Abstract
Antigen-presenting cells are thought to modulate the development of Th1 and Th2 cells by the secretion of interleukin-10 (IL-10) and IL-12. Because glucocorticoids (GC) favor the development of Th2 responses, we determined whether dexamethasone (DEX) and hydrocortisone (HC) have differential effects on lipopolysaccharide-induced IL-10 and IL-12 production in whole-blood cultures. Significant inhibition of IL-12(p40) and IL-12(p70) was found with 10−8 mol/L and 10−9 mol/L DEX respectively, whereas IL-10 was relatively insensitive or even stimulated. Accordingly, the expression of IL-12(p40) and IL-12(p35) mRNA was more sensitive to DEX than IL-10 mRNA. The glucocorticoid receptor (GR) antagonist RU486 enhanced IL-12 production and largely abrogated the inhibition of IL-12 by GC, indicating that this suppression was mainly GR-mediated. High concentrations of RU486 were inhibitory for IL-10, suggesting that GC may exert a positive effect on IL-10. In the presence of neutralizing anti–IL-10 antibodies, DEX was still capable of IL-12 suppression whereas RU486 still enhanced IL-12 production, indicating that GC do not modulate IL-12 via IL-10 exclusively. Taken together these results indicate that GC may favor Th2 development by differential regulation of IL-10 and IL-12.
IT IS WELL-ESTABLISHED that at least two types of T-helper cells are involved in immunoregulation. Th1 cells are supposed to dominate in the regulation of cellular immunity, whereas Th2 cells regulate humoral immunity.1 During a normal immune response both Th1 and Th2 cell types are involved in a crossregulatory fashion. It is suggested that an imbalance between these subsets contributes to the development of disease: a strong Th2 response is thought to play a role in allergic diseases and antibody-mediated autoimmune diseases, whereas a dominating Th1 response might contribute to the development of cell-mediated autoimmune diseases.2,3 The development of Th precursor cells into either Th1 or Th2 cells is dependent on a variety of cytokines. The presence of IL-4 during a developing immune response has been shown to favor Th2 responses.4 On the other hand, IL-12 has been shown to be a crucial factor in the development of Th1 responses.5,6 Therefore, the type of antigen-presenting cell (APC) may be one of the major determinants in the differentiation of naive CD4+ T cells toward Th1 or Th2 cells. Recently it has been shown that human monocytes may be heterogeneous, evidenced by the fact that CD14+/CD16+ cells do not express mRNA for IL-10 in response to lipopolysaccharide (LPS) compared with CD14+/CD16− cells.7 This observation is of importance in view of the fact that IL-10 suppresses IL-12.8 The development of Th1 and Th2 cells may also depend on the activation state of APC, eg, the ability to secrete prostaglandin E2, which was found to suppress IL-12 production and inhibit Th1 cells.9 10
Most likely, glucocorticoids (GC) also play an important role in directing CD4+ T-cell responses. In the mouse it has been shown that dexamethasone (DEX) preferentially suppressed IL-2 and not IL-4, products of Th1 and Th2 cells, respectively.11 Using rat CD4+ T cells, it was found that GC favor Th2 development.12 Also, in humans GC have selective effects on CD4+ T-cell subsets13 which appear to depend on the activation pathway.14 Addition of GC during restimulation of primed human naive CD4+ T cells stimulates IL-4 and IL-10 production and suppresses IL-5 and interferon-γ (IFN-γ) production.15 Accordingly, the synthesis of polyclonal IgE is increased in the presence of GC in vitro.16-18
The selective effect of GC on the Th1-Th2 balance is supported by the in vivo observation that GC play an important role in the development of experimental allergic encephalomyelitis (EAE).19 Lewis rats, which are susceptible for EAE, show an impaired production of GC upon stressful events.20 Moreover, because the relatively resistant PVG rat becomes sensitive to EAE induction after adrenalectomy,21 it is likely that the development of autoimmunity may be related to the integrity of the hypothalamus-pituitary-adrenal (HPA)-axis. This possibility is supported by the observation that patients suffering from reumatoid arthritis display decreased levels of GC as a result of an impaired functioning of the HPA-axis.22
Because the functional characteristics of APC may determine the nature of a developing immune response and since GC seem to favor the development of Th2 responses, the aim of our study was to determine whether GC would have a differential effect on the production of IL-10 and IL-12. Our studies show that IL-10 and IL-12 display a different sensitivity to GC and that different mechanisms are involved in the regulation of these cytokines by GC.
MATERIALS AND METHODS
Antibodies and Reagents
Anti–IL-12 monoclonal antibodies (MoAbs) C11.79, C8.6, and 20C2 were kindly provided by Dr T van der Pouw Kraan (Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, Amsterdam, The Netherlands), J. Wormmeester (Laboratory of Cell Biology and Histology, Academic Medical Center, University of Amsterdam, The Netherlands), and Dr D.H. Presky (Hoffmann-La Roche, Nutley, NJ). These antibodies recognize both IL-12(p40) and the bioactive heterodimer p70, consisting of p40 and p35, as described previously.23
Anti–IL-10 MoAbs (JES3-9D7 and biotinylated JES3-12G8), anti-human tumor necrosis factor-α (TNF-α) MoAbs (MoAb1 and biotinylated MoAb11), and neutralizing anti-human IL-10 MoAb (JES3-9D7) were purchased from Pharmingen (San Diego, CA).
Recombinant human IL-12 was purchased from R&D systems (Abington, UK), recombinant human IL-10 was kindly provided by Dr S. Narula (Schering Plough Research Institute, Kenilworth, NJ), and recombinant human TNF-α was obtained from Pharmingen. Escherichia coli(serotype 0127:B8)-derived LPS was obtained from Sigma (St Louis, MO). The glucocorticoid-receptor (GR) antagonist RU486 (Roussel-UCLAF, Romaineville, France) and the mineralocorticoid-receptor (MR) antagonist spironolactone (Roussel-UCLAF) were a kind gift of Dr Win Sutanto (Division of Medical Pharmacology, LACDR, Leiden, The Netherlands). Recombinant IFN-γ was a kind gift of Peter van der Meide (BPRC, Rijswijk, The Netherlands). Dexamethasone, hydrocortisone, and aldosterone were purchased from Sigma. The following antibodies were used for cell sorting and assessment of cell purity: anti-CD3-FITC (Becton Dickinson, Mountain View, CA), anti–CD19-FITC (Becton Dickinson), and anti–CD14-PE (CLB, Amsterdam, The Netherlands).
Cell Cultures
Whole-blood cultures.
Whole blood was obtained from healthy volunteers by venapuncture and collected in heparinized blood collecting tubes (Becton Dickinson). The blood was 1:5 diluted in Iscoves Modified Dulbecco's Medium (IMDM) supplemented with glutamax (GIBCO, Paisley, UK), 10% fetal calf serum (FCS; Sebak, Gmbh, Aidenbach, Germany), 50 μmol/L β-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin. Fifty microliters of the diluted blood was cultured in 96-well flat-bottomed microtiterplates (Costar, Cambridge, MA) in a final volume of 200 μL per well. Cells were stimulated with 250 ng/mL LPS to induce cytokine production; IL-12(p70) was induced by stimulation with 250 ng/mL LPS in the presence of 1,000 U/mL IFN-γ.
A dose-related response to DEX or HC was studied by the addition of these hormones to the culture wells in a final concentration ranging from 10−10 mol/L to 10−5 mol/L. Stock solutions of 10 mmol/L HC, 20 mmol/L DEX, 0.1 mol/L RU486, and 0.1 mol/L spironolactone were prepared in dimethylsulfoxide (DMSO; Merck, Darmstadt, Germany) and stored at −20°C in 0.2-mL aliquots. Stock solution of aldosterone (2.86 × 10−4 mol/L) was prepared in culture medium and stored at −20°C in 0.5-mL aliquots.
In some experiments endogenous IL-10 was neutralized by the addition of 5 μg/mL anti–IL-10 antibodies. The binding of DEX and HC to the GR was blocked by simultaneous addition of 50 μmol/L or 1 μmol/L RU486 to the culture wells. The binding of HC to the MR was blocked by simultaneous addition of 1 μmol/L spironolactone to the culture wells. The whole blood was put into culture within 2 hours after venapuncture. For assessment of cytokine levels the supernatants of the cultures were obtained after 24 and 48 hours of culture and stored at −20°C.
Cultures of peripheral blood mononuclear cells (PBMC) and purified subsets.
PBMC were isolated by density centrifugation in Histopaque 1.077 (Sigma). For the isolation of subsets two experiments were performed using buffycoats from healthy donors, in which B cells and monocytes were simultaneously labeled with anti–CD19-FITC and anti–CD14-PE, and T cells were labeled with anti–CD3-FITC. Cells of interest were sorted with the use of a FACS Vantage (Becton Dickinson).
Cells were cultured in 24-well flat-bottom tissue culture plates (Costar) at a density of 1 × 106 PBMC/well, 1 × 105 B cells/well, 2 × 105 T cells/well, or 1 × 105 monocytes/well under conditions as described for whole-blood cultures.
Cytokine Assays
For the IL-12(p40) enzyme-linked immunosorbent assay (ELISA), MoAb C11.79 (2 μg/mL in 50 mmol/L NaHCO3, pH 9.5, 50 μL/well) was coated overnight at 4°C on round-bottom microtiterplates with high-binding capacity (Greiner, Nürtingen, Germany). For the IL-12(p70) ELISA plates were coated with MoAb 20C2 (2 μg/mL in 50 mmol/L NaHCO3, pH 9.5, 50 μL/well). As for all subsequent washing steps, the plates were washed six times with phosphate-buffered saline (PBS) containing 0.05% Tween-20. Subsequently the plates were blocked for 1.5 hours with 200 μL PBS containing 0.2% gelatin and 0.05% Tween-20 (PTG). After washing, 50 μL diluted biotinylated MoAb C8.6 in PTG (final concentration, 0.25 μg/mL) was added per well together with 50 μL of the undiluted samples and simultaneously incubated for 2 hours. After washing, the plates were incubated for 1 hour with 75 μL/well poly-streptavidin-horseradish peroxidase (CLB) 1:10,000 diluted in PTG. Finally, after washing, the plates were developed with 100 μL/well 0.1 mol/L 3,5,3′,5′-tetramethyl-benzidine (TMB; Merck) in 0.11 mol/L sodium acetate pH 5.5 containing 0.003% H2O2. The reactions were terminated by the addition of 50 μL of 2 mol/L H2SO4 to each well. The plates were read at 450 nm in a Biorad 3500 platereader (Biorad, Richmond, CA). Recombinant human IL-12 diluted in culture medium was used as a standard, and the standard curves ranged from 4,000 pg/mL to 15 pg/mL.
The IL-10 ELISA was performed in an identical fashion. The plates were coated with JES3-9D7 MoAb (0.5 μg/mL) and biotinylated JES3-12G8 MoAb was used in a concentration of 2 μg/mL. Recombinant human IL-10 diluted in culture medium was used as a standard. The standard curves ranged from 2,500 pg/mL to 10 pg/mL.
For the TNF-α ELISA the plates were coated with 1 μg/mL MoAb1; biotinylated MoAb11 was used in a concentration of 1 μg/mL for detection. The supernatants were tested in a fivefold dilution in culture medium. Recombinant TNF-α diluted in culture medium was used as a standard. The standard curves ranged from 5,000 pg/mL to 19 pg/mL.
Cortisol Measurement
Blood from several individual donors was collected, immediately put on ice and allowed to coagulate. The tubes were spun down for 30 minutes (3,000 rpm, 4°C); serum was collected and immediately stored at −20°C. Cortisol was measured using the fluorescent polarization immunoassay on the TDx from Abbott (Amstelveen, The Netherlands).
RNA Quantitation Using Semi-quantitative Polymerase Chain Reaction (PCR)
Whole blood was stimulated with 250 ng/mL LPS, in the absence or presence of 10−6 mol/L DEX as described above. After 4 or 20 hours of culture—conditions that were found to be optimal for IL-12(p35/p40) and IL-10, respectively—erythrocytes were lysed and mRNA was extracted from the white blood cells using RNAzol B, according to the instructions of the manufacturer (Biotecx Laboratories, Houston, TX). Two micrograms of total mRNA was reverse transcribed using a Reverse Transcription System kit (Promega, Madison, WI) using conditions ensuring optimal cDNA synthesis. cDNA as a readout of the mRNA was quantitated in a PCR using the PQB-3 vector24 as an external standard, which contains primer sequences for IL-10 and β-actin. This vector as well as the PQA-1 vector were kindly provided by Dr D. Shire (Sanofi, Labège, France). To enable quantitation of IL-12(p40) and IL-12(p35) cDNA, two complementary 40-mer sequences each encompassing a 20-mer sequence of IL-12(p40) and of IL-12(p35) were cloned into the HindIII [for IL-12(p40) and (p35) sense primers] and BamHI sites [for IL-12(p40) and (p35) antisense primers] of the PQA-1 vector.24 By means of a parallelism test, PQB-3 and PQA-1/IL-12 were verified to be amplified equally efficient as cDNA from mRNA encoding cytokines or β-actin, when amplified with IL-10, β-actin, IL-12(p40), or (p35) specific primers. cDNA was quantitated in a semi-quantitative fashion by simultaneously amplifying the cDNA in triplicate and the stepfold diluted vector as an external standard in duplicate. Amplification was performed in 50-μL reactions containing 12.5 pmol sense and antisense primer, 0.25 mmol/L dNTPs (GIBCO-BRL, Gaithersburg, MD), 1 U of Taq DNA polymerase (GIBCO-BRL), and PCR buffer II with 2.5 mmol/L MgCl2 (Perkin Elmer, Branchbury, NJ) for IL-12(p40) or (p35) primers and PCR buffer containing 50 mmol/L KCl, 10 mmol/L TRIS/HCl pH 8.3, 2 mmol/L MgCl2, and 60 ng/mL bovine serum albumin (BSA) for IL-10 and β-actin primers. For the amplification the following sense and anti-sense primers (Isogen Bioscience, Maarssen, The Netherlands) were used (given from 5′ → 3′): IL-10 sense: ATGCTTCGAGATCTCCGAGA; IL-10 antisense: AAATCGATGACAGCGCCGTA; IL-12(p40) sense: GGAGTACTCCACATTCCTAC; IL-12(p40) antisense: CCATGGCAACTTGAGAGCTG; IL-12(p35) sense: CAGCAACATGCTCCAGAAGG; IL-12(p35) antisense: CCTAGTTCTTAATCCACATC; β-actin sense: GGGTCAGAAGGATTCCTATG; and β-actin antisense: GGTCTCAAACATGATCTGGG. Cycling conditions were 30 seconds of denaturation at 96°C, 1 minute of annealing at 55°C, and 1 minute of elongation at 72°C during 30 cycles for β-actin and 35 cycles for the cytokines.
PCR products were stained on 1% agarose gels with ethidium bromide or SYBR Green I (Biozym, Landgraaf, The Netherlands). Densities of the amplified vector (known amount in femtograms) and of the amplified cDNA (unknown amount) were analyzed using the Bio-1D digital imaging system version 6 (Vilber Lourmat, Marne La Vallée, France). The comparison of these densities enabled the subsequent calculation of amplified β-actin or cytokine cDNA in femtograms. Results are expressed as a ratio of quantified cytokine product (in femtograms) over β-actin product (in femtograms).
Data Processing and Statistics
The curvefitting option in the Biorad microplatemanager software was applied to calculate the cytokine concentrations in the supernatants. Statistical analysis was performed using the Student's t-test for matched pairs. Differences with a confidence level of 95% or higher were considered to be statistically significant (P < .05).
RESULTS
IL-12 and TNF-α Production Are More Sensitive for DEX Than IL-10 Production
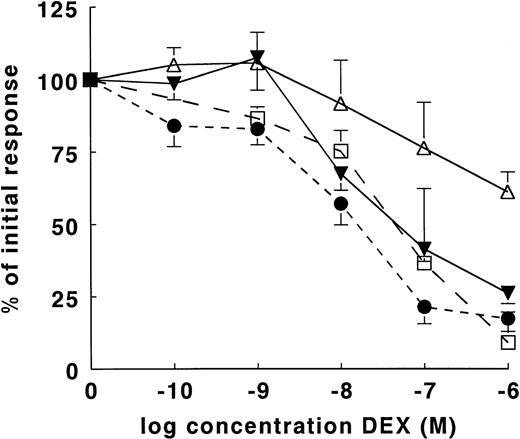
Because GC favor Th2 type of immune responses, we were interested in their effects on IL-10 and IL-12 as cytokines that play a pivotal role in the development of Th1 and Th2 cells. TNF-α was studied as a positive control for inhibition by GC. We used whole-blood cultures because these are more representative for in vivo conditions than cultures of PBMC and because the induction of both IL-10 and IL-12 by LPS is much more efficient in whole blood cultures.9 Figure1 shows the results obtained after 24 hours of stimulation with LPS for IL-10 and IL-12(p40) production (mean of 13 different donors each) and TNF-α production (mean of 9 donors). DEX turned out to have differential suppressive effects on these cytokines. DEX dose-dependently inhibited the LPS-induced IL-12(p40) production to 26% of the initial response at a concentration of 10−6mol/L DEX (P < .01). As shown in Table1, on average 9.1 × 10−8mol/L DEX was needed to achieve 50% inhibition of IL-12(p40). TNF-α was slightly more sensitive in that 50% inhibition was found with 2.6 × 10−8 mol/L DEX (Fig 1 and Table 1).
Dose-dependent effect of DEX on LPS-induced cytokine production in whole-blood cultures. IL-10, IL-12(p40), and TNF-α were induced in whole-blood cultures with 250 ng/mL LPS and in the presence of various concentrations DEX. For the induction of IL-12(p70), 1,000 U/mL IFN-γ was added to the cultures. Supernatants were obtained after 24 hours. The mean cytokine production is expressed as a percentage of the initial response in the absence of DEX. The results of IL-10 (▵, n = 13), IL-12(p40) (▾, n = 13), IL-12(p70) (□, n = 4), and TNF-α (•, n = 9) are expressed as the mean of the percentage (±SEM) of the initial cytokine response. The mean absolute values (±SD) in the absence of DEX for IL-12(p70), IL-12(p40), IL-10, and TNF-α were 127 ± 35 pg/mL, 623 ± 419 pg/mL, 411 ± 611 pg/mL, and 2,318 ± 1,499 pg/mL, respectively.
Dose-dependent effect of DEX on LPS-induced cytokine production in whole-blood cultures. IL-10, IL-12(p40), and TNF-α were induced in whole-blood cultures with 250 ng/mL LPS and in the presence of various concentrations DEX. For the induction of IL-12(p70), 1,000 U/mL IFN-γ was added to the cultures. Supernatants were obtained after 24 hours. The mean cytokine production is expressed as a percentage of the initial response in the absence of DEX. The results of IL-10 (▵, n = 13), IL-12(p40) (▾, n = 13), IL-12(p70) (□, n = 4), and TNF-α (•, n = 9) are expressed as the mean of the percentage (±SEM) of the initial cytokine response. The mean absolute values (±SD) in the absence of DEX for IL-12(p70), IL-12(p40), IL-10, and TNF-α were 127 ± 35 pg/mL, 623 ± 419 pg/mL, 411 ± 611 pg/mL, and 2,318 ± 1,499 pg/mL, respectively.
Relative Sensitivity of Cytokines to DEX
| Donor . | Sex . | TNF-α . | IL-12(p40) . | IL-10 . | |||
|---|---|---|---|---|---|---|---|
| ng/mL . | IC50-150 . | pg/mL . | IC50-150 . | pg/mL . | IC50-150 . | ||
| 4 | M | 1.53 | 0.00001-151 | 360 | 0.011 | 101 | 1.70-151 |
| 5 | M | 1.38 | 0.021 | 98 | 0.0042 | 172 | >100-151 |
| 6 | F | 2.76 | 0.0051 | 696 | 0.0071 | 274 | >100-151 |
| 7 | F | 2.95 | 0.0052 | 617 | 0.0061 | 2,498 | 0.0015 |
| 8 | F | 0.60 | 0.012 | 382 | 0.31 | 235 | >100-151 |
| 9 | M | 5.15 | 0.080 | 308 | 0.082 | 218 | >100-151 |
| 10 | M | 1.29 | 0.015 | 143 | 0.021 | 394 | 0.022 |
| 11 | F | 1.24 | 0.081 | 226 | 0.082 | 382 | 0.33 |
| 12 | F | 3.95 | 0.020 | 772 | 0.033 | 343 | 0.20 |
| 13 | M | ND | ND | 945 | 0.021 | 106 | 4.0-151 |
| 14 | M | ND | ND | 1,321 | 0.12 | 101 | 1.0 |
| 15 | M | ND | ND | 1,498 | 0.063 | 155 | >100-151 |
| 16 | F | ND | ND | 734 | 0.43 | 370 | >100-151 |
| Mean IC50 ± SEM | 0.026 ± 0.010 | 0.091 ± 0.036 | 1.036 ± 0.546-152 | ||||
| Donor . | Sex . | TNF-α . | IL-12(p40) . | IL-10 . | |||
|---|---|---|---|---|---|---|---|
| ng/mL . | IC50-150 . | pg/mL . | IC50-150 . | pg/mL . | IC50-150 . | ||
| 4 | M | 1.53 | 0.00001-151 | 360 | 0.011 | 101 | 1.70-151 |
| 5 | M | 1.38 | 0.021 | 98 | 0.0042 | 172 | >100-151 |
| 6 | F | 2.76 | 0.0051 | 696 | 0.0071 | 274 | >100-151 |
| 7 | F | 2.95 | 0.0052 | 617 | 0.0061 | 2,498 | 0.0015 |
| 8 | F | 0.60 | 0.012 | 382 | 0.31 | 235 | >100-151 |
| 9 | M | 5.15 | 0.080 | 308 | 0.082 | 218 | >100-151 |
| 10 | M | 1.29 | 0.015 | 143 | 0.021 | 394 | 0.022 |
| 11 | F | 1.24 | 0.081 | 226 | 0.082 | 382 | 0.33 |
| 12 | F | 3.95 | 0.020 | 772 | 0.033 | 343 | 0.20 |
| 13 | M | ND | ND | 945 | 0.021 | 106 | 4.0-151 |
| 14 | M | ND | ND | 1,321 | 0.12 | 101 | 1.0 |
| 15 | M | ND | ND | 1,498 | 0.063 | 155 | >100-151 |
| 16 | F | ND | ND | 734 | 0.43 | 370 | >100-151 |
| Mean IC50 ± SEM | 0.026 ± 0.010 | 0.091 ± 0.036 | 1.036 ± 0.546-152 | ||||
Cytokine production in whole-blood cultures was induced with 250 ng/mL LPS. The supernatants were obtained after 24 hours of culture and the cytokines determined by ELISA.
Abbreviations: ND, not done; M, male; F, female.
DEX concentration (μmol/L) required for 50% inhibition of cytokine production.
Results obtained by extrapolation.
N = 7, excluding values >100.
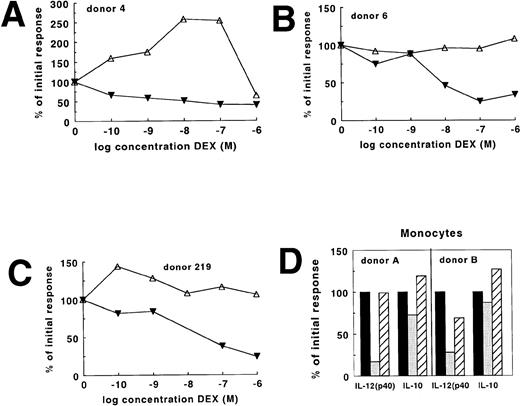
In contrast, IL-10 production was relatively insensitive to DEX: a significant (P < .01) inhibition of IL-10 production to 61% of the initial response was only found with 10−6mol/L DEX. As shown in Fig 2A-C for three individual donors, DEX might not have an effect at all or even stimulate IL-10 production. In 7 of 13 donors it was possible to estimate (partly by extrapolation) that 1.04 × 10−6mol/L DEX would be needed to achieve 50% inhibition of IL-10 production in these donors (Table 1). In 3 donors we found no inhibition at all, whereas in 3 additional donors the inhibition by 10−6 mol/L DEX did not exceed 25%, which made extrapolation impossible. For these donors an IC50 value >100 is given (Table 1). Similar differences in DEX sensitivity between IL-10 and the other cytokines were found after 48 hours of culture (data not shown). Since in several donors IL-10 production was insensitive to DEX, we performed additional experiments to assess if endogenous cortisol determined the sensitivity to DEX in vitro. High endogenous cortisol did not correlate with less suppression of IL-10 or of IL-12(p40) by DEX in vitro (N = 16, data not shown).
Differential regulation of IL-12(p40) and IL-10 in individual donors and monocytes. IL-12(p40) (▾) and IL-10 (▵) were induced in whole-blood cultures with 250 ng/mL LPS and in the presence of various concentrations DEX. The results of three individual donors are shown (A, B, and C). Monocytes stained with PE-conjugated anti-CD14 antibodies were sorted with a flow cytometer to a purity of more than 98% and cultured as described in Materials and Methods (D). The results obtained with two individual donors are shown. Cells were stimulated with LPS in the absence (▪) or presence (□) of 10−6 mol/L DEX or in the presence of 10−6mol/L DEX and 10−6 mol/L RU486 (▨). The results are expressed as a percentage of the initial response with LPS.
Differential regulation of IL-12(p40) and IL-10 in individual donors and monocytes. IL-12(p40) (▾) and IL-10 (▵) were induced in whole-blood cultures with 250 ng/mL LPS and in the presence of various concentrations DEX. The results of three individual donors are shown (A, B, and C). Monocytes stained with PE-conjugated anti-CD14 antibodies were sorted with a flow cytometer to a purity of more than 98% and cultured as described in Materials and Methods (D). The results obtained with two individual donors are shown. Cells were stimulated with LPS in the absence (▪) or presence (□) of 10−6 mol/L DEX or in the presence of 10−6mol/L DEX and 10−6 mol/L RU486 (▨). The results are expressed as a percentage of the initial response with LPS.
In two subsequent experiments we studied B cells, T cells, and monocytes that were positively selected by flow cytometry using FITC-conjugated anti-CD19, PE-conjugated anti-CD14, or FITC-conjugated anti-CD3. The obtained cell populations were more than 98% pure. When stimulated with LPS for 24 hours, production of IL-10 and IL-12(p40) was only detectable in monocyte cultures. These cytokines were not detected in LPS-stimulated cultures of T cells or B cells (data not shown), which is in agreement with recent observations by Guery et al,25 who showed that normal B cells are not capable of IL-12 production. As shown in Fig 2D, IL-12(p40) production by monocytes as opposed to IL-10 production was sensitive to DEX and this effect was antagonized by 1 μmol/L RU486. Effects of this glucocorticoid receptor antagonist will be discussed in more detail below. For the detection of the functional IL-12(p70) protein in our whole-blood culture system it was necessary to add exogenous IFN-γ, which upregulates IL-12(p35) mRNA.26 The mean of results (obtained with whole blood from four different donors) of the effect of DEX on this protein are shown in Fig 1. On average, 50% inhibition was found with 6.4 × 10−8 mol/L DEX. As will be pointed out below it cannot be excluded that IFN-γ altered the sensitivity to DEX. However, additional studies performed in seven donors showed that in the presence of exogenous IFN-γ, 10−6 mol/L DEX caused on average 80% inhibition of IL-12(p70) and on average 25% inhibition of IL-10 (data not shown).
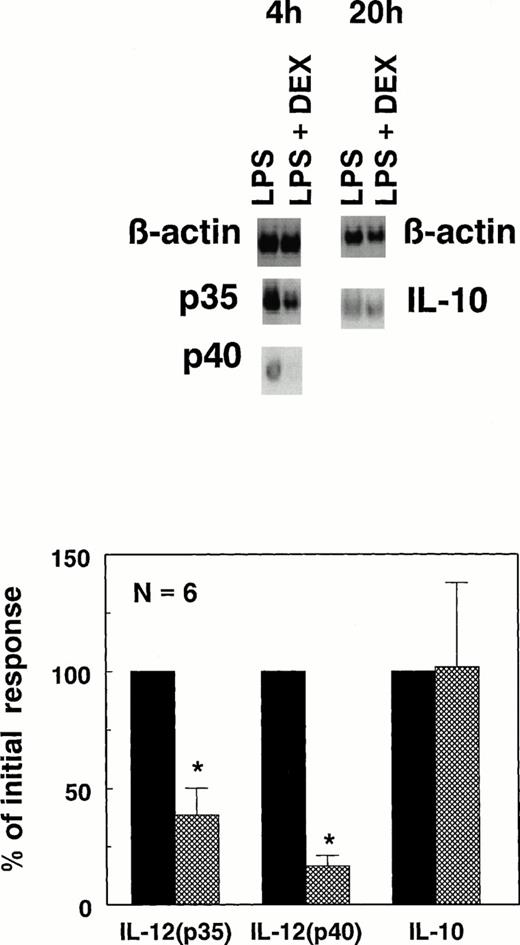
To obtain more insight into the effects of DEX in the absence of IFN-γ, we studied the expression of the p40 and p35 subunits at the mRNA level in whole-blood cultures, using a semi-quantitative PCR. In unstimulated whole blood IL-12(p40) mRNA was below the detection limit whereas a more than 1,000-fold upregulation was found in response to LPS; in contrast, a constitutive expression of IL-12(p35) mRNA was observed which was enhanced threefold in response to LPS (data not shown). As illustrated in Fig 3 for six different donors, DEX consistently suppressed (P < .05) the expression of both IL-12(p40) and IL-12(p35) mRNA. As was already found at the protein level DEX had variable effects on IL-10 mRNA, ranging from inhibition to stimulation, but on average no inhibition was found.
Sensitivity of IL-12(p40), IL-12(p35) mRNA, and IL-10 mRNA to DEX. mRNA was isolated from whole-blood cultures stimulated with 250 ng/mL LPS with (▩) or without (▪) 10−6 mol/L DEX and used to perform semiquantitative RT-PCR assays for IL-12(p40), IL-12(p35), and IL-10 as described in Materials and Methods. mRNA for IL-12(p40) and IL-12(p35) were measured after 4 hours of culture, whereas IL-10 mRNA was measured after 20 hours of culture; these time points were previously established to be optimal for the expression of these particular mRNAs. Results shown are density scans of one typical donor (top) as well as the mean ± SEM of six different healthy donors, expressed as a percentage of the mRNA expression in the absence of DEX (bottom). *P < .05.
Sensitivity of IL-12(p40), IL-12(p35) mRNA, and IL-10 mRNA to DEX. mRNA was isolated from whole-blood cultures stimulated with 250 ng/mL LPS with (▩) or without (▪) 10−6 mol/L DEX and used to perform semiquantitative RT-PCR assays for IL-12(p40), IL-12(p35), and IL-10 as described in Materials and Methods. mRNA for IL-12(p40) and IL-12(p35) were measured after 4 hours of culture, whereas IL-10 mRNA was measured after 20 hours of culture; these time points were previously established to be optimal for the expression of these particular mRNAs. Results shown are density scans of one typical donor (top) as well as the mean ± SEM of six different healthy donors, expressed as a percentage of the mRNA expression in the absence of DEX (bottom). *P < .05.
On the basis of the donors in which inhibition of IL-10 by DEX could be detected (Table 1), we conclude that—as far as DEX has suppressive effects—IL-12 is at least 12-fold more sensitive than IL-10. As will be discussed below, lack of inhibition in the other donors and the potential to even stimulate IL-10 production further support the hypothesis that GC may favor Th2 type of response by differential effects on IL-10 and IL-12.
Suppression of IL-12 by GC Is Mainly Mediated Via GC Receptors
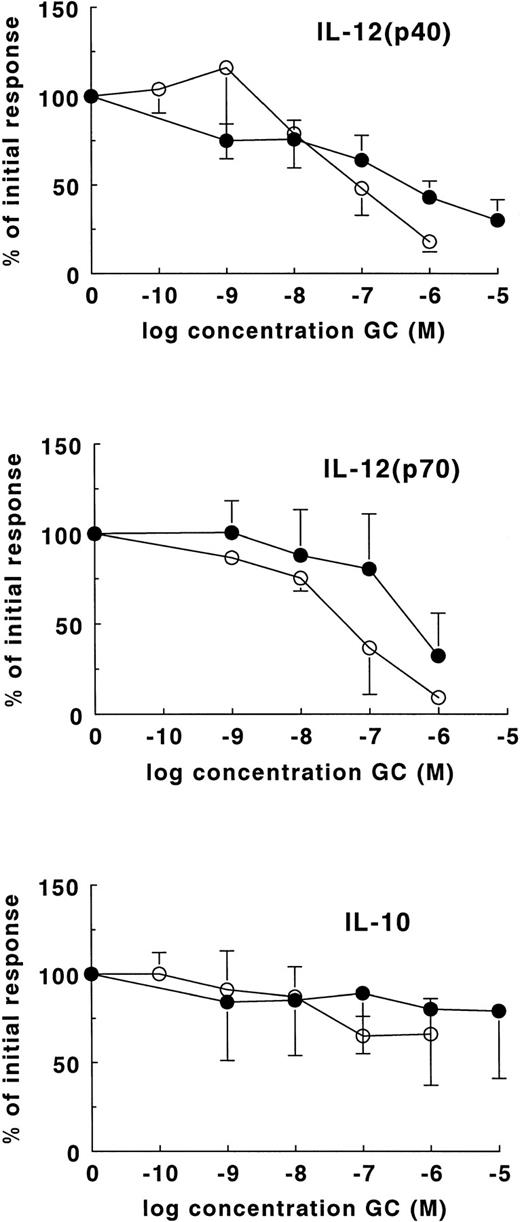
The differences in DEX sensitivity of IL-12 and IL-10 suggested that different receptors may be involved in the regulation of these cytokines. It is known that DEX binds with high affinity to the GC receptor (GR) and with low affinity to the mineralocorticoid receptor (MR).27-29 Because the reverse is true for the physiologic glucocorticoid HC,29 we first compared the efficacy of DEX and HC. As shown in Fig 4 (lower panel) both HC and DEX were relatively ineffective in the inhibition of IL-10. As far as the suppression of IL-12(p40) and IL-12(p70) is concerned, HC was less effective than DEX. On average, a fivefold higher HC concentration was needed for 50% inhibition of IL-12(p40) (Fig 4, upper panel) whereas an eightfold higher concentration was needed for the inhibition of IL-12(p70) (Fig 4, middle panel). These results are in line with the fact that HC has an eightfold lower affinity for the GR as compared with DEX.
Sensitivity of cytokines to DEX and HC. Cytokines were induced in whole-blood cultures with 250 ng/mL LPS in the presence of DEX (○) or HC (•). For the induction of IL-12(p70), 1,000 U/mL IFN-γ was added to the cultures. The data are expressed as a percentage ± SEM of the cytokine production in the absence of GC. The results are the means of the production of IL-12(p40) and IL-10 in whole-blood cultures of 13 different healthy donors. IL-12(p70) production is the mean of the results obtained with four different healthy donors. Cytokines were determined by ELISA in supernatants harvested after 24 hours of culture.
Sensitivity of cytokines to DEX and HC. Cytokines were induced in whole-blood cultures with 250 ng/mL LPS in the presence of DEX (○) or HC (•). For the induction of IL-12(p70), 1,000 U/mL IFN-γ was added to the cultures. The data are expressed as a percentage ± SEM of the cytokine production in the absence of GC. The results are the means of the production of IL-12(p40) and IL-10 in whole-blood cultures of 13 different healthy donors. IL-12(p70) production is the mean of the results obtained with four different healthy donors. Cytokines were determined by ELISA in supernatants harvested after 24 hours of culture.
The role of the GR in the modulation of cytokines by DEX and HC was further addressed by using RU486 as an antagonist. Addition of 1 μmol/L of RU486 antagonized the suppressive actions of both DEX and HC on LPS-induced IL-12(p40), although this antagonizing effect was not complete (Fig 5, upper panel). A concentration of 50 μmol/L RU486, which increased the IL-12(p40) production more than twofold (P < .05), completely abrogated the inhibitory effect of DEX, but not of HC (Fig 5, upper panel). This appeared to be significant on the basis of results obtained in eight different donors (P < .05). Because HC has an eightfold lower affinity for the GR than DEX we had expected a complete antagonizing effect of RU486 on HC suppression. Because this appears not to be the case, HC may also mediate suppressive effects via the MR. Therefore, we studied the effect of the MR agonist aldosterone. The addition of 10−8 mol/L aldosterone caused 17% suppression of IL-12(p40) (n = 15, P < .05; data not shown). This shows that occupation of the MR may indeed contribute to inhibition of IL-12(p40). However, because the MR antagonist spironolactone did not antagonize suppression of IL-12(p40) by HC (data not shown), part of its effect might be mediated by a mechanism different from the GR and MR. RU486 enhanced IL-12(p70) production, probably by antagonizing the inhibitory effects of endogenous cortisol.
The GR antagonist RU486 stimulates IL-12 and inhibits IL-10. Cytokines were induced in whole-blood cultures with 250 ng/mL LPS [for induction of IL-12(p70), 1,000 U/mL IFN-γ was added to the cultures] in the absence (▪) or presence of 10−6 mol/L DEX (▨) or 10−6 mol/L HC (▧). The influence of these GC on IL-12(p40) (upper panel), IL-12(p70) (middle panel), and IL-10 (lower panel) was studied in the absence or presence of 1 or 50 μmol/L RU486. The means of the results ± SEM obtained with eight different donors are shown. IL-12(p70) production is the mean of the results ± SEM obtained with four different healthy donors. The data are expressed as a percentage of the cytokine production in the absence of GC or antagonist. Cytokines were determined by ELISA in supernatants obtained after 24 hours of culture. ND, not done.
The GR antagonist RU486 stimulates IL-12 and inhibits IL-10. Cytokines were induced in whole-blood cultures with 250 ng/mL LPS [for induction of IL-12(p70), 1,000 U/mL IFN-γ was added to the cultures] in the absence (▪) or presence of 10−6 mol/L DEX (▨) or 10−6 mol/L HC (▧). The influence of these GC on IL-12(p40) (upper panel), IL-12(p70) (middle panel), and IL-10 (lower panel) was studied in the absence or presence of 1 or 50 μmol/L RU486. The means of the results ± SEM obtained with eight different donors are shown. IL-12(p70) production is the mean of the results ± SEM obtained with four different healthy donors. The data are expressed as a percentage of the cytokine production in the absence of GC or antagonist. Cytokines were determined by ELISA in supernatants obtained after 24 hours of culture. ND, not done.
Stimulation of IL-10 by GC
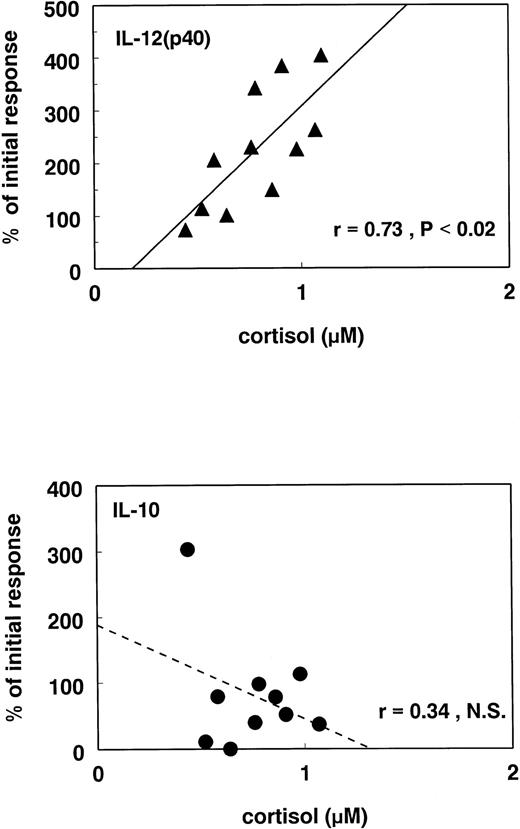
Whereas RU486 significantly enhanced the IL-12 production and antagonized the suppression by GC, different effects were observed with regard to IL-10. Although the slight inhibitory effect of high GC concentrations was antagonized by 1 μmol/L RU486, 50 μmol/L RU486 surprisingly caused a significant inhibition (P < .05) of IL-10 production (Fig 5, lower panel), suggesting that complete inactivation of the GR was inhibitory for this cytokine. Because RU486 has been found to act as an agonist on the progesteron receptor when cyclic adenosine monophosphate (cAMP) levels are increased,31 we first performed additional studies to establish whether the effects of RU486 could be mimicked by equimolar concentrations of progesterone. However, although we could show in whole-blood cultures of four different donors that RU486 stimulated IL-12(p40) and inhibited IL-10, we observed that 50 μmol/L progesterone did not have an effect in these cultures (data not shown). This suggested that the stimulatory effects of high concentrations of RU486 on IL-12, but also the inhibition of IL-10, are caused by inhibition of endogenous cortisol. Indeed, additional experiments with 11 individual donors showed a positive correlation between the stimulatory effects of RU486 on IL-12(p40) production and cortisol levels in serum, whereas for IL-10 such a correlation was not found (Fig 6).
Correlation between antagonizing effects of RU486 and endogenous cortisol. The levels of cortisol were measured in the serum of 11 healthy donors. In these donors whole-blood cultures were performed as described in Fig 1. The effect of 50 μmol/L RU486 was studied on IL-10 and IL-12(p40) production. The level of cortisol in the serum of each donor is plotted against the effect of RU486 on the IL-10 (bottom) and IL-12(p40) (top) production. The effect of RU486 is expressed as the percentage of the initial response with LPS in each donor. Each symbol represents one individual donor. The blood was collected between 8 and 10 am and put into culture immediately after collection.
Correlation between antagonizing effects of RU486 and endogenous cortisol. The levels of cortisol were measured in the serum of 11 healthy donors. In these donors whole-blood cultures were performed as described in Fig 1. The effect of 50 μmol/L RU486 was studied on IL-10 and IL-12(p40) production. The level of cortisol in the serum of each donor is plotted against the effect of RU486 on the IL-10 (bottom) and IL-12(p40) (top) production. The effect of RU486 is expressed as the percentage of the initial response with LPS in each donor. Each symbol represents one individual donor. The blood was collected between 8 and 10 am and put into culture immediately after collection.
IL-10 Is Not an Intermediate in DEX-Mediated Suppression of IL-12 and TNF-α
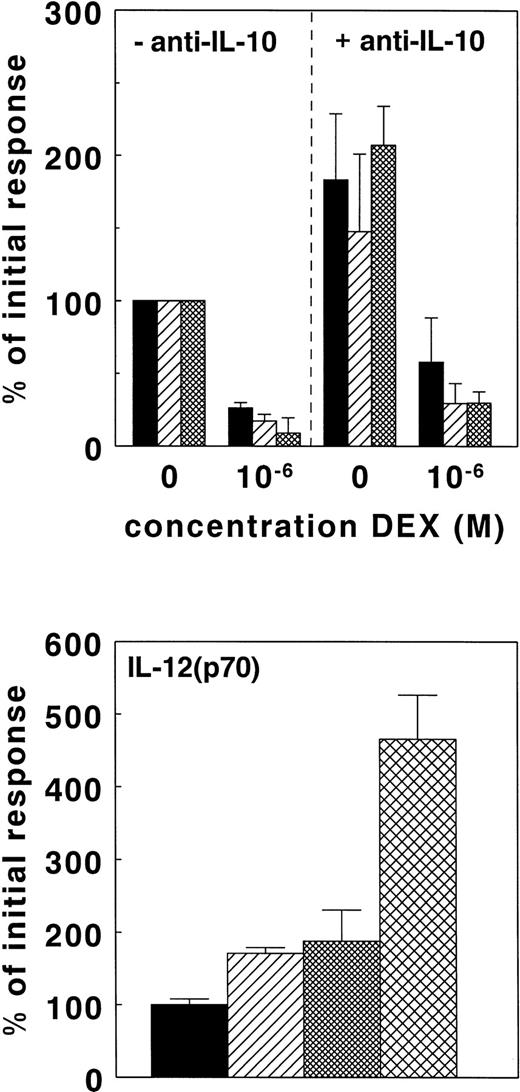
It is well-established that IL-10 can suppress IL-12(p40), IL-12(p70), and TNF-α production.8,9 32 In view of the potential of GC to stimulate IL-10, we investigated whether IL-10 acted as an intermediate in the suppression of IL-12(p40), IL-12(p70), and TNF-α by DEX. As shown in Fig 7 (top), the addition of 5 μg/mL anti–IL-10 to the whole-blood cultures significantly enhanced the LPS-induced IL-12(p40), IL-12(p70), and TNF-α production (P < .05). Using the increased cytokine levels as reference values, 1 μmol/L DEX caused 80% inhibition of TNF-α, 86% inhibition of IL-12(p70), and 68% inhibition of IL-12(p40) in the presence of anti–IL-10. These values did not differ from the extent of inhibition by DEX found in the absence of anti–IL-10. Thus, in the presence of anti–IL-10, DEX was still able to suppress IL-12(p40), IL-12(p70), and TNF-α production. Likewise, we established whether RU486 would stimulate IL-12 under conditions where IL-10 was already neutralized. As shown in Fig 7 (bottom), RU486 enhanced IL-12(p70) production even in the presence of anti–IL-10. Moreover, because RU486 and anti–IL-10 synergistically enhanced the IL-12(p70) it is likely that IL-10 and cortisol suppress IL-12 by separate mechanisms.
IL-10 is not an intermediate in the suppression of IL-12 by GC. Cytokines were induced in whole-blood cultures as described in the legend of Fig 1. Cultures were performed in the absence or presence of 5 μg/mL anti–IL-10 with or without 10−6 mol/L DEX (top panel). Results are expressed as a percentage ± SEM of the cytokine production found in the presence of LPS and in the absence of anti–IL-10. The results are the means of the IL-12(p40) (▪) and TNF-α (▨) production obtained with nine different healthy donors; IL-12(p70) production (▩) is based on results obtained with four different healthy donors. In additional experiments, the effect of 50 μmol/L RU486 (▨), 5 μg/mL anti-IL-10 (▩), or both () on LPS– and IFN-γ–induced IL-12(p70) was studied in nine separate donors (bottom panel). The results are expressed as a percentage ± SEM of the cytokine production in the presence of LPS and IFN-γ alone (▪).
IL-10 is not an intermediate in the suppression of IL-12 by GC. Cytokines were induced in whole-blood cultures as described in the legend of Fig 1. Cultures were performed in the absence or presence of 5 μg/mL anti–IL-10 with or without 10−6 mol/L DEX (top panel). Results are expressed as a percentage ± SEM of the cytokine production found in the presence of LPS and in the absence of anti–IL-10. The results are the means of the IL-12(p40) (▪) and TNF-α (▨) production obtained with nine different healthy donors; IL-12(p70) production (▩) is based on results obtained with four different healthy donors. In additional experiments, the effect of 50 μmol/L RU486 (▨), 5 μg/mL anti-IL-10 (▩), or both () on LPS– and IFN-γ–induced IL-12(p70) was studied in nine separate donors (bottom panel). The results are expressed as a percentage ± SEM of the cytokine production in the presence of LPS and IFN-γ alone (▪).
These results indicate that GC may suppress IL-12 by two complementary mechanisms: direct inhibition and possibly by upregulation of IL-10.
DISCUSSION
GC are generally regarded as immunosuppressive and are therefore widely used for clinical applications ranging from preventing rejection in transplantation to the treatment of allergic asthma. Recent studies have pointed to the possibility that GC may have a selective effect in immune regulation by suppressing IFN-γ production and promoting IL-4 production by CD4+ T cells.12 15 Our data are in support of this hypothesis because they show—on the basis of whole-blood cultures stimulated with LPS—that IL-12 is 10 to 100 times more sensitive to suppression by GC than IL-10, dependent on the presence of IFN-γ.
A comparison between DEX and HC showed that the relative suppressive effects of these GC were consistent with the fact that DEX has a eightfold higher affinity for the GR than HC.27-29Antagonism of the suppressive effects by the GR antagonist RU486 and not by the MR antagonist spironolactone support the conclusion that suppression was largely mediated by the GR. However, because aldosterone showed a modest suppression of IL-12(p40), a minor contribution of MR to the effects of HC cannot be excluded completely. The addition of IFN-γ to the cultures—as a condition required for detectable IL-12(p70) induction in our system—may have affected the sensitivity of this cytokine to GC. As has been shown previously, IL-4 and IL-2 decrease the sensitivity to GC by decreasing the affinity of the GR30; in this system the effect of IL-4 and IL-2 was abolished by IFN-γ. Therefore, IFN-γ may have affected the sensitivity of IL-12 for GC by increasing the affinity of the GR. However, in the absence of exogenous IFN-γ—as illustrated with the use of a semi-quantitative reverse transcriptase-PCR—DEX also suppressed the LPS-induced expression of IL-12(p40) and IL-12(p35) mRNA but had on average no effect on the expression of IL-10 mRNA. T cells may express IL-12(p35) mRNA in the absence of IL-12(p40) mRNA, consistent with the inability of these cells to secrete bioactive IL-12.23 26 Although our data show that expression of mRNA for both subunits can be suppressed by DEX, this does not prove that both mRNAs are equally suppressed in one and the same cell type. Additional studies using isolated monocytes are needed to establish whether suppression of the bioactive IL-12 by DEX is accounted for by an effect on one of the subunits in particular or on both.
With regard to IL-10 we observed in various donors stimulation by GC rather than an inhibitory effect. That this was not a consistent finding in all donors may be due to the fact that in most of the donors occupancy of the GR has already occurred by endogenous cortisol and, consequently, that positive regulation of IL-10 has already been achieved; such a condition will probably be present in the majority of the donors because the whole-blood cultures were performed in the presence of autologous plasma. Preliminary data indicate that stimulation of IL-10 by low concentrations of cortisol is found more frequently using cultures of isolated PBMC. A stimulatory role for GC was in particular evident from the fact that the GR antagonist RU486 inhibited IL-10. This appeared not to be a nonspecific effect because IL-12(p40) and IL-12(p70) were stimulated. Because RU486 may act as an agonist for the progesterone receptor,31 we ruled out that progesterone had inhibitory effects on IL-10 or stimulatory effects on IL-12 (data not shown).
Additional experiments showed that the stimulatory effects of RU486 on IL-12(p40) production correlated with endogenous cortisol, suggesting that this effect of RU486 can be explained by blocking the effects of endogenous cortisol. The fact that we made this observation despite the absence of a direct negative correlation between the IL-12(p40) production capacity and cortisol (data not shown) may be explained by assuming that only part of the hormone is not bound to cortisol-binding protein and available for suppression. In the case of IL-10 any correlation may be difficult to find if occupancy of the GR with low levels of cortisol would lead to stimulation and high concentrations of cortisol with slight suppression of this cytokine.
Our observations are in line with studies showing that hypercortisolemia results in increased plasma IL-10 concentrations in vivo.33,34 However, the mechanism by which these effects may occur are so far unknown. The presence of a GRE in the IL-10 promoter35 points to a potential mechanism of GC in the stimulation of IL-10, although it is unknown thus far whether this GRE is functional.
Apart from having stimulatory effects, high concentrations of GC were inhibitory for IL-10, which has also been shown for DEX on IL-10 production by PBMC and monocytes.36 Mechanisms to be taken into account in these effects are interference at the level of transcription factors. Because IL-12(p40) production is regulated by NF-kB37 this transcription factor may be one of the main targets of GC, for instance via the induction of IkB.38,39The relative resistance of IL-10 to GC would be in agreement with the absence of NF-kB binding sites in the promoter region of the IL-10 gene.35 However, because binding sites for AP-1 and cAMP responsive element binding protein (CREB) are present in the promoter region of IL-10,35 interference with these factors might be one of the mechanisms of IL-10 suppression at pharmacological concentrations of GC. That we did not always observe suppressive effects on IL-10 may be due to the lack of induction of such transcription factors in individual donors. Interestingly, suppression of IL-10 by high concentrations of GC appeared to correlate with the efficiency of LPS to induce IL-10 (data not shown).
Taken together our results indicate that physiological concentrations of GC inhibit IL-12, but do not affect or even stimulate IL-10. The overall outcome of increased levels of GC in vivo may thus be that antigen-presenting cells are modulated to display a functional phenotype that favors the development of a Th2 response. Indeed, recently GC have been found to modulate adherent cells in such a way that they promote Th2 development.40 This bias toward Th2 may be amplified by the direct effects of GC on T cells.11-13,15 The effects of GC on the level of antigen-presenting cells and the development of Th cells might explain why during pregnancy and diseases which are accompanied by excessive release of GC the cellular immunity is suppressed and the humoral immunity is enhanced.41 42 Therefore, GR agonists and antagonists might be of use in selective modulation of Th activity.
ACKNOWLEDGMENT
We are grateful to Dr Kees Lucas for critically reviewing the manuscript and to Bep Blauw and Ellen Siemssen for technical assistance. The assistance of Guus Westra (Department of Hematology, Free University Academical Hospital, Amsterdam, The Netherlands) with the FACS-sorting is highly appreciated. Furthermore, we thank Dr Eef Lentjes (Department of Clinical Chemistry, University Hospital Leiden, Leiden, The Netherlands) for performing the cortisol measurements. We are also grateful to Prof G. Trinchieri (Wistar Institute, Philadelphia, PA) for providing us with the hybridomas C11.79 and C8.6.
Address reprint requests to Lex Nagelkerken, PhD, Division of Immunological and Infectious Diseases, TNO Prevention and Health, PO Box 2215, 2301 CE, Leiden, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 5. The GR antagonist RU486 stimulates IL-12 and inhibits IL-10. Cytokines were induced in whole-blood cultures with 250 ng/mL LPS [for induction of IL-12(p70), 1,000 U/mL IFN-γ was added to the cultures] in the absence (▪) or presence of 10−6 mol/L DEX (▨) or 10−6 mol/L HC (▧). The influence of these GC on IL-12(p40) (upper panel), IL-12(p70) (middle panel), and IL-10 (lower panel) was studied in the absence or presence of 1 or 50 μmol/L RU486. The means of the results ± SEM obtained with eight different donors are shown. IL-12(p70) production is the mean of the results ± SEM obtained with four different healthy donors. The data are expressed as a percentage of the cytokine production in the absence of GC or antagonist. Cytokines were determined by ELISA in supernatants obtained after 24 hours of culture. ND, not done.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/11/10.1182_blood.v91.11.4255/4/m_blod41103005x.jpeg?Expires=1769080741&Signature=RddSpeMTkhmpQF-bF5L~ro3y77ymt6tWYKodf434bO0DkGuJKMESa87~sLi~Yxi8L9XechugvwykI0UX9LyGxEECMneG3kCJmPtEkuspjG4sZd~WwvlptDWBjkMRwRoJ-Iu58WKiZ4F3WL3ETFcD41jekoJvR4LC1GV4~R1q7ek0bZUumgjfju~F1i9VFL0WaHQmdYdh8znCZQV~0OyS7BLGJ-g5CGXlTvVxyh1gILTOqxzJl-ktGKrhhV-BDmcTTY3js8QkgAGfRJ84tlVnrocsXD7r~RDmy5TSujPtmg9YhX7y5l0sensYpPfAQqrRcMO8U9HBhligNGEaJfmhUQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal