Abstract
B-chronic lymphocytic leukemia (B-CLL) is characterized by cellular and humoral immune defects resulting in increased rates of infection and disturbed immune surveillance against cancer cells as well as by the expansion of slowly proliferating tumor cells. We found increased Fas receptor (FasR) expression in peripheral blood CD4+and CD8+ cells of B-CLL patients compared with the equivalent cells of healthy donors. Although increased Fas receptor expression was significant in both T-lymphocytic subsets, only CD4+ cells from B-CLL patients underwent apoptosis after treatment with the agonistic Fas antibody CH11. In CD4+cells of B-CLL patients, the Fas-sensitivity also correlated with a CD4+/CD8+ ratio below the lower threshold of healthy individuals (<1.0). By contrast, FasR expression in the CD19+ fraction of B-CLL patients was downregulated compared with normal controls, and this was associated with an insensitivity to CH11-induced apoptosis. The B-CLL cell line EHEB as well as CD19+ cells from B-CLL patients constitutively expressed Fas ligand (FasL). The FasL was functionally active, as the B-CLL cell line as well as T-cell–depleted CD19+ B-CLL fractions were able to kill target T-acute lymphatic leukemia (T-ALL) cells in vitro. This effect was inhibited by the antagonistic FasR-antibody ZB4, the neutralizing anti-FasL monoclonal antibody (MoAb) NOK-2 or by transfection of the caspase inhibitor crmA. These data point to the fact that expression of FasL on CD19+B-CLL cells, together with enhanced susceptibility of CD4+ T cells toward FasL-bearing effector cells, are causally linked to the relative reduction of CD4+ cells occurring during B-CLL progression. These findings could explain the inversion of the ratio of CD4+/CD8+ cell numbers, which may be causally linked to the immune deficiency observed in these patients and to the expansion of the neoplastic clone in B-CLL.
CHRONIC B-LYMPHOCYTIC leukemia (B-CLL) represents a lymphoproliferative disease with clonal expansion of immunologically immature CD5+ B cells. Clinically, the disease is characterized by a distinct immune deficiency and by the direct consequences of B-cell accumulation, ie, splenomegaly, lymphadenopathy, bone marrow infiltration, and progressive lymphocytosis.1 It has been speculated that an imbalance of immunoregulatory T cells, mainly T-helper cells (CD4+) and T suppressors (CD8+), is the main cause of the immune deficiency.2,3 In fact, an inversion of the ratio of CD4+/CD8+ cell numbers of <1.0 and a deficiency in the function of the T-helper cells were reported by several investigators.2-5 The pathologic T-cell distribution together with the loss of T-cell activities were correlated with the stage of disease and thought to be primarily responsible for the impaired immunoregulation and the increased frequencies of infectious episodes occurring in these patients.2,6,7 However, the precise mechanisms responsible for the imbalance of the T-cell subsets remain obscure. In partial analogy to B-CLL, the primary features of human immunodeficiency virus (HIV) disease are the prominent immune deficiency and a tight correlation between an increasing frequency of infections on the one hand and the inversion of the CD4+/CD8+ratio8 and a progressive depletion of T cells8,9 on the other. Recent data from HIV-infected patients suggest the vital importance of the FAS receptor (FasR)/FAS ligand (FasL) system in the pathogenesis of the above-mentioned clinical and immunologic stigmata associated with this disease.8-10
The FasR (CD95, APO-1) belongs to the tumor necrosis factor (TNF) receptor supergene family, which, after binding of the specific ligand, is capable of mediating cell death.11,12 In resting, naive T cells, both the FasR and the FasL are expressed at low levels but are upregulated upon activation by antigens by T-cell receptor (TCR) signaling.13-15 The expression of FasR renders T cells susceptible to activation-induced cell death (AICD) which, after simultaneous upregulation of FasL, can proceed as “fratricide” and as “suicide” of the FasR+/FasL+T cells.14,15 This cell death program leads to the removal of high-risk cells, either remnants of an immune response or self-reactive T cells in the periphery.16-18 In HIV disease, the viral infection has been shown to lead to abnormal chronic upregulation of FasR and FasL on both CD4+ and CD8+ cells.8-10 In one of these studies, different sensitivities of the purified CD4+ and CD8+ cells to FasR-triggering MoAb CH11 were observed leading in vitro to an inverted CD4+/CD8+ratio.8
Besides expression on T cells, functional FasL expression has recently been demonstrated on normal murine B cells stimulated with phorbol 12-myristate 13-acetate (PMA)/ionomycin.19 These FasL-bearing B lymphocytes were shown to kill FasR+ target A20 B lymphoma cells in vitro.19 In the human system, we recently reported the presence of functionally active FasL on terminally differentiated neoplastic plasma cells.20 Our data on neoplastic plasma cells are part of accumulating evidence that FasL-expressing tumor cells are able to kill target cells in vitro.18 This mechanism of active killing has been implicated in immune escape of tumor cells and by this mechanism T-lymphocyte subsets involved in antitumor responses may be inactivated.
These observations raise the question if the FasR/FasL system might be involved in the pathophysiology of B-CLL, which is associated with B-cell accumulation, immunodeficiency and inversion of the ratio of CD4+/CD8+ cell numbers found in a high percentage of B-CLL patients. Using a B-CLL cell line, native malignant B cells as well as CD4+ and CD8+ T cells derived from the peripheral blood of 33 patients, we studied the interaction of B and T cells with the FasR/FasL system and its contribution to the development of the disturbed homeostasis of CD4+/CD8+ T cells in B-CLL.
MATERIALS AND METHODS
Patients and Controls
Tumor cells were collected during routine examinations from the peripheral blood of 33 patients suffering from B-CLL after obtaining informed patient consent. B-CLL was defined by clinical criteria,1 as well as by cellular morphology and the coexpression of CD19 and CD5 in lymphocytes simultaneously displaying restriction of light-chain rearrangement. Table 1 summarizes the clinical data for the patients. Results from patients were compared with those of 20 age- and sex-matched healthy individuals (mean agehealthy controls: 67 years ± 9.8 [range, 52 to 93], mean ageB-CLL patients: 68 years ± 9.4 [range, 48 to 86]) studied in parallel.
Clinical Features of Patients With B-CLL
| Patient No. . | Sex . | Age . | Rai Stage . | Lymphocytes (×109/L) . | CD4+/CD8+ Ratio . | Pretreatment Status* . |
|---|---|---|---|---|---|---|
| 1 | M | 74 | 2 | 29.4 | 1.20 | — |
| 2 | F | 78 | 2 | 54.5 | 1.00 | — |
| 3 | M | 56 | 0 | 0.5 | 2.00 | — |
| 4 | M | 76 | 0 | 117.0 | 0.67 | — |
| 5 | M | 66 | 2 | 101.0 | 0.50 | — |
| 6 | M | 73 | 1 | 18.6 | 1.35 | — |
| 7 | M | 58 | 2 | 15.0 | 0.20 | — |
| 8 | M | 60 | 2 | 75.0 | 0.25 | -151 (3 yr) |
| 9 | M | 69 | 1 | 13.0 | 0.26 | -151 (2 yr) |
| 10 | F | 72 | 2 | 64.8 | 0.50 | — |
| 11 | F | 49 | 0 | 77.0 | 1.26 | — |
| 12 | M | 52 | 0 | 23.0 | 1.30 | — |
| 13 | F | 58 | 1 | 105.0 | 2.40 | — |
| 14 | F | 74 | 2 | 40.0 | 0.36 | — |
| 15 | F | 64 | 1 | 9.6 | 3.40 | — |
| 16 | F | 75 | 2 | 20.0 | 0.70 | — |
| 17 | M | 55 | 1 | 9.9 | 0.93 | — |
| 18 | F | 70 | 2 | 77.3 | 0.40 | -151 (16 yr) |
| 19 | M | 70 | 2 | 80.3 | 0.40 | -151 (4 yr) |
| 20 | M | 82 | 2 | 108.0 | 0.30 | -151 (2 yr) |
| 21 | M | 56 | 2 | 14.0 | 0.29 | — |
| 22 | M | 85 | 2 | 78.3 | 0.20 | — |
| 23 | F | 78 | 4 | 162.0 | 0.20 | — |
| 24 | F | 68 | 2 | 119.0 | 0.20 | — |
| 25 | F | 61 | 2 | 28.3 | 0.30 | — |
| 26 | M | 86 | 1 | 28.0 | 1.40 | — |
| 27 | M | 71 | 3 | 75.0 | 0.33 | — |
| 28 | M | 72 | 1 | 81.0 | 1.13 | — |
| 29 | M | 72 | 1 | 91.0 | 0.55 | — |
| 30 | M | 58 | 1 | 72.0 | 2.00 | — |
| 31 | F | 73 | 2 | 81.0 | 1.90 | — |
| 32 | F | 79 | 2 | 100.0 | 1.10 | — |
| 33 | F | 62 | 1 | 93.0 | 0.40 | -151 (5 yr) |
| Patient No. . | Sex . | Age . | Rai Stage . | Lymphocytes (×109/L) . | CD4+/CD8+ Ratio . | Pretreatment Status* . |
|---|---|---|---|---|---|---|
| 1 | M | 74 | 2 | 29.4 | 1.20 | — |
| 2 | F | 78 | 2 | 54.5 | 1.00 | — |
| 3 | M | 56 | 0 | 0.5 | 2.00 | — |
| 4 | M | 76 | 0 | 117.0 | 0.67 | — |
| 5 | M | 66 | 2 | 101.0 | 0.50 | — |
| 6 | M | 73 | 1 | 18.6 | 1.35 | — |
| 7 | M | 58 | 2 | 15.0 | 0.20 | — |
| 8 | M | 60 | 2 | 75.0 | 0.25 | -151 (3 yr) |
| 9 | M | 69 | 1 | 13.0 | 0.26 | -151 (2 yr) |
| 10 | F | 72 | 2 | 64.8 | 0.50 | — |
| 11 | F | 49 | 0 | 77.0 | 1.26 | — |
| 12 | M | 52 | 0 | 23.0 | 1.30 | — |
| 13 | F | 58 | 1 | 105.0 | 2.40 | — |
| 14 | F | 74 | 2 | 40.0 | 0.36 | — |
| 15 | F | 64 | 1 | 9.6 | 3.40 | — |
| 16 | F | 75 | 2 | 20.0 | 0.70 | — |
| 17 | M | 55 | 1 | 9.9 | 0.93 | — |
| 18 | F | 70 | 2 | 77.3 | 0.40 | -151 (16 yr) |
| 19 | M | 70 | 2 | 80.3 | 0.40 | -151 (4 yr) |
| 20 | M | 82 | 2 | 108.0 | 0.30 | -151 (2 yr) |
| 21 | M | 56 | 2 | 14.0 | 0.29 | — |
| 22 | M | 85 | 2 | 78.3 | 0.20 | — |
| 23 | F | 78 | 4 | 162.0 | 0.20 | — |
| 24 | F | 68 | 2 | 119.0 | 0.20 | — |
| 25 | F | 61 | 2 | 28.3 | 0.30 | — |
| 26 | M | 86 | 1 | 28.0 | 1.40 | — |
| 27 | M | 71 | 3 | 75.0 | 0.33 | — |
| 28 | M | 72 | 1 | 81.0 | 1.13 | — |
| 29 | M | 72 | 1 | 91.0 | 0.55 | — |
| 30 | M | 58 | 1 | 72.0 | 2.00 | — |
| 31 | F | 73 | 2 | 81.0 | 1.90 | — |
| 32 | F | 79 | 2 | 100.0 | 1.10 | — |
| 33 | F | 62 | 1 | 93.0 | 0.40 | -151 (5 yr) |
*—Chemonaive patients who never had received chemotherapy.
Patients with prior chemotherapy; the time span between collection of cells and previous treatment is given in parentheses.
Preparation of Peripheral Blood Lymphocytes
Blood samples containing 1 mL Kresol-free heparin (5,000 IU/mL; Immuno, Vienna, Austria) were diluted 1:2 in M-199 buffer [50 mL M-199 (10×; Seromed, Berlin, Germany), 430 mL Aqua ad injection, 750 μL heparin (10,000 IU/mL), 100 μg/mL gentamicin, 2.5 μg/mL amphotericin B, 12.5 mL HEPES 1 mol/L, 13 mL NaHCO3]. Mononuclear cells were isolated using Ficoll density-gradient centrifugation (Lymphoprep, Nycomed, Norway). The cells were picked up and washed twice with M-199 buffer, analyzed for cell number, resuspended in a RPMI 1640 culture medium (Seromed) supplemented with 10% (vol/vol) heat-inactivated FCS (Biological Inc, Beth Haemek, Israel), 2 mmol/LL-glutamine (Seromed), and 100 μg/mL gentamicin (GIBCO, Grand Island, NY), and incubated at 37°C in a humidified atmosphere containing 5% CO2. If not used immediately, the cells were resuspended in a solution of FCS and 5% DMSO, frozen, and stored in liquid nitrogen. We found no significant difference either in FasR (PCD4 > .68, PCD8 > .73,PCD19 > .51)/FasL (PCD4 > .42, PCD8 > .25, PCD19 > .35) expression or in Fas-sensitivity (PCD4 > .26, PCD8 > .47, PCD19 > .38) when comparing fresh cells to frozen cells from one and the same patient.
Cell Lines and Culture Conditions
The B-CLL cell line EHEB and the myeloma cell line U266 were obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). The EHEB cell line was established from a patient with B-CLL and immortalized in vitro by means of Epstein-Barr virus (EBV) transformation. The cell line is characterized by an immunophenotype typical of B-CLL disease (CD5+, CD19+, light-chain restriction).21 The U266 cell line was used as a FasR– target cell line.20 A subclone of the CCRF-CEM T-ALL cell line,22 the human T-ALL cell line CEM-C7H2, with further subclones lacking or expressing the cowpoxvirus protein crmA23 was kindly provided by R. Kofler (Department of Experimental Pathology, University of Innsbruck, Innsbruck, Austria). This cell line was used as a FasR+/FasL+control and as a target cell line. All cell lines were cultured as described for the native cell samples (see above).
Flow Cytometric Analysis
FasL staining.
Cells (0.5 × 106) were washed twice in phosphate-buffered saline (PBS), resuspended, and fixed in 1 mL paraformaldehyde (4%) for 15 minutes at 4°C. After an additional wash with PBS, the pellet was resuspended in chilled methanol (70%) and incubated for 5 minutes on ice. Cells were washed twice in PBS, incubated with 1 μg anti-FasL IgG1 MoAb (Transduction Laboratory, Lexington, KY) for 30 minutes at 4°C. Again, cells were washed twice with PBS, and incubated with 3 μL of fluorescein isothiocyanate (FITC)-labeled rabbit anti-mouse (DAKO, Copenhagen, Denmark) for 30 minutes at 4°C. The pellets were resuspended in 200 μL PBS and analyzed immediately. Negative controls were performed simultaneously using a mouse anti-human IgG1 MoAb (DAKO) instead of the anti-FasL MoAb. The FACS data were reported as the mean fluorescence intensity (MFI) ratios. This represents the MFI determined using the anti-FasL MoAb divided by the MFI obtained with the isotype negative control MoAbs. An MFI of >1.5 was considered positive.
CD95 staining.
Cells (0.5 × 106) were incubated with 10 μL FITC-conjugated anti-FasR IgG1 MoAb UB2 (Immunotech, Marseille, France) for 30 minutes at room temperature. Cells were washed twice with PBS, resuspended in 200 μL PBS, and analyzed immediately. Negative controls were performed simultaneously using an FITC-conjugated mouse anti-human IgG1 MoAb (DAKO) instead of the anti-FasR MoAb. The percentage of positive cells was calculated directly from the gated contour blot.
For single-color analysis, the samples were washed twice in PBS and immediately analyzed on a FACScan analyser (Becton Dickinson, Mountain View, CA). Two-color or triple-color flow cytofluorometry were performed by costaining cells using the phenotype MoAbs (CD4, CD8, CD19, or isotype controls, respectively; Becton Dickinson) directly labeled with FITC, RPE, or RPE-Cy5. In all measurements of cell surface antigen expression, gates of the analyzer were set to exclude dead or necrotic cells detected by the forward/side-scatter light analysis. A minimum of 10,000 events were counted for each analysis.
Assay for FasR/FasL-Induced Cell Killing
Target cell death of CEM-C7H2 vector control T-ALL cells resulting from their cocultivation with “effector” B-CLL cells was quantified by measuring target cell DNA fragmentation and loss using the JAM-test.20 24 For this purpose, T-ALL cells were incubated with 10 μCi/mL 3[H]-thymidine (Amersham, Buckinghamshire, UK) for 16 hours, washed three times with PBS, and resuspended in regular culture medium. A total of 100 μL of the T-cell suspension (2 × 104/mL) was cocultivated in 96-well plates with or without 100 μL of the relevant B-CLL cell suspension (2 × 105/mL). Where CD19+B-CLL cells were used as effectors, PBMC of B-CLL patients were depleted of T cells by magnetic cell separation (Miltenyi Biotec, Sunnyvale, CA) according to the manufacturer's protocol. Briefly, PBMC were incubated with a cocktail of mouse anti-CD4 and anti-CD8 antibodies (DAKO), followed by incubation with anti-mouse antibodies bound to magnetic microbeads. T cells were magnetically trapped, resulting in a highly enriched fraction of CD19+ B cells (purity > 98%).
For blocking the Fas pathway in the cocultivation experiments, the labeled T cells were incubated with 0.25 μg/mL of the antagonistic anti-FasR MoAb ZB4 (Immunotech) or 0.75 μg/mL of the neutralizing anti-FasL MoAb NOK-2 (Pharmingen, San Diego, CA). Cocultivation of cells was performed for 72 hours at 37°C. Cells were procured automatically, transferred onto filter papers and washed six times. Incorporated radioactivity of undegraded chromosomal DNA was measured with a β-scintillation counter. The reduction in incorporated radioactivity was used to calculate the percentage of specific target cell killing:
Assay of Apoptosis
The binding of annexinV-FITC was used to follow phosphatidylserine exposition on early apoptotic cells.25 The staining was performed according to manufacturer's instructions. Briefly, 2.5 × 105 cells/mL were incubated with saturating concentrations of annexinV-FITC for 15 to 30 minutes at room temperature and immediately analyzed by flow cytometry.
Data Presentation and Statistical Analysis
Data of Figs 1-3 and 5A and B are depicted in box-blot and whiskers models. The box shows the median and the interquartile range of the data, whereas 25% of the data are within the range of a whisker in one direction. For statistical analysis of data, Pvalues were assessed using a Fisher's PLSD test in the analysis of variance (ANOVA) program of StatView 5.1 (Abacus Concepts Inc, Berkeley, CA). Figure 4 comprises a flow cytometric profile of FasL expression on CD19+ B-CLL cells.
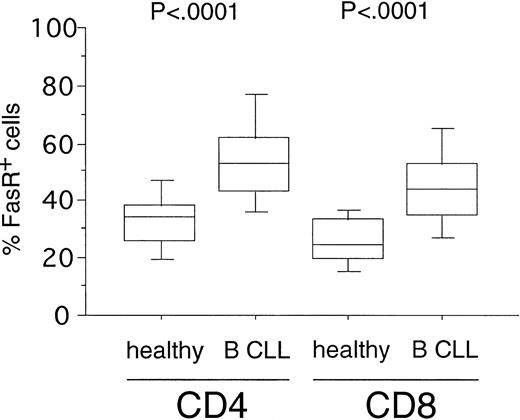
Expression of FasR (CD95) in CD4+ and CD8+ cells from healthy volunteers versus B-CLL patients. PBMC from healthy donors (n = 20) and B-CLL patients (n = 33) were stained with FITC-conjugated anti-FasR MoAb and PE-conjugated anti-CD4 or CD8 MoAb and characterized for specific staining by FACS analysis. The percentage of CD4+ or CD8+ cells reacting with anti-FasR MoAb is shown in a box-blot and whiskers model (see Materials and Methods). Statistical analysis showed the following relationships for differences in FasR expression: CD4+cells: healthy versus B-CLL (P < .0001), CD8+cells: healthy versus B-CLL (P < .0001).
Expression of FasR (CD95) in CD4+ and CD8+ cells from healthy volunteers versus B-CLL patients. PBMC from healthy donors (n = 20) and B-CLL patients (n = 33) were stained with FITC-conjugated anti-FasR MoAb and PE-conjugated anti-CD4 or CD8 MoAb and characterized for specific staining by FACS analysis. The percentage of CD4+ or CD8+ cells reacting with anti-FasR MoAb is shown in a box-blot and whiskers model (see Materials and Methods). Statistical analysis showed the following relationships for differences in FasR expression: CD4+cells: healthy versus B-CLL (P < .0001), CD8+cells: healthy versus B-CLL (P < .0001).
CEM-C7H2 T-ALL cells are killed by B-CLL cells through the Fas/FasL pathway. (A) T-ALL cells carrying the relevant vector control (VC) were incubated with 10 μCi/mL [3H]-thymidine for 16 hours and cocultivated with the indicated B-CLL cells EHEB (□). For blocking experiments, crmA-expressing CEM-C7H2 cells (CRM) were used as target cells. Alternatively, the labeled T-cells (VC) were coincubated with 0.25 μg/mL of the FasR blocking ZB4 MoAb. In a control experiment the FasR– neoplastic B-cell line U266 was used as a target (▨). Cocultivation of cells was performed for 72 hours at 37°C. The reduction of DNA-incorporated radioactivity was used to calculate the percentages of B-CLL–mediated target cell death (n = 12) and is presented in a box-blot and whiskers model. Statistical analysis of the blocking experiments showed the following: specific killing by EHEB: 30% mean inhibition by ZB4 (P < .0001), 66% mean inhibition by crmA expression (P < .0001). (B) CEM-C7H2 T-ALL cells are eliminated by CD19+ cells of B-CLL patients through the Fas/FasL pathway. PBMC of 5 B-CLL patients were depleted of T cells by magnetic cell separation. The resulting CD19+ B-cell fractions (purity > 98%) were cocultivated with [3H]-thymidine–prelabeled CEM-C7H2 target cells for 72 hours at 37°C in a effector/target ratio of 10:1 (four replicates). The reduction of DNA-incorporated radioactivity in the target cells was used to calculate specific target cell killing by CD19+B-CLL cells, which was blocked by preincubation with the antagonistic anti-FasR MoAb ZB4 (0.25 mg/mL, ▧) or the inhibitory anti-FasL MoAb NOK-2 (0.75 mg/mL, ▨). Results of the statistical analysis: 44% mean inhibition by ZB4 MoAb (.0001 < P < .002), 74% mean inhibition by NOK-2 MoAb (P < .0001).
CEM-C7H2 T-ALL cells are killed by B-CLL cells through the Fas/FasL pathway. (A) T-ALL cells carrying the relevant vector control (VC) were incubated with 10 μCi/mL [3H]-thymidine for 16 hours and cocultivated with the indicated B-CLL cells EHEB (□). For blocking experiments, crmA-expressing CEM-C7H2 cells (CRM) were used as target cells. Alternatively, the labeled T-cells (VC) were coincubated with 0.25 μg/mL of the FasR blocking ZB4 MoAb. In a control experiment the FasR– neoplastic B-cell line U266 was used as a target (▨). Cocultivation of cells was performed for 72 hours at 37°C. The reduction of DNA-incorporated radioactivity was used to calculate the percentages of B-CLL–mediated target cell death (n = 12) and is presented in a box-blot and whiskers model. Statistical analysis of the blocking experiments showed the following: specific killing by EHEB: 30% mean inhibition by ZB4 (P < .0001), 66% mean inhibition by crmA expression (P < .0001). (B) CEM-C7H2 T-ALL cells are eliminated by CD19+ cells of B-CLL patients through the Fas/FasL pathway. PBMC of 5 B-CLL patients were depleted of T cells by magnetic cell separation. The resulting CD19+ B-cell fractions (purity > 98%) were cocultivated with [3H]-thymidine–prelabeled CEM-C7H2 target cells for 72 hours at 37°C in a effector/target ratio of 10:1 (four replicates). The reduction of DNA-incorporated radioactivity in the target cells was used to calculate specific target cell killing by CD19+B-CLL cells, which was blocked by preincubation with the antagonistic anti-FasR MoAb ZB4 (0.25 mg/mL, ▧) or the inhibitory anti-FasL MoAb NOK-2 (0.75 mg/mL, ▨). Results of the statistical analysis: 44% mean inhibition by ZB4 MoAb (.0001 < P < .002), 74% mean inhibition by NOK-2 MoAb (P < .0001).
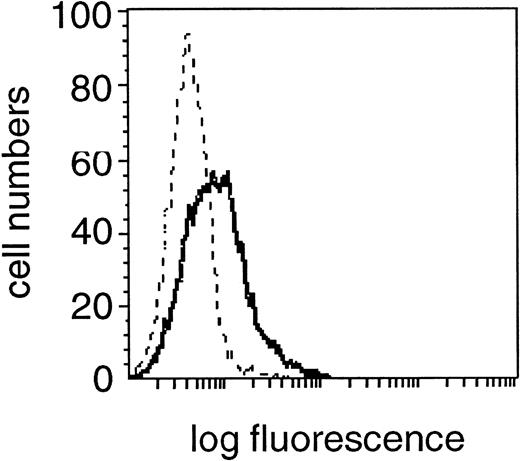
Expression of FasL (CD95L) in the CD19+cell fraction of one representative B-CLL patient. Fixed and permeabilzed PBMC were double-stained with the specific anti-FasL MoAb and CD19 MoAb and characterized for specific staining by means of flow cytometry. A shift in fluorescence intensity was observed in samples stained with the specific anti-FasL MoAb (solid line) compared with samples stained with the relevant isotype control MoAb (dashed line). A flow cytometric profile from one experiment from a single donor, representative of 30 experiments from different donors is shown.
Expression of FasL (CD95L) in the CD19+cell fraction of one representative B-CLL patient. Fixed and permeabilzed PBMC were double-stained with the specific anti-FasL MoAb and CD19 MoAb and characterized for specific staining by means of flow cytometry. A shift in fluorescence intensity was observed in samples stained with the specific anti-FasL MoAb (solid line) compared with samples stained with the relevant isotype control MoAb (dashed line). A flow cytometric profile from one experiment from a single donor, representative of 30 experiments from different donors is shown.
RESULTS
Expression of FasR Antigen on CD4+ and CD8+Cells From Patients With B-CLL and Its Correlation With Fas-Induced Apoptosis
To investigate the susceptibility of T-cell subsets toward Fas-induced apoptosis in B-CLL, we first determined the expression levels of the death receptor molecule FasR. FasR expression could be detected in a fraction of CD4+ cells (mean, 35%; range, 13% to 56%) and CD8+ cells (mean, 27%; range, 13% to 41%) from 20 healthy donors (Fig 1). In B-CLL patients (n = 33), a significantly higher number of FasR+ cells was found in the CD4+ (mean, 53%; range, 23% to 89%,P < .0001) and in the CD8+ subpopulations (mean, 45%; range, 22% to 79%, P < .0001) as compared with the normal equivalent cells (Fig 1).
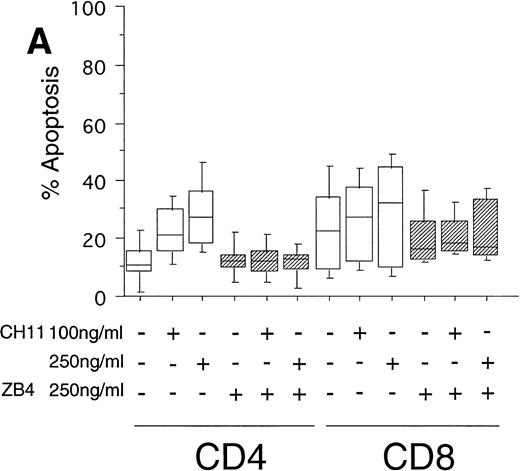
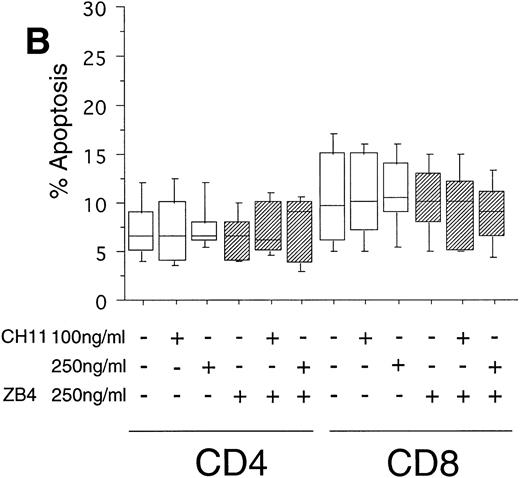
To test the in vivo significance of upregulated FasR expression in T cells of B-CLL patients, we studied the sensitivities of both CD4+ and CD8+ lymphocytic subsets to Fas-induced apoptosis and compared them with those of healthy donors. The peripheral blood mononuclear cells (PBMC) of patients were cultured in the presence of the agonistic anti-FasR antibody CH11 mimicking the function of the physiologic FasL.26 After 24 hours of incubation, the percentages of apoptotic cells in the immunologic subpopulations were determined by double immunofluorescence staining of the CD4+ and CD8+ surface antigens and staining of phosphatidylserine in the outer leaflet of the plasma membrane by means of binding of annexinV-FITC. Externalization of this molecule is reported to be an early, sensitive marker of apoptosis.25The percentage of apoptotic cells in the untreated CD4+fraction of B-CLL patients was already increased as compared with healthy donors (mean: 12% v 7%, P < .05, Fig 2A and B). The addition of the FasR agonist CH11 MoAb led to enhanced apoptosis in CD4+ cells (P < .0002, Fig 2A). This Fas-induced apoptosis was dose dependent and could be blocked by pre-incubation with the antagonistic MoAb ZB4 (P < .002, Fig 2A). Despite high expression levels of FasR in CD8+ cells of B-CLL patients and an elevated percentage of apoptotic cells in untreated CD8+ cells over those of healthy donors (mean, 23% v 10%, P < .02), CH11-incubation of these cells did not result in a significant, dose-dependent enhancement of apoptosis in comparison with untreated CD8+ cells (P > .2, Fig 2A). To answer the question if the insensitivity of the CD8+ fraction of B-CLL patients is the consequence of a delay in apoptosis induction, we cultivated these cells with 0, 100, and 250 ng/mL CH11 MoAb respectively, and measured apoptosis after 3, 5, and 7 days. No significant increase in apoptosis could be found between 24 hours and 7 days of Fas-triggering (P > .6, data not shown). In the unstimulated CD8+ cell fraction from B-CLL patients, the percentage of apoptotic cells was found to be higher as compared with the CD4+ B-CLL cell fraction (P = .002, Fig 2A). Nevertheless, the addition of the inhibitory MoAb ZB4 had no effect on the percentage of spontaneous apoptosis in this cell subpopulation, thus partly arguing against the involvement of the FasR pathway in spontaneous apoptosis of CD8+ B-CLL cells (P > .2, Fig 2A). When we cultured PBMC of normal donors in the presence of the agonistic CH11 MoAb, a significant increase of apoptotic cells was not seen either in the CD4+ (P > .7) or in the CD8+ fraction (P > .7) and preincubation with the ZB4 MoAb showed no effect (Fig 2B). As was the case with the CD8+ fraction of B-CLL patients, incubation with CH11 MoAb for a period longer than 24 hours (ie, 3, 5, and 7 days) did not show any significant increase of apoptosis in these T-cell fractions of healthy controls (P > .8, data not shown).
(A) Induction of apoptosis in CD4+ and CD8+ cells from B-CLL patients (n = 30). PBMC from 30 B-CLL patients were cocultured with the agonistic anti-FasR MoAb CH11 (□) and/or the antagonistic anti-FasR MoAb ZB4 (▨) for 24 hours (up to 7 days, data not shown) in the indicated concentrations. The percentage of apoptosis in the relevant T-cell subpopulations was measured by two-color flow cytometry after staining with PE-conjugated anti-CD4 or anti-CD8 MoAb and AnnexinV-FITC and is shown in a box-blot and whiskers model. Statistical analysis showed the following relationships: CD4+ cells: untreated versus 100 ng CH11-treated (P = .0002), 100 ng CH11- versus 250 ng CH11-treated (P = .02), 250 ng CH11-treated versus 250 ng CH11+ZB4-treated (P < .002); CD8+ cells: untreated versus 250 ng CH11-treated (P > .2), 250 ng CH11-treated versus 250 ng CH11+ZB4-treated (P > .2). (B) Induction of apoptosis in CD4+ and CD8+cells from healthy donors (n = 20). PBMC from healthy volunteers were untreated or cocultured with the agonistic anti-FasR MoAb CH11 (□) and/or the antagonistic anti-FasR MoAb ZB4 (▨) for 24 hours (up to 7 days, data not shown). The percentage of apoptotic cells in CD4+ and CD8+ cell fractions were assessed by two-color flow cytometry as described for (A). Statistical analysis showed the following: CD4+ cells: untreated versus CH11 treated (P > .7), CH11-treated versus CH11+ZB4-treated (P > .8); CD8+ cells: untreated versus CH11 treated (P > .7), CH11-treated versus CH11+ZB4-treated (P > .3).
(A) Induction of apoptosis in CD4+ and CD8+ cells from B-CLL patients (n = 30). PBMC from 30 B-CLL patients were cocultured with the agonistic anti-FasR MoAb CH11 (□) and/or the antagonistic anti-FasR MoAb ZB4 (▨) for 24 hours (up to 7 days, data not shown) in the indicated concentrations. The percentage of apoptosis in the relevant T-cell subpopulations was measured by two-color flow cytometry after staining with PE-conjugated anti-CD4 or anti-CD8 MoAb and AnnexinV-FITC and is shown in a box-blot and whiskers model. Statistical analysis showed the following relationships: CD4+ cells: untreated versus 100 ng CH11-treated (P = .0002), 100 ng CH11- versus 250 ng CH11-treated (P = .02), 250 ng CH11-treated versus 250 ng CH11+ZB4-treated (P < .002); CD8+ cells: untreated versus 250 ng CH11-treated (P > .2), 250 ng CH11-treated versus 250 ng CH11+ZB4-treated (P > .2). (B) Induction of apoptosis in CD4+ and CD8+cells from healthy donors (n = 20). PBMC from healthy volunteers were untreated or cocultured with the agonistic anti-FasR MoAb CH11 (□) and/or the antagonistic anti-FasR MoAb ZB4 (▨) for 24 hours (up to 7 days, data not shown). The percentage of apoptotic cells in CD4+ and CD8+ cell fractions were assessed by two-color flow cytometry as described for (A). Statistical analysis showed the following: CD4+ cells: untreated versus CH11 treated (P > .7), CH11-treated versus CH11+ZB4-treated (P > .8); CD8+ cells: untreated versus CH11 treated (P > .7), CH11-treated versus CH11+ZB4-treated (P > .3).
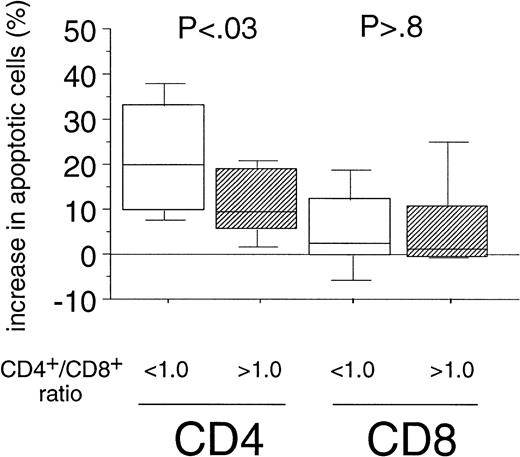
Sensitivity of CD4+ Cells Toward Fas-Mediated Apoptosis Correlates With CD4+/CD8+ Ratio in PBMC of B-CLL Patients
The enhanced sensitivity of the CD4+ cell population over the CD8+ cells to Fas-mediated cell death in B-CLL might be the explanation for the inversion of the CD4+/CD8+ ratio observed during the course of B-CLL.2,3 When determining the CD4+/CD8+ ratios (range, 0.2 to 3.4) in our patients' PBMC by immunophenotyping we also found a correlation between the inversion of the ratio and the Rai stage (P = .01, data not shown). We then assigned two groups, using a CD4+/CD8+ ratio of 1.0 as cutoff level defined as the lower threshold of healthy individuals in accordance with the literature.27 When we compared the percentage of apoptosis induced by the CH11-antibody in vitro in both CD4+ and CD8+ cells of B-CLL patients between the above-mentioned categories, we found a markedly higher sensitivity toward Fas-mediated apoptosis in CD4+ cells derived from patients with a CD4+/CD8+ ratio below 1.0 as compared with the group exhibiting a CD4+/CD8+ ratio within the normal range (P < .03, Fig 3). However, when we analyzed the sensitivity of the CD8+fraction, no correlation between Fas-sensitivity and CD4+/CD8+ ratio could be detected (P> .8, Fig 3).
Correlation of the Fas-sensitivity of CD4+and CD8+ cells of B-CLL patients with the inversion of the CD4+/CD8+ ratio. Experimental data from both T-cell subsets of B-CLL patients (see Fig 2A) were grouped into two catusing a CD4+/CD8+ ratio of 1.0 in PBMC of B-CLL patients as cutoff level (< 1.0: n = 17; >1.0: n = 13). The boxes represent the increase in the percentages of apoptotic cells after 24-hour incubation with CH11 MoAb (250 ng/mL). Results of the statistical analysis CD4+ cells: < 1.0 versus >1.0 (P < .03), CD8+ cells: < 1.0 versus >1.0 (P > .8).
Correlation of the Fas-sensitivity of CD4+and CD8+ cells of B-CLL patients with the inversion of the CD4+/CD8+ ratio. Experimental data from both T-cell subsets of B-CLL patients (see Fig 2A) were grouped into two catusing a CD4+/CD8+ ratio of 1.0 in PBMC of B-CLL patients as cutoff level (< 1.0: n = 17; >1.0: n = 13). The boxes represent the increase in the percentages of apoptotic cells after 24-hour incubation with CH11 MoAb (250 ng/mL). Results of the statistical analysis CD4+ cells: < 1.0 versus >1.0 (P < .03), CD8+ cells: < 1.0 versus >1.0 (P > .8).
FasL Expression in T-Lymphocyte Subsets of Normal Donors and B-CLL Patients
FasL expression has been found in only a small fraction of resting T cells but increases substantially upon stimulation with PMA/ionomycin and TCR signaling.13 The concomitant expression of FasR and FasL is usually considered a feature of activated T-cells.14 15 We could detect only low levels of FasL-expression in CD4+ and CD8+ cells of normal donors, whereas the CD4+ cells of B-CLL patients expressed the antigen significantly higher (P < .009, Table 2). By contrast, in CD8+T cells the FasL expression was not significantly increased over that of CD8+ cells of normal controls (P > .2, Table2).
Determination of FasL Expression (MFI) in Lymphocytic Subsets of B-CLL Patients and Healthy Individuals
| Lymphocytic Subset . | B-CLL Patients . | P . | Healthy Donors . |
|---|---|---|---|
| CD4+ | 4.2 ± .63 | <.009 | 2.1 ± .5 |
| CD8+ | 2.8 ± .29 | >.2 | 2.0 ± .7 |
| CD19+ | 2.4 ± .21 | >.9 | 2.3 ± .1 |
| Lymphocytic Subset . | B-CLL Patients . | P . | Healthy Donors . |
|---|---|---|---|
| CD4+ | 4.2 ± .63 | <.009 | 2.1 ± .5 |
| CD8+ | 2.8 ± .29 | >.2 | 2.0 ± .7 |
| CD19+ | 2.4 ± .21 | >.9 | 2.3 ± .1 |
MFI was determined by immunofluorescence staining and FACS analysis and calculated as the ratio of the MFI achieved with anti FasL MoAb/isotype control Moab. MFI >1.5 was considered positive. Means ± SEM of 30 B-CLL patients and 20 healthy individuals are given.
Expression of FasR in B-CLL Lines and CD19+ Cells From B-CLL Patients and Their Sensitivity to Fas-Induced Apoptosis
In the B-CLL cell line EHEB, a bright fluorescence signal for specific FasR staining could be detected by flow cytometric analysis (data not shown). Nevertheless, this cell line proved to be insensitive toward the death-inducing capacity of the CH11 MoAb. In fact, the mean number of apoptotic cells after 16-hour incubation with the CH11 MoAb did not increase beyond the level of spontaneous apoptosis (n = 4, P> .3, data not shown), while in the FasR+ T-ALL CEM-C7H2 cell line used as Fas-sensitive control,28 a high percentage of cells underwent apoptosis (n = 4, P < .0001, data not shown).
The CD19+ B cells of 33 B-CLL patients showed a decreased FasR expression (mean, 2% positive cells), as compared with CD19+ cells of 20 healthy controls (mean, 9% positive cells; P < .0001; data not shown). We found that CD19+ cells from neither B-CLL patients nor healthy donors were susceptible to Fas-induced cytotoxicity. Stimulation with CH11 MoAb in the concentration range, which effectively induced apoptosis in CD4+ cells of B-CLL patients failed to do so in normal and neoplastic CD19+ cells (normal: n = 20, P > .4, neoplastic: n = 25, P > .7; data not shown) and addition of the inhibitory MoAb ZB4 did not change the percentage of apoptotic cells in this fraction (normal: n = 20, P > .8, neoplastic: n = 25, P > .6; data not shown).
FasL Expression in B-CLL Lines and CD19+ B Lymphocytes of B-CLL Patients
The expression of death-inducing FasL on the surface of cytotoxic cells or malignant tumor cells leads to target-cell killing in various tissues and cell types.18 To study the role of FasL expression on B cells in Fas-mediated apoptosis in the light of the enhanced sensitivity of CD4+ over CD8+ cells of B-CLL patients and over the equivalent cell fractions of healthy donors, we first analyzed surface FasL expression on the B-CLL cell line EHEB in flow cytometry which was found FasL positive with a MFI of 2.3 (mean of three experiments, data not shown). This result could also be confirmed in the CD19+ subpopulation of B-CLL patients, which showed a homogeneous staining profile of FasL in 27 of 30 patients (Table 2, Fig 4). A comparative study of CD19+ cells of B-CLL and normal donors showed no significant difference concerning FasL staining intensity (MFI of 2.4 ± .21 v 2.3 ± .1, P > .9; Table2).
Membrane-Bound FasL Antigen on B-CLL Cells Is Functional
The expression of FasL on cytotoxic T lymphocytes (CTL) is reported to be sufficient for inducing cell death of FasR-bearing target cells.29 Using the JAM assay as a readout system, we examined whether FasL-expressing CD19+ B-CLL cells also exhibited cytolytic activity on the WT and crmA-transfected T-ALL cell line CEM-C7H2.20,22 These cells display features of activated T cells, such as expression of FasR and FasL14 and are susceptible to Fas-induced apoptosis by the agonistic FasR antibody CH11 or FasL+ effector cells.20 CEM-C7H2 cells carrying a vector control (VC) were prelabeled with [3H]thymidine and cocultivated for 72 hours with unlabeled EHEB cells resulting in a significant decrease in T-cell–specific [3H]thymidine content compared with untreated controls. This indicated the induction of DNA fragmentation and cell death in the target cells (Fig5A). The effector/target ratio was tested over a broad range (2:1 to 20:1, data not shown) with T-cell killing most effective at the E/T ratio 10:1. Preincubation of target cells with the antagonistic FasR-antibody ZB4 (0.25 μg/mL) partially protected CEM-C7H2 cells from being killed by B-CLL cells (mean inhibition, 30% [P < .0001]; Fig 5A). crmA-transfected CEM-C7H2 cells were resistant to CH11 MoAb20,28 (and results not shown), reflecting the inhibition of caspase-8, which is involved in Fas-induced signaling.23,30 This target cell subclone (CRM) exhibited a high resistance to the killing mediated by the FasL-positive effector cell line EHEB (mean inhibition, 66%; P < .0001; Fig 5A). As a control experiment, a FasR− B cell line U26620 was used as a target for the FasL+ EHEB cell line in a JAM assay. No killing of the FasR negative target cells could be observed (mean percentage of apoptotic cells, 8%; Fig 5A), thus arguing against an FasL-independent mechanism of killing in these experiments.
Using native neoplastic CD19+ B cells of patients depleted of T cells by magnetic cell separation (purity > 98%) as an effector led to killing of CEM-C7H2 target cells in all five cases tested. This decrease was significantly inhibited in 5 of 5 by preincubation with the antagonistic FasR antibody ZB4 (mean inhibition, 44%; Pvalues ranged from .0001 to .002, Fig 5B) or with the neutralizing FasL antibody NOK-2 (mean inhibition, 74%; P < .0001; Fig 5B).
DISCUSSION
This study showed a pronounced increase of FasR+ cells in CD4+ and CD8+ T-cell fractions of B-CLL patients in comparison to healthy controls (Fig 1). This was associated with a sensitivity of the CD4+ cell fraction toward Fas-mediated apoptosis in vitro (Fig 2a). In the same experimental setting, we found that the CD8+ fraction, despite having a similarly high percentage of FasR+ cells, proved far less sensitive to Fas-mediated cell death triggered by CH11 MoAb in the time frame investigated (after 24 hours, Fig 2A; after up to 7 days, data not shown). By contrast, although FasR expression could be found in a mean of 35% of the CD4+ and 27% of the CD8+fraction of cells of healthy volunteers, neither of the T-cell subsets were susceptible to cell death by the same treatment with CH11 MoAb (Fig 2B). These results in healthy controls are in line with other investigations in which FasR expression was determined in a mean of about 30% in both CD4+ and CD8+ T cells of healthy donors, which nevertheless remained resistant to Fas-mediated apoptosis.8 9
Our data led to several questions: What is the reason for the upregulation of FasR expression on the CD4+ and CD8+ T-cell subsets of B-CLL patients? What could explain the differential sensitivity of these subsets toward FasR ligation?
FasR upregulation has been shown to occur after T-cell activation through TCR stimulation by antigens14,15 or after stimulation with proinflammatory cytokines like TNF-α or interferon-γ.31 In a recent study, FasR expression was observed after exposure of naive peripheral blood cell T lymphocytes to a variety of viral pathogens.32 An increase in FasR expression on both CD4+ and CD8+ cells has also been reported in patients with HIV infection8,9 and with systemic lupus erythematosus (SLE)33; patients with these diseases are known to be susceptible to infection. In B-CLL, repeated activation by infections resulting from the basic immune deficiency characteristic of the disease might lead to chronic T-cell activation accompanied by FasR elevation. Apart from infections, RT-PCR analysis for cytokine RNA production by B-CLL cells showed their frequent production of TNF-α, IL-6, IL-1β, and IL-8.34In addition, an increase in TNF-α serum levels was observed in all stages of B-CLL.35 The impact of TNF-α and IL-1β in FasR upregulation in T cells31 and thyrocytes,36 respectively, has already been shown. These findings might suggest an involvement of a proinflammatory chemokine in the FasR upregulation in T-cells of B-CLL patients, but its precise nature, regulation, and influence on the Fas-dependent T-cell imbalance remain to be determined.
The discrepancy between an elevated FasR expression in CD4+and CD8+ T-cells and a selective resistance toward Fas-induced cell death after ligation in vitro in the CD8+subpopulation is a new observation in B-CLL. However, analogous findings have been described in two other diseases.8,33 One study of HIV-infected individuals8 demonstrated a selective sensitivity of CD4+ over CD8+ T cells to the FasR/FasL apoptotic pathway leading in vitro to a reduced CD4+/CD8+ ratio.8 However, these findings are controversial and could not be confirmed by another group, which found both T-cell subsets to be Fas-sensitive.9 In addition, one study of SLE patients also demonstrated a selective sensitivity of CD4+ over CD8+ T-cells to Fas-mediated apoptosis.33 Furthermore, in the same study the extent of FasR expression on CD4+ T-cell subsets inversely correlated with the absolute size of the CD4+T-cell subset in peripheral blood of SLE patients in vivo and was discussed to be a putative mechanism for lymphopenia.33Finally, both FasR-bearing CD4+ and CD8+tumor-infiltrating lymphocytes (TIL) from an astrocytoma model were susceptible to FasL-dependent apoptosis but, in the case of CD8+ cells, there was a longer delay between activation and apoptosis and a lower proportion of cells was finally killed.18 However, such a delay of apoptosis could not be observed in our study. Together with our data, these reports point to the fact that the constitutive expression as well as the upregulation of FasR on lymphocytes are involved but do not per se predict sensitivity to induction of apoptosis.20,37 38
As a possible explanation for this discrepancy, additional signals might modulate the biologic effects after FasR ligation. In mature murine B cells, for example, CD95 is upregulated by CD40 ligation, but the CD95 signaling pathway and its biologic effects can be disengaged after sIgM receptor crosslinking by foreign antigens39,40or after interleukin-4 receptor (IL4R) activation.41 In addition, FasR signal transduction has been shown to be able to enhance proliferation in T cells responding to mitogens or TCR signaling in vitro,37,38 a finding that points to an additional signaling capacity of CD95 besides that of induction of apoptosis. As another possible explanation for a differential sensitivity toward Fas-induced apoptosis, intrinsic differences in the signaling cascades and/or in the enzymatic equipment could be postulated. Hashimoto et al42 showed that CD4 cross-linking, which led to upregulation of FasR in CD4+ and CD8+ T cells, selectively reduced the expression of the anti-apoptotic factor Bcl-2 in the former T-cell subset, concurrently with the induction of CD4+ T-cell apoptosis. In another study, different proteases were found to be involved in CD4+ and CD8+ T-cell death.9 The fact that we found a difference between the percentage of spontaneous apoptosis in CD8+ cells and CD4+ cells of B-CLL patients (Fig 2A), as well as in the equivalent T-cell subsets of healthy donors (Fig 2B) suggests the involvement of an additional anti-apoptotic factor for the CD8+ fraction in vivo, which underlines possible intrinsic differences of CD4+ and CD8+T cells.
Our in vitro data of a selective sensitivity of CD4+ cells toward Fas-induced apoptosis point to the involvement of this cell death program in the development of an abnormal T-cell distribution in vivo. Our observation of a correlation in individual patients between Fas sensitivity of the CD4+ cell subsets and the relevant CD4+/CD8+ ratio is in agreement with this hypothesis (Fig 3). Our findings strongly suggest the crucial importance of the FasR/FasL system in the gradual inversion of CD4+/CD8+ ratio observed during the course of B-CLL.2,3 In view of the alteration in the normal helper/suppressor T-cell ratio, it has been suggested that regulatory abnormalities of T cells during antibody production occur and may be causally linked to the development of the hypogammaglobulinemia observed in almost all B-CLL patients.3 By contrast to the absolute CD4+ cell depletion described during the progression of acquired immunodeficiency syndrome8-10 and SLE,33 there is an increase in the absolute cell numbers of CD4+ and CD8+ T lymphocytes in B-CLL,2 indicating that the immune deficiency in this disease results from the abnormal T-cell distribution or activity,2 4-6 rather than from the absolute T-cell depletion.
The existence of a sensitive CD4+ fraction of B-CLL patients toward FasR ligation leads to the question of the relevant FasL-bearing effector cells in vivo, which might contribute to the gradual relative reduction of this T-cell subset. There is increasing evidence that CD4+ and CD8+ T lymphocytes exhibit cytolytic activity through FasL.17 In our study, besides FasR expression, the percentage of FasL+cells was significantly higher in CD4+ cells of B-CLL patients when compared with healthy controls. This concomitant expression of both FasR and FasL corresponds to the phenotype of activated T-cells,14,15 which renders T cells susceptible to FasL-mediated cell death by “fratricide” and “suicide.”15 FasL expression has also been shown on B lymphocytes. In resting murine B cells, FasL is nearly undetectable and expression is induced upon stimulation with PMA/ionomycin or bacterial LPS.19 Furthermore, these activated B cells had a FasL-dependent lytic activity since they were capable of killing FasR-expressing target cells.19 In a recent study, we found FasL expression on the surface of neoplastic plasma cells, which also showed a FasL-dependent killing efficacy toward T lymphocytes.20 In the present study, when analyzing PBMC from B-CLL patients, the CD19+ B-cell fraction was found to significantly express FasL which represents a large reservoir of FasL+ potential killer cells in the expanded B-cell fraction. In our experiments, highly enriched CD19+ B-CLL cells (purity > 98%) were able to lyse the target CEM-C7H2 cells through the FasL/FasR pathway in vitro with specificity clearly defined by inhibition with the neutralizing anti-FasL MoAb NOK-2 or the antagonistic anti-FasR MoAb ZB4 (Fig 5B). In addition, we found a constitutive expression of FasL in the EBV-transformed B-CLL cell line EHEB. Concerning the main biologic features examined in this investigation (ie, the expression of FasL and FasR as well as the sensitivity toward Fas-induced cell death [data not shown] and the killing capacity on target T cells [Fig 5A]), this cell line did not differ from the native B-CLL cell samples analyzed in parallel. These results may be considered supportive for the data of the native cell samples of B-CLL patients.
Not only were B-CLL cells capable of killing T cells through FasL, but they also displayed downregulation of their own FasR together with a low sensitivity toward the agonistic FasR-antibody CH11 (data not shown). CD4+ T cells are thought to be involved in tumor cell killing by immune surveillance.17 Our data provide evidence that B-CLL cells might not only resist the attack of CD4+ effector cells using the Fas pathway but might reverse the procedure and send a death signal to “activated” FasR-bearing T cells by constitutively expressing FasL. On the basis of our present knowledge, we cannot exclude the possibility that FasR+/FasL+ CD4+ cells of B-CLL patients also commit Fas-mediated “suicide” or “fratricide” in vivo and that this contributes to the relative reduction of the CD4+ subpopulation. However, the increasing cell number of the malignant B-cell fraction in relation to the absolute T-cell number in B-CLL provides an effector/target ratio that proved to be very effective in T-cell killing in vitro. The continuous presence of FasL on the surface of this expanded B-cell clone, concomitantly with a decreased ability of self-restriction by suicide through FasR downregulation and insensitivity toward FasL-triggering defines a malignant population of aggressive cells. Furthermore, the attack of B-CLL cells on T cells would be especially efficient in case of chronic activation by infections accompanied by FasR upregulation on T cells by antigen or cytokines, secreted either by bystander cells during infection or by B-CLL cells themselves.
Supported by Grant No. 6287 from the Austrian National Bank and by the Province of Tyrol.
Address reprint requests to Richard Greil, MD, Laboratory of Molecular Cytology, Department of Internal Medicine, University of Innsbruck, Anichstrasse 35, A-6020 Innsbruck, Austria.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 5. CEM-C7H2 T-ALL cells are killed by B-CLL cells through the Fas/FasL pathway. (A) T-ALL cells carrying the relevant vector control (VC) were incubated with 10 μCi/mL [3H]-thymidine for 16 hours and cocultivated with the indicated B-CLL cells EHEB (□). For blocking experiments, crmA-expressing CEM-C7H2 cells (CRM) were used as target cells. Alternatively, the labeled T-cells (VC) were coincubated with 0.25 μg/mL of the FasR blocking ZB4 MoAb. In a control experiment the FasR– neoplastic B-cell line U266 was used as a target (▨). Cocultivation of cells was performed for 72 hours at 37°C. The reduction of DNA-incorporated radioactivity was used to calculate the percentages of B-CLL–mediated target cell death (n = 12) and is presented in a box-blot and whiskers model. Statistical analysis of the blocking experiments showed the following: specific killing by EHEB: 30% mean inhibition by ZB4 (P < .0001), 66% mean inhibition by crmA expression (P < .0001). (B) CEM-C7H2 T-ALL cells are eliminated by CD19+ cells of B-CLL patients through the Fas/FasL pathway. PBMC of 5 B-CLL patients were depleted of T cells by magnetic cell separation. The resulting CD19+ B-cell fractions (purity > 98%) were cocultivated with [3H]-thymidine–prelabeled CEM-C7H2 target cells for 72 hours at 37°C in a effector/target ratio of 10:1 (four replicates). The reduction of DNA-incorporated radioactivity in the target cells was used to calculate specific target cell killing by CD19+B-CLL cells, which was blocked by preincubation with the antagonistic anti-FasR MoAb ZB4 (0.25 mg/mL, ▧) or the inhibitory anti-FasL MoAb NOK-2 (0.75 mg/mL, ▨). Results of the statistical analysis: 44% mean inhibition by ZB4 MoAb (.0001 < P < .002), 74% mean inhibition by NOK-2 MoAb (P < .0001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/11/10.1182_blood.v91.11.4273/4/m_blod41125005ax.jpeg?Expires=1769169517&Signature=K3N8GYDS5rnzZ6k5NZsWdMgkAJPSmDzOOi~ahuuLVgmNw5pc5cWNXYRn3cYMkD0Jg2gqurfV~PwS4W2~EtemFhZ0Ck2AnsOyxoT4R5LIYjfDb0o9RgbwGFAHB7L~GgoISkuKIWX7rNdoIyvWt0bTJJ6hhDT~wXRov0TlLZweE46gYiD7Q8NCE~k8vWEjugyYITYuCCyO3FXvkcTP9gh6LyrxkDMLeEuzK0M6y2UqirM95xt5fyQ7hCUFuz-QsBHS8QhxUFd0qQDHMkSm4taKeF-23iWNNXKhVEYlwLIZnjKsMgVy2K0VkfKGk-FtKmiYE0Kr-Z0gifbrTcrtiKPzPA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. CEM-C7H2 T-ALL cells are killed by B-CLL cells through the Fas/FasL pathway. (A) T-ALL cells carrying the relevant vector control (VC) were incubated with 10 μCi/mL [3H]-thymidine for 16 hours and cocultivated with the indicated B-CLL cells EHEB (□). For blocking experiments, crmA-expressing CEM-C7H2 cells (CRM) were used as target cells. Alternatively, the labeled T-cells (VC) were coincubated with 0.25 μg/mL of the FasR blocking ZB4 MoAb. In a control experiment the FasR– neoplastic B-cell line U266 was used as a target (▨). Cocultivation of cells was performed for 72 hours at 37°C. The reduction of DNA-incorporated radioactivity was used to calculate the percentages of B-CLL–mediated target cell death (n = 12) and is presented in a box-blot and whiskers model. Statistical analysis of the blocking experiments showed the following: specific killing by EHEB: 30% mean inhibition by ZB4 (P < .0001), 66% mean inhibition by crmA expression (P < .0001). (B) CEM-C7H2 T-ALL cells are eliminated by CD19+ cells of B-CLL patients through the Fas/FasL pathway. PBMC of 5 B-CLL patients were depleted of T cells by magnetic cell separation. The resulting CD19+ B-cell fractions (purity > 98%) were cocultivated with [3H]-thymidine–prelabeled CEM-C7H2 target cells for 72 hours at 37°C in a effector/target ratio of 10:1 (four replicates). The reduction of DNA-incorporated radioactivity in the target cells was used to calculate specific target cell killing by CD19+B-CLL cells, which was blocked by preincubation with the antagonistic anti-FasR MoAb ZB4 (0.25 mg/mL, ▧) or the inhibitory anti-FasL MoAb NOK-2 (0.75 mg/mL, ▨). Results of the statistical analysis: 44% mean inhibition by ZB4 MoAb (.0001 < P < .002), 74% mean inhibition by NOK-2 MoAb (P < .0001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/11/10.1182_blood.v91.11.4273/4/m_blod41125005bx.jpeg?Expires=1769169517&Signature=j7DC~D8mv9XisEu5WgyaFkXZSFIlUgDPRD3tic3XJy2xzFn7cVp-pCt1lhjst-84-ijP1hr7bw-MhbdeflPEhVuwqT~t8D6EscxrGdRrOMVxrIv18YNxYpLLGUN3pFAoWA4EqykZ1OiPhUKRtFJRFFCnHzY8p5MCKp7R8jlgt-IWTLreW82Uma7BehaRaILGchEkE90LoZp4wU--BkjmpFVmistjsucnKJa6rHtTtfcJzX2iN9jeIOse3eBbjCzq4iH9eLp53jRqGUccDP4jIKayGI5GHoORS39~GMpIqCZBASO5L3--Agmk4VTP-fNhH~MvAsoIv4biEB9SYQiNLg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal