Abstract
Interleukin-15 receptor (IL-15R) and IL-2R have the same β and γ chains, but IL-15R has a specific α chain distinct from that of IL-2Rα, which is indispensable for the high affinity binding of IL-15. In the present study, we examined four IL-2-dependent adult T-cell leukemia (ATL) cell lines for their IL-15R expression. All cell lines bound IL-15, which was not inhibited by a 100-fold excess amount of IL-2, proliferated in response to IL-15 to the same degree as to the stimulation with IL-2, and were maintained without IL-2. The responses to 1L-15 were inhibited by the antibodies against IL-2R β or γ chains but was not by the IL-2R α chain antibody. [125I]–IL-15 exhibited a single high-affinity binding with an apparent kd of 0.17 nmol/L. Reverse transcription–coupled polymerase chain reaction (RT-PCR) showed that the cell lines had the mRNA of IL-15R α. The cell lines also had IL-15 mRNA. Despite the presence of IL-15 mRNA, the cell lines did not secrete IL-15, and the culture supernatants of fresh ATL cells and plasma from the patients did not contain a detectable amount of IL-15 with a few exceptional cases, although fresh ATL cells also responded to IL-15. These results suggest that ATL cells have the complete form of IL-15R and respond to IL-15. Such an IL-15–dependent cell proliferation mechanism might be used in the development of ATL and for the invasion and proliferation of ATL cells in the visceral organs.
INTERLEUKIN-15 (IL-15) has been observed in a culture supernatant of a simian kidney cell line (CV/EBNA) as a factor that activates T cells similarly to IL-2, which is now known to be identical to the IL-T found in the culture supernatant of the cell line HuT-102 described below.1,2 Although IL-15 mRNA is present in a wide variety of cells and tissues including monocytes, placenta, skeletal muscle, kidney, lung, and heart, the IL-15 protein product has rarely been shown in their culture supernatants, except for monocytes.1 It has been reported that T cells do not have IL-15 mRNA.1 IL-15 receptor (IL-15R) and IL-2R have β and γ chains in common,3 but IL-15R has an α chain different from that of IL-2R α, which is essential for the high-affinity binding of IL-15.4 The IL-15R α gene was recently cloned, and it was shown that a variety of cells, including T cells and T-cell lines and many tissues, have the mRNA of IL-15R α, and the expression is enhanced by activation with anti-CD3 antibody or phorbol myristate acetate (PMA) in T cells.4 Interestingly, IL-2R α binds IL-2 with low affinity,5 whereas IL-15R α binds IL-15 with high affinity.6 A high expression of IL-15R α on activated T cells is favorable for their proliferation.
Adult T-cell leukemia (ATL) is a neoplasm of mature helper/inducer T-cell origin7,8 caused by human T-cell leukemia virus type-I (HTLV-I).9-11 Although T cells usually do not produce IL-15, the unique HTLV-I+ T-cell line HuT-102 synthesizes and secretes IL-15 due to the production of a chimeric mRNA joining a segment of the R region of the long terminal repeat (LTR) of HTLV-I and the 5′-untranslated region (UTR) of IL-15.12,13 ATL cells constitutively express IL-2R α due to the transactivation by the p40tax of HTLV-I14 and respond to IL-2 in vitro. In the present study we examined whether four IL-2–dependent ATL cells lines established in our laboratory15-17 bind IL-15 and respond to IL-15. These cell lines are totally dependent on exogenous IL-2 and stop proliferating without IL-2, except for one cell line (OMT), which tends to proliferate autonomously. These cell lines responded to IL-15 to the same degree as to IL-2, and IL-15 could be replaced with IL-2 signal. Because IL-2R β transduces the IL-15 signal,18 there was a possibility that IL-15 bound IL-2R instead of IL-15R and transduced the signal. The results of our inhibition study excluded this possibility. One-hundred–fold excess amount of IL-2 did not inhibit the binding of IL-15. Although anti–IL-2R β and γ chain antibodies inhibited the proliferation induced by IL-15, anti–IL-2R α chain antibody did not. Moreover, the cell line bound [125I]-IL-15 with a single high-affinity binding and had the mRNA of IL-15R α. To our knowledge, this is the first report showing that ATL cells have the complete form of IL-15R and that the IL-2 signal can be replaced with IL-15. We also examined the production of IL-15 from the cell lines and fresh ATL cells, the response of fresh ATL cells to IL-15, and the concentration of IL-15 in plasma from ATL patients. We discussed the pathological role of IL-15 in ATL.
MATERIALS AND METHODS
Cell preparation and IL-15 assay.
Cell lines SO4, ST1, and KK1 are of true ATL cell origin, as confirmed by the concordance of the integration site(s) of the HTLV-I proviral genome and/or the T-cell receptor β chain gene rearrangement profiles with respective original leukemia cells. These cell lines have been maintained in our laboratory with 0.25 Takeda units/mL (100 Japan reference units/mL, 10 ng/mL) of recombinant IL-2 (rIL-2) (kindly provided by Takeda Chemical Industries, Osaka, Japan) for several years. OMT is a recently established cell line and shows helper/inducer T-cell phenotype, but it has not yet been established whether OMT is of true ATL cell origin or HTLV-I–infected normal T-cell origin. SO4, ST1, and KK1 are totally dependent on exogenous IL-2 and stop proliferating without IL-2 and die. OMT tends to proliferate autonomously and is able to survive without IL-2 for several weeks.
Heparinized peripheral blood was drawn from acute or chronic ATL patients, and their mononuclear cells were used as fresh ATL cells. Morphological and marker analyses indicated that the extents of ATL cells in these samples were always more than 80%. Plasma was collected from the same patients. All materials from the patients were obtained after informed consent. The cell lines and fresh ATL cells were cultured with or without rIL-2 at a cell density of 2 × 106/mL for 24 hours, and the culture supernatants were collected. The supernatants and plasma from ATL patients were examined for the concentration of IL-15 protein. The concentration of IL-15 was estimated by an enzyme-linked immunosorbent assay using a IL-15 detection Kit (Quantikine; R&D Systems, Minneapolis, MN). The limit of the detection kit is 1 pg/mL. The culture supernatant of the HuT-102 cell line (which is known to produce IL-15 protein12) was used as a positive control.
Cell proliferation assay.
The cell lines were washed extensively with fresh medium before culturing to remove contaminated IL-2. Then, 1 × 104cells/100 μL were cultured with rIL-2 (0.25 U/mL) or rIL-15 (10 ng/mL; Pharmingen, San Diego, CA) in 96-well tissue culture plates for 48 hours, and the proliferation status of each cell line was estimated by measuring the conversion of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) into water-soluble formazan (CellTiter 96TMAQueous; Promega, Madison, WI). The effects of monoclonal antibodies (MoAbs) against IL-15 (Genzyme Diagnostics, Cambridge, MA), IL-2R α chain (Pharmingen), IL-2R β chain (Pharmingen), or IL-2R γ chain (Sumitomo Electric Industry, Yokohama, Japan) on the proliferation of these cell lines were also examined by the same method. In the case of IL-2R γ antibody, sodium azide added as a preservative was removed by extensive dialysis of the antibody with phosphate-buffered saline (PBS). MoAbs were used at 10 μg/mL of concentration. In the case of fresh ATL cells, the cells were cultured at a cell density of 1 × 105 cells/100 μL with IL-2 or IL-15 for up to 4 days and their proliferation status was compared with the cells cultured without these cytokines. All experiments were performed in triplicate.
Cytofluorometric analysis of the expressions of IL-2R and IL-15R.
The binding of IL-15 on the surface of the ATL cell lines was examined by an indirect immunofluorescence assay. Cells incubated with rIL-15 (50 ng/mL) or PBS as a negative control for 30 minutes at 4°C were examined. Anti–IL-15 antibody (mouse IgG) was used as the first antibody and FITC-conjugated goat anti-mouse IgG antibody (Ortho Diagnostic Systems, Raritan, NJ) as a second antibody. The labeled cells were processed by flow cytometry. To study the inhibition of IL-2 on IL-15 binding, the same assay was done in the presence of 100-fold excess amount of IL-2 (5 μg/mL). The surface expressions of IL-2R α, β, and γ chains were also examined likewise using the antibodies used for the inhibition study.
IL-15 binding assay.
IL-15 was radio-iodinated by the Bolton-Hunter method according to the manufacturer's instructions. In brief, 5 μg of rIL-15 was labeled with 100 μCi of Bolton-Hunter reagent (BHR: NEN; Life Science Products, Boston, MA) in 20 mmol/L K-phosphate buffer (pH 8.0) for 60 minutes on ice. Then, 1 mmol/L of glycine was added to terminate the reaction. The labeled IL-15 was separated from the unreacted BHR and glycine by an ultrafiltration membrane UFC3LCC (Millipore, Bedford, MA). The radiospecific activity of the [125I]-IL-15 was 432 cpm/fmol. ST1 cells, 5 × 105, were incubated for 60 minutes on ice with various concentrations of [125I]-IL-15 (10 pmol/L to 1.25 nmol/L) in the absence (total binding) or presence of a 200-fold excess amount of unlabeled IL-15 (nonspecific binding) in a total volume of 150 μL. Specific binding was calculated by subtracting nonspecifically bound counts per minute from total bound counts per minute. All experiments were performed in triplicate. Dissociation constants (kd) were calculated from the binding data by Scatchard plot analysis.
Reverse transcription-coupled polymerase chain reaction (RT-PCR).
Total RNA was extracted from the four cell lines, fresh ATL cells, the HL-60 myeloid leukemia cell line as a control, and the resting or activated peripheral blood mononuclear cells (PBMC) of healthy adults by the ISOGEN kit (Nippon Gene, Toyama, Japan) and treated with DNase (Message CleanTM Kit; GenHunter, Nashville, TN) to remove contaminated DNA. PBMC were activated by Concanavalin A (Con A; Sigma Chemical Co, St Louis, MO) at a concentration of 10 μg/mL for 48 hours. RT-PCR was performed according to the manufacturer's directions (GeneAmp RNA PCR Kit; Perkin-Elmer, Foster, CA). cDNA was synthesized from 1 μg of RNA by incubation with Moloney murine leukemia virus RT (2.5 U/μL) and 2.5 μmol/L oligo d(T)16 primer for 20 minutes at 42°C. The reaction was stopped by heating the sample to 99°C for 5 minutes. Two microliters of the cDNA obtained was amplified by 35 cycles (for IL-15) or 30 cycles (for IL-15R) with AmpliTaq DNA polymerase. The reaction conditions were 94°C for 30 seconds, 55°C (for IL-15) or 60°C (for IL-15R α) for 30 seconds, and 72°C for 60 seconds. The oligonucleotide primers used were as follows: for IL-15: TAAAACAGAAGCCAACTG (615-632) and CAAGAAGTGTTGATGAACAT (952-971); for IL-15R α: GTCAAGAGCTACAGCTTGTAC (218-238) and GGTGAGCTTTCTCCTGGAG (977-995).19 The expected amplification product sizes of IL-15 and IL-15R α are 357 and 778 bp, respectively. β-Actin was used as a control for the RT-PCR product.
Southern blot.
Because the RT-PCR of IL-15R yielded a few products of unexpected sizes (besides 778 bp), Southern blotting was performed for confirmation of their IL-15R α origin. The PCR products were separated on 2% agarose gels, denatured, and transferred onto a positively charged nylon membrane (Boehringer Mannheim, Mannheim, Germany), and probed with a digoxigenin-labeled internal oligonucleotide, GCTGTGTTGTTTGAGCTGG.19 The membrane was treated with an anti-digoxigenin antibody conjugated with alkaline phosphatase (AP)(Boehringer Mannheim), and the AP was changed to a light signal by CDP-StarTM (Boehringer Mannheim) and exposed to film.
RESULTS
Response of ATL cell lines to IL-2 and IL-15.
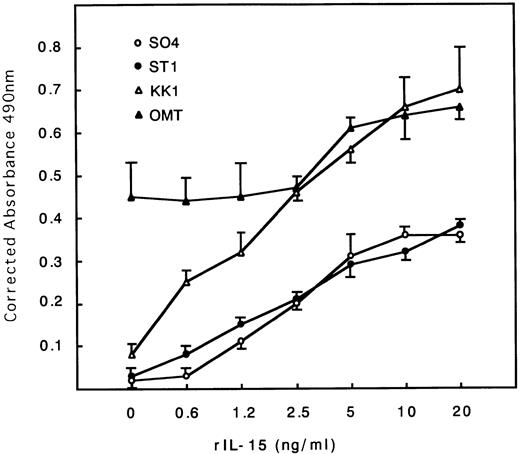
The cell proliferation status of each cell line cultured with or without IL-2 (0.25 U/mL) or IL-15 (10 ng/mL) for 48 hours was estimated. All cell lines except OMT, which tends to proliferate autonomously, stopped proliferation and almost died at 48 hours without IL-2 or IL-15 (Table 1). All cell lines including OMT responded to IL-15 stimulation, proliferated, and were maintained for months with IL-15 alone (data not shown). The extent of the responses to IL-15 were somewhat less than those to IL-2, especially in the SO4 cell line (Table 1). The dose-response curves for IL-15 indicated that the maximum response was obtained at around 10 to 20 ng/mL concentrations in all cell lines (Fig 1). The KK1 cell line responded the most strongly to IL-15 and proliferated at a concentration as little as 0.6 ng/mL.
Response of ATL Cell Lines to IL-2 and IL-15
| Cell Line . | Corrected Absorbance at 490 nm . | ||
|---|---|---|---|
| Nil . | rIL-2 . | rIL-15 . | |
| SO4 | 0.012 ± 0.008 | 0.605 ± 0.013 | 0.350 ± 0.009 |
| ST1 | 0.045 ± 0.012 | 0.826 ± 0.048 | 0.749 ± 0.010 |
| KK1 | 0.054 ± 0.010 | 0.691 ± 0.011 | 0.505 ± 0.067 |
| OMT | 0.302 ± 0.027 | 0.660 ± 0.020 | 0.505 ± 0.036 |
| Cell Line . | Corrected Absorbance at 490 nm . | ||
|---|---|---|---|
| Nil . | rIL-2 . | rIL-15 . | |
| SO4 | 0.012 ± 0.008 | 0.605 ± 0.013 | 0.350 ± 0.009 |
| ST1 | 0.045 ± 0.012 | 0.826 ± 0.048 | 0.749 ± 0.010 |
| KK1 | 0.054 ± 0.010 | 0.691 ± 0.011 | 0.505 ± 0.067 |
| OMT | 0.302 ± 0.027 | 0.660 ± 0.020 | 0.505 ± 0.036 |
Washed 1 × 104 cells/100 μL were cultured with rIL-2 or rIL-15 in 96-well tissue-culture plate for 48 hours, and the proliferation status of each cell line was estimated.
Dose-response curves of IL-15–induced proliferation of ATL cell lines. Cells were cultured with different concentrations of IL-15. All cells responded to IL-15, with the maximum responses at around 10 to 20 ng/mL. KK1 responded at a concentration as little as 0.6 ng/mL and proliferated the most strongly.
Dose-response curves of IL-15–induced proliferation of ATL cell lines. Cells were cultured with different concentrations of IL-15. All cells responded to IL-15, with the maximum responses at around 10 to 20 ng/mL. KK1 responded at a concentration as little as 0.6 ng/mL and proliferated the most strongly.
Cytofluorometric analysis of the IL-15 binding on ATL cell lines.
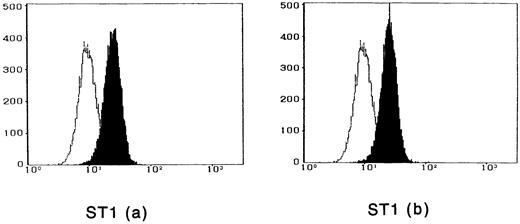
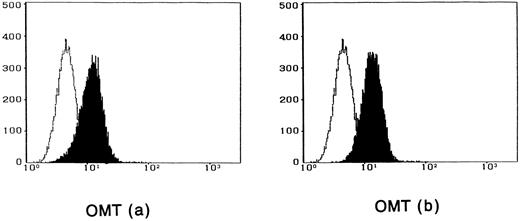
To observe the binding of IL-15 on the cell surface, cells were incubated with IL-15 and the cell-surface–bound IL-15 was detected by the anti–IL-15 antibody. All four ATL cell lines bound enough IL-15 to be visualized by flow cytometry, and the binding was not inhibited by a 100-fold excess amount of IL-2 (Fig 2). The anti–IL-15 antibody inhibited the IL-15–induced cell proliferation but did not inhibit the IL-2–induced cell proliferation (Fig 3), suggesting that the antibody blocks the binding site of IL-15 to the IL-15R. The inhibition of the proliferation was minimal on the OMT cell line because of its autonomous proliferative ability.
Cytofluorometric analysis of the binding of IL-15 on ATL cell lines. After incubation of the cell lines with IL-15 in the absence (a) or presence (b) of 100-fold excess amount of IL-2, the cell-surface–bound IL-15 was detected by an anti–IL-15 MoAb (closed histogram). All cell lines had almost the equivalent amounts of the IL-15 binding sites, which were not inhibited by IL-2. Open hitograms indicate the cells incubated with a control MoAb.
Cytofluorometric analysis of the binding of IL-15 on ATL cell lines. After incubation of the cell lines with IL-15 in the absence (a) or presence (b) of 100-fold excess amount of IL-2, the cell-surface–bound IL-15 was detected by an anti–IL-15 MoAb (closed histogram). All cell lines had almost the equivalent amounts of the IL-15 binding sites, which were not inhibited by IL-2. Open hitograms indicate the cells incubated with a control MoAb.
Inhibition of the IL-15–induced proliferation of ATL cell lines by anti–IL-15 antibody. Anti–IL-15 antibody inhibited the proliferation induced by IL-15 but did not inhibit the proliferation induced by IL-2. The extent of the inhibition was the lowest in OMT cell line.
Inhibition of the IL-15–induced proliferation of ATL cell lines by anti–IL-15 antibody. Anti–IL-15 antibody inhibited the proliferation induced by IL-15 but did not inhibit the proliferation induced by IL-2. The extent of the inhibition was the lowest in OMT cell line.
Inhibition of the IL-15-induced proliferation of ATL cell lines by anti–IL-2R α, β, and γ chain antibodies.
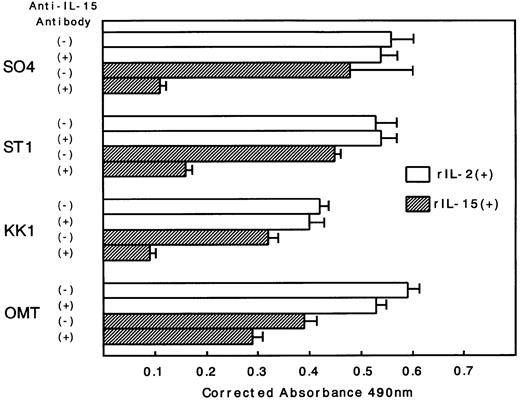
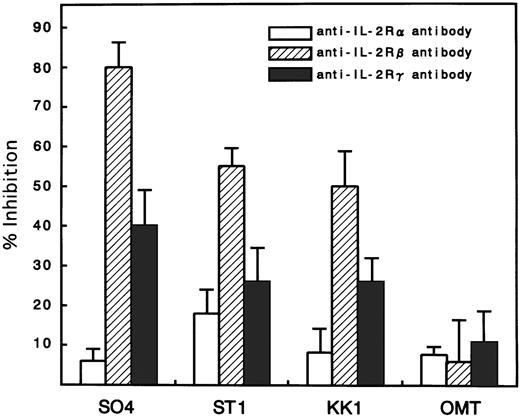
Because there was a possibility that IL-15 used IL-2R instead of IL-15R for the cell-surface binding and signaling for proliferation, an inhibition study using antibodies against IL-2R α, β, and γ chains was performed. All cell lines expressed the α, β, and γ chains, with the strongest expression of α, medium expression of γ, and minimum expression of β (Fig 4). Antibodies against the β and γ chains significantly inhibited the IL-15–induced proliferation with the strongest inhibition by anti-β chain antibody (Fig 5). In contrast, the antibody against the α chain had little effect on the proliferation, suggesting that the binding of IL-15 occurred through IL-15R but not through IL-2R. The proliferation of OMT was not significantly inhibited by any of the antibodies.
Expression of IL-2R α, β, and γ chains on the surface of ATL cell lines. All cell lines expressed the components of IL-2R, with the strongest expression of α, medium expression of γ, and minimum expression of β. Closed histograms indicate the cells incubated with MoAbs against each component of IL-2R. Open histograms indicate the cells incubated with a control MoAb.
Expression of IL-2R α, β, and γ chains on the surface of ATL cell lines. All cell lines expressed the components of IL-2R, with the strongest expression of α, medium expression of γ, and minimum expression of β. Closed histograms indicate the cells incubated with MoAbs against each component of IL-2R. Open histograms indicate the cells incubated with a control MoAb.
Inhibition of IL-15–induced proliferation of ATL cell lines by anti–IL-2R α, β, or γ chain antibodies. The percent inhibition was based on a comparison with cells cultured in IL-15 alone. The anti-β and γ chain antibodies significantly inhibited the proliferation induced by IL-15, with the maximum inhibition of anti-β. The anti-α chain antibody did not inhibit the proliferation. The extent of the inhibition was the smallest in the OMT cell line.
Inhibition of IL-15–induced proliferation of ATL cell lines by anti–IL-2R α, β, or γ chain antibodies. The percent inhibition was based on a comparison with cells cultured in IL-15 alone. The anti-β and γ chain antibodies significantly inhibited the proliferation induced by IL-15, with the maximum inhibition of anti-β. The anti-α chain antibody did not inhibit the proliferation. The extent of the inhibition was the smallest in the OMT cell line.
IL-15 binding assay.
A binding experiment of [125I]-IL-15 was performed using the ST1 cell line. Analysis of the data by the method of Scatchard plot showed that [125I]-IL-15 bound to ST1 cells with an apparent kd of 0.17 nmol/L, a single high-affinity binding (Fig 6). Maximal binding capacity (Bmax) was 0.61 fmol/5 × 105 cells (732 sites/cell).
Binding of [125I]-IL-15 to ST1 cell line.Bmax and kd were determined by the method of Scatchard plot (inset) using 5 × 105 cells. ST1 showed a single high affinity binding for IL-15.
Binding of [125I]-IL-15 to ST1 cell line.Bmax and kd were determined by the method of Scatchard plot (inset) using 5 × 105 cells. ST1 showed a single high affinity binding for IL-15.
Expression of the mRNA of IL-15R α and IL-15.
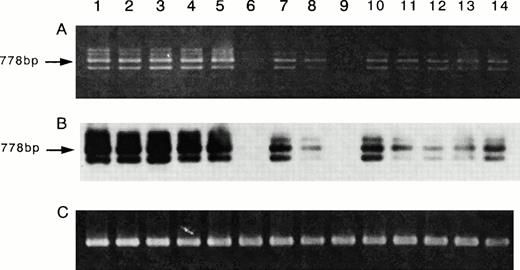
Because the inhibition studies and [125I]-IL-15 binding study suggested the presence of the IL-15R α chain on ATL cell lines, a semiquantitative RT-PCR analysis for the mRNA of IL-15R α was performed. The ATL cell lines and activated PBMC had larger amounts of IL-15R mRNA than those of the fresh ATL cells or resting PBMC (Fig 7A). The myeloid leukemia cell line HL-60 and one sample of fresh ATL cells did not have IL-15R α mRNA. Because the RT-PCR of IL-15R α yielded several unexpected products, a Southern blot analysis was performed for a confirmation of the origin of the products, using an internal oligo probe. The RT-PCR products hybridized with the probe (Fig 7B), suggesting that the bands with sizes other than 778 bp are alternatively spliced isoforms of IL-15R α. Using the same cells, the RT-PCR of IL-15 was also performed. The four ATL cell lines, the fresh ATL cells, and the normal PBMC expressed the mRNA of IL-15, but the HL-60 cell line did not (Fig 8). The HuT-102 cells expressed much more of the RT-PCR product of IL-15 than did the four ATL cell lines (data not shown).
Expression of the mRNA of IL-15R α on ATL cell lines. RT-PCR for IL-15R α mRNA (A) and Southern blot analysis of the products (B), and RT-PCR for β-actin mRNA as a control of PCR products (C) were performed. The results indicated that all four ATL cell lines (lane 2, SO4; lane 3, ST1; lane 4, KK1; lane 5, OMT) had the mRNA of IL-15R α (778 bp). Three samples of fresh ATL cells (lanes 7, 8, and 10) had smaller amounts of the mRNA equivalent to those of the resting normal PBMC (lanes 11 through 14). One sample of fresh ATL cells (lane 9) and the HL-60 cells (lane 6) did not have the IL-15R α mRNA. The activated normal PBMC (lane 1) had an amount of the mRNA larger than that of the resting PBMC.
Expression of the mRNA of IL-15R α on ATL cell lines. RT-PCR for IL-15R α mRNA (A) and Southern blot analysis of the products (B), and RT-PCR for β-actin mRNA as a control of PCR products (C) were performed. The results indicated that all four ATL cell lines (lane 2, SO4; lane 3, ST1; lane 4, KK1; lane 5, OMT) had the mRNA of IL-15R α (778 bp). Three samples of fresh ATL cells (lanes 7, 8, and 10) had smaller amounts of the mRNA equivalent to those of the resting normal PBMC (lanes 11 through 14). One sample of fresh ATL cells (lane 9) and the HL-60 cells (lane 6) did not have the IL-15R α mRNA. The activated normal PBMC (lane 1) had an amount of the mRNA larger than that of the resting PBMC.
Expression of the mRNA of IL-15 on the ATL cell lines. Using the same RNA used for IL-15R α, the RT-PCR for IL-15 mRNA was done. The results showed that the four ATL cell lines (lane 2, SO4; lane 3, ST1; lane 4, KK1; lane 5, OMT), the fresh ATL cells (lanes 7 through 10), the activated PBMC (lane 1), and the resting PBMC (lanes 11 through 14) had almost identical amounts of IL-15 mRNA. The activation of PBMC did not cause an increase of the mRNA. HL-60 (lane 6) did not have any IL-15 mRNA.
Expression of the mRNA of IL-15 on the ATL cell lines. Using the same RNA used for IL-15R α, the RT-PCR for IL-15 mRNA was done. The results showed that the four ATL cell lines (lane 2, SO4; lane 3, ST1; lane 4, KK1; lane 5, OMT), the fresh ATL cells (lanes 7 through 10), the activated PBMC (lane 1), and the resting PBMC (lanes 11 through 14) had almost identical amounts of IL-15 mRNA. The activation of PBMC did not cause an increase of the mRNA. HL-60 (lane 6) did not have any IL-15 mRNA.
Response of fresh ATL cells to IL-15.
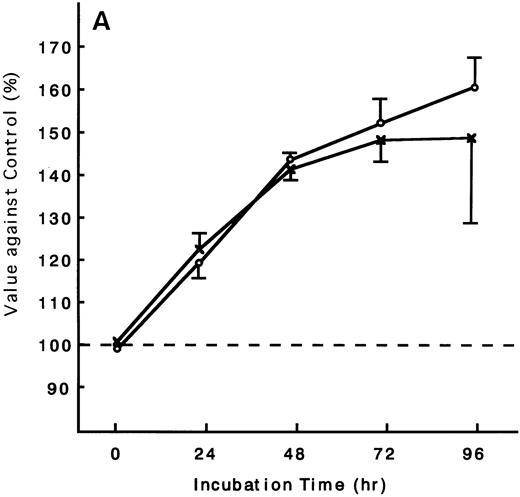
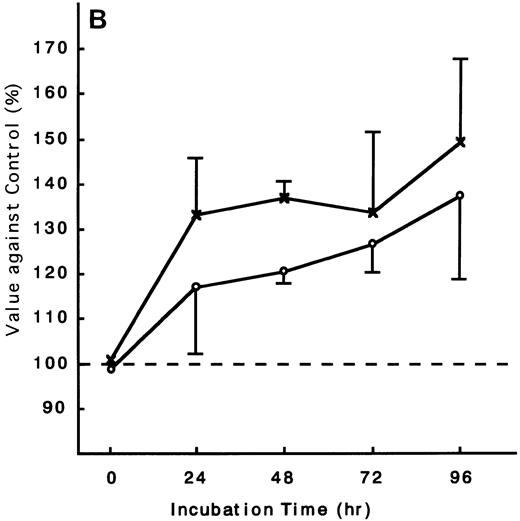
Using the ATL cells from peripheral blood, the response of fresh ATL cells to IL-15 was examined. ATL cells of all patients examined except one responded to IL-15 to the same degree as they did to IL-2 (Fig 9A through C). ATL cells of one patient did not respond to any of the IL-15 or IL-2 (Fig 9D).
Response of fresh ATL cells to IL-15. ATL cells from seven patients were examined for their response to IL-15 (×) or IL-2 (○). The results were expressed as relative values against control cultured without these cytokines. The results of a representative four cases were shown. ATL cells of all patients (A, B, and C) but one (D) responded to IL-15 to the same degree as to IL-2.
Response of fresh ATL cells to IL-15. ATL cells from seven patients were examined for their response to IL-15 (×) or IL-2 (○). The results were expressed as relative values against control cultured without these cytokines. The results of a representative four cases were shown. ATL cells of all patients (A, B, and C) but one (D) responded to IL-15 to the same degree as to IL-2.
Concentration of IL-15 in the culture supernatants of ATL cell lines, fresh ATL cells, and plasma from ATL patients.
Because the cell lines and fresh ATL cells expressed the mRNA of IL-15, the concentrations of IL-15 in their culture supernatants were examined, as was the plasma from ATL patients. None of the culture supernatants from the ATL cell lines contained a detectable amount of IL-15 (the detection limit was 1 pg/mL) (Table 2). The samples from fresh ATL cells without stimulation also did not contain IL-15, but one sample from the fresh ATL cells stimulated with IL-2 contained 1.1 pg/mL of IL-15, although it is not known whether the source of the IL-15 was ATL cells or contaminated normal monocytes. Three of the 31 plasma samples from the ATL patients examined contained 4.9, 1.7, and 1.7 pg/mL of IL-15. The culture supernatant from the HuT-102 cell line as a positive control contained 118 pg/mL of IL-15.
Concentration of IL-15 in Culture Supernatants and Plasma
| Culture Supernatant or Plasma . | IL-15 Concentration (pg/mL) . |
|---|---|
| Culture supernatant from cell lines | |
| HuT-102 | 118 |
| SO4 | <1.0 |
| ST1 | <1.0 |
| KK1 | <1.0 |
| OMT | <1.0 |
| Culture supernatant from fresh ATL cells (n = 17) | |
| Cultured without IL-2 | <1.0 (n = 17) |
| Cultured with IL-2 | <1.0 (n = 16), 1.1 |
| Plasma from ATL patients (n = 31) | <1.0 (n = 28), 4.9, 1.7, 1.7 |
| Culture Supernatant or Plasma . | IL-15 Concentration (pg/mL) . |
|---|---|
| Culture supernatant from cell lines | |
| HuT-102 | 118 |
| SO4 | <1.0 |
| ST1 | <1.0 |
| KK1 | <1.0 |
| OMT | <1.0 |
| Culture supernatant from fresh ATL cells (n = 17) | |
| Cultured without IL-2 | <1.0 (n = 17) |
| Cultured with IL-2 | <1.0 (n = 16), 1.1 |
| Plasma from ATL patients (n = 31) | <1.0 (n = 28), 4.9, 1.7, 1.7 |
Cells were cultured with or without rIL-2 at a cell density of 2 × 106 cells/mL for 24 hours, and the culture supernatants were collected.
DISCUSSION
Although many cytokine receptors have components in common (eg, IL-3R, granulocyte-macrophage colony-stimulating factor receptor [GM-CSFR], and IL-5R have the same β chain, and IL-2R, IL-4R, IL-7R, IL-9R, and IL-13R have a common γ chain), these components may trigger different functions in cells. This suggests that at least some of the consequences of signals are not dependent on the pathway but are rather related to the binding cytokine. IL-15R is unique in that it has two subunits in common with IL-2R, the β and γ chains, which are essential for the signal transduction of IL-2.18,20Consequently, IL-15 mimics the signal of IL-2, stimulates the proliferation of activated T cells,1,2 induces cytotoxic T lymphocytes (CTL) and lymphokine-activated killer (LAK) activity,2,21 and activates natural killer (NK) cells,22 which are all functions of IL-2. The biological activities of IL-15 are not, however, totally redundant to those of IL-2. Although the murine cell line 32D has the β and γ chains of IL-2R and responds to IL-2, it does not respond to IL-15.3 Similarly, although neutrophils express the β and γ chains of IL-2R and are activated by IL-15, they are not activated by IL-2.23 IL-15 and IL-2 differentially affect the differentiation of bipotential T/NK progenitors. IL-15 is a 10 to 50 times more potent inducer of the differentiation of NK cells compared with IL-2.24 25 Such differences in activities seem to be based on the difference of α chains between IL-15R and IL-2R.
In the present study, we showed that the signal for cell proliferation transduced by IL-2 can be replaced with IL-15 in IL-2–dependent ATL cell lines. The maximum proliferation was obtained with as little as 10 ng/mL of IL-15 in all cell lines. However, because the β and γ chains of IL-2R also have some affinity for IL-15, there was a possibility that the IL-15 used IL-2R instead of IL-15R to produce the proliferation. The possibility was disproved by the inhibition study. In cytofluorometric analysis, excess amount of IL-2 did not inhibit the binding of IL-15. Although the anti–IL-2R β and γ chain antibodies inhibited the proliferation of the ATL cell lines induced by IL-15, the anti–IL-2R α chain antibody did not. In addition, Scatchard plot analysis of [125I]-IL-15 binding showed that ST1 cell line had a single high-affinity binding site, which indicates the presence of IL-15R α. The presence of the mRNA of IL-15R α chain in these cell lines also supports the notion that the complete form of IL-15R does exist on the surface of ATL cells. The antibody against IL-2R β was the most effective for inhibition of IL-15–induced proliferation. The result may indicate that the β chain is the most important for the signal transduction of IL-15, as has already been pointed out in a study of B-cell proliferation.26 It has also been reported that IL-15 upregulates the IL-2R α chain but downregulates the high-affinity binding site of IL-15 and rapidly reduces the responsiveness to IL-1527; in addition, the binding affinity of IL-15 with the IL-2R β and γ chains is less stable than that of IL-2.28 However, the responsiveness of the four present ATL cell lines to IL-15 was not transient; these cell lines were maintained by IL-15 for more than several months (data not shown). The fresh ATL cells from three of the four patients examined also had IL-15R α mRNA, although the amounts were smaller than those of the cell lines. The finding that cells from one patient did not have the mRNA may indicate that the expression of IL-15R α on fresh ATL cells differs among ATL patients. This point should be examined more accurately when an antibody against IL-15R α becomes available.
Despite the similarity of the biological functions of IL-2 and IL-15, they have no sequence homology. Moreover, the sources of the two cytokines are quite different. IL-2 is secreted preferentially from T cells.5 The mRNA of IL-15 is detectable in cells and tissues such as placenta, kidney, skeletal muscle, lung, liver, fibroblasts, and monocytes.1 A recent study indicated that the mRNA of IL-15 was also present in T-cell lines.27 The present four ATL cell lines also had IL-15 mRNA. However, the production and secretion of IL-15 protein is tightly regulated at the level of translation by the native leader sequence, and consequently IL-15 protein has rarely been detected in most cells and cell lines, including T cells and T-cell lines.29 This regulation is invalidated in HuT-102 cells by the insertion of the R region of the LTR of HTLV-I at AUG codons, a putative suppresser region for translation upstream from the major ORF of the IL-15 gene.12 13 In the present study, no IL-15 protein was detected in the culture supernatants from the ATL cell lines. None of the culture supernatants from fresh ATL cells contained a detectable amount of IL-15 without stimulation. Only one sample from fresh ATL cells stimulated with IL-2 contained a marginal amount of IL-15. Although three plasma samples contained IL-15, the concentrations were not enough to stimulate the proliferation of ATL cell lines. These results seem to deny the possibility that the proliferation of ATL cells in vivo is regulated by an autocrine mechanism of IL-15.
When we consider the pathological role of IL-15 in the clinical manifestation of ATL, the report of the expression of IL-15 in human skin that is upregulated by ultraviolet B radiation30 is quite suggestive. ATL is well known to show frequent skin invasion. Because IL-15 functions as a chemoattractant for human T cells,31,32 IL-15 produced by the skin may be responsible for the skin involvement in ATL. Such a local production of IL-15 in various tissues may explain the frequent multi-organ involvement in ATL, such as in the lungs,33 liver,34gastrointestinal tract,35 and central nervous system.36 An unknown mechanism that tightly regulates the translation of IL-15 mRNA may be released locally, and the secreted IL-15 may stimulate ATL cells in tissue. Indeed, fresh ATL cells were stimulated for proliferation by IL-15 as shown in the present study. IL-15 also stimulates other lymphoid leukemia cells. It has been shown that IL-15 promotes the proliferation and differentiation of preactivated normal B cells.26 This effect is not limited to normal B cells, but IL-15 stimulates the proliferation of freshly isolated leukemic B cells from chronic lymphocytic leukemia and hairy cell leukemia patients37; however, this stimulation is thought to take place through the IL-2R system and not through the IL-15R system. The proliferation of granular lymphocytes (GL) in patients with lymphoproliferative disease of granular lymphocytes (LDGL) are also stimulated by IL-15.38 Interestingly, GL express the mRNA of IL-15R α, as did the present ATL cell lines, but do not express the mRNA of IL-15, in contrast to our ATL cell lines. Because a variety of cells express the mRNA of IL-15, IL-15 protein produced under specific unknown conditions may trigger and promote leukemic cell growth in vivo.
In conclusion, we showed that the four IL-2–dependent ATL cell lines used expressed a probably complete form of IL-15R with its own α chain, had the mRNA of IL-15, and responded to exogenous IL-15. The proliferation signal induced by IL-2 was replaceable with an IL-15 signal. Because peripheral blood ATL cells from patients also responded to IL-15, IL-15–mediated growth signal may be used for the development of ATL and the invasion and proliferation of ATL cells in tissue.
ACKNOWLEDGMENT
We thank Dr Masahiro Mori (Department of Microbiology and Molecular Pathology, Faculty of Pharmaceutical Sciences, Teikyo University, Kanagawa, Japan) for his helpful suggestions in the radio-iodination of IL-15.
Address reprint requests to Yasuaki Yamada, MD, Department of Laboratory Medicine, Nagasaki University School of Medicine, 1-7-1 Sakamoto-machi, Nagasaki 852, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.




![Fig. 6. Binding of [125I]-IL-15 to ST1 cell line.Bmax and kd were determined by the method of Scatchard plot (inset) using 5 × 105 cells. ST1 showed a single high affinity binding for IL-15.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/11/10.1182_blood.v91.11.4265/4/m_blod41106006x.jpeg?Expires=1769129106&Signature=toju1g~F-Xcd41~3VGddce434M4F0A8Y5TiA924xEN~g-LKTyhWDRW2r4rjtMlBSHhCxkTqNKgwGuGZeKhl0gjy84ikqwALEtW3tdbvfNE8TOdheYgZjYYGRkewwhLU1XP0h~G5P5s9rAe4kU7wWoIG2VRyluzz4moi~oW1RQc8hxQvZN6g3TqDybhVMcT55ZBYj7EMtegVsW5UDE1gnarcjYwhdl5U7wjSkfjx1pec9tzu3Q5FOBi7qQsqWXQBPQxROD~1uzMsoY-m4w91XC52m5~W1yAYxF-hyAZGwgaEeRUhqR-gQhN4cra5kzhzwVViXywyZR2ZTQ6L3sRrOXg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal