequently, under these clearly defined ex vivo conditions, progenitor cells are infectable, but, more importantly for further consideration of the question of marrow failure, HIV-1 infection ex vivo does not impair the capacity of either CFU-GM or BFU-E to undergo clonal replication or differentiation because differentiated colonial progeny contain the provirus.
Notwithstanding the potential for ex vivo infection, unambiguous evidence for the existence of HIV-1–infected progenitor cells from patients has not yet been described. Based on the above observations and a review of the reports outlined in Tables 1 and 2, it is safe to conclude that: (1) a low fraction of progenitor cells are infectable ex vivo by HIV-1 under some conditions, (2) the growth of the few cells infected by HIV-1 may not be impaired as a result of the infection, and (3) in vivo infection of progenitor cells occurs rarely if ever. There is, on the other hand, clear-cut evidence that viral infection of auxiliary cells and viral gene products themselves can indirectly influence survival and growth of hematopoietic progenitors.
Infected Auxiliary Cells and Regenerative Failure
T cells.
The release of hematopoietic growth factors by T cells is variably influenced by HIV-1 infection. Although IL-6 production is increased,34 production of IL-2 is reduced by HIV-135 possibly via the effects of HIV-1 nef,36 which is known to bind protein kinase Cζ (PKCζ),37 an essential signaling factor in T-cell activation.38,39 It was discovered early on that marrow T cells from HIV-1 patients suppress clonal growth of progenitors in vitro40 but this will be discussed below.
Monocyte/macrophages.
HIV-1 tat and gp120 induce cytokine expression in monocytes, but whether HIV-1 infection of monocytes achieves the same level of induction is arguable. Molina et al41 report that peripheral blood mononuclear cells (PBMC) infected with myriad strains of HIV-1 do not express IL-1, IL-6, or tumor necrosis factor-α (TNF-α). IL-12 release is impaired in infected cells,42and reduced production of IL-1 has been reported.43 Taking into account the important capacity of IL-1 to induce multilineage hematopoietic growth factors,44 and of IL-12 to influence interferon-γ (IFN-γ) expression,45,46 substantial effects on the kinetics of myeloid regeneration could result. The most compelling evidence supporting a role for macrophage dysfunction in regenerative failure is work reported recently on the reduced capacity of monocyte/macrophages to support on-demand myelopoieses after HIV-1 infection.47 Specifically, Esser et al47infected PB-derived monocytes on teflon membranes with HIV-1 (HIV-1D117IIII) and quantified both constitutive and endotoxin-induced secretion of a variety of cytokines. They found that while secretion of TNF-α, IL-1β, IL-6, and IL-8 was stimulated, expression of M, G-, and GM-CSF was inhibited.47
Microvascular endothelial cells (MVEC).
MVEC from a variety of organs including the brain,48,49liver,50 kidney,51 and BM3,19 are permissive for HIV-1 infection. Of greater relevance to the issue of regenerative marrow failure, BM MVEC cells are always found to be infected by HIV-1 in seropositive patients regardless of the stage of their disease.19 Moreover, while constitutive production of hematopoietic growth factors by such cells (either alone or admixed with other BM stromal cell types) is normal, IL-1 induced production of G-CSF and IL-6 is significantly reduced.19 This suggests that HIV-1 infection of BM MVEC reduces the capacity of hematopoietic stroma to respond to regulatory signals that normally augment blood cell production during periods of increased demand. The dysfunction in marrow stroma does not seem to derive simply from direct interdiction of IL-1 responses in MVEC because pure populations of MVEC, at least from brain, do not respond abnormally to IL-1 after infection (Moses et al, unpublished, April 1997). Therefore, it is likely that the MVEC infection induces release of factors that influence the behavior of other IL-1–responsive cells in the stroma, most of which are not infected (Fig 1). The mechanisms by which this occurs are currently under investigation in our laboratories. The recent report of hematopoietic support dysfunction of HIV-1–infected mixed BM stromal cell cultures containing only 2% MVEC52 supports the notion that collaboration between infected and uninfected cells results in the failure of the stroma to support myeloid hematopoiesis.
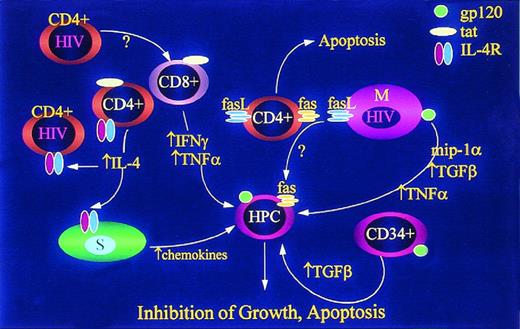
HIV-1 inhibits replication of hematopoietic progenitor cells (HPC) by reducing production of hematopoietic growth factors. HIV-1 infection of mononuclear phagocytes (M) reduces LPS-induced GM-CSF, M-CSF, and IL-1.47 HIV-1 induces expression of IL-10 in mononuclear leukocytes152,153 and IL-10 inhibits LPS induced release of IL-6, IL-1, GM-CSF, and G-CSF.154,155 Infected MVEC inhibit the responsiveness of BM stromal cells to IL-1 such that IL-6 and G-CSF release is inhibited.19 The mechanism of this effect is unknown and may involve release of a repressor factor (marked in the figure as “?”). Finally, infected macrophages release less IL-143 156 and, because IL-1 controls expression of multiple hematopoietic growth factors in auxiliary cells, including endothelial (MVEC) and other stromal cells (S), the responsiveness of HPC to a given set of environmental cues is functionally reduced.
HIV-1 inhibits replication of hematopoietic progenitor cells (HPC) by reducing production of hematopoietic growth factors. HIV-1 infection of mononuclear phagocytes (M) reduces LPS-induced GM-CSF, M-CSF, and IL-1.47 HIV-1 induces expression of IL-10 in mononuclear leukocytes152,153 and IL-10 inhibits LPS induced release of IL-6, IL-1, GM-CSF, and G-CSF.154,155 Infected MVEC inhibit the responsiveness of BM stromal cells to IL-1 such that IL-6 and G-CSF release is inhibited.19 The mechanism of this effect is unknown and may involve release of a repressor factor (marked in the figure as “?”). Finally, infected macrophages release less IL-143 156 and, because IL-1 controls expression of multiple hematopoietic growth factors in auxiliary cells, including endothelial (MVEC) and other stromal cells (S), the responsiveness of HPC to a given set of environmental cues is functionally reduced.
Uninfected (bystander) Auxiliary Cells and Regenerative Marrow Failure
T cells.
The clonal suppressive effect of CD8+ hematopoietic inhibitory T cells (HIT cells) has been recognized for decades.53,54 Such cells have been discovered in HIV-1–infected patients.55 Specifically, clonal growth of CFU-GEMM, BFU-E, and CFU-GM from marrows of HIV-1+ patients significantly increased after depletion of CD8+, γδ+, and d-TCS-1+ T cells. No such effect was seen in cultures of normal marrow samples. Further experiments showed that direct cellular contact between effector and hematopoietic progenitor cells was essential and that IFN-γ and TNF-α were key factors mediating the suppressive effect of the d-TCS-1+cells in HIV+ patients.55 In situ hybridization could not detect mRNA from HIV in d-TCS-1+ cells. Therefore, this subset of T cells is an important inhibitor of hematopoietic progenitor cell growth, but they are not infected by HIV-1 (Fig 2).
HIV-1 inhibits replication of HPC by inducing release of multiple mitotic inhibitory factors and expression of factors known to induce apoptosis. HIV-1–infected CD4+ T cells and HIV-1 tat released from infected cells induce release of IFN-γ96,157 and TNF-α158 from uninfected CD8+ T cells. HIV-1 and HIV-1 tat induce expression of IL-4 (which inhibits release of hematopoietic growth factors by other cells) and IL-4 receptors (IL-4R) thereby enhancing the “blunting” effect in lymphocytes159,160 and possibly stromal cells. IL-4 also induces stromal cells to release TGF-β161 and chemokine inhibitors.162 HIV-1 induction of fas ligand (FasL) in mononuclear phagocytes (M) and CD4+ cells results in apoptosis of uninfected CD4+ cells,163,164 but may also result in apoptosis of HPC which are known to express Fas under a variety of conditions. 57-59,165,166 Infection of macrophages also induces release of TNF-α,167,168TGF-β,169,170 and MIP1α.171 CD34+cells exposed to HIV-1 or HIV-1 gp120 release TGF-β,172 but while this has been called an “autocrine” response, it is not known whether the TGF-producing cells are actually progenitor cells, and is not, therefore, known to be a truly autocrine mechanism of HPC inhibition.
HIV-1 inhibits replication of HPC by inducing release of multiple mitotic inhibitory factors and expression of factors known to induce apoptosis. HIV-1–infected CD4+ T cells and HIV-1 tat released from infected cells induce release of IFN-γ96,157 and TNF-α158 from uninfected CD8+ T cells. HIV-1 and HIV-1 tat induce expression of IL-4 (which inhibits release of hematopoietic growth factors by other cells) and IL-4 receptors (IL-4R) thereby enhancing the “blunting” effect in lymphocytes159,160 and possibly stromal cells. IL-4 also induces stromal cells to release TGF-β161 and chemokine inhibitors.162 HIV-1 induction of fas ligand (FasL) in mononuclear phagocytes (M) and CD4+ cells results in apoptosis of uninfected CD4+ cells,163,164 but may also result in apoptosis of HPC which are known to express Fas under a variety of conditions. 57-59,165,166 Infection of macrophages also induces release of TNF-α,167,168TGF-β,169,170 and MIP1α.171 CD34+cells exposed to HIV-1 or HIV-1 gp120 release TGF-β,172 but while this has been called an “autocrine” response, it is not known whether the TGF-producing cells are actually progenitor cells, and is not, therefore, known to be a truly autocrine mechanism of HPC inhibition.
Monocyte/macrophages.
Apoptosis of lymphoid cells in nodal tissues of patients with HIV-1 infection occurs in cells that are not themselves infected by HIV-1. Priming of the Fas pathway has also been shown in lymphocytes from HIV-1–infected individuals, but cells undergoing apoptosis can be virus free. Badley et al56 have found that HIV-1 induces Fas ligand (FasL) expression in infected monocytes and that this effect may account, indirectly, for apoptotic signals to both lymphocytes and, in our view, possibly progenitor cells (Fig 2). In this report, the investigators argue that this bystander effect, in which HIV-1 induces both Fas and FasL, is a mechanism that can account for lymphocyte depletion during the course of HIV-1 disease. Hematopoietic progenitor cells are also quite susceptible to Fas-induced apoptosis and this particular apoptotic pathway likely accounts for a variety of BM failure states (Fig2).57-59Therefore, it is likely that FasL induction in auxiliary cells may be of substantial relevance, particularly if the Fas pathway is primed by IFN-γ58,60 or chemotherapeutic agents.
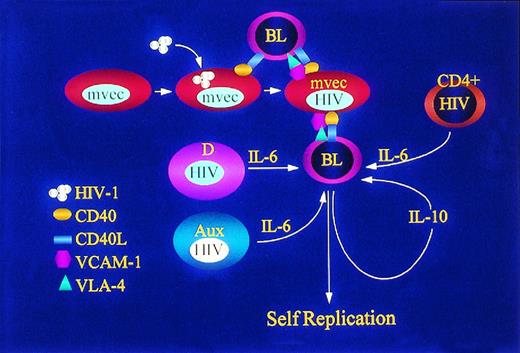
Accessory cells support adherence and growth of poorly differentiated neoplastic pre-B cells (BL). Dendritic cells,95 CD4+ T cells,34 and other auxiliary cells (Aux)97 infected with HIV-1 or exposed to gp120 or gp16034 release IL-6, a potent mitogen for lymphoma cell growth. Lymphoma cells themselves, both those infected with EBV and EBV−, release IL-10 in an autocrine fashion. MVEC infected with HIV-1 express CD40 and bind to lymphoma cell CD40L, a process that activates VCAM-1 expression and subsequent binding with its cognate ligand VLA-4.135Proliferation and survival of lymphoma cells is enhanced following adherence to MVEC. Adherence to MVEC may also facilitate retention of BL cells at sites rich in auxiliary cells for exposure to IL-6.
Accessory cells support adherence and growth of poorly differentiated neoplastic pre-B cells (BL). Dendritic cells,95 CD4+ T cells,34 and other auxiliary cells (Aux)97 infected with HIV-1 or exposed to gp120 or gp16034 release IL-6, a potent mitogen for lymphoma cell growth. Lymphoma cells themselves, both those infected with EBV and EBV−, release IL-10 in an autocrine fashion. MVEC infected with HIV-1 express CD40 and bind to lymphoma cell CD40L, a process that activates VCAM-1 expression and subsequent binding with its cognate ligand VLA-4.135Proliferation and survival of lymphoma cells is enhanced following adherence to MVEC. Adherence to MVEC may also facilitate retention of BL cells at sites rich in auxiliary cells for exposure to IL-6.
BM stromal cells.
Zauli et al33 evaluated the effect of a short-term exposure (2 hours) to two different lymphocytotropic strains of HIV-1 (HIVIIIB and ICR-3) on the survival of a factor-dependent CD34+hematopoietic cell line (TF-1). Although HIV-1–treated TF-1 cells underwent programmed cell death, the response was reversible with optimal doses of IL-3 or GM-CSF or both. Moreover, this group found no signs of productive or latent infection of these cells but did notice that treatment of TF-1 cells with recombinant gp120 plus a polyclonal anti-gp120 antibody, or with anti-CD4 monoclonal antibody (MoAb) plus rabbit anti-mouse IgG, significantly increased the percentage of cells undergoing apoptosis. They reasoned that the apoptotic response was the result of an interaction of gp120 with the CD4 receptor, which was expressed at a low level on the surface of TF-1 cells.
Similarly, in conditions mimicking the steady state in vitro, monocytotropic strains of HIV-1 (Bal, Ada, and JR-FL) did not alter production of TGF-β, TNF-α, MIP-1-α, Steel factor, and IL-6 in long-term BM cultures (LTBMCs).61 Coculture of infected cells with progenitors also failed, at least in one study,61 to alter the number of progenitor cells supported by the stromal layers. The investigators argue that productive and sustained virus replication in the macrophage component of LTBMCs does not significantly alter the profile of major cytokines involved in regulating hematopoiesis at least in the steady state. However, it is important to recognize that this group was unable to infect stromal cells with lymphocytotropic strains,61 likely because the culture conditions were not supportive of microvascular endothelial cell growth. Taking into account the potential importance of stromal MVEC as a reservoir for HIV-1,3,19 these results remained to be confirmed in a long-term system that permits the sustained growth of MVEC. In fact, recently Kohn's group performed studies in which human BM stromal cell cultures were infected ex vivo with a monocytotropic isolate of HIV-1 (JR-FL).52 They reported that stromal cells infected by this isolate fail to normally support the proliferation of hematopoietic progenitor cells but that cells genetically modified to resist HIV-1 infection were fully supportive. Their culture system was not specifically designed to support vascular endothelial cells but did contain 2% MVEC and 2% mononuclear phagocytes. Consequently, mixed marrow stromal cell cultures containing either macrophages or endothelial cells or both are infectable by HIV-1 in vivo and in vitro and such infection reduces the capacity of heterogeneous stromal cells (likely including uninfected ones in the same culture) to support myeloid hematopoiesis. The presence of MVEC in the cultures of this group may account for the differences in the stromal support function described in this study52 compared with other published work.61
Blunted Inductive Responses in Other Auxiliary Cells
Scadden's group tested the hypothesis that HIV-1 infection of Epo-producing cells might blunt the response of those cells to the inductive effect of hypoxia on Epo gene expression.62 Their studies on the hepatoma cell line Hep3B, an Epo-producing cell line in which hypoxia is a reliable inductive stimulus, show that after direct infection Epo production induced by hypoxia was depressed, potentially by translational suppression of Epo mRNA.62 Clearly, because hepatocyte production of Epo in vivo represents a minor component of the response of humans to hypoxia, the effect of HIV-1 on renal Epo production must be similarly evaluated. However, the potential role of HIV-1, or, more likely, specific viral proteins, in repressing the expression of the epo gene is worth pursuing taking into account the frequent occurrence of anemia in patients with AIDS.
THE ROLE OF HIV-1 IN AIDS LYMPHOMAGENESIS
AIDS patients are at increased risk for developing clinically aggressive B-cell non-Hodgkin's lymphomas (NHL).63-66Unlike other AIDS-associated malignancies, the AIDS-associated NHL (AIDS-NHL) develop in every population group at risk for AIDS. Current estimates indicate that 5% to 10% of HIV-1–infected patients develop this life-threatening disease. The etiology of AIDS-NHL is unknown and is likely to be complex. With some interesting exceptions,67,68 the malignant B cells in AIDS-NHL are not directly infected by HIV-1,69-72 suggesting that HIV-1 contributes to B-cell neoplasia via indirect mechanisms. Given the high degree of molecular heterogeneity seen within this group of neoplasms, it is likely that multiple pathways operate individually or in concert within the context of HIV-1 infection to promote lymphomagenesis.
Malignant transformation is the ultimate consequence of sequential genetic changes that occur within a proliferating cell population. Immunocompromised individuals without HIV-1 infection are at increased risk for developing NHL,73 indicating that immunodeficiency per se facilitates transformation. However, NHL arising in immunosuppressed posttransplant patients are generally restricted to the large cell immunoblastic subtype; these patients are not at increased risk for Burkitt's lymphoma.64 This observation suggests that immunosuppression alone is insufficient cause for development of at least the Burkitt's subtype of AIDS-NHL. In addition, AIDS-NHL have a number of distinguishing features including aggressive clinical behavior, extensive extranodal involvement, a high incidence of Epstein-Barr virus (EBV) negativity and a heterogeneous pattern of genetic changes, that suggest the existence of oncogenic mechanisms unique to the HIV-1–infected host. These features are discussed in more detail below.
Distinctive Features of AIDS-NHL
Clinical behavior.
Biggar et al74 recently linked AIDS and cancer registry data for the 1980-1990 decade to evaluate the overall lymphoma risk for persons with AIDS and determined a risk of 348-fold for high-grade lymphomas but only 14-fold for low-grade lymphomas. In another study, over 80% of AIDS-NHL were classified as high-grade tumors while less than 40% of non-AIDS NHL qualified as high-grade neoplasms.75 The aggressive clinical behavior of the AIDS-NHL has necessitated the creation of a separate classification system. Histologically, the majority of systemic AIDS-NHL fall into one of three main categories: (1) high-grade, small noncleaved cell (Burkitt's and Burkitt's-like) lymphomas (SNCCL), (2) high-grade, large cell immunoblastic lymphomas (IBL), and (3) intermediate grade, large noncleaved cell lymphomas (LNCCL). Because of their aggressive clinical behavior, the LNCCL have been functionally classified along with the high-grade IBL as diffuse large cell lymphomas (DLCL). A subtype of “intermediate” lymphomas exhibiting features of both SNCCL and immunoblastic DLCL has also been described.76 The recently described CD30+ anaplastic lymphomas,77 body-cavity–based or primary effusion lymphomas (BCBL/PEL),78,79 and plasmablastic lymphomas (PBL) of the oral cavity80 have also been included in this category, although recent opinion suggests the reclassification of the PEL and PBL, as well as the “intermediate” NHL, as separate pathologic entities.81 Collectively, DLCL comprise about two thirds of the systemic NHL while SNCCL make up approximately one third. T cell, non-T/non-B cell, and low-grade NHL comprise the remainder. The primary central nervous system lymphomas (PCNSL), which account for approximately 20% of all AIDS-NHL, fall almost exclusively into the high-grade immunoblastic subtype of DLCL.
Extranodal involvement.
The extent of extranodal involvement is a characteristic feature of AIDS-NHL. Malignant cells arising in the lymph node may seed to extranodal sites, or the tumor may be exclusively extranodal with no overt involvement of the lymph nodes.82 The most common sites for extranodal involvement include the gastrointestinal tract, liver, BM, and meninges for systemic NHL and perivascular cuffing within the brain parenchyma for the primary CNS NHL.63 83As the name suggests, PEL do not seed from solid tumors but present exclusively in the body cavities as extranodal lymphomatous effusions.
Association with EBV.
While posttransplant NHL arising in immunocompromised patients are invariably EBV-associated, only about half of the AIDS-associated systemic NHL are EBV+.69,70,84-86 Approximately 30% of SNCCL are EBV+ and the transforming antigens EBNA-2 and LMP-1 are not expressed.87 The incidence of EBV among the large cell lymphomas is about 60% to 70%; almost 100% for the immunoblastic subtype where EBNA-2 and LMP-1 are expressed and a lower incidence amongst the LNCCL, which have a latency pattern resembling that of the SNCCL.87 The primary CNS lymphomas are almost exclusively EBV-infected with a latency pattern characterized by expression of EBNA-2 and LMP-1.88 EBV-driven lymphoproliferation in the setting of immunodeficiency is likely to play a central role in the development of EBV+ AIDS-NHL, as it does for posttransplant lymphomas. However, the high frequency of EBV− AIDS-NHL indicates that additional factors must influence lymphoma development.
Genetic diversity.
Genetic lesions characteristic of AIDS-NHL are heterogeneous and tend to segregate with certain histologic subtypes. For example, the c-myc gene rearrangement is found in almost all SNCCL, but occurs in only one quarter of DLCL and is absent in the primary CNS lymphomas.89 Inactivation of the tumor suppressor gene p53 occurs in up to 60% of SNCCL but is seen in only a fraction of DLCL,89 while rearrangements of the bcl-6 gene are seen almost exclusively in DLCL.90 The PEL are generally lacking any of these genetic lesions but are exclusively and consistently infected with HHV-8.78 79 The lack of universality of EBV association and the different patterns of EBV gene expression contribute further to the genetic diversity of the AIDS-NHL.
The high degree of clinical and molecular heterogeneity seen within the AIDS-NHL suggests that multiple pathways exist within the HIV-infected host to promote the development of one or another subtype of lymphoma. The unifying feature of these neoplasms, that they all arise abnormally in the setting of AIDS, argues strongly for a general role for HIV-1 infection that encompasses all of these genetically disparate tumors. Thus, it is likely that in the context of the HIV-1–infected host multiple influences cooperate to induce B-cell hyperplasia and facilitate malignant transformation. These influences include: (1) chronic immune stimulation and polyclonal B-cell activation, (2) the presence of a dysregulated cytokine milieu, (3) inadequate tumor surveillance, and (4) infection with potentially oncogenic agents such as EBV and HHV-8.65,72,91 These pathways provide a mechanism for lymphomagenesis without the need to invoke direct infection of the malignant clone. Indeed, it is generally accepted that the malignant B cells in AIDS-NHL are not directly infected by HIV-1.70-72 89 A more detailed description of the host factors that contribute to the development of AIDS-NHL follows.
Host Factors Contributing to the Development of AIDS-NHL
Chronic immune stimulation.
Primary HIV-1 infection is frequently associated with a persistent and generalized lymphadenopathy (PGL), characterized by expansion of lymph node germinal centers in response to the recruitment, proliferation, differentiation, and apoptotic death of antigen-reactive B cells.92 HIV-1 itself, as well as other environmental or self antigens, may contribute to this polyclonal B-cell hyperplasia and hypergammaglobulinemia. Antigen-driven B-cell hyperproliferation would not only increase the risk for genetic accidents and the emergence of transformed B-cell clones, but would contribute to the expansion of such neoplastic clones. In support of a role for chronic antigen stimulation in the development of AIDS-NHL, Riboldi et al93showed that Burkitt's lymphomas derived from AIDS patients produced self-reactive IgM antibodies, and that somatic mutations within the IgM VH segment were reminiscent of those seen in Ig genes from other autoreactive B-cell clones.
Cytokines.
A characteristic feature of AIDS is the existence of a deregulated cytokine network.92 Some of the key cytokines that regulate B-cell growth and differentiation, such as IL-6, IL-9, and IL-10, are produced by CD4+ T cells,34,94 dendritic cells,95,96 and macrophages47,97-99 following HIV-1 infection or in response to viral proteins, suggesting a potential role for these cytokines in B-cell lymphomagenesis. Indeed, high serum levels of IL-6 may be predictive of the development of NHL.100 Once a lymphoma is established, its growth and survival may then be sustained through paracrine and autocrine growth loops.101,102 Interestingly, studies by Benjamin et al103 have shown that autocrine production of IL-10 is a feature of AIDS-associated Burkitt's lymphomas and is not seen in sporadic or endemic cases.
Impaired immune surveillance.
There is a positive correlation between immunodeficiency, as measured by decreasing CD4 counts, and the development of AIDS-NHL.104 This correlation applies particularly to the risk for developing large cell NHL, because SNCCL may develop when immunity is relatively intact.84,100 Independent of CD4 counts, the duration of the immunodeficent state was also found to increase the risk of developing AIDS-NHL.100 This implies that as patients survive longer with improved retroviral treatment, the incidence of AIDS-NHL may increase.105 Impaired immunosurveillance as a risk factor for AIDS-NHL can be explained by an inability of the host to contain EBV-driven B-cell expansions that may precede malignancy, and the defective response of tumor-infiltrating T cells which are known to play an important role in the containment of NHL.106 In addition, generalized immunodeficiency and consequent secondary infections would exacerbate conditions of chronic B-cell stimulation and cytokine deregulation.
Infection with oncogenic viruses.
Viruses that have been causally linked to AIDS-NHL to date include the herpesviruses EBV and, more recently, HHV-8. The oncogenic potential of EBV is demonstrated by the ability of this virus to transform B cells in vitro107 and the capacity of EBV-infected B cells to cause lymphomas in severe combined immunodeficient (SCID) mice.108 However, because EBV infection is not universal amongst the AIDS-NHL, the absolute role of EBV in the development of these neoplasms is unclear. Importantly, EBV infection is highest in primary CNS lymphomas and systemic IBL which arise in the setting of severe immunodeficiency, and lowest in the SNCCL which frequently present while immune competence is relatively preserved. In addition, expression of the transforming antigens EBNA-2 and LMP-1 is restricted to AIDS-NHL associated with advanced immunodeficiency.87 109 This observation strongly suggests that, while the oncogenic potential of EBV is undisputed, the degree to which EBV contributes to AIDS lymphomagenesis depends largely on the degree to which virus replication and gene expression is influenced by the host immune status.
HHV-8, originally designated as Kaposi's sarcoma–associated herpesvirus (KSHV), is the only infectious agent that has been consistently associated with KS lesions in AIDS patients,110,111 and epidemiological studies have supported a causal role for HHV-8 in the pathogenesis of KS.112 HHV-8 infection has also been consistently shown in the PEL subtype of AIDS-NHL which constitute approximately 3% of this group of neoplasms.113 To date HHV-8 sequences have not been identified in the malignant B cells that comprise any of the other subtypes of AIDS-NHL.114 Although HHV-8+ PEL do not occur exclusively in the setting of AIDS, HIV-1 infection significantly increases the risk for developing this type of malignancy.78 The reproducible and selective infection of PEL by HHV-8 provides a compelling argument for a causal role for viral infection in the development of this type of malignancy. Although a tumorogenic role for HHV-8 has yet to be clearly defined, the virus encodes several genes with oncogenic potential as well as a gene encoding a viral homologue of IL-6 (vIL-6).115 116Therefore, it is conceivable that constitutive or deregulated expression of these genes may induce and/or sustain PEL localization and growth.
A Role for Nonmalignant Accessory Cells in AIDS-NHL
While the consequences of a generalized immunodeficiency are no doubt important for neoplastic transformation, the host microenvironment must nurture the expanding cellular clone(s). Although HIV-1 does not influence lymphomagenesis via direct infection of malignant B cells, microenvironments that favor the characteristic features of AIDS-NHL, namely clinically aggressive growth at extranodal sites, may exist uniquely within the HIV-1–infected host. Such microenvironments may in fact be defined by HIV-1 infection of nonmalignant accessory cells. The concept that viral infection of an accessory cell promotes malignant growth of a distinct cell type was recently proposed to explain the growth of plasma cells in multiple myeloma.117 This study established a positive association between multiple myeloma and the infection of BM dendritic cells with HHV-8. Because dendritic cells play an important role in the growth and differentiation of B cells, the investigators proposed that HHV-8–infected dendritic cells contribute to the development of multiple myeloma via expression of viral genes that support the transformation and growth of malignant plasma cells. HIV infection of accessory cells at extranodal locations with the capacity to influence B-cell growth and development may similarly contribute to B-cell transformation in AIDS-NHL and/or homing and growth of malignant clones.
Hematopoietic Stromal Cells Influence B Lymphopoiesis
An essential accessory cell network for normal B-cell lymphopoiesis and homing in vivo, as well as for B-cell growth in vitro, is provided by the BM stroma.118-122 Depending on the conditions under which cells are cultured, BM stromal cultures can be made to be supportive of either lymphopoiesis, as in the Whitlock-Witte or related systems122-125 or myelopoiesis as in the Dexter system.126,127 Clear differences in growth factor production have not been clarified, and while IL-7 production is produced by the majority of cells in stromal cultures that support B-cell growth and differentiation,123 other factors clearly play a role in the support of B cells by marrow stromal elements. Marrow stromal cells support normal and leukemic B-cell progenitor survival and growth128,129 through mechanisms requiring attachment of the cell populations128 via adhesion molecule/ligand interactions such as VCAM-1/VLA-4,130,131and ICAM-1/LFA-1.132 These interactions represent more than mere physical attachment. By attaching to stromal elements, B lymphoma cells induce tyrosine phosphorylation of a number of proteins in stromal cells and, perhaps of more relevance to a juxtacrine mechanism of B-cell growth, B cells induce the release of IL-6.133
HIV-infected MVEC-enriched stroma supports B-lymphoma cell growth.
Stromal MVEC as well as fibroblasts are effective in supporting B-cell proliferation.134 Indeed, McGinnes et al122reported that MVEC-enriched stroma derived from BM spicules was more effective at supporting B-cell growth than fibroblast-rich aspirate-derived stroma. As previously mentioned, our group and others have shown HIV-1 infection of stromal MVEC within the BM of patients with AIDS and ARC.3,19 More recently, we reported that ex vivo HIV-1 infection of MVEC-enriched stroma isolated from the BM of HIV-1 seronegative lymphoma patients induced the outgrowth and survival of autologous B-lymphoma cells, while such stroma was not supportive of autologous lymphoma growth in the absence of HIV-1 infection.135 In addition, culture of MVEC-enriched stroma isolated from the BM of AIDS patients with B-cell NHL promoted the survival and outgrowth of autologous stromal-dependent malignant B cells. These phenomena were observed for NHL of both the large and small noncleaved B-cell types and included both EBV+ and EBV− categories. Consequently, the unifying feature of these stromal cultures was the requirement for HIV-1–infected MVEC. These studies suggest that HIV-1 infection alters the properties of the stromal microenvironment such that it becomes supportive of the outgrowth and survival of B-cell lymphomas. HIV infection of stromal MVEC may thus play a role in the extranodal growth of AIDS-NHL that arise within and/or localize to the BM (Fig 3).
HIV-infected brain MVEC support B-lymphoma cell growth.
HIV-1 is known to infect brain microvascular endothelial cells (brain MVEC) in AIDS patients48,136 and cultured MVEC from normal brain tissue can be productively infected in vitro.49Recent studies from our laboratory have shown that normal brain MVEC cultured in vitro were moderately supportive of the adhesion and growth of B-lymphoma cells added in coculture (Fig 4). Importantly, HIV-1 infection of these brain MVEC dramatically increased the subsequent adhesion and proliferation of cocultured B-lymphoma cells.135 Physical separation of MVEC and B cells, achieved by culturing B-lymphoma cells in transwell filter chambers over HIV-1–infected MVEC monolayers, suggested that enhanced proliferation was dependent on initial MVEC-B cell attachment. Interactions between the adhesion molecule VCAM-1 and the B-cell integrin VLA-4 play a central role in the adhesion of B cells to MVEC and other stromal elements,118,121,122,134,137,138 implicating VCAM-1 as a potential mediator of the enhanced B-lymphoma cell adhesion. Although VCAM-1 expression on brain MVEC is not induced by HIV-1 infection per se,139 expression of the cytokine receptor CD40 by brain MVEC is upregulated after HIV-1 infection of these cells.135 Recent in vitro studies have shown that CD40 is also expressed on dermal and umbilical vein endothelial cells and that CD40 triggering using soluble CD40 ligand (CD40L) increases the constitutive expression of VCAM-1 by these cells.140-142Studies from our laboratory have shown that while CD40 triggering results in a modest induction of VCAM-1 on uninfected brain MVEC, VCAM-1 is significantly induced following CD40 triggering of HIV-1–infected MVEC.135 The ability of CD40 triggering to preferentially induce VCAM-1 on HIV-1–infected MVEC in vitro suggests that a similar mechanism could operate in vivo. While CD40L expression was first identified on activated T cells,143 additional cell types including macrophages, dendritic cells, smooth muscle cells, endothelial cells, and fibroblasts also express CD40L in certain inflammatory states.144,145 In vivo, interaction of HIV-1–infected MVEC with any of these cell types could induce VCAM-1 expression and create a microenvironment conducive to adhesion and growth of malignant B cells. In addition, because CD40L expression by malignant B cells has also been reported,135,146,147CD40L+ B-lymphoma cells could themselves induce the adhesion phenotype. In support of this hypothesis, the adhesion and growth of a CD40L+, VLA-4+ AIDS-SNCCL cell line on HIV-1–infected MVEC is specifically inhibited by blocking functional CD40-CD40L interactions between the cells.135Interactions between CD40 and CD40L may also play a role in the development of other types of malignancies. For example, van den Oord et al148 analyzed malignant melanoma (MM) lesions for expression of CD40 and CD40L and found that 45% of CD40+MM coexpressed CD40L. Interestingly, patients whose tumors expressed both CD40 and CD40L had a poorer prognosis than those expressing CD40 only. The investigators in this study observed that coexpression of CD40 and CD40L usually occurred in the same area of the tumor, and proposed the existence of CD40-CD40L-mediated autocrine growth loops in the vertical growth phase of MM.
Clusters of Burkitt's lymphoma cells adhere to and proliferate on HIV-1–infected MVEC from autologous human BM (A) and heterologous human brain (C) but not on uninfected brain MVEC (B). (A) Lymphoma cells and stromal cells in (A) were derived from the BM of an HIV-1 seropositive lymphoma patient. The lymphoma cells were EBV−, contained a t(8;14) chromosomal translocation, and proliferated spontaneously in stromal cell cultures that supported MVEC survival. (B) Lymphoma cells from (A) were transferred to pure brain MVEC monolayers. A small percentage of lymphoma cells adhered to brain MVEC, but these brain MVEC did not support sustained adhesion and growth of lymphoma cells. (C) Lymphoma cells from (A) were transferred to pure brain MVEC monolayers infected with HIV-1. Infected MVEC supported the long-term adherence and growth of lymphoma cells.
Clusters of Burkitt's lymphoma cells adhere to and proliferate on HIV-1–infected MVEC from autologous human BM (A) and heterologous human brain (C) but not on uninfected brain MVEC (B). (A) Lymphoma cells and stromal cells in (A) were derived from the BM of an HIV-1 seropositive lymphoma patient. The lymphoma cells were EBV−, contained a t(8;14) chromosomal translocation, and proliferated spontaneously in stromal cell cultures that supported MVEC survival. (B) Lymphoma cells from (A) were transferred to pure brain MVEC monolayers. A small percentage of lymphoma cells adhered to brain MVEC, but these brain MVEC did not support sustained adhesion and growth of lymphoma cells. (C) Lymphoma cells from (A) were transferred to pure brain MVEC monolayers infected with HIV-1. Infected MVEC supported the long-term adherence and growth of lymphoma cells.
Because different lymphoma subtypes express unique classes of regulatory and adhesion molecules,138 HIV-infected brain MVEC likely express additional molecules that enhance MVEC-B lymphoma cell adhesion either directly or via multi-step pathways. For example, the ligand LFA-1 is strongly expressed by certain subsets of NHL138 while its specific counter-receptor, the adhesion molecule ICAM-1, is directly induced on brain MVEC by HIV-1 infection.139 Although our studies to date have not identified any constitutive alteration in cytokine production from HIV-infected brain MVEC, both the physical proximity of B-lymphoma cells to MVEC, as well as the potential for MVEC-B cell adhesion to activate cytokine receptor and cytokine response genes in both cell types, suggest that B cells adherent to HIV-infected MVEC are likely to be optimally responsive to cytokines elaborated from these MVEC, regardless of their absolute amounts.
HIV-1 infection of endothelial cells from a variety of organ systems including the BM,3,19 brain,48,49kidney,51 and liver50 has been reported. The significant correlation between these tissues and extranodal sites targeted for homing and growth of AIDS-NHL strongly suggests that HIV-1–infected endothelial cells function as nonmalignant accessory cells with the capacity to promote the attachment and growth of malignant B cells. The spectrum of phenotypic changes in HIV-1–infected MVEC responsible for enhanced B-lymphoma growth have yet to be fully elucidated, but they will likely be complex. In fact, as would be predicted from the work on uninfected stromal cell/B-cell interactions of LeBien and others,133,134 149 it is very likely that a variety of synergistic mechanisms account for the outgrowths we have seen on HIV-1 infected stromal and brain MVEC (Fig3). Consequently, the inductive changes in adhesion molecule interactions likely represent only the first of many steps in a complex concatenation of induced responses in both accessory and malignant cell elements. However, it is clear that foci of infected endothelial cells, dendritic cells, and macrophages represent a local microenvironment that may, in the setting of AIDS, accommodate a malignant phenotype.
In conclusion, it is generally accepted that in the context of the HIV-1–infected host, the combined consequences of a generalized immunodeficiency, chronic B-cell stimulation, impaired tumor surveillance, and coinfection with oncogenic viruses facilitate the transformation and survival of malignant B cells that are themselves uninfected by HIV-1, and that this mechanism accounts for the high incidence of AIDS-NHL seen within the HIV-1–infected population. In addition, we argue that HIV-1 infection of nonmalignant accessory cells, particularly MVEC, plays an essential role in the homing, growth, and survival of these neoplasms at extranodal sites. While there is still much work to be done to fully elucidate the role of HIV-1 in the development of AIDS-NHL, the clear capacity of HIV-1 to induce changes in the support function of accessory cells for lymphoma cell growth is an essential issue to pursue. Full clarification of the function of the microenvironment in lymphomagenesis will permit the rational design of preventive strategies.
SUMMARY
Patients with HIV-1 infection commonly develop pancytopenia, but the causes are heterogeneous and commonly iatrogenic or multifactorial. The most consistent hematopoietic defects that occur in seropositive patients as a result of HIV-1 infection per se include, first, regenerative BM failure in which on-demand hematopoiesis is suppressed, and second, a high frequency of unusually aggressive, extranodal NHLs. It is clear that neither BM failure nor lymphomagenesis results from infection of stem cells or lymphoid or myeloid progenitor cells in vivo. Indeed, it is clear that infection of such cells is not only rare29,150 151 but that the growth and differentiation of the few cells that may be infected is in no way impaired. However, infection of auxiliary cells, particularly macrophages and microvascular endothelial cells, induces a substantial alteration in the supportive function of the hematopoietic stromal tissues such that myeloid hematopoiesis is suppressed and at the same time, primitive lymphoid cell growth is augmented. We argue that a full molecular clarification of these phenomena should lead to opportunities for the rational design of preventive strategies for both regenerative failure and outgrowths of lymphoid neoplasms.
Supported by grants from the National Institutes of Health (DK49887) and the Department of Veterans Affairs.
Address reprint requests to Grover C. Bagby, Jr, MD, Division of Hematology and Medical Oncology, Oregon Health Sciences University, L580, 3181 SW Sam Jackson Park Road, Portland, OR 97201-3098.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal