Abstract
Variants of the CD44 cell-surface adhesion molecule include additional sequences encoded by combinations of exons from the membrane proximal domain (exons 6–14). Preliminary studies suggest that these additional variable membrane proximal sequences may alter the ligand specificity, glycosylation, and biologic function of CD44. In earlier studies, we found that primary extranodal and widely disseminated aggressive non-Hodgkin's lymphomas (NHLs) and normal activated B cells expressed a directly spliced exon 10–containing variant (CD44ex10), whereas normal resting B cells expressed larger exon 10–containing variants (CD44ex10-14 and CD44ex7-14). To obtain additional information regarding the function of exon 10–containing CD44 variants in aggressive NHL, we generated aggressive NHL transfectants that expressed CD44ex10, CD44ex10-14, CD44ex7-14, the standard CD44 isoform (CD44H), or vector alone, and evaluated the local tumorogenicity, aggregation, and metastatic potential of these transfectants. CD44ex10 aggressive NHL transfectants were more likely to cause local tumor formation in nude mice than transfectants expressing the larger exon 10–containing variants, CD44H, or vector alone. In addition, cell suspensions derived from CD44ex10 local tumors exhibited far greater homotypic aggregation than those obtained from other CD44 or vector-only local tumors. In nude mice that received CD44ex10 transfectants, distant metastases were also significantly more likely to develop than in animals that were given either the CD44ex10-14, CD44ex7-14, CD44H, or vector-only transfectants. These data provide the first evidence that the directly spliced exon 10–containing CD44 variant (CD44ex10) has a unique biologic function in aggressive NHL.
CD44 IS A CELL-SURFACE adhesion molecule expressed by B and T lymphocytes and a variety of other hematopoietic and nonhematopoietic cells.1-3 This cell-surface glycoprotein is the major receptor for hyaluronate, the principal glycosaminoglycan of the extracellular matrix (ECM).4,5CD44 also binds to additional ECM proteins, including fibronectin, collagen types I and VI, and other ligands, including serglycin, osteopontin, the chondroitin sulfate-modified invariant chain, and incompletely characterized cell surface molecules.3,6-11CD44 participates in multiple aspects of lymphoid biology, including early lymphopoiesis, migration, homing, and signal transduction.1,3 12-24
The CD44 adhesion molecule contains three specific domains: (1) an intracellular cytoplasmic tail that associates with cytoskeletal proteins, such as ankyrin, actin, ezrin, radixin, and moesin23,25-28; (2) a transmembrane region; and (3) an extracellular domain with an alternatively spliced membrane-proximal region and an invariant distal segment.2,29-31 The invariant segment of the extracellular domain has significant homology with cartilage link and proteoglycan core proteins.2,29 The alternatively spliced membrane proximal region includes variable numbers of exons 6-14 that are also described as variant (v) exons 2-10.30 31
Normal and malignant lymphocytes express a predominant “standard” (“hematopoietic”) CD44 isoform with no additional exons from the membrane proximal domain.32,33 Lymphocytes and other hematopoietic and nonhematopoietic cells also express CD44 isoforms with additional membrane proximal sequences encoded by specific combinations of exons from the variable region.30-35Preliminary studies suggest that these variable membrane proximal sequences may alter the ligand specificity, glycosylation, and biologic function of the CD44 adhesion molecule.34-46
Specific alternatively spliced CD44 isoforms have been implicated in the development of normal immune responses.33,35,38 In in vivo models in which rodents were exposed to antigen, the resulting activated B and T cells transiently expressed a directly spliced exon 10(v6)–containing CD44 variant.35 In those animals that were pretreated with a CD44ex10 peptide antibody before antigen exposure, normal activated B and T lymphocytes failed to develop.35
Exon 10–containing CD44 isoforms also promote the metastasis of certain hematopoietic and nonhematopoietic malignancies.34,43 In earlier rodent studies, carcinoma cell lines that expressed certain exon 10–containing CD44 variants (CD44ex10-11, CD44ex8-11) metastasized widely.34Nonmetastatic carcinoma cell lines transfected with one of the exon 10–containing isoforms also acquired metastatic potential.34 In additional analyses, a monoclonal antibody (MoAb) directed against the “metastasis” domain (exon 10/v6) retarded the nodal and systemic metastases of rodent carcinoma cell lines expressing exon 10–containing CD44 variants.43 These data, which implicated an exon 10–containing CD44 isoform in the trafficking of rodent tumor cells and normal activated lymphocytes, prompted additional analyses of CD44 isoforms in human tumors, and focused particular attention on human lymphoid malignancies.32,33 47
In studies performed before the identification of alternatively spliced CD44 isoforms, tumors from a series of patients with aggressive non-Hodgkin's lymphoma (NHL) were analyzed with an antibody directed against a CD44 framework epitope.48-51 Patients whose tumors expressed high levels of CD44 were more likely to present with incurable disseminated disease than patients whose tumors expressed low levels of the adhesion molecule(s).49 When additional aggressive NHLs were analyzed with an antibody directed against an exon 10–encoded peptide, a subset of tumors expressed exon 10–containing CD44 variants.33,47 Patients whose tumors expressed exon 10–containing isoforms were also less likely to survive their disease.47 Although these studies implicated exon 10–containing CD44 variants in the clinical behavior of aggressive NHLs, they did not distinguish between potential exon 10–containing variants or identify the specific exon 10–containing isoforms in a given tumor.33 47
In additional studies from our own laboratory, tumors from patients with primary nodal, extranodal, or disseminated aggressive NHL (diffuse large B-cell lymphoma) were evaluated for CD44 variant transcripts using semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR).32 In this small series, tumors from patients with local nodal disease expressed the hematopoietic form of CD44 (CD44H) but lacked additional exon 10–containing CD44 variants.32In contrast, tumors from patients with primary extranodal or widely disseminated disease expressed a directly spliced exon 10–containing variant, CD44ex10.32 These data prompted speculation that CD44ex10 might promote the dissemination of aggressive NHLs. For these reasons, it was of interest that normal activated B cells also expressed CD44ex10, whereas resting peripheral blood lymphocytes lacked CD44ex10 but expressed larger alternatively spliced CD44 isoforms containing exons 10-14 or 7-14 from the membrane proximal variable domain.32
Because the directly spliced CD44ex10 and larger exon 10–containing CD44 isoforms were differentially expressed by clinically relevant subsets of aggressive B-cell lymphomas, activated B cells, and peripheral blood lymphocytes (PBLs), we postulated that these CD44 variants had unique biologic functions. To explore this possibility, the identified CD44ex10, CD44ex10-14, CD44ex7-14, and standard CD44 variants were synthesized and introduced into an aggressive lymphoma cell line. This report compares the in vitro and in vivo behavior of these CD44 isoform-specific transfectants, and directly implicates CD44ex10 in the homotypic aggregation and distant metastasis of these aggressive NHL transfectants.
MATERIALS AND METHODS
Generation of Alternatively Spliced CD44 Constructs
Partial-length alternatively spliced CD44 cDNAs.
Partial-length cDNAs containing bp 518-667 and bp 1811-2134 from the 5′ and 3′ framework regions and one of the intervening alternatively spliced variable regions (exon 10 alone [bp 1154-1282], exons 10-14 [bp 1154-1810], or exons 7-14 [bp 797-1810], Fig 1) were synthesized by RT-PCR. The directly spliced exon 10–containing CD44 variant was obtained from RNA of anti–Ig-activated splenic B cells and the larger exon 10–containing isoforms (CD44ex10-14, CD44ex7-14) were obtained from peripheral blood mononuclear cell RNA.32
Genomic organization of CD44 and the resulting exon 10–containing CD44 splice variants. (a) The genomic organization of CD44 and sizes of individual CD44 exons are shown. Specific functional domains of CD44, including the region of cartilage-link homology, the membrane-proximal variable domain with the “metastasis (meta)” epitope, the transmembrane region and the alternatively spliced cytoplasmic tails are indicated. (b) Schema for synthesis of exon 10–containing CD44 variable regions is shown. The positions of the sense oligonucleotides, FR1S and ex10S, and the antisense oligonucleotides, ex10AS and FR2AS, are indicated. (c) The resulting alternatively spliced exon 10–containing CD44 variants (CD44ex10, CD44ex10-14, CD44ex7-14) and CD44H are shown.
Genomic organization of CD44 and the resulting exon 10–containing CD44 splice variants. (a) The genomic organization of CD44 and sizes of individual CD44 exons are shown. Specific functional domains of CD44, including the region of cartilage-link homology, the membrane-proximal variable domain with the “metastasis (meta)” epitope, the transmembrane region and the alternatively spliced cytoplasmic tails are indicated. (b) Schema for synthesis of exon 10–containing CD44 variable regions is shown. The positions of the sense oligonucleotides, FR1S and ex10S, and the antisense oligonucleotides, ex10AS and FR2AS, are indicated. (c) The resulting alternatively spliced exon 10–containing CD44 variants (CD44ex10, CD44ex10-14, CD44ex7-14) and CD44H are shown.
The partial-length alternatively spliced CD44 cDNAs were synthesized using a two-step strategy (Fig 1b). A sense oligonucleotide from the 5′ CD44 framework region (FR1S, bp 518-539) and an antisense oligonucleotide from ex10 (ex10AS, bp 1231-1208) were used to synthesize the 5′ halves of CD44ex10, CD44ex10-14, and CD44ex7-14 partial-length cDNAs (Fig 1). These cDNA fragments were initially identified by their predicted sizes and hybridization with an appropriate internal oligonucleotide probe. In a second PCR reaction, a sense oligonucleotide from ex10 (ex10S, bp 1153-1176) and an antisense oligonucleotide from the 3′ CD44 framework region (FR2AS, bp 2155-2134) were used to synthesize the 3′ halves of the CD44ex10, CD44ex10-14, and CD44ex7-14 partial-length cDNAs (Fig 1b). These cDNA fragments were also initially identified by their predicted sizes and hybridization with an appropriate internal oligonucleotide probe.
Thereafter, the 5′ and 3′ halves of the partial-length CD44 variant cDNAs were separately subcloned into TA vectors (Invitrogen, Portland, OR) and sequenced to rule out the possibility of PCR-induced mutations. Plasmids containing the 3′ CD44 partial-length variants were subsequently digested with EcoRV, which cleaves the cDNAs at EcoRV sites in exon 10 (bp 1218, Fig 1) and the flanking 3′ vector polylinker. The excised 3′ CD44 partial-length cDNAs were then ligated into the appropriateEcoRV-digested TA-5′ CD44 variant constructs. These religated CD44 cDNAs were then excised from the TA cloning vector withHincII, which cleaves the CD44 variants at bp 567 and 1994, releasing the intact alternatively spliced sequence (Fig 1).
Full-length alternatively spliced CD44 variants.
The full-length CD44H cDNA42 was obtained from I. Stamenkovic (Massachusetts General Hospital, Boston) and subcloned into the Xho I site of the TA vector. Thereafter, TA-CD44H was digested with HincII, releasing the framework bp 567-667/1811-1994 sequence (Fig 1). The appropriateHincII-digested CD44 variable sequences (CD44ex10 [bp 567-667, 1154-1282, 1811-1994], CD44ex10-14 [bp 567-667, 1154-1810, 1811-1994], CD44ex7-14 [bp 567-667, 797-1810, 1811-1994]) were then inserted into the HincII-cut TA-CD44 vector. The newly reconstructed full-length CD44ex10, CD44ex10–14, and CD44ex7-14 cDNAs were then excised with Xho I and cloned into the pRc/CMV expression vector (Invitrogen).
Generation of Alternatively Spliced CD44 Transfectants
The Namalwa aggressive (Burkitt's) NHL cell line, which lacks CD44 expression and grows well in nude mice,42 was used to generate a panel of CD44 isoform-specific transfectants. pRc/CMV-CD44H, pRc/CMV-CD44ex10, pRc/CMV-CD44ex10-14, pRc/CMV-CD44ex7-14, or pRc/CMV alone were linearized with Pvu 1 and introduced into Namalwa cells by electroporation (400 V/960 μF).42
After 48-hour culture in Iscove's Modified Dulbecco's Medium (GIBCO, Grand Island, NY)/20% fetal calf serum (FCS), transfectants were selected for resistance to neomycin (G418 [GIBCO] 750 μg/mL) in media containing RPMI 1640/10% FCS, 2 mmol glutamine, 1 mmol sodium pyruvate, and 10 mmol HEPES buffer. G418-resistant vector-only transfectants were analyzed for pRc/CMV sequences by Southern blotting and hybridization with a pRc/CMV probe. Thereafter, vector-only transfectants were cloned by limiting dilution.
G418-resistant CD44 transfectants were assayed for CD44 cell-surface expression by indirect immunofluorescence using MoAbs directed against a conserved framework epitope of CD44 (αCD44H) or an 8–amino acid (aa) sequence from CD44 exon 10(αCD44v6) (R&D Systems, McKinley Place, MN) and fluorescein-conjugated goat anti-mouse Ig (Coulter, Hialeah, FL). Bulk populations of CD44 isoform-specific Namalwa transfectants were sorted under sterile conditions on a Coulter Epics Elite flow cytometer (Coulter) and cloned by limiting dilution. Clonal CD44 isoform-specific transfectants were subsequently reanalyzed with the CD44 framework and CD44ex10-specific MoAbs and expanded for use in in vitro and in vivo studies. Clonal CD44H, CD44ex10, CD44ex7-14, and CD44ex10-14 transfectants were also reanalyzed at periodic intervals to confirm the stability of their phenotypes.
In Vitro Analysis of CD44 Transfectants
Proliferation and aggregation.
The proliferative rates of individual CD44 isoform-specific and vector-only transfectants were assessed by thymidine incorporation. Individual CD44 isoform-specific and vector-only transfectants were also cultured in 6-well plates at 2 × 103 to 2 × 105 cells/mL in RPMI/10% FCS, 2 mmol glutamine, 1 mmol sodium pyruvate, and 10 mmol HEPES to evaluate cellular morphology and aggregation.
Hyaluronic acid and chondroitin sulfate binding.
Binding studies were performed according to standard protocols38,39 42 with minor modifications. In brief, 24-well plates were coated with 2 mg/mL Rooster comb hyaluronic acid (Sigma Chemical Co, St Louis, MO) or chondroitin sulfate A (Sigma) or PBS alone at 22°C for 18 hours, washed, and blocked with PBS/1% BSA for an additional hour at 37°C. Thereafter, 107cells of each individual transfectant were labeled with 150 μCi of51Cr at 37°C for 2 hours. Cells were subsequently washed and resuspended at a concentration of 1 × 106cells/mL in PBS/0.5% BSA. 8 × 105 cells of each individual transfectant were added to triplicate wells of uncoated plates or plates coated with hyaluronic acid or chondroitin sulfate. Thereafter, plates were centrifuged at 1,200 rpm for 5 minutes, incubated at 37°C in 5% CO2 for 30 minutes, and subsequently washed to remove unbound cells. The remaining adherent cells in the individual wells were lysed with 1% NP-40, and the samples were harvested and analyzed for chromium uptake.
In Vivo Analysis of CD44 Transfectants
Local tumor take.
Multiple independently derived clones from each of the CD44 isoform-specific (CD44H, CD44ex7-14, CD44ex10-14, CD44ex10) or vector-only transfectants were used to assay local tumor take in 4-week-old Ncr/nu nude mice. In each experiment, three animals were injected subcutaneously with 2 × 106 cells from a given clone. Thereafter, mice were evaluated at daily intervals for the onset of palpable and visible local tumors. Tumor-bearing animals were sacrificed when local tumors reached 2 cm in diameter. Local tumors were then excised and single-cell suspensions prepared for in vitro culture at 2 × 105 cells/mL.
Distant metastases.
Multiple independently derived clones from each of the CD44 isoform-specific or vector-only transfectants were used to evaluate metastatic potential in 4-week-old Ncr/nude mice. In each experiment, three animals were injected through the tail vein with 2 × 106 cells from a given clone. Thereafter, mice were followed daily for the onset of hindlimb paralysis, an early indicator of leptomeningeal/CNS infiltration and widely metastatic disease. Animals in which hindlimb paralysis developed were killed; selected animals were also analyzed for evidence of pulmonary and bone marrow metastasis. In brief, lungs and femurs were harvested, fixed in 10% formaldehyde, decalcified, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E).
Statistical Methods
Proportions of mice developing local tumors or remaining free of metastases were compared using Fisher's exact test. There were no adjustments for multiple comparisons.
RESULTS
Generation of Namalwa Transfectants Expressing Alternatively Spliced CD44 Isoforms
To elucidate the function of CD44ex10 in aggressive NHL and to compare CD44ex10 to the larger exon 10–containing isoforms and CD44H, we generated a series of aggressive NHL transfectants that expressed CD44ex10, CD44ex7-14, CD44ex10-14, CD44H, or contained vector (pRc/CMV) alone. The Namalwa aggressive NHL cell line was chosen for these experiments because the line was used in earlier CD44 studies, lacked baseline CD44 expression, and grew well in nude mice.39,42 45 As indicated in Fig 2, control vector-only transfectants did not react with either the CD44 framework or exon 10–specific antibody. CD44H transfectants reacted with the CD44 framework antibody but not the CD44ex10-specific antibody whereas CD44ex10, CD44ex10-14, and CD44ex7-14 transfectants reacted with both the CD44 framework and exon 10–specific MoAbs (Fig 2).
Immunofluorescence analysis of Namalwa transfectants expressing CD44H, CD44ex7-14, CD44ex10-14, CD44ex10, or vector only. Two independently derived representative Namalwa transfectants expressing each of the CD44 variants used (CD44HI and II, CD44ex7-14I and II, CD44ex10-14I and II, CD44ex10I and II) or vector alone were phenotyped with MoAbs directed against a conserved framework CD44 epitope (solid line), an 8aa sequence encoded by CD44ex10/v6 (dotted line), or an isotope-matched negative control antibody (shaded black). CD44H transfectants reacted with the CD44 framework antibody but not the CD44ex10 (v6) antibody whereas CD44ex10, CD44ex10-14, and CD44ex7-14 transfectants reacted with both antibodies. Vector-only transfectants reacted with neither CD44 antibody.
Immunofluorescence analysis of Namalwa transfectants expressing CD44H, CD44ex7-14, CD44ex10-14, CD44ex10, or vector only. Two independently derived representative Namalwa transfectants expressing each of the CD44 variants used (CD44HI and II, CD44ex7-14I and II, CD44ex10-14I and II, CD44ex10I and II) or vector alone were phenotyped with MoAbs directed against a conserved framework CD44 epitope (solid line), an 8aa sequence encoded by CD44ex10/v6 (dotted line), or an isotope-matched negative control antibody (shaded black). CD44H transfectants reacted with the CD44 framework antibody but not the CD44ex10 (v6) antibody whereas CD44ex10, CD44ex10-14, and CD44ex7-14 transfectants reacted with both antibodies. Vector-only transfectants reacted with neither CD44 antibody.
CD44ex10 Transfectants Exhibit Increased Homotypic Aggregation in Vitro
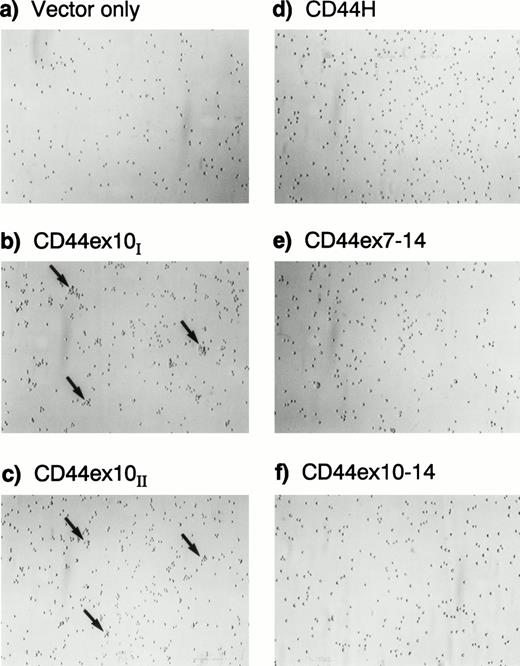
To obtain preliminary information regarding the effect of specific CD44 isoforms on cellular proliferation and aggregation, two independently derived transfectants expressing CD44H, CD44ex10, CD44ex7-14, CD44ex10-14, or containing vector-only were evaluated in vitro. The CD44 isoform-specific and vector-only transfectants had comparable rates of proliferation (data not shown). However, the directly spliced CD44ex10–containing Namalwa transfectants exhibited a subtle increase in homotypic aggregation that was not apparent in the other CD44 or vector-only transfectants (Fig 3).
CD44ex10 transfectants exhibit a subtle increase in homotypic aggregation in vitro. Two independently derived representative CD44ex10–containing Namalwa transfectants (CD44ex10I and II) and additional representative CD44H, CD44ex7-14, CD44ex10-14, and vector-only transfectants are shown. The directly spliced CD44ex10–containing Namalwa transfectants exhibited a subtle increase in homotypic aggregation that was not apparent in other CD44 or vector-only transfectants.
CD44ex10 transfectants exhibit a subtle increase in homotypic aggregation in vitro. Two independently derived representative CD44ex10–containing Namalwa transfectants (CD44ex10I and II) and additional representative CD44H, CD44ex7-14, CD44ex10-14, and vector-only transfectants are shown. The directly spliced CD44ex10–containing Namalwa transfectants exhibited a subtle increase in homotypic aggregation that was not apparent in other CD44 or vector-only transfectants.
CD44ex10 Transfectants Are More Likely to Develop Local Tumors in Nude Mice
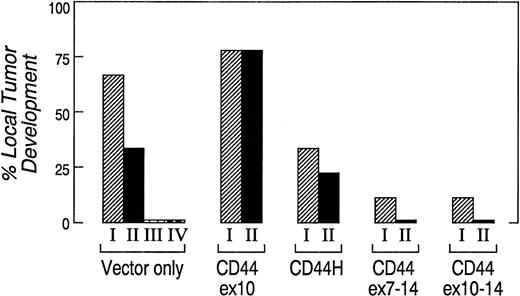
To compare their ability to form local tumors in vivo, two to four independently derived clones expressing CD44H, CD44ex10, CD44ex7-14, CD44ex10-14, or vector-only were separately injected subcutaneously (SQ) into cohorts of nude mice. A summary of the data from three separate experiments is shown in Fig 4. The incidence of local tumor development was significantly greater in animals that received CD44ex10 transfectants than in animals that were given vector-only transfectants (CD44ex10 78% v vector-only 38%, P = .01). In marked contrast, animals that received either CD44ex7-14 or CD44ex10-14 transfectants had significantly lower rates of local tumor development than those of animals administered vector-only transfectants (CD44ex7-14, 6% or CD44ex10-14, 6%v vector-only 38%, P = .03, Fig 4).
CD44ex10 transfectants are more likely to form local tumors in nude mice. Two to four independently derived vector-only, CD44ex10, CD44H, CD44ex7-14, or CD44ex10-14 Namalwa transfectants were injected SQ into nude mice. Animals were subsequently followed for the development of local tumors. The data represent a summary of three identical experiments in which a cohort of three animals received one of the indicated independently derived Namalwa transfectants (vector-onlyI, II, II or IV, CD44ex10I or II, CD44HI or II, CD447-14I or II, or CD44ex10–14I or II). The incidence of local tumor development was significantly greater in animals that received CD44ex10 transfectants than in animals administered vector-only tranfectants (CD44ex10, 78% v vector-only, 38%, P = .01), whereas animals injected with either CD44ex7-14 or CD44ex10-14 transfectants had a significantly lower rate of local tumor development than that of animals administered vector-only transfectants (CD44ex7-14 6% or CD44ex10-14, 6% v vector-only, 38, P = .03). The incidence of local tumor development associated with a given CD44 isoform or vector-only was determined by averaging the rates of local tumor development of multiple independently derived clones: vector-only, four clones; CD44ex10, two clones; CD44ex7-14, two clones; CD44ex10-14, two clones; CD44H, two clones. P values were calculated using a 2 × 2 Fisher's exact test.
CD44ex10 transfectants are more likely to form local tumors in nude mice. Two to four independently derived vector-only, CD44ex10, CD44H, CD44ex7-14, or CD44ex10-14 Namalwa transfectants were injected SQ into nude mice. Animals were subsequently followed for the development of local tumors. The data represent a summary of three identical experiments in which a cohort of three animals received one of the indicated independently derived Namalwa transfectants (vector-onlyI, II, II or IV, CD44ex10I or II, CD44HI or II, CD447-14I or II, or CD44ex10–14I or II). The incidence of local tumor development was significantly greater in animals that received CD44ex10 transfectants than in animals administered vector-only tranfectants (CD44ex10, 78% v vector-only, 38%, P = .01), whereas animals injected with either CD44ex7-14 or CD44ex10-14 transfectants had a significantly lower rate of local tumor development than that of animals administered vector-only transfectants (CD44ex7-14 6% or CD44ex10-14, 6% v vector-only, 38, P = .03). The incidence of local tumor development associated with a given CD44 isoform or vector-only was determined by averaging the rates of local tumor development of multiple independently derived clones: vector-only, four clones; CD44ex10, two clones; CD44ex7-14, two clones; CD44ex10-14, two clones; CD44H, two clones. P values were calculated using a 2 × 2 Fisher's exact test.
Taken together, these data suggest that CD44ex10 enhances the development of local tumors, whereas larger ex10-containing variants (CD44ex7-14 and CD44ex10-14) do not have a similar effect (Fig 4). Furthermore, these studies provide the first functional evidence that in aggressive NHLs, the biologic consequences of the exon 10–encoded amino acid sequence differ when exon 10 is included in the directly spliced CD44ex10 variant or the larger alternatively spliced CD44 isoforms (CD44ex10-14 and CD44ex7-14) (Fig 4).
CD44ex10 Transfectants Are More Likely to Develop Distant Metastases in Nude Mice
Although the above-mentioned studies implicate CD44ex10 in the development of subcutaneous tumors, aggressive NHLs do not characteristically originate in subcutaneous tissue. For these reasons, the more relevant functional parameter of hematogenous dissemination was evaluated in an additional series of animals. In these experiments, two to four independently derived clones expressing CD44ex10, CD44ex7-14, CD44ex10-14, CD44H, or vector-only were separately injected into the tail veins of nude mice. Thereafter, the mice were followed daily for the onset of hindlimb paralysis, an early indicator of leptomeningeal/CNS infiltration and widely metastatic disease. Animals in which hindlimb paralysis developed were killed at the onset of symptoms and evaluated for additional histologic evidence of disseminated lymphoma.
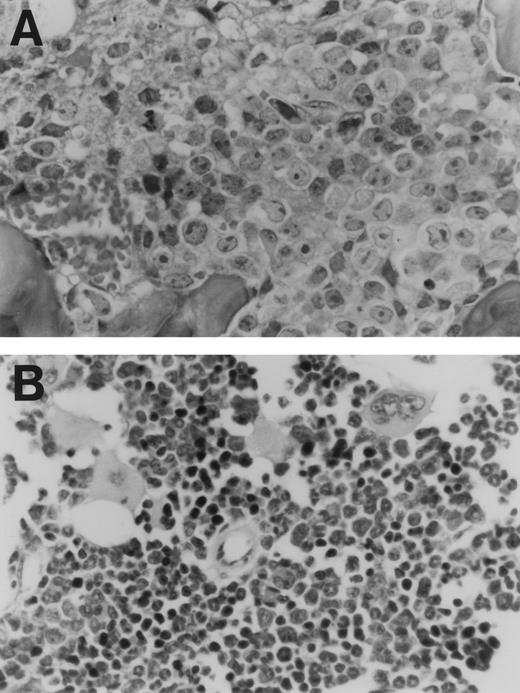
The data from two separate experiments are summarized in Fig 5. As indicated, animals that received CD44ex10 transfectants were significantly less likely to remain free of metastases than animals that were given vector-only transfectants (CD44ex10 v vector-only, 11% v 79%, metastasis-free,P < .0001). Animals that received CD44ex10 transfectants and developed hindlimb paralysis had obvious evidence of widely metastatic disease (Fig 6). For example, the bone marrow of a representative animal injected with CD44ex10 transfectants was almost completely replaced with aggressive NHL (Fig 6).
CD44ex10 transfectants are more likely to cause systemic metastases in nude mice. Two to four independently derived CD44ex10, CD44ex7-14, CD44ex10-14, CD44H, or vector-only Namalwa transfectants were injected into the tail veins of nude mice. Animals were subsequently followed daily for the onset of hindlimb paralysis, an early indicator of leptomeningeal/CNS infiltration and widely metastatic disease. Animals in which hindlimb paralysis developed were sacrificed and evaluated for additional histologic evidence of disseminated lymphoma. The data represent a summary of two identical experiments in which a cohort of three animals was injected with each of the indicated independently derived Namalwa transfectants (vector-onlyI, II, III, or IV, CD44ex10I, II, or III, CD44ex7-14I or II, CD44ex10-14I or II, or CD44HI or II). Data are plotted as percentage of injected animals without metastasis over time (50 days). Animals that received CD44ex10 transfectants were significantly less likely to remain free of metastases than animals that were given vector-only transfectants (CD44ex10, 11% v vector-only, 79% metastasis-free, P < .0001). By contrast, animals that received the larger exon 10–containing isoforms or CD44H were as likely to remain free of metastases as vector-only control animals (CD44ex7-14, 83%, CD44ex10-14, 83%, CD44H, 67% vvector-only, 79% metastasis-free, all P values NS). The likelihood of remaining free of metastasis after receiving a given CD44 or vector-only transfectant was determined by averaging the percentages of metastasis-free animals that received each independently derived clone: vector-only, four clones; CD44ex10, three clones; CD44ex7-14, two clones; CD44ex10-14, two clones; CD44H, two clones. Pvalues were calculated using a 2 × 2 Fisher's exact test.
CD44ex10 transfectants are more likely to cause systemic metastases in nude mice. Two to four independently derived CD44ex10, CD44ex7-14, CD44ex10-14, CD44H, or vector-only Namalwa transfectants were injected into the tail veins of nude mice. Animals were subsequently followed daily for the onset of hindlimb paralysis, an early indicator of leptomeningeal/CNS infiltration and widely metastatic disease. Animals in which hindlimb paralysis developed were sacrificed and evaluated for additional histologic evidence of disseminated lymphoma. The data represent a summary of two identical experiments in which a cohort of three animals was injected with each of the indicated independently derived Namalwa transfectants (vector-onlyI, II, III, or IV, CD44ex10I, II, or III, CD44ex7-14I or II, CD44ex10-14I or II, or CD44HI or II). Data are plotted as percentage of injected animals without metastasis over time (50 days). Animals that received CD44ex10 transfectants were significantly less likely to remain free of metastases than animals that were given vector-only transfectants (CD44ex10, 11% v vector-only, 79% metastasis-free, P < .0001). By contrast, animals that received the larger exon 10–containing isoforms or CD44H were as likely to remain free of metastases as vector-only control animals (CD44ex7-14, 83%, CD44ex10-14, 83%, CD44H, 67% vvector-only, 79% metastasis-free, all P values NS). The likelihood of remaining free of metastasis after receiving a given CD44 or vector-only transfectant was determined by averaging the percentages of metastasis-free animals that received each independently derived clone: vector-only, four clones; CD44ex10, three clones; CD44ex7-14, two clones; CD44ex10-14, two clones; CD44H, two clones. Pvalues were calculated using a 2 × 2 Fisher's exact test.
Histologic evidence of disseminated lymphoma in a nude mouse injected with a CD44ex10 Namalwa transfectant. This representative animal received a tail vein injection of CD44ex10 Namalwa cells and subsequently developed hindlimb paralysis. Thereafter, the animal was sacrificed and evaluated for additional histologic evidence of disseminated lymphoma. The bone marrow (A) is almost completely replaced with aggressive NHL. (B) The bone marrow of a control animal is included for comparison.
Histologic evidence of disseminated lymphoma in a nude mouse injected with a CD44ex10 Namalwa transfectant. This representative animal received a tail vein injection of CD44ex10 Namalwa cells and subsequently developed hindlimb paralysis. Thereafter, the animal was sacrificed and evaluated for additional histologic evidence of disseminated lymphoma. The bone marrow (A) is almost completely replaced with aggressive NHL. (B) The bone marrow of a control animal is included for comparison.
Although animals that received CD44ex10 transfectants were significantly less likely to remain free of metastases, those that received CD44ex7-14, CD44ex10-14, or CD44H transfectants were as likely to remain metastasis-free as control animals (CD44ex7-14, 83%, CD44ex10-14, 83%, CD44H, 67% v vector-only, 79% metastasis-free, all P values non significant [NS], Fig 5). Therefore, in this experimental model, the directly spliced CD44ex10 variant promoted the dissemination of aggressive NHLs, whereas the larger exon 10–containing isoforms had no similar effect. These data provide further in vivo evidence of the unique functions of CD44ex10 and the larger exon 10–containing isoforms.
CD44ex10 Transfectants Do Not Exhibit Increased Binding to Hyaluronic Acid or Chondroitin Sulfate
To determine whether the unique properties of CD44ex10 transfectants resulted from an increased affinity to previously described major CD44 ligands, the hyaluronic acid and chondroitin sulfate binding of CD44ex10 transfectants was compared with that of the other CD44 or vector-only transfectants. Specifically, two to three independently derived vector-only, CD44H, CD44ex7-14, CD44ex10-14, and CD44ex10 transfectants were evaluated for adherence to immobilized hyaluronic acid or chondroitin sulfate A (Table 1). As indicated, the hyaluronic acid binding of CD44ex10 transfectants was similar to that of the other CD44 transfectants (Table 1). The chondroitin sulfate A binding of CD44ex10 transfectants was also comparable to that of vector-only transfectants (Table 1). Taken together, these data suggest that altered hyaluronic acid or chondroitin sulfate A binding is unlikely to explain the increased tumorigenicity and distant metastasis of CD44ex10 transfectants.
Hyaluronic Acid and Chondroitin Sulfate A Binding of Vector-Only and CD44 Transfectants
| Transfectants . | No Addition . | cpm Bound . | Chondroitin Sulfate A . | (Fold Increase) . | |
|---|---|---|---|---|---|
| Hyaluronic Acid . | (Fold Increase) . | ||||
| Vector-only | |||||
| I | 33,564 ± 854 | 38,229 ± 2204 | (1.1×) | 39,352 ± 1399 | (1.2×) |
| II | 21,034 ± 699 | 32,161 ± 1550 | (1.5×) | 36,633 ± 912 | (1.7×) |
| CD44H | |||||
| I | 30,113 ± 938 | 65,419 ± 2418 | (2.2×) | 41,669 ± 1109 | (1.4×) |
| II | 22,525 ± 576 | 79,882 ± 889 | (3.5×) | 65,398 ± 2027 | (2.9×) |
| CD44ex7-14 | |||||
| I | 27,560 ± 1727 | 101,766 ± 2358 | (3.6×) | 85,804 ± 2537 | (3.1×) |
| II | 26,912 ± 1617 | 108,794 ± 6525 | (4.0×) | 50,506 ± 569 | (1.9×) |
| CD44ex10-14 | |||||
| I | 42,789 ± 872 | 136,912 ± 9765 | (3.2×) | 37,136 ± 755 | (0.9×) |
| II | 31,673 ± 958 | 91,457 ± 847 | (2.9×) | 39,096 ± 755 | (1.2×) |
| CD44ex10 | |||||
| I | 29,523 ± 2593 | 89,353 ± 427 | (3.0×) | 38,233 ± 1657 | (1.3×) |
| II | 40,586 ± 389 | 123,748 ± 5973 | (3.0×) | 51,581 ± 608 | (1.3×) |
| III | 43,009 ± 1242 | 124,258 ± 1713 | (2.8×) | 56,236 ± 3002 | (1.3×) |
| Transfectants . | No Addition . | cpm Bound . | Chondroitin Sulfate A . | (Fold Increase) . | |
|---|---|---|---|---|---|
| Hyaluronic Acid . | (Fold Increase) . | ||||
| Vector-only | |||||
| I | 33,564 ± 854 | 38,229 ± 2204 | (1.1×) | 39,352 ± 1399 | (1.2×) |
| II | 21,034 ± 699 | 32,161 ± 1550 | (1.5×) | 36,633 ± 912 | (1.7×) |
| CD44H | |||||
| I | 30,113 ± 938 | 65,419 ± 2418 | (2.2×) | 41,669 ± 1109 | (1.4×) |
| II | 22,525 ± 576 | 79,882 ± 889 | (3.5×) | 65,398 ± 2027 | (2.9×) |
| CD44ex7-14 | |||||
| I | 27,560 ± 1727 | 101,766 ± 2358 | (3.6×) | 85,804 ± 2537 | (3.1×) |
| II | 26,912 ± 1617 | 108,794 ± 6525 | (4.0×) | 50,506 ± 569 | (1.9×) |
| CD44ex10-14 | |||||
| I | 42,789 ± 872 | 136,912 ± 9765 | (3.2×) | 37,136 ± 755 | (0.9×) |
| II | 31,673 ± 958 | 91,457 ± 847 | (2.9×) | 39,096 ± 755 | (1.2×) |
| CD44ex10 | |||||
| I | 29,523 ± 2593 | 89,353 ± 427 | (3.0×) | 38,233 ± 1657 | (1.3×) |
| II | 40,586 ± 389 | 123,748 ± 5973 | (3.0×) | 51,581 ± 608 | (1.3×) |
| III | 43,009 ± 1242 | 124,258 ± 1713 | (2.8×) | 56,236 ± 3002 | (1.3×) |
Two or three independently derived vector-only, CD44H, CD44ex7-14, CD44ex10-14, and CD44ex10 transfectants were evaluated for adherence to immobilized hyaluronic acid, chondroitin sulfate A or plastic alone (no addition). Values are mean ± SE bound cpm for triplicate samples. The increase in hyaluronic acid or chondroitin sulfate A adhesion over background adhesion (no addition) for each transfectant is also shown (fold increase). Data are derived from one of three similar experiments.
Cells Derived From CD44ex10 Local Tumors Show Increased Homotypic Aggregation In Vitro
To explore additional mechanisms for the increased local tumor take and distant metastasis of CD44ex10 transfectants, we prepared single-cell suspensions of the CD44ex10 local tumors and the less common vector-only, CD44H, CD44ex7-14, and CD44ex10-14 local tumors. Thereafter, these tumor-derived single-cell suspensions were plated, cultured, and monitored for changes in cellular morphology and aggregation in vitro (Fig 7). Cells derived from CD44ex10 local tumors exhibited dramatically increased homotypic aggregation when compared with cells derived from vector-only, CD44H, CD44ex7-14, or CD44ex10-14 local tumors (Fig 7). Taken together with the previous in vitro analyses (Fig 3), these data (Fig 7) suggest that CD44ex10 also modulates adhesion to an as yet unidentified cell-surface ligand(s) in aggressive NHLs.
Cells derived from CD44ex10 local tumors exhibit increased homotypic aggregation in vitro. Single-cell suspensions of the CD44ex10 local tumors and the less common vector-only, CD44H, CD44ex7-14, and CD44ex10-14 local tumors were prepared, plated, cultured, and monitored for changes in cellular morphology and aggregation in vitro. Cell suspensions from local tumors of representative independently derived CD44ex10 Namalwa transfectants (I or II) or vector-only, CD44H, and CD44ex7-14 transfectants are shown. As indicated, cell suspensions from CD44ex10 local tumors exhibited dramatically increased homotypic aggregation.
Cells derived from CD44ex10 local tumors exhibit increased homotypic aggregation in vitro. Single-cell suspensions of the CD44ex10 local tumors and the less common vector-only, CD44H, CD44ex7-14, and CD44ex10-14 local tumors were prepared, plated, cultured, and monitored for changes in cellular morphology and aggregation in vitro. Cell suspensions from local tumors of representative independently derived CD44ex10 Namalwa transfectants (I or II) or vector-only, CD44H, and CD44ex7-14 transfectants are shown. As indicated, cell suspensions from CD44ex10 local tumors exhibited dramatically increased homotypic aggregation.
DISCUSSION
The differential expression of CD44ex10 and larger exon 10–containing CD44 isoforms by clinically relevant subsets of aggressive B-cell lymphomas, activated B cells, and PBLs32prompted us to explore the functions of these unique CD44 variants in aggressive NHL. In initial in vitro analyses, CD44ex10 transfectants exhibited a subtle increase in homotypic aggregation (Fig 3). These CD44ex10 transfectants were also more likely to develop local tumors in nude mice than transfectants expressing the larger ex10–containing variants (CD44ex7-14 and CD44ex10-14), CD44H, or vector alone (Fig 4). Cell suspensions derived from CD44ex10 local tumors also exhibited far greater homotypic aggregation than those obtained from the other CD44 or vector-only transfectants (Fig 7). Of additional interest, distant metastases were significantly more likely to develop in nude mice injected with CD44ex10 transfectants than in animals that received either the CD44ex10-14, CD44ex7-14, CD44H, or vector-only transfectants (Fig 5). These data provide the first evidence that the major exon 10–containing CD44 variant in aggressive NHL (CD44ex10)32 has a unique biologic function. Furthermore, these studies underscore the importance of specifically identifying the relevant exon 10–containing CD44 isoforms expressed in primary aggressive NHLs.
Our observations regarding the less efficient growth of larger exon 10–containing (CD44ex7-14 and CD44ex10-14) aggressive NHL transfectants are largely consistent with previous studies.39,45 In recent analyses in SCID mice, single CD44ex7-14 (v3-10) or CD44ex10-14 (v6-10) Namalwa transfectants developed local tumors more slowly than CD44−parental cells.45 In these animals, a Namalwa transfectant expressing CD44ex10-14 (v6-10) also metastasized less efficiently than the CD44− parental cell lines, although a single Namalwa transfectant expressing CD44ex7-14 (v3-10) disseminated more rapidly.45 Both the CD44ex7-14 (v3-10) and CD44ex10-14 (v6-10) Namalwa transfectants adhered weakly to hyaluronan-coated surfaces.39 45 In our own studies there was modest variation in the tumorogenicity and metastatic potential of independently derived clones expressing specific CD44 variants or vector alone (Figs 4 and 5). This modest clonal variation emphasizes the importance of obtaining multiple independently derived CD44 or vector-only transfectants to identify consistent differences in their behavior. Our own studies also suggest that the increased metastatic potential of CD44ex10 Namalwa transfectants is unlikely to be attributable to increased hyaluronic acid or chondroitin sulfate A binding.
Although CD44ex7-14 and CD44ex10-14 aggressive NHL Namalwa transfectants have been evaluated in previous in vitro and in vivo assays,39,45 the directly spliced CD44ex10 has not been similarly examined. We focused on CD44ex10 because it was preferentially expressed by primary extranodal and widely metastatic aggressive NHLs in our earlier studies.32 The fact that CD44ex10 is also expressed by normal activated human and rodent lymphocytes32,33,35 38 prompts speculation regarding a common function of CD44ex10 in normal and malignant activated lymphocytes.
In recent studies, transgenic mice expressing CD44v4-7 (ex8-11) under the control of a T-cell promoter exhibited more rapid immune responses to T-cell mitogens and T-dependent antigens.46 These accelerated immune responses required v6/exon 10–encoded sequence because a neutralizing v6/exon 10 antibody reduced the immune responses to those of control animals.46 In additional analyses, an activating v6/exon 10 antibody promoted Ca2+ mobilization and CD3-dependent signaling of mitogen-stimulated T cells.38 Taken together, these data suggest that when CD44 v6/exon 10–encoded sequence is expressed within the appropriate context and microenvironment, it may confer ligand-dependent proliferative advantages.
For these reasons, our additional observations regarding CD44ex10-specific homotypic aggregation are of particular interest. Although the standard form of CD44 and an additional alternatively spliced variant (CD44R1 [CD44ex12-14]) have been associated with homotypic aggregation in selected settings,11 37 only the CD44ex10 aggressive NHL transfectants exhibited significantly increased aggregation in the current studies (Fig 7). Furthermore, single-cell suspensions of local CD44ex10 tumors displayed more striking homotypic aggregation than CD44ex10 transfectants maintained in vitro (Figs 5 and7). These data suggest that CD44ex10 recognizes an additional cell-surface ligand that may be upregulated in vivo and that the interaction between CD44ex10, and this ligand directly or indirectly increases the self-adhesion of aggressive NHL cells. Because CD44ex10 significantly increases the metastatic potential of aggressive NHLs in this model system, identification of the candidate CD44ex10 ligand may lead to novel therapeutic strategies.
ACKNOWLEDGMENT
We thank Donna Favreau for manuscript preparation.
Supported by National Institutes of Health Grant No. CA66996. M.A.S. is the recipient of a Leukemia Society Scholar Award.
Address reprint requests to Margaret A. Shipp, MD, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal